Do Patients with Parkinson’s Disease Benefit from Dynamic Body Weight Support? A Pilot Study on the Emerging Role of Rysen
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population and Setting
2.3. Procedures
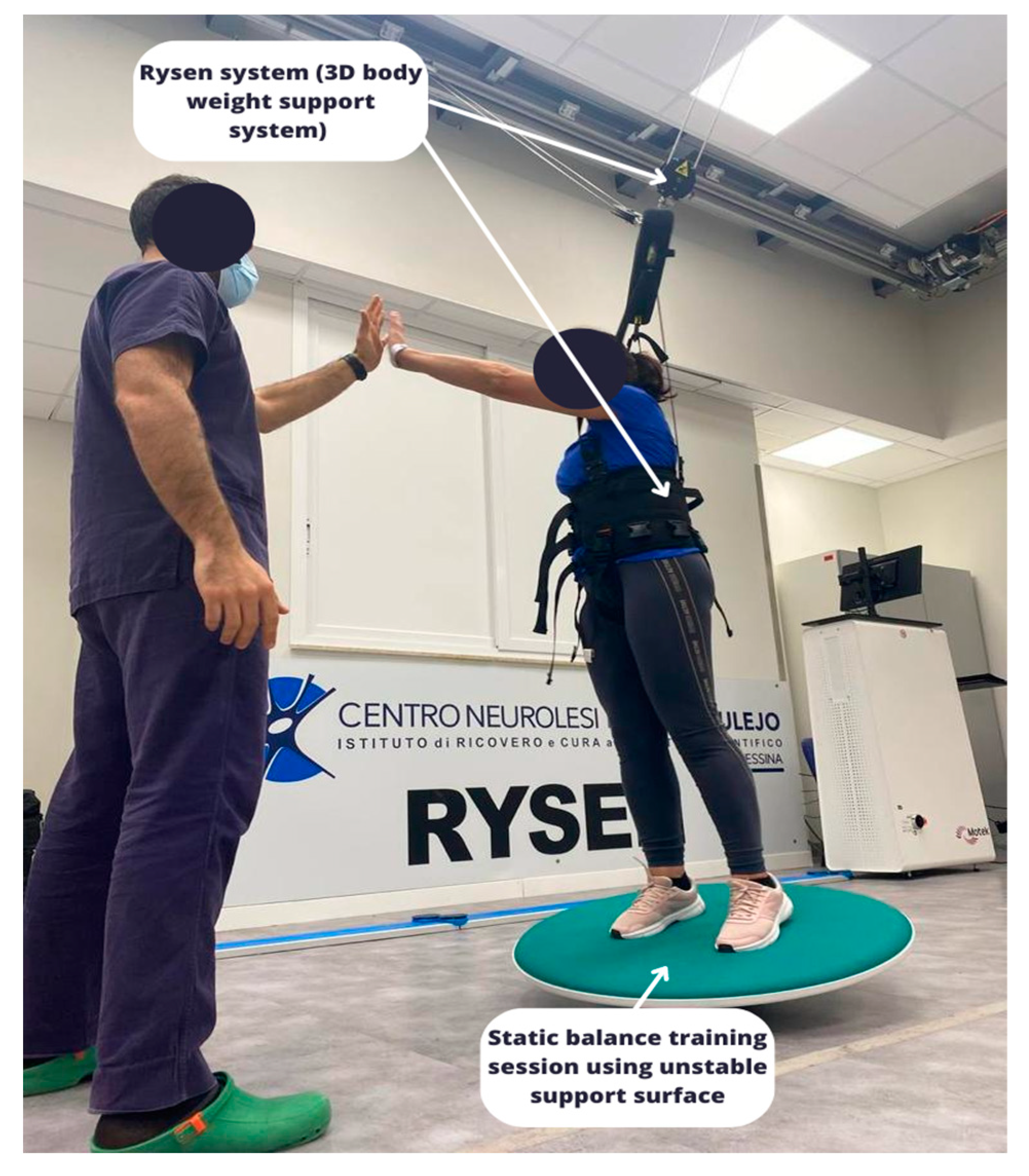
2.3.1. The Rysen System
- Stand up: this mode was used to initiate a training session by gently bringing the individual into a standing position in the longitudinal direction of the room, in order to perform a postural alignment with the aid of the BWS.
