Sensory Modulation Abilities in Healthy Preterm-Born Children: An Observational Study Using the Sensory Processing and Self-Regulation Checklist (SPSRC)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling and Data Collection
2.2. Sensory Processing and Self-Regulation Checklist
2.3. Statistical Analysis
3. Results
3.1. Differences between the Two Groups
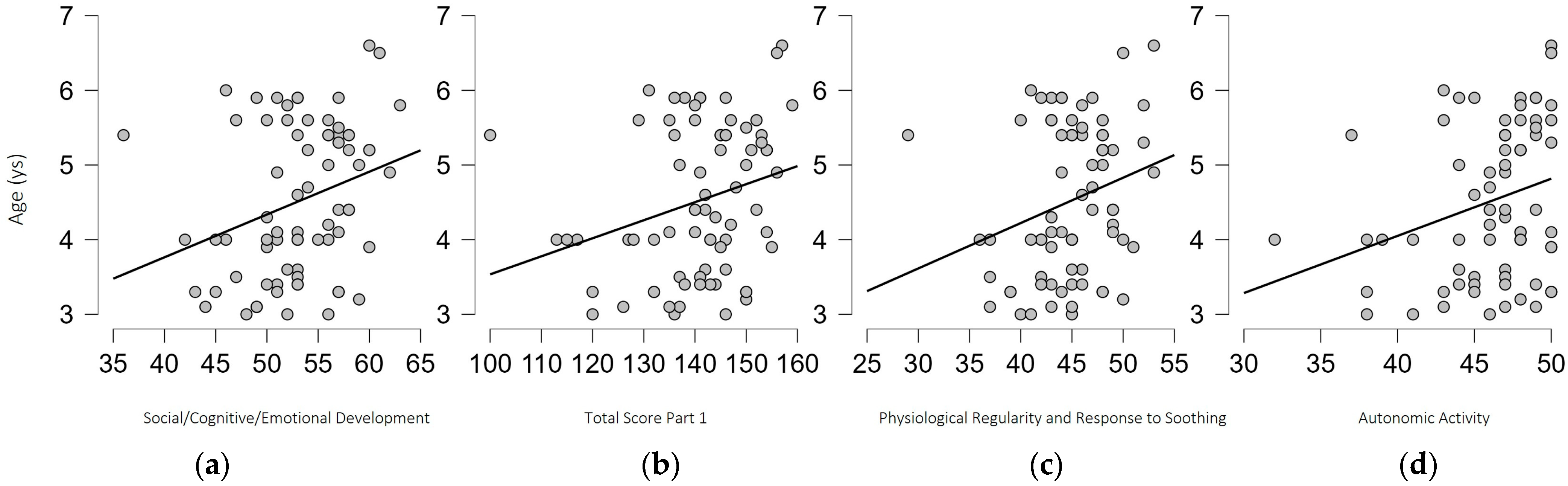
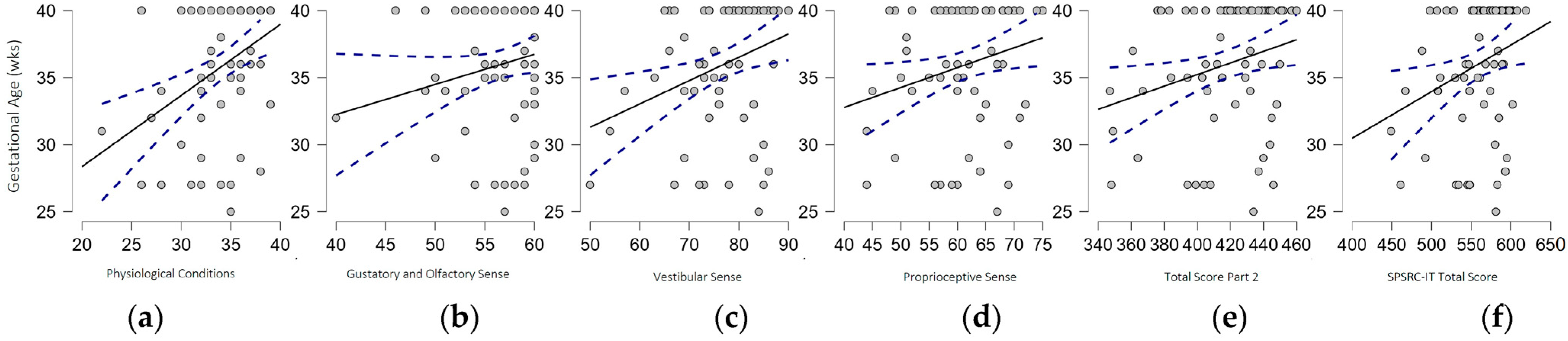
3.2. Correlations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miller, L.J.; Anzalone, M.E.; Lane, S.J.; Cermak, S.A.; Osten, E.T. Concept evolution in sensory integration: A proposed nosology for diagnosis. Am. J. Occup. Ther. 2007, 61, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Stein, B.E.; Perrault, T.J.; Stanford, T.R.; Rowland, B.A. Postnatal experiences influence how the brain integrates information from different senses. Front. Integr. Neurosci. 2009, 3, 21. [Google Scholar] [CrossRef]
- Hornix, B.E.; Havekes, R.; Kas, M.J.H. Multisensory cortical processing and dysfunction across the neuropsychiatric spectrum. Neurosci. Biobehav. Rev. 2019, 97, 138–151. [Google Scholar] [CrossRef]
- Dionne-Dostie, E.; Paquette, N.; Lassonde, M.; Gallagher, A. Multisensory Integration and Child Neurodevelopment. Brain Sci. 2015, 5, 32–57. [Google Scholar] [CrossRef]
- Morrongiello, B.A.; Lasenby, J.; Lee, N. Infants’ learning, memory, and generalization of learning for bimodal events. J. Exp. Child Psychol. 2003, 84, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Purpura, G.; Cioni, G.; Tinelli, F. Multisensory-based rehabilitation approach: Translational insights from animal models to early intervention. Front. Neurosci. 2017, 11, 430. [Google Scholar] [CrossRef] [PubMed]
