Abstract
With technological applications, especially in genetic testing, new diseases have been discovered and new disease concepts have been proposed in recent years; however, the pathogenesis and treatment of these rare diseases are not as well established as those of common diseases. To demonstrate the importance of rare disease research, in this paper we focus on our research topic, Perry disease (Perry syndrome). Perry disease is a rare autosomal dominant neurodegenerative disorder clinically characterized by parkinsonism, depression/apathy, weight loss, and respiratory symptoms including central hypoventilation and central sleep apnea. The pathological classification of Perry disease falls under TAR DNA-binding protein 43 (TDP-43) proteinopathies. Patients with Perry disease exhibit DCTN1 mutations, which is the causative gene for the disease; they also show relatively uniform pathological and clinical features. This review summarizes recent findings regarding Perry disease from both basic and clinical perspectives. In addition, we describe technological innovations and outline future challenges and treatment prospects. We discuss the expansion of research from rare diseases to common diseases and the importance of collaboration between clinicians and researchers. Here, we highlight the importance of researching rare diseases as it contributes to a deeper understanding of more common diseases, thereby opening up new avenues for scientific exploration.
1. Introduction
The low incidence of rare diseases influences patient resources, diagnostic and therapeutic approaches, and research on mechanisms and potential treatments [1]. For many rare diseases, collaboration among researchers is often difficult and guidelines do not exist. Against this background, we focus on one rare disease, Perry disease/syndrome, and discuss strategies for research into other rare diseases.
2. History of Perry Disease Research
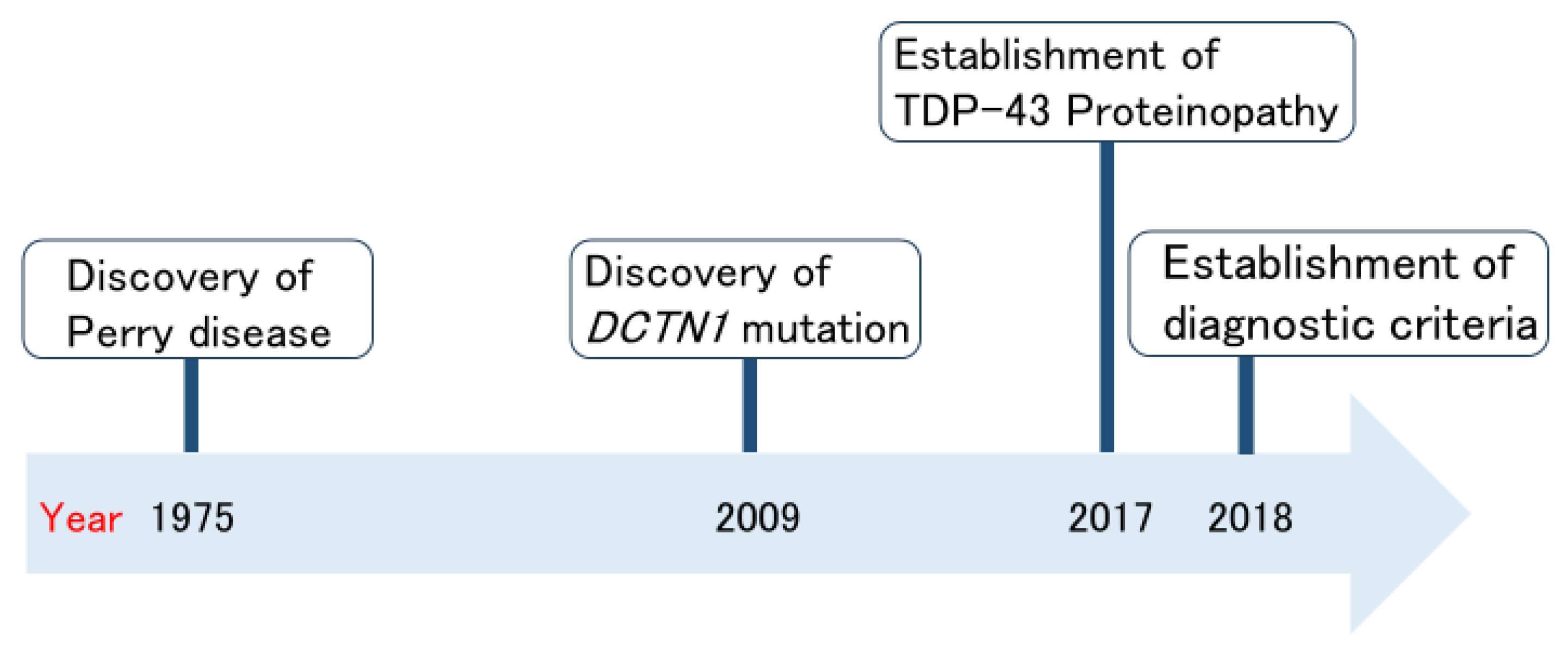
Perry disease is a neurodegenerative disease; it is rare, autosomal dominant, and patients display the clinical symptoms of parkinsonism, respiratory symptoms, depression/apathy, and weight loss [2,3]. Figure 1 shows the history of Perry disease research. In 1975, Perry disease was initially reported and named by Perry et al. [4] in Canada. The identification of DCTN1 mutations in 2009 accelerated Perry disease research [5]. Just after the establishment of a new TAR DNA-binding protein 43 (TDP-43) proteinopathy [6,7], in 2018, international diagnostic criteria for Perry disease were created [8]. Perry disease generally produces uniform clinical features and characteristic pathological findings; therefore, in the same year, we proposed a name change from Perry syndrome to Perry disease [8]. Here, we summarize Perry disease research to date from the clinician’s perspective. We also discuss the importance of bench to bedside strategies regarding Perry disease. Based on this understanding, we would like to highlight the often-overlooked value of rare disease research, particularly its potential in contributing to the elucidation of common disease pathogenesis.
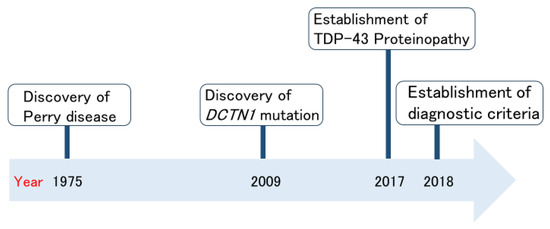
Figure 1.
Milestones of Perry disease research.
3. Importance of Early Diagnosis in Perry Disease
All diseases, including rare diseases, benefit from early diagnosis. In this section, we discuss the necessity of early diagnosis using Perry disease as our example. Early diagnosis is always critical during therapeutic intervention, and the publication of international diagnostic criteria enables the early diagnosis of Perry disease [8]. Indeed, prior to the development of the diagnostic criteria, there were only about 20 reported families with Perry disease; however, in the approximately five years since the publication of the diagnostic criteria (see Table 1 [8]), this number has increased to over 30. Parkinsonism, family history, and DCTN1 mutation are sufficient to diagnose Perry disease, with an emphasis on family history. For early diagnosis, genetic testing is recommended if an individual has parkinsonism and a family history of parkinsonism or respiratory symptoms. This is particularly important given that patients with Perry disease often present before the age of 50 and show significant disease progression, with respiratory failure and sudden death occurring within just 5 years of disease onset. Although the previously reported case involving p.K56R mutation in the DCTN1 gene with slow progression [9] is important, it did not meet the proposed Perry disease diagnostic criteria due to the lack of family history and should be discussed in future studies [8]. Since Perry disease is often difficult to diagnose in the early stages, key points for clinical features are listed in Table 2.

Table 1.
Diagnostic criteria for Perry disease.

Table 2.
Comparison of clinical features of Perry disease, Parkinson’s disease, multiple system atrophy, progressive supranuclear palsy, and frontotemporal lobar degeneration.
Next-generation sequencing technologies have now revealed gene mutations in patients without a family history [10,11] and genetic testing is being more widely used in the diagnosis of neurological diseases [12]. Of particular importance are compound heterozygous mutations. Many case reports have been published, and one example shows that novel heterozygous mutations in the SYNE1 gene have also been reported in juvenile amyotrophic lateral sclerosis (ALS) [13]. Regarding the DCTN1 gene, a case of parkinsonism with two trans variants has been reported [14], and we predict the diagnosis of more such cases in the future. Therefore, a revision of the diagnostic criteria of Perry disease may be needed. As mentioned above, revisions in diagnostic criteria for all diseases should be ongoing in order to keep pace with technological innovations.
4. Pathology of Perry Disease
Neurodegenerative diseases characteristically involve neuronal loss and the progressive degeneration in different parts of the nervous system, and coexist with proteinopathies such as tauopathy, synucleinopathy, and TDP-43 proteinopathy [15,16]. Therefore, pathological analysis is essential for elucidating the pathogenesis of these diseases and for developing treatments.
