Efficiency of Direct Transcutaneous Electroneurostimulation of the Median Nerve in the Regression of Residual Neurological Symptoms after Carpal Tunnel Decompression Surgery
Abstract
:1. Introduction
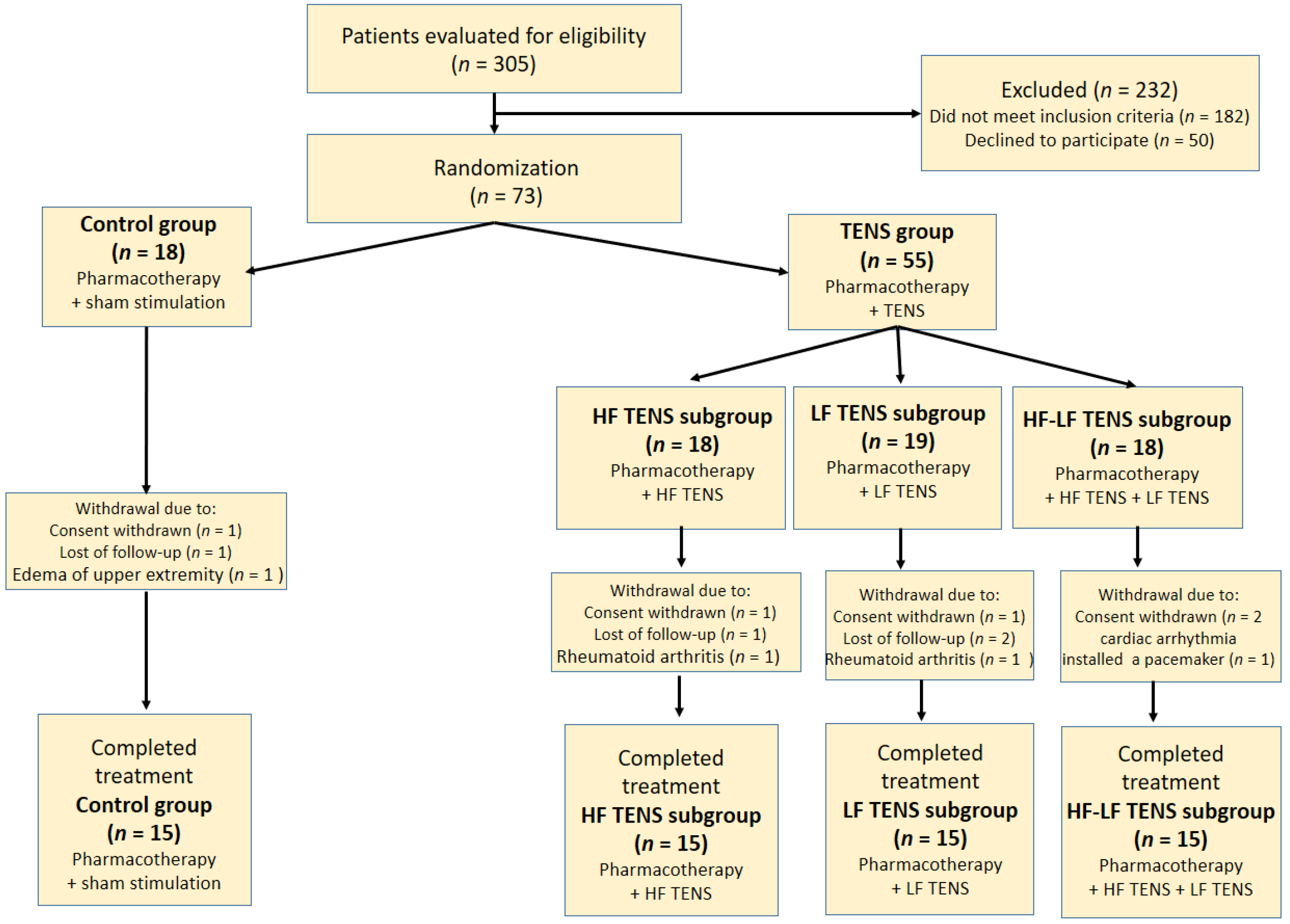
2. Materials and Methods
2.1. Participants
- European patients;
- Adult patients (male: 21 to 60 years old, female: 20 to 55 years old) according to World Health Organization classification;
- Neurophysiological parameters of the median nerve without deterioration;
- Amplitude compound muscle action potential of the median nerve > 2.5 mV;
- History of CTS before CTDS of less than 5 years;
- CTDS older than 6 months but less than 9 months;
- The severity of paresthesia was 5 points or more;
- Signed voluntary informed consent to participate in this study.
- Epilepsy and uncontrolled seizure disorder;
- Severely cognitive disorders or mental illness;
- Hereditary polyneuropathy or history of cervical radiculopathy C6;
- Distal polyneuropathy of the upper extremities;
- Damage to the median nerve anywhere along its path from the brachial plexus to the carpal canal;
- History of stroke, spinal cord injury, traumatic brain injury, multiple sclerosis, or edema of the upper extremities;
- History of cardiac arrythmias or hemodynamic instability;
- Cardiac pacemaker or other implanted electronic system;
- Botulinum toxin injections to any muscle of hand in the previous 3 months;
- Evidence of deep venous thrombosis or other forms of venous thromboembolism;
- Rheumatoid arthritis, gout, psoriasis, and arthrosis of the joints of the upper extremities;
- Type 1 or type 2 diabetes mellitus;
- Vascular atherosclerosis of the upper extremities;
- Raynaud’s disease and vibration white finger;
- De Quervain’s tenosynovitis, deformity and dislocation of the carpometacarpal joint of the thumb, and wrist arthritis;