- Dunn, W. Supporting children to participate successfully in everyday life by using sensory processing knowledge. Infants Young Child. 2007, 20, 84–101. [Google Scholar] [CrossRef]
- Dunn, W. The sensations of everyday life: Empirical, theoretical, and pragmatic considerations. Am. J. Occup. Ther. 2001, 55, 608–620. [Google Scholar] [CrossRef] [PubMed]
- De Gangi, G.A.; Breinbauer, C.; Roosevelt, J.D.; Porges, S.; Greenspan, S. Prediction of childhood problems at three years in children experiencing disorders of regulation during infancy. Infant Ment. Health J. 2000, 21, 156–175. [Google Scholar] [CrossRef]
- Mikami, M.; Hirota, T.; Takahashi, M.; Adachi, M.; Saito, M.; Koeda, S.; Yoshida, K.; Sakamoto, Y.; Kato, S.; Nakamura, K.; et al. Atypical Sensory Processing Profiles and Their Associations With Motor Problems In Preschoolers with Developmental Coordination Disorder. Child Psychiatry Hum. Dev. 2021, 52, 311–320. [Google Scholar] [CrossRef]
- Mulligan, S.; Douglas, S.; Armstrong, C. Characteristics of Idiopathic Sensory Processing Disorder in Young Children. Front. Integr. Neurosci. 2021, 15, 647928. [Google Scholar] [CrossRef]
- Ptak, A.; Miękczyńska, D.; Dębiec-Bąk, A.; Stefańska, M. The Occurrence of the Sensory Processing Disorder in Children Depending on the Type and Time of Delivery: A Pilot Study. Int. J. Environ. Res. Public Health 2022, 19, 6893. [Google Scholar] [CrossRef]
- DC:0–5TM: Diagnostic Classification of Mental Health and Developmental Disorders of Infancy and Early Childhood (Version 2.0); ZERO TO THREE: Washington, DC, USA, 2021; (Original work published 2016).
