Safety and Metabolic Tolerance of Citrate Anticoagulation in Critically Ill Polytrauma Patients with Acute Kidney Injury Requiring an Early Continuous Kidney Replacement Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection and Management of Trauma
2.3. Management and Technique of CKRT
2.4. Anticoagulation in Group Heparin
2.5. Anticoagulation in Group Citrate
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Survivors and Non-Survivors
3.2. Baseline Characteristics and Outcome of Patients According to Citrate and Heparin Anticoagulation
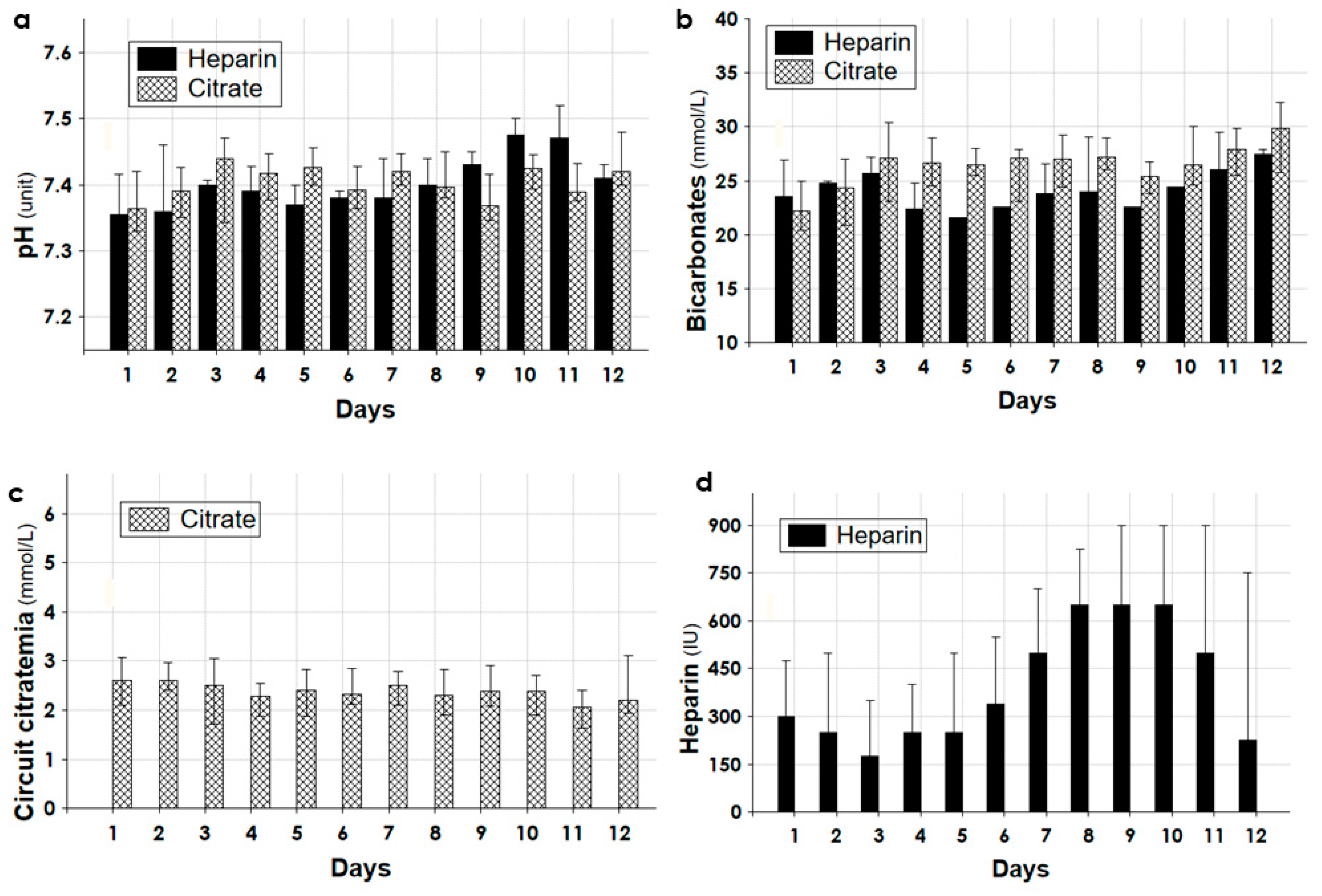
3.3. Dialytic and Clinical Data during the First 12 Days of CKRT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haines, R.W.; Fowler, A.J.; Kirwan, C.J.; Prowle, J.R. The Incidence and Associations of Acute Kidney Injury in Trauma Patients Admitted to Critical Care: A Systematic Review and Meta-Analysis. J. Trauma Acute Care Surg. 2019, 86, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Golino, G.; Greco, M.; Rigobello, A.; Danzi, V.; De Cal, M.; Malchiorna, N.; Zannella, M.; Navalesi, P.; Costa-Pinto, R.; Ronco, C.; et al. Incidence of Acute Kidney Injury in Polytrauma Patients and Predictive Performance of TIMP2 × IGFBP7 Biomarkers for Early Identification of Acute Kidney Injury. Diagnostics 2022, 12, 2481. [Google Scholar] [CrossRef]
- Søvik, S.; Isachsen, M.S.; Nordhuus, K.M.; Tveiten, C.K.; Eken, T.; Sunde, K.; Brurberg, K.G.; Beitland, S. Acute Kidney Injury in Trauma Patients Admitted to the ICU: A Systematic Review and Meta-Analysis. Intensive Care Med. 2019, 45, 407–419. [Google Scholar] [CrossRef]
- Bosch, X.; Poch, E.; Grau, J.M. Rhabdomyolysis and Acute Kidney Injury. N. Engl. J. Med. 2009, 361, 62–72. [Google Scholar] [CrossRef]
- Harrois, A.; Libert, N.; Duranteau, J. Acute Kidney Injury in Trauma Patients. Curr. Opin. Crit. Care 2017, 23, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Farhat, A.; Grigorian, A.; Nguyen, N.T.; Smith, B.; Williams, B.J.; Schubl, S.D.; Joe, V.; Elfenbein, D.; Nahmias, J. Obese Trauma Patients Have Increased Need for Dialysis. Eur. J. Trauma Emerg. Surg. 2020, 46, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Gomes, E.; Antunes, R.; Dias, C.; Araújo, R.; Costa-Pereira, A. Acute Kidney Injury in Severe Trauma Assessed by RIFLE Criteria: A Common Feature without Implications on Mortality? Scand. J. Trauma Resusc. Emerg. Med. 2010, 18, 1. [Google Scholar] [CrossRef]