- Walking: when the walking mode was selected, all kinds of gait exercises were performed by editing the vertical and horizontal supports (see Figure 2).
- Static and dynamic balance: the subject shifted his weight from side to side to find the equilibrium position. There were three-dimensional virtual boundaries within which the subject tried to keep his balance. When the subject leaned beyond one of the boundaries, the support increased. Editable parameters: vertical support (see Figure 1).
- Stairs: virtual boundaries on both sides of the subject in the longitudinal direction of the room were used to simulate stairs. Thanks to lateral resistance, the subject was supported to maintain the walking position, reducing the difficulty of lateral balance. The therapist can personalize the vertical and horizontal supports.
- Sit down: this was used at the end of a training session to return the individual to a sitting position, and as an exercise during the training session.
2.3.2. Conventional Rehabilitation Treatment
2.4. Outcome Measures
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Armstrong, M.J.; Okun, M.S. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA 2020, 323, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J. Parkinson’s disease: Clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Liang, S.; Wang, Y.; Zhao, Y.; Zhao, J.; Li, H.; Wu, J.; Cheng, Y.; Wu, F.; Wu, J. Quantitative Analysis of Postural Instability in Patients with Parkinson’s Disease. Park. Dis. 2021, 2021, 5681870. [Google Scholar] [CrossRef]
- Bonanno, M.; De Nunzio, A.M.; Quartarone, A.; Militi, A.; Petralito, F.; Calabrò, R.S. Gait Analysis in Neurorehabilitation: From Research to Clinical Practice. Bioengineering 2023, 10, 785. [Google Scholar] [CrossRef] [PubMed]
- Tibar, H.; El Bayad, K.; Bouhouche, A.; Ait Ben Haddou, E.H.; Benomar, A.; Yahyaoui, M.; Benazzouz, A.; Regragui, W. Non-Motor Symptoms of Parkinson’s Disease and Their Impact on Quality of Life in a Cohort of Moroccan Patients. Front. Neurol. 2018, 9, 170. [Google Scholar] [CrossRef] [PubMed]
- Radder, D.L.M.; Lígia Silva de Lima, A.; Domingos, J.; Keus, S.H.J.; van Nimwegen, M.; Bloem, B.R.; de Vries, N.M. Physiotherapy in Parkinson’s Disease: A Meta-Analysis of Present Treatment Modalities. Neurorehabil. Neural Repair 2020, 34, 871–880. [Google Scholar] [CrossRef]
- Farrell, J.W., 3rd; Merkas, J.; Pilutti, L.A. The Effect of Exercise Training on Gait, Balance, and Physical Fitness Asymmetries in Persons With Chronic Neurological Conditions: A Systematic Review of Randomized Controlled Trials. Front. Physiol. 2020, 11, 585765. [Google Scholar] [CrossRef] [PubMed]
- Pang, M.Y. Physiotherapy management of Parkinson’s disease. J. Physiother. 2021, 67, 163–176. [Google Scholar] [CrossRef]
- Lihala, S.; Mitra, S.; Neogy, S.; Datta, N.; Choudhury, S.; Chatterjee, K.; Mondal, B.; Halder, S.; Roy, A.; Sengupta, M.; et al. Dance movement therapy in rehabilitation of Parkinson’s disease—A feasibility study. J. Bodyw. Mov. Ther. 2021, 26, 12–17. [Google Scholar] [CrossRef]
- Wang, Y.T.; Huang, G.; Duke, G.; Yang, Y. Tai Chi, Yoga, and Qigong as Mind-Body Exercises. Evid. Based Complement. Alternat. Med. 2017, 2017, 8763915. [Google Scholar] [CrossRef] [PubMed]
- Möller, J.C.; Zutter, D.; Riener, R. Technology-Based Neurorehabilitation in Parkinson’s Disease—A Narrative Review. Clin. Transl. Neurosci. 2021, 5, 23. [Google Scholar] [CrossRef]
