Abstract
(1) Background: Patients with sepsis following surgical intervention may exhibit fundamental distinctions from those experiencing sepsis without prior surgery. Despite the potential clinical importance of distinguishing these two sepsis subpopulations, dissimilarities, particularly in outcome, between surgical and non-surgical patients have been subject to limited scientific investigations in the existing literature. This study aimed to investigate the differences in mortality and sepsis-associated organ dysfunction between these two groups. (2) Methods: A retrospective analysis was conducted using data from a large cohort of prospectively enrolled patients with sepsis (n = 737) admitted to three intensive care units at University Medical Center Goettingen; patients were categorized into surgical (n = 582) and non-surgical sepsis groups (n = 155). The primary outcomes assessed were 28- and 90-day mortality rates, and secondary endpoints were multiple clinical parameters and measures of sepsis-associated organ dysfunction. (3) Results: Non-surgical patients presented a significantly higher 90-day mortality (37%) compared to surgical sepsis patients (30%, p = 0.0457). Moreover, the non-surgical sepsis group exhibited increased sepsis-associated organ dysfunction, as evidenced by higher average SOFA scores (p < 0.001), elevated levels of serum Procalcitonin (p = 0.0102), and a higher utilization of organ replacement therapies such as ventilation (p < 0.001), vasopressor treatment (p < 0.001), and renal replacement therapy (p = 0.0364). Additionally, non-surgical sepsis patients had higher organ-specific SOFA respiratory (p < 0.001), cardiovascular (p < 0.001), renal (p < 0.001), coagulation (0.0335), and central nervous system (p = 0.0206) subscores. (4) Conclusions: These results suggested that patients with non-surgical sepsis may face distinct challenges and a higher risk of adverse outcomes compared to patients with sepsis following surgical intervention. These findings have important implications for clinical decision-making, patient management, and resource allocation in sepsis care.
1. Introduction
Sepsis is a severe medical condition characterized by infection-induced dysregulated host immune response and subsequent organ dysfunction [1]. It remains a major global health concern, contributing to substantial morbidity and mortality rates [2,3,4]. While extensive research has focused on understanding the impact of sepsis on outcomes, limited research has directly compared the outcomes between surgical and non-surgical patients.
As sepsis is a prevalent and multifaceted medical condition encountered across various departments of the hospital, sepsis patients encompass a highly heterogeneous patient cohort [5,6]. Patients, for instance, vary inter-individually by the involved pathogens, the prior site of infection, preexisting comorbidities, or status of immune response, whereas therapy regimes remain limited to untailored supportive care [6].
The subgroup of patients with postoperative sepsis represents a distinct subset within this broader sepsis population. The nature of surgical intervention introduces unique factors, such as surgical site infections, perioperative stress, and alterations in immune response that contribute to the complexity and clinical characteristics of postoperative sepsis patients [7,8,9].
In contrast, patients with sepsis unrelated to surgery often present with severe underlying medical conditions that can exacerbate the severity of their septic condition [10]. These comorbidities, which may include chronic respiratory, cardiovascular, renal, and neurologic diseases, can further compromise their immune response and increase the risk of adverse outcomes [11,12].
Investigating the differences in mortality and sepsis-associated organ dysfunction between postoperative sepsis and non-interventional sepsis patients is crucial for understanding the distinct challenges faced by each group. This retrospective analysis of data from a large cohort of prospectively enrolled patients with sepsis aimed to reveal significant differences in mortality and sepsis-associated organ disfunction between surgical and non-surgical patients with sepsis.
2. Materials and Methods
The present study was performed at the University Medical Center Goettingen, Ger- many, between 2012 and 2019. All investigations and study protocols were approved under the ethical project identification code 1/15/12 by the responsible institutional ethics committee of the University of Goettingen. The study was performed in accordance with the provisions of the Helsinki Declaration. Written informed consent was obtained from all patients or their legal representatives.
The study adhered to and followed the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guideline regulations throughout the investigation [13].