- Mariano, F.; Tedeschi, L.; Morselli, M.; Stella, M.; Triolo, G. Normal Citratemia and Metabolic Tolerance of Citrate Anticoagulation for Hemodiafiltration in Severe Septic Shock Burn Patients. Intensive Care Med. 2010, 36, 1735–1743. [Google Scholar] [CrossRef]
- Mariano, F.; Morselli, M.; Holló, Z.; Agostini, F.; Stella, M.; Biancone, L. Citrate Pharmacokinetics at High Levels of Circuit Citratemia during Coupled Plasma Filtration Adsorption. Nephrol. Dial Transplant. 2015, 30, 1911–1919. [Google Scholar] [CrossRef]
- Kellum, J.A.; Lameire, N.; KDIGO AKI Guideline Work Group. Diagnosis, Evaluation, and Management of Acute Kidney Injury: A KDIGO Summary (Part 1). Crit. Care 2013, 17, 204. [Google Scholar] [CrossRef]
- Khadzhynov, D.; Dahlinger, A.; Schelter, C.; Peters, H.; Kindgen-Milles, D.; Budde, K.; Lehner, L.J.; Halleck, F.; Staeck, O.; Slowinski, T. Hyperlactatemia, Lactate Kinetics and Prediction of Citrate Accumulation in Critically Ill Patients Undergoing Continuous Renal Replacement Therapy With Regional Citrate Anticoagulation. Crit. Care Med. 2017, 45, e941–e946. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.-N.; Haroon, S.W.P.; Mukhopadhyay, A.; Lau, T.; Murali, T.M.; Phua, J.; Tan, Z.-Y.; Lee, N.; Chua, H.-R. Hyperlactatemia Predicts Citrate Intolerance With Regional Citrate Anticoagulation During Continuous Renal Replacement Therapy. J. Intensive Care Med. 2019, 34, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Hou, F.F.; Wu, Q.; Chen, P.Y.; Xie, D.; Zhang, X. Natural History and Impact on Outcomes of Acute Kidney Injury in Patients with Road Traffic Injury. Clin. Nephrol. 2009, 71, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Beitland, S.; Moen, H.; Os, I. Acute Kidney Injury with Renal Replacement Therapy in Trauma Patients. Acta Anaesthesiol. Scand. 2010, 54, 833–840. [Google Scholar] [CrossRef]
- Skinner, D.L.; Hardcastle, T.C.; Rodseth, R.N.; Muckart, D.J.J. The Incidence and Outcomes of Acute Kidney Injury amongst Patients Admitted to a Level I Trauma Unit. Injury 2014, 45, 259–264. [Google Scholar] [CrossRef]
