Double-Negative T (DNT) Cells in Patients with Systemic Lupus Erythematosus
Abstract
1. Introduction
2. DNT Cells and Lupus in Mice
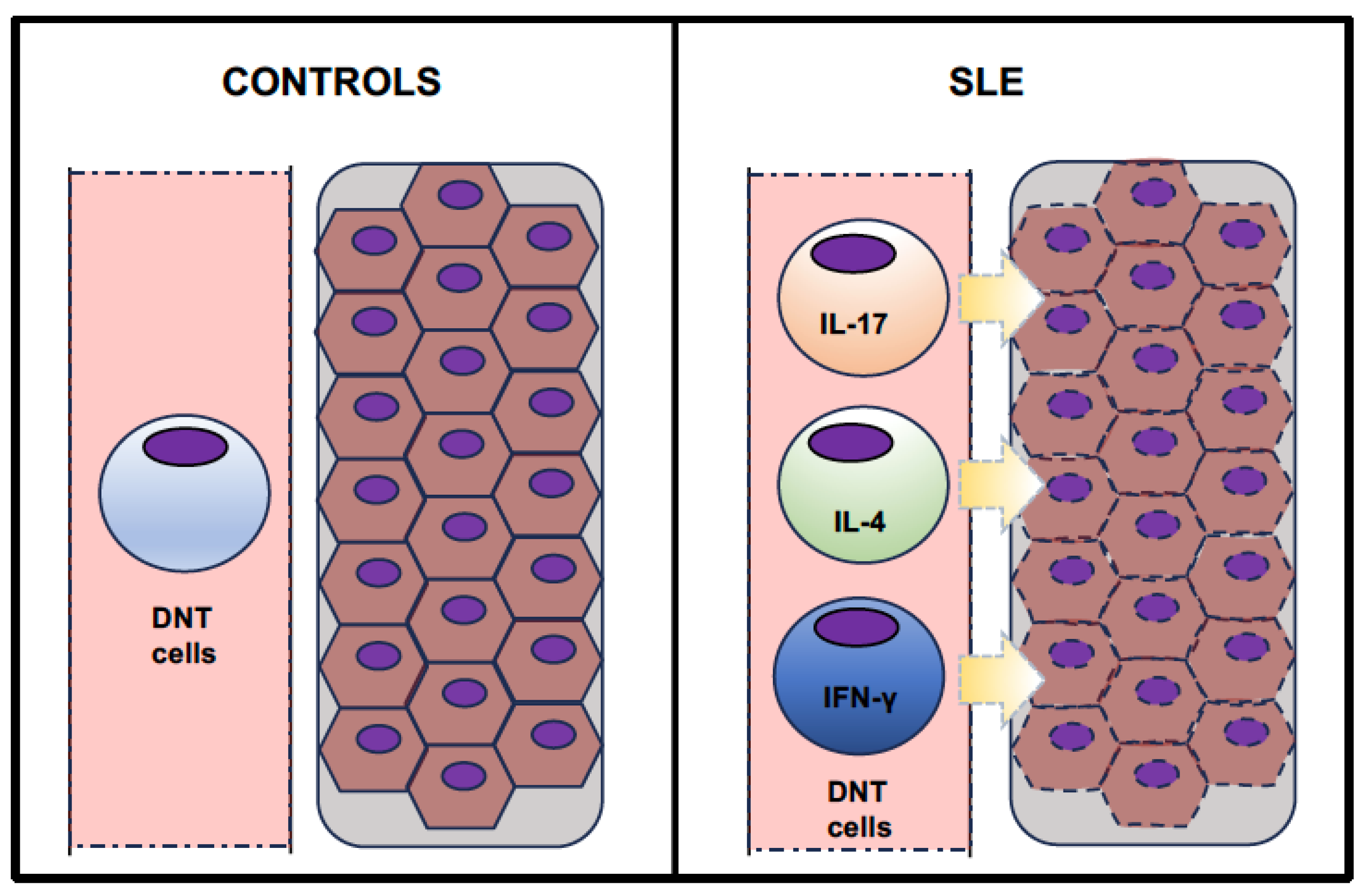
3. DNT Cells and Systemic Lupus Erythematosus
4. Knowledge Gaps and Perspectives on Human DNT Cells
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Velikkakam, T.; Gollob, K.J.; Dutra, W.O. Double-Negative T Cells: Setting the Stage for Disease Control or Progression. Immunology 2022, 165, 371–385. [Google Scholar] [CrossRef]
- Li, H.; Boulougoura, A.; Endo, Y.; Tsokos, G.C. Abnormalities of T cells in systemic lupus erythematosus: New insights in pathogenesis and therapeutic strategies. J. Autoimmun. 2022, 132, 102870. [Google Scholar] [CrossRef]
- Crispín, J.C.; Tsokos, G.C. Human TCR-alpha beta+CD4−CD8− T cells can derive from CD8+ T cells and display an inflammatory effector phenotype. J. Immunol. 2009, 183, 4675–4681. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zheng, Y.; Sheng, J.; Han, Y.; Yang, Y.; Pan, H.; Yao, J. CD3+CD4−CD8− (Double-Negative) T Cells in Inflammation, Immune Disorders and Cancer. Front. Immunol. 2022, 13, 816005. [Google Scholar] [CrossRef] [PubMed]
- Lambert, M.P. Presentation and Diagnosis of Autoimmune Lymphoproliferative Syndrome (ALPS). Expert Rev. Clin. Immunol. 2021, 17, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.B.; Bleesing, J.J.; Dianzani, U.; Fleisher, T.A.; Jaffe, E.S.; Lenardo, M.J.; Rieux-Laucat, F.; Siegel, R.M.; Su, H.C.; Teachey, D.T.; et al. Revised Diagnostic Criteria and Classification for the Autoimmune Lymphoproliferative Syndrome (ALPS): Report from the 2009 NIH International Workshop. Blood 2010, 116, e35–e40. [Google Scholar] [CrossRef]
- Bride, K.; Teachey, D. Autoimmune Lymphoproliferative Syndrome: More than a FAScinating Disease. F1000Research 2017, 6, 1928. [Google Scholar] [CrossRef]
- Teachey, D.T.; Seif, A.E.; Grupp, S.A. Advances in the Management and Understanding of Autoimmune Lymphoproliferative Syndrome (ALPS). Br. J. Haematol. 2010, 148, 205–216. [Google Scholar] [CrossRef]
- Morand, E.F.; Fernandez-Ruiz, R.; Blazer, A.; Niewold, T.B. Advances in the Management of Systemic Lupus Erythematosus. BMJ 2023, 383, e073980. [Google Scholar] [CrossRef]
- Fanouriakis, A.; Tziolos, N.; Bertsias, G.; Boumpas, D.T. Update on the Diagnosis and Management of Systemic Lupus Erythematosus. Ann. Rheum. Dis. 2021, 80, 14–25. [Google Scholar] [CrossRef]
- Omarjee, O.; Picard, C.; Frachette, C.; Moreews, M.; Rieux-Laucat, F.; Soulas-Sprauel, P.; Viel, S.; Lega, J.-C.; Bader-Meunier, B.; Walzer, T.; et al. Monogenic Lupus: Dissecting Heterogeneity. Autoimmun. Rev. 2019, 18, 102361. [Google Scholar] [CrossRef] [PubMed]
- Abdolmaleki, F.; Farahani, N.; Gheibi Hayat, S.M.; Pirro, M.; Bianconi, V.; Barreto, G.E.; Sahebkar, A. The Role of Efferocytosis in Autoimmune Diseases. Front. Immunol. 2018, 9, 1645. [Google Scholar] [CrossRef] [PubMed]
- Crow, M.K. Pathogenesis of Systemic Lupus Erythematosus: Risks, Mechanisms and Therapeutic Targets. Ann. Rheum. Dis. 2023, 82, 999–1014. [Google Scholar] [CrossRef] [PubMed]
- Belot, A.; Cimaz, R. Monogenic Forms of Systemic Lupus Erythematosus: New Insights into SLE Pathogenesis. Pediatr. Rheumatol. Online J. 2012, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Dossybayeva, K.; Abdukhakimova, D.; Poddighe, D. Basophils and Systemic Lupus Erythematosus in Murine Models and Human Patients. Biology 2020, 9, 308. [Google Scholar] [CrossRef] [PubMed]
