The Impact of GLP-1 RAs and DPP-4is on Hospitalisation and Mortality in the COVID-19 Era: A Two-Year Observational Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Demographic Data
2.3. Statistical Analysis
3. Results
3.1. Antidiabetic Prescriptions
3.2. Population Characteristics
3.3. Antidiabetic Drugs and Outcomes
3.4. One-versus-One Comparisons
3.5. Logistic Regression Analyses
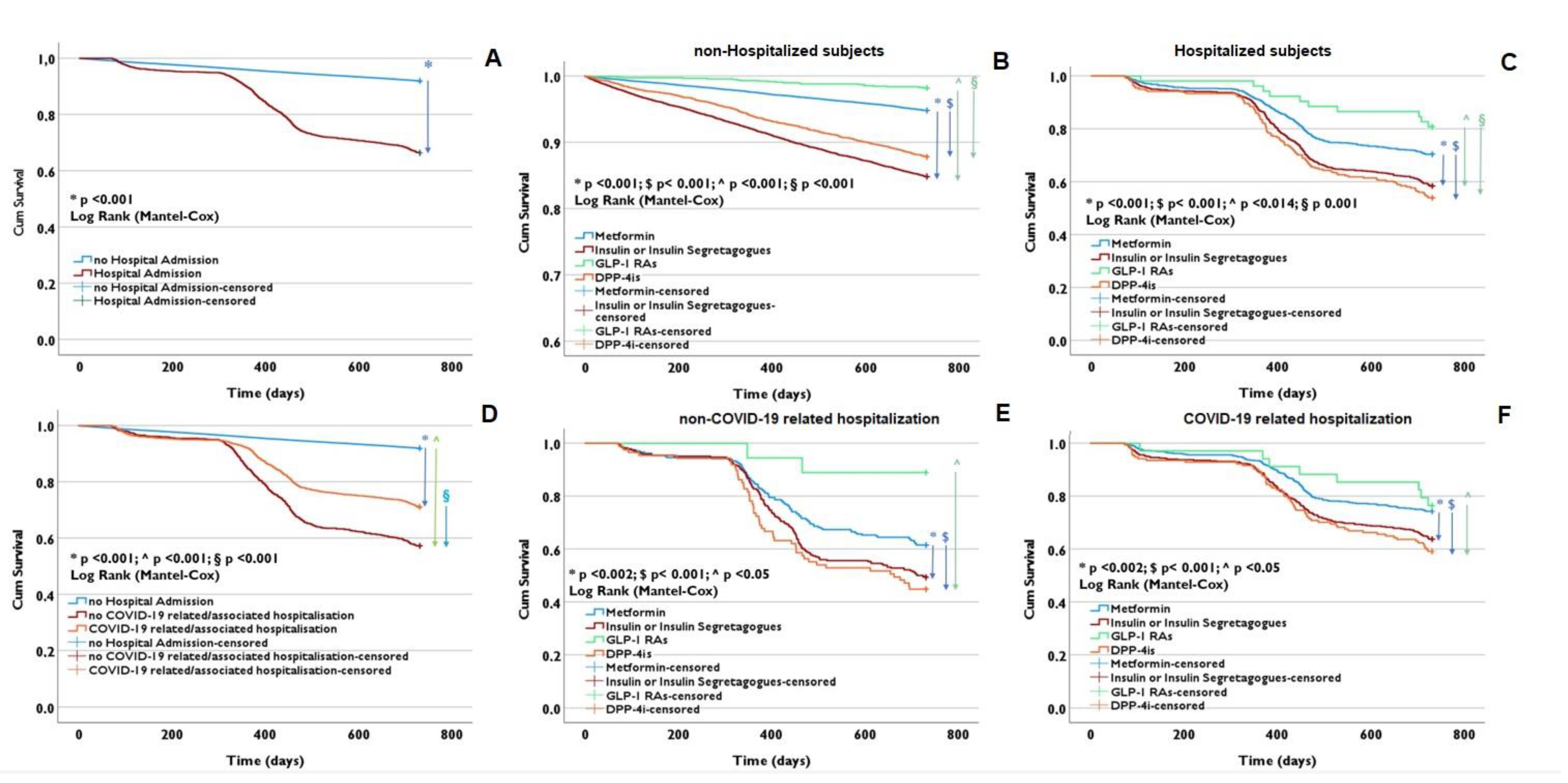
3.6. Survival Estimates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dal Canto, E.; Ceriello, A.; Rydén, L.; Ferrini, M.; Hansen, T.B.; Schnell, O.; Standl, E.; Beulens, J.W. Diabetes as a cardiovascular risk factor: An overview of global trends of macro and micro vascular complications. Eur. J. Prev. Cardiol. 2019, 26, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Kim, L.; Garg, S.; O’halloran, A.; Whitaker, M.; Pham, H.; Anderson, E.J.; Armistead, I.; Bennett, N.M.; Billing, L.; Como-Sabetti, K.; et al. Risk Factors for Intensive Care Unit Admission and In-hospital Mortality Among Hospitalized Adults Identified through the US Coronavirus Disease 2019 (COVID-19)-Associated Hospitalization Surveillance Network (COVID-NET). Clin. Infect. Dis. 2021, 72, e206–e214. [Google Scholar] [CrossRef]
- Holman, N.; Knighton, P.; Kar, P.; O’Keefe, J.; Curley, M.; Weaver, A.; Barron, E.; Bakhai, C.; Khunti, K.; Wareham, N.J.; et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: A population-based cohort study. Lancet Diabetes Endocrinol. 2020, 8, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Feldman, E.L.; Savelieff, M.G.; Hayek, S.S.; Pennathur, S.; Kretzler, M.; Pop-Busui, R. COVID-19 and Diabetes: A Collision and Collusion of Two Diseases. Diabetes 2020, 69, 2549–2565. [Google Scholar] [CrossRef] [PubMed]
- Corrao, S.; Pinelli, K.; Vacca, M.; Raspanti, M.; Argano, C. Type 2 Diabetes Mellitus and COVID-19: A Narrative Review. Front. Endocrinol. 2021, 12, 609470. [Google Scholar] [CrossRef] [PubMed]
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef]
- Solerte, S.B.; Di Sabatino, A.; Galli, M.; Fiorina, P. Dipeptidyl peptidase-4 (DPP4) inhibition in COVID-19. Acta Diabetol. 2020, 57, 779–783. [Google Scholar] [CrossRef]
- Mirabelli, M.; Chiefari, E.; Puccio, L.; Foti, D.P.; Brunetti, A. Potential Benefits and Harms of Novel Antidiabetic Drugs During COVID-19 Crisis. Int. J. Environ. Res. Public Health 2020, 17, 3664. [Google Scholar] [CrossRef]
- Monda, V.M.; Porcellati, F.; Strollo, F.; Gentile, S. ACE2 and SARS-CoV-2 Infection: Might GLP-1 Receptor Agonists Play a Role? Diabetes Ther. 2020, 11, 1909–1914. [Google Scholar] [CrossRef]
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef]
- Kuba, K.; Imai, Y.; Rao, S.; Gao, H.; Guo, F.; Guan, B.; Huan, Y.; Yang, P.; Zhang, Y.; Deng, W.; et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nat. Med. 2005, 11, 875–879. [Google Scholar] [CrossRef] [PubMed]
