Immediate Effects of Anti-Spastic Epidural Cervical Spinal Cord Stimulation on Functional Connectivity of the Central Motor System in Patients with Stroke- and Traumatic Brain Injury-Induced Spasticity: A Pilot Resting-State Functional Magnetic Resonance Imaging Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Ethics Statement
2.3. Study Design
2.4. Spasticity Assessment and Analysis
2.5. SCS Therapy: Temporary Electrode Installation and Stimulation Protocol
2.6. Functional MRI Scanning Parameters
2.7. Resting State Data Preprocessing Pipeline
2.8. Functional Connectivity Analysis
3. Results
3.1. Spasticity Elimination
3.2. Seed-Based Functional Connectivity Analysis
3.3. ROI-to-ROI Analysis
3.4. Whole-Brain Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Doussoulin, A.; Rivas, C.; Bacco, J.; Sepúlveda, P.; Carvallo, G.; Gajardo, C.; Soto, A.; Rivas, R. Prevalence of Spasticity and Postural Patterns in the Upper Extremity Post Stroke. J. Stroke Cerebrovasc. Dis. 2020, 29, 105253. [Google Scholar] [CrossRef] [PubMed]
- Dorňák, T.; Justanová, M.; Konvalinková, R.; Říha, M.; Mužík, J.; Hoskovcová, M.; Srp, M.; Navrátilová, D.; Otruba, P.; Gál, O.; et al. Prevalence and Evolution of Spasticity in Patients Suffering from First-Ever Stroke with Carotid Origin: A Prospective, Longitudinal Study. Eur. J. Neurol. 2019, 26, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Schinwelski, M.J.; Sitek, E.J.; Wąż, P.; Sławek, J.W. Prevalence and Predictors of Post-Stroke Spasticity and Its Impact on Daily Living and Quality of Life. Neurol. Neurochir. Pol. 2019, 53, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Sommerfeld, D.K.; Eek, E.U.B.; Svensson, A.K.; Holmqvist, L.W.; Von Arbin, M.H. Spasticity after Stroke: Its Occurrence and Association with Motor Impairments and Activity Limitations. Stroke 2004, 35, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Urban, P.P.; Wolf, T.; Uebele, M.; Marx, J.J.; Vogt, T.; Stoeter, P.; Bauermann, T.; Weibrich, C.; Vucurevic, G.D.; Schneider, A.; et al. Occurence and Clinical Predictors of Spasticity after Ischemic Stroke. Stroke 2010, 41, 2016–2020. [Google Scholar] [CrossRef] [PubMed]
- Nakase-Richardson, R.; McNamee, S.; Howe, L.L.; Massengale, J.; Peterson, M.; Barnett, S.D.; Harris, O.; McCarthy, M.; Tran, J.; Scott, S.; et al. Descriptive Characteristics and Rehabilitation Outcomes in Active Duty Military Personnel and Veterans with Disorders of Consciousness with Combat- and Noncombat-Related Brain Injury. Arch. Phys. Med. Rehabil. 2013, 94, 1861–1869. [Google Scholar] [CrossRef] [PubMed]
- Thibaut, F.A.; Chatelle, C.; Wannez, S.; Deltombe, T.; Stender, S.; Schnakers, S.; Laureys, S. Spasticity in Disorders of Consciousness: A Behavioral Study. Eur. J. Phys. Rehabil. Med. 2015, 51, 389–397. [Google Scholar] [PubMed]
- Lance, J.W. Symposium Synopsis. In Spasticity: Disordered Motor Control; Feldman, R., Young, R., Koella, W., Eds.; Symposia Specialists: Chicago, IL, USA, 1980. [Google Scholar]
- Mukherjee, A.; Chakravarty, A. Spasticity Mechanisms—For the Clinician. Front. Neurol. 2010, 1, 149. [Google Scholar] [CrossRef]