- Undergoing physiotherapy treatment or acupuncture after CTDS.
2.2. Sample Size Calculation
2.3. Neurological Examination
2.3.1. Pain Syndrome
2.3.2. Positive Sensory Symptoms
2.3.3. Negative Sensory Symptoms
2.3.4. Motor Disorders
2.4. Neurophysiology Examination
2.5. Ultrasonography of Carpal Canal
2.6. Pharmacotherapy
2.7. Surgery of Carpal Tunnel Syndrome
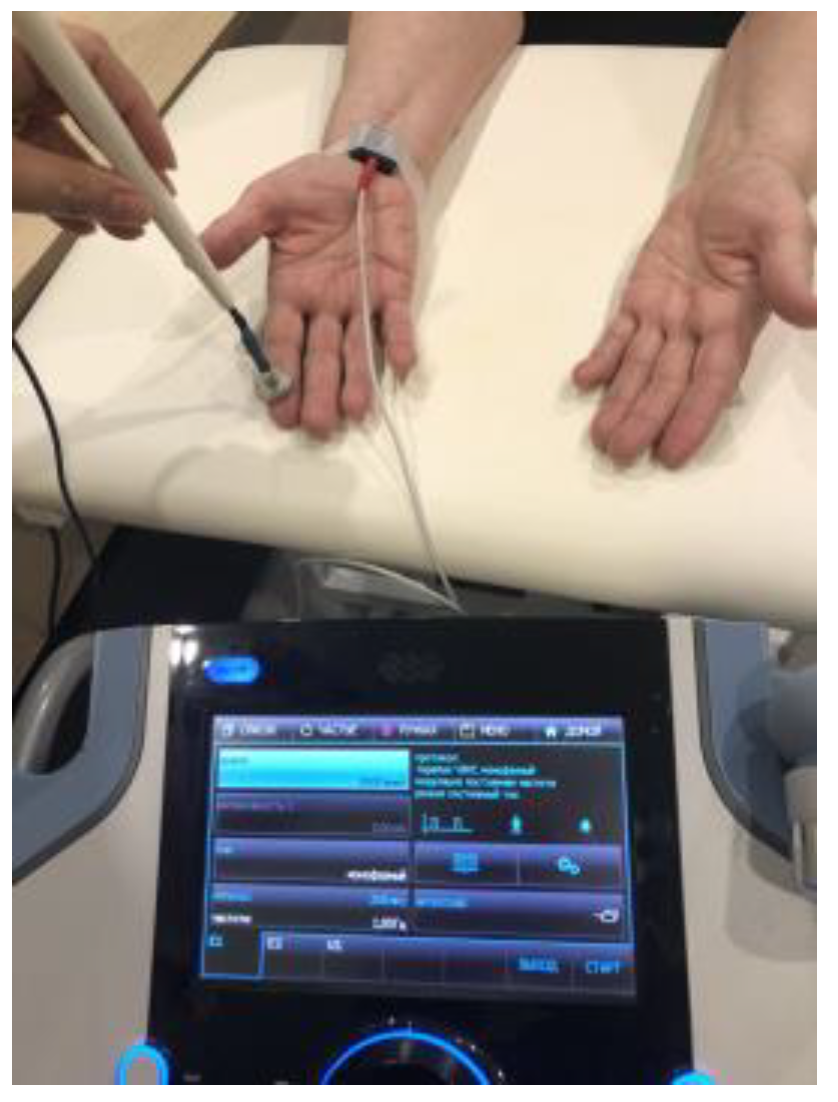
2.8. Transcutaneous Electroneurostimulation
2.8.1. Protocol of Sham Stimulation
2.8.2. Protocol of High-Frequency Low-Amplitude Transcutaneous Electroneurostimulation
2.8.3. Protocol of Low-Frequency High-Amplitude Transcutaneous Electroneurostimulation
2.8.4. Protocol of Co-Administration of High-Frequency Low-Amplitude Transcutaneous Electroneurostimulation and Low-Frequency High-Amplitude Transcutaneous Electroneurostimulation
2.8.5. Transcutaneous Electroneurostimulation Technique
2.9. Statistical Analysis
3. Results
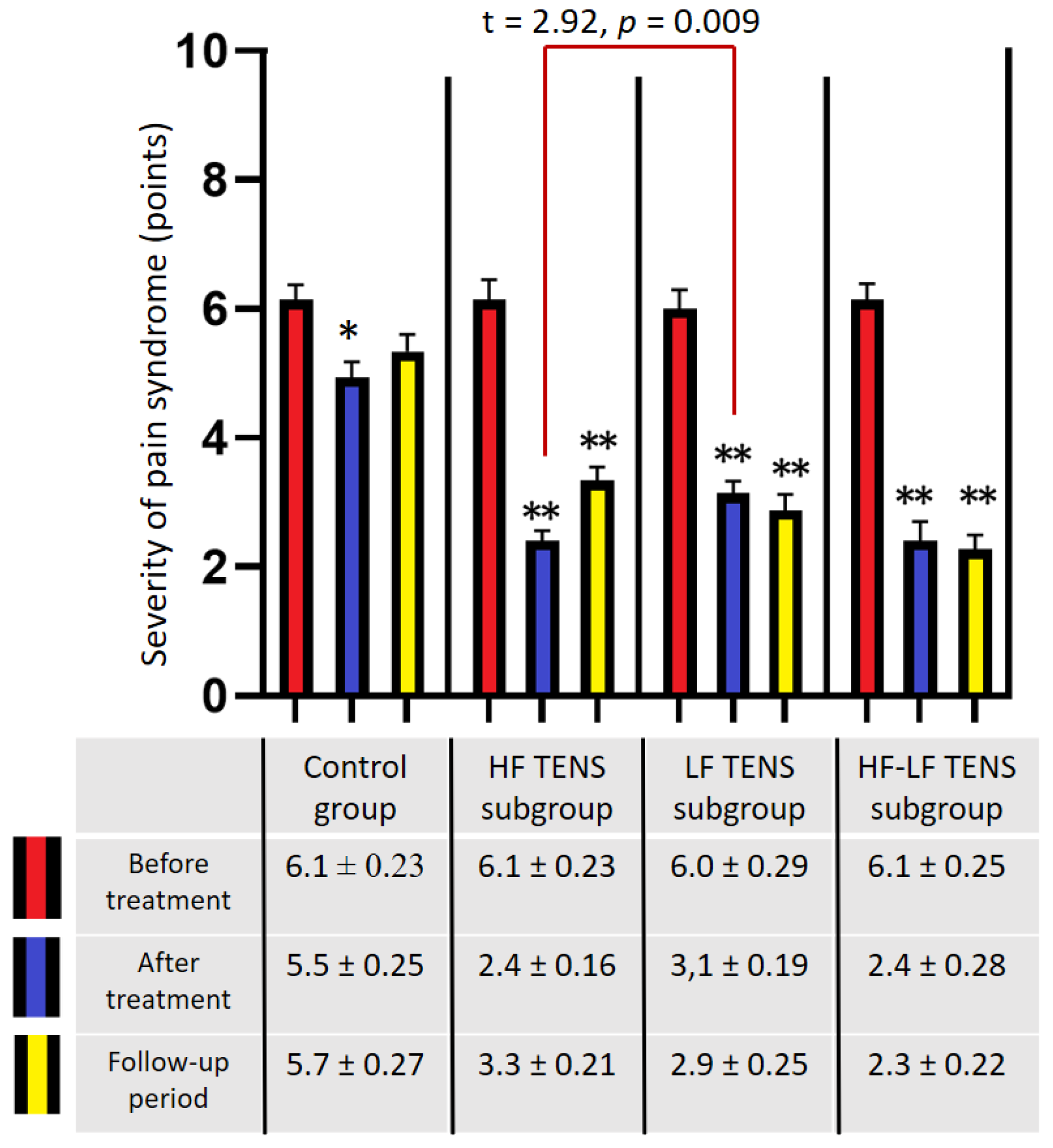
3.1. Assessment of Pain Syndrome
3.1.1. Visual Analogue Scale
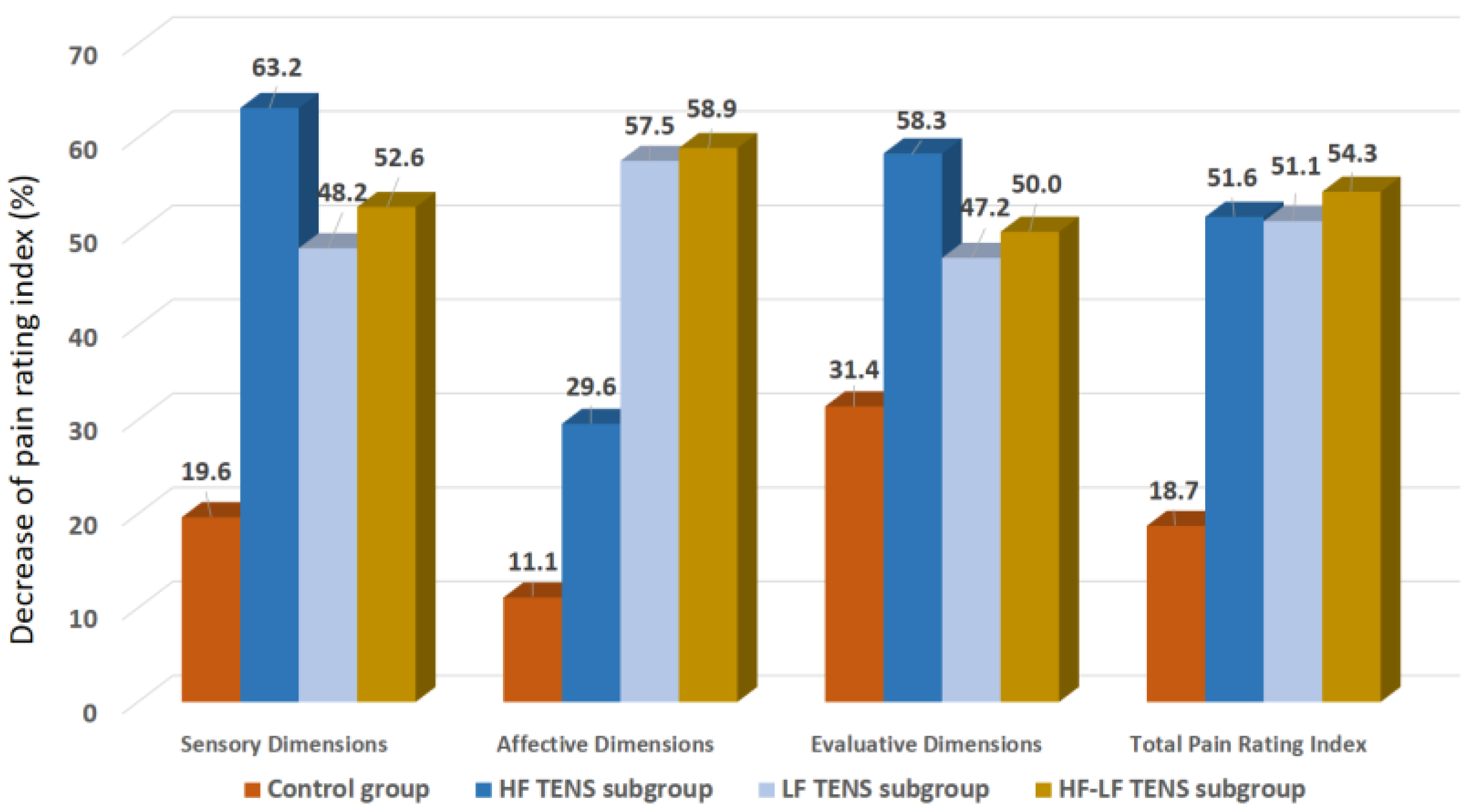
3.1.2. Pain Assessment Using McGill Pain Questionnaire
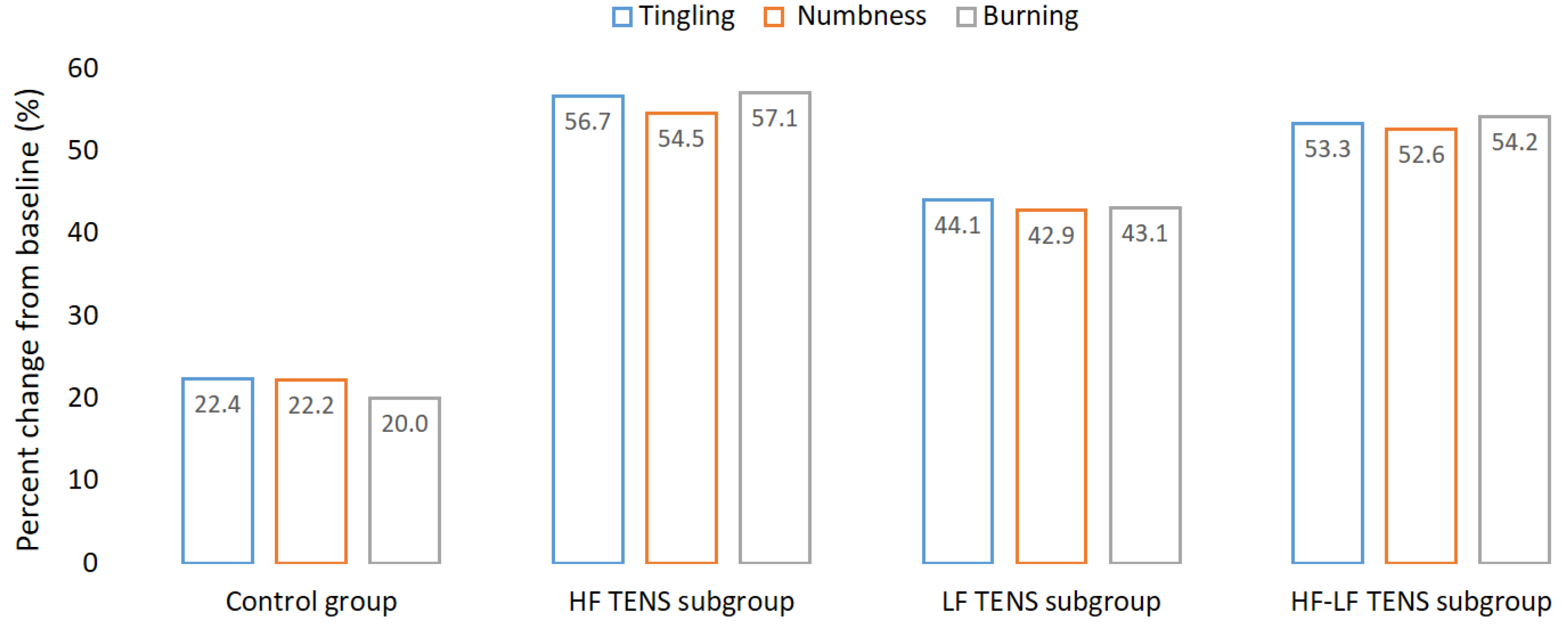
3.2. Assessment of Positive Sensory
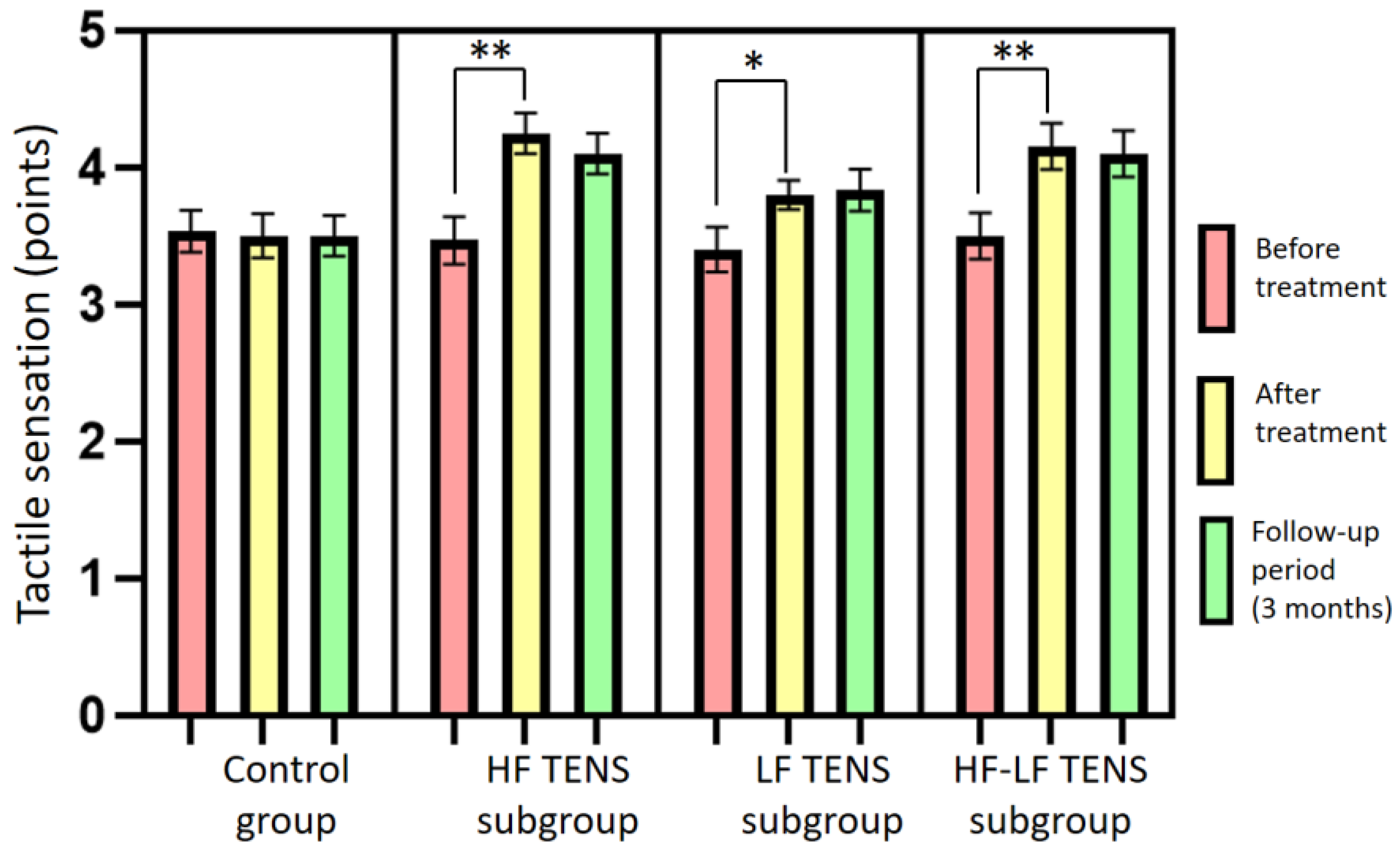
3.3. Assessment of Tactile Sensation
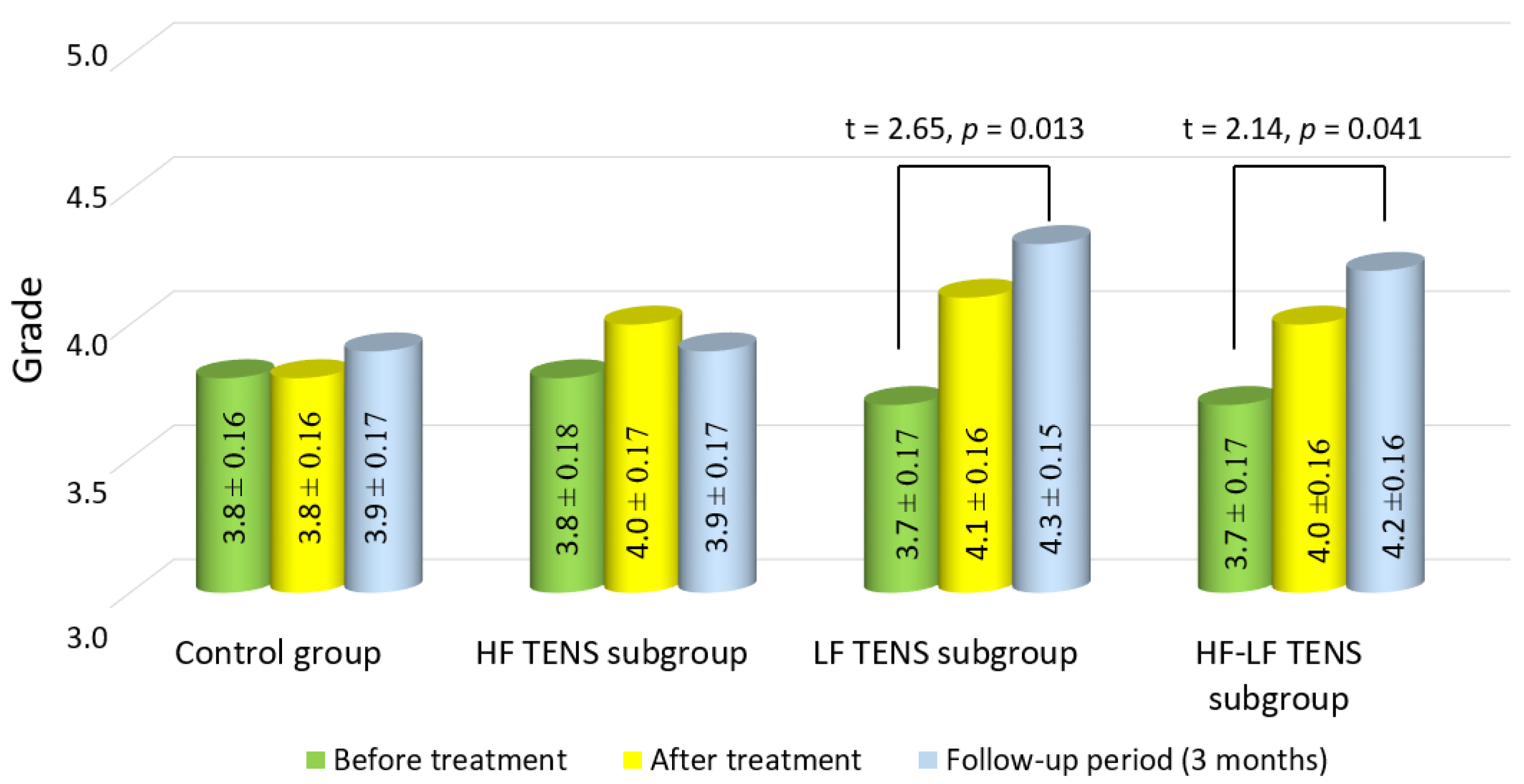
3.4. Assessment of Muscle Strength in the Affected Hand
3.5. Assessment of Fine Movement Skills by the Jebsen–Taylor Test
3.6. Electrodiagnostic Evaluation of Median Nerve
4. Discussion
5. Limitations
- We did not have the opportunity to study the morphological changes of the median nerves using ultrasonography after TENS treatment, because in our study we used only clinical and neurophysiological methods of examination to appraise the effectiveness of treatment. In the next studies, we plan to conduct a control examination of TENS treatment of residual neurological symptoms after CTDS with ultrasonography.