- Engel-Yeger, B.; Hardal-Nasser, R.; Gal, E. Sensory processing dysfunctions as expressed among children with different severities of intellectual developmental disabilities. Res. Dev. Disabil. 2011, 32, 1770–1775. [Google Scholar] [CrossRef] [PubMed]
- Little, L.M.; Dean, E.; Tomchek, S.; Dunn, W. Sensory Processing Patterns in Autism, Attention Deficit Hyperactivity Disorder, and Typical Development. Phys. Occup. Ther. Pediatr. 2018, 38, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Valagussa, G.; Purpura, G.; Nale, A.; Pirovano, R.; Mazzucchelli, M.; Grossi, E.; Perin, C. Sensory Profile of Children and Adolescents with Autism Spectrum Disorder and Tip-Toe Behavior: Results of an Observational Pilot Study. Children 2022, 9, 1336. [Google Scholar] [CrossRef] [PubMed]
- Niutanen, U.; Harra, T.; Lano, A.; Metsäranta, M. Systematic review of sensory processing in preterm children reveals abnormal sensory modulation, somatosensory processing and sensory-based motor processing. Acta Paediatr. Int. J. Paediatr. 2020, 109, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Bröring, T.; Oostrom, K.J.; Lafeber, H.N.; Jansma, E.P.; Oosterlaan, J. Sensory modulation in preterm children: Theoretical perspective and systematic review. PLoS ONE 2017, 12, e0170828. [Google Scholar] [CrossRef]
- Ream, M.A.; Lehwald, L. Neurologic Consequences of Preterm Birth. Curr. Neurol. Neurosci. Rep. 2017, 18, 48. [Google Scholar] [CrossRef] [PubMed]
- Bröring, T.; Königs, M.; Oostrom, K.J.; Lafeber, H.N.; Brugman, A.; Oosterlaan, J. Sensory processing difficulties in school-age children born very preterm: An exploratory study. Early Hum. Dev. 2018, 117, 22–31. [Google Scholar] [CrossRef]
- Rahkonen, P.; Lano, A.; Pesonen, A.K.; Heinonen, K.; Räikkönen, K.; Vanhatalo, S.; Autti, T.; Valanne, L.; Andersson, S.; Metsäranta, M. Atypical sensory processing is common in extremely low gestational age children. Acta Paediatr. Int. J. Paediatr. 2015, 104, 522–528. [Google Scholar] [CrossRef]
- Ryckman, J.; Hilton, C.; Rogers, C.; Pineda, R. Sensory processing disorder in preterm infants during early childhood and relationships to early neurobehavior. Early Hum. Dev. 2017, 113, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Eeles, A.L.; Anderson, P.J.; Brown, N.C.; Lee, K.J.; Boyd, R.N.; Spittle, A.J.; Doyle, L.W. Sensory profiles of children born <30weeks’ gestation at 2years of age and their environmental and biological predictors. Early Hum. Dev. 2013, 89, 727–732. [Google Scholar] [CrossRef]
- Eeles, A.L.; Anderson, P.J.; Brown, N.C.; Lee, K.J.; Boyd, R.N.; Spittle, A.J.; Doyle, L.W. Sensory profiles obtained from parental reports correlate with independent assessments of development in very preterm children at 2years of age. Early Hum. Dev. 2013, 89, 1075–1080. [Google Scholar] [CrossRef]
- Purpura, G.; Lai, C.Y.Y.; Previtali, G.; Gomez, I.N.B.; Yung, T.W.K.; Tagliabue, L.; Cerroni, F.; Carotenuto, M.; Nacinovich, R. Psychometric Properties of the Italian Version of Sensory Processing and Self-Regulation Checklist (SPSRC). Healthcare 2023, 11, 92. [Google Scholar] [CrossRef] [PubMed]