- Mayer, N.H.; Esquenazi, A. Muscle Overactivity and Movement Dysfunction in the Upper Motoneuron Syndrome. Phys. Med. Rehabil. Clin. N. Am. 2003, 14, 855–883. [Google Scholar] [CrossRef]
- Sheean, G. The Pathophysiology of Spasticity. Eur. J. Neurol. 2002, 9 (Suppl. S1), 3–61. [Google Scholar] [CrossRef]
- Burke, D.; Gillies, J.D.; Lance, J.W. The Quadriceps Stretch Reflex in Human Spasticity. J. Neurol. Neurosurg. Psychiatry 1970, 33, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Tardieu, G.; Tardieu, C.; Colbeau-Justin, P.; Bret, M.D. Effects of Muscle Length on an Increased Stretch Reflex in Children with Cerebral Palsy. J. Neurol. Neurosurg. Psychiatry 1982, 45, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Trompetto, C.; Marinelli, L.; Mori, L.; Pelosin, E.; Currà, A.; Molfetta, L.; Abbruzzese, G. Pathophysiology of Spasticity: Implications for Neurorehabilitation. Biomed Res. Int. 2014, 2014, 354906. [Google Scholar] [CrossRef]
- Burke, D.; Wissel, J.; Donnan, G.A. Pathophysiology of Spasticity in Stroke. Neurology 2013, 80, S20–S26. [Google Scholar] [CrossRef] [PubMed]
- Gracies, J.M. Pathophysiology of Spastic Paresis. II: Emergence of Muscle Overactivity. Muscle Nerve 2005, 31, 552–571. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, A. Control of Spinal Mechanism from the Brain. In The Nervous System; Tower, D., Bradley, R., Eds.; Raven Press: New York, NY, USA, 1975; pp. 253–265. [Google Scholar]
- Li, S.; Francisco, G.E. New Insights into the Pathophysiology of Post-Stroke Spasticity. Front. Hum. Neurosci. 2015, 9, 192. [Google Scholar] [CrossRef] [PubMed]
- Li, S. Spasticity, Motor Recovery, and Neural Plasticity after Stroke. Front. Neurol. 2017, 8, 120. [Google Scholar] [CrossRef]
- Sherman, S.J.; Koshland, G.F.; Laguna, J.F. Hyper-Reflexia without Spasticity after Unilateral Infarct of the Medullary Pyramid. J. Neurol. Sci. 2000, 175, 145–155. [Google Scholar] [CrossRef]
- Bucy, P.C.; Keplinger, J.E.; Siqueira, E.B. Destruction of the “Pyramidal Tract” in Man. J. Neurosurg. 1964, 21, 285–298. [Google Scholar] [CrossRef]
- Goldstein, L.B. Common Drugs May Influence Motor Recovery after Stroke. Neurology 1995, 45, 865–871. [Google Scholar] [CrossRef]
- Verrotti, A.; Greco, R.; Spalice, A.; Chiarelli, F.; Iannetti, P. Pharmacotherapy of Spasticity in Children with Cerebral Palsy. Pediatr. Neurol. 2006, 34, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kolaski, K.; Ajizian, S.J.; Passmore, L.; Pasutharnchat, N.; Koman, L.A.; Smith, B.P. Safety Profile of Multilevel Chemical Denervation Procedures Using Phenol or Botulinum Toxin or Both in a Pediatric Population. Am. J. Phys. Med. Rehabil. 2008, 87, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Zafonte, R.; Elovic, E.P.; Lombard, L. Acute Care Management of Post-TBI Spasticity. J. Head Trauma Rehabil. 2004, 19, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Aquilina, K.; Graham, D.; Wimalasundera, N. Selective Dorsal Rhizotomy: An Old Treatment Re-Emerging. Arch. Dis. Child. 2015, 100, 798–802. [Google Scholar] [CrossRef]