- We were unable to compare the efficacy of TENS with other physical therapy treatments. First, this was not the purpose of our study, and second, we compared the effectiveness of TENS with a (sham stimulation) placebo. The effectiveness of various physiotherapeutic methods, such as manual therapy [17,18], low-intensity laser therapy [19,20,21], acupuncture [22,23,24], electroacupuncture [25], and extracorporeal shock wave therapy [26] has been proven in many studies in the treatment of patients with residual neurological symptoms after CTDS, but TENS has never been studied in the treatment of this disease. Thus, before comparing TENS with other methods of treatment, it was necessary first to prove the effectiveness of TENS in the treatment of patients with residual neurological symptoms after CTDS with the determination of optimal current parameters and appropriate modalities to achieve the maximum therapeutic effect. However, in the next works, we plan to compare TENS with ultrasound and laser therapy in the treatment of patients with residual neurological symptoms after CTDS.
6. Conclusions
7. Declaration of Patient Consent
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Erfanifam, T.; Anaraki, P.H.; Vahedi, L.; Nourmohammadi, J.; Emami, B.; Khameneh, A. The outcomes of carpal tunnel decompression based on electro-diagnostic approaches and clinical symptoms in patients suffering from carpal tunnel syndrome (CTS). J. Family Med. Prim. Care. 2022, 11, 2411–2416. [Google Scholar] [CrossRef]
- Amadio, P.C. The first carpal tunnel release? J. Hand Surg. Br. 1995, 20, 40–41. [Google Scholar] [CrossRef] [PubMed]