- Eriksson, M.; Brattström, O.; Mårtensson, J.; Larsson, E.; Oldner, A. Acute Kidney Injury Following Severe Trauma: Risk Factors and Long-Term Outcome. J. Trauma Acute Care Surg. 2015, 79, 407–412. [Google Scholar] [CrossRef]
- Stewart, I.J.; Sosnov, J.A.; Howard, J.T.; Chung, K.K. Acute Kidney Injury in Critically Injured Combat Veterans: A Retrospective Cohort Study. Am. J. Kidney Dis. 2016, 68, 564–570. [Google Scholar] [CrossRef]
- Fujinaga, J.; Kuriyama, A.; Shimada, N. Incidence and Risk Factors of Acute Kidney Injury in the Japanese Trauma Population: A Prospective Cohort Study. Injury 2017, 48, 2145–2149. [Google Scholar] [CrossRef]
- Haines, R.W.; Lin, S.-P.; Hewson, R.; Kirwan, C.J.; Torrance, H.D.; O’Dwyer, M.J.; West, A.; Brohi, K.; Pearse, R.M.; Zolfaghari, P.; et al. Acute Kidney Injury in Trauma Patients Admitted to Critical Care: Development and Validation of a Diagnostic Prediction Model. Sci. Rep. 2018, 8, 3665. [Google Scholar] [CrossRef]
- Pape, H.-C.; Lefering, R.; Butcher, N.; Peitzman, A.; Leenen, L.; Marzi, I.; Lichte, P.; Josten, C.; Bouillon, B.; Schmucker, U.; et al. The Definition of Polytrauma Revisited: An International Consensus Process and Proposal of the New “Berlin Definition”. J. Trauma Acute Care Surg. 2014, 77, 780–786. [Google Scholar] [CrossRef]
- Kortbeek, J.B.; Al Turki, S.A.; Ali, J.; Antoine, J.A.; Bouillon, B.; Brasel, K.; Brenneman, F.; Brink, P.R.; Brohi, K.; Burris, D.; et al. Advanced Trauma Life Support, 8th Edition, the Evidence for Change. J. Trauma 2008, 64, 1638–1650. [Google Scholar] [CrossRef] [PubMed]
- Brochard, L.; Abroug, F.; Brenner, M.; Broccard, A.F.; Danner, R.L.; Ferrer, M.; Laghi, F.; Magder, S.; Papazian, L.; Pelosi, P.; et al. An Official ATS/ERS/ESICM/SCCM/SRLF Statement: Prevention and Management of Acute Renal Failure in the ICU Patient: An International Consensus Conference in Intensive Care Medicine. Am. J. Respir. Crit. Care Med. 2010, 181, 1128–1155. [Google Scholar] [CrossRef] [PubMed]
- Galvagno, S.M.; Nahmias, J.T.; Young, D.A. Advanced Trauma Life Support® Update 2019: Management and Applications for Adults and Special Populations. Anesthesiol. Clin. 2019, 37, 13–32. [Google Scholar] [CrossRef] [PubMed]