2.1. Patient Enrollment
This study comprised a secondary analysis of data from a previous prospective, observational cohort study, which aimed to investigate the association of genetic and clinical data with mortality and patient-centered clinical outcomes in individuals diagnosed with sepsis and septic shock. The study population consisted of 737 patients with clinically defined sepsis that were enrolled from three anesthesiologic ICUs at University Medical Center Goettingen, Germany, between 2012 and 2019. The cohort comprised individuals from various settings, including patients with severe infections in the community, patients with extended medical treatment on general wards (e.g., pneumonia, NSTEMI, and coronary heart disease), and postoperative patients from diverse surgical disciplines. The surgical cases involved a wide range of conditions, including general surgery (cholecystitis, esophageal rupture, mediastinitis, perforated appendicitis, necrotizing pancreatitis, ileus, etc.), trauma surgery (polytrauma and periprosthetic infections), thoracic, cardiac, and vascular surgery (aortocoronary bypass, valve surgeries, etc.), neurosurgery (intracranial bleeding, spondylodiscitis, etc.), and ear, nose, and throat (ENT) surgery (retropharyngeal abscess, tongue base cancer, and hypopharynx cancer).
Continuous patient enrollment adhered to the most up-to-date internationally recognized consensus guidelines and definitions for sepsis and septic shock at this time [1,14]. Eligible patients were identified and monitored for a maximum duration of 28 days, unless they were discharged or experienced mortality prior to that period. The assessment of mortality rates was conducted through telephone follow-up or written request from the local registry, at the 28- and 90-day timepoints.
Previously described exclusion criteria were applied [15,16,17,18,19,20,21,22]:
- -
- Age below 18 years;
- -
- Pregnancy or breastfeeding;
- -
- Immunosuppressive drugs and/or chemotherapy within six months prior to enrollment;
- -
- History of myocardial infarction within six weeks before recruitment;
- -
- New York Heart Association stage IV chronic heart failure;
- -
- Human immunodeficiency virus (HIV) infection and/or hepatitis B/C infection;
- -
- End-stage incurable disease;
- -
- Persistent vegetative state (apallic syndrome);
- -
- “Do Not Treat” or “Do Not Resuscitate” order;
- -
- Participation in interventional studies;
- -
- Family member of a study-site employee.
Eligible patients were categorized according to their recent surgical history into surgical patients (including elective and emergency surgery) and non-surgical patients that did not undergo any type of surgical intervention. Surgical history included cardiac and non-cardiac surgery, including neurosurgery.
2.2. Data Collection
All clinical and patient baseline data were extracted from the electronic patient record system, specifically the IntelliSpace Critical Care and Anesthesia (ICCA) software developed by Philips Healthcare, Andover, MA, USA. The data collection was performed using standardized clinical report forms (CRFs).
Upon enrollment, pertinent baseline characteristics were collected, including comorbidities, preexisting medication, as well as the initial Sequential Organ Failure Assessment (SOFA) and Acute Physiology and Chronic Health Evaluation (APACHE II) scores.
Patients were subsequently monitored for a period of 90 days, with 28- and 90-day mortality being recorded as the primary outcome parameters.
Throughout the initial 28 days following sepsis onset, significant clinical data were generated including SOFA score-relevant organ dysfunction variables, information about organ replacement therapies, and inflammatory, kidney, and liver values.
2.3. Statistical Analysis
For the statistical analysis of the present study STATISTICA 13 software (version 13.0, StatSoft, Tulsa, OK, USA) was used. A p-value < 0.05 was considered statistically significant. For the Kaplan–Meier survival analysis, a log-rank test was applied. Continuous variables were analyzed using the Mann–Whitney U-Test, whereas categorical variables were assessed using either Pearson’s chi-square-test or two-sided Fisher’s exact test, if applicable. Categorical variables are presented as absolute numbers or percentages and continuous variables are presented as mean ± standard deviation or median and interquartile ranges, respectively.
To adjust for the effect of confounders on survival, multivariate Cox regression analyses were conducted. They were divided into one analysis involving relevant epidemiologic baseline characteristics and parameters that differed significantly between the two groups at baseline and another analysis that also included time-varying covariates that differed during observation.