- Vidailhet, M.; Vercueil, L.; Houeto, J.L.; Krystkowiak, P.; Lagrange, C.; Yelnik, J.; Bardinet, E.; Benabid, A.L.; Navarro, S.; Dormont, D.; et al. Bilateral, Pallidal, Deep-Brain Stimulation in Primary Generalised Dystonia: A Prospective 3 Year Follow-up Study. Lancet Neurol. 2007, 6, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Arredondo, A.; Cázares-Ramírez, E.; Carrillo-Mora, P.; Martínez-Vargas, M.; Cárdenas-Rodríguez, N.; Coballase-Urrutia, E.; Alemón-Medina, R.; Sampieri, A.; Navarro, L.; Carmona-Aparicio, L. Baclofen in the Therapeutic of Sequele of Traumatic Brain Injury: Spasticity. Clin. Neuropharmacol. 2016, 39, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Korzhova, J.; Sinitsyn, D.; Chervyakov, A.; Poydasheva, A.; Zakharova, M.; Suponeva, N.; Chernikova, L.; Piradov, M. Transcranial and Spinal Cord Magnetic Stimulation in Treatment of Spasticity: A Literature Review and Meta-Analysis. Eur. J. Phys. Rehabil. Med. 2018, 54, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Krames, E.S.; Hunter Peckham, P.; Rezai, A.R.; Aboelsaad, F. What Is Neuromodulation? Neuromodulation 2009, 1, 3–8. [Google Scholar] [CrossRef]
- Dooley, D.M.; Sharkey, J. Electrostimulation of the Nervous System for Patients with Demyelinating and Degenerative Diseases of the Nervous System and Vascular Diseases of the Extremities. Stereotact. Funct. Neurosurg. 1977, 40, 208–217. [Google Scholar] [CrossRef]
- Siegfried, J.; Krainick, J.U.; Haas, H.; Adorjani, C.; Meyer, M.; Thoden, U. Electrical Spinal Cord Stimulation for Spastic Movement Disorders. Stereotact. Funct. Neurosurg. 1978, 41, 134–141. [Google Scholar] [CrossRef]
- Siegfried, J.; Lazorthes, Y.; Broggi, G. Electrical Spinal Cord Stimulation for Spastic Movement Disorders. Appl. Neurophysiol. 1981, 44, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Waltz, J.M.; Reynolds, L.O.; Riklan, M. Multi-Lead Spinal Cord Stimulation for Control of Motor Disorders. Stereotact. Funct. Neurosurg. 1981, 44, 244–257. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.; Gray, E.; Kudzma, J. Beneficial Augmentation Following Dorsal Column Stimulation in Some Neurological Diseases. Appl. Neurophysiol. 1981, 44, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, S.; Nanivadekar, A.C.; McKernan, G.; Helm, E.R.; Boninger, M.L.; Collinger, J.L.; Gaunt, R.A.; Fisher, L.E. Sensory Restoration by Epidural Stimulation of the Lateral Spinal Cord in Upper-Limb Amputees. Elife 2020, 9, e54349. [Google Scholar] [CrossRef] [PubMed]
- Nagel, S.J.; Wilson, S.; Johnson, M.D.; Machado, A.; Frizon, L.; Chardon, M.K.; Reddy, C.G.; Gillies, G.T.; Howard, M.A. Spinal Cord Stimulation for Spasticity: Historical Approaches, Current Status, and Future Directions. Neuromodulation 2017, 20, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Cook, A.W.; Weinstein, S.P. Chronic Dorsal Column Stimulation in Multiple Sclerosis. Preliminary Report. N. Y. State J. Med. 1973, 73, 2868–2872. [Google Scholar]