- Herrada, A.A.; Escobedo, N.; Iruretagoyena, M.; Valenzuela, R.A.; Burgos, P.I.; Cuitino, L.; Llanos, C. Innate Immune Cells’ Contribution to Systemic Lupus Erythematosus. Front. Immunol. 2019, 10, 772. [Google Scholar] [CrossRef]
- Gupta, S.; Kaplan, M.J. Bite of the Wolf: Innate Immune Responses Propagate Autoimmunity in Lupus. J. Clin. Investig. 2021, 131. [Google Scholar] [CrossRef] [PubMed]
- Perry, D.; Sang, A.; Yin, Y.; Zheng, Y.-Y.; Morel, L. Murine Models of Systemic Lupus Erythematosus. J. Biomed. Biotechnol. 2011, 2011, 271694. [Google Scholar] [CrossRef]
- Theofilopoulos, A.N.; Dixon, F.J. Etiopathogenesis of Murine SLE. Immunol. Rev. 1981, 55, 179–216. [Google Scholar] [CrossRef]
- Furukawa, F. Experimental Models of Lupus Erythematosus. In Cutaneous Lupus Erythematosus; Kuhn, A., Lehmann, P., Ruzicka, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 221–238. [Google Scholar] [CrossRef]
- Satoh, M.; Reeves, W.H. Induction of Lupus-Associated Autoantibodies in BALB/c Mice by Intraperitoneal Injection of Pristane. J. Exp. Med. 1994, 180, 2341–2346. [Google Scholar] [CrossRef]
- Brandt, D.; Hedrich, C.M. TCRαβ+CD3+CD4−CD8− (Double Negative) T Cells in Autoimmunity. Autoimmun. Rev. 2018, 17, 422–430. [Google Scholar] [CrossRef]
- Datta, S.K.; Patel, H.; Berry, D. Induction of a Cationic Shift in IgG Anti-DNA Autoantibodies. Role of T Helper Cells with Classical and Novel Phenotypes in Three Murine Models of Lupus Nephritis. J. Exp. Med. 1987, 165, 1252–1268. [Google Scholar] [CrossRef] [PubMed]
- Shivakumar, S.; Tsokos, G.C.; Datta, S.K. T Cell Receptor Alpha/Beta Expressing Double-Negative (CD4−/CD8−) and CD4+ T Helper Cells in Humans Augment the Production of Pathogenic Anti-DNA Autoantibodies Associated with Lupus Nephritis. J. Immunol. 1989, 143, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tsokos, G.C. Double-Negative T Cells in Autoimmune Diseases. Curr. Opin. Rheumatol. 2021, 33, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Amarilyo, G.; Lourenço, E.V.; Shi, F.-D.; La Cava, A. IL-17 Promotes Murine Lupus. J. Immunol. 2014, 193, 540–543. [Google Scholar] [CrossRef]
- Qiao, G.; Yang, L.; Li, Z.; Williams, J.W.; Zhang, J. A77 1726, the Active Metabolite of Leflunomide, Attenuates Lupus Nephritis by Promoting the Development of Regulatory T Cells and Inhibiting IL-17-Producing Double Negative T Cells. Clin. Immunol. 2015, 157, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Kyttaris, V.C.; Tsokos, G.C. The Role of IL-23/IL-17 Axis in Lupus Nephritis. J. Immunol. 2009, 183, 3160–3169. [Google Scholar] [CrossRef]
- Dai, H.; He, F.; Tsokos, G.C.; Kyttaris, V.C. IL-23 Limits the Production of IL-2 and Promotes Autoimmunity in Lupus. J. Immunol. 2017, 199, 903–910. [Google Scholar] [CrossRef]
- Mizui, M.; Koga, T.; Lieberman, L.A.; Beltran, J.; Yoshida, N.; Johnson, M.C.; Tisch, R.; Tsokos, G.C. IL-2 Protects Lupus-Prone Mice from Multiple End-Organ Damage by Limiting CD4−CD8− IL-17-Producing T Cells. J. Immunol. 2014, 193, 2168–2177. [Google Scholar] [CrossRef]
- Schmidt, T.; Paust, H.-J.; Krebs, C.F.; Turner, J.-E.; Kaffke, A.; Bennstein, S.B.; Koyro, T.; Peters, A.; Velden, J.; Hünemörder, S.; et al. Function of the Th17/Interleukin-17A Immune Response in Murine Lupus Nephritis. Arthritis Rheumatol. 2015, 67, 475–487. [Google Scholar] [CrossRef]
- Richard, M.L.; Gilkeson, G. Mouse Models of Lupus: What They Tell Us and What They Don’t. Lupus Sci. Med. 2018, 5, e000199. [Google Scholar] [CrossRef]
- Liu, M.F.; Li, J.S.; Weng, T.H.; Lei, H.Y. Double-Negative (CD4−CD8−) TCRalphabeta+ Cells in Patients with Systemic Lupus Erythematosus. Scand. J. Rheumatol. 1998, 27, 130–134. [Google Scholar] [CrossRef]
