Chronic Wound Management: From Gauze to Homologous Cellular Matrix
Abstract
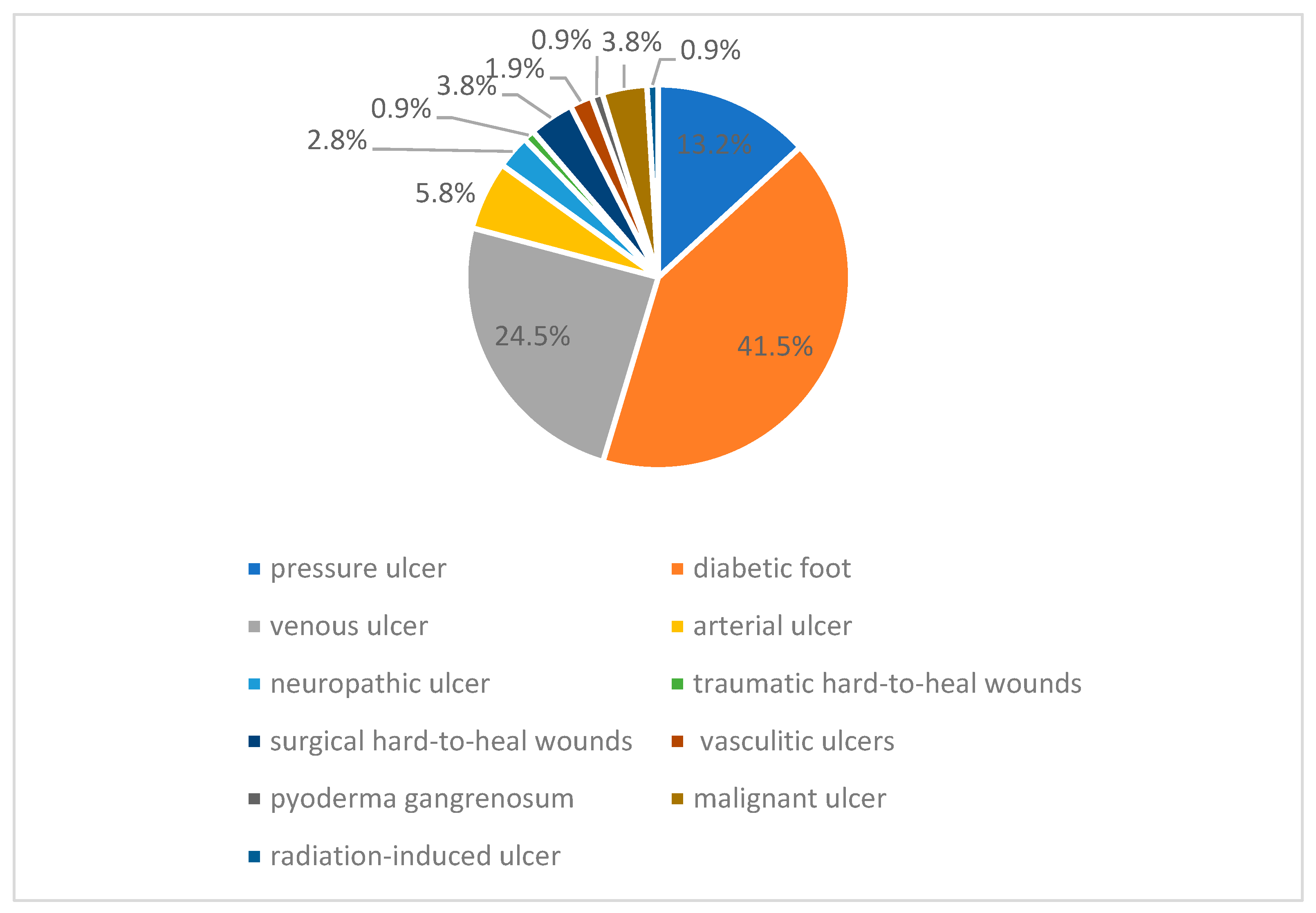
:1. Introduction
2. Materials and Methods
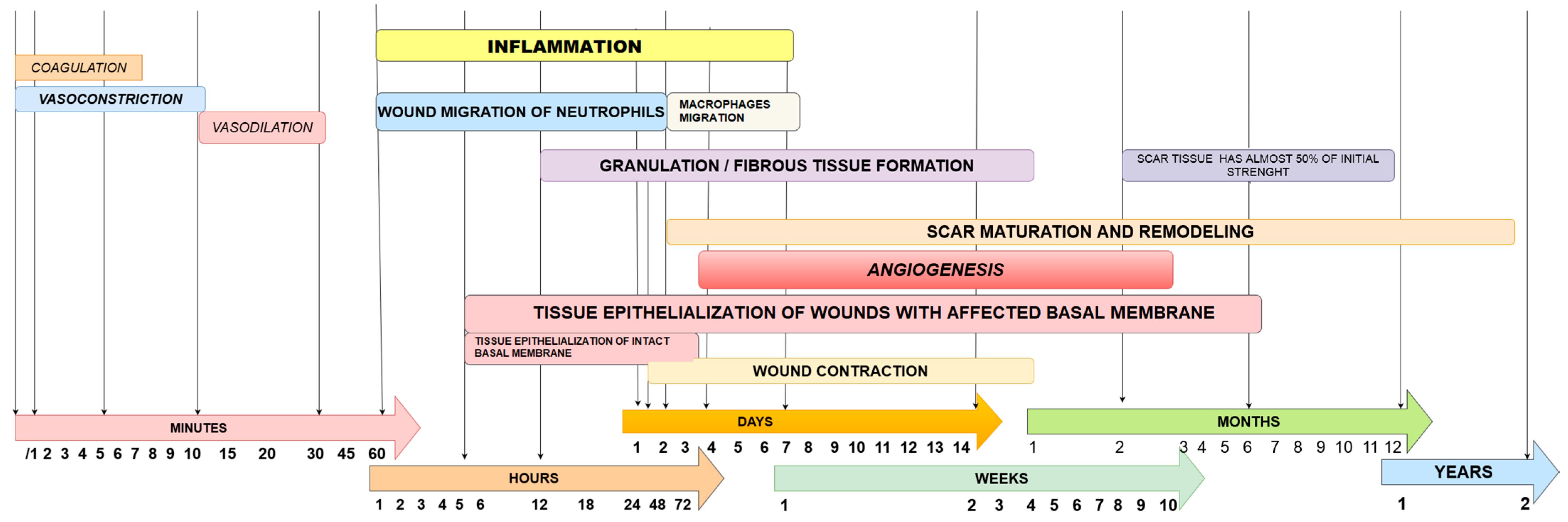
3. Wound Healing
3.1. Wound Assessement
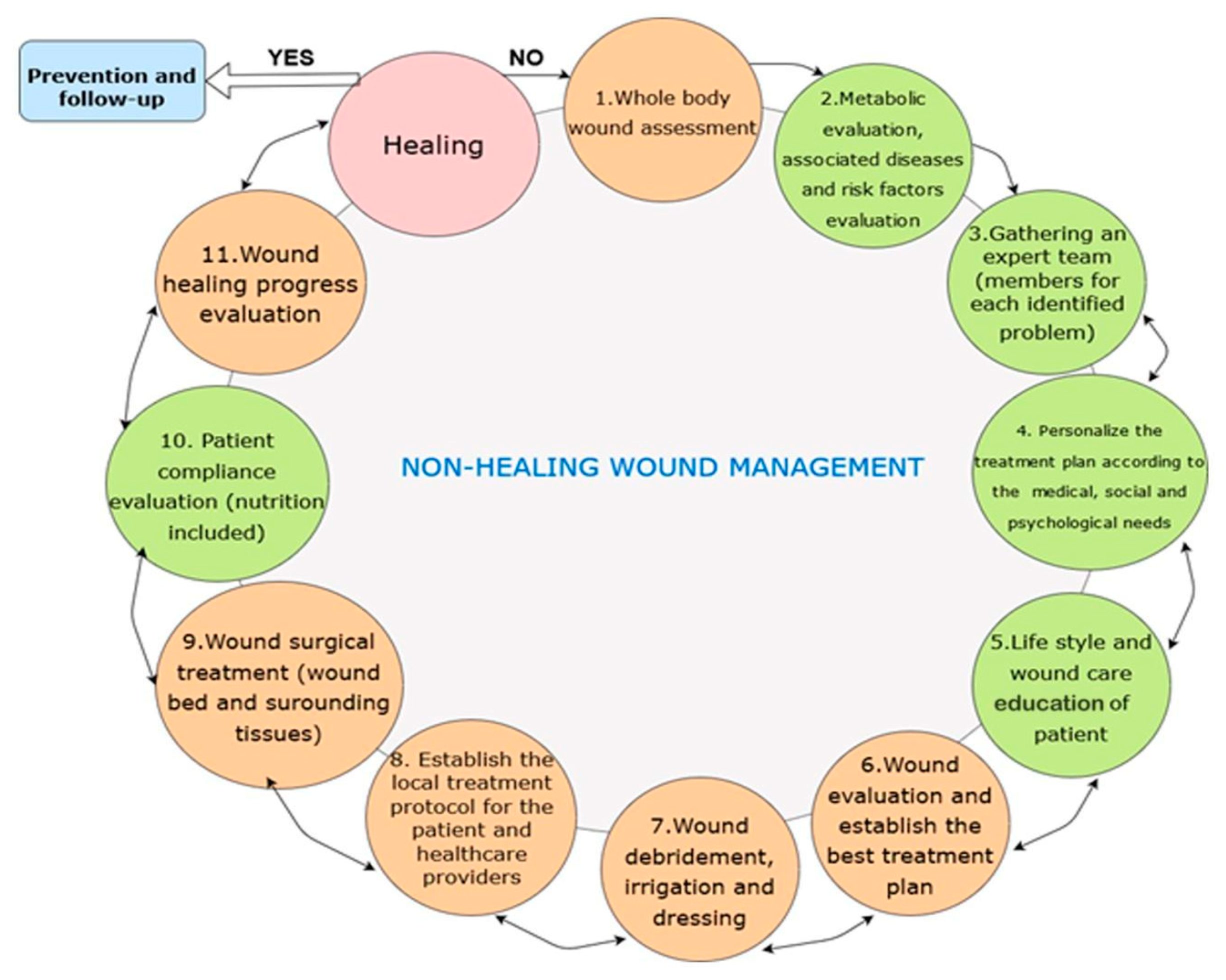
3.2. Standard Wound Management Principles
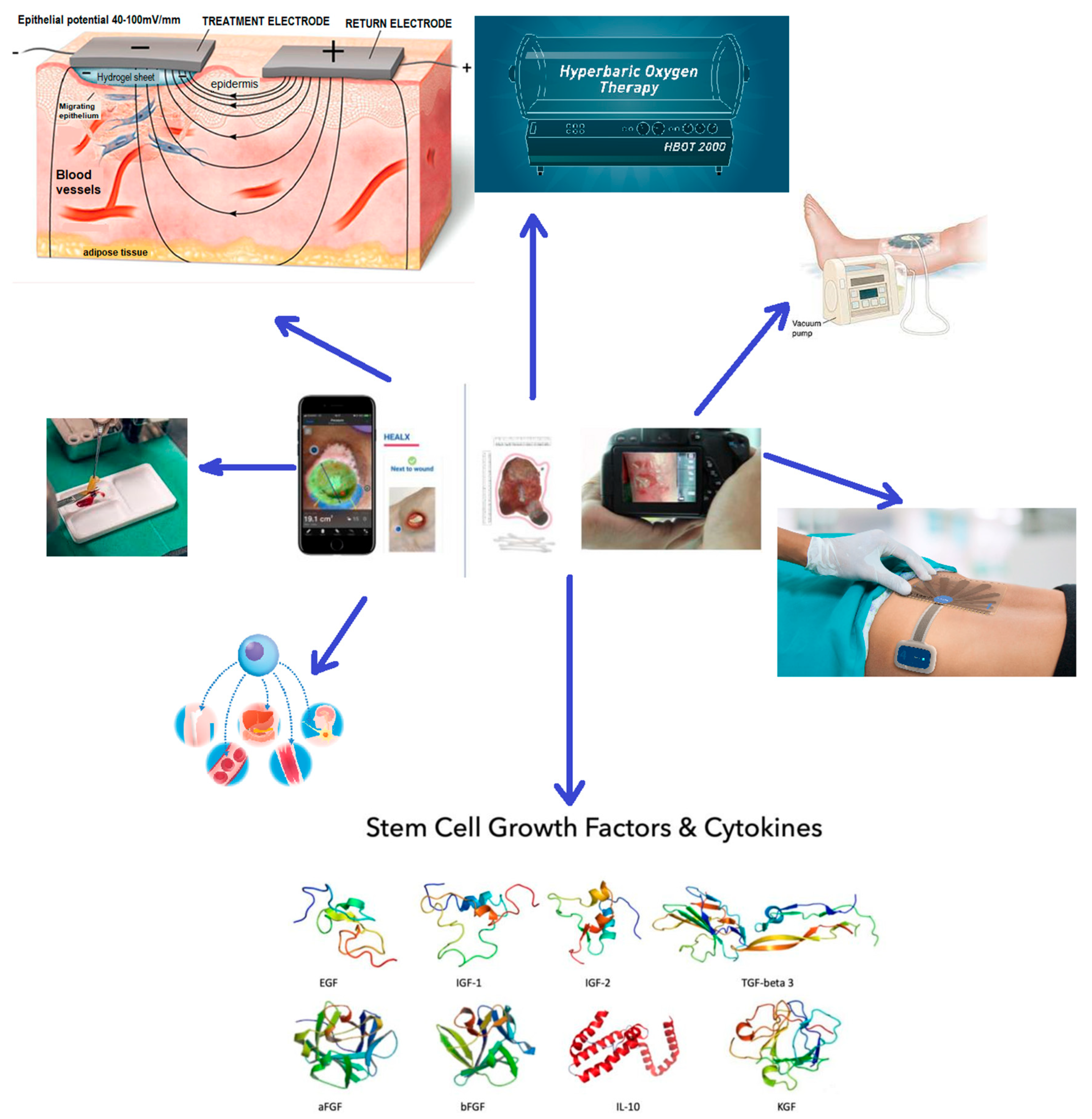
4. Novel Therapies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Järbrink, K.; Ni, G.; Sönnergren, H.; Schmidtchen, A.; Pang, C.; Bajpai, R.; Car, J. Prevalence and incidence of chronic wounds and related complications: A protocol for a systematic review. Syst. Rev. 2016, 5, 152. [Google Scholar] [CrossRef] [PubMed]
- Nelson, E.A.; Adderley, U. Venous leg ulcers. BMJ Clin. Evid. 2016, 2016, 1902. [Google Scholar] [PubMed]
- Gould, L.; Abadir, P.; Brem, H.; Carter, M.; Conner-Kerr, T.; Davidson, J.; DiPietro, L.; Falanga, V.; Fife, C.; Gardner, S.; et al. Chronic wound repair and healing in older adults: Current status and future research. Wound Repair Regen. 2015, 23, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lindholm, C.; Searle, R. Wound management for the 21st century: Combining effectiveness and efficiency. Int. Wound J. 2016, 13 (Suppl. S2), 5–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Lu, J.; Jing, Y.; Tang, S.; Zhu, D.; Bi, Y. Global epidemiology of diabetic foot ulceration: A systematic review and meta-analysis. Ann. Med. 2017, 49, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Martins-Mendes, D.; Monteiro-Soares, M.; Boyko, E.J.; Ribeiro, M.; Barata, P.; Lima, J.; Soares, R. The independent contribution of diabetic foot ulcer on lower extremity amputation and mortality risk. J. Diabetes Its Complicat. 2014, 28, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Troxler, M.; Vowden, K.; Vowden, P. Integrating adjunctive therapy into practice: The importance of recognising ‘hard-to-heal’ wounds. J. Community Nurs. 2006, 32, 99–105. [Google Scholar]
- Olsson, M.; Järbrink, K.; Divakar, U.; Bajpai, R.; Upton, Z.; Schmidtchen, A.; Car, J. The humanistic and economic burden of chronic wounds: A systematic review. Wound Repair Regen. 2019, 27, 114–125. [Google Scholar] [CrossRef]
- Al-Gharibi, K.A.; Sharstha, S.; Al-Faras, M.A. Cost-Effectiveness of Wound Care: A concept analysis. Sultan Qaboos Univ. Med. J. 2018, 18, e433–e439. [Google Scholar] [CrossRef]