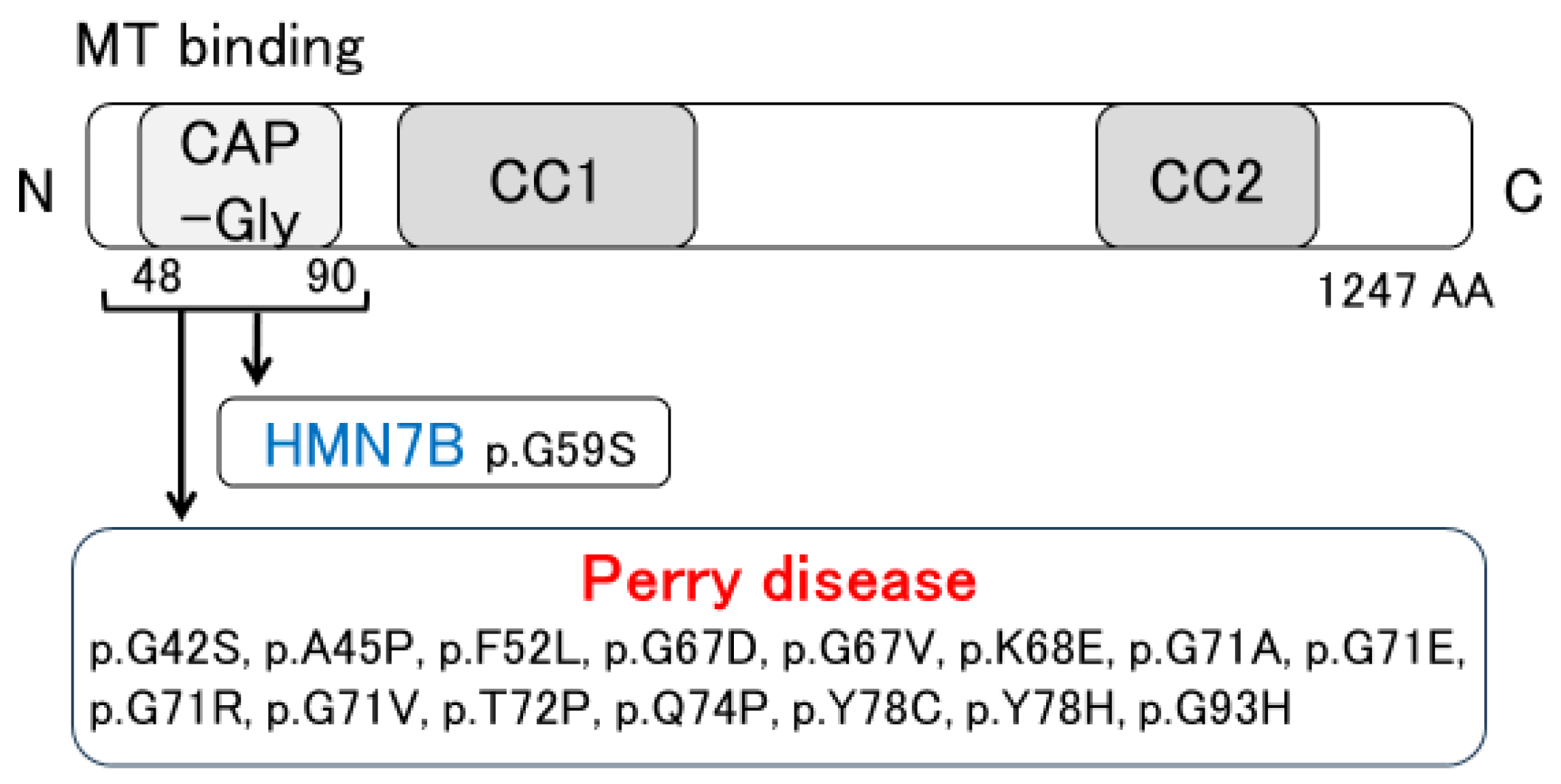
The pathological features of Perry disease have revealed substantial neuronal loss and gliosis, with few or no Lewy bodies or neurofibrillary tangles present in the substantia nigra [4,6,7,17,18,19,20,21,22,23]. Neuronal loss is detected in the lentiform nucleus, the locus coeruleus, the dorsal raphe nucleus, the periaqueductal gray matter, the hypothalamus, and the brainstem, including putative respiratory neurons in the medulla [6,20,21,24]. In Perry disease patients, TDP-43 pathology is noted in the extrapyramidal system and brainstem [6,7]. Such distribution differs from that of other TDP-43 proteinopathies, such as ALS and frontotemporal lobar degeneration (FTLD) with TDP-43 inclusions (FTLD-TDP) [7]. Furthermore, as described below, our pathological research presents Perry disease as a distinctive type of TDP-43 proteinopathy. Distal hereditary motor neuropathy 7B (HMN7B) is one type of motor neuron disease. As shown in Figure 2, a missense mutation in the DCTN1 gene also causes HMN7B. The pathology of HMN7B shows no TDP-43 pathology but evidence of dynactin aggregates. Specifically, neuronal cytoplasmic inclusions (NCIs) that are positive for the dynactin subunit p50 (DCTN2) have been observed. Similar inclusions have also been seen in Perry disease [25,26]. Therefore, we set out to evaluate the distribution and morphology of TDP-43 and dynactin pathology in patients with Perry disease, HMN7B, ALS, FTLD-MND, and hippocampal sclerosis (HpScl) in order to clarify Perry disease pathology [7]. Perry disease exhibited a unique TDP-43 pathology, which included abundant NCIs, dystrophic neurites, spheroids, and perivascular astrocytic inclusions. These findings differed from the TDP-43 pathology noted in ALS, FTLD-MND, and HpScl. An immunoelectron microscopic study demonstrated the difference between TDP-43-positive NCIs in Perry disease and those of ALS or FTLD-TDP. In contrast, no TDP-43 pathology was detected in HMN7B. Furthermore, dynactin pathology and p50-positive NCIs remained unseen in ALS, FTLD-MND, and HpScl [7].
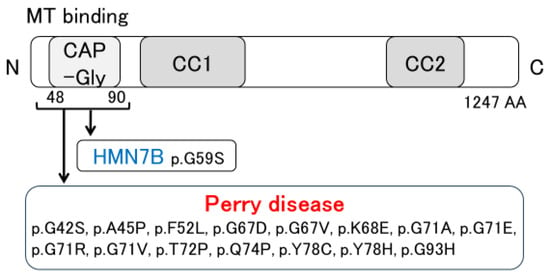
Figure 2.
Discovery of DCTN1 mutations. MT: microtubule; CAP-Gly: cytoskeleton-associated protein glycine-rich; CC: coiled-coil; HMN7B: distal hereditary motor neuropathy 7B.
Recently, multiple neuropathologies have received much attention [27,28]. A study of aged persons revealed that about 80% of participants had comorbid multiple neuropathologies, including TDP-43 pathology, at autopsy [29]. ALS/parkinsonism dementia complex (ALS/PDC) is also known as a multiple proteinopathy (tauopathy, synucleinopathy, and TDP-43 proteinopathy); however, it remains unclear whether there is a coexistence of proteinopathies [30,31,32,33,34]. Regarding Perry disease, Honda et al. reported that an elderly Perry disease patient harboring a p.F52L mutation with a long-term course had tau and synuclein aggregates at autopsy. This is thought to be due to the possibility that Perry disease exhibits multiple neuropathologies over a long period of time and the effects of aging [35]. Chung et al. also revealed the presence of neurofibrillary tangles in the parahippocampal gyrus during aging in Perry disease [36]. Therefore, analysis of Perry disease pathology may shed light on the study of multiple neuropathologies.
Cryo-electron microscopy (cryo-EM) technology has improved significantly in recent years, making it now possible to determine the atomic coordinates of many biomolecules [37]. New findings in cryo-EM analysis in 2022 from three research groups suggest a potential etiologic commonality of transmembrane protein 106B (TMEM106B) among distinct proteinopathies [38,39,40]. An analysis of the cryo-EM structure of TDP-43 and TMEM106B in patients with Perry disease is needed to further elucidate the pathophysiology.
In summary, we have updated findings regarding Perry disease pathology. Most of these results were performed by a collaboration between our team and the Mayo Clinic Brain Bank team. We emphasize the importance of gathering samples and data from patients with rare diseases and having these examined by rare disease teams.
5. Expansion of DCTN1 Mutations
Recent advances in clinical and molecular genetics have led to the discovery of many genes responsible for single gene disorders and disease susceptibility genes, and new disease concepts have been proposed. For example, cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), a typical inherited systemic arterial vessel disease that causes vascular dementia and lacunar infarction, is caused by mutations in the NOTCH3 gene in an autosomal dominant manner [41,42,43]. Regarding the NOTCH3 gene, an analysis of 200,000 cases in the U.K. revealed that NOTCH3 variants are common in the general population and are also susceptibility genes for isolated stroke and vascular dementia [42]. In addition, a recent study conducted in Japan reported that the RNF213 p.R4810K variant, a susceptibility gene for moyamoya disease, is associated with intracranial arterial stenosis, resulting in atherothrombotic stroke, and impacts endovascular therapy for large vessel occlusion stroke [44,45]. Therefore, the authors proposed the concept of RNF213-related vasculopathy [46]. In the area of neurodegenerative diseases, Yabe et al. found changes in the bassoon (BSN) gene, which translates the BSN in the active zone of presynaptic neurotransmitter release sites, in progressive supranuclear palsy (PSP)-like syndrome patients [47]. They also showed that tauopathy is a novel condition in which tau proteins with three and four repeats accumulate [47]. The BSN gene has also been reported to be associated with other neurological disorders, such as multiple sclerosis, and the disease concept of bassoon proteinopathy has been proposed [48].
DCTN1, the causative gene for Perry disease, encodes the largest subunit of dynactin (DCTN1/p150Glued) [5,49,50,51,52,53,54,55,56]. Dynactin is a multimeric complex that acts as an essential cofactor of the microtubule-based motor cytoplasmic dynein [49,50,51,52,53,54,55,56]. Dynein and dynactin together form the principal motor machinery driving the retrograde transport of cargo within cells [57,58]. DCTN1 is an essential factor in the initiation of dynein-dependent retrograde transport from microtubule plus-ends [59,60]. DCTN1 contains basic domains, a cytoskeleton-associated protein glycine-rich (CAP-Gly) domain, and the coiled-coil 1 (CC1) and CC2 domains. The CC1 and CC2 domains interact with other dynactin subunits and the dynein intermediate chain. The CAP-Gly and basic domains have microtubule binding affinity [60,61,62,63]. Most mutations in Perry disease are located within CAP-Gly domains [8,23,64,65,66,67,68,69,70]. Importantly, a DCTN1 mutation (p.G59S mutation) causes HMN7B [25,26,71] (Figure 2). Furthermore, DCTN1 is a risk gene for ALS [72,73,74], and the p.G59R mutation may cause dHMN and ALS [75]. Interestingly, it is reported that a novel p.Q93H mutation in DCTN1 causes a motor neuron disease phenotype and Perry disease [64]. Wszolek et al. have proposed the disease concept of DCTN1-related neurodegeneration [73,74], which is expected to be established along with revised diagnostic criteria.