- Sieling, P.A.; Porcelli, S.A.; Duong, B.T.; Spada, F.; Bloom, B.R.; Diamond, B.; Hahn, B.H. Human Double-Negative T Cells in Systemic Lupus Erythematosus Provide Help for IgG and Are Restricted by CD1c. J. Immunol. 2000, 165, 5338–5344. [Google Scholar] [CrossRef]
- Crispín, J.C.; Oukka, M.; Bayliss, G.; Cohen, R.A.; Van Beek, C.A.; Stillman, I.E.; Kyttaris, V.C.; Juang, Y.-T.; Tsokos, G.C. Expanded Double Negative T Cells in Patients with Systemic Lupus Erythematosus Produce IL-17 and Infiltrate the Kidneys. J. Immunol. 2008, 181, 8761–8766. [Google Scholar] [CrossRef]
- Lai, Z.-W.; Hanczko, R.; Bonilla, E.; Caza, T.N.; Clair, B.; Bartos, A.; Miklossy, G.; Jimah, J.; Doherty, E.; Tily, H.; et al. N-Acetylcysteine Reduces Disease Activity by Blocking Mammalian Target of Rapamycin in T Cells from Systemic Lupus Erythematosus Patients: A Randomized, Double-Blind, Placebo-Controlled Trial. Arthritis Rheum. 2012, 64, 2937–2946. [Google Scholar] [CrossRef]
- Lai, Z.-W.; Borsuk, R.; Shadakshari, A.; Yu, J.; Dawood, M.; Garcia, R.; Francis, L.; Tily, H.; Bartos, A.; Faraone, S.V.; et al. Mechanistic Target of Rapamycin Activation Triggers IL-4 Production and Necrotic Death of Double-Negative T Cells in Patients with Systemic Lupus Erythematosus. J. Immunol. 2013, 191, 2236–2246. [Google Scholar] [CrossRef]
- Lai, Z.-W.; Kelly, R.; Winans, T.; Marchena, I.; Shadakshari, A.; Yu, J.; Dawood, M.; Garcia, R.; Tily, H.; Francis, L.; et al. Sirolimus in Patients with Clinically Active Systemic Lupus Erythematosus Resistant to, or Intolerant of, Conventional Medications: A Single-Arm, Open-Label, Phase ½ Trial. Lancet 2018, 391, 1186–1196. [Google Scholar] [CrossRef]
- Dean, G.S.; Anand, A.; Blofeld, A.; Isenberg, D.A.; Lydyard, P.M. Characterization of CD3+CD4−CD8− (double negative) T cells in patients with systemic lupus erythematosus: Production of IL-4. Lupus 2002, 11, 501–507. [Google Scholar] [CrossRef]
- Anand, A.; Dean, G.S.; Quereshi, K.; Isenberg, D.A.; Lydyard, P.M. Characterization of CD3+CD4−CD8− (double negative) T cells in patients with systemic lupus erythematosus: Activation markers. Lupus 2002, 11, 493–500. [Google Scholar] [CrossRef]
- Tarbox, J.A.; Keppel, M.P.; Topcagic, N.; Mackin, C.; Ben Abdallah, M.; Baszis, K.W.; White, A.J.; French, A.R.; Cooper, M.A. Elevated Double Negative T Cells in Pediatric Autoimmunity. J. Clin. Immunol. 2014, 34, 594–599. [Google Scholar] [CrossRef]
- Wang, H.-X.; Chu, S.; Li, J.; Lai, W.-N.; Wang, H.-X.; Wu, X.-J.; Kang, X.; Qiu, Y.-R. Increased IL-17 and IL-21 Producing TCRαβ+CD4−CD8− T Cells in Chinese Systemic Lupus Erythematosus Patients. Lupus 2014, 23, 643–654. [Google Scholar] [CrossRef]
- El-Sayed, Z.A.; El-Owaidy, R.H.; Mohamed, N.L.; Shehata, B.A. Alpha Beta Double Negative T Cells in Children with Systemic Lupus Erythematosus: The Relation to Disease Activity and Characteristics. Mod. Rheumatol. 2018, 28, 654–660. [Google Scholar] [CrossRef]
- Alexander, J.J.; Jacob, A.; Chang, A.; Quigg, R.J.; Jarvis, J.N. Double Negative T Cells, a Potential Biomarker for Systemic Lupus Erythematosus. Precis. Clin. Med. 2020, 3, 34–43. [Google Scholar] [CrossRef]
- Stratigou, V.; Doyle, A.F.; Carlucci, F.; Stephens, L.; Foschi, V.; Castelli, M.; McKenna, N.; Cook, H.T.; Lightstone, L.; Cairns, T.D.; et al. Altered Expression of Signalling Lymphocyte Activation Molecule Receptors in T-Cells from Lupus Nephritis Patients-a Potential Biomarker of Disease Activity. Rheumatology 2017, 56, 1206–1216. [Google Scholar] [CrossRef]