- Gomez, I.N.B.; Calsa, A.P.; Esguerra, J.T.; Penetrante, P.J.H.; Porlucas, K.; Santos, M.E.; Umali, C.B.; Lai, C.Y.Y. Psychometric Properties of the Sensory Processing and Self-Regulation Checklist: English Version. Occup. Ther. Int. 2021, 2021, 6658786. [Google Scholar] [CrossRef]
- Gomez, I.N.B.; Morato-Espino, P.G.G.; Lai, C.Y. Examining the Linguistic Equivalency and Cross-Cultural Adaptation of the Sensory Processing and Self-Regulation Checklist- Tagalog Version. Asian J. Occup. Ther. 2021, 17, 57–63. [Google Scholar] [CrossRef]
- Lai, C.Y.Y.; Chiu, A.S.M. Sensory Processing and Self-Regulation Checklist; Heep Hong Society: Hong Kong, China, 2013. [Google Scholar]
- Ayres, J.A. Characteristics of types of sensory integrative dysfunction. Am. J. Occup. Ther. 1971, 25, 329–334. [Google Scholar]
- Pavão, S.L.; Rocha, N.A.C.F. Sensory processing disorders in children with cerebral palsy. Infant. Behav. Dev. 2017, 46, 1–6. [Google Scholar] [CrossRef]
- Will, E.; Daunhauer, L.; Fidler, D.; Raitano Lee, N.; Rosenberg, C.R.; Hepburn, S. Sensory Processing and Maladaptive Behavior: Profiles Within the Down Syndrome Phenotype. Phys. Occup. Ther. Pediatr. 2019, 39, 461–476. [Google Scholar] [CrossRef]
- Battajon, N.; Bechini, C.; De Osti, F.; Galletti, A.; Frigo, A.C.; Lago, P. Neurodevelopmental outcomes of very low birth weight preterms in preschool childhood: A prospective cohort study. Ital. J. Pediatr. 2023, 49, 56. [Google Scholar] [CrossRef]
- Longo, S.; Caporali, C.; Pisoni, C.; Borghesi, A.; Perotti, G.; Tritto, G.; Olivieri, I.; La Piana, R.; Tonduti, D.; Decio, A.; et al. Neurodevelopmental outcome of preterm very low birth weight infants admitted to an Italian tertiary center over an 11-year period. Sci. Rep. 2021, 11, 16316. [Google Scholar] [CrossRef]
- Saha, A.K.; Mukherjee, S. Neurodevelopment outcome of late prematurity: A retrospective cohort study from a developing country. Eur. J. Pediatr. 2023, 182, 2715–2722. [Google Scholar] [CrossRef] [PubMed]
- Rogers, E.E.; Hintz, S.R. Early neurodevelopmental outcomes of extremely preterm infants. Semin. Perinatol. 2016, 40, 497–509. [Google Scholar] [CrossRef] [PubMed]
- McGowan, J.E.; Alderdice, F.A.; Holmes, V.A.; Johnston, L. Early childhood development of late-preterm infants: A systematic review. Pediatrics 2011, 127, 1111–1124. [Google Scholar] [CrossRef]
- Brumbaugh, J.E.; Conrad, A.L.; Lee, J.K.; DeVolder, I.J.; Zimmerman, M.B.; Magnotta, V.A.; Axelson, E.D.; Nopoulos, P.C. Altered brain function, structure, and developmental trajectory in children born late preterm. Pediatr. Res. 2016, 80, 197–203. [Google Scholar] [CrossRef]
- Guzzetta, A.; Tinelli, F.; Del Viva, M.M.; Bancale, A.; Arrighi, R.; Pascale, R.R.; Cioni, G. Motion perception in preterm children: Role of prematurity and brain damage. Neuroreport 2009, 20, 1339–1343. [Google Scholar] [CrossRef]
- Tinelli, F.; Bulgheroni, S.; Mazzotti, S.; Vago, C.; Groppo, M.; Scaramuzzo, R.T.; Riva, D.; Cioni, G. Ventral stream sensitivity in “healthy” preterm-born adolescents: Psychophysical and neuropsychological evaluation. Early Hum. Dev. 2014, 90, 45–49. [Google Scholar] [CrossRef]
- Wickremasinghe, A.C.; Rogers, E.E.; Johnson, B.C.; Shen, A.; Barkovich, A.J.; Marco, E.J. Children born prematurely have atypical Sensory Profiles. J. Perinatol. 2013, 33, 631–635. [Google Scholar] [CrossRef]
