Development and Validation of a High-Performance Liquid Chromatography with Tandem Mass Spectrometry (HPLC-MS/MS) Method for Quantification of Major Molnupiravir Metabolite (β-D-N4-hydroxycytidine) in Human Plasma
Abstract
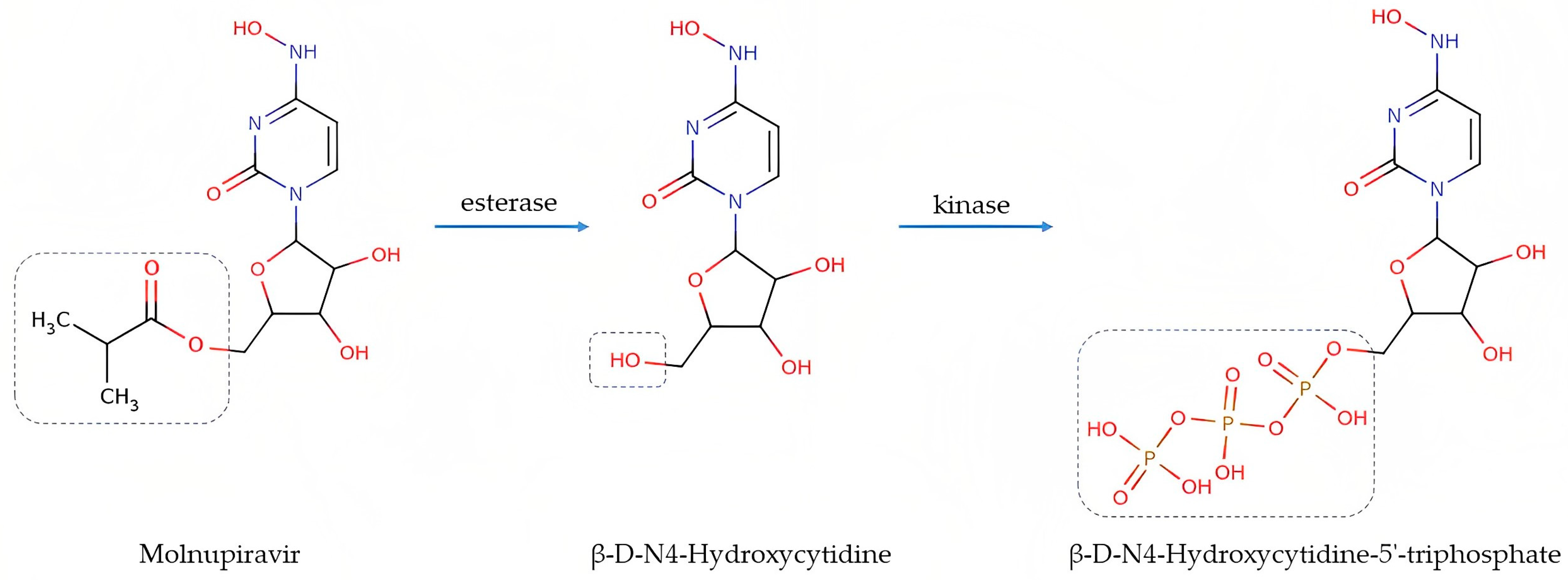
:1. Introduction
2. Materials and Methods
2.1. Solutions and Reagents
2.2. Preparation of Stock and Working Solutions
2.3. Preparation of Calibration Standards and Quality Control Samples
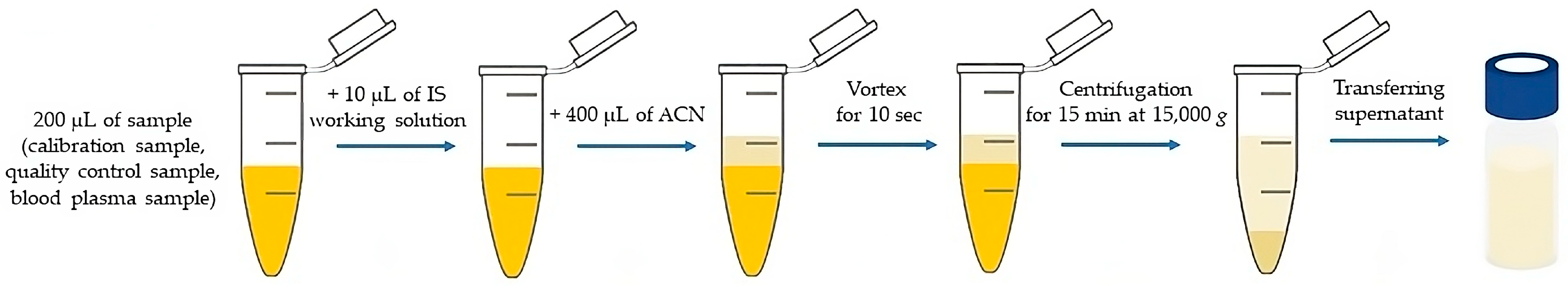
2.4. Sample Preparation
2.5. Equipment
2.6. Chromatographic Conditions
2.7. MS/MS Conditions
2.8. Validation of Analytical Method
2.8.1. Selectivity
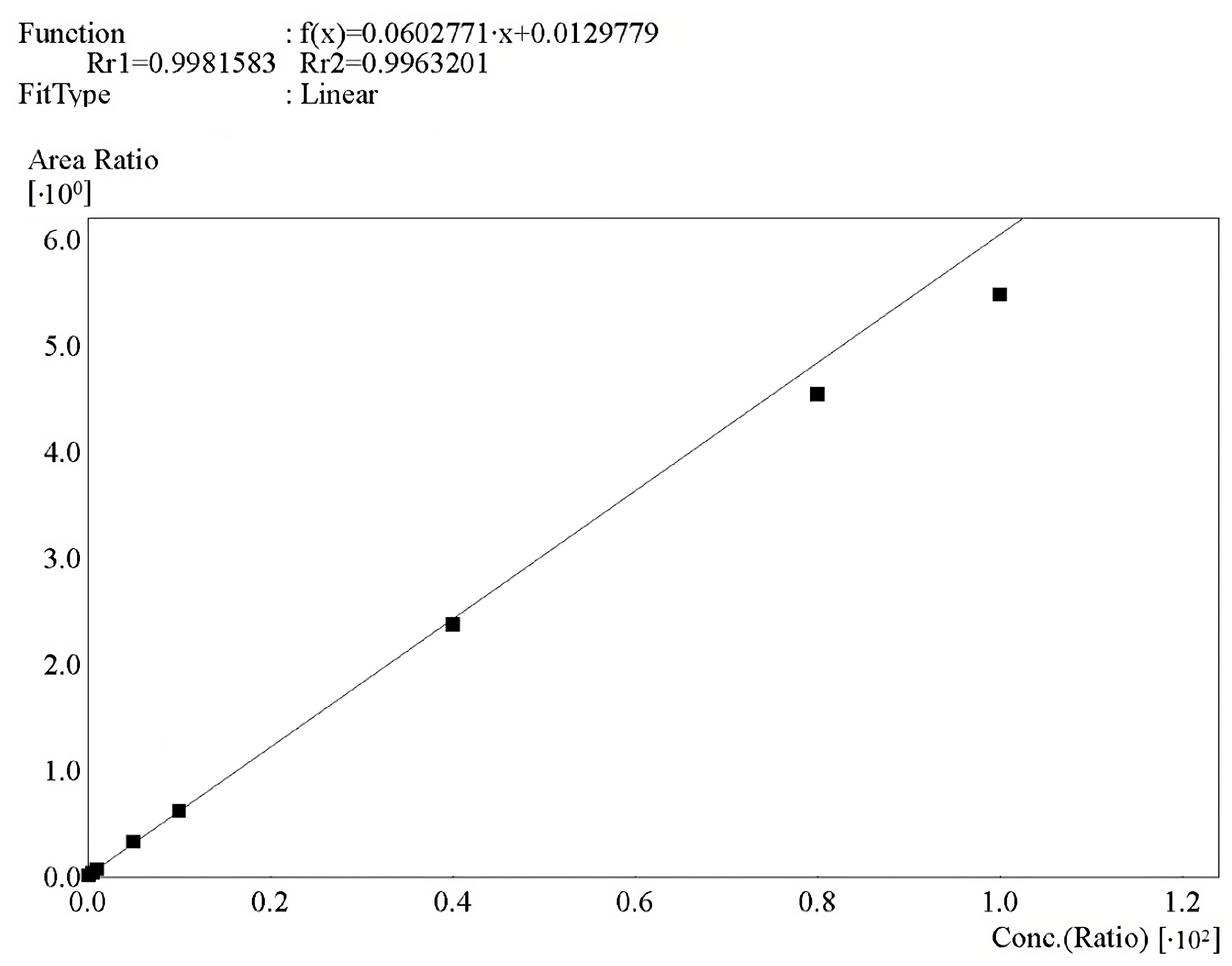
2.8.2. Calibration Curve
2.8.3. Accuracy and Precision
2.8.4. Lower Limit of Quantification
2.8.5. Suitability of Standard Sample
2.8.6. Recovery
2.8.7. Matrix Effect
2.8.8. Stability
- Bench-top stability
- Post-preparative stability
- Freeze and thaw stability
- Stability of stock and working standard solutions
- Long-term stability
2.8.9. Carryover
3. Results
3.1. Method Development
3.2. Method Validation
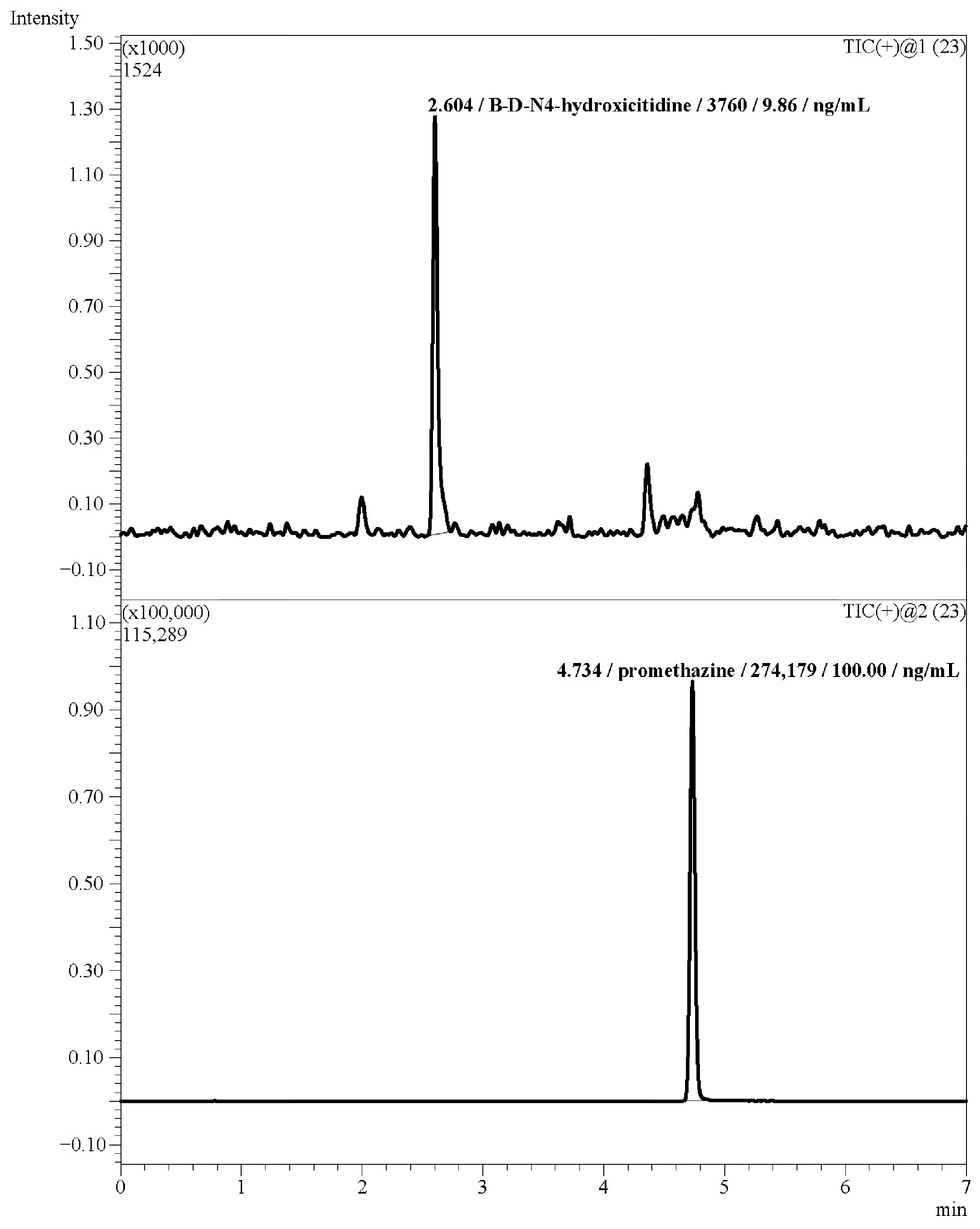
3.2.1. Selectivity
3.2.2. Calibration Curve
3.2.3. Accuracy and Precision
3.2.4. Lower Limit of Quantification
3.2.5. Suitability of Standard Sample
3.2.6. Recovery
3.2.7. Matrix Effect
3.2.8. Stability
3.2.9. Carryover
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, C.; Lu, T.L.; Lin, L. Real-World Clinical Outcomes of Molnupiravir for the Treatment of Mild to Moderate COVID-19 in Adult Patients during the Dominance of the Omicron Variant: A Meta-Analysis. Antibiotics 2023, 12, 393. [Google Scholar] [CrossRef] [PubMed]
- Garg, I.; Shekhar, R.; Sheikh, A.B.; Pal, S. Impact of COVID-19 on the changing patterns of respiratory syncytial virus infections. Infect. Dis. Rep. 2022, 14, 558–568. [Google Scholar] [CrossRef] [PubMed]
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 11 April 2023).