- Raj, V.S.; Mou, H.; Smits, S.L.; Dekkers, D.H.; Müller, M.A.; Dijkman, R.; Muth, D.; Demmers, J.A.; Zaki, A.; Fouchier, R.A.; et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature 2013, 495, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Parker, H.E.; Reimann, F.; Gribble, F.M. Molecular mechanisms underlying nutrient-stimulated incretin secretion. Expert Rev. Mol. Med. 2010, 12, e1. [Google Scholar] [CrossRef] [PubMed]
- Rowlands, J.; Heng, J.; Newsholme, P.; Carlessi, R. Pleiotropic Effects of GLP-1 and Analogs on Cell Signaling, Metabolism, and Function. Front. Endocrinol. 2018, 9, 672. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-S.; Jun, H.-S. Anti-Inflammatory Effects of GLP-1-Based Therapies beyond Glucose Control. Mediat. Inflamm. 2016, 2016, 3094642. [Google Scholar] [CrossRef]
- Lontchi-Yimagou, E.; Sobngwi, E.; Matsha, T.E.; Kengne, A.P. Diabetes Mellitus and Inflammation. Curr. Diabetes Rep. 2013, 13, 435–444. [Google Scholar] [CrossRef]
- Martin, J.H.; Deacon, C.F.; Gorrell, M.D.; Prins, J.B. Incretin-based therapies—Review of the physiology, pharmacology and emerging clinical experience. Intern. Med. J. 2011, 41, 299–307. [Google Scholar] [CrossRef]
- Hedrington, M.S.; Davis, S.N. Oral semaglutide for the treatment of type 2 diabetes. Expert Opin. Pharmacother. 2019, 20, 133–141. [Google Scholar] [CrossRef]
- Iacobellis, G.; Fricke, A.C.V. Effects of Semaglutide Versus Dulaglutide on Epicardial Fat Thickness in Subjects with Type 2 Diabetes and Obesity. J. Endocr. Soc. 2020, 4, bvz042. [Google Scholar] [CrossRef]
- Lima-Martínez, M.M.; Paoli, M.; Rodney, M.; Balladares, N.; Contreras, M.; D’marco, L.; Iacobellis, G. Effect of sitagliptin on epicardial fat thickness in subjects with type 2 diabetes and obesity: A pilot study. Endocrine 2016, 51, 448–455. [Google Scholar] [CrossRef]
- Ma, X.; Liu, Z.; Ilyas, I.; Little, P.J.; Kamato, D.; Sahebka, A.; Chen, Z.; Luo, S.; Zheng, X.; Weng, J.; et al. GLP-1 receptor agonists (GLP-1RAs): Cardiovascular actions and therapeutic potential. Int. J. Biol. Sci. 2021, 17, 2050–2068. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Yuan, G.; Cheng, F.; Zhang, J.; Guo, X.; Zeman, M.; Vecka, M.; Burda, M.; Tvrzická, E.; Staňková, B.; et al. Mast Cell and M1 Macrophage Infiltration and Local Pro-Inflammatory Factors Were Attenuated with Incretin-Based Therapies in Obesity-Related Glomerulopathy. Metab. Syndr. Relat. Disord. 2017, 15, 344–353. [Google Scholar] [CrossRef]
- Solerte, S.B.; D’addio, F.; Trevisan, R.; Lovati, E.; Rossi, A.; Pastore, I.; Dell’acqua, M.; Ippolito, E.; Scaranna, C.; Bellante, R.; et al. Sitagliptin Treatment at the Time of Hospitalization Was Associated with Reduced Mortality in Patients with Type 2 Diabetes and COVID-19: A Multicenter, Case-Control, Retrospective, Observational Study. Diabetes Care 2020, 43, 2999–3006. [Google Scholar] [CrossRef] [PubMed]
- Zein, A.F.M.Z.; Raffaello, W.M. Dipeptidyl peptidase-4 (DPP-IV) inhibitor was associated with mortality reduction in COVID-19—A systematic review and meta-analysis. Prim. Care Diabetes 2022, 16, 162–167. [Google Scholar] [CrossRef]
