Comparative Study between the Diagnostic Effectiveness of Brain SPECT with [123I]Ioflupane and [123I]MIBG Scintigraphy in Multiple System Atrophy
Abstract
1. Introduction
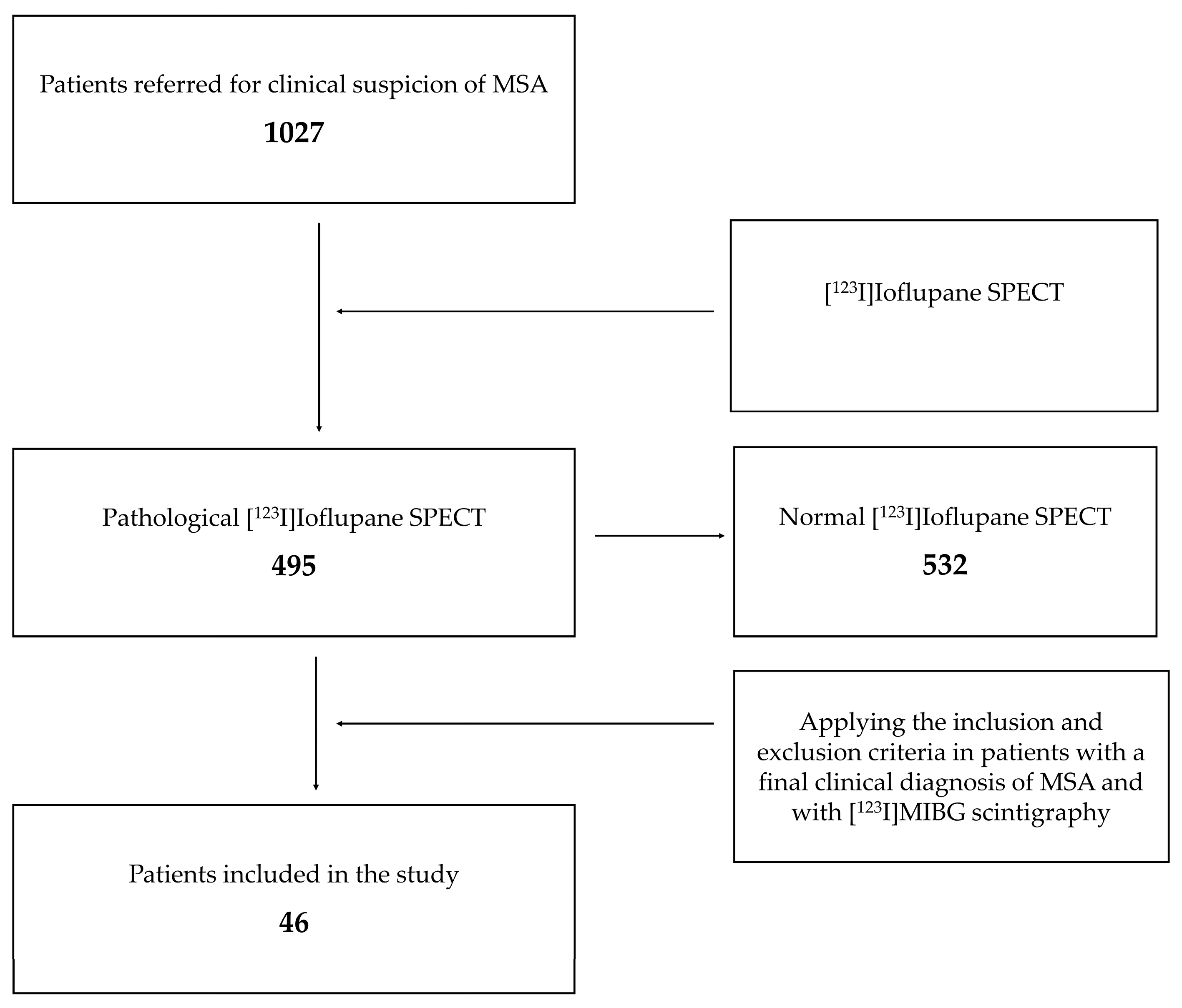
2. Materials and Methods
2.1. Inclusion Criteria
2.2. Exclusion Criteria
2.3. Ethical Considerations
2.4. Statistical Analysis
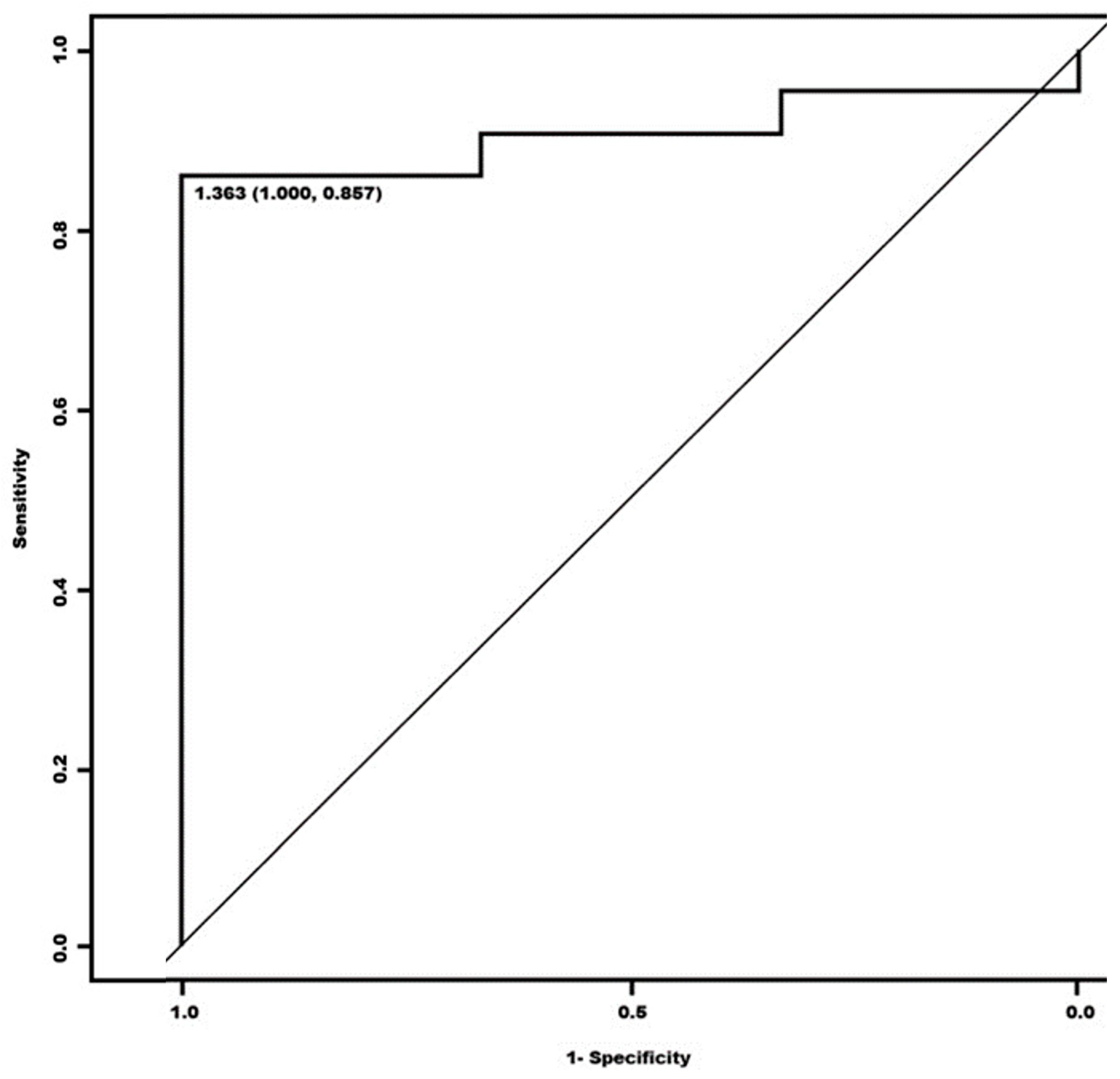
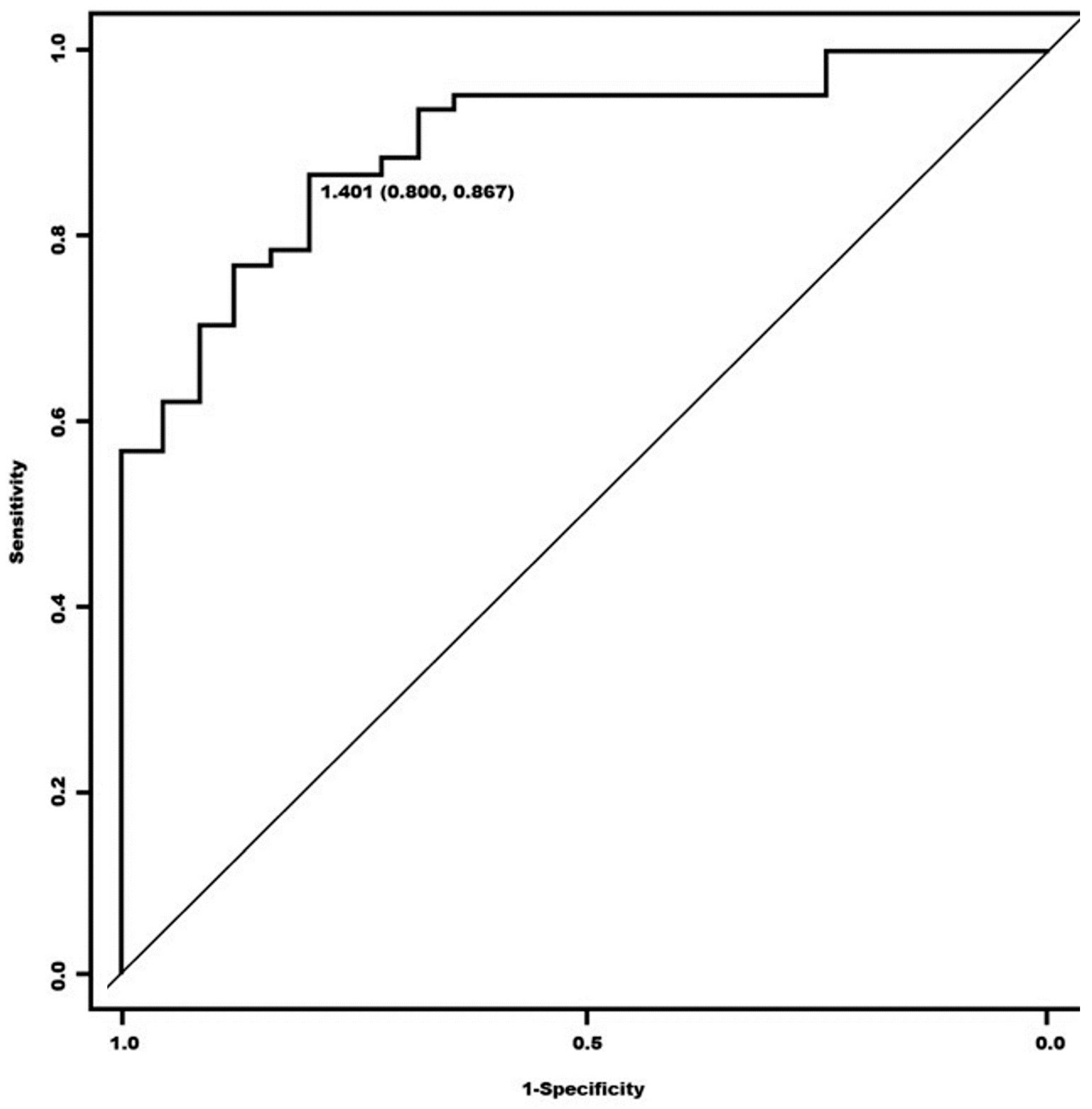
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Ghio, S.; Camilleri, A.; Caruana, M.; Ruf, V.C.; Schmidt, F.; Leonov, A.; Ryazanov, S.; Griesinger, C.; Cauchi, R.J.; Kamp, F.; et al. Cardiolipin Promotes Pore-Forming Activity of Alpha-Synuclein Oligomers in Mitochondrial Membranes. ACS Chem. Neurosci. 2019, 10, 3815–3829. [Google Scholar] [CrossRef] [PubMed]
- Bernal-Conde, L.D.; Ramos-Acevedo, R.; Reyes-Hernández, M.A.; Balbuena-Olvera, A.J.; Morales-Moreno, I.D.; Argüero-Sánchez, R.; Schüle, B.; Guerra-Crespo, M. Alpha-Synuclein Physiology and Pathology: A Perspective on Cellular Structures and Organelles. Front. Neurosci. 2020, 13, 1399. [Google Scholar] [CrossRef] [PubMed]
- Lantos, P.L. The Definition of Multiple System Atrophy: A Review of Recent Developments. J. Neuropathol. Exp. Neurol. 1998, 57, 1099–1111. [Google Scholar] [CrossRef] [PubMed]
- Bhidayasiri, R.; Ling, H. Multiple System Atrophy. Neurologist 2008, 14, 224–237. [Google Scholar] [CrossRef] [PubMed]
- Stefanova, N.; Bücke, P.; Duerr, S.; Wenning, G.K. Multiple System Atrophy: An Update. Lancet Neurol. 2009, 8, 1172–1178. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Vaquero, M.; Heras-Garvin, A.; Krismer, F.; Deleanu, R.; Boesch, S.; Wenning, G.K.; Stefanova, N. Signs of Early Cellular Dysfunction in Multiple System Atrophy. Neuropathol. Appl. Neurobiol. 2021, 47, 268–282. [Google Scholar] [CrossRef]
