Amyloid-Beta Peptides Trigger Premature Functional and Gene Expression Alterations in Human-Induced Neurons
Abstract
:1. Introduction
2. Materials and Methods
2.1. Maintenance of hiPSCs and Neural Induction
2.2. Differentiation of hiNPCs and Mixed Cultures of hiNs and hiAs
2.3. Cell-Secreted Aβ Peptides Treatment
2.4. Synthetic Aβ1–42 Treatment
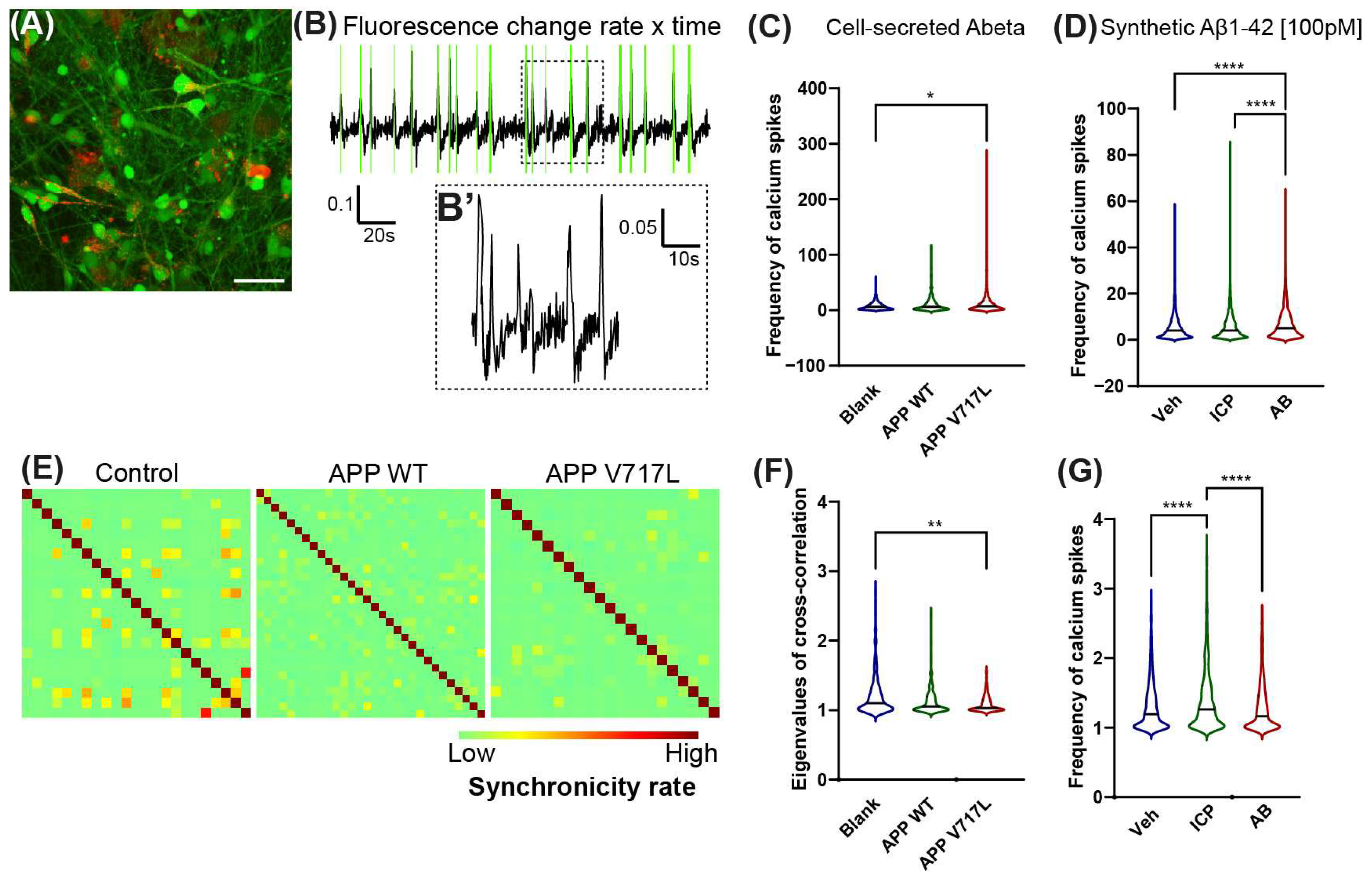
2.5. Calcium Imaging Experiments
2.6. Analyses of Calcium Transients
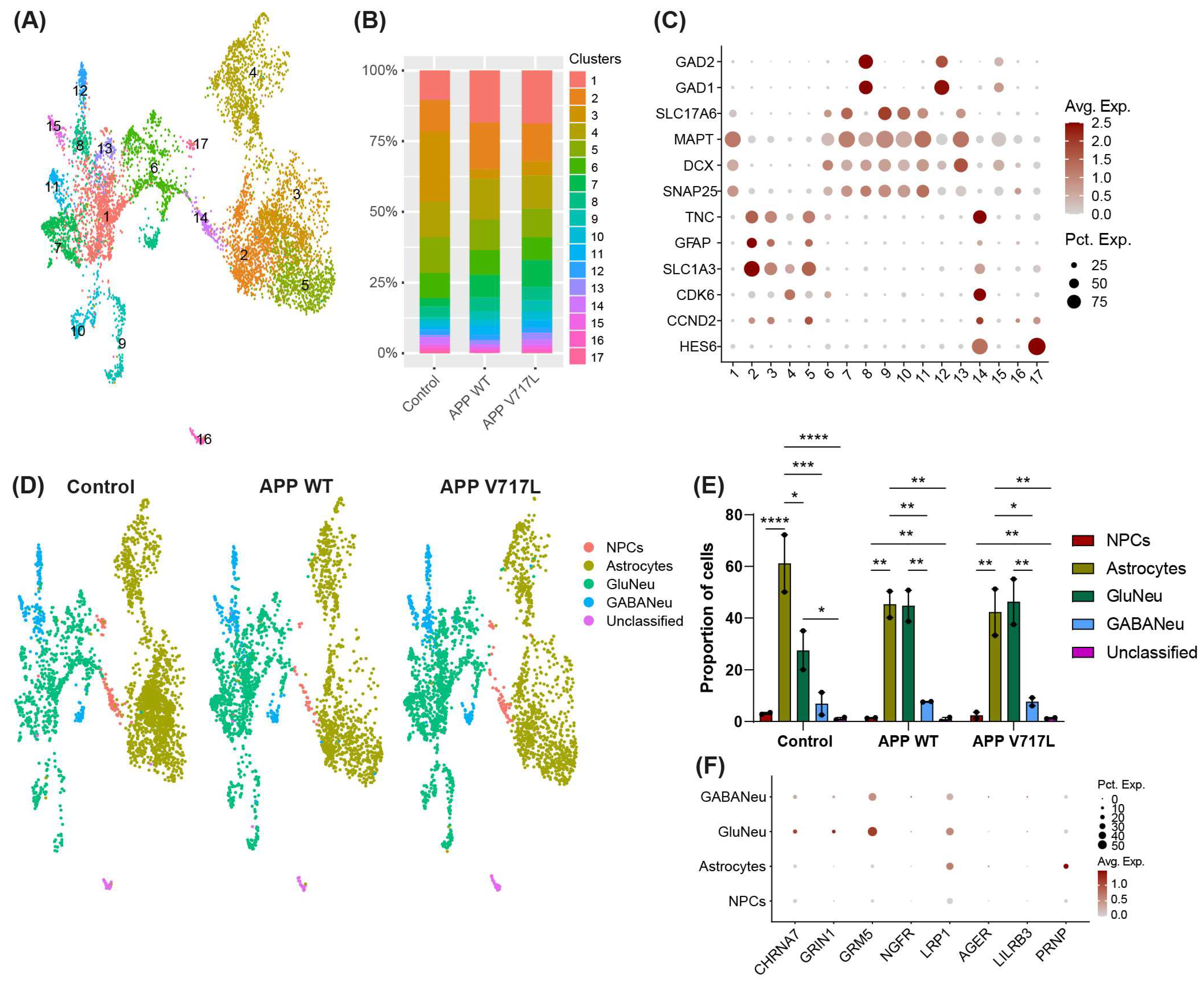
2.7. Single-Nucleus RNA Sequencing
2.8. Statistical Analysis
3. Results
3.1. Exposure to Low Concentrations of Aβ Peptides Increases Electrical Activity and Reduces Synchronicity of hiNs
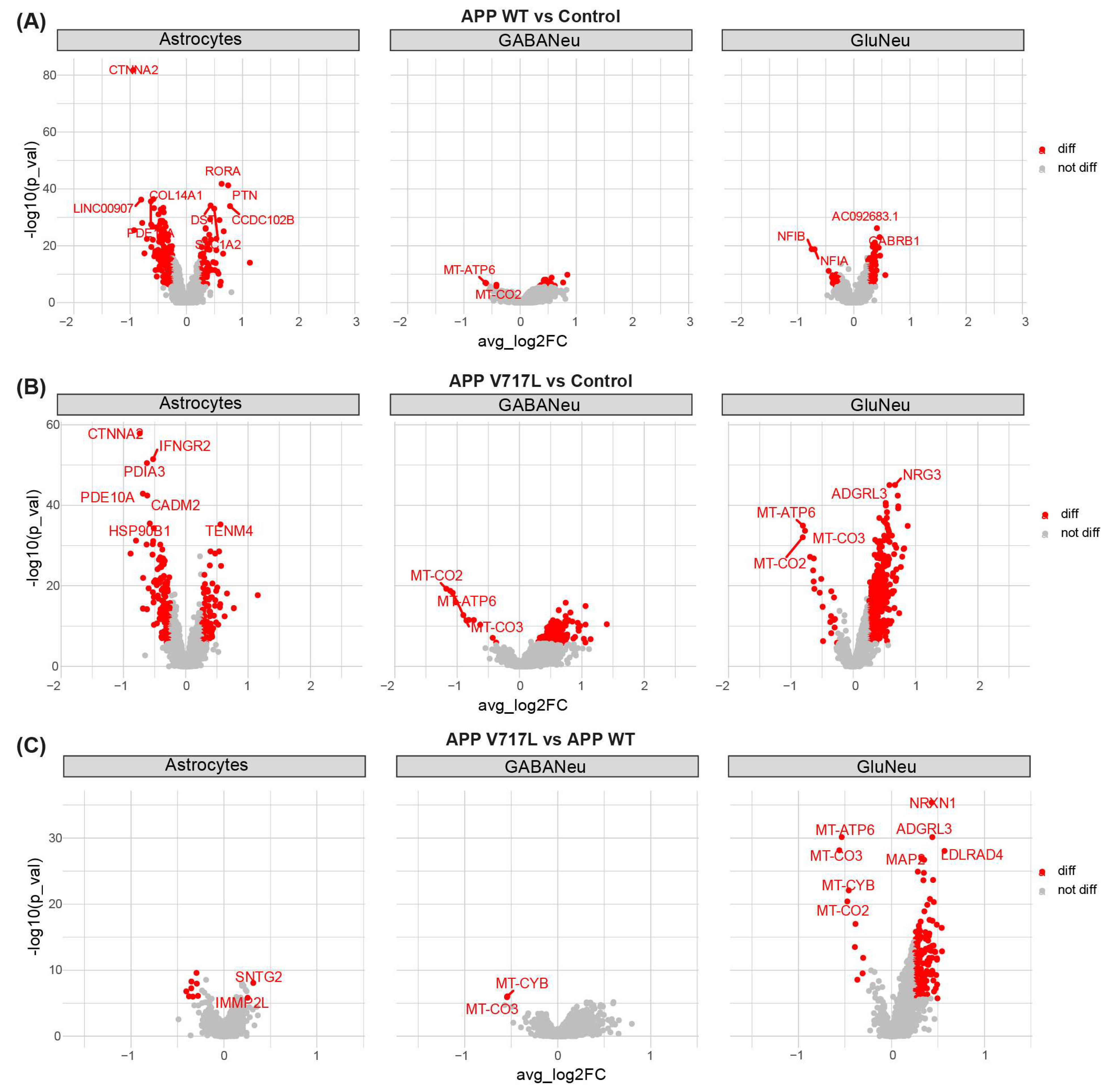
3.2. Treatment with Conditioned Media Containing Higher Concentrations of Aβ Peptides Elicits Transcriptional Changes Mainly in hiNs
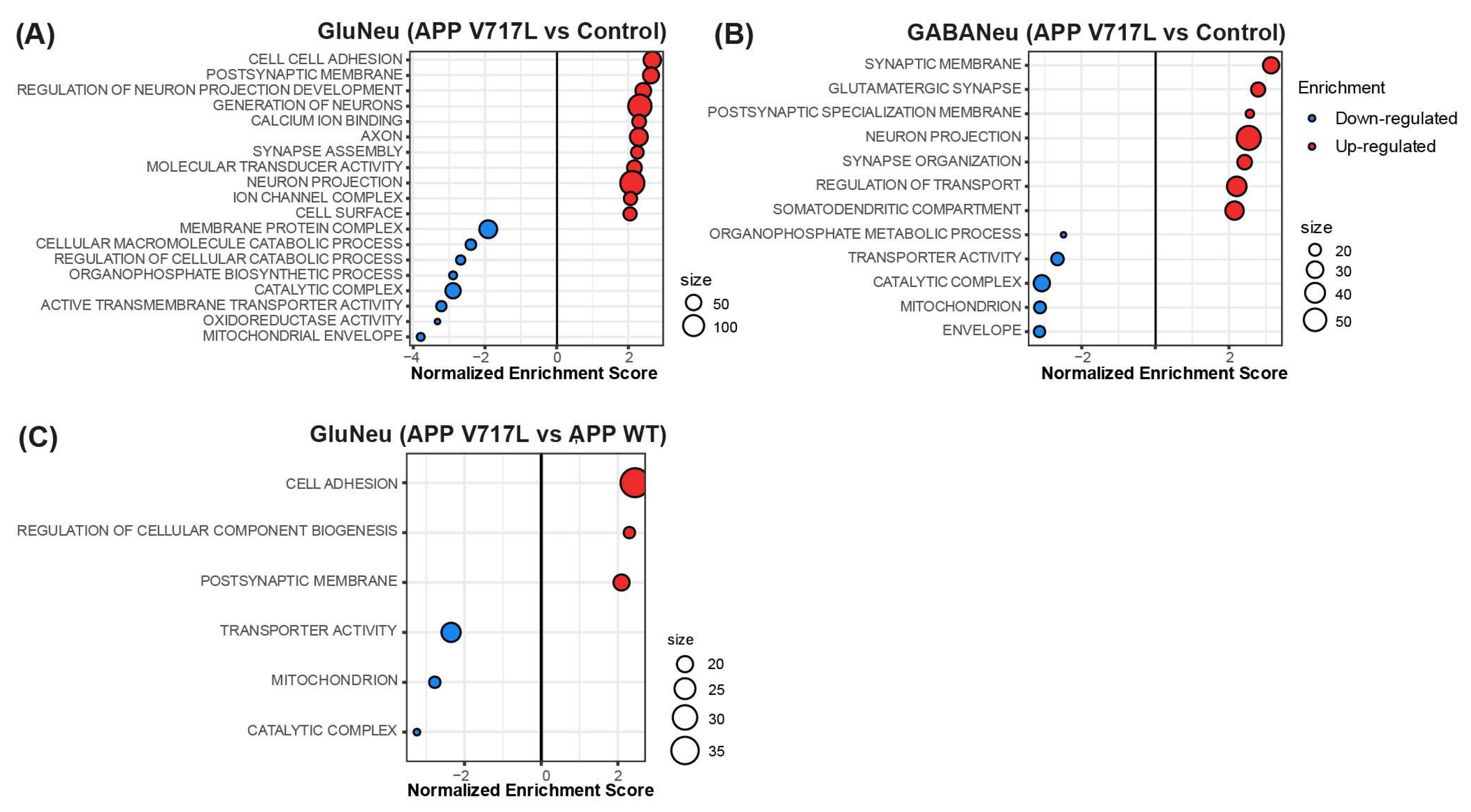
3.3. Genes Modulated by Aβ Peptides Are Mainly Associated with Oxidative Stress and Synapse Transmission
3.4. Selective Effect of Aβ on Synaptic Processes of Glutamatergic Neurons
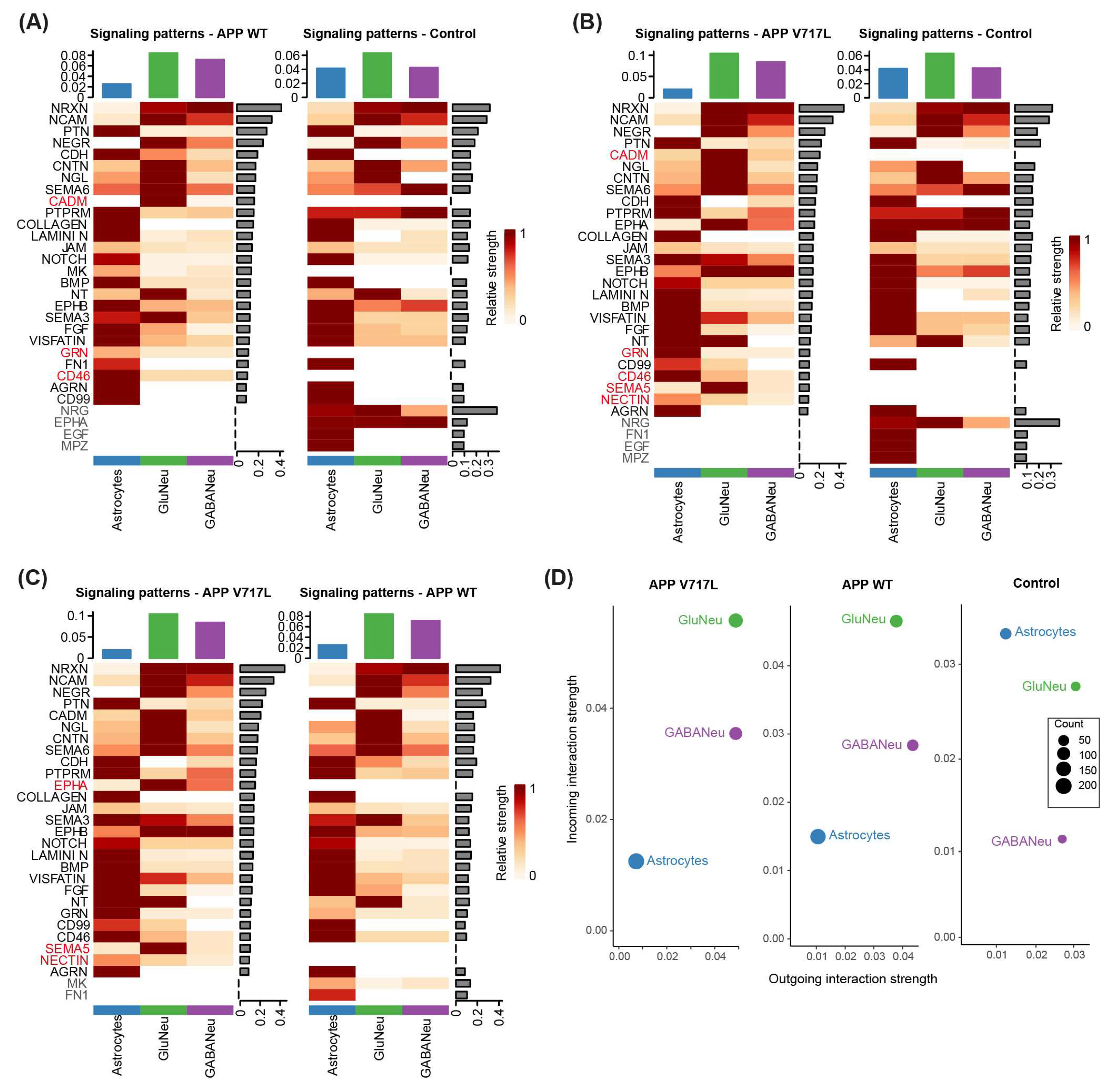
3.5. Exposure to Aβ Peptides Alters Neuroglial Communication
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prince, M.; Ali, G.C.; Guerchet, M.; Prina, A.M.; Albanese, E.; Wu, Y.T. Recent Global Trends in the Prevalence and Incidence of Dementia, and Survival with Dementia. Alzheimer’s Res. Ther. 2016, 8, 23. [Google Scholar] [CrossRef]
- Karran, E.; Mercken, M.; Strooper, B. De The Amyloid Cascade Hypothesis for Alzheimer’s Disease: An Appraisal for the Development of Therapeutics. Nat. Rev. Drug Discov. 2011, 10, 698–712. [Google Scholar] [CrossRef] [PubMed]
- Lacorte, E.; Ancidoni, A.; Zaccaria, V.; Remoli, G.; Tariciotti, L.; Bellomo, G.; Sciancalepore, F.; Corbo, M.; Lombardo, F.L.; Bacigalupo, I.; et al. Safety and Efficacy of Monoclonal Antibodies for Alzheimer’s Disease: A Systematic Review and Meta-Analysis of Published and Unpublished Clinical Trials. J. Alzheimer’s Dis. 2022, 87, 101–129. [Google Scholar] [CrossRef] [PubMed]