- Burnett, A.C.; Anderson, P.J.; Lee, K.J.; Roberts, G.; Doyle, L.W.; Cheong, J.L.Y. Trends in Executive Functioning in Extremely Preterm Children Across 3 Birth Eras. Pediatrics 2018, 141, e20171958. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, C.; Adrian, J.; Bakeman, R.; Fuller, M.; Akshoomoff, N. Self-regulation task in young school age children born preterm: Correlation with early academic achievement. Early Hum. Dev. 2021, 157, 105362. [Google Scholar] [CrossRef] [PubMed]
- Walton, K.; Daniel, A.I.; Mahood, Q.; Vaz, S.; Law, N.; Unger, S.L.; O’Connor, D.L. Eating Behaviors, Caregiver Feeding Interactions, and Dietary Patterns of Children Born Preterm: A Systematic Review and Meta-Analysis. Adv. Nutr. 2022, 13, 875–912. [Google Scholar] [CrossRef] [PubMed]
- De Rose, P.; Albamonte, E.; Laganà, V.; Sivo, S.; Pisoni, S.; Gallini, F.; Serrao, F.; Tinelli, F.; Purpura, G.; Ometto, A.; et al. Perceptual-motor abilities in pre-school preterm children. Early Hum. Dev. 2013, 89, 809–814. [Google Scholar] [CrossRef]
- Edwards, J.; Berube, M.; Erlandson, K.; Haug, S.; Johnstone, H.; Meagher, M.; Sarkodee-Adoo, S.; Zwicker, J.G. Developmental Coordination Disorder in School-Aged Children Born Very Preterm and/or at Very Low Birth Weight: A Systematic Review. J. Dev. Behav. Pediatr. 2011, 32, 678–687. [Google Scholar] [CrossRef] [PubMed]
- van Hoorn, J.F.; Schoemaker, M.M.; Stuive, I.; Dijkstra, P.U.; Rodrigues Trigo Pereira, F.; van der Sluis, C.K.; Hadders-Algra, M. Risk factors in early life for developmental coordination disorder: A scoping review. Dev. Med. Child Neurol. 2021, 63, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.T.; Li, Y.C.; Lin, H.Y.; Lee, S.D.; Wang, P.J. Sensory Processing Impairments in Children with Developmental Coordination Disorder. Children 2022, 9, 1443. [Google Scholar] [CrossRef]
- Chien, C.W.; Rodger, S.; Copley, J.; Branjerdporn, G.; Taggart, C. Sensory Processing and Its Relationship with Children’s Daily Life Participation. Phys. Occup. Ther. Pediatr. 2016, 36, 73–87. [Google Scholar] [CrossRef]
- Infante-Rivard, C.; Jacques, L. Empirical study of parental recall bias. Am. J. Epidemiol. 2000, 152, 480–486. [Google Scholar] [CrossRef]
- Bond, T.; Yan, Z.; Heene, M. Applying the Rasch Model: Fundamental Measurement in the Human Sciences; Routledge: Abingdon, UK, 2020. [Google Scholar]
- Piscitelli, D.; Pellicciari, L. Responsiveness: Is it time to move beyond ordinal scores and approach interval measurements? Clin. Rehabil. 2018, 32, 1426–1427. [Google Scholar] [CrossRef]
- Tesio, L.; Caronni, A.; Kumbhare, D.; Scarano, S. Interpreting results from Rasch analysis 1. The “most likely” measures coming from the model. Disabil. Rehabil. 2023, 1–13. [Google Scholar] [CrossRef]
- Geri, T.; Piscitelli, D.; Meroni, R.; Bonetti, F.; Giovannico, G.; Traversi, R.; Testa, M. Rasch analysis of the Neck Bournemouth Questionnaire to measure disability related to chronic neck pain. J. Rehabil. Med. 2015, 47, 836–843. [Google Scholar] [CrossRef]