3. Results
3.1. Demographics and Patient Baseline Characteristics
For this study, a total of 737 patients were enrolled. At baseline, the average age of the study population was 63 years. Two thirds (66%) of them were male, and the average Body Mass Index (BMI) was 28. More than half of the patients (51%) were in septic shock during the observation.
The patients were categorized into a surgical sepsis group (n = 582) and a non-surgical sepsis group (n = 155). At baseline, significant differences between the two groups were observed in terms of BMI (27 ± 6 vs. 30 ± 10 kg/m2, p = 0.0354), initial severity scores, the prevalence of common comorbidities, preexisting medication, and the primary site of infection (p < 0.001).
Non-surgical patients exhibited significantly higher day 1 SOFA (11 ± 4 vs. 9 ± 4, p < 0.001) and APACHE II scores (23 ± 7 vs. 21 ± 7, p < 0.001) compared to surgical sepsis patients.
The prevalence of chronic obstructive pulmonary disease (COPD; 21% vs. 13%, p = 0.0208) and bronchial asthma (5% vs. 2%, p = 0.0136) was higher in the non-surgical cohort, whereas cancer was more frequent in the surgical cohort (16% vs. 7%, p = 0.0062).
Regarding the preexisting medication, surgical patients had a higher rate of statin use compared to non-surgical patients (25% vs. 17%, p = 0.0268).
All presented results can be obtained from Table 1.

Table 1.
Patient baseline characteristics with regard to surgical history.
3.2. Survival Analysis
The conducted Kaplan–Meier survival analyses showed similar trends. The survival analysis conducted for the 28-day observation period indicated a higher survival rate for patients who underwent surgery (80% vs. 74%). However, this result did not reach statistical significance (p = 0.0554, Figure 1).

Figure 1.
Kaplan–Meier 28-day survival analysis with regard to surgical history.
For the 90-day observation period, surgical sepsis patients exhibited a favorable survival rate of 70% compared to non-surgical patients with a survival rate of only 63% (p = 0.0457, Figure 2).
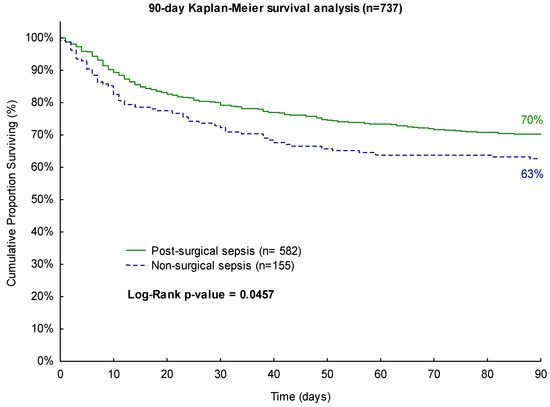
Figure 2.
Kaplan–Meier 90-day survival analysis with regard to surgical history.
3.3. Disease Severity Analysis
Non-surgical patients demonstrated a significantly higher sepsis disease severity compared to surgical patients, as indicated by several objective measures (Table 2). This included higher average SOFA scores (p < 0.001), a longer duration in septic shock (p = 0,0285), elevated levels of serum Procalcitonin (p = 0.0102), and a greater utilization of organ replacement therapies, such as ventilation (p < 0.001), vasopressor treatment (p < 0.001), and dialysis (p = 0.0364).

Table 2.
Disease severity with regard to surgical history.
Additionally, non-surgical sepsis patients exhibited higher organ-specific SOFA respiratory (p < 0.001), cardiovascular (p < 0.001), renal (p < 0.001), coagulation (p = 0.0335) and central nervous system (p = 0.0206) subscores.
Regarding their renal functions, non-surgical patients presented higher serum creatinine values (p < 0.001), lower urin outputs per day (p < 0.001), and per kg body weight per hour (p < 0.001).
With an average of 41 compared to 32 days, the hospital length of stay was longer in surgical patients compared to non-surgical sepsis patients (p < 0.001).