- Vivino, G.; Antonelli, M.; Moro, M.L.; Cottini, F.; Conti, G.; Bufi, M.; Cannata, F.; Gasparetto, A. Risk Factors for Acute Renal Failure in Trauma Patients. Intensive Care Med. 1998, 24, 808–814. [Google Scholar] [CrossRef]
- Ostermann, M.; Bellomo, R.; Burdmann, E.A.; Doi, K.; Endre, Z.H.; Goldstein, S.L.; Kane-Gill, S.L.; Liu, K.D.; Prowle, J.R.; Shaw, A.D.; et al. Controversies in Acute Kidney Injury: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Conference. Kidney Int. 2020, 98, 294–309. [Google Scholar] [CrossRef]
- Monchi, M.; Berghmans, D.; Ledoux, D.; Canivet, J.-L.; Dubois, B.; Damas, P. Citrate vs. Heparin for Anticoagulation in Continuous Venovenous Hemofiltration: A Prospective Randomized Study. Intensive Care Med. 2004, 30, 260–265. [Google Scholar] [CrossRef]
- Mariano, F.; Mella, A.; Rumbolo, F.; Holló, Z.; Bergamo, D.; Congiu, G.; Mengozzi, G.; Berardino, M.; Stella, M.; Biancone, L. Clearance of NT-ProBNP and Procalcitonin during Continuous Venovenous Hemodialysis with the Medium Cutoff Filter in Patients with Rhabdomyolysis-Associated Early Acute Kidney Injury. Blood Purif. 2023, 52, 446–454. [Google Scholar] [CrossRef]
- Mariano, F.; Morselli, M.; Bergamo, D.; Hollo, Z.; Scella, S.; Maio, M.; Tetta, C.; Dellavalle, A.; Stella, M.; Triolo, G. Blood and Ultrafiltrate Dosage of Citrate as a Useful and Routine Tool during Continuous Venovenous Haemodiafiltration in Septic Shock Patients. Nephrol. Dial. Transplant. 2011, 26, 3882–3888. [Google Scholar] [CrossRef]
- Mariano, F.; Hollo’, Z.; Depetris, N.; Malvasio, V.; Mella, A.; Bergamo, D.; Pensa, A.; Berardino, M.; Stella, M.; Biancone, L. Coupled-Plasma Filtration and Adsorption for Severe Burn Patients with Septic Shock and Acute Kidney Injury Treated with Renal Replacement Therapy. Burns 2020, 46, 190–198. [Google Scholar] [CrossRef]
- Brown, C.V.R.; Rhee, P.; Chan, L.; Evans, K.; Demetriades, D.; Velmahos, G.C. Preventing Renal Failure in Patients with Rhabdomyolysis: Do Bicarbonate and Mannitol Make a Difference? J. Trauma 2004, 56, 1191–1196. [Google Scholar] [CrossRef]