- Palop, J.J.; Mucke, L. Network Abnormalities and Interneuron Dysfunction in Alzheimer Disease. Nat. Rev. Neurosci. 2016, 17, 777–792. [Google Scholar] [CrossRef] [PubMed]
- Vossel, K.A.; Tartaglia, M.C.; Nygaard, H.B.; Zeman, A.Z.; Miller, B.L. Review Epileptic Activity in Alzheimer’s Disease: Causes and Clinical Relevance. Lancet Neurol. 2017, 16, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Canter, R.G.; Penney, J.; Tsai, L.H. The Road to Restoring Neural Circuits for the Treatment of Alzheimer’s Disease. Nature 2016, 539, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Styr, B.; Slutsky, I. Imbalance between Firing Homeostasis and Synaptic Plasticity Drives Early-Phase Alzheimer’s Disease. Nat. Neurosci. 2018, 21, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.S.; Wolf, F.; De Strooper, B.; Busche, M.A. Tipping the Scales: Peptide-Dependent Dysregulation of Neural Circuit Dynamics in Alzheimer’s Disease. Neuron 2020, 107, 417–435. [Google Scholar] [CrossRef]
- Gulisano, W.; Melone, M.; Ripoli, C.; Tropea, M.R.; Li Puma, D.D.; Giunta, S.; Cocco, S.; Marcotulli, D.; Origlia, N.; Palmeri, A.; et al. Neuromodulatory Action of Picomolar Extracellular Aβ42 Oligomers on Presynaptic and Postsynaptic Mechanisms Underlying Synaptic Function and Memory. J. Neurosci. 2019, 39, 5986–6000. [Google Scholar] [CrossRef]
- Zott, B.; Simon, M.M.; Hong, W.; Unger, F.; Chen-Engerer, H.J.; Frosch, M.P.; Sakmann, B.; Walsh, D.M.; Konnerth, A. A Vicious Cycle of β Amyloid−dependent Neuronal Hyperactivation. Science 2019, 365, 559–565. [Google Scholar] [CrossRef]
- Cirrito, J.R.; Yamada, K.A.; Finn, M.B.; Sloviter, R.S.; Bales, K.R.; May, P.C.; Schoepp, D.D.; Paul, S.M.; Mennerick, S.; Holtzman, D.M. Synaptic Activity Regulates Interstitial Fluid Amyloid-β Levels in Vivo. Neuron 2005, 48, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Bero, A.W.; Yan, P.; Roh, J.H.; Cirrito, J.R.; Stewart, F.R.; Raichle, M.E.; Lee, J.M.; Holtzman, D.M. Neuronal Activity Regulates the Regional Vulnerability to Amyloid-β 2 Deposition. Nat. Neurosci. 2011, 14, 750–756. [Google Scholar] [CrossRef]
