Quantification of Squalene and Lactic Acid in Hair Bulbs with Damaged Sheaths: Are They Metabolic Wastes in Alopecia?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bulbs Sampling
2.3. Bulbs Observation under Polarised Microscope
2.4. Inclusion Criteria
2.5. Squalene and Lactic Acid Extraction from Bulbs
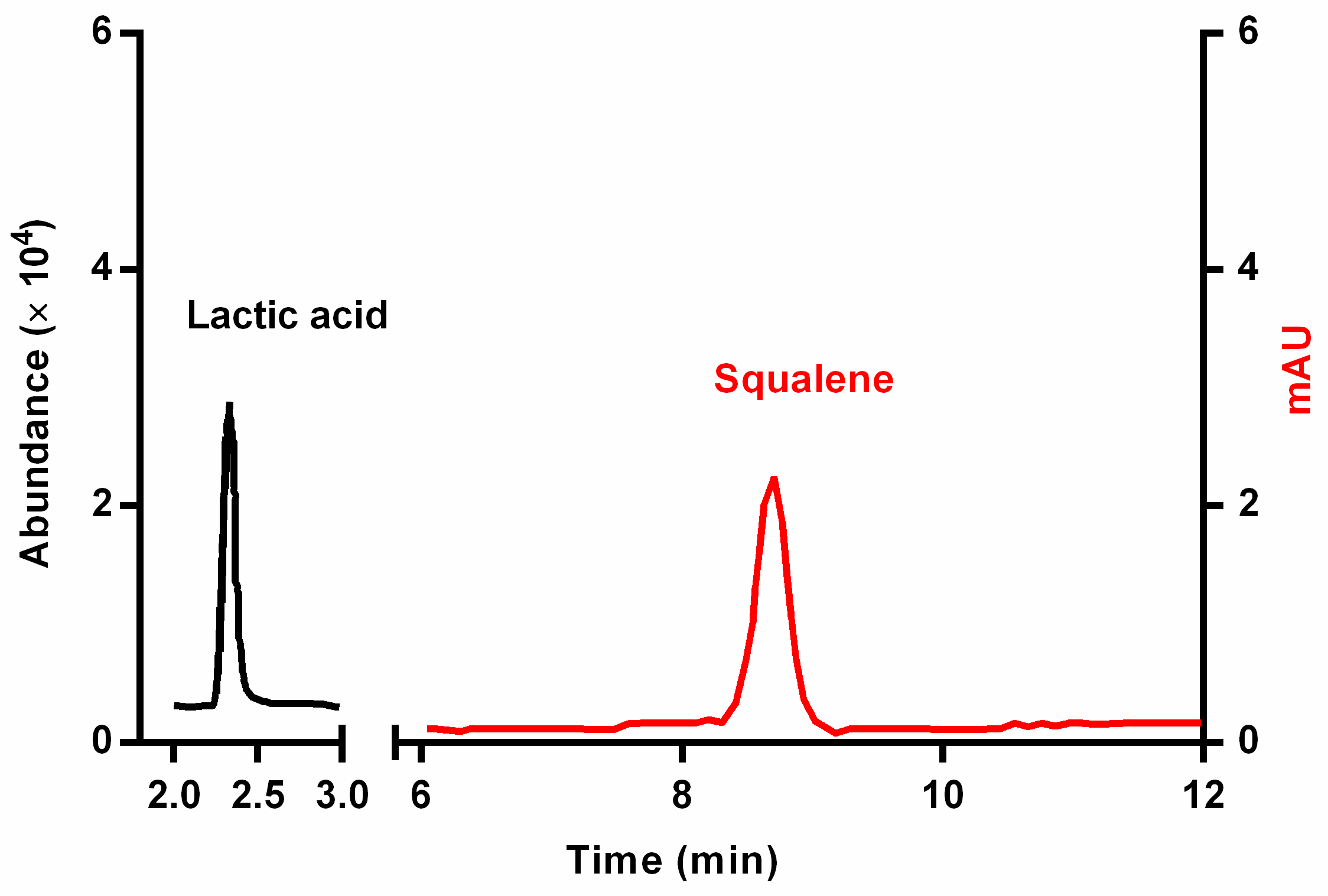
2.6. Quantification of Squalene through HPLC-DAD Analysis
2.7. Quantification of Lactic Acid through HPLC-MS Analysis
2.8. Validation of the HPLC Quantitative Methods
2.9. Linearity
2.10. Limit of Detection (LOD) and Limit of Quantification (LOQ)
2.11. Repeatability
2.12. Recovery
3. Results
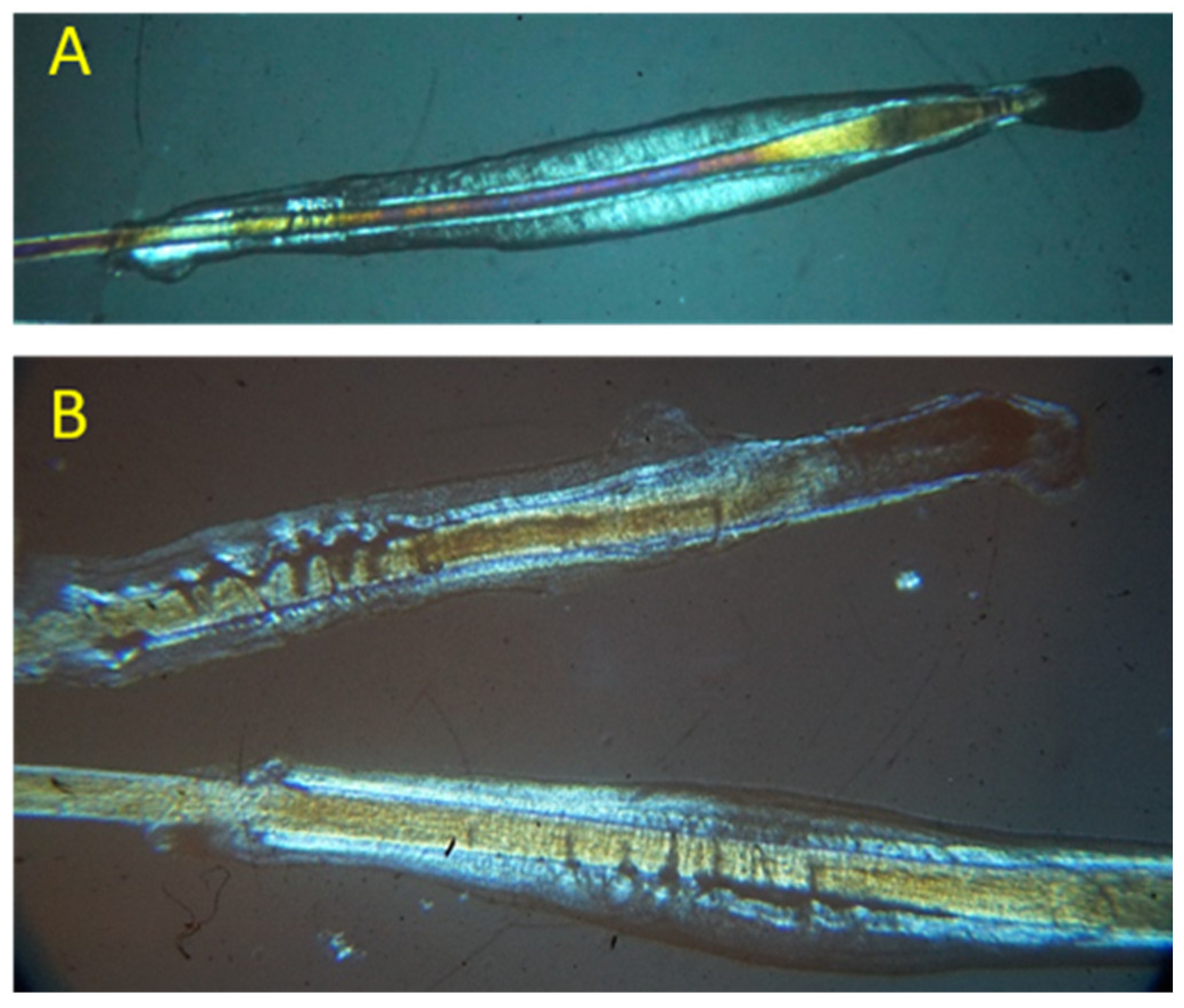
3.1. Evaluation of the Bulb Sheaths Damage through Polarised Light Microscopy
3.2. Selection of the Extraction Method
3.3. Validation of the Analytical Method
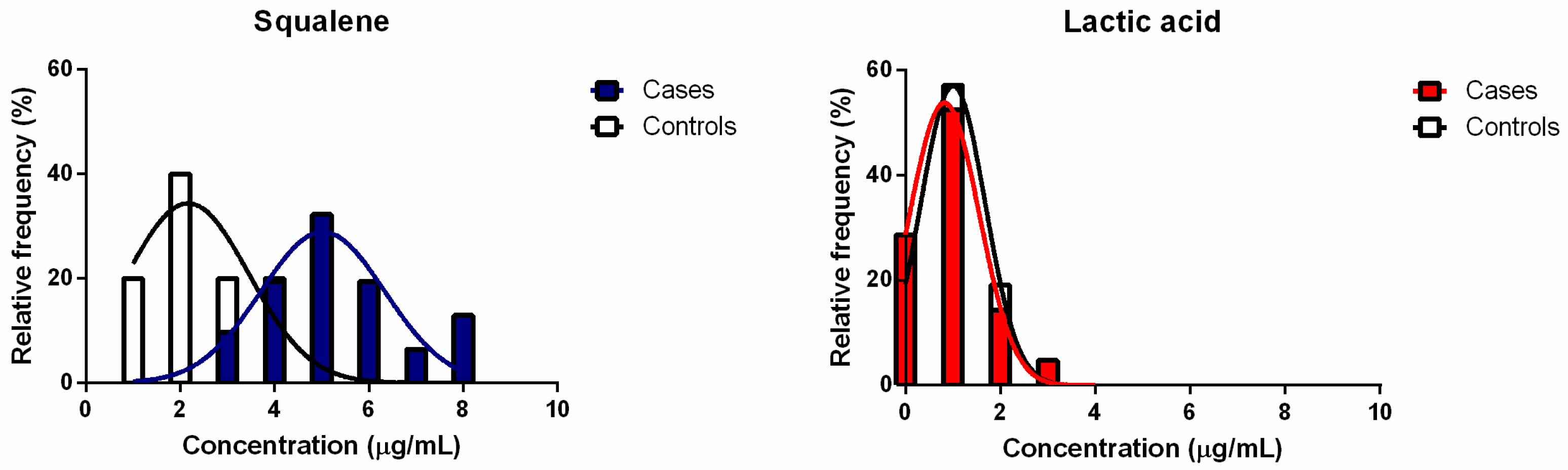
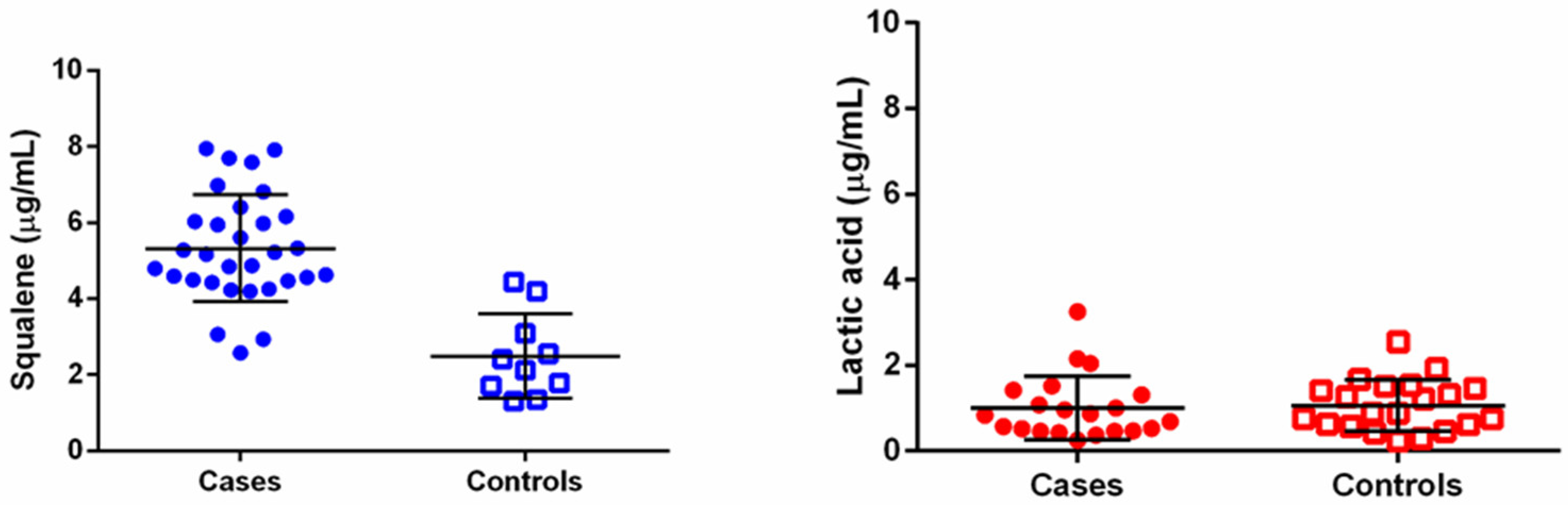
3.4. Quantification of Squalene and Lactic Acid in Hair Bulbs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Phillips, T.G.; Slomiany, W.P.; Allison, R. Hair Loss: Common Causes and Treatment. Am. Fam. Physician 2017, 96, 371–378. [Google Scholar] [PubMed]
- Trüeb, R.M.; Dias, M.F.R.G. Alopecia Areata: A Comprehensive Review of Pathogenesis and Management. Clin. Rev. Allergy Immunol. 2018, 54, 68–87. [Google Scholar] [CrossRef] [PubMed]
- Lolli, F.; Pallotti, F.; Rossi, A.; Fortuna, M.C.; Caro, G.; Lenzi, A.; Sansone, A.; Lombardo, F. Androgenetic alopecia: A review. Endocrine 2017, 57, 9–17. [Google Scholar] [CrossRef]