- Jones, A.R.; Bush, H.M.; Frazier, S.K. Injury Severity, Sex, and Transfusion Volume, but Not Transfusion Ratio, Predict Inflammatory Complications after Traumatic Injury. Heart Lung 2017, 46, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Morgera, S.; Schneider, M.; Slowinski, T.; Vargas-Hein, O.; Zuckermann-Becker, H.; Peters, H.; Kindgen-Milles, D.; Neumayer, H.-H. A Safe Citrate Anticoagulation Protocol with Variable Treatment Efficacy and Excellent Control of the Acid-Base Status. Crit. Care Med. 2009, 37, 2018–2024. [Google Scholar] [CrossRef] [PubMed]
- Malbrain, M.L.N.G.; Martin, G.; Ostermann, M. Everything You Need to Know about Deresuscitation. Intensive Care Med. 2022, 48, 1781–1786. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, I.; Bellomo, R.; Koch, B. Blood Flow Reductions during Continuous Renal Replacement Therapy and Circuit Life. Intensive Care Med. 2004, 30, 2074–2079. [Google Scholar] [CrossRef] [PubMed]
- Brain, M.; Winson, E.; Roodenburg, O.; McNeil, J. Non Anti-Coagulant Factors Associated with Filter Life in Continuous Renal Replacement Therapy (CRRT): A Systematic Review and Meta-Analysis. BMC Nephrol. 2017, 18, 69. [Google Scholar] [CrossRef]
- Schiffl, H. Intensity of Renal Replacement Therapy and Outcomes in Critically Ill Patients with Acute Kidney Injury: Critical Appraisal of the Dosing Recommendations. Ther. Apher. Dial. 2020, 24, 620–627. [Google Scholar] [CrossRef]
- Zanza, C.; Romenskaya, T.; Racca, F.; Rocca, E.; Piccolella, F.; Piccioni, A.; Saviano, A.; Formenti-Ujlaki, G.; Savioli, G.; Franceschi, F.; et al. Severe Trauma-Induced Coagulopathy: Molecular Mechanisms Underlying Critical Illness. Int. J. Mol. Sci. 2023, 24, 7118. [Google Scholar] [CrossRef]
- Oudemans-van Straaten, H.M. Hemostasis and Thrombosis in Continuous Renal Replacement Treatment. Semin. Thromb. Hemost. 2015, 41, 91–98. [Google Scholar] [CrossRef]
- Ricci, Z.; Tolwani, A.; Lumlertgul, N. Precision Renal Replacement Therapy. Curr. Opin. Crit. Care 2020, 26, 574–580. [Google Scholar] [CrossRef]
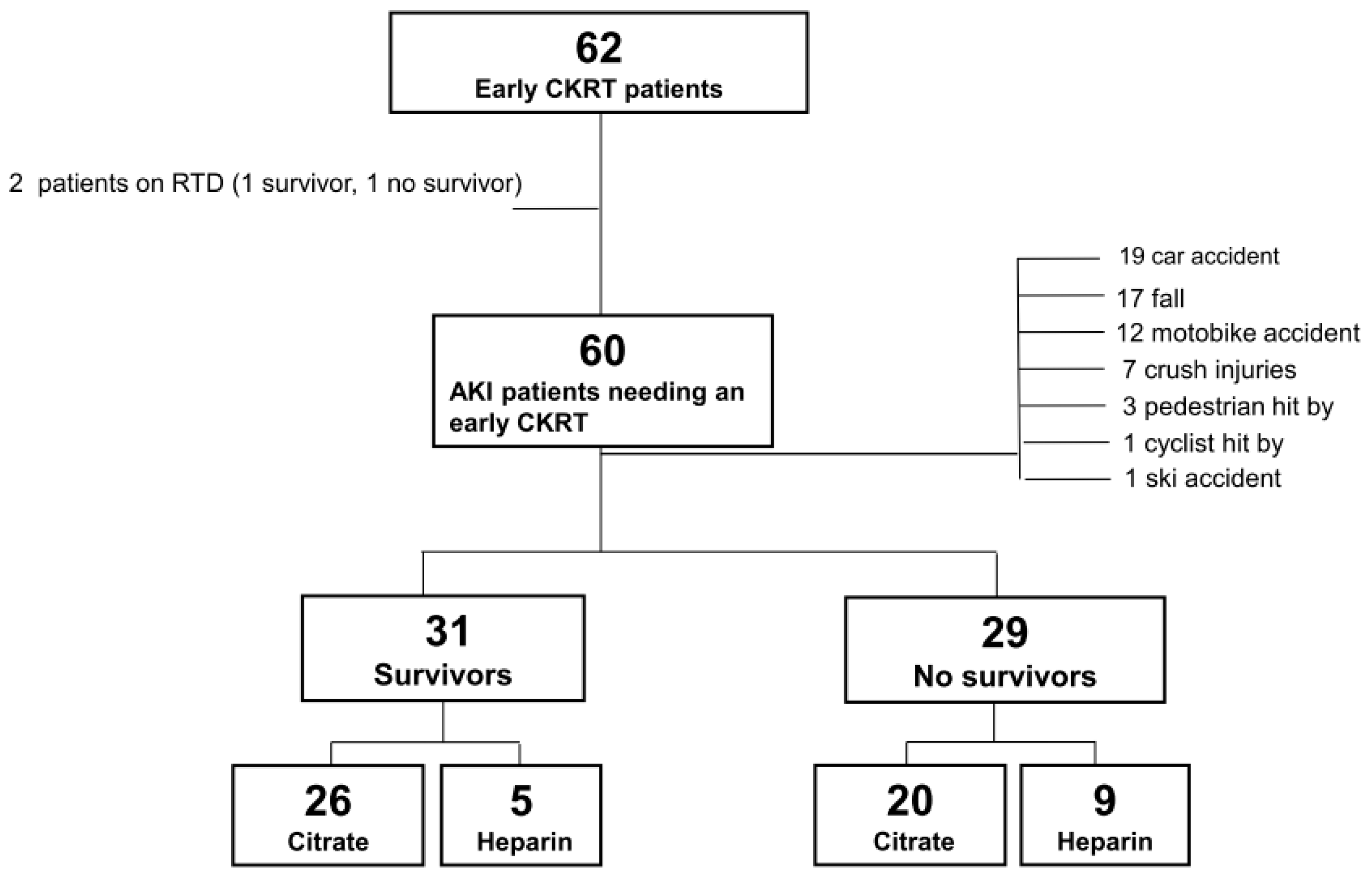
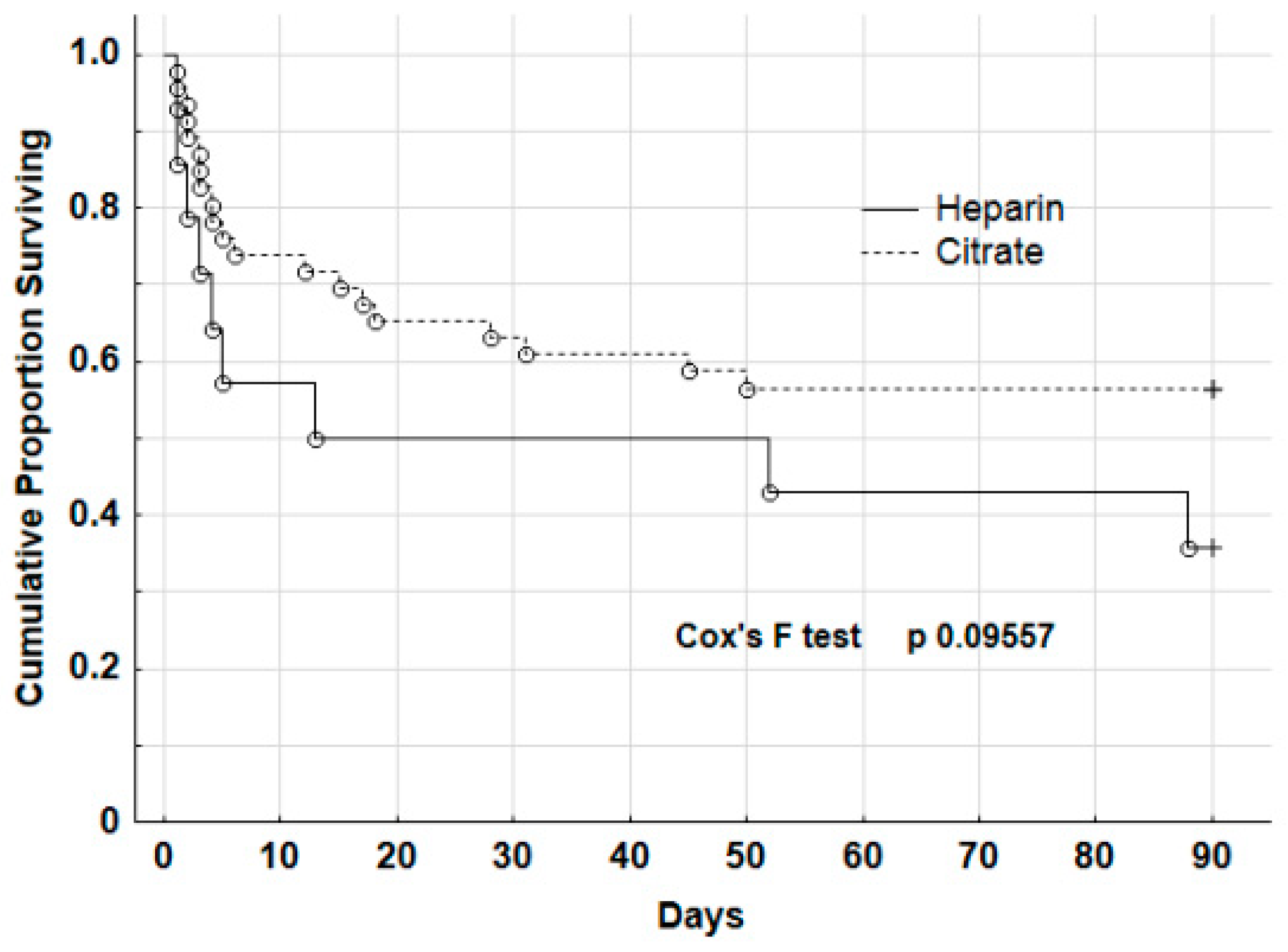
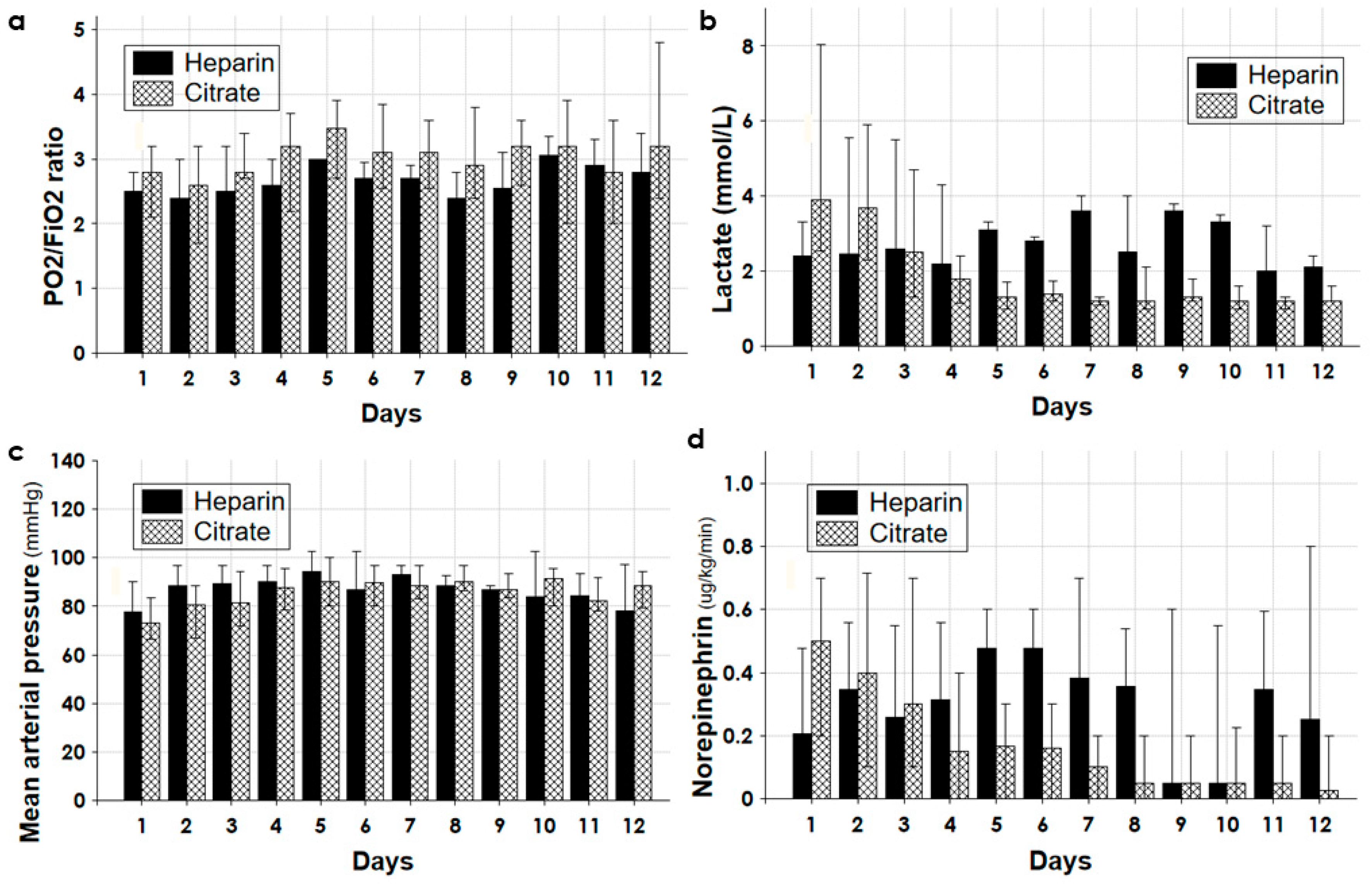
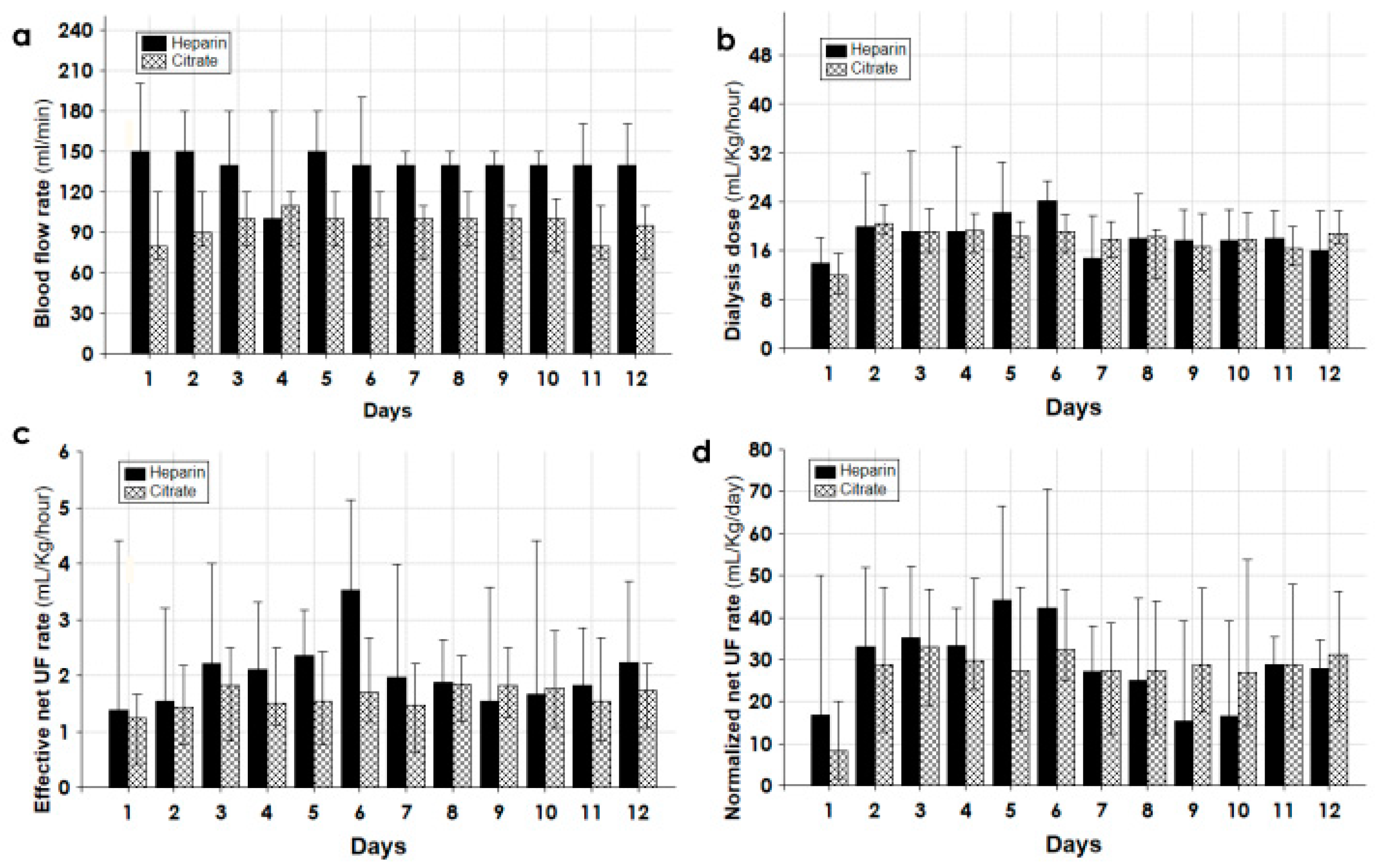
| All | Survivors | Non-Survivors | p | |
|---|---|---|---|---|
| Patients (%, n) | 60 | 51.7%, 31 | 48.3%, 29 | - |
| Age (years) | 54 (38.5–72) | 45.0 (31–68) | 68.0 (49–78) | 0.002 |