- Orhurhu, V.; Orman, S.; Peck, J.; Urits, I.; Orhurhu, M.S.; Jones, M.R.; Manchikanti, L.; Kaye, A.D.; Odonkor, C.; Hirji, S.; et al. Carpal tunnel release surgery—A systematic review of open and endoscopic approaches. Anesth. Pain Med. 2020, 10, e112291. [Google Scholar] [CrossRef] [PubMed]
- Ise, M.; Saito, T.; Katayama, Y.; Nakahara, R.; Shimamura, Y.; Hamada, M.; Senda, M.; Ozaki, T. Relationship between clinical outcomes and nerve conduction studies before and after surgery in patients with carpal tunnel syndrome. BMC Musculoskelet Disord. 2021, 22, 882. [Google Scholar] [CrossRef]
- Guyette, T.M.; Wilgis, E.F. Timing of improvement after carpal tunnel release. J Surg Orthop Adv. 2004, 13, 206–209. [Google Scholar]
- Aydin, M.; Argun, G.; Acar, B.; Arikan, M.; Toğral, G.; Cinaroglu, S.; Mert, A.; Demi Rtas, M. Residual symptoms after carpal tunnel decompression and treatment with gabapentin: A multicenter study. Cureus 2021, 13, e17638. [Google Scholar] [CrossRef]
- Botte, M.J.; von Schroeder, H.P.; Abrams, R.A.; Gellman, H. Recurrent carpal tunnel syndrome. Hand Clin. 1996, 12, 731–743. [Google Scholar] [PubMed]
- Jones, N.F.; Ahn, H.C.; Eo, S. Revision surgery for persistent and recurrent carpal tunnel syndrome and for failed carpal tunnel release. Plast. Reconstr. Surg. 2012, 129, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Campagna, R.; Pessis, E.; Feydy, A.; Guerini, H.; Le Vie, D.; Corlobé, P.; Drapé, J.L. MRI assessment of recurrent carpal tunnel syndrome after open surgical release of the median nerve. AJR Am. J. Roentgenol. 2009, 193, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Al-Zamil, M.K. Differential diagnosis of carpal tunnel syndrome. Bull. Med. Stomatol. Inst. 2022, 2, 61–71. Available online: https://elibrary.ru/item.asp?id=49864221 (accessed on 1 June 2022).