- Romaní-Pérez, M.; Outeiriño-Iglesias, V.; Moya, C.M.; Santisteban, P.; González-Matías, L.C.; Vigo, E.; Mallo, F. Activation of the GLP-1 Receptor by Liraglutide Increases ACE2 Expression, Reversing Right Ventricle Hypertrophy, and Improving the Production of SP-A and SP-B in the Lungs of Type 1 Diabetes Rats. Endocrinology 2015, 156, 3559–3569. [Google Scholar] [CrossRef]
- Pal, R.; Bhansali, A. COVID-19, diabetes mellitus and ACE2: The conundrum. Diabetes Res. Clin. Pract. 2020, 162, 108132. [Google Scholar] [CrossRef]
- Chaudhry, F.; Lavandero, S.; Xie, X.; Sabharwal, B.; Zheng, Y.-Y.; Correa, A.; Narula, J.; Levy, P. Manipulation of ACE2 expression in COVID-19. Open Heart 2020, 7, e001424. [Google Scholar] [CrossRef]
- Shajahan, A.; Pepi, L.E.; Rouhani, D.S.; Heiss, C.; Azadi, P. Glycosylation of SARS-CoV-2: Structural and functional insights. Anal. Bioanal. Chem. 2021, 413, 7179–7193. [Google Scholar] [CrossRef]
- Pai, W.-Y.; Lo, W.-Y.; Hsu, T.; Peng, C.-T.; Wang, H.-J. Angiotensin-(1-7) Inhibits Thrombin-Induced Endothelial Phenotypic Changes and Reactive Oxygen Species Production via NADPH Oxidase 5 Downregulation. Front. Physiol. 2017, 8, 994. [Google Scholar] [CrossRef]
- Fraga-Silva, R.A.; Costa-Fraga, F.P.; De Sousa, F.B.; Alenina, N.; Bader, M.; Sinisterra, R.D.; Santos, R.A.S. An orally active formulation of angiotensin-(1-7) produces an antithrombotic effect. Clinics 2011, 66, 837–841. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Stavrou, E.; Schmaier, A.A.; Grobe, N.; Morris, M.; Chen, A.; Nieman, M.T.; Adams, G.N.; LaRusch, G.; Zhou, Y.; et al. Angiotensin 1-7 and Mas decrease thrombosis in Bdkrb2−/− mice by increasing NO and prostacyclin to reduce platelet spreading and glycoprotein VI activation. Blood 2013, 121, 3023–3032. [Google Scholar] [CrossRef] [PubMed]
- Becker, R.C. COVID-19 update: COVID-19-associated coagulopathy. J. Thromb. Thrombolysis 2020, 50, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Verdecchia, P.; Cavallini, C.; Spanevello, A.; Angeli, F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur. J. Intern. Med. 2020, 76, 14–20. [Google Scholar] [CrossRef]
- Asakura, H.; Ogawa, H. COVID-19-associated coagulopathy and disseminated intravascular coagulation. Int. J. Hematol. 2021, 113, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Helms, J.; Tacquard, C.; Severac, F.; Leonard-Lorant, I.; Ohana, M.; Delabranche, X.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; Gandet, F.F.; et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: A multicenter prospective cohort study. Intensive Care Med. 2020, 46, 1089–1098. [Google Scholar] [CrossRef]
- Shukla, A.K.; Banerjee, M. Angiotensin-Converting-Enzyme 2 and Renin-Angiotensin System Inhibitors in COVID-19: An Update. High Blood Press. Cardiovasc. Prev. 2021, 28, 129–139. [Google Scholar] [CrossRef]
- Kahkoska, A.R.; Abrahamsen, T.J.; Alexander, G.C.; Bennett, T.D.; Chute, C.G.; Haendel, M.A.; Klein, K.R.; Mehta, H.; Miller, J.D.; Moffitt, R.A.; et al. Association Between Glucagon-Like Peptide 1 Receptor Agonist and Sodium–Glucose Cotransporter 2 Inhibitor Use and COVID-19 Outcomes. Diabetes Care 2021, 44, 1564–1572. [Google Scholar] [CrossRef]