- Hofstoetter, U.S.; Freundl, B.; Lackner, P.; Binder, H. Transcutaneous Spinal Cord Stimulation Enhances Walking Performance and Reduces Spasticity in Individuals with Multiple Sclerosis. Brain Sci. 2021, 11, 472. [Google Scholar] [CrossRef] [PubMed]
- Koulousakis, A.; Buchhaas, U.; Nittner, K. Application of SCS for Movement Disorders and Spasticity. Acta Neurochir. Suppl. 1987, 39, 112–116. [Google Scholar] [CrossRef]
- Broseta, J.; Garcia-March, G.; Sánchez-Ledesma, M.J.; Barberá, J.; González-Darder, J. High-Frequency Cervical Spinal Cord Stimulation in Spasticity and Motor Disorders. Acta Neurochir. Suppl. 1987, 39, 106–111. [Google Scholar] [CrossRef]
- Cioni, B.; Meglio, M.; Prezioso, A.; Talamonti, G.; Tirendi, M. Spinal Cord Stimulation (SCS) in Spastic Hemiparesis. Pacing Clin. Electrophysiol. 1989, 12, 739–742. [Google Scholar] [CrossRef]
- Barolat-Romana, G.; Myklebust, J.B.; Hemmy, D.C.; Wenninger, W. Immediate Effects of Spinal Cord Stimulation in Spinal Spasticity. J. Neurosurg. 1985, 62, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Dekopov, A.V.; Shabalov, V.A.; Tomsky, A.A.; Hit, M.V.; Salova, E.M. Chronic Spinal Cord Stimulation in the Treatment of Cerebral and Spinal Spasticity. Stereotact. Funct. Neurosurg. 2015, 93, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Dimitrijevic, M.R.; Illis, L.S.; Nakajima, K.; Sharkey, P.C.; Sherwood, A.M. Spinal Cord Stimulation for the Control of Spasticity in Patients with Chronic Spinal Cord Injury: II. Neurophysiologic Observations. Cent. Nerv. Syst. Trauma 1986, 3, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Hofstoetter, U.S.; Freundl, B.; Danner, S.M.; Krenn, M.J.; Mayr, W.; Binder, H.; Minassian, K. Transcutaneous Spinal Cord Stimulation Induces Temporary Attenuation of Spasticity in Individuals with Spinal Cord Injury. J. Neurotrauma 2020, 37, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Terao, T.; Taya, K.; Sawauchi, S.; Numoto, T.R.; Murakami, S.; Abe, T.; Hashimoto, T. Therapeutic Effect of Spinal Cord Stimulation for a Patient Suffering Spasticity after Hypoxia of the Brain. Neurol. Surg. 2004, 32, 613–618. [Google Scholar]
- Gybels, J.; Van Roost, D. Spinal Cord Stimulation for Spasticity. In Neurosurgery for Spasticity; Sindou, M.P., Abbott, I.R., Keravel, Y., Eds.; Springer: Vienna, Austira, 1991; pp. 73–81. [Google Scholar] [CrossRef]
- Taccola, G.; Barber, S.; Horner, P.J.; Bazo, H.A.C.; Sayenko, D. Complications of Epidural Spinal Stimulation: Lessons from the Past and Alternatives for the Future. Spinal Cord 2020, 58, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-L.; Li, J.; Xu, H.-C.; Liu, Y.-C.; Yang, T.-T.; Yuan, H. Progress in the Efficacy and Mechanism of Spinal Cord Stimulation in Neuropathological Pain. Ibrain 2022, 8, 23–36. [Google Scholar] [CrossRef]
- Stančák, A.; Kozák, J.; Vrba, I.; Tintěra, J.; Vrána, J.; Poláček, H.; Stančák, M. Functional Magnetic Resonance Imaging of Cerebral Activation during Spinal Cord Stimulation in Failed Back Surgery Syndrome Patients. Eur. J. Pain 2008, 12, 137–148. [Google Scholar] [CrossRef]