- Sevy, J.O.; Varacallo, M. Carpal Tunnel Syndrome. 2022. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Kronlage, S.C.; Menendez, M.E. The benefit of carpal tunnel release in patients with electrophysiologically moderate and severe disease. J. Hand Surg. Am. 2015, 40, 438–444.e1. [Google Scholar] [CrossRef]
- Fowler, J.R.; Munsch, M.; Huang, Y.; Hagberg, W.C.; Imbriglia, J.E. Pre-operative electrodiagnostic testing predicts time to resolution of symptoms after carpal tunnel release. J. Hand Surg. 2016, 41, 137–142. [Google Scholar] [CrossRef]
- Matsis, R.; Chou, J.; Clode, N. Outcome of carpal tunnel decompression with pre-surgical diagnosis determined on general practitioner assessment and nerve conduction study. J. Clin. Orthop. Trauma. 2020, 13, 15–18. [Google Scholar] [CrossRef]
- Sun, P.O.; Selles, R.W.; Jansen, M.C.; Slijper, H.P.; Ulrich, D.J.O.; Walbeehm, E.T. Recurrent and persistent carpal tunnel syndrome: Predicting clinical outcome of revision surgery. J. Neurosurg. 2019, 132, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Kilinc, F.; Behmanesh, B.; Seifert, V.; Marquardt, G. Does Recurrence of Carpal Tunnel Syndrome (CTS) after Complete Division of the Transverse Ligament Really Exist? J. Clin. Med. 2021, 10, 4208. [Google Scholar] [CrossRef]
- Mohamed, F.I.; Hassan, A.A.; Abdel-Magied, R.A.; Wageh, R.N. Manual therapy intervention in the treatment of patients with carpal tunnel syndrome: Median nerve mobilization versus medical treatment. Egypt. Rheumatol. Rehabil. 2016, 43, 27–34. [Google Scholar] [CrossRef]
- Wolny, T.; Linek, P. Is manual therapy based on neurodynamic techniques effective in the treatment of carpal tunnel syndrome? A randomized controlled trial. Clin. Rehabil. 2019, 33, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Pratelli, E.; Pintucci, M.; Cultrera, P.; Baldini, E.; Stecco, A.; Petrocelli, A.; Pasquetti, P. Conservative treatment of carpal tunnel syndrome: Comparison between laser therapy and Fascial Manipulation((R)). J. Bodyw. Mov. Ther. 2015, 19, 113–118. [Google Scholar] [PubMed]
- Chang, W.D.; Wu, J.H.; Jiang, J.A.; Yeh, C.Y.; Tsai, C.T. Carpal tunnel syndrome treated with a diode laser: A controlled treatment of the transverse carpal ligament. Photomed. Laser Surg. 2008, 26, 551–557. [Google Scholar] [CrossRef]
- Güner, A.; Altan, L.; Kasapoğlu Aksoy, M. The effectiveness of the low-power laser and kinesiotaping in the treatment of carpal tunnel syndrome, a pilot study. Rheumatol. Int. 2018, 38, 895–904. [Google Scholar] [CrossRef]
- Cabýoglu, M.T.; Ergene, N.; Tan, U. The mechanism of acupuncture and clinical applications. Int. J. Neurosci. 2006, 16, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Bahrami-Taghanaki, H.; Azizi, H.; Hasanabadi, H.; Jokar, M.H.; Iranmanesh, A.; Khorsand-Vakilzadeh, A.; Badiee-Aval, S. Acupuncture for Carpal Tunnel Syndrome: A Randomized Controlled Trial Studying Changes in Clinical Symptoms and Electrodiagnostic Tests. Altern. Ther. Health Med. 2020, 26, 10–16. [Google Scholar]
- Ho, C.Y.; Lin, H.C.; Lee, Y.C.; Chou, L.W.; Kuo, T.W.; Chang, H.W.; Chen, Y.S.; Lo, S.F. Clinical effectiveness of acupuncture for carpal tunnel syndrome. Am. J. Chin. Med. 2014, 42, 303–314. [Google Scholar] [CrossRef]
- Li, T.; Yan, J.; Hu, J.; Liu, X.; Wang, F. Efficacy and safety of electroacupuncture for carpal tunnel syndrome (CTS): A systematic review and meta-analysis of randomized controlled trials. Front. Surg. 2022, 9, 952361. [Google Scholar] [CrossRef]
- Kim, J.C.; Jung, S.H.; Lee, S.U.; Lee, S.Y. Effect of extracorporeal shockwave therapy on carpal tunnel syndrome: A systematic review and meta-analysis of randomized controlled trials. Medicine 2019, 98, e16870. [Google Scholar] [CrossRef] [PubMed]
- Chapman, R.C.; Wilson, M.E.; Gehrig, J.D. Comparative effects of acupuncture and transcutaneous stimulation on the perception of painful dental stimuli. Pain 1976, 2, 265–283. [Google Scholar] [CrossRef] [PubMed]
- Melzack, R.; Wall, P.D. Acupuncture and transcutaneous electrical nerve stimulation. Postgrad. Med. J. 1984, 60, 893–896. [Google Scholar] [CrossRef]
- Sluka, K.A.; Deacon, M.; Stibal, A.; Strissel, S.; Terpstra, A. Spinal blockade of opioid receptors prevents the analgesia produced by TENS in arthritic rats. J. Pharmacol. Exp. Ther. 1999, 289, 840–846. [Google Scholar]
- DeSantana, J.M.; Da Silva, L.F.; De Resende, M.A.; Sluka, K.A. Transcutaneous electrical nerve stimulation at both high and low frequencies activates ventrolateral periaqueductal grey to decrease mechanical hyperalgesia in arthritic rats. Neuroscience 2009, 163, 1233–1241. [Google Scholar] [CrossRef]