- Natarelli, N.; Gahoonia, N.; Sivamani, R.K. Integrative and Mechanistic Approach to the Hair Growth Cycle and Hair Loss. J. Clin. Med. 2023, 12, 893. [Google Scholar] [CrossRef] [PubMed]
- Kidangazhiathmana, A.; Santhosh, P. Pathogenesis of androgenetic alopecia. Clin. Dermatol. Rev. 2022, 6, 69. [Google Scholar] [CrossRef]
- Peyravian, N.; Deo, S.; Daunert, S.; Jimenez, J.J. The Inflammatory Aspect of Male and Female Pattern Hair Loss. J. Inflamm. Res. 2020, 13, 879–881. [Google Scholar] [CrossRef]
- Heilmann-Heimbach, S.; Hochfeld, L.M.; Henne, S.K.; Nöthen, M.M. Hormonal regulation in male androgenetic alopecia—Sex hormones and beyond: Evidence from recent genetic studies. Exp. Dermatol. 2020, 29, 814–827. [Google Scholar] [CrossRef]
- Brajac, I.; Tkalčić, M.; Dragojević, D.M.; Gruber, F. Roles of Stress, Stress Perception and Trait-Anxiety in the Onset and Course of Alopecia Areata. J. Dermatol. 2003, 30, 871–878. [Google Scholar] [CrossRef]
- Choi, S.; Zhang, B.; Ma, S.; Gonzalez-Celeiro, M.; Stein, D.; Jin, X.; Kim, S.T.; Kang, Y.L.; Besnard, A.; Rezza, A.; et al. Corticosterone inhibits GAS6 to govern hair follicle stem-cell quiescence. Nature 2021, 592, 428–432. [Google Scholar] [CrossRef]
- Chien Yin, G.O.; Siong-See, J.L.; Wang, E.C.E. Telogen Effluvium—A review of the science and current obstacles. J. Dermatol. Sci. 2021, 101, 156–163. [Google Scholar] [CrossRef]
- Panchaprateep, R.; Korkij, W.; Asawanonda, P. Brain-derived nerve factor and neurotrophins in androgenetic alopecia. Br. J. Dermatol. 2011, 165, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Shin, J.M.; Kim, D.; Park, S.; Hong, D.; Jung, K.E.; Kim, C.D.; Seo, Y.J.; Lee, Y. Role of Substance P in Regulating Micro-Milieu of Inflammation in Alopecia Areata. Ann. Dermatol. 2022, 34, 270–277. [Google Scholar] [CrossRef]