- Li, H.; Adamopoulos, I.E.; Moulton, V.R.; Stillman, I.E.; Herbert, Z.; Moon, J.J.; Sharabi, A.; Krishfield, S.; Tsokos, M.G.; Tsokos, G.C. Systemic Lupus Erythematosus Favors the Generation of IL-17 Producing Double Negative T Cells. Nat. Commun. 2020, 11, 2859. [Google Scholar] [CrossRef]
- Alunno, A.; Bistoni, O.; Bartoloni, E.; Caterbi, S.; Bigerna, B.; Tabarrini, A.; Mannucci, R.; Falini, B.; Gerli, R. IL-17-producing CD4−CD8− T cells are expanded in the peripheral blood, infiltrate salivary glands and are resistant to corticosteroids in patients with primary Sjogren’s syndrome. Ann. Rheum. Dis. 2013, 72, 286–292. [Google Scholar] [CrossRef]
- Alunno, A.; Carubbi, F.; Bistoni, O.; Caterbi, S.; Bartoloni, E.; Bigerna, B.; Pacini, R.; Beghelli, D.; Cipriani, P.; Giacomelli, R.; et al. CD4−CD8− T-cells in primary Sjogren’s syndrome: Association with the extent of glandular involvement. J. Autoimmun. 2014, 51, 38–43. [Google Scholar] [CrossRef]
- Brandt, D.; Sergon, M.; Abraham, S.; Mabert, K.; Hedrich, C.M. TCR+CD3+CD4−CD8− effector T cells in psoriasis. Clin. Immunol. 2017, 181, 51–59. [Google Scholar] [CrossRef]
- Bafor, E.E.; Valencia, J.C.; Young, H.A. Double Negative T Regulatory Cells: An Emerging Paradigm Shift in Reproductive Immune Tolerance? Front. Immunol. 2022, 13, 886645. [Google Scholar] [CrossRef]
- Achita, P.; Dervovic, D.; Ly, D.; Lee, J.B.; Haug, T.; Joe, B.; Hirano, N.; Zhang, L. Infusion of ex-vivo expanded human TCR-αβ+ double-negative regulatory T cells delays onset of xenogeneic graft-versus-host disease. Clin. Exp. Immunol. 2018, 193, 386–399. [Google Scholar] [CrossRef]
- Ford, M.S.; Chen, W.; Wong, S.; Li, C.; Vanama, R.; Elford, A.R.; Asa, S.L.; Ohashi, P.S.; Zhang, L. Peptide-activated double-negative T cells can prevent autoimmune type-1 diabetes development. Eur. J. Immunol. 2007, 37, 2234–2241. [Google Scholar] [CrossRef] [PubMed]
- McIver, Z.; Serio, B.; Dunbar, A.; O’Keefe, C.L.; Powers, J.; Wlodarski, M.; Jin, T.; Sobecks, R.; Bolwell, B.; Maciejewski, J.P. Double-negative regulatory T cells induce allotolerance when expanded after allogeneic haematopoietic stem cell transplantation. Br. J. Haematol. 2008, 141, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Fumi, M.; Villarroel, V.; Katz, S.I. Identification of CD3+CD4−CD8− T Cells as Potential Regulatory Cells in an Experimental Murine Model of Graft vs. Host Skin Disease (GvHD). J. Investig. Dermatol. 2013, 133, 2538–2545. [Google Scholar] [CrossRef]
- Neyt, K.; GeurtsvanKessel, C.; Lambrecht, B. Double-negative T resident memory cells of the lung react to influenza virus infection via CD11chi dendritic cells. Mucosal Immunol. 2016, 9, 999–1014. [Google Scholar] [CrossRef]
- Passos, L.S.A.; Koh, C.C.; Magalhães, L.M.D.; Nunes, M.D.C.P.; Gollob, K.J.; Dutra, W.O. Distinct CD4−CD8− (Double-Negative) Memory T-Cell Subpopulations Are Associated with Indeterminate and Cardiac Clinical Forms of Chagas Disease. Front. Immunol. 2021, 12, 761795. [Google Scholar] [CrossRef] [PubMed]
- Bekbossynova, M.; Akhmaltdinova, L.; Dossybayeva, K.; Tauekelova, A.; Smagulova, Z.; Tsechoeva, T.; Turebayeva, G.; Sailybayeva, A.; Kalila, Z.; Mirashirova, T.; et al. Central and effector memory T cells in peripheral blood of patients with interstitial pneumonia: Preliminary clues from a COVID-19 study. Respir. Res. 2022, 23, 278. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Wang, Y.; Wu, Y.; Feng, M.; Zhao, X.; Gao, C.; Guo, H.; Luo, J. Double-negative T cells are absolutely elevated in patients with antineutrophil cytoplasmic autoantibody-associated vasculitis. Mol. Immunol. 2021, 132, 250–259. [Google Scholar] [CrossRef]
