In Vitro Evaluation of the Antimicrobial Properties of Nanoparticles as New Agents Used in Teat Sealants for Mastitis Prevention in Dry Cows
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Homogenous Metal NPs and Their Complexes Using the Self-Organization Phenomenon
2.2. Physicochemical Properties of Metal NPs
2.3. The In Vitro Culture of BME-UV1 Cells
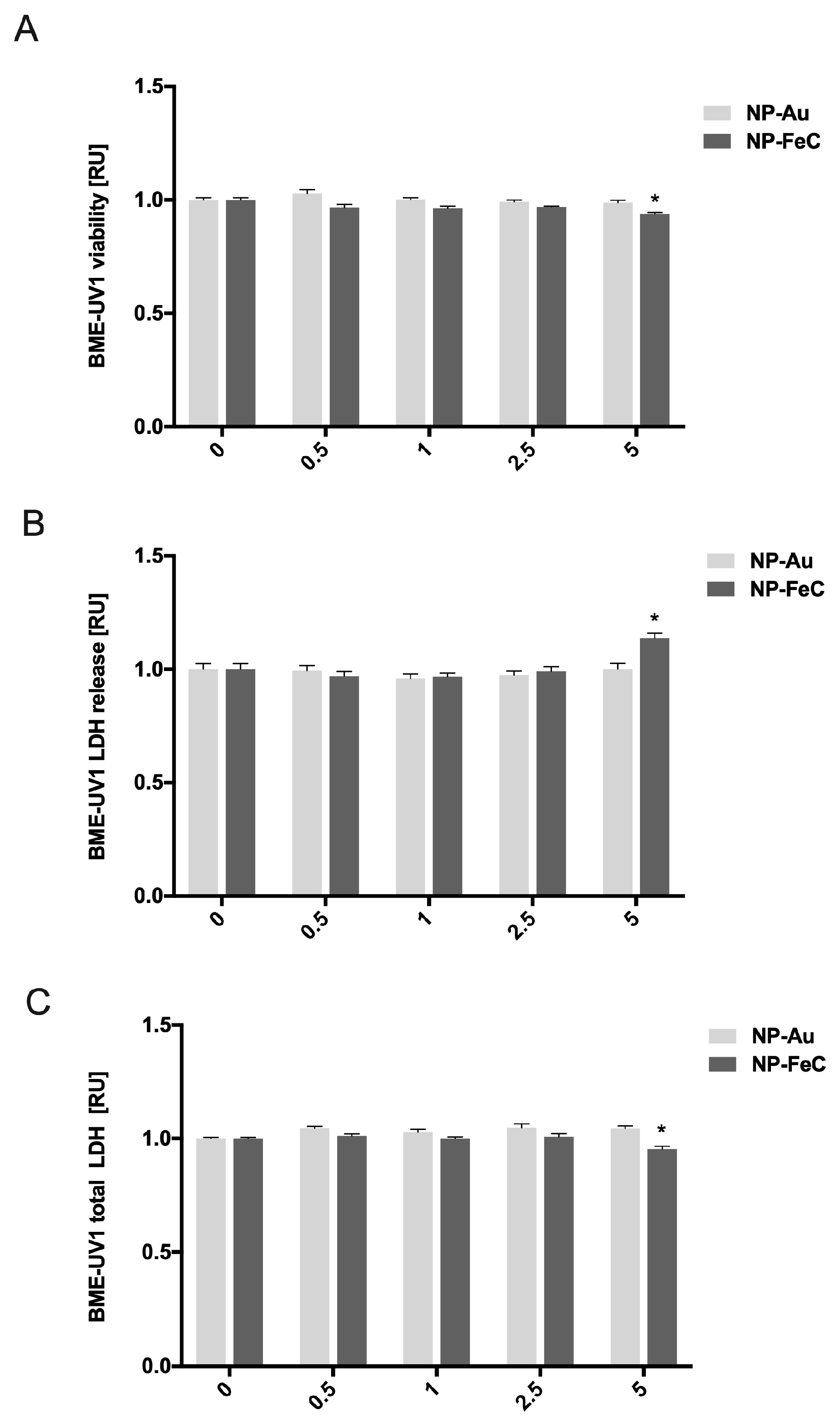
2.4. The Viability of BME-UV1 Cells after Incubation with AuNPs and NP-FeCs
2.5. The In Vitro Membrane Integrity of BME-UV1 Cells after Incubation with AuNPs and NP-FeCs
2.6. The In Vitro Estimation of the Number of BME-UV1 Cells after Incubation with AuNPs and NP-FeCs
2.7. Statistical Analysis
2.8. In Vitro Bacterial and Fungi Cultures
2.9. Preliminary NP Concentrations
2.10. Preparation of Mixture and Wax with the Addition of NPs
3. Results
3.1. The Estimated Viability of the BME-UV1 Cells after Incubation with AuNPs and NP-FeCs
3.2. The Cytotoxic Effect of AgNPs, CuNPs, AuNPs, and NP-FeCs on Pathogen Viability
3.3. The Cytotoxic Effect of the AgCuAuNP Complex on Pathogen Viability
3.4. The In Vitro Antimicrobial Properties and Cytotoxic Effect of the AgCuAuNP Complex
4. Discussion
4.1. Nanotechnology and NPs’ Properties
4.2. The Influence of NPs on the Viability of Bovine Mammary Gland Cells
4.3. The Antimicrobial Properties of NPs
4.4. Teat Sealant in Dry Cow Therapy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruegg, P.L. A 100-Year Review: Mastitis detection, management, and prevention. J. Dairy Sci. 2017, 100, 10381–10397. [Google Scholar] [CrossRef] [PubMed]
- Kalińska, A.; Gołębiewski, M.; Wójcik, A. Mastitis pathogens in dairy cattle—A review. World Sci. News 2017, 89, 22–31. [Google Scholar]
- Cobirka, M.; Tancin, V.; Slama, P. Epidemiology and Classification of Mastitis. Animals 2020, 10, 2212. [Google Scholar] [CrossRef]
- Sukumar, K.; James, P.C. Incidence of fungal mastitis in cattle. Tamilnadu J. Vet. Anim. Sci. 2012, 8, 356–359. [Google Scholar]
- Schwarz, S.; Kehrenberg, C.; Walsh, T.R. Use of antimicrobial agents in veterinary medicine and food animal production. Int. J. Antimicrob. Agents 2001, 17, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Singer, R.S.; Finch, R.; Wegener, H.C.; Bywater, R.; Walters, J.; Lipsitch, M. Antibiotic resistance—The interplay between antibiotic use in animals and human beings. Lancet Infect. Dis. 2003, 3, 47–51. [Google Scholar] [CrossRef]
- Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016, 4, 1–24. [Google Scholar] [CrossRef]
- Krömker, V.; Leimbach, S. Mastitis treatment—Reduction in antibiotic usage in dairy cows. Reprod. Domest. Anim. 2017, 52, 21–29. [Google Scholar] [CrossRef]
- Høiby, N.; Ciofu, O.; Johansen, H.K.; Song, Z.; Moser, C.; Jensen, P.Ø.; Molin, S.; Givskov, M.; Tolker-Nielsen, T.; Bjarnsholt, T. The clinical impact of bacterial biofilms. Int. J. Oral Sci. 2011, 3, 55–65. [Google Scholar] [CrossRef]
- Durán, N.; Marcato, P.D.; Conti, R.D.; Alves, O.L.; Costa, T.M.; Brocchi, M. Potential Use of Silver Nanoparticles on Pathogenic Bacteria, their Toxicity and Possible Mechanisms of Action. J. Braz. Chem. Soc. 2010, 21, 949–959. [Google Scholar] [CrossRef]