- Vance, C.G.; Dailey, D.L.; Rakel, B.A.; Sluka, K.A. Using TENS for pain control: The state of the evidence. Pain Manag. 2014, 4, 197–209. [Google Scholar] [CrossRef]
- Johnson, M.I.; Bjordal, J.M. Transcutaneous electrical nerve stimulation for the management of painful conditions: Focus on neuropathic pain. Expert. Rev. Neurother. 2011, 11, 735–753. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.T.; Sluka, K.A. Reduction in inflammation-induced sensitization of dorsal horn neurons by transcutaneous electrical nerve stimulation in anesthetized rats. Exp. Brain Res. 2001, 137, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Baptista, A.F.; Gomes, J.R.; Oliveira, J.T.; Santos, S.M.; Vannier-Santos, M.A.; Martinez, A.M. High- and low-frequency transcutaneous electrical nerve stimulation delay sciatic nerve regeneration after crush lesion in the mouse. J. Peripher. Nerv. Syst. 2008, 13, 71–80. [Google Scholar] [CrossRef]
- Bersch, I.; Fridén, J. Electrical stimulation alters muscle morphological properties in denervated upper limb muscles. Ebiomedicine 2021, 74, 103737. [Google Scholar] [CrossRef]
- Bahadori, S.; Immins, T.; Wainwright, T.W. The effect of calf neuromuscular electrical stimulation and intermittent pneumatic compression on thigh microcirculation. Microvasc. Res. 2017, 111, 37–41. [Google Scholar] [CrossRef]
- Al-Zamil, M.; Minenko, I.A.; Kulikova, N.G.; Alade, M.; Petrova, M.M.; Pronina, E.A.; Romanova, I.V.; Narodova, E.A.; Nasyrova, R.F.; Shnayder, N.A. Clinical experience of high frequency and low frequency TENS in treatment of diabetic neuropathic pain in Russia. Healthcare 2022, 10, 250. [Google Scholar] [CrossRef]
- Al-Zamil, M.K.; Kulikova, N.G. Transcutaneous electroneurostimulation in treatment patients with diabetic neuropathy. Russ. J. Physiother. Balneol. Rehabil. 2021, 20, 119–124. [Google Scholar] [CrossRef]
- Al-Zamil, M.K.; Minenko, I.A. Algorithm for the treatment of carpal syndrome in patients with type 2 diabetes mellitus using acupuncture and transcutaneous electrical nerve stimulation in combination with drug therapy. Bull. New Med. Technol. Electron. Ed. 2016, 2, 2. Available online: http://www.medtsu.tula.ru/VNMT/Bulletin/E2016-2/2-2.pdf (accessed on 1 June 2016). [CrossRef]
- Forst, T.; Nguyen, M.; Forst, S.; Disselhoff, B.; Pohlmann, T.; Pfützner, A. Impact of low frequency transcutaneous electrical nerve stimulation on symptomatic diabetic neuropathy using the new Salutaris device. Diabetes Nutr. Metab. 2004, 17, 163–168. [Google Scholar]
- Sears, E.D.; Chung, K.C. Validity and responsiveness of the Jebsen-Taylor Hand Function Test. J. Hand Surg. Am. 2010, 35, 30–37. [Google Scholar] [CrossRef]
- Lin, T.Y.; Chang, K.V.; Wu, W.T.; Özçakar, L. Ultrasonography for the diagnosis of carpal tunnel syndrome: An umbrella review. J. Neurol. 2022, 269, 4663–4675. [Google Scholar] [CrossRef] [PubMed]
- Tu, I.T.; Jou, I.M.; Ko, P.Y.; Lee, J.S.; Kuo, L.C.; Li, C.Y.; Wu, P.T. Diagnosis of carpal tunnel syndrome in patients without diabetes with hemodialysis using ultrasonography: Is it a useful adjunctive tool? Arch. Phys. Med. Rehabil. 2022, 103, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.W.H.; Griffith, J.F.; Tsoi, C.; Fong, R.C.W.; Mak, M.C.K.; Tse, W.L.; Ho, P.C. Ultrasonography Findings of the Carpal Tunnel after Endoscopic Carpal Tunnel Release for Carpal Tunnel Syndrome. Korean J. Radiol. 2021, 22, 1132–1141. [Google Scholar] [CrossRef]
- Pace, V.; Marzano, F.; Placella, G. Update on surgical procedures for carpal tunnel syndrome: What is the current evidence and practice? What are the future research directions? World J. Orthop. 2023, 14, 6–12. [Google Scholar] [CrossRef]
- Jebsen, R.H.; Taylor, N.; Trieschmann, R.B.; Trotter, M.J.; Howard, L.A. An objective and standardized test of hand function. Arch. Phys. Med. Rehabil. 1969, 50, 311–319. [Google Scholar]
- Somers, D.L.; Clemente, F.R. Contralateral high or a combination of high- and low-frequency transcutaneous electrical nerve stimulation reduces mechanical allodynia and alters dorsal horn neurotransmitter content in neuropathic rats. J. Pain 2009, 10, 221–229. [Google Scholar] [CrossRef]
- Vance, C.G.; Radhakrishnan, R.; Skyba, D.A.; Sluka, K.A. Transcutaneous electrical nerve stimulation at both high and low frequencies reduces primary hyperalgesia in rats with joint inflammation in a time-dependent manner. Phys. Ther. 2007, 87, 44–51. [Google Scholar] [CrossRef]
- Yuan, C.S.; Attele, A.S.; Dey, L.; Lynch, J.P.; Guan, X. Transcutaneous electrical acupoint stimulation potentiates analgesic effect of morphine. J. Clin. Pharmacol. 2002, 42, 899–903. [Google Scholar] [CrossRef]