- Deogaonkar, M.; Sharma, M.; Oluigbo, C.; Nielson, D.M.; Yang, X.; Vera-Portocarrero, L.; Molnar, G.F.; Abduljalil, A.; Sederberg, P.B.; Knopp, M.; et al. Spinal Cord Stimulation (SCS) and Functional Magnetic Resonance Imaging (FMRI): Modulation of Cortical Connectivity with Therapeutic SCS. Neuromodulation 2016, 19, 142–152. [Google Scholar] [CrossRef]
- Kishima, H.; Saitoh, Y.; Oshino, S.; Hosomi, K.; Ali, M.; Maruo, T.; Hirata, M.; Goto, T.; Yanagisawa, T.; Sumitani, M.; et al. Modulation of Neuronal Activity after Spinal Cord Stimulation for Neuropathic Pain; H215O PET Study. Neuroimage 2010, 49, 2564–2569. [Google Scholar] [CrossRef]
- Bentley, L.D.; Duarte, R.V.; Furlong, P.L.; Ashford, R.L.; Raphael, J.H. Brain Activity Modifications Following Spinal Cord Stimulation for Chronic Neuropathic Pain: A Systematic Review. Eur. J. Pain 2016, 20, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.P.; Brownstone, R.M. Mechanisms of Spinal Cord Stimulation for the Treatment of Pain: Still in the Dark after 50 Years. Eur. J. Pain 2019, 23, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W.; Smith, M.B. Interrater Reliability of a Modified Ashworth Scale of Muscle Spasticity. Phys. Ther. 1987, 67, 206–207. [Google Scholar] [CrossRef] [PubMed]
- Ashworth, B. Preliminary Trial of Carisoprodol in Multiple Sclerosis. Practitioner 1964, 192, 540–542. [Google Scholar] [PubMed]
- Rorden, C.; Brett, M. Stereotaxic Display of Brain Lesions. Behav. Neurol. 2000, 12, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.H.; Kim, T.; Min, J.H.; Kim, M.; Ko, H.Y.; Shin, Y.-I. Corticoreticular Pathway in Post-Stroke Spasticity: A Diffusion Tensor Imaging Study. J. Pers. Med. 2021, 11, 1151. [Google Scholar] [CrossRef]
- Jang, S.H.; Lee, S.J. Corticoreticular Tract in the Human Brain: A Mini Review. Front. Neurol. 2019, 10, 1188. [Google Scholar] [CrossRef]
- Alves, I.; Tedim Cruz, V.; Grebe, H.P. Spasticity as the First Manifestation of Ischaemic Lesions Involving the Cingulum. Case Rep. Neurol. Med. 2013, 2013, 534243. [Google Scholar] [CrossRef]
- Lemon Roger, N. Descending Pathways in Motor Control. Annu. Rev. Neurosci. 2008, 31, 195–218. [Google Scholar] [CrossRef]
- Gilman, S.; Marco, L.A.; Ebel, H.C. Effects of Medullary Pyramidotomy in the Monkey: II. Abnormalities of Spindle Afferent Responses. Brain 1971, 94, 515–530. [Google Scholar] [CrossRef]
- Gallivan, J.P.; McLean, D.A.; Valyear, K.F.; Pettypiece, C.E.; Culham, J.C. Decoding Action Intentions from Preparatory Brain Activity in Human Parieto-Frontal Networks. J. Neurosci. 2011, 31, 9599–9610. [Google Scholar] [CrossRef] [PubMed]
- Carpaneto, J.; Umiltà, M.A.; Fogassi, L.; Murata, A.; Gallese, V.; Micera, S.; Raos, V. Decoding the Activity of Grasping Neurons Recorded from the Ventral Premotor Area F5 of the Macaque Monkey. Neuroscience 2011, 188, 80–94. [Google Scholar] [CrossRef] [PubMed]
- Karabanov, A.N.; Chao, C.C.; Paine, R.; Hallett, M. Mapping Different Intra-Hemispheric Parietal-Motor Networks Using Twin Coil TMS. Brain Stimul. 2013, 6, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Koch, G.; Oliveri, M.; Cheeran, B.; Ruge, D.; Gerfo, E.L.; Salerno, S.; Torriero, S.; Marconi, B.; Mori, F.; Driver, J.; et al. Hyperexcitability of Parietal-Motor Functional Connections in the Intact Left-Hemisphere of Patients with Neglect. Brain 2008, 131, 3147–3155. [Google Scholar] [CrossRef] [PubMed]