- Watanabe, H.; Saito, Y.; Terao, S.; Ando, T.; Kachi, T.; Mukai, E.; Aiba, I.; Abe, Y.; Tamakoshi, A.; Doyu, M.; et al. Progression and Prognosis in Multiple System Atrophy: An Analysis of 230 Japanese Patients. Brain 2002, 125, 1070–1083. [Google Scholar] [CrossRef]
- Fanciulli, A.; Stankovic, I.; Krismer, F.; Seppi, K.; Levin, J.; Wenning, G.K. Multiple System Atrophy. Int. Rev. Neurobiol. 2019, 149, 137–192. [Google Scholar] [CrossRef] [PubMed]
- Bower, J.H.; Maraganore, D.M.; McDonnell, S.K.; Rocca, W.A. Incidence of Progressive Supranuclear Palsy and Multiple System Atrophy in Olmsted County, Minnesota, 1976 to 1990. Neurology 1997, 49, 1284–1288. [Google Scholar] [CrossRef]
- Jellinger, K.A. Multiple System Atrophy: An Oligodendroglioneural Synucleinopathy. J. Alzheimer’s Dis. 2018, 62, 1141–1179. [Google Scholar] [CrossRef] [PubMed]
- Schrag, A.; Ben-Shlomo, Y.; Quinn, N.P. Prevalence of Progressive Supranuclear Palsy and Multiple System Atrophy: A Cross-Sectional Study. Lancet 1999, 354, 1771–1775. [Google Scholar] [CrossRef] [PubMed]
- Vidal, J.S.; Vidailhet, M.; Elbaz, A.; Derkinderen, P.; Tzourio, C.; Alpérovitch, A. Risk Factors of Multiple System Atrophy: A Case-Control Study in French Patients. Mov. Disord. 2008, 23, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Hara, K.; Momose, Y.; Tokiguchi, S.; Shimohata, M.; Terajima, K.; Onodera, O.; Kakita, A.; Yamada, M.; Takahashi, H.; Hirasawa, M.; et al. Multiplex Families with Multiple System Atrophy. Arch. Neurol. 2007, 64, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Filosto, M.; Tonin, P.; Vattemi, G.; Scarpelli, M.; Baronchelli, C.; Broglio, L.; Tentorio, M.; Cotelli, M.; Padovani, A.; Tomelleri, G. Definite Multiple System Atrophy in a German Family. J. Neurol. Neurosurg. Psychiatry 2009, 80, 449–450. [Google Scholar]