- Posnett, J.; Gottrup, F.; Lundgren, H.; Saal, G. The resource impact of wounds on health-care providers in Europe. J. Wound Care 2009, 18, 154–161. [Google Scholar] [CrossRef]
- Defez, C.; Fabbro-Peray, P.; Cazaban, M.; Boudemaghe, T.; Sotto, A.; Daurès, J.P. Additional direct medical costs of nosocomial infections: An estimation from a cohort of patients in a French university hospital. J. Hosp. Infect. 2008, 68, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Zimlichman, E.; Henderson, D.; Tamir, O.; Franz, C.; Song, P.; Yamin, C.K.; Keohane, C.; Denham, C.R.; Bates, D.W. Health care-associated infections: A meta-analysis of costs and financial impact on the US health care system. JAMA Intern. Med. 2013, 173, 2039–2046. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Guest, J.F.; Ayoub, N.; McIlwraith, T.; Uchegbu, I.; Gerrish, A.; Weidlich, D.; Vowden, K.; Vowden, P. Health economic burden that different wound types impose on the UK’s National Health Service. Int. Wound J. 2017, 14, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.R.; Bernstein, J.M. Chronic wound infection: Facts and controversies. Clin. Dermatol. 2010, 28, 519–526. [Google Scholar] [CrossRef]
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef] [PubMed]
- Dubhashi, S.P.; Sindwani, R.D. A Comparative Study of Honey and Phenytoin Dressings for Chronic Wounds. Indian J. Surg. 2015, 77, 1209–1213. [Google Scholar] [CrossRef]
- Martin, P.; Nunan, R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br. J. Dermatol. 2015, 173, 370–378. [Google Scholar] [CrossRef]
- Leaper, D.J.; Durani, P. Topical antimicrobial therapy of chronic wounds healing by secondary intention using iodine products. Int. Wound J. 2008, 5, 361–368. [Google Scholar] [CrossRef]
- Krzyszczyk, P.; Schloss, R.; Palmer, A.; Berthiaume, F. The Role of Macrophages in Acute and Chronic Wound Healing and Interventions to Promote Pro-wound Healing Phenotypes. Front. Physiol. 2018, 9, 419. [Google Scholar] [CrossRef]
- Qing, C. The molecular biology in wound healing & non-healing wound. Chin. J. Traumatol. 2017, 20, 189–193. [Google Scholar] [PubMed]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in Chronic Wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef] [PubMed]