- Siebenhaar, F.; Sharov, A.A.; Peters, E.M.J.; Sharova, T.Y.; Syska, W.; Mardaryev, A.N.; Freyschmidt-Paul, P.; Sundberg, J.P.; Maurer, M.; Botchkarev, V.A. Substance P as an Immunomodulatory Neuropeptide in a Mouse Model for Autoimmune Hair Loss (Alopecia Areata). J. Investig. Dermatol. 2007, 127, 1489–1497. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, R.; Wen, L.; Fan, Z.; Guo, Y.; Hu, Z.; Miao, Y. Recent Progress in the Understanding of the Effect of Sympathetic Nerves on Hair Follicle Growth. Front. Cell Dev. Biol. 2021, 9, 736738. [Google Scholar] [CrossRef] [PubMed]
- Philpott, M.P.; Sanders, D.; Westgate, G.E.; Kealey, T. Human hair growth in vitro: A model for the study of hair follicle biology. J. Dermatol. Sci. 1994, 7, S55–S72. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, G.; Ji, C.; Hoptroff, M.; Jones, A.; Collins, L.Z.; Janssen, H.G. Gas chromatography-mass spectrometry and Raman imaging measurement of squalene content and distribution in human hair. Anal. Bioanal. Chem. 2016, 408, 2357–2362. [Google Scholar] [CrossRef] [PubMed]
- Goetz, N.; Burgaud, H.; Berrebi, C. Analysis of the lipid content of single hair bulbs. Comparison with the content of the sebaceous gland and with surface lipids. J. Soc. Cosmet. Chem 1984, 35, 411–422. [Google Scholar]
- Ferri, A.; Franzoia, R.; Martínez Sánchez, G. Hair Analysis by Polarized Light Microscopy, a New Tool in Medical Research. J. Toxicol. Curr. Res. 2018, 2, 004. [Google Scholar] [CrossRef]