- Israelsen, S.B.; Pottegård, A.; Sandholdt, H.; Madsbad, S.; Thomsen, R.W.; Benfield, T. Comparable COVID-19 outcomes with current use of GLP-1 receptor agonists, DPP-4 inhibitors or SGLT-2 inhibitors among patients with diabetes who tested positive for SARS-CoV-2. Diabetes Obes. Metab. 2021, 23, 1397–1401. [Google Scholar] [CrossRef]
- Khunti, K.; Knighton, P.; Zaccardi, F.; Bakhai, C.; Barron, E.; Holman, N.; Kar, P.; Meace, C.; Sattar, N.; Sharp, S.; et al. Prescription of glucose-lowering therapies and risk of COVID-19 mortality in people with type 2 diabetes: A nationwide observational study in England. Lancet Diabetes Endocrinol. 2021, 9, 293–303. [Google Scholar] [CrossRef]
- Hariyanto, T.I.; Intan, D.; Hananto, J.E.; Putri, C.; Kurniawan, A. Pre-admission glucagon-like peptide-1 receptor agonist (GLP-1RA) and mortality from coronavirus disease 2019 (COVID-19): A systematic review, meta-analysis, and meta-regression. Diabetes Res. Clin. Pract. 2021, 179, 109031. [Google Scholar] [CrossRef]
- Nguyen, N.N.; Ho, D.S.; Nguyen, H.S.; Ho, D.K.N.; Li, H.-Y.; Lin, C.-Y.; Chiu, H.-Y.; Chen, Y.-C. Preadmission use of antidiabetic medications and mortality among patients with COVID-19 having type 2 diabetes: A meta-analysis. Metabolism 2022, 131, 155196. [Google Scholar] [CrossRef]
- Chen, Y.; Lv, X.; Lin, S.; Arshad, M.; Dai, M. The Association Between Antidiabetic Agents and Clinical Outcomes of COVID-19 Patients with Diabetes: A Bayesian Network Meta-Analysis. Front. Endocrinol. 2022, 13, 895458. [Google Scholar] [CrossRef] [PubMed]
- Roussel, R.; Darmon, P.; Pichelin, M.; Goronflot, T.; Abouleka, Y.; Bachir, L.A.; Allix, I.; Ancelle, D.; Barraud, S.; Bordier, L.; et al. Use of dipeptidyl peptidase-4 inhibitors and prognosis of COVID-19 in hospitalized patients with type 2 diabetes: A propensity score analysis from the CORONADO study. Diabetes Obes. Metab. 2021, 23, 1162–1172. [Google Scholar] [CrossRef]
- Pérez-Belmonte, L.M.; Torres-Peña, J.D.; López-Carmona, M.D.; Ayala-Gutiérrez, M.M.; Fuentes-Jiménez, F.; Huerta, L.J.; Muñoz, J.A.; Rubio-Rivas, M.; Madrazo, M.; Garcia, M.G.; et al. Mortality and other adverse outcomes in patients with type 2 diabetes mellitus admitted for COVID-19 in association with glucose-lowering drugs: A nationwide cohort study. BMC Med. 2020, 18, 359. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.Y.; Lee, J.; Nam, H.; Kyoung, D.-S.; Shin, D.W.; Kim, D.J. Effects of a DPP-4 Inhibitor and RAS Blockade on Clinical Outcomes of Patients with Diabetes and COVID-19. Diabetes Metab. J. 2021, 45, 251–259. [Google Scholar] [CrossRef]
- Mirani, M.; Favacchio, G.; Carrone, F.; Betella, N.; Biamonte, E.; Morenghi, E.; Mazziotti, G.; Lania, A.G. Impact of Comorbidities and Glycemia at Admission and Dipeptidyl Peptidase 4 Inhibitors in Patients with Type 2 Diabetes with COVID-19: A Case Series from an Academic Hospital in Lombardy, Italy. Diabetes Care 2020, 43, 3042–3049. [Google Scholar] [CrossRef]