- Lei, H.; Tian, M.; Zhang, X.; Liu, X.; Wang, B.; Wu, R.; Lv, Y. Expansion of Double-Negative T Cells in Patients before Liver Transplantation Correlates with Post-Transplant Infections. J. Clin. Med. 2022, 11, 3502. [Google Scholar] [CrossRef]
- Chen, X.; Wang, D.; Zhu, X. Application of double-negative T cells in haematological malignancies: Recent progress and future directions. Biomark. Res. 2022, 10, 11. [Google Scholar] [CrossRef]
- Lee, J.; Minden, M.D.; Chen, W.C.; Streck, E.; Chen, B.; Kang, H.; Arruda, A.; Ly, D.; Der, S.D.; Kang, S.; et al. Allogeneic Human Double Negative T Cells as a Novel Immunotherapy for Acute Myeloid Leukemia and Its Underlying Mechanisms. Clin. Cancer Res. 2018, 24, 370–382. [Google Scholar] [CrossRef]
- Tan, Y.; Zou, S.; Guo, W.; Xiang, Y.; Dong, Y.; Zhu, Q.; Wu, S.; Luo, M.; Shen, L.; Liang, K. Frequency and functional profile of circulating TCRαβ+ double negative T cells in HIV/TB co-infection. BMC Infect. Dis. 2022, 22, 890. [Google Scholar] [CrossRef] [PubMed]
- Cowley, S.C.; Meierovics, A.I.; Frelinger, J.A.; Iwakura, Y.; Elkins, K.L. Lung CD4−CD8− double-negative T cells are prominent producers of IL-17A and IFN-gamma during primary respiratory murine infection with Francisella tularensis live vaccine strain. J. Immunol. 2010, 184, 5791–5801. [Google Scholar] [CrossRef] [PubMed]
- Poddighe, D.; Dossybayeva, K.; Bexeitov, Y.; Mukusheva, Z. Basophils in autoimmunity: Systemic lupus erythematosus and more? Autoimmun. Rev. 2021, 20, 102790. [Google Scholar] [CrossRef] [PubMed]
- Dossybayeva, K.; Bexeitov, Y.; Mukusheva, Z.; Almukhamedova, Z.; Assylbekova, M.; Abdukhakimova, D.; Rakhimzhanova, M.; Poddighe, D. Analysis of Peripheral Blood Basophils in Pediatric Systemic Lupus Erythematosus. Diagnostics 2022, 12, 1701. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Tang, Y.; Fu, S.; Lv, J.; Liu, B.; Feng, M.; Li, J.; Lai, D.; Wan, X.; Xu, A. Basophil count, a marker for disease activity in systemic lupus erythematosus. Clin. Rheumatol. 2015, 34, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Chen, J.; Zhao, Y.; Liu, Y.; Xu, H.; Mo, X. Basophils as a potential marker of lupus nephritis by flow cytometry. Future Sci. OA 2021, 7, FSO690. [Google Scholar] [CrossRef]
- Zhong, W.; Su, W.; Zhang, Y.; Liu, Q.; Wu, J.; Di, C.; Zhang, Z.; Xia, Z. Basophils as a primary inducer of the T helper type 2 immunity in ovalbumin-induced allergic airway inflammation. Immunology 2014, 142, 202–215. [Google Scholar] [CrossRef]
- Poddighe, D.; Mathias, C.B.; Freyschmidt, E.J.; Kombe, D.; Caplan, B.; Marseglia, G.L.; Oettgen, H.C. Basophils are rapidly mobilized following initial aeroallergen encounter in naïve mice and provide a priming source of IL-4 in adaptive immune responses. J. Biol. Regul. Homeost Agents 2014, 28, 91–103. [Google Scholar]
- Erard, F.; Wild, M.T.; Garcia-Sanz, J.A.; Le Gros, G. Switch of CD8 T cells to noncytolytic CD8−CD4− cells that make TH2 cytokines and help B cells. Science 1993, 260, 1802–1805. [Google Scholar] [CrossRef]
- Kienzle, N.; Buttigieg, K.; Groves, P.; Kawula, T.; Kelso, A. A clonal culture system demonstrates that IL-4 induces a subpopulation of noncytolytic T cells with low CD8, perforin, and granzyme expression. J. Immunol. 2002, 168, 1672–1681. [Google Scholar] [CrossRef]