Genetic counseling, especially predictive genetic counseling, is necessary for the early diagnosis and treatment of hereditary diseases. Since genetic counseling for neurodegenerative diseases began primarily with Huntington’s disease (HD), the HD genetic counseling protocol has been adopted as the standard [76]; however, Perry disease, which is also a severe autosomal dominant disorder and with the aforementioned expansion of the disease concept, may provide a new model for genetic counseling for neurodegenerative diseases.
In this section, we discussed the connection between single gene disorders and common diseases. We predict the expansion of such ideas by many clinicians and researchers. In the future, the frequency of genetic testing is set to increase in clinical settings. Therefore, it is important that genetic counselors are included in clinical teams, as described later.
6. Basic Research of Perry Disease
In genetic diseases, the discovery of genetic mutations is essential to the development of research. Next, disease models are required. The reproduction of aggregates is important in neurodegenerative diseases. Regarding Perry disease, dynactin and TDP-43 are targets, and many studies have reproduced dynactin aggregates in cultured cells [77,78,79,80,81]. Our research revealed that truncated mutant forms of DCTN1 cause TDP-43 mislocalization and aggregates. Furthermore, induced pluripotent stem cells (iPSCs) were generated from a patient with Perry disease (p.F52L mutation) and they were differentiated into tyrosine hydroxylase (TH)-positive neurons; dynactin aggregates were identified in the cytoplasm of the patient’s TH-positive neurons, and this partially recapitulated Perry disease pathology [79]. Using biochemical analysis with a panel of truncated mutants, we also found that DCTN1 binds to TDP-43 and demonstrated that the DCTN1 CAP-Gly-basic supradomain, dynactin domain, and C-terminal region interacted with TDP-43, preferentially through its C-terminal region [81]. In mouse models, we have focused on the p.G71A mutation, which presents a typical Perry disease phenotype, and generated transgenic (Tg) and knock-in mice [82,83]. DCTN1G71A transgenic mice showed behavioral defects, parallel apathy-like symptoms, and parkinsonism [82]; furthermore, these knock-in mice displayed a decrease in TH immunoreactivity in the neurons of the substantia nigra [83]. Yu J et al. generated DCTN1 conditional knockout mice by deleting DCTN1 in midbrain dopaminergic neurons [84,85]. These mice showed impaired motor coordination and a loss of dopaminergic neurons [85]; however, none of the model mice were able to reproduce the complete clinical phenotypes and pathology of Perry disease patients.
In contrast, the DCTN1 p.G59S mutation of HMN7B models has also been reported. The p.G59S mutant cells exhibit dynactin aggregates and a reduced binding affinity for microtubules [5,86,87]. Both p.G59S Tg and knock-in mice exhibited the phenotype of motor neuron disease [88,89,90]. Furthermore, the HMN7B mutation was found to disrupt transport within murine primary dorsal root ganglion (DRG) neurons [62,91]. In Perry disease mutations, reductions in microtubule binding affinity for microtubules, dynactin aggregates, autophagic insufficiency, and apoptotic changes were noted in non-neuronal cells as well as the HMN7B mutant [5,77,78,79,80,87]. In the motor neurons of Drosophila, HMN7B mutation showed an accumulation of dynein at synaptic termini; however, this was not found in Perry disease mutants, although the latter disrupted flux from the distal neurite (p.Q74P and p.G71R) and a p.Q74P mutation was impaired in inhibiting microtubule catastrophe in murine primary DRG neurons [62,63,91]. These results suggest the possibility of a greater fragility in dopaminergic neurons than in motor neurons in Perry disease. To address this question, similar platform phenotypic comparisons between Perry disease mutations and the HMN7B mutation, using dopaminergic neurons and/or motor neurons differentiated from iPSCs, will be needed [3].
As for treatment approaches, Hosaka et al. reported that reduced TDP-43 protein levels improved neuronal activities in a Drosophila model of Perry disease [92]. This approach may be one option for studying the therapeutic treatment of Perry disease [3]. With the development of genome-editing technologies, genetic therapy is being attempted in several neurodegenerative diseases [93,94,95]. Gene therapy is also expected to be used in Perry disease; however, the time at which intervention should start needs to be discussed. It is impossible to follow the natural history of the disease prior to its onset in many patients because Perry disease is rare. Although it is important to analyze the disease in animal models, a Perry disease model has not yet been established; this is required for the future development of research on other diseases. Tacik et al. reported the implantation of a diaphragmatic pacemaker in a patient with Perry disease as a treatment for respiratory symptoms [96]. In the future, it is expected that diaphragmatic pacemakers will be improved, first in animal studies and then by applying techniques such as deep brain stimulation.
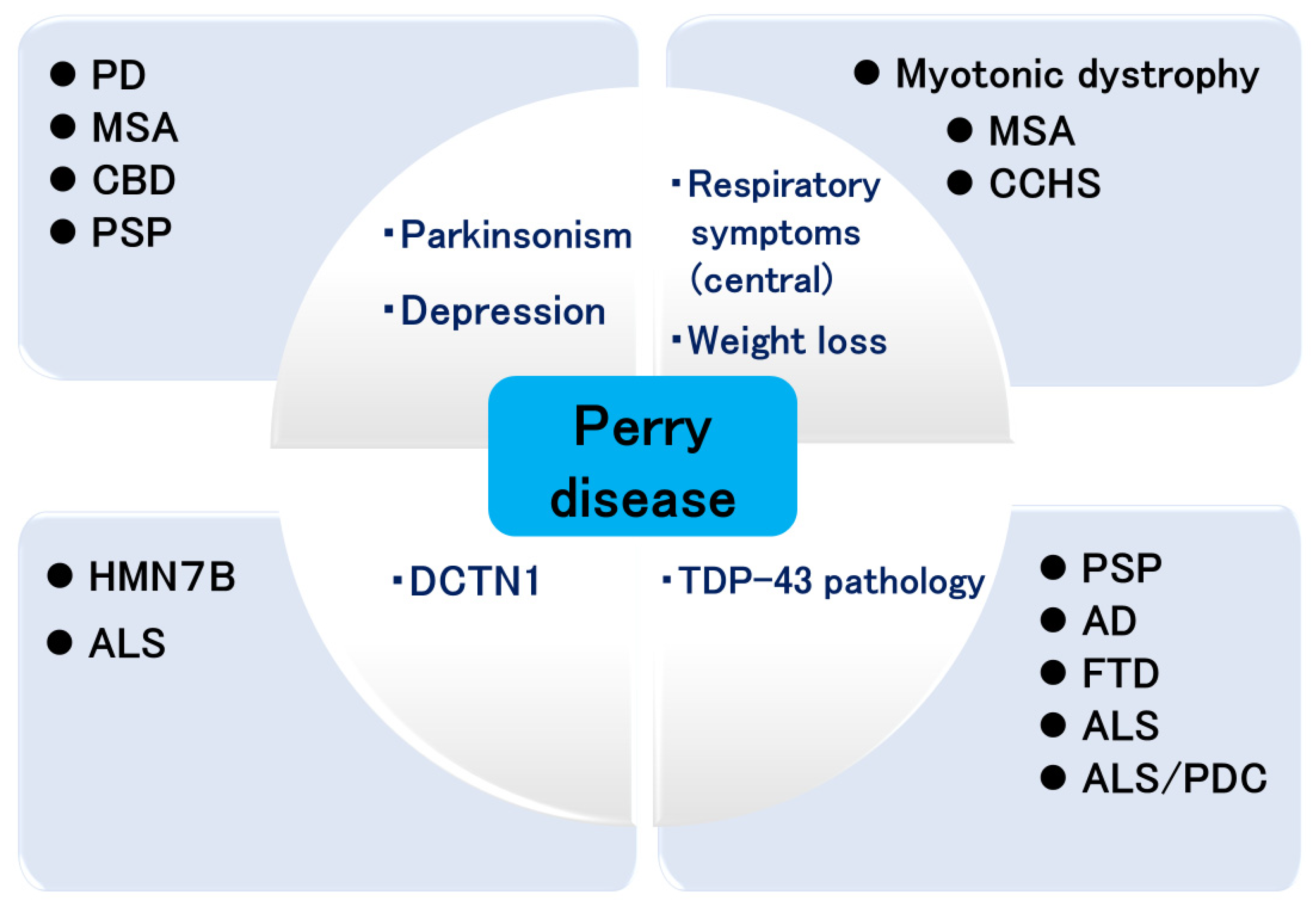
The attempts in achieving breakthroughs in Perry disease research have led to the study of various diseases (Figure 3). Clinically, such research advances the study of PD and Parkinsonian syndromes, respiratory symptoms, multiple system atrophy, congenital central hypoventilation syndrome [97], and myotonic dystrophy. Genetically, it contributes to the study of HMN7B and ALS, and pathologically it will merge with the studies of other neurodegenerative diseases.
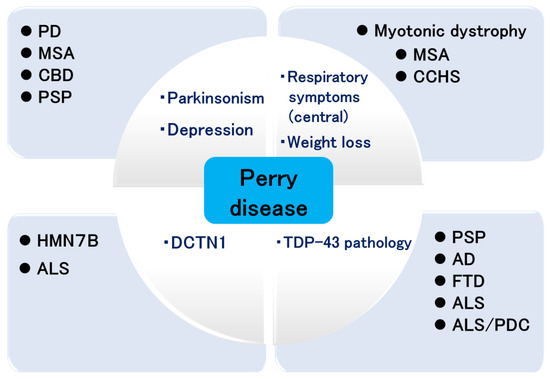
Figure 3.
Expansion of Perry disease research. PD: Parkinson’s disease; MSA: multiple system atrophy; CBD: corticobasal degeneration; PSP: progressive supranuclear palsy; HMN7B: distal hereditary motor neuropathy 7B; ALS: amyotrophic lateral sclerosis; CCHS: congenital central hypoventilation syndrome; AD: Alzheimer’s disease; FTD: frontotemporal dementia; PDC: parkinsonism dementia complex.