- Astokorki, A.H.Y.; Mauger, A.R. Transcutaneous electrical nerve stimulation reduces exercise-induced perceived pain and improves endurance exercise performance. Eur. J. Appl. Physiol. 2017, 117, 483–492. [Google Scholar] [CrossRef]
- Han, J.S.; Chen, X.H.; Sun, S.L.; Xu, X.J.; Yuan, Y.; Yan, S.C.; Hao, J.X.; Terenius, L. Effect of low- and high-frequency TENS on Met-enkephalin-Arg-Phe and dynorphin A immunoreactivity in human lumbar CSF. Pain 1991, 47, 295–298. [Google Scholar] [CrossRef]
- Francis, R.P.; Johnson, M.I. The characteristics of acupuncture-like transcutaneous electrical nerve stimulation (acupuncture-like TENS): A literature review. Acupunct. Electrother. Res. 2011, 36, 231–258. [Google Scholar] [CrossRef]
- Wang, X.; Chan, S.T.; Fang, J.; Nixon, E.E.; Liu, J.; Kwong, K.K.; Rosen, B.R.; Hui, K.K. Neural encoding of acupuncture needling sensations: Evidence from a FMRI study. Evid. Based Complement. Alternat Med. 2013, 2013, 483105. [Google Scholar] [CrossRef] [PubMed]
- Han, J.S. Acupuncture: Neuropeptide release produced by electrical stimulation of different frequencies. Trends Neurosci. 2003, 26, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Al-Zamil, M.K.; Kulikova, N.G. TENS and acupuncture in treatment of carpal tunnel syndrome. Int. J. Pharmacogn. Chin. Med. 2021, 5, 000210. [Google Scholar] [CrossRef]
- Gozani, S.N. Remote analgesic effects of conventional transcutaneous electrical nerve stimulation: A scientific and clinical review with a focus on chronic pain. J. Pain Res. 2019, 12, 3185–3201. [Google Scholar] [CrossRef]
- Dailey, D.L.; Rakel, B.A.; Vance, C.G. Transcutaneous electrical nerve stimulation reduces pain, fatigue and hyperalgesia while restoring central inhibition in primary fibromyalgia. Pain 2013, 154, 2554–2562. [Google Scholar] [CrossRef]
- Kulikova, N.G.; Konchugova, T.V.; Astakhova, K.A.; Nesterova, E.V.; Al-Zamil, M.K. Indicators of bioelectrical activity of the brain in patients with distal polyneuropathy after the use of transcutaneous methods of electrical nerve stimulation of the median nerves. Quest. Curortol. Physiother. Ther. Phys. Cult. 2021, 98, 108–109. [Google Scholar] [CrossRef]
- Sherry, J.E.; Oehrlein, K.M.; Hegge, K.S.; Morgan, B.J. Effect of burst-mode transcutaneous electrical nerve stimulation on peripheral vascular resistance. Phys. Ther. 2001, 81, 1183–1191. [Google Scholar] [CrossRef]
- Sandberg, M.L.; Sandberg, M.K.; Dahl, J. Blood flow changes in the trapezius muscle and overlying skin following transcutaneous electrical nerve stimulation. Phys. Ther. 2007, 87, 1047–1055. [Google Scholar] [CrossRef]
- do Carmo Almeida, T.C.; Dos Santos Figueiredo, F.W.; Barbosa Filho, V.C.; de Abreu, L.C.; Fonseca, F.L.A.; Adami, F. Effects of transcutaneous electrical nerve stimulation on proinflammatory cytokines: Systematic review and meta-analysis. Mediat. Inflamm. 2018, 2018, 1094352. [Google Scholar] [CrossRef]
- Beckwée, D.; De Hertogh, W.; Lievens, P.; Bautmans, I.; Vaes, P. Effect of tens on pain in relation to central sensitization in patients with osteoarthritis of the knee: Study protocol of a randomized controlled trial. Trials 2012, 13, 21. [Google Scholar] [CrossRef] [PubMed]
- Haastert-Talini, K.; Grothe, C. Electrical stimulation for promoting peripheral nerve regeneration. Int. Rev. Neurobiol. 2013, 109, 111–124. [Google Scholar] [CrossRef]
- Mendonça, A.C.; Barbieri, C.H.; Mazzer, N. Directly applied low intensity direct electric current enhances peripheral nerve regeneration in rats. J. Neurosci. Methods 2003, 129, 183–190. [Google Scholar] [CrossRef]
- Gordon, T. Electrical stimulation to enhance axon regeneration after peripheral nerve injuries in animal models and humans. Neurotherapeutics 2016, 13, 295–310. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.C.; Silva, V.Z.M.D.; Júnior, G.C.; Liebano, R.E.; Durigan, J.L.Q. Transcutaneous electrical nerve stimulation and interferential current demonstrate similar effects in relieving acute and chronic pain: A systematic review with meta-analysis. Braz. J. Phys. Ther. 2018, 22, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Gibson, W.; Wand, B.M.; O’Connell, N.E. Transcutaneous electrical nerve stimulation (TENS) for neuropathic pain in adults. Cochrane Database Syst. Rev. 2017, 9, CD011976. [Google Scholar] [CrossRef]
- Al-Zamil, M.K.; Kulikova, N.G.; Vasileva, E.S.; Ephimov, M.A. Dynamics of allodynia in the treatment of patients with diabetic polyneuropathy using transdermal electrical nerve stimulation. Russ. J. Physiother. Balneol. Rehabil. 2021, 20, 187–193. [Google Scholar] [CrossRef]