| Gender ratio (male/female) | 55/5 | 28/3 | 27/2 | 0.699 |
| Mechanical ventilation (%, n) | 100%, 60 | 100%, 31 | 100%, 29 | - |
| Rhabdomyolysis (%, n) | 65.0%, 39 | 77.4%, 24 | 51.7%, 15 | 0.037 |
| Urine output (mL/day) | 180 (0–935) | 390 (0–2400) | 0.0 (0–250) | 0.003 |
| CKRT days | 768 | 606 | 162 | - |
| Median CKRT time (days) | 10 (3–18) | 11 (5–22) | 3 (2–13) | 0.035 |
| CKRT interval (days after admission) | 2 (2–3) | 2 (2–3) | 2 (2–3) | 0.810 |
| Citrate anticoagulation (%, n) | 76.7%, 46 | 81.2%, 26 | 71.2%,20 | 0.369 |
| Exitus interval (days after admission) | - | - | 4.0 (2–17) | - |
| on 1st day of CKRT a | ||||
| SOFA score | 13 (11–15) | 12 (11–14) | 15 (13–16) | 0.006 |
| Cardiovascular | 4 (4–4) | 4 (1.5–4) | 4 (4–4) | 0.015 |
| Respiratory | 2 (1–3) | 2 (1–2) | 2 (1.5–3) | 0.011 |
| Hematologic | 2 (1–2) | 2 (1–2) | 2 (1–2) | 0.431 |
| Liver | 2 (1–2) | 1 (1–2) | 2 (2–2) | 0.060 |
| Norepinephrine (ug/kg/min) | 0.4 (0.2–0.7) | 0.35 (0.1–0.5) | 0.5 (0.3–1.0) | 0.025 |
| Lactate (mmol/L) | 3.4 (2.1–8.0) | 2.8 (1.9–4.6) | 4.6 (2.4–10.1) | 0.014 |
| Bicarbonates (mmol/L) | 22.4 (19.4–25.0) | 23.0 (21.6–25.4) | 20.8 (16.9–25.0) | 0.005 |
| Odds Ratio | Confidence Intervals | p-Value | ||
|---|---|---|---|---|
| −95% CL | +95% CL | |||
| SOFA score (on the 1st day) | 1.472 | 1.076 | 2.014 | 0.013 |
| Age | 1.049 | 1.008 | 1.092 | 0.016 |
| Norepinephrine | 1.459 | 0.284 | 7.494 | 0.643 |
| Lactates | 1.020 | 0.831 | 1.252 | 0.843 |
| Bicarbonates | 0.886 | 0.746 | 1.052 | 0.159 |
| Group Citrate | Group Heparin | ||||||
|---|---|---|---|---|---|---|---|
| All | Dead | Alive | All | Dead | Alive | p | |
| Patients (%, n) | 46 | 43.5%, 20 | 56.5%, 26 | 14 | 57.1%, 9 | 42.9%, 5 | 0.121 |
| Age (years) | 54.5 (41–72) | 66 (54–79) | 46 (38–68) | 50 (26–71) | 71 (35–73) | 26 (25–54) | 0.371 |
| Sex ratio (male/female) | 42/4 | 19/1 | 23/3 | 13/1 | 8/1 | 5/0 | 0.854 |
| Mechanical ventilation (%, n) | 100%, 46 | 100%, 20 | 100%, 26 | 100%, 14 | 100%, 9 | 100%, 5 | - |
| Rhabdomyolysis (%, n) | 63.1%, 29 | 50.0%, 10 | 73.1%, 19 | 71.4%, 10 | 55.5%, 5 | 80.0%, 4 | 0.565 |
| Median CKRT time (days) | 11.0 (4–21) | 4.5 (3–18) | 12.5 (5–22) | 7.0 (1–13) | 3.0 (1–12) | 10 (8–13) | 0.781 |