- Hohler, A.D.; Singh, V.J. Probable Hereditary Multiple System Atrophy-Autonomic (MSA-A) in a Family in the United States. J. Clin. Neurosci. 2012, 19, 479–480. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shlomo, Y.; Wenning, G.K.; Tison, F.; Quinn, N.P. Survival of Patients with Pathologically Proven Multiple System Atrophy: A Meta-Analysis. Neurology 1997, 48, 384–393. [Google Scholar] [CrossRef]
- Geser, F.; Seppi, K.; Stampfer-Kountchev, M.; Köllensperger, M.; Diem, A.; Ndayisaba, J.P.; Ostergaard, K.; Dupont, E.; Cardozo, A.; Tolosa, E.; et al. The European Multiple System Atrophy-Study Group (EMSA-SG). J. Neural Transm. 2005, 112, 1677–1686. [Google Scholar] [CrossRef]
- Wüllner, U.; Schmitz-Hübsch, T.; Abele, M.; Antony, G.; Bauer, P.; Eggert, K. Features of Probable Multiple System Atrophy Patients Identified among 4770 Patients with Parkinsonism Enrolled in the Multicentre Registry of the German Competence Network on Parkinson’s Disease. J. Neural Transm. 2007, 114, 1161–1165. [Google Scholar] [CrossRef]
- Köllensperger, M.; Geser, F.; Ndayisaba, J.P.; Boesch, S.; Seppi, K.; Ostergaard, K.; Dupont, E.; Cardozo, A.; Tolosa, E.; Abele, M.; et al. Presentation, Diagnosis, and Management of Multiple System Atrophy in Europe: Final Analysis of the European Multiple System Atrophy Registry. Mov. Disord. 2010, 25, 2604–2612. [Google Scholar] [CrossRef]
- Wenning, G.K.; Geser, F.; Krismer, F.; Seppi, K.; Duerr, S.; Boesch, S.; Köllensperger, M.; Goebel, G.; Pfeiffer, K.P.; Barone, P.; et al. The Natural History of Multiple System Atrophy: A Prospective European Cohort Study. Lancet Neurol. 2013, 12, 264–274. [Google Scholar] [CrossRef]
- Wenning, G.K.; Shlomo, Y.B.; Magalhães, M.; Danie, S.E.; Quinn, N.P. Clinical Features and Natural History of Multiple System Atrophy. An Analysis of 100 Cases. Brain 1994, 117 Pt 4, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Vanacore, N. Epidemiological Evidence on Multiple System Atrophy. J. Neural Transm. 2005, 112, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Gilman, S.; Wenning, G.K.; Low, P.A.; Brooks, D.J.; Mathias, C.J.; Trojanowski, J.Q.; Wood, N.W.; Colosimo, C.; Durr, A.; Fowler, C.J.; et al. Second Consensus Statement on the Diagnosis of Multiple System Atrophy. Neurology 2008, 71, 670–676. [Google Scholar] [CrossRef]