- Grey, J.E.; Enoch, S.; Harding, K.G. Wound assessment. BMJ Clin. Res. Ed. 2006, 332, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Harries, R.L.; Bosanquet, D.C.; Harding, K.G. Wound bed preparation: TIME for an update. Int. Wound J. 2016, 13 (Suppl. S3), 8–14. [Google Scholar] [CrossRef] [PubMed]
- Ligresti, C.; Bo, F. Wound bed preparation of difficult wounds: An evolution of the principles of TIME. Int. Wound J. 2007, 4, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Fong, K.Y.; Lai, T.P.; Chan, K.S.; See, I.J.L.; Goh, C.C.; Muthuveerappa, S.; Tan, A.H.; Liang, S.; Lo, Z.J. Clinical validation of a smartphone application for automated wound measurement in patients with venous leg ulcers. Int. Wound J. 2023, 20, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Sussman, C.; Bates-Jensen, B.M. Wound Care: A Collaborative Practice Manual; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007. [Google Scholar]
- Mukherjee, R.; Manohar, D.D.; Das, D.K.; Achar, A.; Mitra, A.; Chakraborty, C. Automated tissue classification framework for reproducible chronic wound assessment. BioMed Res. Int. 2014, 2014, 851582. [Google Scholar] [CrossRef]
- Taradaj, J.J.F.M.; Review, P.C. Prevention and treatment of pressure ulcers by newest recommendations from European Pressure Ulcer Advisory Panel (EPUAP): Practical reference guide for GPs. Fam. Med. Prim. Care Rev. 2017, 19, 81–83. [Google Scholar] [CrossRef]
- Eklöf, B.; Rutherford, R.B.; Bergan, J.J.; Carpentier, P.H.; Gloviczki, P.; Kistner, R.L.; Meissner, M.H.; Moneta, G.L.; Myers, K.; Padberg, F.T.; et al. Revision of the CEAP classification for chronic venous disorders: Consensus statement. J. Vasc. Surg. 2004, 40, 1248–1252. [Google Scholar] [CrossRef]
- Armstrong, D.G.; Lavery, L.A.; Harkless, L.B. Validation of a diabetic wound classification system. The contribution of depth, infection, and ischemia to risk of amputation. Diabetes Care 1998, 21, 855–859. [Google Scholar] [CrossRef]
- Schaper, N.C. Diabetic foot ulcer classification system for research purposes: A progress report on criteria for including patients in research studies. Diabetes/Metab. Res. Rev. 2004, 20 (Suppl. S1), S90–S95. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, M.; Yafi, A.; Cinat, M.; Choi, B.; Durkin, A.J. Noninvasive assessment of burn wound severity using optical technology: A review of current and future modalities. Burn. J. Int. Soc. Burn. Inj. 2011, 37, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Newton, D.J. Laser Doppler imaging in the investigation of lower limb wounds. Int. J. Low. Extrem. Wounds 2003, 2, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.X.; Huang, K.; Qin, R.; Huang, J.; Xu, J.S.; Ding, L.; Gnyawali, U.S.; Gordillo, G.M.; Gnyawali, S.C.; Sen, C.K. Dual-mode imaging of cutaneous tissue oxygenation and vascular function. J. Vis. Exp. JoVE 2010, 46, 2095. [Google Scholar] [CrossRef]
- Yudovsky, D.; Nouvong, A.; Pilon, L. Hyperspectral imaging in diabetic foot wound care. J. Diabetes Sci. Technol. 2010, 4, 1099–1113. [Google Scholar] [CrossRef] [PubMed]
- Löndahl, M.; Katzman, P.; Nilsson, A.; Hammarlund, C. Hyperbaric oxygen therapy facilitates healing of chronic foot ulcers in patients with diabetes. Diabetes Care 2010, 33, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, T.F., Jr.; Passman, M.A.; Marston, W.A.; Ennis, W.J.; Dalsing, M.; Kistner, R.L.; Lurie, F.; Henke, P.K.; Gloviczki, M.L.; Eklöf, B.G.; et al. Management of venous leg ulcers: Clinical practice guidelines of the Society for Vascular Surgery ® and the American Venous Forum. J. Vasc. Surg. 2014, 60, 3s–59s. [Google Scholar] [CrossRef] [PubMed]
- Lipsky, B.A.; Berendt, A.R.; Cornia, P.B.; Pile, J.C.; Peters, E.J.; Armstrong, D.G.; Deery, H.G.; Embil, J.M.; Joseph, W.S.; Karchmer, A.W.; et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin. Infect. Dis. 2012, 54, e132–e173. [Google Scholar] [CrossRef]
- Granick, M.; Boykin, J.; Gamelli, R.; Schultz, G.; Tenenhaus, M. Toward a common language: Surgical wound bed preparation and debridement. Wound Repair Regen. 2006, 14 (Suppl. S1), S1–S10. [Google Scholar] [CrossRef]
- Falanga, V.; Brem, H.; Ennis, W.J.; Wolcott, R.; Gould, L.J.; Ayello, E.A. Maintenance debridement in the treatment of difficult-to-heal chronic wounds. Recommendations of an expert panel. Ostomy/Wound Manag. 2008, (Suppl. 2–13), quiz 14–15. [Google Scholar]
- Tian, X.; Liang, X.M.; Song, G.M.; Zhao, Y.; Yang, X.L. Maggot debridement therapy for the treatment of diabetic foot ulcers: A meta-analysis. J. Wound Care 2013, 22, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Leaper, D.J.; Schultz, G.; Carville, K.; Fletcher, J.; Swanson, T.; Drake, R. Extending the TIME concept: What have we learned in the past 10 years? Int. Wound J. 2012, 9 (Suppl. S2), 1–19. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.G.; Nguyen, H.C.; Lavery, L.A.; van Schie, C.H.; Boulton, A.J.; Harkless, L.B. Off-loading the diabetic foot wound: A randomized clinical trial. Diabetes Care 2001, 24, 1019–1022. [Google Scholar] [CrossRef] [PubMed]