- Miglior, F.; Fleming, A.; Malchiodi, F.; Brito, L.F.; Martin, P.; Baes, C.F. A 100-Year Review: Identification and genetic selection of economically important traits in dairy cattle. J. Dairy Sci. 2017, 100, 10251–10271. [Google Scholar] [CrossRef] [PubMed]
- Khodashenas, B. The Influential Factors on Antibacterial Behaviour of Copper and Silver Nanoparticles. Indian Chem. Eng. 2016, 58, 224–239. [Google Scholar] [CrossRef]
- Kalińska, A.; Jaworski, S.; Wierzbicki, M.; Gołębiewski, M. Silver and Copper Nanoparticles—An Alternative in Future Mastitis Treatment and Prevention? Int. J. Mol. Sci. 2019, 20, 1672. [Google Scholar] [CrossRef] [PubMed]
- Kot, M.; Kalińska, A.; Jaworski, S.; Wierzbicki, M.; Smulski, S.; Gołębiewski, M. In Vitro Studies of Nanoparticles as a Potentially New Antimicrobial Agent for the Prevention and Treatment of Lameness and Digital Dermatitis in Cattle. Int. J. Mol. Sci. 2023, 24, 6146. [Google Scholar] [CrossRef] [PubMed]
- Kalińska, A.; Jaworski, S.; Wierzbicki, M.; Kot, M.; Radzikowski, D.; Smulski, S.; Gołębiewski, M. Silver and Copper Nanoparticles as the New Biocidal Agents Used in Pre- and Post-Milking Disinfectants with the Addition of Cosmetic Substrates in Dairy Cows. Int. J. Mol. Sci. 2023, 24, 1658. [Google Scholar] [CrossRef]
- Ostaszewska, T.; Chojnacki, M.; Kamaszewski, M.; Sawosz-Chwalibóg, E. Histopathological effects of silver and copper nanoparticles on the epidermis, gills, and liver of Siberian sturgeon. Environ. Sci. Pollut. Res. 2016, 23, 1621–1633. [Google Scholar] [CrossRef]
- Ostaszewska, T.; Śliwiński, J.; Kamaszewski, M.; Sysa, P.; Chojnacki, M. Cytotoxicity of silver and copper nanoparticles on rainbow trout (Oncorhynchus mykiss) hepatocytes. Environ. Sci. Pollut. Res. 2017, 25, 908–915. [Google Scholar] [CrossRef]
- Szudrowicz, H.; Kamaszewski, M.; Adamski, A.; Skrobisz, M.; Frankowska-Łukawska, J.; Wójcik, M.; Bochenek, J.; Kawalski, K.; Martynow, J.; Bujarski, P.; et al. The Effects of Seven-Day Exposure to Silver Nanoparticles on Fertility and Homeostasis of Zebrafish (Danio rerio). Int. J. Mol. Sci. 2022, 23, 11239. [Google Scholar] [CrossRef]
- Matuszewski, A.; Łukasiewicz, M.; Niemiec, J.; Kamaszewski, M.; Jaworski, S.; Domino, M.; Jasiński, T.; Chwalibóg, A.; Sawosz, E. Calcium Carbonate nanoparticles—Toxicity and effect of in ovo inoculation on chicken embryo development, broiler performance and bone status. Animals 2021, 11, 932. [Google Scholar] [CrossRef]
- Jagielski, T.; Bakuła, Z.; Pleń, M.; Kamiński, M.; Nowakowska, J.; Bielecki, J.; Wolska, K.; Grudniak, A.M. The activity of silver nanoparticles against microalgae of the Prototheca genus. Nanomedicine 2018, 13, 1025–1036. [Google Scholar] [CrossRef]
- Ahamed, M.; AlSalhi, M.S.; Siddiqui, M.K.J. Silver nanoparticle applications and human health. Clin. Chim. Acta 2010, 411, 1841–1848. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.-J.; Kim, H.; Liu, Y.; Han, H.-K.; Kwon, K.; Chang, K.-H.; Park, K.; Kim, Y.; Shim, K.; An, S.S.A.; et al. Incompatibility of silver nanoparticles with lactate dehydrogenase leakage assay for cellular viability test is attributed to protein binding and reactive oxygen species generation. Toxicol. Lett. 2014, 225, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Oliver, S.P.; Murinda, S.E. Antimicrobial resistance of mastitis pathogens. Vet. Clin. Food Anim. Pract. 2012, 28, 165–185. [Google Scholar] [CrossRef] [PubMed]