- Lu, H.-T.; Jiang, Y.; Chen, F. Determination of Squalene Using High-Performance Liquid Chromatography with Diode Array Detection. Chromatographia 2004, 59, 367–371. [Google Scholar] [CrossRef]
- Norton, D.; Crow, B.; Bishop, M.; Kovalcik, K.; George, J.; Bralley, J.A. High performance liquid chromatography–tandem mass spectrometry (HPLC/MS/MS) assay for chiral separation of lactic acid enantiomers in urine using a teicoplanin based stationary phase. J. Chromatogr. B 2007, 850, 190–198. [Google Scholar] [CrossRef]
- Huber, L. Chapter 3—Validation overview. In Good Laboratory Practice and Current Good Manufacturing Practice; Agilent Technologies: Boeblingen, Germany, 2000. [Google Scholar]
- Auwärter, V.; Kießling, B.; Pragst, F. Squalene in hair—A natural reference substance for the improved interpretation of fatty acid ethyl ester concentrations with respect to alcohol misuse. Forensic Sci. Int. 2004, 145, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, J.V.; Bishop, J.J.; Benner, B.A. Forensic analysis of hair surface components using off-line supercritical fluid extraction and large volume injection. J. Sep. Sci. 2003, 26, 137–141. [Google Scholar] [CrossRef]
- Lee, W.-S. Integral hair lipid in human hair follicle. J. Dermatol. Sci. 2011, 64, 153–158. [Google Scholar] [CrossRef]
- Coderch, L.; Oliver, M.A.; Martínez, V.; Manich, A.M.; Rubio, L.; Martí, M. Exogenous and endogenous lipids of human hair. Ski. Res. Technol. 2017, 23, 479–485. [Google Scholar] [CrossRef]
- Lee, W.-S.; Oh, T.H.; Chun, S.H.; Jeon, S.Y.; Lee, E.Y.; Lee, S.; Park, W.-S.; Hwang, S. Integral Lipid in Human Hair Follicle. J. Investig. Dermatol. Symp. Proc. 2005, 10, 234–237. [Google Scholar] [CrossRef]