- Atan, T.; Özyemişci Taşkıran, Ö.; Bora Tokçaer, A.; Kaymak Karataş, G.; Karakuş Çalışkan, A.; Karaoğlan, B. Effects of different percentages of body weight-supported treadmill training in Parkinson’s disease: A double-blind randomized controlled trial. Turk. J. Med. Sci. 2019, 49, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Plooij, M.; Keller, U.; Sterke, B.; Komi, S.; Vallery, H.; von Zitzewitz, J. Design of RYSEN: An Intrinsically Safe and Low-Power Three-Dimensional Overground Body Weight Support. IEEE Rob. Aut. Lett. 2018, 3, 2253–2260. [Google Scholar] [CrossRef]
- Lorenzo-García, P.; Cavero-Redondo, I.; Torres-Costoso, A.I.; Guzmán-Pavón, M.J.; Núñez de Arenas-Arroyo, S.; Álvarez-Bueno, C. Body Weight Support Gait Training for Patients with Parkinson Disease: A Systematic Review and Meta-analyses. Arch. Phys. Med. Rehabil. 2021, 102, 2012–2021. [Google Scholar] [CrossRef]
- Miranda-Cantellops, N.; Tiu, T.K. Berg Balance Testing. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Caronni, A.; Picardi, M.; Redaelli, V.; Antoniotti, P.; Pintavalle, G.; Aristidou, E.; Gilardone, G.; Carpinella, I.; Lencioni, T.; Arcuri, P.; et al. The Falls Efficacy Scale International is a valid measure to assess the concern about falling and its changes induced by treatments. Clin. Rehabil. 2022, 36, 558–570. [Google Scholar] [CrossRef] [PubMed]
- Scura, D.; Munakomi, S. Tinetti Gait and Balance Test. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK578181/ (accessed on 20 November 2022).
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement Disorder Society UPDRS Revision Task Force. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Disord. 2008, 23, 2129–2170. [Google Scholar] [CrossRef]
- Ravaud, J.F.; Delcey, M.; Yelnik, A. Construct validity of the functional independence measure (FIM): Questioning the unidimensionality of the scale and the “value” of FIM scores. Scand. J. Rehabil. Med. 1999, 31, 31–41. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 6 June 2023).
- Koyanagi, Y.; Fukushi, I.; Nakamura, M.; Suzuki, K.; Oda, N.; Aita, T.; Seki, H. The effect of body weight-supported overground gait training for patients with Parkinson’s disease: A retrospective case-control observational study. PLoS ONE 2021, 16, e0254415. [Google Scholar] [CrossRef]
- Lee, H.K.; Altmann, L.J.; McFarland, N.; Hass, C.J. The relationship between balance confidence and control in individuals with Parkinson’s disease. Park. Relat. Disord. 2016, 26, 24–28. [Google Scholar] [CrossRef][Green Version]
- Massion, J. Postural control system. Curr. Opin. Neurobiol. 1994, 4, 877–887. [Google Scholar] [CrossRef]
- Rasman, B.G.; Forbes, P.A.; Tisserand, R.; Blouin, J.S. Sensorimotor Manipulations of the Balance Control Loop-Beyond Imposed External Perturbations. Front. Neurol. 2018, 9, 899. [Google Scholar] [CrossRef] [PubMed]
- Ampar, N.; Mehta, A.; Mahale, R.R.; Javali, M.; Pradeep, R.; Acharya, P.; Srinivasa, R. Electrophysiological Evaluation of Audiovestibular Pathway Dysfunction in Parkinson’s Disease and Its Correlates: A Case Control Study. Ann. Indian Acad. Neurol. 2021, 24, 531–535. [Google Scholar] [CrossRef]
- Smith, P.F. Vestibular Functions and Parkinson’s Disease. Front. Neurol. 2018, 9, 1085. [Google Scholar] [CrossRef] [PubMed]