- Puppel, K.; Kalińska, A.; Kot, M.; Slósarz, J.; Kunowska-Slósarz, M.; Grodkowski, G.; Kuczyńska, B.; Solarczyk, P.; Przysucha, T.; Gołębiewski, M. The Effect of Staphylococcus spp., Streptococcus spp. and Enterobacteriaceae on the Development of Whey Protein Levels and Oxidative Stress Markers in Cows with Diagnosed Mastitis. Animals 2020, 10, 1591. [Google Scholar] [CrossRef]
- Radzikowski, D.; Kalińska, A.; Ostaszewska, U.; Gołębiewski, M. Alternative solutions to antibiotics in mastitis treatment for dairy cows—A review. Anim. Sci. Pap. Rep. 2020, 38, 117–133. [Google Scholar]
- Lange, A.; Grzenia, A.; Wierzbicki, M.; Strojny-Cieslak, B.; Kalińska, A.; Gołębiewski, M.; Radzikowski, D.; Sawosz, E.; Jaworski, S. Silver and Copper Nanoparticles Inhibit Biofilm Formation by Mastitis Pathogens. Animals 2021, 11, 1884. [Google Scholar] [CrossRef]
- Tamrakar, S.; Nishida, M.; Amen, Y.; Tran, H.B.; Suhara, H.; Fukami, K.; Parajuli, G.P.; Shimizu, K. Antibacterial activity of Nepalese wild mushrooms against Staphylococcus aureus and Propionibacterium acnes. J. Wood Sci. 2017, 63, 379–387. [Google Scholar] [CrossRef]
- Zhao, D.L.; Wang, D.; Tian, X.Y.; Cao, F.; Li, Y.Q.; Zhang, C.S. Anti-phytopathogenic and cytotoxic activities of crude extracts and secondary metabolites of marine-derived fungi. Mar. Drugs 2018, 16, 36. [Google Scholar] [CrossRef]
- Wernicki, A.; Puchalski, A.; Urban-Chmiel, R.; Dec, M.; Stegierska, D.; Dudzic, A.; Wojcik, A. Antimicrobial properties of gold, silver, copper and platinum nanoparticles against selected microorganisms isolated from cases of mastitis in cattle. Med. Weter 2014, 70, 564–567. [Google Scholar]
- Gołębiewski, M. Evaluation of changes in technical and production indicators on farms covered by the “Healthy Cow” program. In Proceedings of the Conference “Heathy Cow” Oral Presentation, Warsaw University of Life Sciences, Warsaw, Poland, 4 December 2013. [Google Scholar]
- van Soest, F.J.; Santman-Berends, I.M.; Lam, T.J.; Hogeveen, H. Failure and preventive costs of mastitis on Dutch dairy farms. J. Dairy Sci. 2016, 99, 8365–8374. [Google Scholar] [CrossRef]
- Doehring, C.; Sundrum, A. The informative value of an overview on antibiotic consumption, treatment efficacy and cost of clinical mastitis at farm level. Prev. Vet. Med. 2019, 165, 63–70. [Google Scholar] [CrossRef]
- Hogeveen, H.; Steeneveld, W.; Wolf, C.A. Production diseases reduce the efficiency of dairy production: A review of the results, methods, and approaches regarding the economics of mastitis. Annu. Rev. Resour. Econ. 2019, 11, 289–312. [Google Scholar] [CrossRef]
- Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2021, European Medicines Agency, 2022, Luxemburg. Available online: https://www.ema.europa.eu/en/documents/report/sales-veterinary-antimicrobial-agents-31-european-countries-2021-trends-2010-2021-twelfth-esvac_en.pdf (accessed on 15 May 2023).