- Quinn, N.P. How to Diagnose Multiple System Atrophy. Mov. Disord. 2005, 20 (Suppl. S1), S5–S10. [Google Scholar] [CrossRef] [PubMed]
- Wenning, G.K.; Tison, F.; Seppi, K.; Sampiao, C.; Diem, A.; Yekhlef, F.; Ghorayeb, I.; Ory, F.; Galitzky, M.; Scaravilli, T.; et al. Development and Validation of the Unified Multiple System Atrophy Rating Scale (UMSARS). Mov. Disord. 2004, 19, 1391–1402. [Google Scholar] [CrossRef] [PubMed]
- Kashihara, K.; Ohno, M.; Tomita, S. Dropped Head Syndrome in Parkinson’s Disease. Mov. Disord. 2006, 21, 1213–1216. [Google Scholar] [CrossRef] [PubMed]
- Spuler, S.; Krug, H.; Klein, C.; Medialdea, I.C.; Jakob, W.; Ebersbach, G.; Gruber, D.; Hoffmann, K.T.; Trottenberg, T.; Kupsch, A. Myopathy Causing Camptocormia in Idiopathic Parkinson’s Disease: A Multidisciplinary Approach. Mov. Disord. 2010, 25, 552–559. [Google Scholar] [CrossRef]
- Ozawa, T.; Paviour, D.; Quinn, N.P.; Josephs, K.A.; Sangha, H.; Kilford, L.; Healy, D.G.; Wood, N.W.; Lees, A.J.; Holton, J.L.; et al. The Spectrum of Pathological Involvement of the Striatonigral and Olivopontocerebellar Systems in Multiple System Atrophy: Clinicopathological Correlations. Brain 2004, 127, 2657–2671. [Google Scholar] [CrossRef]
- Gilman, S.; Low, P.; Quinn, N.; Albanese, A.; Ben-Shlomo, Y.; Fowler, C.; Kaufmann, H.; Klockgether, T.; Lang, A.; Lantos, P.; et al. Consensus Statement on the Diagnosis of Multiple System Atrophy. Clin. Auton. Res. 1998, 8, 359–362. [Google Scholar] [CrossRef]