- Komarov, T.N.; Karnakova, P.K.; Archakova, O.A.; Shchelgacheva, D.S.; Bagaeva, N.S.; Shohin, I.E.; Zaslavskaya, K.Y.; Bely, P.A. Development and Validation of HPLC-UV Method for the Determination of Favipiravir in Human Plasma. Drug Dev. 2022, 11, 220–229. (In Russian) [Google Scholar] [CrossRef]
- Komarov, T.N.; Karnakova, P.K.; Archakova, O.A.; Shchelgacheva, D.S.; Bagaeva, N.S.; Shohin, I.E.; Zaslavskaya, K.Y.; Bely, P.A. Simultaneous Determination of Major Molnupiravir Metabolite (β-D-N4-hydroxycytidine) and Favipiravir in Human Plasma by HPLC-MS/MS. Drug Dev. 2023, 12, 215–226. [Google Scholar] [CrossRef]
- Zabidi, N.Z.; Liew, H.L.; Farouk, I.A.; Puniyamurti, A.; Yip, A.J.W.; Wijesinghe, V.N.; Low, Z.Y.; Tang, J.W.; Chow, V.T.K.; Lal, S.K. Evolution of SARS-CoV-2 Variants: Implications on Immune Escape, Vaccination, Therapeutic and Diagnostic Strategies. Viruses 2023, 15, 944. [Google Scholar] [CrossRef] [PubMed]
- Popovic, M.E. XBB. 1.5 Kraken cracked: Gibbs energies of binding and biosynthesis of the XBB. 1.5 variant of SARS-CoV-2. Microbiol. Res. 2023, 270, 127337. [Google Scholar] [CrossRef]
- Wang, C.; Liu, B.; Zhang, S.; Huang, N.; Zhao, T.; Lu, Q.B.; Cui, F. Differences in incidence and fatality of COVID-19 by SARS-CoV-2 Omicron variant versus Delta variant in relation to vaccine coverage: A world-wide review. J. Med. Virol. 2023, 95, e28118. [Google Scholar] [CrossRef]
- Antonacci, F.; Petroncini, M.; Salvaterra, E.; Bertoglio, P.; Daddi, N.; Lai, G.; Brandolini, J.; Solli, P.; Dolci, G. Lung Transplant Recipients and COVID-19: Report of Two Cases. J. Clin. Med. 2023, 12, 4287. [Google Scholar] [CrossRef]
- Chatterjee, S.; Bhattacharya, M.; Nag, S.; Dhama, K.; Chakraborty, C. A detailed overview of SARS-CoV-2 omicron: Its sub-variants, mutations and pathophysiology, clinical characteristics, immunological landscape, immune escape, and therapies. Viruses. 2023, 15, 167. [Google Scholar] [CrossRef]
- Lee, C.C.; Hsieh, C.C.; Ko, W.C. Molnupiravir—A novel oral anti-SARS-CoV-2 agent. Antibiotics 2021, 10, 1294. [Google Scholar] [CrossRef]