| CKRT interval (days after admission) | 2.0 (2–3) | 2.0 (2–3) | 2.0 (2–3) | 3.0 (2–3) | 2.0 (1–3) | 3.0 (3–3) | 0.745 |
| Exitus interval (days post-CRRT) | - | 4.5 (2.5–17.5) | - | - | 4.0 (2.0–13) | - | 0.466 |
| Urine output (mL/day) | 158 (0–890) | 375 (0–2400) | 0 (0–230) | 290 (0–1600) | 150 (0–400) | 1600 (860–2400) | 0.551 |
| on 1st day of CKRT a | |||||||
| SOFA score | 13.0 (12–15) | 15.0 (13–16) | 12.0 (10–14) | 13.5 (11–16) | 15.0 (13–16) | 12.0 (10–14) | 0.368 |
| Cardiovascular | 4 (4–4) | 4 (4–4) | 4 (3–4) | 4 (4–4) | 4 (4–4) | 4 (0–4) | 0.542 |
| Respiratory | 2 (1–3) | 2 (1–3) | 1 (1–2) | 2 (2–3) | 2 (2–3) | 2 (2–3) | 0.059 |
| Hematologic | 2 (1–2) | 2 (1–2.5) | 2 (1–2) | 2 (1–2) | 2 (1–2) | 2 (2–2) | 0.676 |
| Liver | 2 (1–2) | 2 (1–2) | 1.0 (1–2) | 2 (1–2) | 2 (1–2) | 2 (1–2) | 0.683 |
| Norepinephrine (μg/kg/min) a | 0.43 (0.2–0.7) | 0.5 (0.3–1.0) | 0.38 (0.1–0.6) | 0.35 (0.1–0.7) | 0.47(0.2–0.7) | 0.18 (0.1–0.5) | 0.385 |
| Lactate (mmol/L) a | 3.9 (2.1–8.0) | 5.1 (2.6–10.1) | 3.0 (1.9–4.6) | 2.5 (2.1–4.8) | 2.2 (2.2–2.6) | 3.1 (2.4–8.1) | 0.966 |
| Bicarbonates (mmol/L) a | 22.1 (18–25) | 20.5 (16–25) | 22.6 (22–25) | 23.0 (21–25) | 21.6 (17–23) | 25.0 (24–25) | 0.685 |
| All | Group Citrate | Group Heparin | p | |
|---|---|---|---|---|
| CKRT days | 418 | 306 | 112 | - |
| Effective time of CKRT (h/day) | 20.0 (11–23) | 20.0 (12–23) | 16.0 (7–22) | 0.002 |
| Filter lifespan (h) | 36.0 (24–48) | 38.5 (24–49) | 33.0 (24–41) | 0.250 |
| Blood flow rate (mL/min) | 100 (80–120) | 100 (80–120) | 140 (100–180) | 0.002 |
| Delivered dialysis dose (mL/kg/h) | 18.0 (13.2–22.2) | 18.1 (13.3–21.2) | 17.8 (12.8–24.0) | 0.075 |
| Normalized net fluid removal (mL/kg/day) | 28.0 (11.1–46.0) | 27.5 (11.8–45.7) | 33.1 (9.8–49.7) | 0.308 |
| Effective net fluid removal (mL/kg/h) | 1.61 (0.83–2.50) | 1.53 (0.76–2.35) | 2.03 (1.40–3.39) | 0.002 |
| Circuit citratemia (mmol/L) | - | 3.5 (2.9–4.0) | - | - |
| Unfractionated heparin (units/h) | - | - | 350 (150–650) | - |
| Hemorrhagic episodes (1/days, n) | 0.103, 43 | 0.045, 14 | 0.273, 29 | 0.002 |
| Parameter | Citrate | Heparin |
|---|---|---|
| Daily effective time | high, dialysis more continuous | decreased, with more down time |