- Hariyanto, T.I.; Kurniawan, A. Dipeptidyl peptidase 4 (DPP4) inhibitor and outcome from coronavirus disease 2019 (COVID-19) in diabetic patients: A systematic review, meta-analysis, and meta-regression. J. Diabetes Metab. Disord. 2021, 20, 543–550. [Google Scholar] [CrossRef]
- Nyland, J.E.; Raja-Khan, N.T.; Bettermann, K.; Haouzi, P.A.; Leslie, D.L.; Kraschnewski, J.L.; Parent, L.J.; Grigson, P.S. Diabetes, Drug Treatment, and Mortality in COVID-19: A Multinational Retrospective Cohort Study. Diabetes 2021, 70, 2903–2916. [Google Scholar] [CrossRef]
- Rakhmat, I.I.; Kusmala, Y.Y.; Handayani, D.R.; Juliastuti, H.; Nawangsih, E.N.; Wibowo, A.; Lim, M.A.; Pranata, R. Dipeptidyl peptidase-4 (DPP-4) inhibitor and mortality in coronavirus disease 2019 (COVID-19)—A systematic review, meta-analysis, and meta-regression. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Banerjee, M.; Mukherjee, S.; Bhogal, R.S.; Kaur, A.; Bhadada, S.K. Dipeptidyl peptidase-4 inhibitor use and mortality in COVID-19 patients with diabetes mellitus: An updated systematic review and meta-analysis. Ther. Adv. Endocrinol. Metab. 2021, 12, 204201882199648. [Google Scholar] [CrossRef]
- Bonora, B.M.; Avogaro, A.; Fadini, G.P. Disentangling conflicting evidence on DPP-4 inhibitors and outcomes of COVID-19: Narrative review and meta-analysis. J. Endocrinol. Investig. 2021, 44, 1379–1386. [Google Scholar] [CrossRef] [PubMed]
- Panchapakesan, U.; Pegg, K.; Gross, S.; Komala, M.G.; Mudaliar, H.; Forbes, J.; Pollock, C.; Mather, A. Effects of SGLT2 Inhibition in Human Kidney Proximal Tubular Cells—Renoprotection in Diabetic Nephropathy? PLoS ONE 2013, 8, e54442. [Google Scholar] [CrossRef]
- Bonnet, F.; Scheen, A.J. Effects of SGLT2 inhibitors on systemic and tissue low-grade inflammation: The potential contribution to diabetes complications and cardiovascular disease. Diabetes Metab. 2018, 44, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Vallon, V.; Gerasimova, M.; Rose, M.A.; Masuda, T.; Satriano, J.; Mayoux, E.; Koepsell, H.; Thomson, S.C.; Rieg, T.; Layton, A.T.; et al. SGLT2 inhibitor empagliflozin reduces renal growth and albuminuria in proportion to hyperglycemia and prevents glomerular hyperfiltration in diabetic Akita mice. Am. J. Physiol. Physiol. 2014, 306, F194–F204. [Google Scholar] [CrossRef] [PubMed]
- La Grotta, R.; de Candia, P.; Olivieri, F.; Matacchione, G.; Giuliani, A.; Rippo, M.R.; Tagliabue, E.; Mancino, M.; Rispoli, F.; Ferroni, S.; et al. Anti-inflammatory effect of SGLT-2 inhibitors via uric acid and insulin. Cell. Mol. Life Sci. 2022, 79, 273. [Google Scholar] [CrossRef]
- Garvey, W.T.; Van Gaal, L.; Leiter, L.A.; Vijapurkar, U.; List, J.; Cuddihy, R.; Ren, J.; Davies, M.J. Effects of canagliflozin versus glimepiride on adipokines and inflammatory biomarkers in type 2 diabetes. Metabolism 2018, 85, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Packer, M. Do sodium-glucose co-transporter-2 inhibitors prevent heart failure with a preserved ejection fraction by counterbalancing the effects of leptin? A novel hypothesis. Diabetes Obes. Metab. 2018, 20, 1361–1366. [Google Scholar] [CrossRef]