- Pourkarim, F.; Pourtaghi-Anvarian, S.; Rezaee, H. Molnupiravir: A new candidate for COVID-19 treatment. Pharmacol. Res. Perspect. 2022, 10, e00909. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.J.; Tchesnokov, E.P.; Schinazi, R.F.; Götte, M. Molnupiravir promotes SARS-CoV-2 mutagenesis via the RNA template. J. Biol. Chem. 2021, 297, 100770. [Google Scholar] [CrossRef] [PubMed]
- Malone, B.; Campbell, E.A. Molnupiravir: Coding for catastrophe. Nat. Struct. Mol. Biol. 2021, 28, 706–708. [Google Scholar] [CrossRef]
- Vicenti, I.; Zazzi, M.; Saladini, F. SARS-CoV-2 RNA-dependent RNA polymerase as a therapeutic target for COVID-19. Expert. Opin. Ther. Pat. 2021, 31, 325–337. [Google Scholar] [CrossRef]
- Saravolatz, L.D.; Depcinski, S.; Sharma, M. Molnupiravir and nirmatrelvir-ritonavir: Oral coronavirus disease 2019 antiviral drugs. Clin. Infect. Dis. 2023, 76, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Peng, W.Y.; Lee, W.H.; Lin, T.Y.; Yang, M.H.; Dalley, J.W.; Tsai, T.H. Transfer and biotransformation of the COVID-19 prodrug molnupiravir and its metabolite β-D-N4-hydroxycytidine across the blood-placenta barrier. EBioMedicine. 2023, 95, 104748. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.G.; Strizki, J.M.; Brown, M.L.; Wan, H.; Shamsuddin, H.H.; Ramgopal, M.; Florescu, D.F.; Delobel, P.; Khaertynova, I.; Flores, J.; et al. Molnupiravir for the treatment of COVID-19 in immunocompromised participants: Efficacy, safety, and virology results from the phase 3 randomized, placebo-controlled MOVe-OUT trial. Infection 2023, 51, 1–12. [Google Scholar] [CrossRef]
- Teli, D.; Balar, P.; Patel, K.; Sharma, A.; Chavda, V.; Vora, L. Molnupiravir: A Versatile Prodrug against SARS-CoV-2 Variants. Metabolites 2023, 13, 309. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Available online: https://www.fda.gov/media/155241/download (accessed on 11 April 2023).