- Koch, G.; Del Olmo, M.F.; Cheeran, B.; Ruge, D.; Schippling, S.; Caltagirone, C.; Rothwell, J.C. Focal Stimulation of the Posterior Parietal Cortex Increases the Excitability of the Ipsilateral Motor Cortex. J. Neurosci. 2007, 27, 6815–6822. [Google Scholar] [CrossRef] [PubMed]
- Wolpert, D.M.; Flanagan, J.R. Computations Underlying Sensorimotor Learning. Curr. Opin. Neurobiol. 2016, 37, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Shadmehr, R.; Smith, M.A.; Krakauer, J.W. Error Correction, Sensory Prediction, and Adaptation in Motor Control. Annu. Rev. Neurosci. 2010, 33, 89–108. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.B.; Christensen, M.S.; Farmer, S.F.; Lorentzen, J. Spastic Movement Disorder: Should We Forget Hyperexcitable Stretch Reflexes and Start Talking about Inappropriate Prediction of Sensory Consequences of Movement? Exp. Brain Res. 2020, 238, 1627–1636. [Google Scholar] [CrossRef]
- Veverka, T.; Hok, P.; Kaňovský, P.; Hluštík, P. Changes of the Brain Functional Connectivity in Post-Stroke Spasticity: A Pilot Study. J. Neurol. Sci. 2019, 405, 47. [Google Scholar] [CrossRef]
- Šenkárová, Z.; Hluštík, P.; Otruba, P.; Herzig, R.; Kaňovský, P. Modulation of Cortical Activity in Patients Suffering from Upper Arm Spasticity Following Stroke and Treated with Botulinum Toxin A: An FMRI Study. J. Neuroimaging 2010, 20, 9–15. [Google Scholar] [CrossRef]
- Veverka, T.; Hluštík, P.; Hok, P.; Otruba, P.; Zapletalová, J.; Tüdös, Z.; Krobot, A.; Kaňovský, P. Sensorimotor Modulation by Botulinum Toxin A in Post-Stroke Arm Spasticity: Passive Hand Movement. J. Neurol. Sci. 2016, 362, 14–20. [Google Scholar] [CrossRef]
- Veverka, T.; Hluštík, P.; Hok, P.; Otruba, P.; Tüdös, Z.; Zapletalová, J.; Krobot, A.; Kaňovský, P. Cortical Activity Modulation by Botulinum Toxin Type A in Patients with Post-Stroke Arm Spasticity: Real and Imagined Hand Movement. J. Neurol. Sci. 2014, 346, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Tomášová, Z.; Hluštík, P.; Král, M.; Otruba, P.; Herzig, R.; Krobot, A.; Kaňovský, P. Cortical Activation Changes in Patients Suffering from Post-Stroke Arm Spasticity and Treated with Botulinum Toxin A. J. Neuroimaging 2013, 23, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Enzinger, C.; Johansen-Berg, H.; Dawes, H.; Bogdanovic, M.; Collett, J.; Guy, C.; Ropele, S.; Kischka, U.; Wade, D.; Fazekas, F.; et al. Functional MRI Correlates of Lower Limb Function in Stroke Victims with Gait Impairment. Stroke 2008, 39, 1507–1513. [Google Scholar] [CrossRef]