- Gager, G.M.; von Lewinski, D.; Sourij, H.; Jilma, B.; Eyileten, C.; Filipiak, K.; Hülsmann, M.; Kubica, J.; Postula, M.; Siller-Matula, J.M. Effects of SGLT2 Inhibitors on Ion Homeostasis and Oxidative Stress associated Mechanisms in Heart Failure. Biomed. Pharmacother. 2021, 143, 112169. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Nagata, N.; Nagashimada, M.; Zhuge, F.; Ni, Y.; Chen, G.; Mayoux, E.; Kaneko, S.; Ota, T. SGLT2 Inhibition by Empagliflozin Promotes Fat Utilization and Browning and Attenuates Inflammation and Insulin Resistance by Polarizing M2 Macrophages in Diet-induced Obese Mice. Ebiomedicine 2017, 20, 137–149. [Google Scholar] [CrossRef]
- Xu, L.; Ota, T. Emerging roles of SGLT2 inhibitors in obesity and insulin resistance: Focus on fat browning and macrophage polarization. Adipocyte 2017, 7, 121–128. [Google Scholar] [CrossRef]
- Boye, K.S.; Erdemir, E.T.; Zimmerman, N.; Reddy, A.; Benneyworth, B.D.; Dabora, M.C.; Hankosky, E.R.; Bethel, M.A.; Clark, C.; Lensing, C.J.; et al. Risk Factors Associated with COVID-19 Hospitalization and Mortality: A Large Claims-Based Analysis Among People with Type 2 Diabetes Mellitus in the United States. Diabetes Ther. 2021, 12, 2223–2239. [Google Scholar] [CrossRef] [PubMed]
- Kan, C.; Zhang, Y.; Han, F.; Xu, Q.; Ye, T.; Hou, N.; Sun, X. Mortality Risk of Antidiabetic Agents for Type 2 Diabetes with COVID-19: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2021, 12, 708494. [Google Scholar] [CrossRef] [PubMed]
- Kow, C.S.; Hasan, S.S. Mortality risk with preadmission metformin use in patients with COVID-19 and diabetes: A meta-analysis. J. Med. Virol. 2021, 93, 695–697. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wei, Q.; Li, W.X.; McCowen, K.C.; Xiong, W.; Liu, J.; Jiang, W.; Marin, T.; Thomas, R.L.; He, M.; et al. Metformin Use in Diabetes Prior to Hospitalization: Effects on Mortality in COVID-19. Endocr. Pract. 2020, 26, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Ghany, R.; Palacio, A.; Dawkins, E.; Chen, G.; McCarter, D.; Forbes, E.; Chung, B.; Tamariz, L. Metformin is associated with lower hospitalizations, mortality and severe coronavirus infection among elderly medicare minority patients in 8 states in USA. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 513–518. [Google Scholar] [CrossRef]
- Scheen, A. Metformin and COVID-19: From cellular mechanisms to reduced mortality. Diabetes Metab. 2020, 46, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Bramante, C.T.; Ingraham, N.E.; Murray, T.A.; Marmor, S.; Hovertsen, S.; Gronski, J.; McNeil, C.; Feng, R.; Guzman, G.; Abdelwahab, N.; et al. Metformin and risk of mortality in patients hospitalised with COVID-19: A retrospective cohort analysis. Lancet Healthy Longev. 2021, 2, e34–e41. [Google Scholar] [CrossRef]
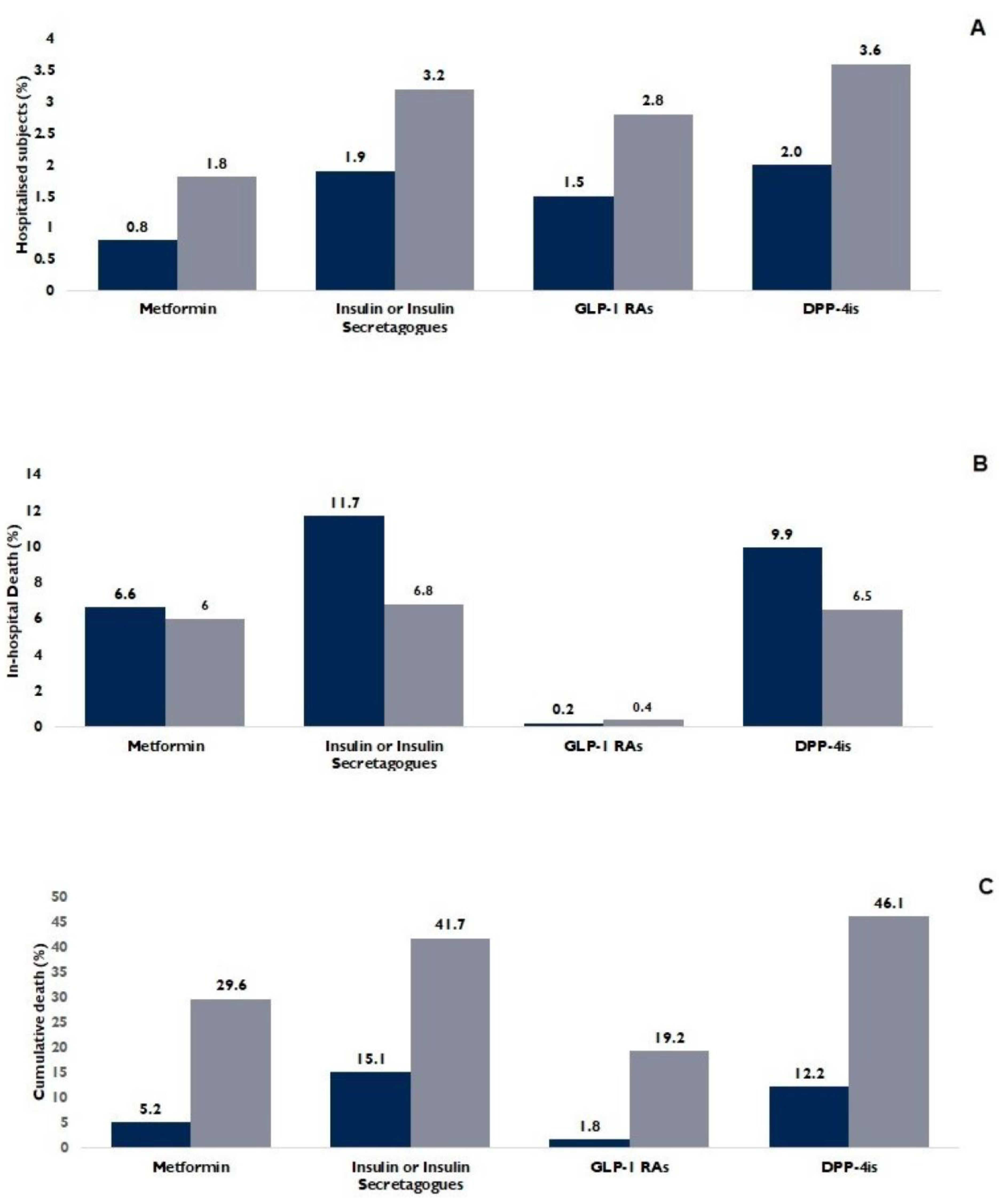

| Variables | Total | Non-Hospitalized Subjects | Hospitalized Subjects (n = 2910) | p Value | ||
|---|---|---|---|---|---|---|
| Non-COVID-19 Hospitalization | COVID-19 Hospitalization | |||||