- Tian, F.; Feng, Q.; Chen, Z. Efficacy and safety of molnupiravir treatment for COVID-19: A systematic review and meta-analysis of randomized controlled trials. Int. J. Antimicrob. Agents. 2023, 26, 106870. [Google Scholar] [CrossRef]
- Jonsdottir, H.R.; Siegrist, D.; Julien, T.; Padey, B.; Bouveret, M.; Terrier, O.; Pizzorno, A.; Huang, S.; Samby, K.; Wells, T.N.; et al. Molnupiravir combined with different repurposed drugs further inhibits SARS-CoV-2 infection in human nasal epithelium in vitro. Biomed. Pharmacother. 2022, 150, 113058. [Google Scholar] [CrossRef]
- Abdelnabi, R.; Foo, C.S.; Kaptein, S.J.; Zhang, X.; Do, T.N.; Langendries, L.; Vangeel, L.; Breuer, J.; Pang, J.; Williams, R.; et al. The combined treatment of Molnupiravir and Favipiravir results in a potentiation of antiviral efficacy in a SARS-CoV-2 hamster infection model. EBioMedicine. 2021, 72, 103595. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Green, A.C.; Tazare, J.; Curtis, H.J.; Fisher, L.; Nab, L.; Schultze, A.; Mahalingasivam, V.; Parker, E.P.; Hulme, W.J.; et al. Comparative effectiveness of sotrovimab and molnupiravir for prevention of severe COVID-19 outcomes in patients in the community: Observational cohort study with the OpenSAFELY platform. BMJ 2022, 379, e071932. [Google Scholar] [CrossRef] [PubMed]
- Gouda, A.S.; Marzouk, H.M.; Rezk, M.R.; Salem, A.M.; Morsi, M.I.; Nouman, E.G.; Abdallah, Y.M.; Hassan, A.Y.; Abdel-Megied, A.M. A validated LC-MS/MS method for determination of antiviral prodrug molnupiravir in human plasma and its application for a pharmacokinetic modeling study in healthy Egyptian volunteers. J. Chromatogr. B. Biomed. Appl. 2022, 1206, 123363. [Google Scholar] [CrossRef] [PubMed]
- Amara, A.; Penchala, S.D.; Else, L.; Hale, C.; FitzGerald, R.; Walker, L.; Lyons, R.; Fletcher, T.; Khoo, S. The development and validation of a novel LC-MS/MS method for the simultaneous quantification of Molnupiravir and its metabolite ß-d-N4-hydroxycytidine in human plasma and saliva. J. Pharm. Biomed. Anal. 2021, 206, 114356. [Google Scholar] [CrossRef] [PubMed]