- Busche, M.A.; Chen, X.; Henning, H.A.; Reichwald, J.; Staufenbiel, M.; Sakmann, B.; Konnerth, A. Critical Role of Soluble Amyloid-β for Early Hippocampal Hyperactivity in a Mouse Model of Alzheimer’s Disease. Proc. Natl. Acad. Sci. USA 2012, 109, 8740–8745. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.V.; Skotte, N.H.; Christensen, S.K.; Polli, F.S.; Shabani, M.; Markussen, K.H.; Haukedal, H.; Westi, E.W.; Diaz-delCastillo, M.; Sun, R.C.; et al. Hippocampal Disruptions of Synaptic and Astrocyte Metabolism Are Primary Events of Early Amyloid Pathology in the 5xFAD Mouse Model of Alzheimer’s Disease. Cell Death Dis. 2021, 12, 954. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ma, X.; Wei, J.; Shakir, N.; Zhang, J.K.; Zhang, L.; Nehme, A.; Cui, Y.; Ferguson, D.; Bai, F.; et al. Early Impairment of Cortical Circuit Plasticity and Connectivity in the 5XFAD Alzheimer’s Disease Mouse Model. Transl. Psychiatry 2022, 12, 371. [Google Scholar] [CrossRef]
- Ghatak, S.; Dolatabadi, N.; Trudler, D.; Zhang, X.; Wu, Y.; Mohata, M.; Ambasudhan, R.; Talantova, M.; Lipton, S.A. Mechanisms of Hyperexcitability in Alzheimer’s Disease HiPSC-Derived Neurons and Cerebral Organoids vs. Isogenic Control. eLife 2019, 8, e50333. [Google Scholar] [CrossRef]
- Chambers, S.M.; Fasano, C.A.; Papapetrou, E.P.; Tomishima, M.; Sadelain, M.; Studer, L. Highly Efficient Neural Conversion of Human ES and IPS Cells by Dual Inhibition of SMAD Signaling. Nat. Biotechnol. 2009, 27, 275–280. [Google Scholar] [CrossRef]
- Bardy, C.; Van Den Hurk, M.; Eames, T.; Marchand, C.; Hernandez, R.V.; Kellogg, M.; Gorris, M.; Galet, B.; Palomares, V.; Brown, J.; et al. Neuronal Medium That Supports Basic Synaptic Functions and Activity of Human Neurons in Vitro. Proc. Natl. Acad. Sci. USA 2015, 112, E2725–E2734. [Google Scholar] [CrossRef]