- Peng, W.W.; Tang, Z.Y.; Zhang, F.R.; Li, H.; Kong, Y.Z.; Iannetti, G.D.; Hu, L. Neurobiological mechanisms of TENS-induced analgesia. Neuroimage 2019, 195, 396–408. [Google Scholar] [CrossRef]
- Alarcón, J.B.; Chuhuaicura, P.B.; Sluka, K.A.; Vance, C.G.T.; Fazan, V.P.S.; Godoy, K.A.; Fuentes, R.E.; Dias, F.J. Transcutaneous Electrical Nerve Stimulation in Nerve Regeneration: A Systematic Review of In Vivo Animal Model Studies. Neuromodulation 2022, 25, 1248–1258. [Google Scholar] [CrossRef]
- Su, H.L.; Chiang, C.Y.; Lu, Z.H.; Cheng, F.C.; Chen, C.J.; Sheu, M.L.; Sheehan, J.; Pan, H.C. Late administration of high-frequency electrical stimulation increases nerve regeneration without aggravating neuropathic pain in a nerve crush injury. BMC Neurosci. 2018, 19, 37. [Google Scholar] [CrossRef]
- Ni, L.; Yao, Z.; Zhao, Y.; Zhang, T.; Wang, J.; Li, S.; Chen, Z. Electrical stimulation therapy for peripheral nerve injury. Front. Neurol. 2023, 14, 1081458. [Google Scholar] [CrossRef] [PubMed]

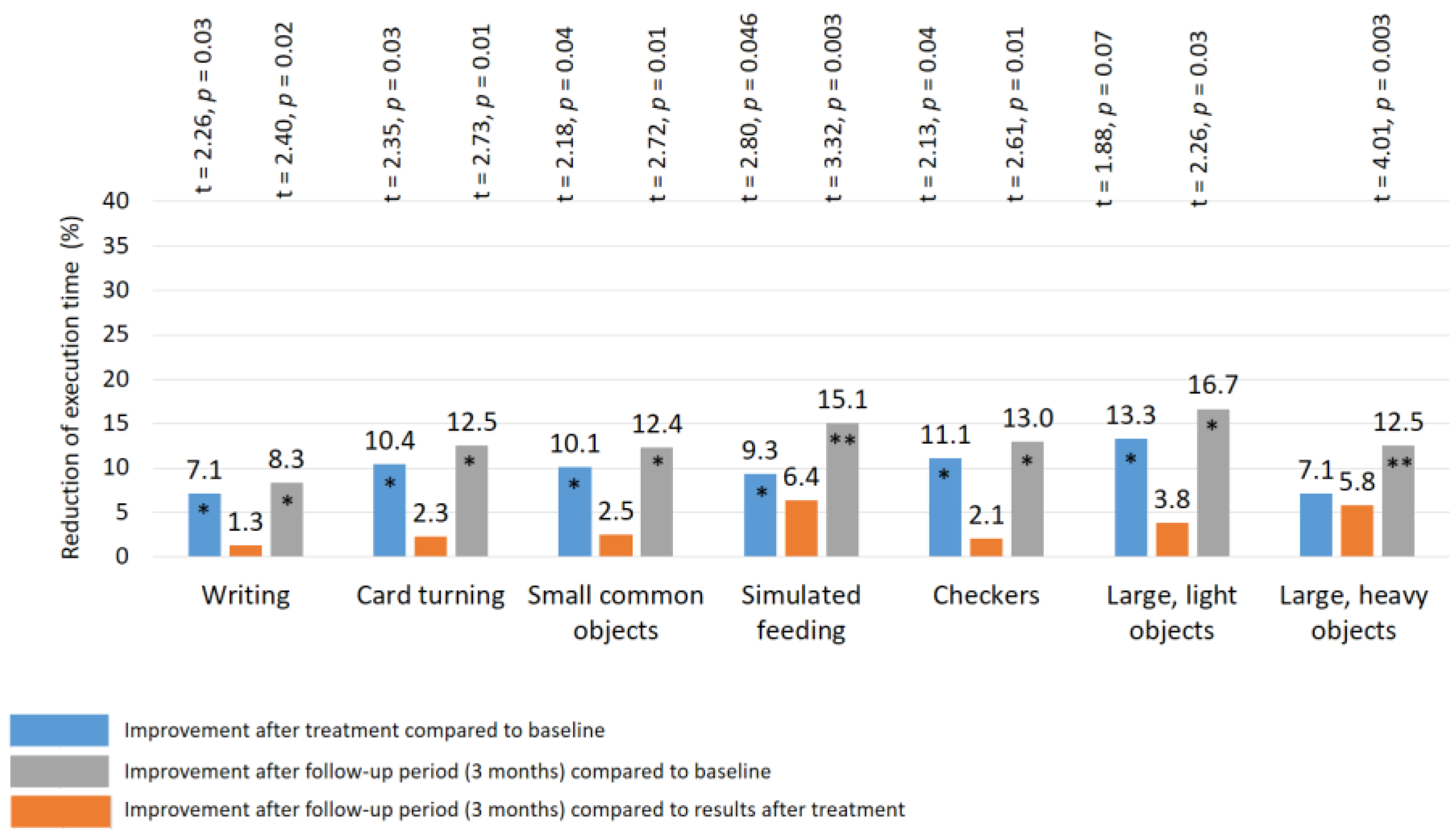
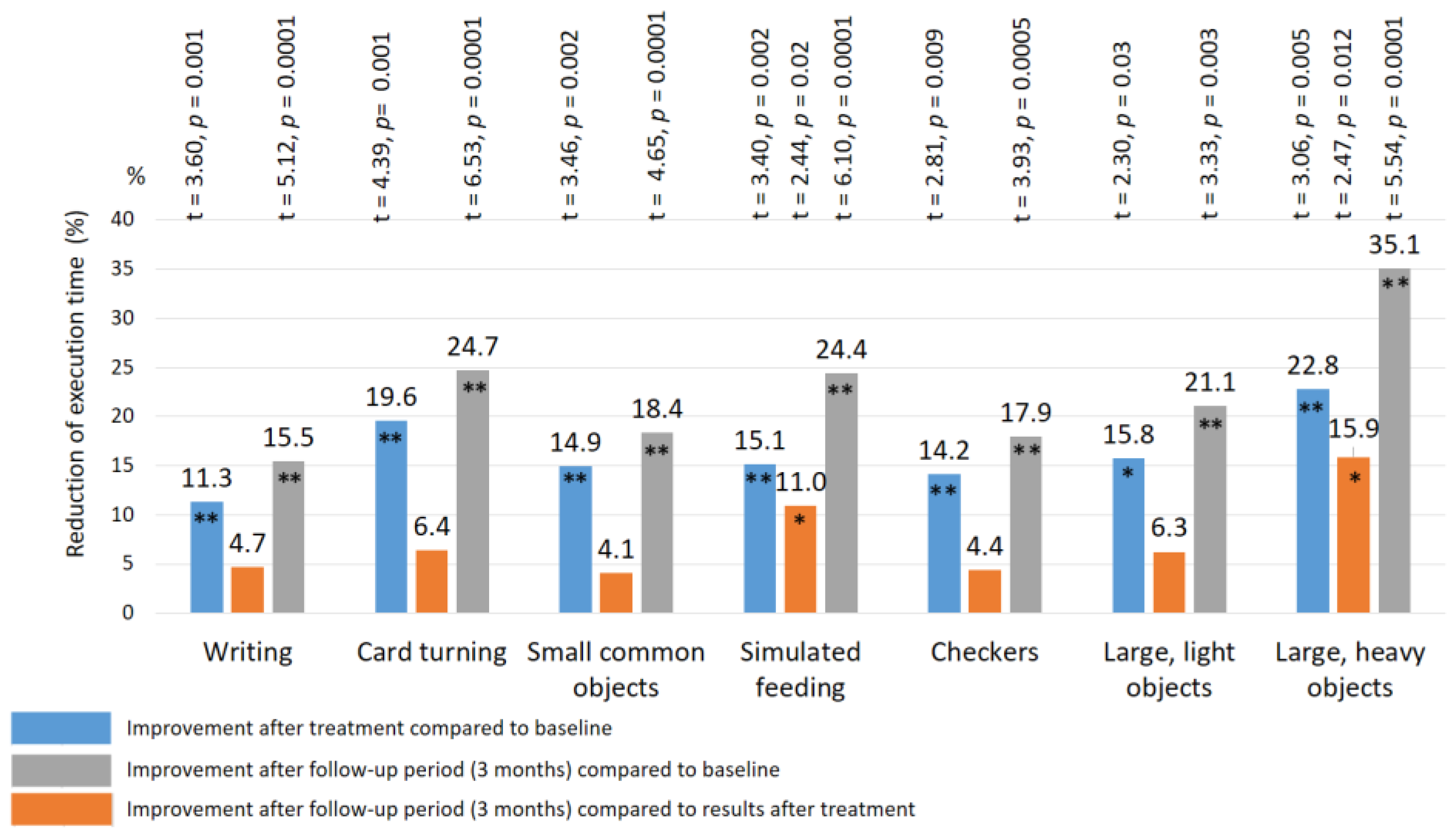
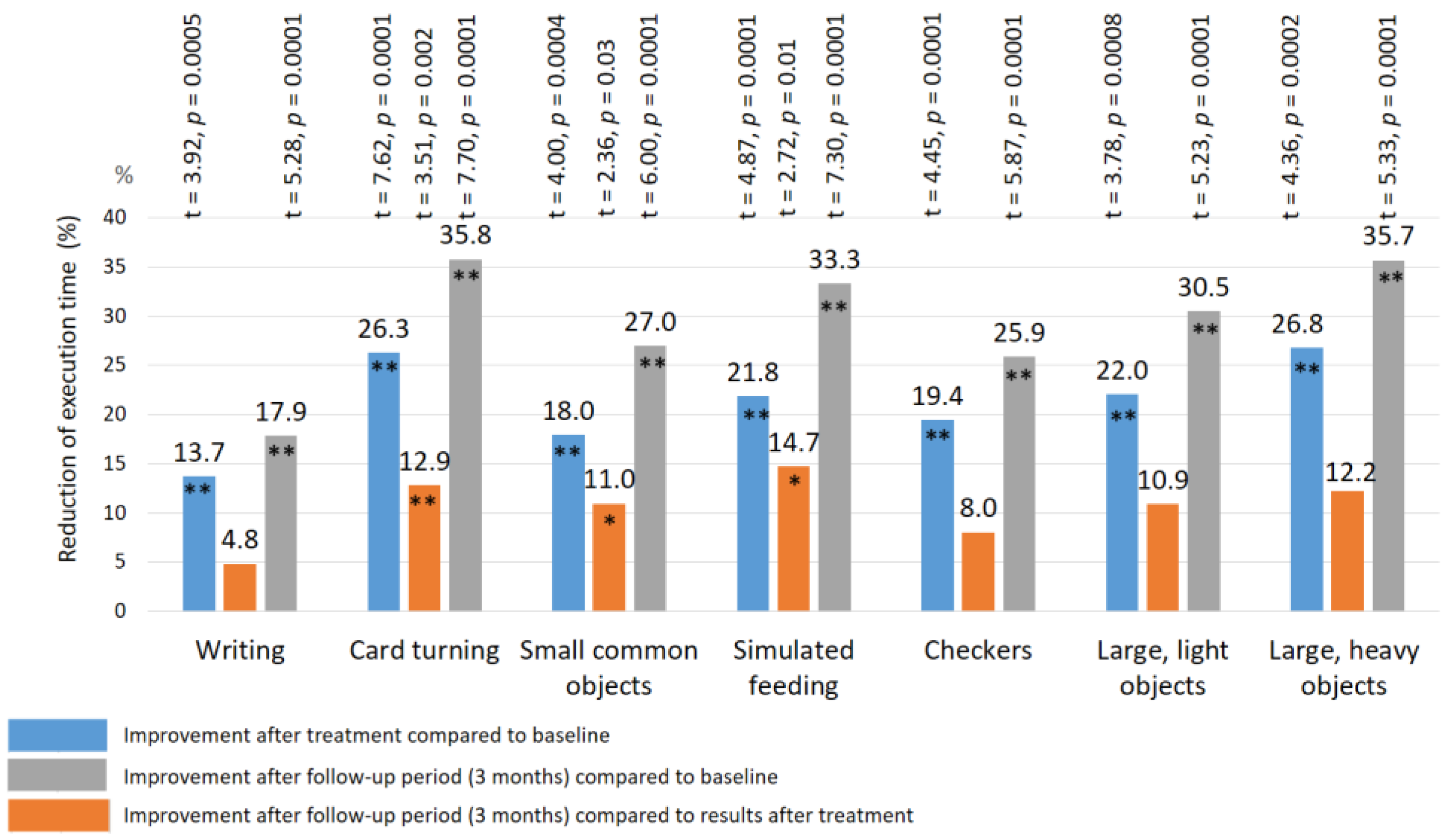
| Reflex | Control Group | TENS Group | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HF TENS Subgroup | LF TENS Subgroup | HF-LF TENS Subgroup | Normal [46] | ||||||||||
| Writing | 16.9± 0.49 | 16.2 ± 0.46 | 17.2 ± 0.48 | 16.8 ± 0.45 | 15.6 ± 0.38 * | 15.4 ± 0.37 * | 16.8 ± 0.41 | 14.9 ± 0.33 ** | 14.2 ± 0.30 ** | 16.8 ± 0.47 | 14.5 ± 0.35 ** | 13.8 ± 0.32 ** | 11.7 ± 2.1 |
| Card turning | 9.4 ± 0.28 | 9.1 ± 0.26 | 9.5 ± 0.28 | 9.6 ± 0.30 | 8.6 ± 0.30 * | 8.4 ± 0.32 * | 9.7 ± 0.32 | 7.8 ± 0.29 ** | 7.3 ± 0.18 ** | 9.5 ± 0.26 | 7.0 ± 0.20 ** | 6.1 ± 0.16 **## | 4.3 ± 1.4 |
| Small common objects | 8.7 ± 0.30 | 8.5 ± 0.28 | 9.2 ± 0.30 | 8.9 ± 0.26 | 8.0 ± 0.32 * | 7.8 ± 0.31 * | 8.7 ± 0.28 | 7.4 ± 0.25 ** | 7.1 ± 0.20 ** | 8.9 ± 0.32 | 7.3 ± 0.24 ** | 6.5 ± 0.24 **# | 5.5 ± 1.0 |
| Simulated feeding | 8.4 ± 0.32 | 8.5 ± 0.30 | 8.2 ± 0.30 | 8.6 ± 0.30 | 7.8 ± 0.24 * | 7.3 ± 0.25 * | 8.6 ± 0.28 | 7.3 ± 0.26 ** | 6.5 ± 0.20 **# | 8.7 ± 0.30 | 6.8 ± 0.25 ** | 5.8 ± 0.26 **# | 6.7 ± 1.1 |
| Checkers | 5.5 ± 0.38 | 5.7 ± 0.35 | 5.6 ± 0.37 | 5.8 ± 0.35 | 4.9 ± 0.25 * | 4.7 ± 0.26 * | 5.4 ± 0.34 | 4.3 ± 0.30 ** | 4.1 ± 0.20 ** | 5.7 ± 0.30 | 4.5 ± 0.25 ** | 4.1 ± 0.20 ** | 3.3 ± 0.6 |
| Large light objects | 5.9 ± 0.30 | 6.0 ± 0.32 | 5.8 ± 0.30 | 6.0 ± 0.30 | 5.2 ± 0.30 * | 5.0 ± 0.28 * | 5.7 ± 0.31 | 4.8 ± 0.25 * | 4.5 ± 0.20 ** | 5.9 ± 0.28 | 4.6 ± 0.20 ** | 4.1 ± 0.20 ** | 3.1 ± 0.5 |
| Large heavy objects | 5.4 ± 0.29 | 5.1 ± 0.28 | 5.2 ± 0.30 | 5.6 ± 0.30 | 5.2 ± 0.30 | 4.9 ± 0.25 ** | 5.7 ± 0.32 | 4.4 ± 0.28 ** | 3.7 ± 0.22 **# | 5.6 ± 0.28 | 4.1 ± 0.20 ** | 3.6 ± 0.25 ** | 3.2 ± 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Zamil, M.; Minenko, I.A.; Kulikova, N.G.; Mansur, N.; Nuvakhova, M.B.; Khripunova, O.V.; Shurygina, I.P.; Topolyanskaya, S.V.; Trefilova, V.V.; Petrova, M.M.; et al. Efficiency of Direct Transcutaneous Electroneurostimulation of the Median Nerve in the Regression of Residual Neurological Symptoms after Carpal Tunnel Decompression Surgery. Biomedicines 2023, 11, 2396. https://doi.org/10.3390/biomedicines11092396
Al-Zamil M, Minenko IA, Kulikova NG, Mansur N, Nuvakhova MB, Khripunova OV, Shurygina IP, Topolyanskaya SV, Trefilova VV, Petrova MM, et al. Efficiency of Direct Transcutaneous Electroneurostimulation of the Median Nerve in the Regression of Residual Neurological Symptoms after Carpal Tunnel Decompression Surgery. Biomedicines. 2023; 11(9):2396. https://doi.org/10.3390/biomedicines11092396
Chicago/Turabian StyleAl-Zamil, Mustafa, Inessa A. Minenko, Natalia G. Kulikova, Numman Mansur, Margarita B. Nuvakhova, Olga V. Khripunova, Irina P. Shurygina, Svetlana V. Topolyanskaya, Vera V. Trefilova, Marina M. Petrova, and et al. 2023. "Efficiency of Direct Transcutaneous Electroneurostimulation of the Median Nerve in the Regression of Residual Neurological Symptoms after Carpal Tunnel Decompression Surgery" Biomedicines 11, no. 9: 2396. https://doi.org/10.3390/biomedicines11092396
APA StyleAl-Zamil, M., Minenko, I. A., Kulikova, N. G., Mansur, N., Nuvakhova, M. B., Khripunova, O. V., Shurygina, I. P., Topolyanskaya, S. V., Trefilova, V. V., Petrova, M. M., Narodova, E. A., Soloveva, I. A., Nasyrova, R. F., & Shnayder, N. A. (2023). Efficiency of Direct Transcutaneous Electroneurostimulation of the Median Nerve in the Regression of Residual Neurological Symptoms after Carpal Tunnel Decompression Surgery. Biomedicines, 11(9), 2396. https://doi.org/10.3390/biomedicines11092396