- Parsons, T.L.; Kryszak, L.A.; Marzinke, M.A. Development and validation of assays for the quantification of β-D-N4-hydroxycytidine in human plasma and β-D-N4-hydroxycytidine-triphosphate in peripheral blood mononuclear cell lysates. J. Chromatogr. B. Biomed. Appl. 2021, 1182, 122921. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Wazir, P.; Chibber, P.; Kapoor, N.; Gautam, A. Re-Validation of New Develop Highly Sensitive, Simple LCMS/MS Method for the Estimation of Rohitukine and its Application in ADME/Pre-Clinical Pharmacokinetics. Mass Spectrom. Purif. Tech. 2017, 3, 2. [Google Scholar] [CrossRef]
- N4-Hydroxycytidine. Drugbank. Available online: https://go.drugbank.com/drugs/DB15660 (accessed on 15 April 2023).
- Promethazine. Drugbank. Available online: https://go.drugbank.com/drugs/DB01069 (accessed on 15 April 2023).
- Rules for Conducting Bioequivalence Studies of Medicinal Products within the Eurasian Economic Union (Approved by Decision N° 85 of the Council of the Eurasian Economic Commission of 03.11.2016). Available online: https://docs.cntd.ru/document/456026107/ (accessed on 10 April 2023).
- European Medicines Agency. Available online: https://www.ema.europa.eu/en/bioanalytical-method-validation/ (accessed on 10 April 2023).
- Food and Drug Administration. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/bioanalytical-method-validation-guidance-industry/ (accessed on 10 April 2023).
| Time, min | Eluent A, % | Eluent B, % | Flow Rate of Mobile Phase, mL/min |
|---|---|---|---|
| 0.00 | 95.00 | 5.00 | 1.00 |
| 2.00 | 95.00 | 5.00 | 1.00 |
| 2.50 | 20.00 | 80.00 | 1.00 |
| 3.00 | 0.00 | 100.00 | 1.00 |
| 3.50 | 0.00 | 100.00 | 1.00 |
| 3.60 | 95.00 | 5.00 | 1.00 |
| 4.40 | 95.00 | 5.00 | 1.00 |
| 4.50 | 95.00 | 5.00 | 1.20 |
| 4.90 | 95.00 | 5.00 | 1.20 |
| 5.00 | 95.00 | 5.00 | 1.00 |
| 7.00 | 95.00 | 5.00 | 1.00 |