| Prescribed fluid removal (mL/kg/daily) | potentially high | potentially high |
| Effective net fluid removal (mL/kg/h) | low, and constant | variable, and higher |
| Blood flow rate (mL/min) | low, enough at 80–100 mL/min | higher, at 120–140 to increase circuit patency |
| Metabolic tolerance | good, with reduced blood flow rate (60–80 mL/min) and low circuit citratemia (2 mmol/L) | good |
| Hemodynamic stability | good, constant over time | variable, depending on prescribed UF and blood flow rate |
| Circuit anticoagulation efficacy | high, predictable | less predictable (depending on platelet count, ATIII), poor with no heparin/saline flushes |
| Hemorrhagic episodes | low incidence | increased incidence |
| Nursing workload | Low | Increased |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariano, F.; Mella, A.; Randone, P.; Agostini, F.; Bergamo, D.; Berardino, M.; Biancone, L. Safety and Metabolic Tolerance of Citrate Anticoagulation in Critically Ill Polytrauma Patients with Acute Kidney Injury Requiring an Early Continuous Kidney Replacement Therapy. Biomedicines 2023, 11, 2570. https://doi.org/10.3390/biomedicines11092570
Mariano F, Mella A, Randone P, Agostini F, Bergamo D, Berardino M, Biancone L. Safety and Metabolic Tolerance of Citrate Anticoagulation in Critically Ill Polytrauma Patients with Acute Kidney Injury Requiring an Early Continuous Kidney Replacement Therapy. Biomedicines. 2023; 11(9):2570. https://doi.org/10.3390/biomedicines11092570
Chicago/Turabian StyleMariano, Filippo, Alberto Mella, Paolo Randone, Fulvio Agostini, Daniela Bergamo, Maurizio Berardino, and Luigi Biancone. 2023. "Safety and Metabolic Tolerance of Citrate Anticoagulation in Critically Ill Polytrauma Patients with Acute Kidney Injury Requiring an Early Continuous Kidney Replacement Therapy" Biomedicines 11, no. 9: 2570. https://doi.org/10.3390/biomedicines11092570
APA StyleMariano, F., Mella, A., Randone, P., Agostini, F., Bergamo, D., Berardino, M., & Biancone, L. (2023). Safety and Metabolic Tolerance of Citrate Anticoagulation in Critically Ill Polytrauma Patients with Acute Kidney Injury Requiring an Early Continuous Kidney Replacement Therapy. Biomedicines, 11(9), 2570. https://doi.org/10.3390/biomedicines11092570