- Guillot-Sestier, M.V.; Sunyach, C.; Ferreira, S.T.; Marzolo, M.P.; Bauer, C.; Thevenet, A.; Checler, F. α-Secretase-Derived Fragment of Cellular Prion, N1, Protects against Monomeric and Oligomeric Amyloid β(Aβ)-Associated Cell Death. J. Biol. Chem. 2012, 287, 5021–5032. [Google Scholar] [CrossRef]
- Radstake, F.D.W.; Raaijmakers, E.A.L.; Luttge, R.; Zinger, S.; Frimat, J.P. CALIMA: The Semi-Automated Open-Source Calcium Imaging Analyzer. Comput. Methods Programs Biomed. 2019, 179, 104991. [Google Scholar] [CrossRef]
- Umakantha, A.; Morina, R.; Cowley, B.R.; Snyder, A.C.; Smith, M.A.; Yu, B.M. Bridging Neuronal Correlations and Dimensionality Reduction. Neuron 2021, 109, 2740–2754.e12. [Google Scholar] [CrossRef] [PubMed]
- Korsunsky, I.; Millard, N.; Fan, J.; Slowikowski, K.; Zhang, F.; Wei, K.; Baglaenko, Y.; Brenner, M.; Loh, P.-R. Fast, sensitive and accurate integration of single-cell data with Harmony. Nat. Methods 2019, 16, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Hafemeister, C.; Satija, R. Normalization and variance stabilization of single-cell RNA-seq data using regularized negative binomial regression. Genome Biol. 2019, 20, 296. [Google Scholar] [CrossRef] [PubMed]
- Kilinc, D.; Vreulx, A.-C.; Mendes, T.; Flaig, A.; Marques-Coelho, D.; Verschoore, M.; Demiautte, F.; Amouyel, P.; Eysert, F.; Dourlen, P.; et al. Pyk2 Overexpression in Postsynaptic Neurons Blocks Amyloid Β1–42-Induced Synaptotoxicity in Microfluidic Co-Cultures. Brain Commun. 2020, 2, fcaa139. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.F.; Xu, T.H.; Yan, Y.; Zhou, Y.R.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid Beta: Structure, Biology and Structure-Based Therapeutic Development. Acta Pharmacol. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef] [PubMed]
- Koopmans, F.; van Nierop, P.; Andres-Alonso, M.; Byrnes, A.; Cijsouw, T.; Coba, M.P.; Cornelisse, L.N.; Farrell, R.J.; Goldschmidt, H.L.; Howrigan, D.P.; et al. SynGO: An Evidence-Based, Expert-Curated Knowledge Base for the Synapse. Neuron 2019, 103, 217–234.e4. [Google Scholar] [CrossRef]