- Dolibog, P.; Franek, A.; Taradaj, J.; Polak, A.; Dolibog, P.; Blaszczak, E.; Wcislo, L.; Hrycek, A.; Urbanek, T.; Ziaja, J.; et al. A randomized, controlled clinical pilot study comparing three types of compression therapy to treat venous leg ulcers in patients with superficial and/or segmental deep venous reflux. Ostomy Wound Manag. 2013, 59, 22–30. [Google Scholar]
- Sakarya, S.; Gunay, N.; Karakulak, M.; Ozturk, B.; Ertugrul, B.J.W. Hypochlorous acid: An ideal wound care agent with powerful microbicidal, antibiofilm, and wound healing potency. Wounds 2014, 26, 342–350. [Google Scholar] [PubMed]
- Burks, R.I. Povidone-iodine solution in wound treatment. Phys. Ther. 1998, 78, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.; Granick, M.S.; Tomaselli, N.L. Wound Dressings and Comparative Effectiveness Data. Adv. Wound Care 2014, 3, 511–529. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef]
- Gilca, M.; Tiplica, G.S.; Salavastru, C.M. Traditional and ethnobotanical dermatology practices in Romania and other Eastern European countries. Clin. Dermatol. 2018, 36, 338–352. [Google Scholar] [CrossRef]
- Skórkowska-Telichowska, K.; Czemplik, M.; Kulma, A.; Szopa, J. The local treatment and available dressings designed for chronic wounds. J. Am. Acad. Dermatol. 2013, 68, e117–e126. [Google Scholar] [CrossRef]
- Yager, D.R.; Kulina, R.A.; Gilman, L.A. Wound fluids: A window into the wound environment? Int. J. Low. Extrem. Wounds 2007, 6, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Andersen, C.; Black, J.; de Leon, J.; Fife, C.; Lantis Ii, J.C.; Niezgoda, J.; Snyder, R.; Sumpio, B.; Tettelbach, W.; et al. Management of Chronic Wounds: Diagnosis, Preparation, Treatment, and Follow-up. Wounds A Compend. Clin. Res. Pract. 2017, 29, S19–S36. [Google Scholar]
- Chaby, G.; Senet, P.; Vaneau, M.; Martel, P.; Guillaume, J.C.; Meaume, S.; Téot, L.; Debure, C.; Dompmartin, A.; Bachelet, H.; et al. Dressings for acute and chronic wounds: A systematic review. Arch. Dermatol. 2007, 143, 1297–1304. [Google Scholar] [CrossRef] [PubMed]