We have summarized the basic research regarding Perry disease in this section. In rare disease research, findings of common points between rare diseases and common diseases may require collaboration with other researchers. Although Perry disease research is in the process of development, this research trajectory may impact the research of other rare diseases in the future.
7. Team Approach for Perry Disease
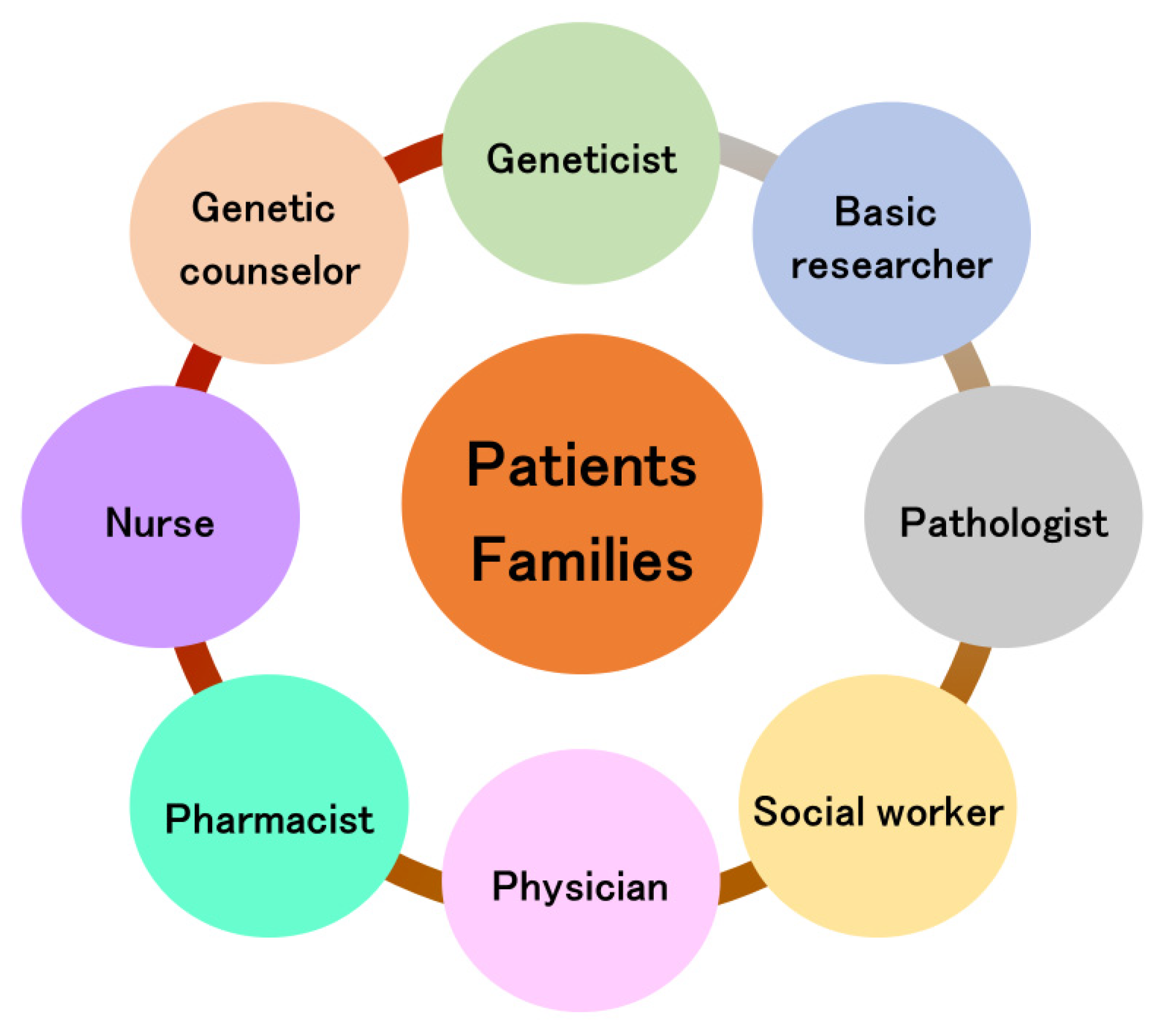
The importance of the bench to bedside concept has been described in many research fields [98,99,100]. In particular, Hampton suggests the key clinical breakthrough of bench to bedside and back again [101]. Thus, the circulation of information is needed (Figure 4), and it takes teams of clinicians and basic researchers worldwide to achieve such breakthroughs [102,103]. In the clinical setting, a team approach in device-based therapies is widespread [104,105], and the need for a team approach is recognized in the treatment of Parkinson’s disease [106]. Rare neurodegenerative diseases require the involvement of even more professions. The team approach for rare neurodegenerative diseases is shown in Figure 5. In genetic diseases, the role of genetic counselors is important [76], especially in diagnosis, where the involvement of geneticists and basic researchers is essential in assessing the presence or absence of pathological variants. Although the ACMG guidelines are used to evaluate pathological variants [107], these pathological variants may be evaluated for each disease and should be evaluated in a conference including clinicians and geneticists and basic researchers.
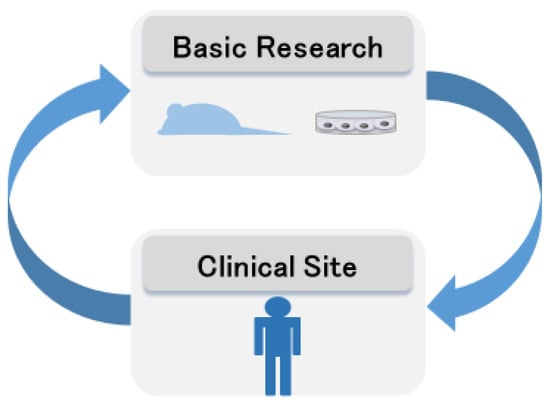
Figure 4.
Bench to bedside circulation.
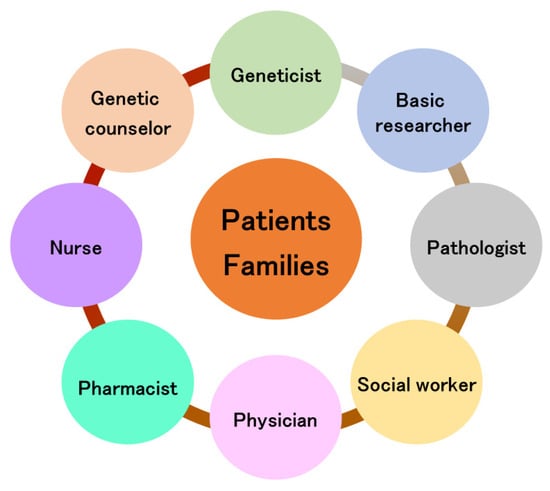
Figure 5.
Team approach for rare neurodegenerative diseases.
Regarding Perry disease, an international conference in Tokyo, Japan, was held from 22 to 23 February 2011 [8]. This meeting led to international collaborative research on Perry disease and the development of international diagnostic criteria [8]. We have just organized a second international conference on Perry disease in Fukuoka, Japan, bringing together clinicians and basic researchers. At this meeting, a revision of diagnostic criteria and the concept of DCTN1-related neurodegeneration was discussed. It is highly desirable to establish an international consortium involving various professions on many rare diseases to make breakthroughs in the future.
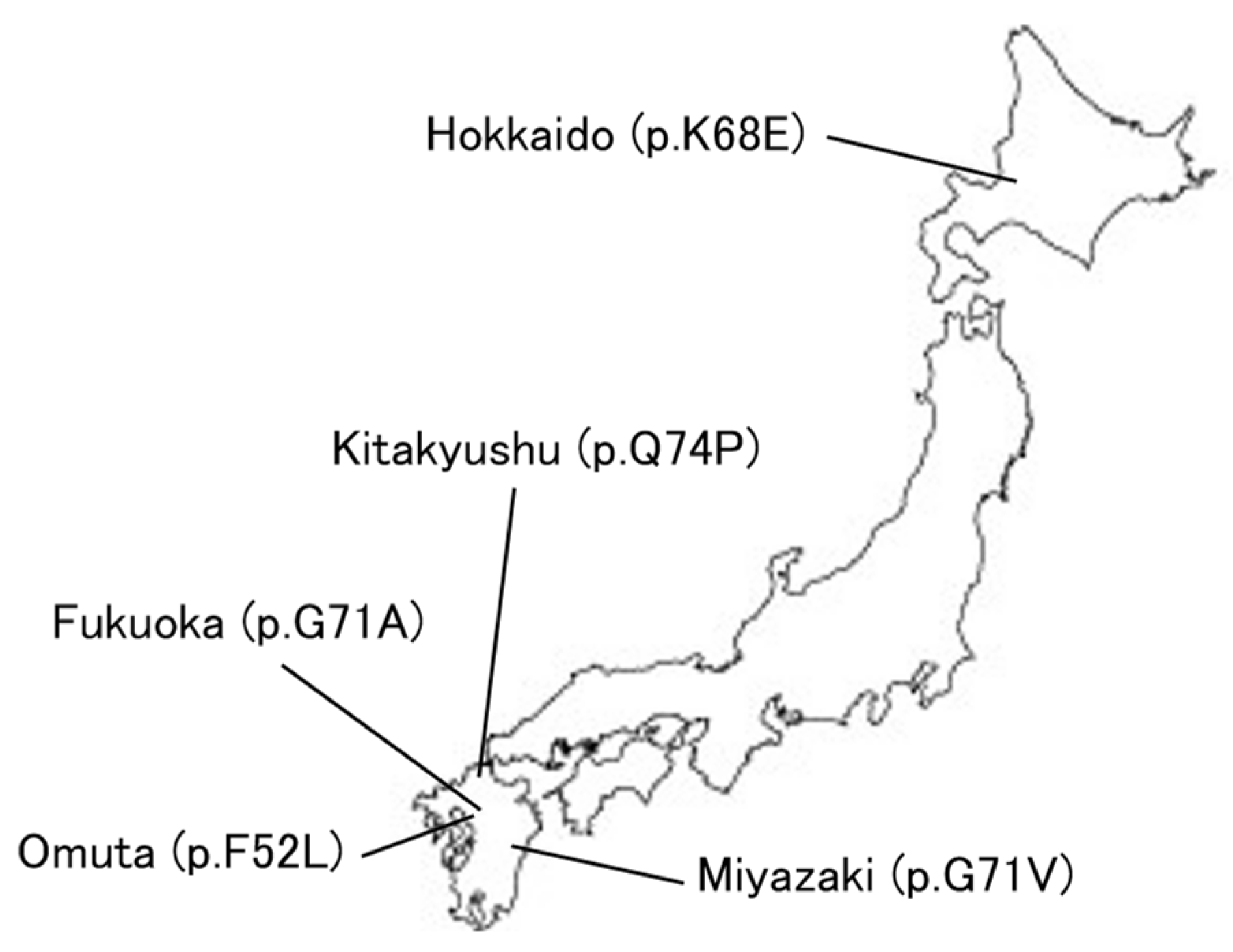
COVID-19 has revolutionized medical systems and telemedicine is expanding [106]. Figure 6 shows the location of the current Perry disease families in Japan, and in fact, we are also providing remote genetic counseling in cooperation with other facilities to families of patients with Perry disease who live far away from home. The development of telemedicine may be essential for the treatment of rare diseases and the development of research in this context.