- Benarroch, E.E. Brainstem in Multiple System Atrophy: Clinicopathological Correlations. Cell. Mol. Neurobiol. 2003, 23, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Sakakibara, R.; Kuno, S.; Ishizuka, O.; Kitta, T.; Yoshimura, N. Prevalence and Treatment of LUTS in Patients with Parkinson Disease or Multiple System Atrophy. Nat. Rev. Urol. 2017, 14, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Plazzi, G.; Corsini, R.; Provini, F.; Pierangeli, G.; Martinelli, P.; Montagna, P.; Lugaresi, E.; Cortelli, P. REM Sleep Behavior Disorders in Multiple System Atrophy. Neurology 1997, 48, 1094–1097. [Google Scholar] [CrossRef]
- Giannini, G.; Mastrangelo, V.; Provini, F.; Droghini, A.; Cecere, A.; Barletta, G.; Mignani, F.; Guaraldi, P.; Cortelli, P.; Calandra-Buonaura, G. Progression and Prognosis in Multiple System Atrophy Presenting with REM Behavior Disorder. Neurology 2020, 94, E1828–E1834. [Google Scholar] [CrossRef] [PubMed]
- Rekik, S.; Martin, F.; Dodet, P.; Redolfi, S.; Leu-Semenescu, S.; Corvol, J.C.; Grabli, D.; Arnulf, I. Stridor Combined with Other Sleep Breathing Disorders in Multiple System Atrophy: A Tailored Treatment? Sleep Med. 2018, 42, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Wenning, G.K.; Stankovic, I.; Vignatelli, L.; Fanciulli, A.; Calandra-Buonaura, G.; Seppi, K.; Palma, J.A.; Meissner, W.G.; Krismer, F.; Berg, D.; et al. The Movement Disorder Society Criteria for the Diagnosis of Multiple System Atrophy. Mov. Disord. 2022, 37, 1131–1148. [Google Scholar] [CrossRef] [PubMed]
- Villena-Salinas, J.; Ortega-Lozano, S.J.; Amrani-Raissouni, T.; Agüera, E.; Caballero-Villarraso, J. Follow-Up Findings in Multiple System Atrophy from [123I]Ioflupane Single-Photon Emission Computed Tomography (SPECT): A Prospective Study. Biomedicines 2023, 11, 2893. [Google Scholar] [CrossRef]