- Dolibog, P.; Franek, A.; Taradaj, J.; Dolibog, P.; Blaszczak, E.; Polak, A.; Brzezinska-Wcislo, L.; Hrycek, A.; Urbanek, T.; Ziaja, J.; et al. A comparative clinical study on five types of compression therapy in patients with venous leg ulcers. Int. J. Med. Sci. 2014, 11, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Wang, C.; Liu, H.; Li, Q.; Li, R.; Zhang, Y.; Liu, Y.; Shao, Y.; Wang, J. Selection of Appropriate Wound Dressing for Various Wounds. Front. Bioeng. Biotechnol. 2020, 8, 182. [Google Scholar] [CrossRef] [PubMed]
- Brett, D. A Review of Collagen and Collagen-based Wound Dressings. Wounds A Compend. Clin. Res. Pract. 2008, 20, 347–356. [Google Scholar]
- Britto, E.J.; Nezwek, T.A.; Popowicz, P.; Robins, M. Wound dressings. In StatPearls [Internet]; StatPearls Publishing: St. Petersburg, FL, USA, 2022. [Google Scholar]
- Peinemann, F.; Sauerland, S. Negative-pressure wound therapy: Systematic review of randomized controlled trials. Dtsch. Arztebl. Int. 2011, 108, 381–389. [Google Scholar] [CrossRef]
- Everts, P.; Onishi, K.; Jayaram, P.; Lana, J.F.; Mautner, K. Platelet-Rich Plasma: New Performance Understandings and Therapeutic Considerations in 2020. Int. J. Mol. Sci. 2020, 21, 7794. [Google Scholar] [CrossRef]
- Naik, B.; Karunakar, P.; Jayadev, M.; Marshal, V.R. Role of Platelet rich fibrin in wound healing: A critical review. J. Conserv. Dent. JCD 2013, 16, 284–293. [Google Scholar] [CrossRef]
- Urbanczyk, M.; Layland, S.L.; Schenke-Layland, K.J.M.B. The role of extracellular matrix in biomechanics and its impact on bioengineering of cells and 3D tissues. Matrix Biol. 2020, 85, 1–14. [Google Scholar] [CrossRef]
- Pikuła, M.; Marek-Trzonkowska, N.; Wardowska, A.; Renkielska, A.; Trzonkowski, P. Adipose tissue-derived stem cells in clinical applications. Expert Opin. Biol. Ther. 2013, 13, 1357–1370. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Zhao, J.; Zhang, J.; Li, B.; Gou, Z.; Gou, M.; Li, X. Bioprinting of skin constructs for wound healing. Burn. Trauma 2018, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Park, B.S.; Sung, J.H.; Yang, J.M.; Park, S.B.; Kwak, S.J.; Park, J.S. Wound healing effect of adipose-derived stem cells: A critical role of secretory factors on human dermal fibroblasts. J. Dermatol. Sci. 2007, 48, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Raghuram, A.C.; Yu, R.P.; Lo, A.Y.; Sung, C.J.; Bircan, M.; Thompson, H.J.; Wong, A.K. Role of stem cell therapies in treating chronic wounds: A systematic review. World J. Stem Cells 2020, 12, 659–675. [Google Scholar] [CrossRef] [PubMed]