- Ymazaki, J.; Maeda, K. Analysis of Lipids in the Medulla of Japanese Hair and Their Function. Cosmetics 2018, 5, 27. [Google Scholar] [CrossRef]
- Wei, X.; Wang, X.; Fang, Y.; Huang, Q. Comparison of hair from rectum cancer patients and from healthy persons by Raman microspectroscopy and imaging. J. Mol. Struct. 2013, 1048, 83–87. [Google Scholar] [CrossRef]
- Hou, S.-W.; He, S.-Y.; Xie, J.; Li, M.-Y.; Hong, M.-S.; Guan, F.-L.; Hu, Y.-L.; Huang, Y.-L.; Xu, C.-H. Integral characterization of normal and alopecic hair at different degeneration stages by in-situ visible and chemical imaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 235, 118315. [Google Scholar] [CrossRef]
- Cho, S.-H.; Choi, M.H.; Sim, W.Y.; Lee, W.-Y.; Chung, B.C. Metabolic alterations of DHEA and cholesterol sulphates in the hair of patients with acne measured by liquid chromatography–mass spectrometry. Exp. Dermatol. 2010, 19, 694–696. [Google Scholar] [CrossRef]
- Csuka, D.A.; Csuka, E.A.; Juhász, M.L.W.; Sharma, A.N.; Mesinkovska, N.A. A systematic review on the lipid composition of human hair. Int. J. Dermatol. 2023, 62, 404–415. [Google Scholar] [CrossRef]
- Masukawa, Y.; Tsujimura, H.; Imokawa, G. A systematic method for the sensitive and specific determination of hair lipids in combination with chromatography. J. Chromatogr. B 2005, 823, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Palmer, M.A.; Blakeborough, L.; Harries, M.; Haslam, I.S. Cholesterol homeostasis: Links to hair follicle biology and hair disorders. Exp. Dermatol. 2020, 29, 299–311. [Google Scholar] [CrossRef] [PubMed]
| Conc. Range (ppm) | R2 | LOD (ppm) | LOQ (ppm) | Repeatability (RSD %, n = 3) | Recovery (%, n = 3) | ||