| Analytical Method (Ionization Source; Ionization (+/−), MRM) | Object | Sample Preparation | Column | Mobile Phase, Elution | Analytical Range, ng/mL | Ref. |
|---|---|---|---|---|---|---|
| HPLC-MS/MS (electrospray; +, 260.1 → 128.1) | Human plasma | Protein precipitation by ACN | Agilent Zorbax Eclipse plus C18 4.6 × 150 mm; 5 µm | 0.2% CH₃COOH—MeOH, isocratic elution | 20–10,000 | [25] |
| HPLC-MS/MS (electrospray; −, (258.0 → 125.9) | Human plasma, salvia | Protein precipitation by ACN | Waters Atlantis dC18 2.1 × 100 mm; 3 µm | NH4CH3CO2 in H2O (pH = 4.3)— 1 mM NH4CH3CO2 in ACN, gradient elution | 2.5–5000 | [26] |
| UPLC-MS/MS (electrospray; +, 260.2 → 128.0) | Human plasma | Ultrafiltration | Scherzo SM-C18 3 × 50 mm; 3 µm | 50 mM NH4HCO2:5 mM NH₄OH—80 mM NH4HCO2:8 mM NH₄OH in H2O:ACN [80:20], gradient elution | 1–5000 | [27] |
| Day | Linear Equation | Correlation Coefficient (r) |
|---|---|---|
| 1 | y = 0.053x + 0.009 | 0.995 |
| 2 | y = 0.067x + 0.007 | 0.997 |
| 3 | y = 0.070x + 0.011 | 0.996 |
| 4 | y = 0.111x + 0.018 | 0.997 |
| 5 | y = 0.060x + 0.013 | 0.998 |
| Inter-Day 1 (n = 5) | Inter-Day 2 (n = 5) | Inter-Day 3 (n = 5) | Inter-Day 4 (n = 5) | Inter-Day 5 (n = 5) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Average | RSD, % | E, % | Average | RSD, % | E, % | Average | RSD, % | E, % | Average | RSD, % | E, % | Average | RSD, % | E, % | |
| LLOQ | 8.45 | 3.66 | −15.50 | 10.64 | 9.55 | 6.44 | 9.46 | 15.44 | −5.40 | 9.46 | 4.26 | −5.42 | 10.01 | 8.56 | 0.12 |
| L | 28.85 | 9.65 | −3.85 | 32.28 | 2.22 | 7.59 | 32.52 | 4.99 | 8.40 | 30.44 | 6.51 | 1.48 | 32.92 | 4.36 | 9.74 |
| M1 | 2023.51 | 2.27 | 1.18 | 2099.69 | 2.68 | 4.98 | 2143.62 | 1.49 | 7.18 | 2184.11 | 0.74 | 9.21 | 1988.31 | 1.21 | −0.58 |
| M2 | 4847.18 | 6.88 | −3.06 | 4974.07 | 1.38 | −0.52 | 5159.72 | 1.68 | 3.19 | 5198.78 | 2.91 | 3.98 | 4738.69 | 4.44 | −5.23 |
| H | 7007.04 | 2.15 | −6.57 | 7161.09 | 2.76 | −4.52 | 7046.84 | 1.46 | −6.04 | 7605.53 | 2.63 | 1.41 | 6915.28 | 5.28 | −7.80 |