- Wiśniewski, P.; Marks-Bielska, R. The importance of the implementation of the European Green Deal for the Polish countryside and agriculture. In Polska Wieś 2022: Raport o Stanie Wsi; Jerzy, W., Andrzej, H., Eds.; Fundacja na rzecz Rozwoju Polskiego Rolnictwa: Warsaw, Poland, 2022; pp. 119–132. ISBN 978-83-66849-54-9. [Google Scholar]
- Meaney, W.J. Effect of a dry period teat seal on bovine udder infection. Ir. J. Agric. Res. 1977, 16, 293–299. [Google Scholar]
- Molina, L.R.; Costa, H.; Leão, J.M.; Malacco, V.M.; Facury, E.J.; Carvalho, A.U.; Lage, C.F. Efficacy of an internal teat seal associated with a dry cow intramammary antibiotic for prevention of intramammary infections in dairy cows during the dry and early lactation periods. Pesq. Vet. Bras. 2017, 37, 465–470. [Google Scholar] [CrossRef]
- Freu, G.; Tomazi, T.; Monteiro, C.P.; Barcelos, M.M.; Alves, B.G.; dos Santos, M.V. Internal teat sealant administered at drying off reduces intramammary infections during the dry and early lactation periods of dairy cows. Animals 2020, 10, 1522. [Google Scholar] [CrossRef]
- Lavery, A.; Craig, A.L.; Gordon, A.W.; Ferris, C.P. Impact of adopting non-antibiotic dry-cow therapy on the performance and udder health of dairy cows. Vet. Rec. 2022, 190, e1731. [Google Scholar] [CrossRef]
- ElAshmawy, W.R.; Okello, E.; Williams, D.R.; Anderson, R.J.; Karle, B.; Lehenbauer, T.W.; Aly, S.S. Effectiveness of Intramammary Antibiotics, Internal Teat Sealants, or Both at Dry-Off in Dairy Cows: Milk Production and Somatic Cell Count Outcomes. Vet. Sci. 2022, 9, 559. [Google Scholar] [CrossRef]
- Clabby, C.; McParland, S.; Dillon, P.; Arkins, S.; Flynn, J.; Murphy, J.; Boloña, P.S. Internal teat sealants alone or in combination with antibiotics at dry-off–the effect on udder health in dairy cows in five commercial herds. Animal 2022, 16, 100449. [Google Scholar] [CrossRef]