- Iwabuchi, Y.; Kameyama, M.; Matsusaka, Y.; Narimatsu, H.; Hashimoto, M.; Seki, M.; Ito, D.; Tabuchi, H.; Yamada, Y.; Jinzaki, M. A Diagnostic Strategy for Parkinsonian Syndromes Using Quantitative Indices of DAT SPECT and MIBG Scintigraphy: An Investigation Using the Classification and Regression Tree Analysis. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1833–1841. [Google Scholar] [CrossRef]
- Eckhardt, C.; Krismer, F.; Donnemiller, E.; Eschlböck, S.; Fanciulli, A.; Raccagni, C.; Bösch, S.; Mair, K.; Scherfler, C.; Djamshidian, A.; et al. Cardiac Sympathetic Innervation in Parkinson’s Disease versus Multiple System Atrophy. Clin. Auton. Res. 2022, 32, 103–114. [Google Scholar] [CrossRef]
- Nicolas, N.; Valentina, G.; Burkhard Pierre, R. The Role of Molecular Imaging in Assessing Degenerative Parkinsonism—An Updated Review. Swiss Med. Wkly. 2018, 148, w14621. [Google Scholar] [CrossRef]
- Brooks, D.J.; Tambasco, N. Imaging Synucleinopathies. Mov. Disord. 2016, 31, 814–829. [Google Scholar] [CrossRef]
- Wenning, G.K. Parkinsonism and Dysautonomia: Multiple System Atrophy? Parkinsonism Relat. Disord. 2020, 77, 150–151. [Google Scholar] [CrossRef] [PubMed]
- Brumberg, J.; Isaias, I.U. SPECT Molecular Imaging in Atypical Parkinsonism. In International Review of Neurobiology; Elsevier Inc.: Amsterdam, The Netherlands, 2018; Volume 142, pp. 37–65. ISBN 9780128151419. [Google Scholar]
- Ortega Lozano, S.J.; Martínez Del Valle Torres, M.D.; Jiménez-Hoyuela García, J.M.; Delgado García, A.; Campos Arillo, V. Aplicación Del FP-CIT-I-123 En La Práctica Clínica de Pacientes Con Parkinsonismo. Rev. Esp. Med. Nucl. 2005, 24, 224–233. [Google Scholar] [CrossRef]
- Morbelli, S.; Esposito, G.; Arbizu, J.; Barthel, H.; Boellaard, R.; Bohnen, N.I. EANM Practice Guideline/SNMMI Procedure Standard for Dopaminergic Imaging in Parkinsonian Syndromes 1.0. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1885–1912. [Google Scholar] [CrossRef] [PubMed]