|---|---|---|---|---|---|---|---|
| Intraday | Interday | ||||||
| Squalene | 5–250 | 0.9999 | 1 | 3 | 4.2 | 3.6 | 89.4/92.7 |
| Lactic acid | 0.1–10 | 0.9981 | 0.07 | 0.22 | 2.5 | 3.1 | 93.0/101 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perinelli, D.R.; Cambriani, A.; Antognini, G.; Agostinacchio, G.; Marliani, A.; Cespi, M.; Torregiani, E.; Bonacucina, G. Quantification of Squalene and Lactic Acid in Hair Bulbs with Damaged Sheaths: Are They Metabolic Wastes in Alopecia? Biomedicines 2023, 11, 2493. https://doi.org/10.3390/biomedicines11092493
Perinelli DR, Cambriani A, Antognini G, Agostinacchio G, Marliani A, Cespi M, Torregiani E, Bonacucina G. Quantification of Squalene and Lactic Acid in Hair Bulbs with Damaged Sheaths: Are They Metabolic Wastes in Alopecia? Biomedicines. 2023; 11(9):2493. https://doi.org/10.3390/biomedicines11092493
Chicago/Turabian StylePerinelli, Diego Romano, Alessandra Cambriani, Gianluigi Antognini, Gaetano Agostinacchio, Andrea Marliani, Marco Cespi, Elisabetta Torregiani, and Giulia Bonacucina. 2023. "Quantification of Squalene and Lactic Acid in Hair Bulbs with Damaged Sheaths: Are They Metabolic Wastes in Alopecia?" Biomedicines 11, no. 9: 2493. https://doi.org/10.3390/biomedicines11092493
APA StylePerinelli, D. R., Cambriani, A., Antognini, G., Agostinacchio, G., Marliani, A., Cespi, M., Torregiani, E., & Bonacucina, G. (2023). Quantification of Squalene and Lactic Acid in Hair Bulbs with Damaged Sheaths: Are They Metabolic Wastes in Alopecia? Biomedicines, 11(9), 2493. https://doi.org/10.3390/biomedicines11092493