- Jin, S.; Guerrero-Juarez, C.F.; Zhang, L.; Chang, I.; Ramos, R.; Kuan, C.H.; Myung, P.; Plikus, M.V.; Nie, Q. Inference and Analysis of Cell-Cell Communication Using CellChat. Nat. Commun. 2021, 12, 1088. [Google Scholar] [CrossRef]
- Gulisano, W.; Melone, M.; Li Puma, D.D.; Tropea, M.R.; Palmeri, A.; Arancio, O.; Grassi, C.; Conti, F.; Puzzo, D. The Effect of Amyloid-β Peptide on Synaptic Plasticity and Memory Is Influenced by Different Isoforms, Concentrations, and Aggregation Status. Neurobiol. Aging 2018, 71, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Kazim, S.F.; Seo, J.H.; Bianchi, R.; Larson, C.S.; Sharma, A.; Wong, R.K.S.; Gorbachev, K.Y.; Pereira, A.C. Neuronal Network Excitability in Alzheimer’s Disease: The Puzzle of Similar versus Divergent Roles of Amyloid B and TAU. eNeuro 2021, 8, 0418-20. [Google Scholar] [CrossRef]
- Willem, M.; Tahirovic, S.; Busche, M.A.; Ovsepian, S.V.; Chafai, M.; Kootar, S.; Hornburg, D.; Evans, L.D.B.; Moore, S.; Daria, A.; et al. σ-Secretase Processing of APP Inhibits Neuronal Activity in the Hippocampus. Nature 2015, 526, 443–447. [Google Scholar] [CrossRef]
- Spires-Jones, T.L.; Hyman, B.T. The Intersection of Amyloid Beta and Tau at Synapses in Alzheimer’s Disease. Neuron 2014, 82, 756–771. [Google Scholar] [CrossRef] [PubMed]
- Kwart, D.; Gregg, A.; Scheckel, C.; Murphy, E.; Paquet, D.; Duffield, M.; Fak, J.; Olsen, O.; Darnell, R.; Tessier-Lavigne, M. A Large Panel of Isogenic APP and PSEN1 Mutant Human IPSC Neurons Reveals Shared Endosomal Abnormalities Mediated by APP β-CTFs, Not Aβ. Neuron 2019, 104, 256–270.e5. [Google Scholar] [CrossRef] [PubMed]
- Mitew, S.; Kirkcaldie, M.T.K.; Dickson, T.C.; Vickers, J.C. Altered Synapses and Gliotransmission in Alzheimer’s Disease and AD Model Mice. Neurobiol. Aging 2013, 34, 2341–2351. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.; Gsell, W.; Wahis, J.; Luckett, E.S.; Jamoulle, T.; Vermaercke, B.; Preman, P.; Moechars, D.; Hendrickx, V.; Jaspers, T.; et al. Astrocyte Calcium Dysfunction Causes Early Network Hyperactivity in Alzheimer’s Disease. Cell Rep. 2022, 40, 111280. [Google Scholar] [CrossRef]