| Authors, Year, Country | Study Design | Primary Study Aim | SLE Pts. (n) | SLE pts. (Gender, Age) | SLE pts. Disease Duration | SLE Groups | SLE Groups (n, Age) | SLE Groups’ Disease Duration | Controls [n; Gender; Age] | DNT Cells Immuno-phenotype | DNT Cells [% CD3+] | Flow Cytometry Equipment | Therapy | DNT Cell-Related Findings |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Shivakumar et al., 1989, USA [24] | Prospective Cross- sectional | - To investigate the production of cationic anti-DNA IgG in lupus nephritis and cellular mechanisms regulating this process. | 20 | M:F = 3:18 Age n/a | n/a | - Active (with nephritis) - Inactive | n = 12 range 22–34 yrs. n = 8 range 35–55 yrs. | range 0.5–12 yrs. range 8–12 yrs. | n = 8 M:F = 3:5 range 20–30 yrs. | CD3+ CD4− CD8− TCRαβ+ | Mean ± SD Active: 2.7 ± 0.80 Inactive: 0.9 ± 0.06 Controls: 0.27 ± 0.09 I vs. C [p < 0.001] A vs. C [p< 0.001] A vs. I [p< 0.001] | FACScan | n/a | - DNT cells were markedly expanded in SLE patients and, along with CD4+T cells, supported the production of pathogenic anti-DNA IgG with cationic charge. |
| Liu et al., 1998, Taiwan [33] | Prospective Cross- sectional | - To investigate DNT cells in the peripheral blood mononuclear cells of SLE patients. | 47 | M:F = 4:43 Mean (range) 30 yrs. (12.0–58.0) | n/a | - Active (with nephritis) -Inactive | n = 26 n = 21 | n/a n/a | n = 44 M:F = 3:41 “Similar age” | CD3+ CD4− CD8− TCRαβ+ | Mean ± SD SLE: 1.14 ± 0.88 Controls: 0.88 ± 0.54 | FACSsort | - “Majority of patients were taking variable doses of steroids”. - Cytotoxic drugs (n = 21) | - Increased number of DNT cells was found in SLE patients, but neither association with lupus nephritis nor correlation with disease activity and anti-DNA titers was observed. |
| Sieling et al., 2000, USA [34] | Prospective Cross- sectional | - To investigate DNT cells and mechanisms leading to IgG autoantibody production in SLE. | 20 | M:F = 2:18 Mean (range) 39.1 yrs. (13–68) | Mean 8.7 yrs (0.5–25) | - | - | - | Yes (n = n/a) F = 57% Mean 32 yrs. | CD3+ CD4− CD8− TCRαβ+ | Mean ± SD SLE: 3.0 ± 0.4 Matched donors: 0.6 ± 0.1 Unmatched donors 1.0 ± 0.2 [p <0.005] | n/a | Prednisone (0–40 mg/die) Cytotoxic drugs (n = 6) | - DNT cells from SLE patients produced both IL-4 and IFN-γ and supported CD1c1+ B cells to produce IgG antibodies. |
| Dean et al., 2002, UK [39] | Prospective Cross- sectional | - To assess the percentage of IL-4+ DNT cells from patients with SLE and compare them with conventional T lymphocytes. | 50 | M:F = 1:49 Mean (range) 37.2 yrs. (17–66) | n/a | Variable disease activity | - | - | n = 16 M:F = 3:13 Mean (range) 36.1 yrs. (21–57) | CD3+ CD4− CD8− TCRαβ+ | n/a $ | FACScan | - SLE patients were on steroid and/or Immuno- suppressive drugs, but no detailed information. | - IL-4+DNT cells were more frequent in peripheral blood of patients with SLE than healthy controls. |
| Crispin et al., 2008, USA [35] | Prospective Cross- sectional | - To investigate DNT cells and their cytokine production in patients with SLE. | 24 | M:F = 0:24 Mean (range) 40.2 yrs. (25–57) | n/a | Variable disease activity | - | - | n = 16 n/a | CD3+ CD4− CD8− TCRαβ+ | n/a $ | FACSAria | “Prednisone was discontinued at least 24 h before venipuncture”. | - DNT cells from SLE patients can produce IL-17 and IFN-γ. In detail, IL-17 producing cells and DNT cells are present in kidney biopsies of SLE patients. |