3.4. Multivariate Cox Proportional Hazards Regression Analysis
The multivariate Cox regression analyses for the 90-day and 28-day mortality, including variables that varied at baseline, revealed that age (p < 0.001) and the initial SOFA score (p < 0.001) significantly impacted both 90-day and 28-day mortality (Table 3). BMI only had a significant impact on 28-day mortality (HR = 0.97; 95%-CI: 0.94–1; p = 0.0243) but did not reach statistical significance for 90-day mortality. History of cancer significantly affected 90-day mortality (HR = 1.44; 95%-CI = 1.01–2.03; p = 0.0418).

Table 3.
Multivariate Cox proportional hazards regression analysis, including variables that varied as baseline.
Prior surgical intervention emerged as a significant prognostic variable for 90-day mortality (p = 0.0428) with an adjusted hazard ration of 0.73 (95%-CI = 0.53–0.99).
The multivariate Cox regression model, including parameters that differed between the two investigated groups at baseline as well as time-varying covariates that differed during observation, revealed the following results (Table 4): Age (p < 0.001), SOFA day 1 (p < 0.001), medical history of cancer (90-day mortality: p < 0.001, 28-day mortality: 0.0229), hospital length of stay (p < 0.001), SOFA cardiovascular subscore (90-day mortality: p < 0.001, 28-day mortality: 0.0092), SOFA central nervous system subscore (p < 0.001), SOFA renal score (90-day mortality: p = 0.0032, 28-day mortality: 0.018), and the fraction of dialysis days during observation days (90-day mortality: p < 0.001, 28-day mortality: 0.0143) significantly impacted either 28- and 90-day mortality. The SOFA respiratory subscore (p = 0.022) only had a significant impact on 90-day mortality, whereas BMI (p = 0.0421) and days in septic shock (p = 0.0494) were shown to be relevant prognostic factors for 28-day mortality.

Table 4.
Multivariate Cox proportional hazards regression analysis including time-varying covariates.
Surgical intervention was neither shown to be a relevant predictor for the 90-day mortality (HR = 1.08; 95%-CI = 0.78–1.5; p = 0.6321) nor the 28-day mortality (HR = 0.98; 95%-CI = 0.64–1.47; p = 0.9052) in the multivariate analysis, including time-varying covariates.
4. Discussion
This study aimed to investigate differences in mortality and sepsis-associated organ disfunction between surgical and non-surgical patients with sepsis.
The primary finding of this investigation was that non-surgical patients had a significantly higher 90-day mortality rate (37%) compared to surgical sepsis patients (30%, p = 0.0457). After adjusting for relevant confounders in the multivariate Cox regression analysis, surgical intervention remained an independent prognostic variable for 90-day survival (HR 0.73, 95%-CI 0.53–0.99, p = 0.0428), considering variables that varied between the two investigated groups at baseline. This result could not be replicated in the following multivariate analysis that also included time-varying covariates that differed during observation. This result suggests that the above-mentioned time-varying covariates might be confounding or mediating the relationship between baseline variables and 28- and 90-day survival.
Regarding the secondary endpoints, it was shown that non-surgical patients exhibited a significantly higher disease severity, as indicated by general inflammatory values (i.e., serum Procalcitonin) and specific parameters for nearly every major organ system (respiratory, cardiovascular, renal, and central nervous system). However, hospital length of stay was increased in surgical sepsis patients.
Our results suggest a potentially worse prognosis of non-surgical patients in terms of 90-day survival and sepsis-associated disease severity compared to surgical patients. These findings may be attributed to differences in baseline conditions such as comorbidities or preexisting medications (i.e., BMI, COPD, bronchial asthma, and use of statin therapy in our study population).
These findings align with the assumption that medical sepsis patients often present with severe chronic comorbidities, whereas surgeons are less likely to operate on severely debilitated patients with high perioperative morbidity and mortality risk [23]. Additionally, early diagnosis and treatment of sepsis, which is of pivotal importance for patient outcomes, may be limited in non-surgical patients. They frequently present to the hospital at a later stage of the disease, whereas surgical patients are often extensively evaluated preoperatively, admitted to the hospital beforehand, and sepsis often manifests during their clinical stay post-intervention [8,24].