- Meroni, R.; Piscitelli, D.; Bonetti, F.; Zambaldi, M.; Guccione, A.A.; Pillastrini, P. Rasch analysis of the Italian version of fear avoidance beliefs questionnaire (FABQ-I). Disabil. Rehabil. 2015, 37, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Tesio, L.; Caronni, A.; Simone, A.; Kumbhare, D.; Scarano, S. Interpreting results from Rasch analysis 2. Advanced model applications and the data-model fit assessment. Disabil. Rehabil. 2023, 1–14. [Google Scholar] [CrossRef] [PubMed]
| Preterm Children (n = 37) | |
|---|---|
| Extremely preterm babies (≤28 weeks GA) | 9 (24.3%) |
| Very preterm babies (29–31 weeks GA) | 4 (10.8%) |
| Moderate preterm babies (32–34 weeks GA) | 10 (27.1%) |
| Late preterm babies (35–36 weeks GA) | 14 (37.8%) |
| FT Group (n = 37) | PT Group (n = 37) | |
|---|---|---|
| Sex (M; F) | 17; 20 | 17; 20 |
| Age (mean; SD) | 4.5 (1.0) | 4.5 (1.0) |
| Age range | 3–6.6 years | 3–6.5 years |
| Gestational Age (mean; SD) | 39.8 (0.7) weeks | 32.2 (3.6) weeks |
| Weight at birth (mean; SD) | 3195 (406.8) g | 1705 (649.9) g |
| English Items | Italian (Cross-Cultural-Adapted) Items [25] | |
|---|---|---|
| Part 1: Self-Regulation | ||
| Section 1A: physiological | Falls asleep easily at night (e.g., falls asleep after lying on bed within 20 min) | Si addormenta facilmente di notte (ad es. si addormenta dopo essersi sdraiato sul letto entro venti minuti). |
| Section 1B: social/cognitive/emotional | Unable to comprehend adults’ intentions or requests by observing their facial expressions, gestures, body languages, or speeches | È incapace di comprendere le intenzioni o le richieste degli adulti osservando il loro viso, espressioni, gesti, linguaggi del corpo o discorsi |
| Section 1C: facing changes or challenges | Throws temper tantrum or cries when he/she is asked to switch from one activity to another without advance notice during play or in family gathering | Quando gli viene chiesto di passare da un’attività ad un’altra senza preavviso mentre gioca o è in compagnia dei familiari fa i capricci o piange. |
| Part 2: Sensory Processing | ||
| Scale 2A: Auditory | Appears excessively nervous, distressed, covers ears or complains about unexpected sounds (e.g., sounds produced by radio broadcast at MTR, alarm clocks or hand dryers) | Appare eccessivamente nervoso, angosciato, si copre le orecchie o si lamenta di suoni inaspettati (ad es. suoni della radio, sveglie o asciugamani ad emissione d’aria). |
| Scale 2B: Vision | Unable to notice or shows no response to flashing lights (e.g., neon lights or lights of Christmas decorations) | È incapace di notare o non mostra alcuna risposta alle luci lampeggianti (ad es. luci al neon o luci degli addobbi natalizi). |
| Scale 2C: Tactile | Appears excessively nervous, distressed or makes complaints while walking barefoot on a rough mat or grass mat | Quando cammina a piedi nudi su un tappeto ruvido o sull’erba appare eccessivamente nervoso, angosciato o si lamenta. |
| Scale 2D: Gustatory/Olfactory | Sniffs before manipulating objects or playing with toys | Annusa prima di manipolare oggetti o giocare con i giochi |
| Scale 2E: Vestibular | Unable to notice or shows no response when he/she is about to fall | È incapace di notare o non mostra alcuna risposta quando sta per cadere |