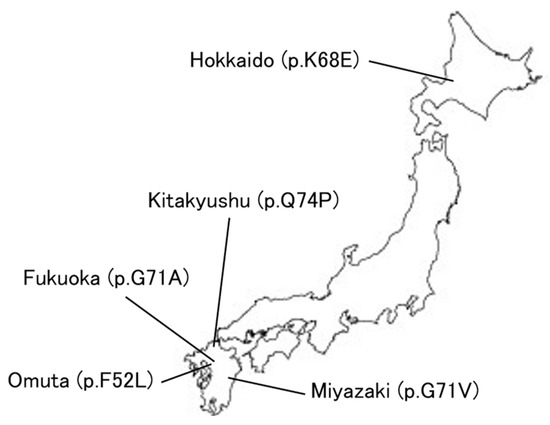
Figure 6.
Perry disease in Japan.
We have discussed a team approach based on Perry disease. It is important that a team approach is consistently implemented from diagnosis through to treatment in clinical settings. For research, a team approach is essential to archive bench to bedside and back again. We expect that team approaches will be seamlessly conducted in the future alongside technological advances.
8. Discussion
In the previous sections, we described the history of research regarding Perry disease. It began with Perry, who reported the disease from a family study [4], and the discovery of the DCTN1 mutation [5], which was the first step toward understanding the pathogenesis of the disease, thanks to international collaboration. Such collaborations led to an elucidation of the pathology [7] and the establishment of the diagnostic criteria of Perry disease [8]. We also described how Perry disease research can guide the study of common diseases through sections on pathology, genetics, and basic research. In the team approach section, we also proposed collaboration on new devices tailored to the social context.
We discussed the importance of research on rare diseases and, needless to say, the study of common diseases helps in the study of rare diseases. Many studies that have been performed on common diseases have not been implemented for rare diseases. This is also true for Perry disease research. Perry disease is rare, so the analysis of its pathogenesis is poor. For example, research into the affected neural circuits and brain regions associated with Perry disease will provide a clearer understanding of the disease’s symptoms. In particular, analyses of specific regions and their role in affecting motor control, respiratory function, and emotional processing could shed light on the neurological basis of the symptoms. In addition, studies of neurodegenerative processes in affected brain regions, such as neuronal loss, gliosis, and protein aggregates, will clarify pathophysiological mechanisms [108,109]. Future research regarding how these processes relate to symptoms and disease progression will strengthen the links between pathophysiology and clinical presentation [110,111].
Several limitations of this review need to be acknowledged. First, this manuscript is a review of only one disease, Perry disease. Second, a treatment for Perry disease has not been established, and it cannot yet be made a model case for rare disease research. Despite these limitations, this is a report to demonstrate the importance of research on rare diseases through research on Perry disease. It is expected that, in the future, other researchers will publish similar reviews for other rare diseases.
9. Conclusions
We reviewed our research topic, Perry disease, to reiterate the importance of research regarding rare diseases. Clinical and basic research regarding Perry disease has been described, including research history and collaboration. Rare diseases, like Perry disease, contribute to the study of common diseases. We hope that the research trajectory of Perry disease will serve as a model case for research on other rare neurodegenerative diseases. Far fewer researchers are involved in the study of rare diseases, so barriers to collaboration do exist. Nevertheless, there is collaboration among researchers, particularly clinicians and basic researchers; this is essential for research development. There is a need to establish an international collaboration system for solving these problems, and this may be the key to breakthroughs. The establishment of this system along with disease research requires support from governments and the industrial sector. Therefore, further development of industry–government–academia collaboration is proposed.
Author Contributions
T.M.: Conducted the project, wrote the first draft; J.Y.-K.: Review and critique; S.F.: Revised the manuscript; Y.T.: Conception and organization of the project, edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by JSPS KAKENHI (T.M., 23K06937), Grant of The Clinical Research Promotion Foundation 2021 (T.M.) and Research on rare and intractable diseases, Health and Labor Sciences Research Grants (Y.T., T.M.).
Informed Consent Statement
Patient consent was waived as there are no new data in this review article.
Data Availability Statement
No new data were created.
Acknowledgments
We are grateful to the patients with Perry disease and their families who cooperated with our study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Haendel, M.; Vasilevsky, N.; Unni, D.; Bologa, C.; Harris, N.; Rehm, H.; Hamosh, A.; Baynam, G.; Groza, T.; McMurry, J.; et al. How many rare diseases are there? Nat. Rev. Drug Discov. 2020, 19, 77–78. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, Y.; Mishima, T.; Fujioka, S. Perry Disease: Concept of a new disease and clinical diagnostic criteria. J. Mov. Disord. 2021, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mishima, T.; Fujioka, S.; Tsuboi, Y. Perry Disease: Recent advances and perspectives. Expert Opin. Orphan Drugs 2019, 7, 253–259. [Google Scholar] [CrossRef]
- Perry, T.L.; Bratty, P.J.; Hansen, S.; Kennedy, J.; Urquhart, N.; Dolman, C.L. Hereditary mental depression and Parkinsonism with taurine deficiency. Arch. Neurol. 1975, 32, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Farrer, M.J.; Hulihan, M.M.; Kachergus, J.M.; Dächsel, J.C.; Stoessl, A.J.; Grantier, L.L.; Calne, S.; Calne, D.B.; Lechevalier, B.; Chapon, F.; et al. DCTN1 Mutations in Perry syndrome. Nat. Genet. 2009, 41, 163–165. [Google Scholar] [CrossRef]
- Wider, C.; Dickson, D.W.; Stoessl, A.J.; Tsuboi, Y.; Chapon, F.; Gutmann, L.; Lechevalier, B.; Calne, D.B.; Personett, D.A.; Hulihan, M.; et al. Pallidonigral TDP-43 Pathology in Perry syndrome. Parkinsonism Relat. Disord. 2009, 15, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Mishima, T.; Koga, S.; Lin, W.L.; Kasanuki, K.; Castanedes-Casey, M.; Wszolek, Z.K.; Oh, S.J.; Tsuboi, Y.; Dickson, D.W. Perry Syndrome: A distinctive type of TDP-43 proteinopathy. J. Neuropathol. Exp. Neurol. 2017, 76, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Mishima, T.; Fujioka, S.; Tomiyama, H.; Yabe, I.; Kurisaki, R.; Fujii, N.; Neshige, R.; Ross, O.A.; Farrer, M.J.; Dickson, D.W.; et al. Establishing diagnostic criteria for Perry syndrome. J. Neurol. Neurosurg. Psychiatry 2018, 89, 482–487. [Google Scholar] [CrossRef]
- Gustavsson, E.K.; Trinh, J.; Guella, I.; Szu-Tu, C.; Khinda, J.; Lin, C.H.; Wu, R.M.; Stoessl, J.; Appel-Cresswell, S.; McKeown, M.; et al. DCTN1 p.K56R in Progressive supranuclear palsy. Parkinsonism Relat. Disord. 2016, 28, 56–61. [Google Scholar] [CrossRef]
- Rexach, J.; Lee, H.; Martinez-Agosto, J.A.; Németh, A.H.; Fogel, B.L. Clinical application of next-generation sequencing to the practice of neurology. Lancet Neurol. 2019, 18, 492–503. [Google Scholar] [CrossRef]
- Koyama, S.; Okabe, Y.; Suzuki, Y.; Igari, R.; Sato, H.; Iseki, C.; Tanji, K.; Suzuki, K.; Ohta, Y. Differing clinical features between Japanese siblings with cerebrotendinous xanthomatosis with a novel compound heterozygous CYP27A1 mutation: A case report. BMC Neurol. 2022, 22, 193. [Google Scholar] [CrossRef]
- Klein, C.J.; Foroud, T.M. Neurology individualized medicine: When to use next-generation sequencing panels. Mayo Clin. Proc. 2017, 92, 292–305. [Google Scholar] [CrossRef] [PubMed]