Furthermore, differences in the primary site of infection between the two groups should be considered as a potential influencing factor in the study’s results. Pulmonary infections were more common in non-surgical patients, whereas abdominal and surgical-site infections were more frequent in surgical patients. Previous studies have reported the prognostic role of the primary site of infection, with pulmonary infections associated with worse hospital mortality and adverse patient-centered outcomes compared to other infection types [25,26,27].
This study has limitations. It is a single-center investigation conducted at a large university medical center in Germany. The generalizability of the results would have been improved by conducting the investigation in an international multi-center design. Although the data were collected prospectively, the analyses performed were carried out in a retrospective study design. Additionally, we acknowledge the potential impact of unmeasured variables on the observed outcomes, such as the specific type and timing of surgical interventions, as well as variations in clinical management protocols. Furthermore, we must acknowledge certain limitations arising from the absence of analyses pertaining to source control and the etiology of sepsis. While our investigation focused on comparing outcomes and characteristics between surgical and non-surgical sepsis patients, we did not investigate the specific management of infection sources or identify the causative pathogens responsible for sepsis in our cohorts. Due to the inherent heterogeneity typical of sepsis cohorts, analyses of the individual reasons for intervention and patient-specific type of surgery could not be conducted in this study.
Nevertheless, we consider our findings to be important as investigations of differences in sepsis outcome between surgical and non-surgical patients are scarce. Our results underscore the need to consider patient-individual factors, such as recent surgical intervention or baseline medical condition, in sepsis treatment and care. Clear differentiation between sepsis endotypes and risk stratification based on recent surgical history are crucial in clinical decision-making and patient care.
Author Contributions
Conceptualization, C.M., B.B., J.H., M.Q. and A.M.; Data curation, C.M., J.R., C.B. and J.H.; Formal analysis, C.M., J.R., C.B., M.N. and A.M.; Funding acquisition, J.H., M.Q. and A.M.; Investigation, C.M., J.R., C.B., M.Q. and A.M.; Methodology, C.M., B.B., J.H. and A.M.; Project administration, C.M., B.B., M.N. and A.M.; Resources, B.B., M.N., J.H., M.Q. and A.M.; Software, J.H.; Supervision, J.H. and A.M.; Validation, B.B. and J.H.; Visualization, C.M., J.R. and C.B.; Writing—original draft, C.M., J.R., C.B. and A.M.; Writing—review and editing, B.B., M.N., J.H. and M.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Volkswagen Stiftung, grant number ZN3168. We acknowledge support from the Open Access Publication Funds of Goettingen University.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of the University of Goettingen, Germany (protocol code 1/15/12).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent was obtained from the patients or legal representatives to publish this paper.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Markwart, R.; Saito, H.; Harder, T.; Tomczyk, S.; Cassini, A.; Fleischmann-Struzek, C.; Reichert, F.; Eckmanns, T.; Allegranzi, B. Epidemiology and Burden of Sepsis Acquired in Hospitals and Intensive Care Units: A Systematic Review and Meta-Analysis. Intensive Care Med. 2020, 46, 1536–1551. [Google Scholar] [CrossRef]