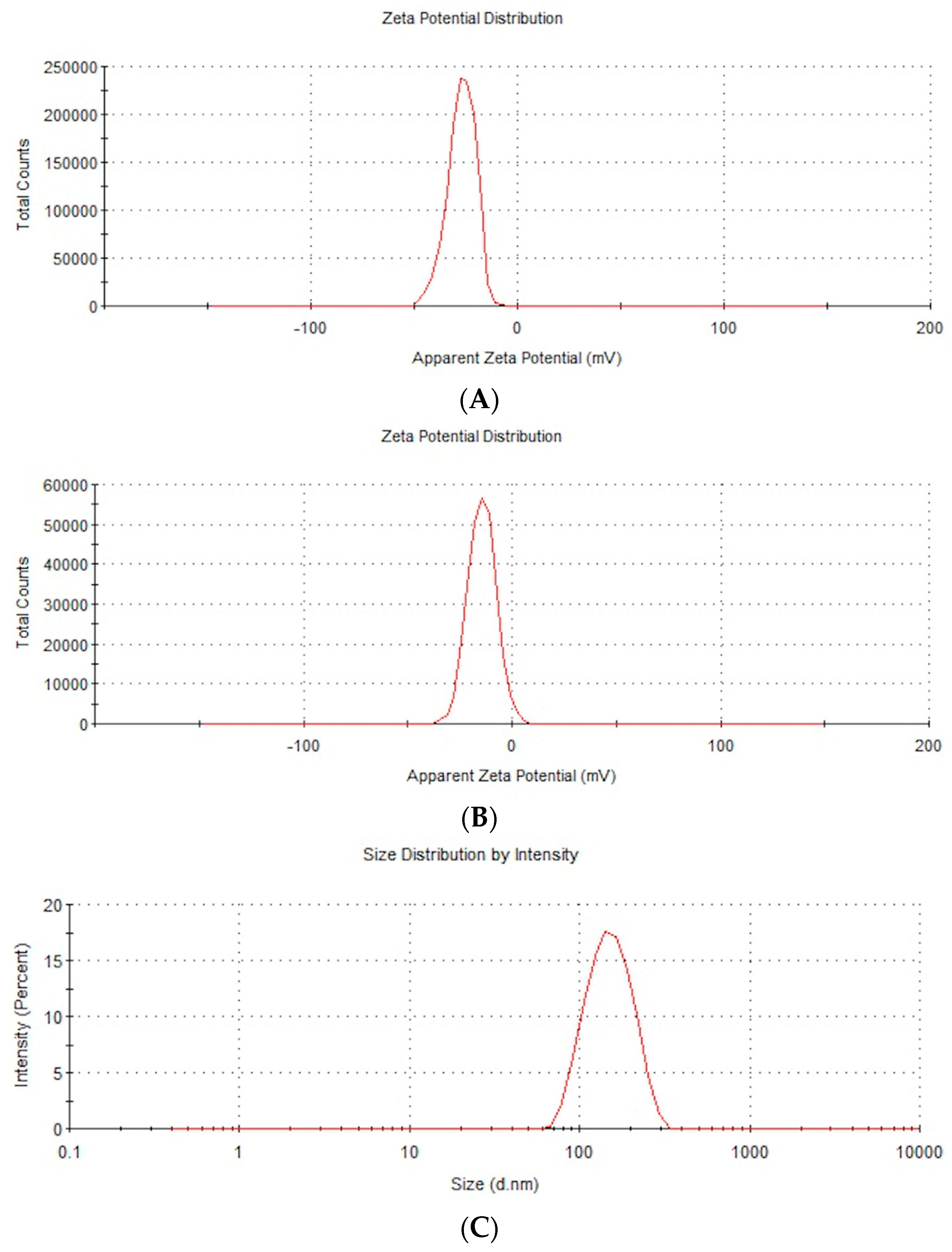
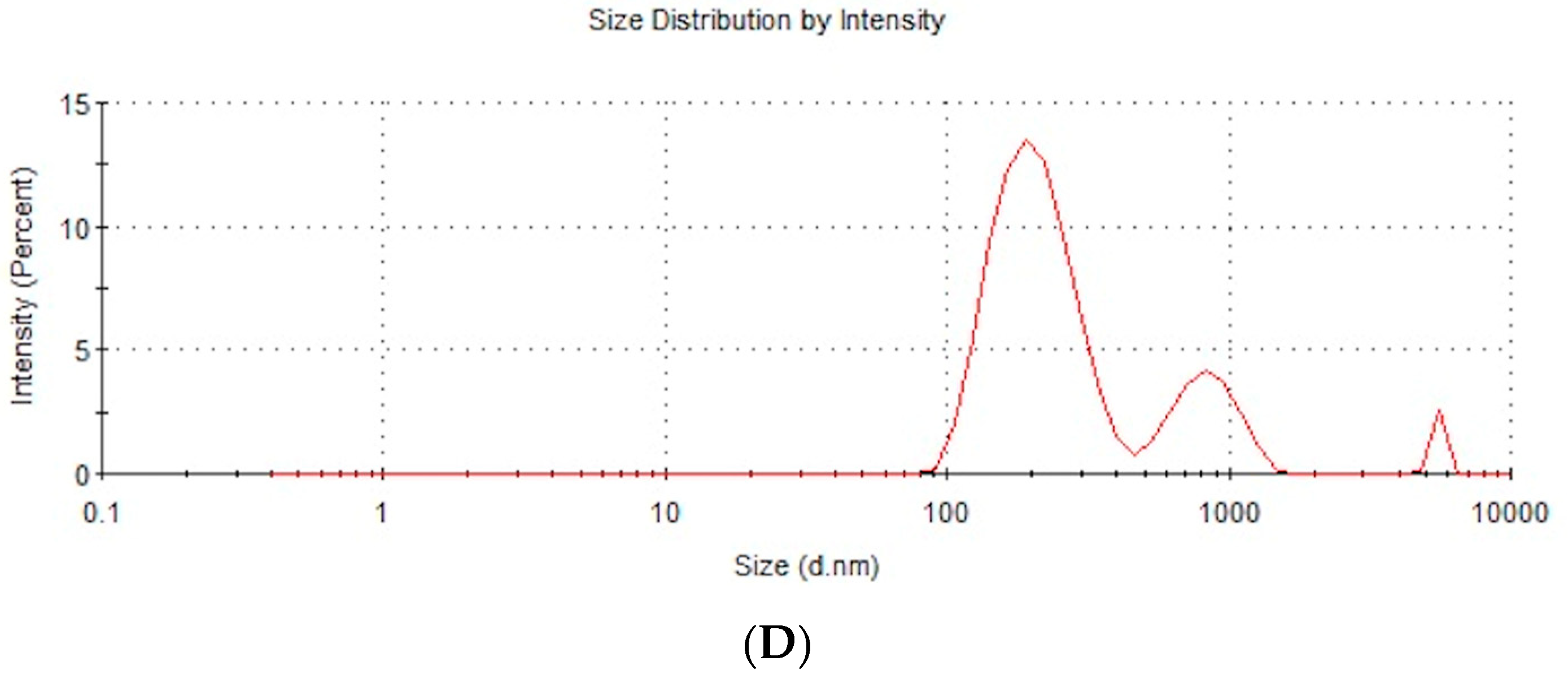
| Zeta Potential | Hydrodynamic Size | NP Diameter | NP Structure | |
|---|---|---|---|---|
| (mV) | (nm) | (nm) | ||
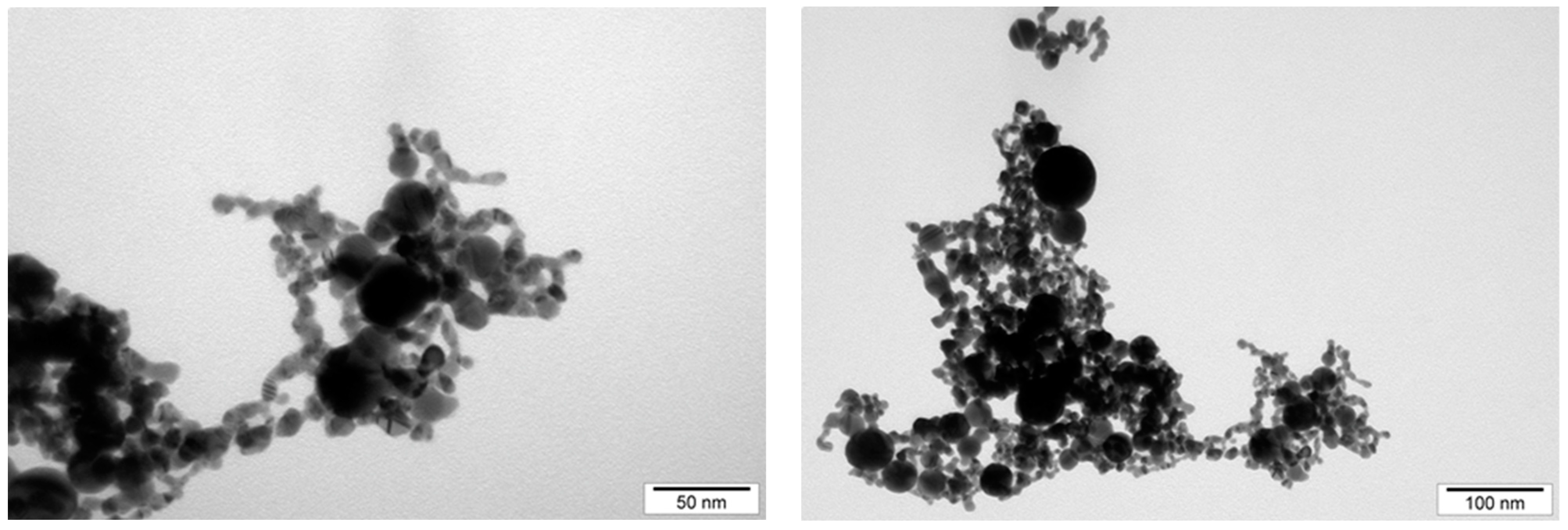
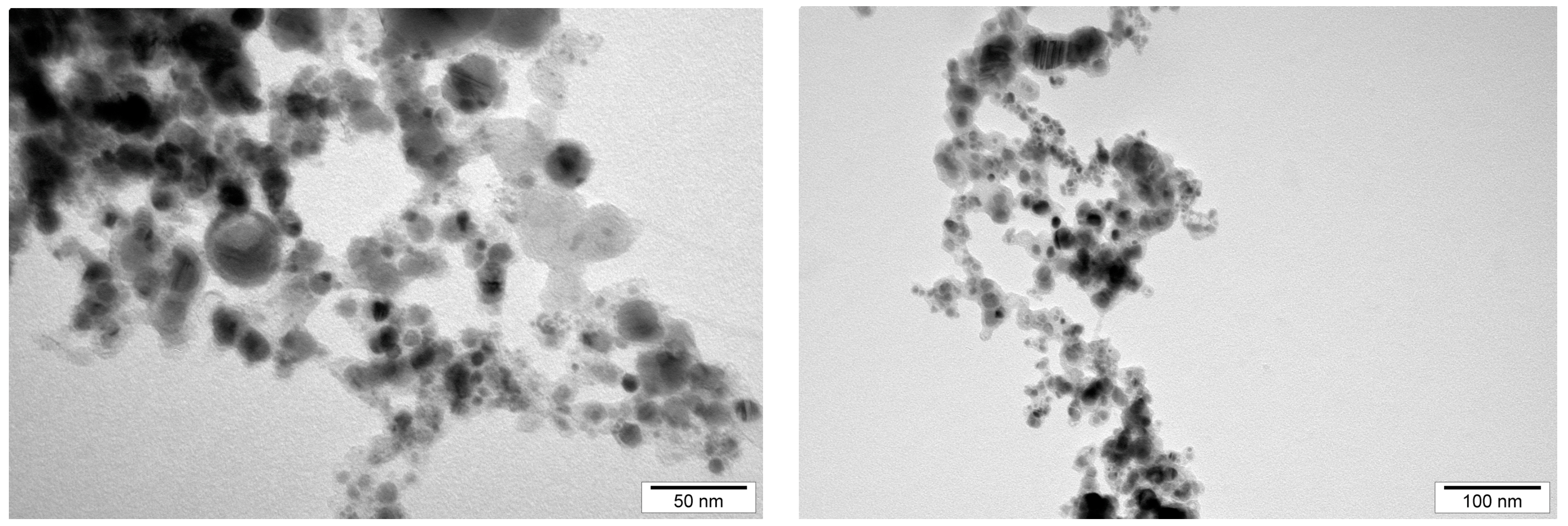
| Measurement method | Mobility | Dynamic light scattering (DLS) | Transmission electron microscopy | Transmission electron microscopy |
| Defined parameter | Zeta potential | Average size of agglomerate | Size of single NPs | NP form |
| AuNPs | −28.4 | 148.3 | 15–75 | Spherical |
| NP-FeCs | −18.5 | 342.9 | 10–80 | Spherical |
| Staphylococcus aureus | Escherichia coli | Streptococcus agalactiae | Streptococcus uberis | Enterococcus faecalis | Enterobacter cloacae | Pseudomonas aeruginosa | Candida albicans | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Average | SE | Average | SE | Average | SE | Average | SE | Average | SE | Average | SE | Average | SE | Average | SE | |
| Control group | 100.00 | 0.013 | 100.00 | 0.050 | 100.00 | 0.076 | 100.00 | 0.036 | 100.00 | 0.027 | 100.00 | 0.038 | 100.00 | 0.029 | 100.00 | 0.022 |
| AgNPs0.5 | 79.32 | 0.047 | 72.20 | 0.020 | 80.53 | 0.009 | 63.94 | 0.035 | 91.66 | 0.041 | 80.07 | 0.043 | 84.99 | 0.037 | 88.97 | 0.026 |
| AgNPs1 | 77.54 | 0.056 | 75.43 | 0.039 | 76.47 | 0.025 | 58.36 | 0.042 | 72.78 | 0.019 | 63.38 | 0.053 | 82.05 | 0.062 | 78.20 | 0.030 |
| AgNPs2 | 74.87 | 0.034 | 58.46 | 0.031 | 64.64 | 0.026 | 56.50 | 0.025 | 69.31 | 0.067 | 55.45 | 0.013 | 81.37 | 0.013 | 77.42 | 0.033 |
| AgNPs5 | 63.91 | 0.069 | 64.76 | 0.046 | 48.42 | 0.030 | 49.44 | 0.015 | 52.24 | 0.061 | 64.35 | 0.020 | 73.59 | 0.040 | 73.00 | 0.039 |