- Konstantinidis, E.; Portal, B.; Mothes, T.; Beretta, C.; Lindskog, M.; Erlandsson, A. Intracellular Deposits of Amyloid-Beta Influence the Ability of Human IPSC-Derived Astrocytes to Support Neuronal Function. J. Neuroinflammation 2023, 20, 3. [Google Scholar] [CrossRef]
- d’Errico, P.; Ziegler-Waldkirch, S.; Aires, V.; Hoffmann, P.; Mezö, C.; Erny, D.; Monasor, L.S.; Liebscher, S.; Ravi, V.M.; Joseph, K.; et al. Microglia Contribute to the Propagation of Aβ into Unaffected Brain Tissue. Nat. Neurosci. 2022, 25, 20–25. [Google Scholar] [CrossRef]
- Joshi, P.; Turola, E.; Ruiz, A.; Bergami, A.; Libera, D.D.; Benussi, L.; Giussani, P.; Magnani, G.; Comi, G.; Legname, G.; et al. Microglia Convert Aggregated Amyloid-β into Neurotoxic Forms through the Shedding of Microvesicles. Cell Death Differ. 2014, 21, 582–593. [Google Scholar] [CrossRef]
- Iohan, L.D.C.C.; Lambert, J.-C.; Costa, M.R. Analysis of Modular Gene Co-Expression Networks Reveals Molecular Pathways Underlying Alzheimer’s Disease and Progressive Supranuclear Palsy. PLoS ONE 2022, 17, e0266405. [Google Scholar] [CrossRef]
- Shao, F.; Wang, M.; Guo, Q.; Zhang, B.; Wang, X. Characterization of Alzheimer’s Disease-Associated Excitatory Neurons via Single-Cell RNA Sequencing Analysis. Front. Aging Neurosci. 2021, 13, 742176. [Google Scholar] [CrossRef]
- Wan, Y.W.; Al-Ouran, R.; Mangleburg, C.G.; Perumal, T.M.; Lee, T.V.; Allison, K.; Swarup, V.; Funk, C.C.; Gaiteri, C.; Allen, M.; et al. Meta-Analysis of the Alzheimer’s Disease Human Brain Transcriptome and Functional Dissection in Mouse Models. Cell Rep. 2020, 32, 107908. [Google Scholar] [CrossRef]
- Mathys, H.; Davila-Velderrain, J.; Peng, Z.; Gao, F.; Mohammadi, S.; Young, J.Z.; Menon, M.; He, L.; Abdurrob, F.; Jiang, X.; et al. Single-Cell Transcriptomic Analysis of Alzheimer’s Disease. Nature 2019, 570, 332–337. [Google Scholar] [CrossRef]