- Van Meer, M.P.A.; Otte, W.M.; van der Marel, K.; Nijboer, C.H.; Kavelaars, A.; van der Sprenkel, J.W.B.; Viergever, M.A.; Dijkhuizen, R.M. Extent of Bilateral Neuronal Network Reorganization and Functional Recovery in Relation to Stroke Severity. J. Neurosci. 2012, 32, 4495–4507. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Nakada, T. Cortical Reorganization in Patients with Subcortical Hemiparesis: Neural Mechanisms of Functional Recovery and Prognostic Implication. J. Neurosurg. 2003, 98, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Sunnerhagen, K.S.; Opheim, A.; Alt Murphy, M. Onset, Time Course and Prediction of Spasticity after Stroke or Traumatic Brain Injury. Ann. Phys. Rehabil. Med. 2019, 62, 431–434. [Google Scholar] [CrossRef]
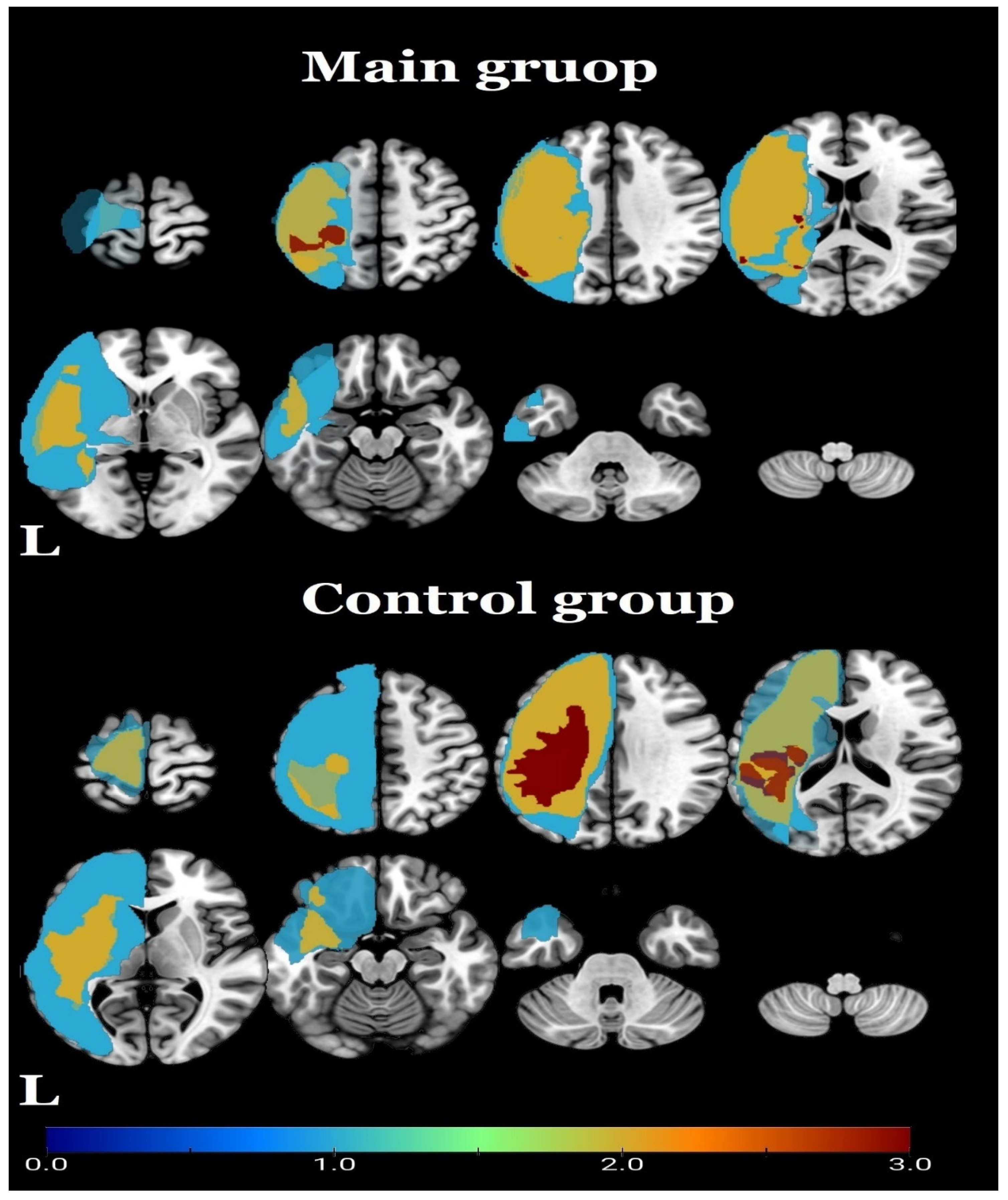

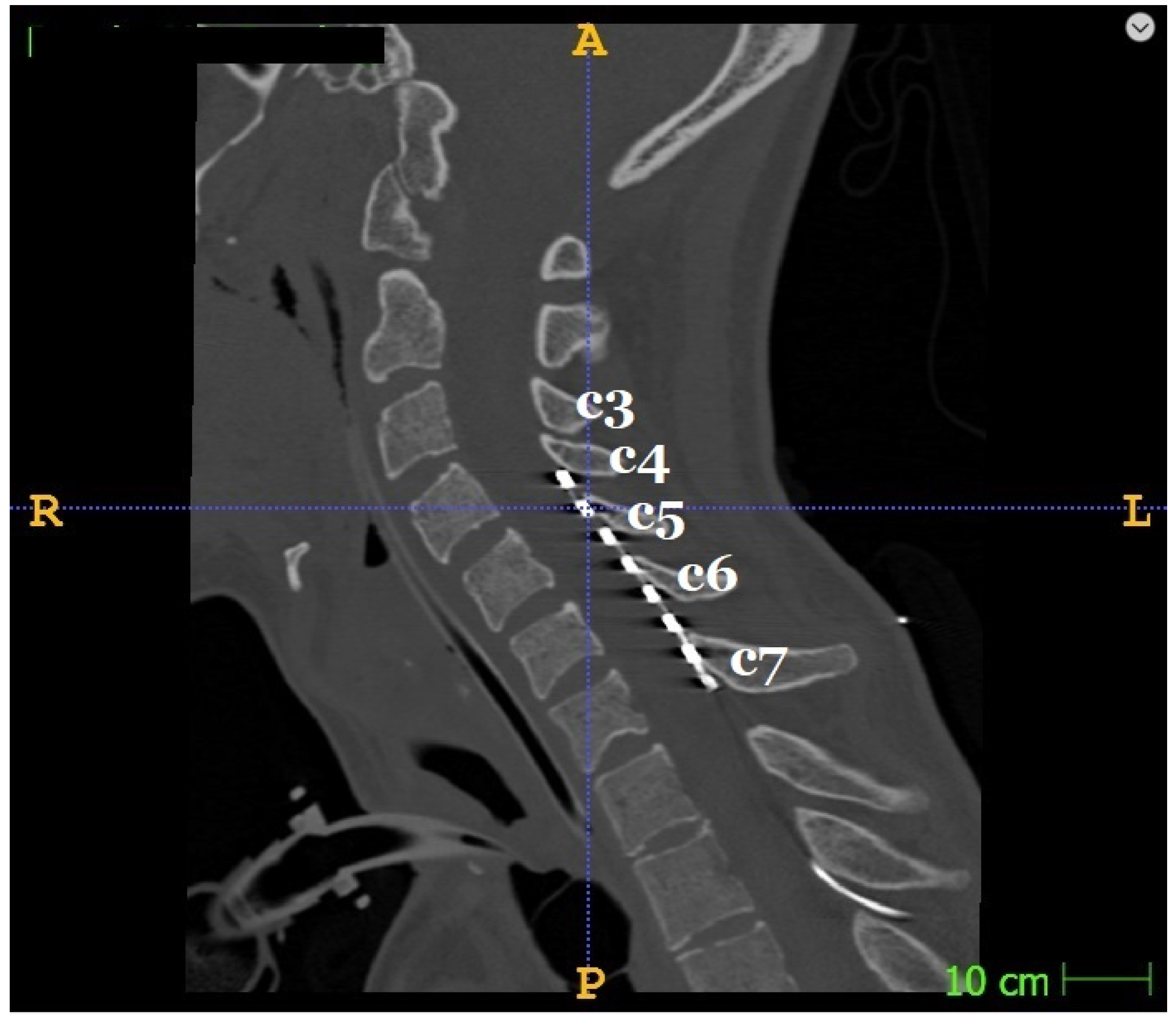


| Patient | Age | Sex | Handedness | Etiology | Lesion Side | Brain Tissue Damage Localisation | Lesion Volume (cm3) | Time since Stroke (days) | AS Hand | AS Leg | SCS Duration Days (hours per day) | SCS Mode | Time between fMRI Sessions (days) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Main group | |||||||||||||
| P01 | 35 | M | R | Hemorrhage | L | CS | 284.18 | 703 | 4 | 3 | 4 (12) | Tonic | 7 |
| P02 | 50 | F | R | IS | L | CS | 157.99 | 190 | 2 | 2 | 3 (12) | Tonic | 7 |
| P03 | 66 | M | R | Hemorrhage | L | S | 2.44 | 81 | 2 | 2 | 5 (12) | Tonic | 5 |
| P04 | 51 | M | R | IS | L | CS | 29.74 | 22 | 3 | 3 | 5 (12) | Burst | 5 |
| P05 | 25 | F | R | Anoxic | - | - | - | 1426 | 3 | 3 | 5 (12) | Burst | 6 |
| P06 | 50 | M | R | TBI | L | C | 15.92 | 65 | 2 | 2 | 1 (12) | Tonic | 4 |
| P07 | 19 | M | R | TBI | L | CS | 152.46 | 65 | 3 | - | 4 (12) | Burst | 4 |
| P08 | 32 | F | R | TBI | L | S | 0.46 | 3794 | 4 | 4 | 6 (12) | Tonic, burst | 6 |
| Control group | |||||||||||||
| P09 | 57 | M | R | IS | L | CS | 176.58 | 269 | - | - | - | - | 5 |
| P10 | 24 | F | R | TBI | L | C | 2.328 | 1094 | 4 | 3 | - | - | 5 |
| P11 | 75 | M | R | IS | L | CS | 217.26 | 65 | 2 | 2 | - | - | 7 |
| P12 | 58 | M | R | IS | L | CS | 48.65 | 524 | - | - | - | - | 3 |
| P13 | 54 | M | R | IS | L | S | 78.86 | 2230 | 2 | 2 | - | - | 6 |
| P14 | 66 | M | R | IS | _ | _ | _ | 20 | 1 | 1 | - | - | 4 |
| P15 | 55 | M | R | IS | _ | _ | _ | 251 | 1 | 1 | - | - | 3 |
| P16 | 63 | F | R | IS | L | S | 5.9 | 361 | 1 | 1 | - | - | 4 |
| Main Group (n = 8) | Control Group (n = 8) | p-Value | |
|---|---|---|---|
| Age, years | 42.5 (24) | 56.5 (11) | 0.046 |
| Female, % | 27.5 | 25 | - |
| Disease duration, days | 135.5 (1180.25) | 315 (840) | 0.795 |
| Lesion volume, cm3 | 22.8 (155.63) | 27.8 (151.57) | 0.873 |
| AS hand | 3 (2) | 1 (4) | >0.05 |
| AS leg | 2.5 (4) | 1 (3) | >0.05 |
| Time between 1st and 2nd fMRI | 5.5 (2.5) | 4.5 (2.5) | 0.226 |
| Seed | Region | MNI (x, y, z) | Cluster Size | F(2,14) | p-FDR |
|---|---|---|---|---|---|
| Brainstem | Temporal pole r | 52, 8, −32 | 725 | 32.02 | 0.000 |
| Angular g. r | 54, −64, 38 | 250 | 28.19 | 0.000 | |
| Orbital part of inferior frontal g. r | 46, 30, 0 | 248 | 18.38 | 0.000 | |
| Precentral g. r | Anterior prefrontal cortex r | 10, 46, 12 | 313 | 22.24 | 0.000 |
| Precentral g. l | No significant effect | ||||
| Postcentral g. r | Cerebellum | −2, −62, −50 | 427 | 25.86 | 0.000 |
| Postcentral g. l | No significant effect | ||||
| Supplementary motor cortex r | No significant effect | ||||