| Lai et al., 2012, USA [36] | Prospective, Controlled, Double-blind trial | - To assess the safety, tolerance, and efficacy of the GSH precursor NAC and its related immunobiologcal impact. | 36 | M:F = 2:34 Mean ± SEM (range) 44.6 ± 1.8 yrs. (25–64) | n/a | Inactive (or stable disease) | - | - | n = 42 M:F = 3:39 Mean ± SEM (range) 44.4 ± 1.7 yrs. (22–63) | CD3+ CD4− CD8− | Mean ± SD Baseline: 6.2 ± 0.5 After 3-mo NAC: 5.3 ± 0.5 [p = 0.043] | n/a | n/a | - “The mean±SEM 1.35± 0.12-fold DNT cells in patients with SLE compared to matched healthy controls (p = 0.008) was eliminated by NAC treatment, which also increased the mitochondrial hyper-polarization, mass, and apoptosis of DNT cells in SLE patients”. |
| Lai et al., 2013, USA [37] | Prospective Longitudinal | - To assess the mitochondrial dysfunction and mTOR activation in peripheral blood mononuclear cells from SLE patients. | 59 | M:F = 3:56 Mean ± SEM (range) 43.1 ± 1.6 yrs. (20–65) | n/a | - | - | - | n = 54 M:F = 7:47 Mean ± SEM (range) 39.1 ± 1.8 yrs. (20–62) | CD3+ CD4− CD8− | n/a $ | n/a | n/a | - mTOR activation increases the production of IL-4 and necrosis of CD3+/CD42/ CD82 DNT cells. |
| Tarbox et al., 2014, USA [41] | Prospective Cross- sectional | - To assess DNT cells in several pediatric autoimmune diseases, including SLE. | 23 | M:F = 5:18 Mean ± SD (range) 13 ± 5 yrs. (2–25) | n/a | - | - | - | n = 28 M:F = 7:21 Mean ± SD (range) 17 ± 5 yrs. (7–25) | CD3+ CD56− CD4− CD8− TCRαβ+ TCRγδ− | Mean ± SD (range) SLE: 2.2 ± 0.9 (0.4–4.5) | n/a | - No cytotoxic drugs (n = 19) - Cytotoxic drugs (n = 17) - Steroids only (n = 3) - Steroids + cytotoxic drug (n = 15) | - A portion (34.8%, slightly higher than other rheumatic disease, but not significantly) of SLE patients showed increased number of DNT cells. In general, patients with increased DNT cell percentages showed increased CD45RA expression. |
| Wang et al., 2014, China [42] | Prospective Cross- sectional | - To assess DNT cells, their Fas expression, and intracellular cytokine levels in SLE patients. | 120 | M:F = 9:111 Mean ± SEM (range) 29.6 ± 1.1 yrs. (9–63) | n/a | - Active - Inactive | n = 82 n = 38 | n/a n/a | n = 43 M:F = 3:40 Mean ± SEM (range) 30.6 ± 1.4 yrs. (7–25) | CD3+ CD4− CD8− TCRαβ+ | Mean ± SEM SLE: 2.32 ± 0.12 Active: 2.68 ± 0.16 Inactive: 1.55 ± 0.11 Control: 1.03 ± 0.09 I vs. C [p < 0.001] A vs. C [p < 0.001] A vs. I [p < 0.001] | FACS Calibur | n/a | - DNT cells are increased in SLE patients and their value positively correlated with disease activity. - Abnormal Fas expression was observed in DNT cells. |
| El Sayed et al., 2017, Egypt [43] | Prospective Longitudinal | - To assess peripheral DNT cells in pediatric SLE and their potential correlation with disease activity and different organ damage. | 21 | M:F = 0:21 Mean ± SD (range) 13 ± 2 yrs. (10–17) | n/a | - new diagnosis (active) - previous diagnosis (active) | n = 12 n = 9 | 0 yrs. (diagnosis) range 0.5–3 yrs. | n = 20 M:F = 0:20 Mean ± SD (range) 14 ± 2 yrs. [11,12,13,14,15,16,17] | CD3+ CD4− CD8− TCRαβ+ | Median (IQR) Disease activity: 3.7 (3.0–5.7) Disease remission: 1.4 (1.2–1.8) Controls: 1.0 (0.5–1.4) Active New SLE: 5.0 (3.7–5.9) Active Old SLE: 2.8 (1.7–3.4) | Epics XLTM Navios | - “All patients received corticosteroid treatment during the period of follow-up” - CPM (n = 7) - MMF (n = 7) - Rituximab (n = 3) | - DNT cell percentage was significantly higher in proliferative nephritis than in non-proliferative nephritis but was comparable between patients with and without nephritis. - Active patients had more frequent DNT cell increase than those in remission. - DNT cell percentages showed a significant and positive correlation with SLEDAI-2K score and were higher in newly diagnosed SLE patients. |