- Fleischmann-Struzek, C.; Rose, N.; Freytag, A.; Spoden, M.; Prescott, H.C.; Schettler, A.; Wedekind, L.; Ditscheid, B.; Storch, J.; Born, S.; et al. Epidemiology and Costs of Postsepsis Morbidity, Nursing Care Dependency, and Mortality in Germany, 2013 to 2017. JAMA Netw. Open 2021, 4, e2134290. [Google Scholar] [CrossRef]
- Gaieski, D.F.; Edwards, J.M.; Kallan, M.J.; Carr, B.G. Benchmarking the Incidence and Mortality of Severe Sepsis in the United States. Crit. Care Med. 2013, 41, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.M.; Evans, L.E.; Rhodes, A. The Surviving Sepsis Campaign Bundle: 2018 Update. Intensive Care Med. 2018, 44, 925–928. [Google Scholar] [CrossRef] [PubMed]
- Varon, J.; Baron, R.M. Sepsis Endotypes: The Early Bird Still Gets the Worm. eBioMedicine 2022, 76, 103832. [Google Scholar] [CrossRef] [PubMed]
- Cohen, N.S.; Bock, J.M.; May, A.K. Sepsis and Postoperative Surgical Site Infections. Surgery 2023, 174, 403–405. [Google Scholar] [CrossRef]
- Vogel, T.R.; Dombrovskiy, V.Y.; Lowry, S.F. Trends in Postoperative Sepsis: Are We Improving Outcomes? Surg. Infect 2009, 10, 71–78. [Google Scholar] [CrossRef]
- Chen, P.-Y.; Luo, C.-W.; Chen, M.-H.; Yang, M.-L.; Kuan, Y.-H. Epidemiological Characteristics of Postoperative Sepsis. Open Med. 2019, 14, 928–938. [Google Scholar] [CrossRef]
- Rhee, C.; Dantes, R.; Epstein, L.; Murphy, D.J.; Seymour, C.W.; Iwashyna, T.J.; Kadri, S.S.; Angus, D.C.; Danner, R.L.; Fiore, A.E.; et al. Incidence and Trends of Sepsis in US Hospitals Using Clinical vs Claims Data, 2009–2014. JAMA 2017, 318, 1241–1249. [Google Scholar] [CrossRef]
- Sinapidis, D.; Kosmas, V.; Vittoros, V.; Koutelidakis, I.M.; Pantazi, A.; Stefos, A.; Katsaros, K.E.; Akinosoglou, K.; Bristianou, M.; Toutouzas, K.; et al. Progression into Sepsis: An Individualized Process Varying by the Interaction of Comorbidities with the Underlying Infection. BMC Infect. Dis. 2018, 18, 242. [Google Scholar] [CrossRef] [PubMed]
- Spoden, M.; Hartog, C.S.; Schlattmann, P.; Freytag, A.; Ostermann, M.; Wedekind, L.; Storch, J.; Reinhart, K.; Günster, C.; Fleischmann-Struzek, C. Occurrence and Risk Factors for New Dependency on Chronic Care, Respiratory Support, Dialysis and Mortality in the First Year After Sepsis. Front. Med. 2022, 9, 878337. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.M.; Fink, M.P.; Marshall, J.C.; Abraham, E.; Angus, D.; Cook, D.; Cohen, J.; Opal, S.M.; Vincent, J.-L.; Ramsay, G.; et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit. Care Med. 2003, 31, 1250–1256. [Google Scholar] [CrossRef]
- Mewes, C.; Runzheimer, J.; Böhnke, C.; Büttner, B.; Hinz, J.; Quintel, M.; Mansur, A. Association of Sex Differences with Mortality and Organ Dysfunction in Patients with Sepsis and Septic Shock. J. Pers. Med. 2023, 13, 836. [Google Scholar] [CrossRef] [PubMed]
- Hinz, J.; Büttner, B.; Kriesel, F.; Steinau, M.; Popov, A.F.; Ghadimi, M.; Beissbarth, T.; Tzvetkov, M.; Bergmann, I.; Mansur, A. The FER Rs4957796 TT Genotype Is Associated with Unfavorable 90-Day Survival in Caucasian Patients with Severe ARDS Due to Pneumonia. Sci. Rep. 2017, 7, 9887. [Google Scholar] [CrossRef] [PubMed]