- Palakurthi, B.; Burugupally, S.P. Postural Instability in Parkinson’s Disease: A Review. Brain Sci. 2019, 9, 239. [Google Scholar] [CrossRef] [PubMed]
- Stiles, L.; Smith, P.F. The vestibular-basal ganglia connection: Balancing motor control. Brain Res. 2015, 1597, 180–188. [Google Scholar] [CrossRef]
- Goetschalckx, M.; Van Geel, F.; Meesen, R.; Moumdjian, L.; Geraerts, M.; Feys, P. Rhythmic interlimb coordination of the lower limbs in multiple sclerosis during auditory pacing to three different frequencies. Gait Posture 2021, 86, 334–340. [Google Scholar] [CrossRef]
- Klarner, T.; Zehr, E.P. Sherlock Holmes and the curious case of the human locomotor central pattern generator. J. Neurophysiol. 2018, 120, 53–77. [Google Scholar] [CrossRef]
- Bardakan, M.M.; Fink, G.R.; Zapparoli, L.; Bottini, G.; Paulesu, E.; Weiss, P.H. Imaging the neural underpinnings of freezing of gait in Parkinson’s disease. Neuroimage Clin. 2022, 35, 103123. [Google Scholar] [CrossRef]
- Ginis, P.; Nackaerts, E.; Nieuwboer, A.; Heremans, E. Cueing for people with Parkinson’s disease with freezing of gait: A narrative review of the state-of-the-art and novel perspectives. Ann. Phys. Rehabil. Med. 2018, 61, 407–413. [Google Scholar] [CrossRef]
- Berra, E.; de Icco, R.; Avenali, M.; Dagna, C.; Cristina, S.; Pacchetti, C.; Fresia, M.; Sandrini, G.; Tassorelli, C. Body Weight Support Combined with Treadmill in the Rehabilitation of Parkinsonian Gait: A Review of Literature and New Data from a Controlled Study. Front. Neurol. 2019, 9, 1066. [Google Scholar] [CrossRef]
- Muthukrishnan, N.; Abbas, J.J.; Shill, H.A.; Krishnamurthi, N. Cueing Paradigms to Improve Gait and Posture in Parkinson’s Disease: A Narrative Review. Sensors 2019, 19, 5468. [Google Scholar] [CrossRef] [PubMed]
- Yiou, E.; Caderby, T.; Delafontaine, A.; Fourcade, P.; Honeine, J.L. Balance control during gait initiation: State-of-the-art and research perspectives. World J. Orthop. 2017, 8, 815–828. [Google Scholar] [CrossRef] [PubMed]


| All | EG | CG | p-Value | |
|---|---|---|---|---|
| Participants: N | 30 | 15 | 15 | - |
| Male: N (%) | 15 (50.0) | 10 (66.7) | 5 (33.3) | 0.14 |
| Female: N (%) | 15 (50.0) | 5 (33.3) | 10 (66.7) | 0.14 |
| Age, years: median (IR) | 68 (9.5) | 68 (8.5) | 68 (8.5) | 0.85 |
| DD, years: median (IR) | 11.5 (8.75) | 8 (9.5) | 13 (7.5) | 0.72 |
| H&Y: median (IR) | 3.5 (1) | 3 (1) | 3.5 (0.7) | 0.23 |
| Time | Objective | EG Exercise Training | CG Exercise Training |
|---|---|---|---|
| 5’ | Postural stability | Postural alignment with BWS | Postural alignment with the aid of a therapist |
| 15’ | Specific gait training | Forward/sideways/backward stepping at different speeds, with frequent and rapid changes in direction, while performing a dual-task activity to train shared attention (i.e., walking and counting backwards; walking and throwing a ball in the air), or walking along a path on surfaces of different textures (foam rubber mats, sandbags, wooden tablets, etc.), with the support provided by the Rysen system in addition to the supervision of a therapist. | Exercises for gait initiation comprised: weight shifting between lower limbs, stepping training over levels, heel strike/limb-loading acceptance, and push-off/initial swing of the moving limb. Overground gait exercises included: walking over obstacles with different sizes and colours and walking over different surfaces (e.g., foam rubber mats, sandbags, wooden tablets, etc.), always with the assistance of the physiotherapist. |