- Villena-Salinas, J.; Ortega-Lozano, S.J.; Amrani-Raissouni, T.; Agüera, E.; Caballero-Villarraso, J. Diagnostic Effectiveness of [123I]Ioflupane Single Photon Emission Computed Tomography (SPECT) in Multiple System Atrophy. J. Clin. Med. 2023, 12, 3478. [Google Scholar] [CrossRef] [PubMed]
- Catalan, M.; Dore, F.; Polverino, P.; Bertolotti, C.; Sartori, A.; Antonutti, L.; Cucca, A.; Furlanis, G.; Capitanio, S.; Manganotti, P. 123I-Metaiodobenzylguanidine Myocardial Scintigraphy in Discriminating Degenerative Parkinsonisms. Mov. Disord. Clin. Pract. 2021, 8, 717–724. [Google Scholar] [CrossRef]
- Skowronek, C.; Zange, L.; Lipp, A. Cardiac 123I-MIBG Scintigraphy in Neurodegenerative Parkinson Syndromes: Performance and Pitfalls in Clinical Practice. Front. Neurol. 2019, 10, 152. [Google Scholar] [CrossRef]
- Brandl, S.J.; Braune, S. Sensitivity and Specificity of Cardiac Metaiodobenzylguanidine Scintigraphy in the Early Diagnosis of Parkinson’s Disease. Clin. Auton. Res. 2019, 29, 567–574. [Google Scholar] [CrossRef]
- Shin, D.H.; Lee, P.H.; Bang, O.Y.; Joo, I.S.; Huh, K. Clinical Implications of Cardiac-MIBG SPECT in the Differentiation of Parkinsonian Syndromes. J. Clin. Neurol. 2006, 2, 51. [Google Scholar] [CrossRef]
- Giannoccaro, M.P.; Donadio, V.; Giannini, G.; Devigili, G.; Rizzo, G.; Incensi, A.; Cason, E.; Calandra-Buonaura, G.; Eleopra, R.; Cortelli, P.; et al. Comparison of 123I-MIBG Scintigraphy and Phosphorylated α-Synuclein Skin Deposits in Synucleinopathies. Park. Relat. Disord. 2020, 81, 48–53. [Google Scholar] [CrossRef]

| [123I]Ioflupane SPECT | [123I]MIBG Scintigraphy | |
|---|---|---|
| Sensitivity | 95.24% (87.61–100) | 78.57% (64.97–92.17) |
| Specificity | 25% (0.00–79.93) | 50% (0.00–100) |
| PPV | 93.02% (84.25–100) | 94.29% (85.17–100) |
| PNV | 33.33% (0.00–100) | 18.18% (0.00–45.52) |
| Accuracy | 89.13% | 76.09% |
| S/O Indexes | Healthy | Sick | p Value |
|---|---|---|---|
| Global | = 1.429 (S = 0.056) | = 0.658 (S = 0.672) | * |