- Grubman, A.; Chew, G.; Ouyang, J.F.; Sun, G.; Choo, X.Y.; McLean, C.; Simmons, R.K.; Buckberry, S.; Vargas-Landin, D.B.; Poppe, D.; et al. A Single-Cell Atlas of Entorhinal Cortex from Individuals with Alzheimer’s Disease Reveals Cell-Type-Specific Gene Expression Regulation. Nat. Neurosci. 2019, 22, 2087–2097. [Google Scholar] [CrossRef] [PubMed]
- Leng, K.; Li, E.; Eser, R.; Piergies, A.; Sit, R.; Tan, M.; Neff, N.; Li, S.H.; Rodriguez, R.D.; Suemoto, C.K.; et al. Molecular Characterization of Selectively Vulnerable Neurons in Alzheimer’s Disease. Nat. Neurosci. 2021, 24, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Marques-Coelho, D.; Iohan, L.D.C.C.; Melo de Farias, A.R.; Flaig, A.; Letournel, F.; Martin-Négrier, M.L.; Chapon, F.; Faisant, M.; Godfraind, C.; Maurage, C.A.; et al. Differential Transcript Usage Unravels Gene Expression Alterations in Alzheimer’s Disease Human Brains. npj Aging Mech. Dis. 2021, 7, 2. [Google Scholar] [CrossRef]
- Klein, R. Bidirectional Modulation of Synaptic Functions by Eph/Ephrin Signaling. Nat. Neurosci. 2009, 12, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, K.; Miyata, M.; Shiotani, H.; Kameyama, T.; Takai, Y. Nectin-2 in General and in the Brain. Mol. Cell. Biochem. 2022, 477, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Carulli, D.; de Winter, F.; Verhaagen, J. Semaphorins in Adult Nervous System Plasticity and Disease. Front. Synaptic Neurosci. 2021, 13, 672891. [Google Scholar] [CrossRef]
- O’Connor, T.P.; Cockburn, K.; Wang, W.; Tapia, L.; Currie, E.; Bamji, S.X. Semaphorin 5B Mediates Synapse Elimination in Hippocampal Neurons. Neural Dev. 2009, 4, 18. [Google Scholar] [CrossRef]
- Lambert, J.C.; Ibrahim-Verbaas, C.A.; Harold, D.; Naj, A.C.; Sims, R.; Bellenguez, C.; Jun, G.; DeStefano, A.L.; Bis, J.C.; Beecham, G.W.; et al. Meta-Analysis of 74,046 Individuals Identifies 11 New Susceptibility Loci for Alzheimer’s Disease. Nat. Genet. 2013, 45, 1452–1458. [Google Scholar] [CrossRef]