| Intra-Day(n = 15) | Intra-Day (n = 20) | Intra-Day (n = 25) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Average | RSD, % | E, % | Average | RSD, % | E, % | Average | RSD, % | E, % | |
| LLOQ | 9.52 | 14.07 | −4.82 | 9.50 | 12.26 | −4.97 | 9.60 | 13.94 | −3.95 |
| L | 31.21 | 7.93 | 4.05 | 31.02 | 7.53 | 3.40 | 31.40 | 7.88 | 4.67 |
| M1 | 2088.94 | 3.19 | 4.45 | 2112.73 | 3.38 | 5.64 | 2087.85 | 3.19 | 4.39 |
| M2 | 4993.66 | 4.61 | −0.13 | 5044.94 | 4.53 | 0.90 | 4983.69 | 4.62 | −0.33 |
| H | 7071.66 | 2.25 | −5.71 | 7205.13 | 4.00 | −3.93 | 7147.16 | 2.22 | −4.70 |
| Biological Matrix | Blank Plasma | Hemolyzed Blank Plasma | Hyperlipidemic Blank Plasma |
|---|---|---|---|
| L | 132.76 | 117.59 | 117.17 |
| 109.40 | 119.95 | 125.84 | |
| 120.81 | 106.27 | 124.73 | |
| M1 | 105.20 | 116.60 | 117.52 |
| 106.61 | 120.73 | 104.35 | |
| 100.94 | 117.37 | 108.28 | |
| M2 | 105.57 | 118.52 | 98.63 |
| 105.75 | 110.09 | 99.02 | |
| 105.57 | 113.89 | 91.38 | |
| H | 114.50 | 112.51 | 113.90 |
| 111.77 | 113.30 | 114.67 | |
| 117.51 | 112.90 | 114.90 | |
| Average | 112.30 | ||
| SD | 8.46 | ||
| RSD, % | 7.54 | ||
| Biological Matrix | Blank Plasma | Hemolyzed Blank Plasma | Lipemic Blank Plasma | |||
|---|---|---|---|---|---|---|
| L | H | L | H | L | H | |
| 1.89 | 1.29 | 2.68 | 1.39 | 2.45 | 1.50 | |
| 2.01 | 1.21 | 2.53 | 1.31 | 2.23 | 1.48 | |
| 1.81 | 1.29 | 2.57 | 1.42 | 2.17 | 1.54 | |
| 2.14 | 1.28 | 2.82 | 1.37 | 2.42 | 1.51 | |
| 1.77 | 1.30 | 2.69 | 1.37 | 2.23 | 1.60 | |
| 1.92 | 1.27 | 2.67 | 1.39 | 2.30 | 1.58 | |
| Average | 1.92 | 1.27 | 2.66 | 1.38 | 2.30 | 1.53 |
| RSD, % | 6.96 | 2.54 | 3.80 | 2.63 | 4.99 | 2.93 |
| Bench-Top | Post-Preparative | Freeze–Thaw | Long-Term 1 | Long-Term 2 | Stock Solution | Work Solution | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L | H | L | H | L | H | L | H | L | H | L | H | L | H | |
| Average | 33.10 | 8126.07 | 32.33 | 6958.93 | 32.98 | 6938.47 | 32.76 | 6844.23 | 29.55 | 7164.52 | 31.02 | 6968.54 | 32.64 | 6947.63 |