| AuNPs0.5 | 97.08 | 0.026 | 91.04 | 0.076 | 97.43 | 0.026 | 91.11 | 0.035 | 93.72 | 0.044 | 84.11 | 0.024 | 87.98 | 0.035 | 98.10 | 0.047 |
| AuNPs1 | 87.98 | 0.129 | 86.14 | 0.036 | 90.44 | 0.023 | 86.84 | 0.018 | 82.61 | 0.005 | 67.15 | 0.041 | 86.25 | 0.029 | 94.37 | 0.011 |
| AuNPs2 | 84.24 | 0.081 | 88.57 | 0.008 | 88.59 | 0.058 | 84.40 | 0.017 | 80.66 | 0.021 | 64.10 | 0.026 | 84.10 | 0.011 | 86.62 | 0.038 |
| AuNPs5 | 76.27 | 0.052 | 87.46 | 0.036 | 82.84 | 0.011 | 78.57 | 0.042 | 60.05 | 0.026 | 46.89 | 0.003 | 60.43 | 0.037 | 76.82 | 0.034 |
| CuNPs0.5 | 92.64 | 0.055 | 85.94 | 0.032 | 95.51 | 0.023 | 89.47 | 0.038 | 86.74 | 0.036 | 85.50 | 0.025 | 90.80 | 0.020 | 97.35 | 0.036 |
| CuNPs1 | 88.75 | 0.089 | 83.08 | 0.034 | 91.73 | 0.012 | 73.90 | 0.015 | 80.11 | 0.017 | 72.54 | 0.030 | 79.40 | 0.018 | 91.04 | 0.029 |
| CuNPs2 | 81.05 | 0.144 | 78.75 | 0.028 | 85.67 | 0.037 | 70.97 | 0.031 | 67.30 | 0.018 | 60.36 | 0.042 | 70.81 | 0.019 | 86.17 | 0.033 |
| CuNPs5 | 84.07 | 0.099 | 71.05 | 0.004 | 79.60 | 0.036 | 54.41 | 0.048 | 55.86 | 0.021 | 70.35 | 0.029 | 67.11 | 0.031 | 88.28 | 0.036 |
| NP-FeC0.5 | 96.05 | 0.044 | 101.02 | 0.030 | 130.37 | 0.069 | 134.41 | 0.154 | 104.20 | 0.025 | 125.42 | 0.066 | 99.58 | 0.050 | 138.43 | 0.054 |
| NP-FeC 1 | 99.97 | 0.052 | 110.80 | 0.052 | 137.72 | 0.112 | 159.15 | 0.050 | 159.60 | 0.033 | 125.60 | 0.043 | 104.35 | 0.030 | 123.08 | 0.018 |
| NP-FeC 2 | 96.91 | 0.112 | 100.12 | 0.047 | 128.33 | 0.081 | 132.87 | 0.041 | 144.87 | 0.033 | 129.78 | 0.050 | 108.90 | 0.070 | 130.89 | 0.089 |
| NP-FeC 5 | 98.69 | 0.170 | 97.82 | 0.016 | 125.65 | 0.019 | 139.84 | 0.082 | 146.80 | 0.062 | 132.55 | 0.054 | 122.71 | 0.053 | 135.51 | 0.040 |
| p < 0.05 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | |||||||||