| Stratigou et al., 2017, United Kingdom [45] | Prospective Longitudinal | - To investigate the expression of SLAM-family receptors on T lymphocytes, including DNT cells, from SLE patients with different disease activity. | 30 | M:F = n/a Median 34.5 yrs. | Median (range) 8 yrs. (0–35) | -Active (with nephritis) -Inactive | n = 19 n = 11 | n/a n/a | n = 20 M:F = 4:16 Median (range) 34 yrs. (24–54) | CD3+ CD4− CD8− | Mean ± SEM Active: 5.75 ± 3.43 Inactive: 3.68 ± 1.77 Control: 5.25 ± 3.34 I vs. C [p = ns] A vs. C [p = ns] A vs. I [p = ns] | FACSVerse | MMF (n = 16) HCQ (n = 23) AZA (n = 6) Pred (n = 14) None (n = 1) | - The frequency of DNT cells expressing SLAMF2/4/7 receptors was markedly altered in SLE patients, but these differences did not correlate with disease activity. - SLAMF6 expression on DNT cells could correlate with the response to B-cell depletion after rituximab. |
| Lai et al., 2018, USA [38] | Prospective, Single-arm, Open-label, Phase 1/2 trial | - To assess sirolimus in active SLE patients that were intolerant or resistant to conventional drugs. | 40 | M:F = 2:38 Mean ± SD (range) 45.4 ± 14.3 yrs. (18–71) | n/a | - | - | - | 43 Mean ± SD (range) 45.3 ± 12.7 yrs. Matched for Gender and Ethnicity | CD3+ CD4− CD8− | n/a $ | n/a | n/a | - Increased production of IL-4 and-IL-17 by CD4+ T cells and DNT cells at baseline, which was reduced after 12 months of treatment with sirolimus. IFN-γ production increased during sirolimus treatment in both CD4+ and DNT cells. Mean mitochondrial mass in DNT cells was higher in patients than in controls at baseline, and there was a decrease trend during sirolimus treatment. |
| Alexander et al., 2020, USA [44] | Prospective Cross-sectional | - To investigate the role of DNT cells in SLE and their potential impact on kidney disease. | 50 | M:F = n/a Range 7–15 yrs. | n/a | - | - | - | Yes n/a | CD3+ CD4− CD8− | Mean ± SD SLE: 10.0 ± 6.1 Controls: 6.5 ± 1.0 | LSRII Contessa | n/a | - DNT cells were increased in kidneys of active SLE patients and correlated with kidney function, in terms of BUN levels. |
| Li et al., 2020, USA [46] | Prospective Cross-sectional | - to study the interaction between marginal-zone macrophages and DNT cells. | n/a | n/a | n/a | - | - | - | Yes n/a | CD3+ CD4− CD8− CD56- TCRαβ+ | Done $ | n/a | n/a | - DNT cells were significantly increased in blood from SLE patients compared with healthy controls. - Moreover, Ki67+ DNT cells were also more represented in SLE patients (both in blood and kidney biopsies). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poddighe, D.; Dossybayeva, K.; Kozhakhmetov, S.; Rozenson, R.; Assylbekova, M. Double-Negative T (DNT) Cells in Patients with Systemic Lupus Erythematosus. Biomedicines 2024, 12, 166. https://doi.org/10.3390/biomedicines12010166
Poddighe D, Dossybayeva K, Kozhakhmetov S, Rozenson R, Assylbekova M. Double-Negative T (DNT) Cells in Patients with Systemic Lupus Erythematosus. Biomedicines. 2024; 12(1):166. https://doi.org/10.3390/biomedicines12010166
Chicago/Turabian StylePoddighe, Dimitri, Kuanysh Dossybayeva, Samat Kozhakhmetov, Rafail Rozenson, and Maykesh Assylbekova. 2024. "Double-Negative T (DNT) Cells in Patients with Systemic Lupus Erythematosus" Biomedicines 12, no. 1: 166. https://doi.org/10.3390/biomedicines12010166
APA StylePoddighe, D., Dossybayeva, K., Kozhakhmetov, S., Rozenson, R., & Assylbekova, M. (2024). Double-Negative T (DNT) Cells in Patients with Systemic Lupus Erythematosus. Biomedicines, 12(1), 166. https://doi.org/10.3390/biomedicines12010166