| E, % | 10.34 | 8.35 | 7.77 | −7.21 | 9.94 | −7.49 | 9.21 | −8.74 | −1.51 | −4.47 | 3.39 | −7.09 | 8.79 | −7.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komarov, T.; Karnakova, P.; Archakova, O.; Shchelgacheva, D.; Bagaeva, N.; Popova, M.; Karpova, P.; Zaslavskaya, K.; Bely, P.; Shohin, I. Development and Validation of a High-Performance Liquid Chromatography with Tandem Mass Spectrometry (HPLC-MS/MS) Method for Quantification of Major Molnupiravir Metabolite (β-D-N4-hydroxycytidine) in Human Plasma. Biomedicines 2023, 11, 2356. https://doi.org/10.3390/biomedicines11092356
Komarov T, Karnakova P, Archakova O, Shchelgacheva D, Bagaeva N, Popova M, Karpova P, Zaslavskaya K, Bely P, Shohin I. Development and Validation of a High-Performance Liquid Chromatography with Tandem Mass Spectrometry (HPLC-MS/MS) Method for Quantification of Major Molnupiravir Metabolite (β-D-N4-hydroxycytidine) in Human Plasma. Biomedicines. 2023; 11(9):2356. https://doi.org/10.3390/biomedicines11092356
Chicago/Turabian StyleKomarov, Timofey, Polina Karnakova, Olga Archakova, Dana Shchelgacheva, Natalia Bagaeva, Mariia Popova, Polina Karpova, Kira Zaslavskaya, Petr Bely, and Igor Shohin. 2023. "Development and Validation of a High-Performance Liquid Chromatography with Tandem Mass Spectrometry (HPLC-MS/MS) Method for Quantification of Major Molnupiravir Metabolite (β-D-N4-hydroxycytidine) in Human Plasma" Biomedicines 11, no. 9: 2356. https://doi.org/10.3390/biomedicines11092356
APA StyleKomarov, T., Karnakova, P., Archakova, O., Shchelgacheva, D., Bagaeva, N., Popova, M., Karpova, P., Zaslavskaya, K., Bely, P., & Shohin, I. (2023). Development and Validation of a High-Performance Liquid Chromatography with Tandem Mass Spectrometry (HPLC-MS/MS) Method for Quantification of Major Molnupiravir Metabolite (β-D-N4-hydroxycytidine) in Human Plasma. Biomedicines, 11(9), 2356. https://doi.org/10.3390/biomedicines11092356