| Staphylococcus aureus | Escherichia coli | Streptococcus agalactiae | Streptococcus uberis | Enterococcus faecalis | Enterobacter cloacae | Pseudomonas aeruginosa | Candida albicans | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Average | SE | Average | SE | Average | SE | Average | SE | Average | SE | Average | SE | Average | SE | Average | SE | |
| Control group | 100.00 | 0.021 | 100.00 | 0.077 | 100.00 | 0.022 | 100.00 | 0.062 | 100.00 | 0.043 | 100.00 | 0.028 | 100.00 | 0.035 | 100.00 | 0.041 |
| AgAuCuNPs0.5 | 80.40 | 0.036 | 76.21 | 0.109 | 79.76 | 0.021 | 80.09 | 0.025 | 87.70 | 0.029 | 91.28 | 0.045 | 81.79 | 0.031 | 85.01 | 0.041 |
| AgAuCuNPs1 | 72.21 | 0.031 | 57.11 | 0.021 | 60.75 | 0.020 | 65.53 | 0.058 | 57.75 | 0.030 | 73.03 | 0.018 | 74.38 | 0.028 | 86.22 | 0.061 |
| AgAuCuNPs2 | 70.88 | 0.033 | 61.91 | 0.053 | 58.77 | 0.026 | 55.54 | 0.014 | 58.99 | 0.042 | 78.35 | 0.054 | 77.40 | 0.025 | 82.18 | 0.013 |
| AgAuCuNPs5 | 70.38 | 0.016 | 57.99 | 0.051 | 56.67 | 0.032 | 53.99 | 0.022 | 60.03 | 0.018 | 80.37 | 0.044 | 75.21 | 0.035 | 75.45 | 0.010 |
| p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.05 | |||||||||
| Staphylococcus aureus | Escherichia coli | Streptococcus agalactiae | Candida albicans | |||||
|---|---|---|---|---|---|---|---|---|
| Average | SE | Average | SE | Average | SE | Average | SE | |
| Control group | 100.00 | 0.15 | 100.00 | 0.11 | 100.00 | 0.07 | 100.00 | 0.03 |
| Wax | 91.81 | 0.07 | 84.94 | 0.08 | 79.76 | 0.07 | 92.68 | 0.06 |
| AgNPs1+ wax | 85.10 | 0.19 | 78.65 | 0.04 | 53.73 | 0.02 | 73.22 | 0.03 |
| AuNPs1+ wax | 92.14 | 0.05 | 84.11 | 0.04 | 53.73 | 0.02 | 77.67 | 0.03 |
| CuNPs1+ wax | 86.70 | 0.04 | 81.12 | 0.09 | 58.43 | 0.02 | 86.90 | 0.03 |