| Supplementary motor cortex l | Cerebellum | −12, −50, −56 | 84 | 46.96 | 0.000 |
| Seed | Region | BA | MNI (x, y, z) | Cluster Size | T | p-FDR |
|---|---|---|---|---|---|---|
| Main group | ||||||
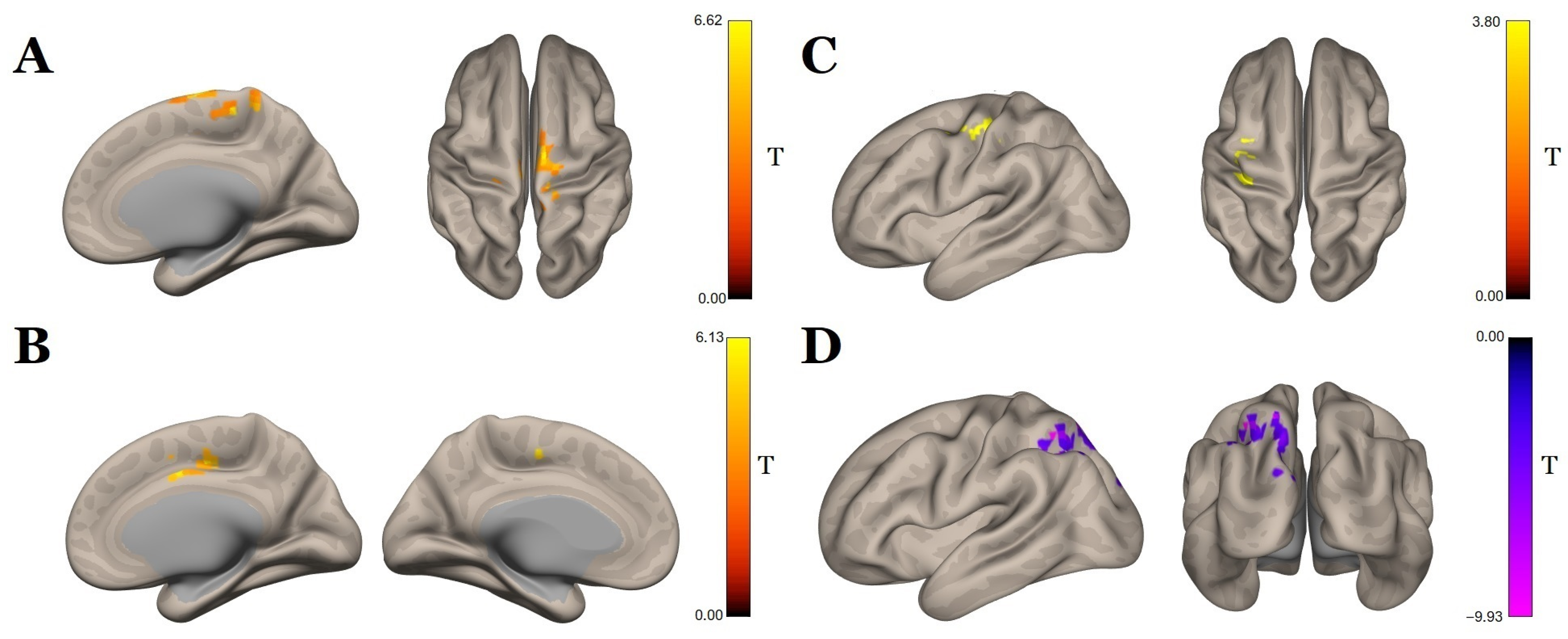
| Brainstem | Premotor cortex r Supplementary motor cortex r | 6 | 18, −12, 74 | 669 | 9.19 | 0.007 |
| Precentral g. r | Ventral anterior cingulate cortex r | 24 | 14, −16, 46 | 376 | 6.70 | 0.049 |
| Postcentral g. l | Visuo-motor area l | 7 | −30, −56, 50 | 722 | −12.76 | 0.000 |
| Supplementary motor cortex r | Primary somatosensory cortex l | 1 | −38, −24, 46 | 360 | 7.81 | 0.041 |
| Precentral g. l | No significant effect | |||||
| Postcentral g. r | ||||||
| Supplementary motor cortex l | ||||||
| Control group | ||||||
| Brainstem | Temporal pole r | 38 | 46, 14, −28 | 1275 | −8.75 | 0.000 |
| Orbital part of inferior frontal g. r | 47 | 46, 28, −4 | 481 | −5.23 | 0.001 | |
| Postcentral g. r | Cerebellum | - | 0, −74, −40 | 662 | −6.91 | 0.000 |
| Precentral g. r | No significant effect | |||||
| Precentral g. l | ||||||
| Postcentral g. l | ||||||
| Supplementary motor cortex r | ||||||
| Supplementary motor cortex l | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mayorova, L.; Radutnaya, M.; Varyukhina, M.; Vorobyev, A.; Zhdanov, V.; Petrova, M.; Grechko, A. Immediate Effects of Anti-Spastic Epidural Cervical Spinal Cord Stimulation on Functional Connectivity of the Central Motor System in Patients with Stroke- and Traumatic Brain Injury-Induced Spasticity: A Pilot Resting-State Functional Magnetic Resonance Imaging Study. Biomedicines 2023, 11, 2266. https://doi.org/10.3390/biomedicines11082266
Mayorova L, Radutnaya M, Varyukhina M, Vorobyev A, Zhdanov V, Petrova M, Grechko A. Immediate Effects of Anti-Spastic Epidural Cervical Spinal Cord Stimulation on Functional Connectivity of the Central Motor System in Patients with Stroke- and Traumatic Brain Injury-Induced Spasticity: A Pilot Resting-State Functional Magnetic Resonance Imaging Study. Biomedicines. 2023; 11(8):2266. https://doi.org/10.3390/biomedicines11082266
Chicago/Turabian StyleMayorova, Larisa, Margarita Radutnaya, Maria Varyukhina, Alexey Vorobyev, Vasiliy Zhdanov, Marina Petrova, and Andrey Grechko. 2023. "Immediate Effects of Anti-Spastic Epidural Cervical Spinal Cord Stimulation on Functional Connectivity of the Central Motor System in Patients with Stroke- and Traumatic Brain Injury-Induced Spasticity: A Pilot Resting-State Functional Magnetic Resonance Imaging Study" Biomedicines 11, no. 8: 2266. https://doi.org/10.3390/biomedicines11082266
APA StyleMayorova, L., Radutnaya, M., Varyukhina, M., Vorobyev, A., Zhdanov, V., Petrova, M., & Grechko, A. (2023). Immediate Effects of Anti-Spastic Epidural Cervical Spinal Cord Stimulation on Functional Connectivity of the Central Motor System in Patients with Stroke- and Traumatic Brain Injury-Induced Spasticity: A Pilot Resting-State Functional Magnetic Resonance Imaging Study. Biomedicines, 11(8), 2266. https://doi.org/10.3390/biomedicines11082266