- Naruse, H.; Ishiura, H.; Mitsui, J.; Takahashi, Y.; Matsukawa, T.; Toda, T.; Tsuji, S. Juvenile amyotrophic lateral sclerosis with complex phenotypes associated with novel SYNE1 mutations. Amyotroph Lateral Scler. Front. Degener. 2021, 22, 576–578. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Neshige, S.; Morino, H.; Maruyama, H. Extubation failure due to atypical Parkinsonism with negligible motor and variable non-motor symptoms associated with a variant of DCTN1. Intern. Emerg. Med. 2023, 18, 329–331. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, J.D.; Fão, L.; Vilaça, R.; Cardoso, S.M.; Rego, A.C. Macroautophagy and mitophagy in neurodegenerative disorders: Focus on therapeutic interventions. Biomedicines 2021, 9, 1625. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.J.; Lin, C.H.; Lane, H.Y. From Menopause to Neurodegeneration—Molecular Basis and Potential Therapy. Int. J. Mol. Sci. 2021, 22, 8654. [Google Scholar] [CrossRef]
- Perry, T.L.; Wright, J.M.; Berry, K.; Hansen, S.; Perry, T.L., Jr. Dominantly inherited apathy, central hypoventilation, and Parkinson’s syndrome: Clinical, biochemical, and neuropathologic studies of 2 new cases. Neurology 1990, 40, 1882–1887. [Google Scholar] [CrossRef]
- Roy, E.P., III; Riggs, J.E.; Martin, J.D.; Ringel, R.A.; Gutmann, L. Familial Parkinsonism, apathy, weight loss, and central hypoventilation: Successful long-term management. Neurology 1988, 38, 637–639. [Google Scholar] [CrossRef]
- Bhatia, K.P.; Daniel, S.E.; Marsden, C.D. Familial Parkinsonism with depression: A clinicopathological study. Ann. Neurol. 1993, 34, 842–847. [Google Scholar] [CrossRef]
- Tsuboi, Y.; Wszolek, Z.K.; Kusuhara, T.; Doh-ura, K.; Yamada, T. Japanese family with Parkinsonism, depression, weight loss, and central hypoventilation. Neurology 2002, 58, 1025–1030. [Google Scholar] [CrossRef]
- Kim, D.D.; Alghefari, H.; Jenkins, M.; Ang, L.C.; Pasternak, S.H. Neuropathology of Perry syndrome: Evidence of medullary and hypothalamic involvement. Mov. Disord. Clin. Pract. 2021, 8, 713–716. [Google Scholar] [CrossRef] [PubMed]
- Dulski, J.; Koga, S.; Prudencio, M.; Tipton, P.W.; Ali, S.; Strongosky, A.J.; Rose, J.H.; Parrales, Z.A.; Dunmore, J.A.; Jansen-West, K.; et al. Perry syndrome: Novel DCTN1 mutation in a large kindred and first observation of prodromal disease. Parkinsonism Relat. Disord. 2023, 112, 105481. [Google Scholar] [CrossRef] [PubMed]
- Dulski, J.; Koga, S.; Liberski, P.P.; Sitek, E.J.; Butala, A.A.; Sławek, J.; Dickson, D.W.; Wszolek, Z.K. Perry disease: Expanding the genetic basis. Mov. Disord. Clin. Pract. 2023, 10, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, Y.; Dickson, D.W.; Nabeshima, K.; Schmeichel, A.M.; Wszolek, Z.K.; Yamada, T.; Benarroch, E.E. Neurodegeneration involving putative respiratory neurons in Perry syndrome. Acta Neuropathol. 2008, 115, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Puls, I.; Jonnakuty, C.; LaMonte, B.H.; Holzbaur, E.L.; Tokito, M.; Mann, E.; Floeter, M.K.; Bidus, K.; Drayna, D.; Oh, S.J.; et al. Mutant dynactin in motor neuron disease. Nat. Genet. 2003, 33, 455–456. [Google Scholar] [CrossRef] [PubMed]
- Puls, I.; Oh, S.J.; Sumner, C.J.; Wallace, K.E.; Floeter, M.K.; Mann, E.A.; Kennedy, W.R.; Wendelschafer-Crabb, G.; Vortmeyer, A.; Powers, R.; et al. Distal spinal and bulbar muscular atrophy caused by dynactin mutation. Ann. Neurol. 2005, 57, 687–694. [Google Scholar] [CrossRef]
- Alafuzoff, I.; Libard, S. Mixed brain pathology is the most common cause of cognitive impairment in the elderly. J. Alzheimers Dis. 2020, 78, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Stein, T.D.; Crary, J.F. Chronic traumatic encephalopathy and neuropathological comorbidities. Semin. Neurol. 2020, 40, 384–393. [Google Scholar] [CrossRef]
- Yu, L.; Boyle, P.A.; Dawe, R.J.; Bennett, D.A.; Arfanakis, K.; Schneider, J.A. Contribution of TDP and hippocampal sclerosis to hippocampal volume loss in older-old persons. Neurology 2020, 94, e142–e152. [Google Scholar] [CrossRef]
- Hasegawa, M.; Arai, T.; Akiyama, H.; Nonaka, T.; Mori, H.; Hashimoto, T.; Yamazaki, M.; Oyanagi, K. TDP-43 is deposited in the Guam Parkinsonism-dementia complex brains. Brain 2007, 130, 1386–1394. [Google Scholar] [CrossRef]
- Miklossy, J.; Steele, J.C.; Yu, S.; McCall, S.; Sandberg, G.; McGeer, E.G.; McGeer, P.L. Enduring involvement of tau, beta-amyloid, alpha-synuclein, ubiquitin and TDP-43 pathology in the amyotrophic lateral sclerosis/parkinsonism-dementia complex of Guam (ALS/PDC). Acta Neuropathol. 2008, 116, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Geser, F.; Winton, M.J.; Kwong, L.K.; Xu, Y.; Xie, S.X.; Igaz, L.M.; Garruto, R.M.; Perl, D.P.; Galasko, D.; Lee, V.M.; et al. Pathological TDP-43 in parkinsonism–dementia complex and amyotrophic lateral sclerosis of Guam. Acta Neuropathol. 2008, 115, 133–145. [Google Scholar] [CrossRef]
- Mimuro, M.; Yoshida, M.; Kuzuhara, S.; Kokubo, Y. Amyotrophic lateral sclerosis and Parkinsonism-dementia complex of the Hohara focus of the Kii Peninsula: A multiple proteinopathy? Neuropathology 2018, 38, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Condello, C.; Ayers, J.I.; Dalgard, C.L.; Garcia Garcia, M.M.; Rivera, B.M.; Seeley, W.W.; Perl, D.P.; Prusiner, S.B. Guam ALS-PDC is a distinct double-prion disorder featuring both tau and aβ prions. Proc. Natl. Acad. Sci. USA 2023, 120, e2220984120. [Google Scholar] [CrossRef] [PubMed]
- Honda, H.; Sasagasako, N.; Shen, C.; Shijo, M.; Hamasaki, H.; Suzuki, S.O.; Tsuboi, Y.; Fujii, N.; Iwaki, T. DCTN1 F52L mutation case of Perry syndrome with progressive supranuclear palsy-like tauopathy. Parkinsonism Relat. Disord. 2018, 51, 105–110. [Google Scholar] [CrossRef]
- Chung, E.J.; Kim, S.J.; Kim, E.J.; Ahn, J.W.; Huh, G.Y.; Cho, H.J.; Cairns, N.J. Neuropathological findings in a south Korean patient with Perry syndrome. Clin. Neuropathol. 2020, 39, 80–85. [Google Scholar] [CrossRef]
- Tafur, L.; Hinterndorfer, K.; Gabus, C.; Lamanna, C.; Bergmann, A.; Sadian, Y.; Hamdi, F.; Kyrilis, F.L.; Kastritis, P.L.; Loewith, R. Cryo-EM structure of the SEA complex. Nature 2022, 611, 399–404. [Google Scholar] [CrossRef]
- Schweighauser, M.; Arseni, D.; Bacioglu, M.; Huang, M.; Lövestam, S.; Shi, Y.; Yang, Y.; Zhang, W.; Kotecha, A.; Garringer, H.J.; et al. Age-dependent formation of TMEM106B amyloid filaments in human brains. Nature 2022, 605, 310–314. [Google Scholar] [CrossRef]
- Chang, A.; Xiang, X.; Wang, J.; Lee, C.; Arakhamia, T.; Simjanoska, M.; Wang, C.; Carlomagno, Y.; Zhang, G.; Dhingra, S.; et al. Homotypic fibrillization of TMEM106B across diverse neurodegenerative diseases. Cell 2022, 185, 1346–1355.e15. [Google Scholar] [CrossRef]
- Jiang, Y.X.; Cao, Q.; Sawaya, M.R.; Abskharon, R.; Ge, P.; DeTure, M.; Dickson, D.W.; Fu, J.Y.; Ogorzalek Loo, R.R.; Loo, J.A.; et al. Amyloid fibrils in FTLD-TDP are composed of TMEM106B and not TDP-43. Nature 2022, 605, 304–309. [Google Scholar] [CrossRef]
- Lee, Y.C.; Chung, C.P.; Chang, M.H.; Wang, S.J.; Liao, Y.C. NOTCH3 Cysteine-altering variant is an important risk factor for stroke in the Taiwanese population. Neurology 2020, 94, e87–e96. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.P.H.; Nannoni, S.; Harshfield, E.L.; Tozer, D.; Gräf, S.; Bell, S.; Markus, H.S. NOTCH3 variants are more common than expected in the general population and associated with stroke and vascular dementia: An analysis of 200 000 participants. J. Neurol. Neurosurg. Psychiatry 2021, 92, 694–701. [Google Scholar] [CrossRef]