| 15’ | Static and dynamic balance | Static balance activities included: standing with a decreased base of support or on an unstable support surface, controlling heel–toe imbalances, and shifting the centre of gravity. Dynamic balance activities included: walking with frequent stops and changes in direction, and postural variations. Static–dynamic balance activities included: walking while holding a ball, a plate, or a tower of glasses in the palm of the hand during stable balance conditions. All these exercises were performed with the BWS system provided by Rysen, in addition to the supervision of a therapist. | Static balance activities included: standing with different variations in the base of support, tandem standing, and shifting the centre of gravity using an oscillating platform. Dynamic balance activities included: standing up and sitting down from a chair with or without using hands, walking in tandem, lateral weight shifting, stationary stepping, walking with frequent stops and changes in direction, and postural variations. All these exercises were performed with the continuous manual and visual assistance of a therapist. |
| 5’ | Return to the sitting position | Sit-down exercise was used in order to return to the sitting position, with the assistance of the Rysen system. | Sit-to-stand exercise and squats were used in order to return to the sitting position with the manual assistance provided by a therapist. |
| Clinical Assessment | Group Coefficient | Adjusted R2 | |||
|---|---|---|---|---|---|
| Estimate | Std. Error | t-Value | p-Value | ||
| BBS | 3.60 | 2.19 | 1.64 | 0.112 | 0.70 |
| FES-I | −5.46 | 3.21 | −1.70 | 0.101 | 0.14 |
| POMA | 1.32 | 1.88 | 0.70 | 0.487 | 0.48 |
| UPDRS | −4.66 | 2.12 | −2.20 | 0.036 | 0.59 |
| FIM | 12.36 | 3.36 | 3.67 | 0.001 | 0.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciatto, L.; Pullia, M.; Tavilla, G.; Dauccio, B.; Messina, D.; De Cola, M.C.; Quartarone, A.; Cellini, R.; Bonanno, M.; Calabrò, R.S. Do Patients with Parkinson’s Disease Benefit from Dynamic Body Weight Support? A Pilot Study on the Emerging Role of Rysen. Biomedicines 2023, 11, 2148. https://doi.org/10.3390/biomedicines11082148
Ciatto L, Pullia M, Tavilla G, Dauccio B, Messina D, De Cola MC, Quartarone A, Cellini R, Bonanno M, Calabrò RS. Do Patients with Parkinson’s Disease Benefit from Dynamic Body Weight Support? A Pilot Study on the Emerging Role of Rysen. Biomedicines. 2023; 11(8):2148. https://doi.org/10.3390/biomedicines11082148
Chicago/Turabian StyleCiatto, Laura, Massimo Pullia, Graziana Tavilla, Biagio Dauccio, Daniela Messina, Maria Cristina De Cola, Angelo Quartarone, Roberta Cellini, Mirjam Bonanno, and Rocco Salvatore Calabrò. 2023. "Do Patients with Parkinson’s Disease Benefit from Dynamic Body Weight Support? A Pilot Study on the Emerging Role of Rysen" Biomedicines 11, no. 8: 2148. https://doi.org/10.3390/biomedicines11082148
APA StyleCiatto, L., Pullia, M., Tavilla, G., Dauccio, B., Messina, D., De Cola, M. C., Quartarone, A., Cellini, R., Bonanno, M., & Calabrò, R. S. (2023). Do Patients with Parkinson’s Disease Benefit from Dynamic Body Weight Support? A Pilot Study on the Emerging Role of Rysen. Biomedicines, 11(8), 2148. https://doi.org/10.3390/biomedicines11082148