| Subjects, n (%) | 76,764 | 73,854 (96.2) | 988 (34.0) | 1922 (66.0) | <0.001 | |
| Age (years), mean ± SD | 70 ± 13 | 70 ± 13 | 74 ± 13 | 73 ± 13 | 0.05 | |
| Age < 60 years, n (%) | 14,861 (19.4) | 14,435 (19.5) | 135 (13.7) | 291 (15.1) | 0.36 | |
| Age 60–69 years, n (%) | 18,291 (23.8) | 17,789 (24.1) | 164 (16.6) | 338 (17.6) | 0.57 | |
| Age 70–79 years, n (%) | 24,278 (31.6) | 23,372 (31.6) | 288 (29.1) | 618 (32.2) | 0.23 | |
| Age ≥ 80 years, n (%) | 19,334 (25.2) | 18,258 (24.7) | 401 (40.6) | 675 (35.1) | 0.05 | |
| Sex, n (%) | Female | 35,418 (46.1) | 34,231 (46.5) | 410 (41.5) | 777 (40.4) | <0.001 |
| Male | 41,251 (53.9) | 39,528 (53.5) | 578 (58.5) | 1145 (59.6) | ||
| Days of hospital stay, mean ± SD | - | - | 18.5 ± 16.3 | 18.2 ± 17.7 | 0.73 | |
| In-hospital death, n (%) | 739 (1.0) | - | 318 (32.2) | 421 (21.9) | <0.001 | |
| Cumulative death, n (%) | 6926 (9.0) | 5947 (8.1) | 423 (42.8) | 556 (28.9) | <0.001 | |
| Cardiovascular events | ||||||
| Non-fatal MI, n (%) | 573 (0.7) | 524 (0.7) | 22 (2.2) | 27 (1.4) | 0.11 | |
| Non-fatal CVA, n (%) | 1470 (1.9) | 1277 (1.7) | 81 (8.2) | 112 (5.8) | 0.023 | |
| Heart failure (HF), n (%) | 1898 (2.5) | 1589 (2.2) | 125 (12.7) | 184 (9.6) | 0.022 | |
| Malignant dysrhythmia (MD), n (%) | 323 (0.4) | 287 (0.4) | 14 (1.4) | 22 (1.1) | 0.53 | |
| Cardiac shock (CS), n (%) | 50 (0.1) | 40 (0.1) | 0 (0.0) | 10 (0.5) | 0.019 | |
| 4-point MACE, n (%) | 3794 (4.9) | 3263 (4.4) | 215 (21.8) | 316 (16.4) | 0.004 | |
| Antidiabetic drugs | ||||||
| Metformin, n (%) | 30,238 (39.4) | 29,455 (39.9) | 239 (24.2) | 544 (28.3) | 0.07 | |
| Insulin or insulin secretagogues, n (%) | 14,739 (19.2) | 13,976 (18.9) | 284 (28.7) | 479 (24.9) | 0.09 | |
| GLP-1 RAs, n (%) | 1227 (1.6) | 1175 (1.6) | 18 (1.8) | 34 (1.8) | 0.92 | |
| GLP-1 RAs + metformin, n (%) | 910 (1.2) | 875 (1.2) | 9 (0.9) | 26 (1.4) | 0.31 | |
| GLP-1 RA alone, n (%) | 127 (0.2) | 122 (0.2) | 2 (0.2) | 3 (0.2) | 0.78 | |
| GLP-1 RAs + insulin or insulin secretagogues, n (%) | 190 (0.2) | 178 (0.2) | 7 (0.7) | 5 (0.3) | 0.08 | |
| DPP-4is, n (%) | 4301 (5.6) | 4060 (5.5) | 87 (8.8) | 154 (8.0) | 0.50 | |
| DPP-4i alone, n (%) | 1169 (1.5) | 1078 (1.5) | 38 (3.8) | 53 (2.8) | 0.12 | |
| DPP-4is + metformin, n (%) | 2037 (2.7) | 1972 (2.7) | 16 (1.6) | 49 (2.5) | 0.12 | |
| DPP-4is + insulin or insulin secretagogues, n (%) | 1095 (1.4) | 1010 (1.4) | 33 (3.3) | 52 (2.7) | 0.35 | |
| Other drug combinations or other drugs, n (%) | 26,259 (34.2) | 25,188 (34.1) | 360 (36.4) | 711 (37.0) | 0.84 | |
| B | S.E. | Wald | df | p Value | OR | 95% CI (Upper-Lower) | |
|---|---|---|---|---|---|---|---|
| Hospital admission | |||||||
| Sex (F/M) | −0.27 | 0.05 | 29.63 | 1 | <0.001 | 0.77 | 0.70–0.84 |
| Age < 60 years | - | - | 82.01 | 3 | <0.001 | - | - |
| Age 60–69 years | −0.01 | 0.09 | 0.01 | 1 | 0.99 | 1.00 | 0.84–1.18 |
| Age 70–79 years | 0.23 | 0.08 | 8.87 | 1 | 0.003 | 1.26 | 1.08–1.46 |
| Age ≥ 80 years | 0.55 | 0.08 | 51.96 | 1 | <0.001 | 1.73 | 1.49–2.01 |
| 4-point MACE | 1.31 | 0.07 | 396.51 | 1 | <0.001 | 3.72 | 3.27–4.24 |