- Kristof, K.; Büttner, B.; Grimm, A.; Mewes, C.; Schmack, B.; Popov, A.F.; Ghadimi, M.; Beissbarth, T.; Hinz, J.; Bergmann, I.; et al. Anaemia Requiring Red Blood Cell Transfusion Is Associated with Unfavourable 90-Day Survival in Surgical Patients with Sepsis. BMC Res. Notes 2018, 11, 879. [Google Scholar] [CrossRef]
- Mewes, C.; Böhnke, C.; Alexander, T.; Büttner, B.; Hinz, J.; Popov, A.-F.; Ghadimi, M.; Beißbarth, T.; Raddatz, D.; Meissner, K.; et al. Favorable 90-Day Mortality in Obese Caucasian Patients with Septic Shock According to the Sepsis-3 Definition. J. Clin. Med. 2019, 9, 46. [Google Scholar] [CrossRef]
- Mewes, C.; Büttner, B.; Hinz, J.; Alpert, A.; Popov, A.F.; Ghadimi, M.; Beissbarth, T.; Tzvetkov, M.; Shen-Orr, S.; Bergmann, I.; et al. The CTLA-4 Rs231775 GG Genotype Is Associated with Favorable 90-Day Survival in Caucasian Patients with Sepsis. Sci. Rep. 2018, 8, 15140. [Google Scholar] [CrossRef]
- Mewes, C.; Alexander, T.; Büttner, B.; Hinz, J.; Alpert, A.; Popov, A.-F.; Ghadimi, M.; Beißbarth, T.; Tzvetkov, M.; Grade, M.; et al. TIM-3 Genetic Variants Are Associated with Altered Clinical Outcome and Susceptibility to Gram-Positive Infections in Patients with Sepsis. Int. J. Mol. Sci. 2020, 21, 8318. [Google Scholar] [CrossRef]
- Mewes, C.; Alexander, T.; Büttner, B.; Hinz, J.; Alpert, A.; Popov, A.-F.; Beißbarth, T.; Tzvetkov, M.; Grade, M.; Quintel, M.; et al. Effect of the Lymphocyte Activation Gene 3 Polymorphism Rs951818 on Mortality and Disease Progression in Patients with Sepsis-A Prospective Genetic Association Study. J. Clin. Med. 2021, 10, 5302. [Google Scholar] [CrossRef] [PubMed]
- Runzheimer, J.; Mewes, C.; Büttner, B.; Hinz, J.; Popov, A.-F.; Ghadimi, M.; Kristof, K.; Beissbarth, T.; Schamroth, J.; Tzvetkov, M.; et al. Lack of an Association between the Functional Polymorphism TREM-1 Rs2234237 and the Clinical Course of Sepsis among Critically Ill Caucasian Patients–A Monocentric Prospective Genetic Association Study. J. Clin. Med. 2019, 8, 301. [Google Scholar] [CrossRef] [PubMed]
- Brakenridge, S.C.; Efron, P.A.; Cox, M.C.; Stortz, J.A.; Hawkins, R.B.; Ghita, G.; Gardner, A.; Mohr, A.M.; Anton, S.D.; Moldawer, L.L.; et al. Current Epidemiology of Surgical Sepsis: Discordance Between Inpatient Mortality and 1-Year Outcomes. Ann. Surg. 2019, 270, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Finks, J.F.; Osborne, N.H.; Birkmeyer, J.D. Trends in Hospital Volume and Operative Mortality for High-Risk Surgery. N. Engl. J. Med. 2011, 364, 2128–2137. [Google Scholar] [CrossRef]
- Motzkus, C.A.; Luckmann, R. Does Infection Site Matter? A Systematic Review of Infection Site Mortality in Sepsis. J. Intensive Care Med. 2017, 32, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Leligdowicz, A.; Dodek, P.M.; Norena, M.; Wong, H.; Kumar, A.; Kumar, A.; Co-operative Antimicrobial Therapy of Septic Shock Database Research Group. Association between Source of Infection and Hospital Mortality in Patients Who Have Septic Shock. Am. J. Respir. Crit. Care Med. 2014, 189, 1204–1213. [Google Scholar] [CrossRef]
- Stortz, J.A.; Cox, M.C.; Hawkins, R.B.; Ghita, G.L.; Brumback, B.A.; Mohr, A.M.; Moldawer, L.L.; Efron, P.A.; Brakenridge, S.C.; Moore, F.A. Phenotypic Heterogeneity by Site of Infection in Surgical Sepsis: A Prospective Longitudinal Study. Crit. Care 2020, 24, 203. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).