| Right | = 1.421 (S = 0.063) | = 1.329 (S = 0.127) | >0.05 |
| Left | = 1.437 (S = 0.056) | = 1.304 (S = 0.133) | >0.05 |
| Visual Scintigraphy * | ||||
|---|---|---|---|---|
| Suspected MSA | Non-Suspected MSA | p Value | ||
| Visual SPECT ** | Negative 3 (6.5%) | 3 (8.6%) | 0 | p > 0.05 |
| Positive 43 (93.5%) | 32 (91.4%) | 11 (100%) | ||
| Diagnostic classification | Possible 13 (28.3%) | 11 (31.4%) | 2 (18.2%) | p > 0.05 |
| Probable 33 (71.7%) | 24 (68.6%) | 9 (81.8%) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villena-Salinas, J.; Ortega-Lozano, S.J.; Amrani-Raissouni, T.; Agüera-Morales, E.; Caballero-Villarraso, J. Comparative Study between the Diagnostic Effectiveness of Brain SPECT with [123I]Ioflupane and [123I]MIBG Scintigraphy in Multiple System Atrophy. Biomedicines 2024, 12, 102. https://doi.org/10.3390/biomedicines12010102
Villena-Salinas J, Ortega-Lozano SJ, Amrani-Raissouni T, Agüera-Morales E, Caballero-Villarraso J. Comparative Study between the Diagnostic Effectiveness of Brain SPECT with [123I]Ioflupane and [123I]MIBG Scintigraphy in Multiple System Atrophy. Biomedicines. 2024; 12(1):102. https://doi.org/10.3390/biomedicines12010102
Chicago/Turabian StyleVillena-Salinas, Javier, Simeón José Ortega-Lozano, Tomader Amrani-Raissouni, Eduardo Agüera-Morales, and Javier Caballero-Villarraso. 2024. "Comparative Study between the Diagnostic Effectiveness of Brain SPECT with [123I]Ioflupane and [123I]MIBG Scintigraphy in Multiple System Atrophy" Biomedicines 12, no. 1: 102. https://doi.org/10.3390/biomedicines12010102
APA StyleVillena-Salinas, J., Ortega-Lozano, S. J., Amrani-Raissouni, T., Agüera-Morales, E., & Caballero-Villarraso, J. (2024). Comparative Study between the Diagnostic Effectiveness of Brain SPECT with [123I]Ioflupane and [123I]MIBG Scintigraphy in Multiple System Atrophy. Biomedicines, 12(1), 102. https://doi.org/10.3390/biomedicines12010102