- Kim, Y.; Bae, J.S.; Lee, J.Y.; Song, H.K.; Lee, J.H.; Lee, M.; Kim, C.; Lee, S.H. Genotype and phenotype differences in CADASIL from an Asian perspective. Int. J. Mol. Sci. 2022, 23, 11506. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, S.; Yoshimoto, T.; Ohara, M.; Takagaki, M.; Nakamura, H.; Watanabe, K.; Gon, Y.; Todo, K.; Sasaki, T.; Araki, H.; et al. Effect of the RNF213 p.R4810K variant on the progression of intracranial artery stenosis: A 15-year follow-up study. Neurol. Genet. 2022, 8, e200029. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, T.; Tanaka, K.; Koga, J.; Saito, S.; Yamagami, H.; Nakaoku, Y.; Ogata, S.; Nishimura, K.; Yamaguchi, E.; Chiba, T.; et al. Impact of the RNF213 p.R4810K variant on endovascular therapy for large-vessel occlusion stroke. Stroke Vasc. Interv. Neurol. 2022, 2, e000396. [Google Scholar] [CrossRef]
- Ihara, M.; Yamamoto, Y.; Hattori, Y.; Liu, W.; Kobayashi, H.; Ishiyama, H.; Yoshimoto, T.; Miyawaki, S.; Clausen, T.; Bang, O.Y.; et al. Moyamoya disease: Diagnosis and interventions. Lancet Neurol. 2022, 21, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Yabe, I.; Yaguchi, H.; Kato, Y.; Miki, Y.; Takahashi, H.; Tanikawa, S.; Shirai, S.; Takahashi, I.; Kimura, M.; Hama, Y.; et al. Mutations in bassoon in individuals with familial and sporadic progressive supranuclear palsy-like syndrome. Sci. Rep. 2018, 8, 819. [Google Scholar] [CrossRef]
- Schattling, B.; Engler, J.B.; Volkmann, C.; Rothammer, N.; Woo, M.S.; Petersen, M.; Winkler, I.; Kaufmann, M.; Rosenkranz, S.C.; Fejtova, A.; et al. Bassoon proteinopathy drives neurodegeneration in multiple sclerosis. Nat. Neurosci. 2019, 22, 887–896. [Google Scholar] [CrossRef]
- Schroer, T.A. Dynactin. Annu. Rev. Cell Dev. Biol. 2004, 20, 759–779. [Google Scholar] [CrossRef]
- Holzbaur, E.L.F.; Hammarback, J.A.; Paschal, B.M.; Kravit, N.G.; Pfister, K.K.; Vallee, R.B. Homology of a 150 K cytoplasmic dynein-associated polypeptide with the drosophila gene glued. Nature 1991, 351, 579–583. [Google Scholar] [CrossRef]
- Gill, S.R.; Schroer, T.A.; Szilak, I.; Steuer, E.R.; Sheetz, M.P.; Cleveland, D.W. Dynactin, a conserved, ubiquitously expressed component of an activator of vesicle motility mediated by cytoplasmic dynein. J. Cell Biol. 1991, 115, 1639–1650. [Google Scholar] [CrossRef]
- Karki, S.; Holzbaur, E.L.F. Affinity chromatography demonstrates a direct binding between cytoplasmic dynein and the dynactin complex. J. Biol. Chem. 1995, 270, 28806–28811. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, K.T.; Vallee, R.B. Cytoplasmic dynein binds dynactin through a direct interaction between the intermediate chains and p150 Glued. J. Cell Biol. 1995, 131, 1507–1516. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Foster, H.E.; Rondelet, A.; Lacey, S.E.; Bahi-Buisson, N.; Bird, A.W.; Carter, A.P. Cryo-EM reveals how human cytoplasmic dynein is auto-inhibited and activated. Cell 2017, 169, 1303–1314.e18. [Google Scholar] [CrossRef]
- Urnavicius, L.; Lau, C.K.; Elshenawy, M.M.; Morales-Rios, E.; Motz, C.; Yildiz, A.; Carter, A.P. Cryo-EM shows how dynactin recruits two dyneins for faster movement. Nature 2018, 554, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Olenick, M.A.; Holzbaur, E.L.F. Dynein activators and adaptors at a glance. J. Cell Sci. 2019, 132, jcs227132. [Google Scholar] [CrossRef] [PubMed]
- Reck-Peterson, S.L.; Redwine, W.B.; Vale, R.D.; Carter, A.P. The cytoplasmic dynein transport machinery and its many cargoes. Nat. Rev. Mol. Cell Biol. 2018, 19, 382–398. [Google Scholar] [CrossRef]
- Canty, J.T.; Yildiz, A. Activation and regulation of cytoplasmic dynein. Trends Biochem. Sci. 2020, 45, 440–453. [Google Scholar] [CrossRef]
- Waterman-Storer, C.M.; Karki, S.; Holzbaur, E.F.L. The p150Glued component of the dynactin complex binds to both microtubules and the actin-related protein centractin (Arp-1). Proc. Natl. Acad. Sci. USA 1995, 92, 1634–1638. [Google Scholar] [CrossRef]
- Vaughan, K.T.; Tynan, S.H.; Faulkner, N.E.; Echeverri, C.J.; Vallee, R.B. Colocalization of cytoplasmic dynein with dynactin and CLIP-170 at microtubule distal ends. J. Cell Sci. 1999, 112, 1437–1447. [Google Scholar] [CrossRef]
- Culver–Hanlon, T.L.; Lex, S.A.; Stephens, A.D.; Quintyne, N.J.; King, S.J. A microtubule-binding domain in dynactin increases dynein processivity by skating along microtubules. Nat. Cell Biol. 2006, 8, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Moughamian, A.J.; Holzbaur, E.L. Dynactin is required for transport initiation from the distal axon. Neuron 2012, 74, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, T.E.; Machamer, J.; O’Hara, K.; Kim, J.H.; Collins, S.E.; Wong, M.Y.; Sahin, B.; Imlach, W.; Yang, Y.; Levitan, E.S.; et al. The p150Glued CAP-Gly domain regulates initiation of retrograde transport at synaptic termini. Neuron 2012, 74, 344–360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, H.; Liu, W.; Wang, J.; Zhang, J.; Chang, X.; Huang, S.; Pang, X.; Guo, J.; Wang, Q.; et al. A novel Q93H missense mutation in DCTN1 caused distal hereditary motor neuropathy type 7B and Perry syndrome from a Chinese family. Neurol. Sci. 2021, 42, 3695–3705. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, P.; Sarma, G.R.K.; Murgod, U.; Srinivas, M.; Roy, A.K. Perry syndrome with a novel mutation and a rare presentation: First report from India. Ann. Indian Acad. Neurol. 2022, 25, 703–706. [Google Scholar] [CrossRef] [PubMed]
- Boardman, J.; Mascareno Ponte, M.; Chaouch, A.; Kobylecki, C. Perry syndrome with intrafamilial heterogeneity in presentation and survival including acute respiratory failure: Case series. Mov. Disord. Clin. Pract. 2022, 9, 816–820. [Google Scholar] [CrossRef] [PubMed]
- Dulski, J.; Cerquera-Cleves, C.; Milanowski, L.; Kwiatek-Majkusiak, J.; Koziorowski, D.; Ross, O.A.; Pentela-Nowicka, J.; Sławek, J.; Wszolek, Z.K. L-Dopa response, choreic dyskinesia, and dystonia in Perry syndrome. Parkinsonism Relat. Disord. 2022, 100, 19–23. [Google Scholar] [CrossRef]
- Stoker, T.B.; Dostal, V.; Cochius, J.; Williams-Gray, C.H.; Scherzer, C.R.; Wang, J.; Liu, G.; Coyle-Gilchrist, I. DCTN1 mutation associated Parkinsonism: Case series of three new families with Perry syndrome. J. Neurol. 2022, 269, 6667–6672. [Google Scholar] [CrossRef]
- Silva, E.; Itzcovich, T.; Niikado, M.; Caride, A.; Fernández, E.; Vázquez, J.C.; Romorini, L.; Marazita, M.; Sevlever, G.; Martinetto, H.; et al. Perry disease in an Argentine family due to the DCTN1 p.G67D variant. Parkinsonism Relat. Disord. 2022, 97, 63–64. [Google Scholar] [CrossRef]
- Pan, X.; Hong, Q.; Lu, X.; Li, Z.; Wang, L.; Chen, W.; Pan, S. A Chinese pedigree with Perry disease caused by the p.Y78H mutation in DCTN1: A 6-year clinical follow-up. Behav. Brain Res. 2023, 441, 114284. [Google Scholar] [CrossRef]
- Hwang, S.H.; Kim, E.J.; Hong, Y.B.; Joo, J.; Kim, S.M.; Nam, S.H.; Hong, H.D.; Kim, S.H.; Oh, K.; Lim, J.G.; et al. Distal hereditary motor neuropathy type 7B with dynactin 1 mutation. Mol. Med. Rep. 2016, 14, 3362–3368. [Google Scholar] [CrossRef] [PubMed]
- Münch, C.; Sedlmeier, R.; Meyer, T.; Homberg, V.; Sperfeld, A.D.; Kurt, A.; Prudlo, J.; Peraus, G.; Hanemann, C.O.; Stumm, G.; et al. Point mutations of the P150 subunit of dynactin (DCTN1) gene in ALS. Neurology 2004, 63, 724–726. [Google Scholar] [CrossRef] [PubMed]
- Konno, T.; Ross, O.A.; Teive, H.A.G.; Sławek, J.; Dickson, D.W.; Wszolek, Z.K. DCTN1-related neurodegeneration: Perry syndrome and beyond. Parkinsonism Relat. Disord. 2017, 41, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Dulski, J.; Konno, T.; Wszolek, Z. DCTN1-related neurodegeneration. In GeneReviews® [Internet]; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 2010. Available online: https://www.ncbi.nlm.nih.gov/books/NBK47027/ (accessed on 17 December 2023).