- Bellenguez, C.; Küçükali, F.; Jansen, I.E.; Kleineidam, L.; Moreno-Grau, S.; Amin, N.; Naj, A.C.; Campos-Martin, R.; Grenier-Boley, B.; Andrade, V.; et al. New Insights into the Genetic Etiology of Alzheimer’s Disease and Related Dementias. Nat. Genet. 2022, 54, 412–436. [Google Scholar] [CrossRef]
- Lee, S.H.; Kang, J.; Ho, A.; Watanabe, H.; Bolshakov, V.Y.; Shen, J. APP Family Regulates Neuronal Excitability and Synaptic Plasticity but Not Neuronal Survival. Neuron 2020, 108, 676–690.e8. [Google Scholar] [CrossRef] [PubMed]
- Costa, M. Single-nulceus RNA sequencing of human-induced neuron and astrocytes treated with cell-secreted amyloid beta peptides. Mendeley Data 2023. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melo de Farias, A.R.; Pelletier, A.; Iohan, L.C.C.; Saha, O.; Bonnefond, A.; Amouyel, P.; Delahaye, F.; Lambert, J.-C.; Costa, M.R. Amyloid-Beta Peptides Trigger Premature Functional and Gene Expression Alterations in Human-Induced Neurons. Biomedicines 2023, 11, 2564. https://doi.org/10.3390/biomedicines11092564
Melo de Farias AR, Pelletier A, Iohan LCC, Saha O, Bonnefond A, Amouyel P, Delahaye F, Lambert J-C, Costa MR. Amyloid-Beta Peptides Trigger Premature Functional and Gene Expression Alterations in Human-Induced Neurons. Biomedicines. 2023; 11(9):2564. https://doi.org/10.3390/biomedicines11092564
Chicago/Turabian StyleMelo de Farias, Ana Raquel, Alexandre Pelletier, Lukas Cruz Carvalho Iohan, Orthis Saha, Amélie Bonnefond, Philippe Amouyel, Fabien Delahaye, Jean-Charles Lambert, and Marcos R. Costa. 2023. "Amyloid-Beta Peptides Trigger Premature Functional and Gene Expression Alterations in Human-Induced Neurons" Biomedicines 11, no. 9: 2564. https://doi.org/10.3390/biomedicines11092564
APA StyleMelo de Farias, A. R., Pelletier, A., Iohan, L. C. C., Saha, O., Bonnefond, A., Amouyel, P., Delahaye, F., Lambert, J.-C., & Costa, M. R. (2023). Amyloid-Beta Peptides Trigger Premature Functional and Gene Expression Alterations in Human-Induced Neurons. Biomedicines, 11(9), 2564. https://doi.org/10.3390/biomedicines11092564