- Mazini, L.; Rochette, L.; Admou, B.; Amal, S.; Malka, G. Hopes and Limits of Adipose-Derived Stem Cells (ADSCs) and Mesenchymal Stem Cells (MSCs) in Wound Healing. Int. J. Mol. Sci. 2020, 21, 1306. [Google Scholar] [CrossRef] [PubMed]
- Marino, G.; Moraci, M.; Armenia, E.; Orabona, C.; Sergio, R.; De Sena, G.; Capuozzo, V.; Barbarisi, M.; Rosso, F.; Giordano, G.; et al. Therapy with autologous adipose-derived regenerative cells for the care of chronic ulcer of lower limbs in patients with peripheral arterial disease. J. Surg. Res. 2013, 185, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, R.; Kishi, K. Skin graft. Plast. Surg. Int. 2012, 2012, 563493. [Google Scholar] [CrossRef]
- Glavan, A.; Marian, C. Cognitive Edge Computing through Artificial Intelligence. In Proceedings of the 2020 13th International Conference on Communications (COMM), Bucharest, Romania, 18–20 June 2020; pp. 285–290. [Google Scholar]
- Jun, Y.J.; Shin, D.; Choi, W.J.; Hwang, J.H.; Kim, H.; Kim, T.G.; Lee, H.B.; Oh, T.S.; Shin, H.W.; Suh, H.S.; et al. A Mobile Application for Wound Assessment and Treatment: Findings of a User Trial. Int. J. Low. Extrem. Wounds 2016, 15, 344–353. [Google Scholar] [CrossRef]
- Marian, C.V.; Croitoru, V.; Pavaloiu, I.B. Automatic Allocation Mechanism for Virtual Machines Interface Addresses IP Address Interfaces Provisioning. In Proceedings of the 2018 International Conference on Communications (COMM), Bucharest, Romania, 14–16 June 2018; pp. 403–406. [Google Scholar]
- Lungu, R.S.; Marian, C.V. Data Collection and Command Mechanism for Management of Network Resources. In Proceedings of the 2022 14th International Conference on Electronics, Computers and Artificial Intelligence (ECAI), Ploiesti, Romania, 30 June–1 July 2022; pp. 1–5. [Google Scholar]
- Marian, C.V. DNS Records Secure Provisioning Mechanism for Virtual Machines automatic management in high density data centers. In Proceedings of the 2021 IEEE International Black Sea Conference on Communications and Networking (BlackSeaCom), Virtual, 24–28 May 2021; pp. 1–5. [Google Scholar]