- He, J.; Yu, W.; Liu, X.; Fan, D. An identical DCTN1 mutation in two Chinese siblings manifest as dHMN and ALS respectively: A case report. Amyotroph Lateral Scler Front. Degener. 2022, 23, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J.S. Predictive genetic counseling for neurodegenerative diseases: Past, present, and future. Cold Spring Harb. Perspect. Med. 2020, 10, a036525. [Google Scholar] [CrossRef]
- Wider, C.; Dachsel, J.C.; Farrer, M.J.; Dickson, D.W.; Tsuboi, Y.; Wszolek, Z.K. Elucidating the genetics and pathology of Perry syndrome. J. Neurol. Sci. 2010, 289, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K.; Saiki, S.; Furuya, N.; Yamada, D.; Imamichi, Y.; Li, Y.; Kawajiri, S.; Sasaki, H.; Koike, M.; Tsuboi, Y.; et al. P150glued-associated disorders are caused by activation of intrinsic apoptotic pathway. PLoS ONE 2014, 9, e94645. [Google Scholar] [CrossRef] [PubMed]
- Mishima, T.; Ishikawa, T.; Imamura, K.; Kondo, T.; Koshiba, Y.; Takahashi, R.; Takahashi, J.; Watanabe, A.; Fujii, N.; Tsuboi, Y.; et al. Cytoplasmic aggregates of dynactin in iPSC-derived tyrosine hydroxylase-positive neurons from a patient with Perry syndrome. Parkinsonism Relat. Disord. 2016, 30, 67–72. [Google Scholar] [CrossRef]
- Ishikawa, K.I.; Saiki, S.; Furuya, N.; Imamichi, Y.; Tsuboi, Y.; Hattori, N. P150glued deficiency impairs effective fusion between autophagosomes and lysosomes due to their redistribution to the cell periphery. Neurosci. Lett. 2019, 690, 181–187. [Google Scholar] [CrossRef]
- Deshimaru, M.; Kinoshita-Kawada, M.; Kubota, K.; Watanabe, T.; Tanaka, Y.; Hirano, S.; Ishidate, F.; Hiramoto, M.; Ishikawa, M.; Uehara, Y.; et al. DCTN1 binds to TDP-43 and regulates TDP-43 aggregation. Int. J. Mol. Sci. 2021, 22, 3985. [Google Scholar] [CrossRef]
- Mishima, T.; Deshimaru, M.; Watanabe, T.; Kubota, K.; Kinoshita-Kawada, M.; Yuasa-Kawada, J.; Takasaki, K.; Uehara, Y.; Jinno, S.; Iwasaki, K.; et al. Behavioral defects in a DCTN1G71A transgenic mouse model of Perry syndrome. Neurosci. Lett. 2018, 666, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Deshimaru, M.; Mishima, T.; Watanabe, T.; Kubota, K.; Hosoi, M.; Kinoshita-Kawada, M.; Yuasa-Kawada, J.; Ikeda, M.; Mori, M.; Murata, Y.; et al. Behavioral profile in a Dctn1G71A knock-in mouse model of Perry disease. Neurosci. Lett. 2021, 764, 136234. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Lai, C.; Shim, H.; Xie, C.; Sun, L.; Long, C.X.; Ding, J.; Li, Y.; Cai, H. Genetic ablation of dynactin P150Glued in postnatal neurons causes preferential degeneration of spinal motor neurons in aged mice. Mol. Neurodegener. 2018, 13, 10. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yang, X.; Zheng, J.; Sgobio, C.; Sun, L.; Cai, H. Deficiency of Perry syndrome-associated P150Glued in midbrain dopaminergic neurons leads to progressive neurodegeneration and endoplasmic reticulum abnormalities. NPJ Parkinsons Dis. 2023, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.R.; Sumner, C.J.; Caviston, J.P.; Tokito, M.K.; Ranganathan, S.; Ligon, L.A.; Wallace, K.E.; LaMonte, B.H.; Harmison, G.G.; Puls, I.; et al. A motor neuron disease-associated mutation in p150Glued perturbs dynactin function and induces protein aggregation. J. Cell Biol. 2006, 172, 733–745. [Google Scholar] [CrossRef] [PubMed]
- Araki, E.; Tsuboi, Y.; Daechsel, J.; Milnerwood, A.; Vilarino-Guell, C.; Fujii, N.; Mishima, T.; Oka, T.; Hara, H.; Fukae, J.; et al. A novel DCTN1 mutation with late-onset Parkinsonism and frontotemporal atrophy. Mov. Disord. 2014, 29, 1201–1204. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.; Lin, X.; Chandran, J.; Shim, H.; Yang, W.J.; Cai, H. The G59S mutation in p150glued causes dysfunction of dynactin in mice. J. Neurosci. 2007, 27, 13982–13990. [Google Scholar] [CrossRef] [PubMed]
- Chevalier-Larsen, E.S.; Wallace, K.E.; Pennise, C.R.; Holzbaur, E.L. Lysosomal proliferation and distal degeneration in motor neurons expressing the G59S mutation in the P150Glued subunit of dynactin. Hum. Mol. Genet. 2008, 17, 1946–1955. [Google Scholar] [CrossRef]
- Laird, F.M.; Farah, M.H.; Ackerley, S.; Hoke, A.; Maragakis, N.; Rothstein, J.D.; Griffin, J.; Price, D.L.; Martin, L.J.; Wong, P.C. Motor neuron disease occurring in a mutant dynactin mouse model is characterized by defects in vesicular trafficking. J. Neurosci. 2008, 28, 1997–2005. [Google Scholar] [CrossRef]
- Lazarus, J.E.; Moughamian, A.J.; Tokito, M.K.; Holzbaur, E.L. Dynactin subunit P150Glued is a neuron-specific anti-catastrophe factor. PLoS Biol. 2013, 11, e1001611. [Google Scholar] [CrossRef]
- Hosaka, Y.; Inoshita, T.; Shiba-Fukushima, K.; Cui, C.; Arano, T.; Imai, Y.; Hattori, N. Reduced TDP-43 expression improves neuronal activities in a drosophila model of Perry syndrome. eBioMedicine 2017, 21, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Gaj, T.; Sirk, S.J.; Shui, S.L.; Liu, J. Genome-editing technologies: Principles and applications. Cold Spring Harb. Perspect. Biol. 2016, 8, a023754. [Google Scholar] [CrossRef] [PubMed]
- Bulaklak, K.; Gersbach, C.A. The once and future gene therapy. Nat. Commun. 2020, 11, 5820. [Google Scholar] [CrossRef] [PubMed]
- Horton, R.H.; Saade, D.; Markati, T.; Harriss, E.; Bönnemann, C.G.; Muntoni, F.; Servais, L. A systematic review of adeno-associated virus gene therapies in neurology: The need for consistent safety monitoring of a promising treatment. J. Neurol. Neurosurg. Psychiatry 2022, 93, 1276–1288. [Google Scholar] [CrossRef] [PubMed]
- Tacik, P.; Fiesel, F.C.; Fujioka, S.; Ross, O.A.; Pretelt, F.; Castañeda Cardona, C.; Kidd, A.; Hlavac, M.; Raizis, A.; Okun, M.S.; et al. Three families with Perry syndrome from distinct parts of the world. Parkinsonism Relat. Disord. 2014, 20, 884–888. [Google Scholar] [CrossRef] [PubMed]
- Trang, H.; Samuels, M.; Ceccherini, I.; Frerick, M.; Garcia-Teresa, M.A.; Peters, J.; Schoeber, J.; Migdal, M.; Markstrom, A.; Ottonello, G.; et al. Guidelines for diagnosis and management of congenital central hypoventilation syndrome. Orphanet J. Rare Dis. 2020, 15, 252. [Google Scholar] [CrossRef] [PubMed]
- Mishima, T.; Fujioka, S.; Fukae, J.; Yuasa-Kawada, J.; Tsuboi, Y. Modeling Parkinson’s disease and atypical Parkinsonian syndromes using induced pluripotent stem cells. Int. J. Mol. Sci. 2018, 19, 3870. [Google Scholar] [CrossRef]
- Shad, M.U. Recent developments in pharmacotherapy of depression: Bench to bedside. J. Pers. Med. 2023, 13, 773. [Google Scholar] [CrossRef]
- Louis, E.D. Essential tremor: From bedside to bench and back to bedside. Curr. Opin. Neurol. 2014, 27, 461–467. [Google Scholar] [CrossRef]
- Hampton, T. Bench to bedside and back again may be key to clinical breakthroughs. JAMA 2017, 318, 16–17. [Google Scholar] [CrossRef]
- Manolio, T.A.; Fowler, D.M.; Starita, L.M.; Haendel, M.A.; MacArthur, D.G.; Biesecker, L.G.; Worthey, E.; Chisholm, R.L.; Green, E.D.; Jacob, H.J.; et al. Bedside back to bench: Building bridges between basic and clinical genomic research. Cell 2017, 169, 6–12. [Google Scholar] [CrossRef]
- Vollstedt, E.J.; Kasten, M.; Klein, C.; MJFF Global Genetic Parkinson’s Disease Study Group. Using global team science to identify genetic Parkinson’s disease worldwide. Ann. Neurol. 2019, 86, 153–157. [Google Scholar] [CrossRef]
- Lupușoru, G.; Ailincăi, I.; Frățilă, G.; Ungureanu, O.; Andronesi, A.; Lupușoru, M.; Banu, M.; Văcăroiu, I.; Dina, C.; Sinescu, I. Tumor lysis syndrome: An endless challenge in onco-nephrology. Biomedicines 2022, 10, 1012. [Google Scholar] [CrossRef]
- Boarescu, P.M.; Roşian, A.N.; Roşian, Ş.H. Transvenous lead extraction procedure-indications, methods, and complications. Biomedicines 2022, 10, 2780. [Google Scholar] [CrossRef]
- Mishima, T.; Fujioka, S.; Morishita, T.; Inoue, T.; Tsuboi, Y. Personalized medicine in Parkinson’s disease: New options for advanced treatments. J. Pers. Med. 2021, 11, 650. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- Battaglia, S.; Nazzi, C.; Thayer, J.F. Heart’s tale of trauma: Fear-conditioned heart rate changes in post-traumatic stress disorder. Acta Psychiatr. Scand. 2023, 148, 463–466. [Google Scholar] [CrossRef]
- Tran, K.N.; Nguyen, N.P.K.; Nguyen, L.T.H.; Shin, H.M.; Yang, I.J. Screening for neuroprotective and rapid antidepressant-like effects of 20 essential oils. Biomedicines 2023, 11, 1248. [Google Scholar] [CrossRef]
- Polyák, H.; Galla, Z.; Nánási, N.; Cseh, E.K.; Rajda, C.; Veres, G.; Spekker, E.; Szabó, Á.; Klivényi, P.; Tanaka, M.; et al. The tryptophan-kynurenine metabolic system is suppressed in cuprizone-induced model of demyelination simulating progressive multiple sclerosis. Biomedicines 2023, 11, 945. [Google Scholar] [CrossRef]
- Battaglia, S.; Schmidt, A.; Hassel, S.; Tanaka, M. Editorial: Case reports in neuroimaging and stimulation. Front. Psychiatry 2023, 14, 1264669. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).