| Metformin | - | - | 115.75 | 3 | <0.001 | - | - |
| Insulin or insulin secretagogues | 0.51 | 0.05 | 88.79 | 1 | <0.001 | 1.66 | 1.50–1.85 |
| GLP-1 RAs | 0.64 | 0.15 | 18.37 | 1 | <0.001 | 1.69 | 1.41–2.52 |
| DPP-4is | 0.58 | 0.08 | 55.97 | 1 | <0.001 | 1.78 | 1.53–2.08 |
| In-hospital death | |||||||
| Sex (F/M) | −0.50 | 0.09 | 29.09 | 1 | <0.001 | 0.61 | 0.51–0.72 |
| Age < 60 years | 179.07 | 3 | <0.001 | - | - | ||
| Age 60–69 years | 0.67 | 0.30 | 4.88 | 1 | 0.03 | 1.95 | 1.08–3.53 |
| Age 70–79 years | 1.74 | 0.27 | 43.04 | 1 | <0.001 | 5.68 | 3.38–9.53 |
| Age ≥ 80 years | 2.45 | 0.26 | 88.65 | 1 | <0.001 | 11.62 | 6.97–19.35 |
| 4-point MACE | 1.55 | 0.10 | 229.20 | 1 | <0.001 | 4.71 | 3.85–5.75 |
| Metformin | 42.07 | 3 | <0.001 | - | - | ||
| Insulin or insulin secretagogues | 0.59 | 0.10 | 33.33 | 1 | <0.001 | 1.81 | 1.48–2.20 |
| GLP-1 RAs | 0.80 | 0.35 | 5.33 | 1 | 0.021 | 1.84 | 1.13–4.42 |
| DPP-4is | 0.67 | 0.14 | 23.59 | 1 | <0.001 | 1.96 | 1.49–2.56 |
| Cumulative death | |||||||
| Sex (F/M) | −0.24 | 0.03 | 53.59 | 1 | <0.001 | 0.79 | 0.74–0.84 |
| Age < 60 years | - | - | 2269.81 | 3 | <0.001 | - | - |
| Age 60–69 years | 1.02 | 0.10 | 107.46 | 1 | <0.001 | 2.76 | 2.28–3.35 |
| Age 70–79 years | 1.72 | 0.09 | 368.67 | 1 | <0.001 | 5.57 | 4.67–6.64 |
| Age ≥ 80 years | 2.84 | 0.09 | 1066.26 | 1 | <0.001 | 17.14 | 14.45–20.33 |
| 4-point MACE | 1.16 | 0.05 | 578.85 | 1 | <0.001 | 3.20 | 2.91–3.51 |
| Metformin | - | - | 565.10 | 3 | <0.001 | - | - |
| Insulin or insulin secretagogues | 0.82 | 0.04 | 545.72 | 1 | <0.001 | 2.28 | 2.13–2.44 |
| GLP-1 RAs | −0.20 | 0.19 | 1.12 | 1 | 0.29 | 0.82 | 0.57–1.18 |
| DPP-4is | 0.60 | 0.05 | 124.76 | 1 | <0.001 | 1.81 | 1.63–2.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greco, S.; Monda, V.M.; Valpiani, G.; Napoli, N.; Crespini, C.; Pieraccini, F.; Marra, A.; Passaro, A. The Impact of GLP-1 RAs and DPP-4is on Hospitalisation and Mortality in the COVID-19 Era: A Two-Year Observational Study. Biomedicines 2023, 11, 2292. https://doi.org/10.3390/biomedicines11082292
Greco S, Monda VM, Valpiani G, Napoli N, Crespini C, Pieraccini F, Marra A, Passaro A. The Impact of GLP-1 RAs and DPP-4is on Hospitalisation and Mortality in the COVID-19 Era: A Two-Year Observational Study. Biomedicines. 2023; 11(8):2292. https://doi.org/10.3390/biomedicines11082292
Chicago/Turabian StyleGreco, Salvatore, Vincenzo M. Monda, Giorgia Valpiani, Nicola Napoli, Carlo Crespini, Fabio Pieraccini, Anna Marra, and Angelina Passaro. 2023. "The Impact of GLP-1 RAs and DPP-4is on Hospitalisation and Mortality in the COVID-19 Era: A Two-Year Observational Study" Biomedicines 11, no. 8: 2292. https://doi.org/10.3390/biomedicines11082292
APA StyleGreco, S., Monda, V. M., Valpiani, G., Napoli, N., Crespini, C., Pieraccini, F., Marra, A., & Passaro, A. (2023). The Impact of GLP-1 RAs and DPP-4is on Hospitalisation and Mortality in the COVID-19 Era: A Two-Year Observational Study. Biomedicines, 11(8), 2292. https://doi.org/10.3390/biomedicines11082292