- Sfat, R.; Marian, C.V. Medical Systems Open Data Exchange Interconnection and Web Questionnaires Based on the HL7 FHIR Standards. In Proceedings of the 2022 E-Health and Bioengineering Conference (EHB), Iasi, Romania, 17–18 November 2022; pp. 1–4. [Google Scholar]
- Iordache, C.A.; Marian, C.V. Enhanced Accesability and Anti-Fraudulent System for Polling Stations and Mobile Voting in Hospitals. In Proceedings of the 2022 E-Health and Bioengineering Conference (EHB), Iasi, Romania, 17–18 November 2022; pp. 1–4. [Google Scholar]
- Marian, C.V. How to Use Auto-Adaptive Detection Algorithm and Area Under the Curve for Better Patients’ Evaluation in a Continuous Glucose Monitoring Sensors Based Decision Expert System. In Proceedings of the 2022 E-Health and Bioengineering Conference (EHB), Iasi, Romania, 17–18 November 2022; pp. 1–4. [Google Scholar]
- Marian, C.V. Real Time Notification Messaging (Using Area Under the Curve) for Diabetes Patients’ Management in a Continuous Glucose Monitoring Sensors based Decision Support System. In Proceedings of the 2022 E-Health and Bioengineering Conference (EHB), Iasi, Romania, 17–18 November 2022; pp. 1–4. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popescu, V.; Cauni, V.; Petrutescu, M.S.; Rustin, M.M.; Bocai, R.; Turculet, C.R.; Doran, H.; Patrascu, T.; Lazar, A.M.; Cretoiu, D.; et al. Chronic Wound Management: From Gauze to Homologous Cellular Matrix. Biomedicines 2023, 11, 2457. https://doi.org/10.3390/biomedicines11092457
Popescu V, Cauni V, Petrutescu MS, Rustin MM, Bocai R, Turculet CR, Doran H, Patrascu T, Lazar AM, Cretoiu D, et al. Chronic Wound Management: From Gauze to Homologous Cellular Matrix. Biomedicines. 2023; 11(9):2457. https://doi.org/10.3390/biomedicines11092457
Chicago/Turabian StylePopescu, Valentin, Victor Cauni, Marius Septimiu Petrutescu, Maria Madalina Rustin, Raluca Bocai, Cristina Rachila Turculet, Horia Doran, Traian Patrascu, Angela Madalina Lazar, Dragos Cretoiu, and et al. 2023. "Chronic Wound Management: From Gauze to Homologous Cellular Matrix" Biomedicines 11, no. 9: 2457. https://doi.org/10.3390/biomedicines11092457
APA StylePopescu, V., Cauni, V., Petrutescu, M. S., Rustin, M. M., Bocai, R., Turculet, C. R., Doran, H., Patrascu, T., Lazar, A. M., Cretoiu, D., Varlas, V. N., & Mastalier, B. (2023). Chronic Wound Management: From Gauze to Homologous Cellular Matrix. Biomedicines, 11(9), 2457. https://doi.org/10.3390/biomedicines11092457