| AgAuCuNPs1+ wax | 70.78 | 0.03 | 55.09 | 0.03 | 57.74 | 0.04 | 66.30 | 0.04 |
| Mix | 85.67 | 0.09 | 90.22 | 0.06 | 90.14 | 0.04 | 96.76 | 0.05 |
| AgNPs1+ mix | 79.83 | 0.02 | 75.30 | 0.07 | 57.81 | 0.02 | 77.27 | 0.04 |
| AuNPs1+ mix | 84.31 | 0.06 | 84.11 | 0.10 | 58.34 | 0.03 | 85.89 | 0.05 |
| CuNPs1+ mix | 85.29 | 0.08 | 81.12 | 0.12 | 82.08 | 0.04 | 83.26 | 0.03 |
| AgAuCuNPs1+ mix | 77.76 | 0.11 | 57.38 | 0.04 | 62.66 | 0.08 | 64.44 | 0.03 |
| p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radzikowski, D.; Kalińska, A.; Kot, M.; Jaworski, S.; Wierzbicki, M.; Gołębiewski, M. In Vitro Evaluation of the Antimicrobial Properties of Nanoparticles as New Agents Used in Teat Sealants for Mastitis Prevention in Dry Cows. Biomedicines 2023, 11, 2291. https://doi.org/10.3390/biomedicines11082291
Radzikowski D, Kalińska A, Kot M, Jaworski S, Wierzbicki M, Gołębiewski M. In Vitro Evaluation of the Antimicrobial Properties of Nanoparticles as New Agents Used in Teat Sealants for Mastitis Prevention in Dry Cows. Biomedicines. 2023; 11(8):2291. https://doi.org/10.3390/biomedicines11082291
Chicago/Turabian StyleRadzikowski, Daniel, Aleksandra Kalińska, Magdalena Kot, Sławomir Jaworski, Mateusz Wierzbicki, and Marcin Gołębiewski. 2023. "In Vitro Evaluation of the Antimicrobial Properties of Nanoparticles as New Agents Used in Teat Sealants for Mastitis Prevention in Dry Cows" Biomedicines 11, no. 8: 2291. https://doi.org/10.3390/biomedicines11082291
APA StyleRadzikowski, D., Kalińska, A., Kot, M., Jaworski, S., Wierzbicki, M., & Gołębiewski, M. (2023). In Vitro Evaluation of the Antimicrobial Properties of Nanoparticles as New Agents Used in Teat Sealants for Mastitis Prevention in Dry Cows. Biomedicines, 11(8), 2291. https://doi.org/10.3390/biomedicines11082291