| Scale 2F: Proprioceptive | Likes to walk on tiptoes | Gli piace camminare in punta di piedi |
| Scales/Factor | FT Group Mean (SD) | PT Group Mean (SD) | p-Value |
|---|---|---|---|
| PART 1—Self Regulation | |||
| Section 1A: Physiological Condition | 35.4 (2.9) | 33.3 (3.8) | 0.008 ** |
| Section 1B: Social/Cognitive/Emotional Development | 53.6 (4.5) | 52.9 (5.6) | 0.745 |
| Section 1C: Behaviors When Facing Changes or Challenges | 54.1 (4.0) | 53.2 (4.7) | 0.375 |
| SPSRC-IT Score Part 1 | 143.1 (8.9) | 138.6 (12.7) | 0.119 |
| Factor 1: Emotional Regulation–Facing Challenges | 24.7 (3.2) | 23.6 (3.4) | 0.077 |
| Factor 2: Emotional Regulation–Facing Changes | 26.7 (2.2) | 25.2 (2.7) | 0.023 * |
| Factor 3: Physiological Regularity and Response to Soothing | 45.4 (3.9) | 44.5 (4.6) | 0.621 |
| Factor 4: Autonomic Activity | 46.3 (3.2) | 46.0 (3.9) | 0.896 |
| PART 2—Sensory Processing | |||
| Section 2A: Auditory Sense | 70.8 (4.7) | 68.1 (6.1) | 0.062 |
| Section 2B: Vision Sense | 63.1 (2.9) | 62.5 (3.1) | 0.331 |
| Section 2C: Tactile Sense | 90.6 (5.5) | 89.4 (6.2) | 0.582 |
| Section 2D: Gustatory and Olfactory Sense | 57.6 (3.5) | 55.6 (4.3) | 0.012 * |
| Section 2E: Vestibular Sense | 79.4 (6.8) | 74.5 (8.6) | 0.011 * |
| Section 2F: Proprioceptive Sense | 63.5 (7.3) | 59.7 (7.7) | 0.031 * |
| SPSRC-IT Score Part 2 | 425.1 (24.4) | 409.9 (30.0) | 0.024 * |
| Factor 1: Sensory Seeking Behavior | 112.4 (5.6) | 111.2 (5.6) | 0.075 |
| Factor 2: Sensory Under-Responsivity | 139.6 (5.9) | 135.9 (8.1) | 0.044 * |
| Factor 3: Sensory Over-Responsivity | 145.3 (16.2) | 136.7 (16.9) | 0.031 * |
| Factor 4: Stability of Sensory Responsivity | 27.9 (3.2) | 26.0 (4.4) | 0.034 * |
| SPSRC-IT Total Score | 568.2 (30.3) | 548.5 (39.1) | 0.022 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Previtali, G.; Lai, C.Y.Y.; Valvassori Bolgè, M.; Cavallini, A.; Nacinovich, R.; Piscitelli, D.; Purpura, G. Sensory Modulation Abilities in Healthy Preterm-Born Children: An Observational Study Using the Sensory Processing and Self-Regulation Checklist (SPSRC). Biomedicines 2023, 11, 2319. https://doi.org/10.3390/biomedicines11082319
Previtali G, Lai CYY, Valvassori Bolgè M, Cavallini A, Nacinovich R, Piscitelli D, Purpura G. Sensory Modulation Abilities in Healthy Preterm-Born Children: An Observational Study Using the Sensory Processing and Self-Regulation Checklist (SPSRC). Biomedicines. 2023; 11(8):2319. https://doi.org/10.3390/biomedicines11082319
Chicago/Turabian StylePrevitali, Giulia, Cynthia Y. Y. Lai, Maria Valvassori Bolgè, Anna Cavallini, Renata Nacinovich, Daniele Piscitelli, and Giulia Purpura. 2023. "Sensory Modulation Abilities in Healthy Preterm-Born Children: An Observational Study Using the Sensory Processing and Self-Regulation Checklist (SPSRC)" Biomedicines 11, no. 8: 2319. https://doi.org/10.3390/biomedicines11082319
APA StylePrevitali, G., Lai, C. Y. Y., Valvassori Bolgè, M., Cavallini, A., Nacinovich, R., Piscitelli, D., & Purpura, G. (2023). Sensory Modulation Abilities in Healthy Preterm-Born Children: An Observational Study Using the Sensory Processing and Self-Regulation Checklist (SPSRC). Biomedicines, 11(8), 2319. https://doi.org/10.3390/biomedicines11082319





