Effect of Leflunomide–Metal Complexes on ROS, TNF, and Brain Indolamines in Comparison with Anti-Depressants as Adjunct Therapy in Rheumatoid Arthritic Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Induction of Arthritis
2.3. Animal Groups
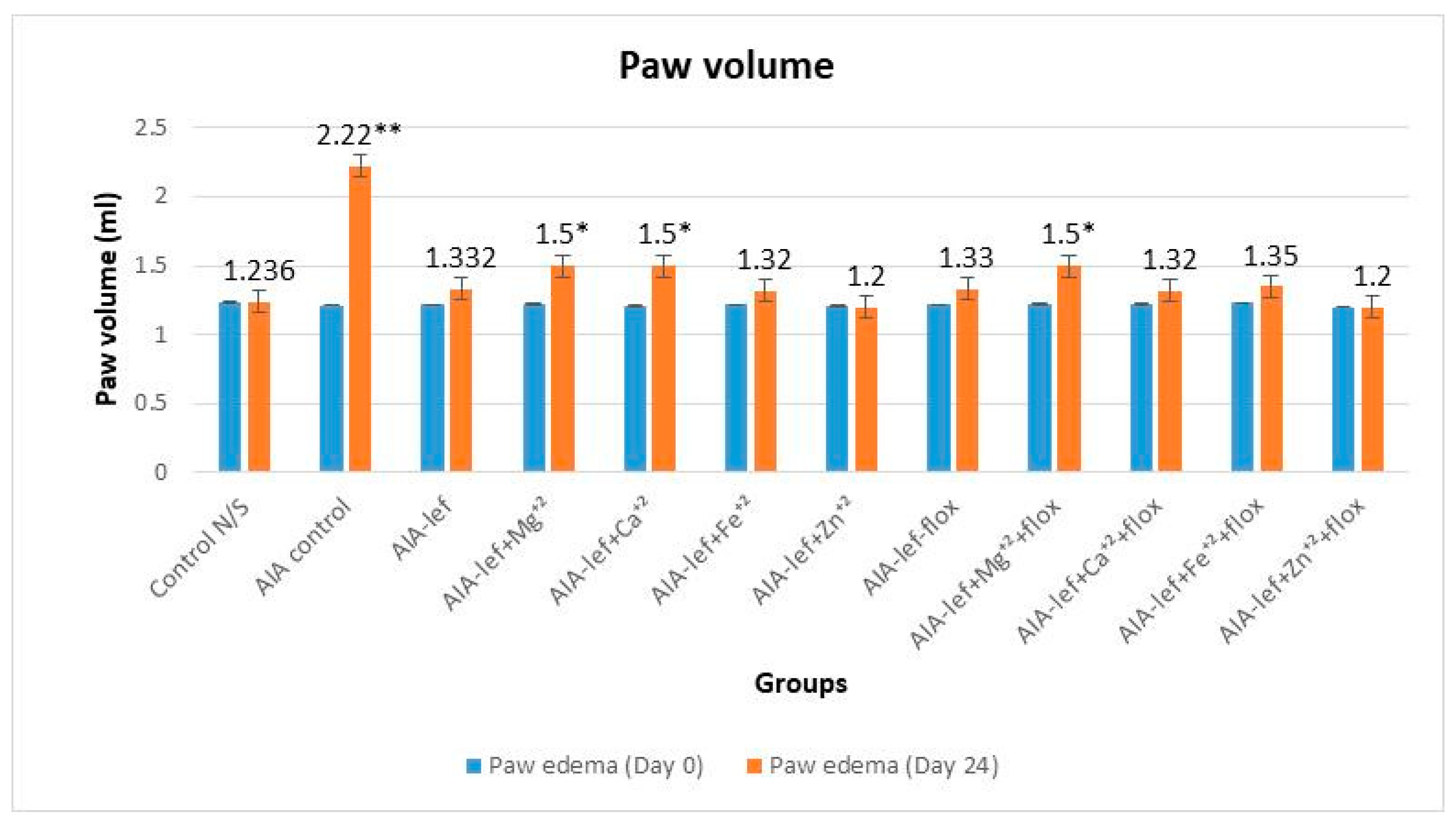
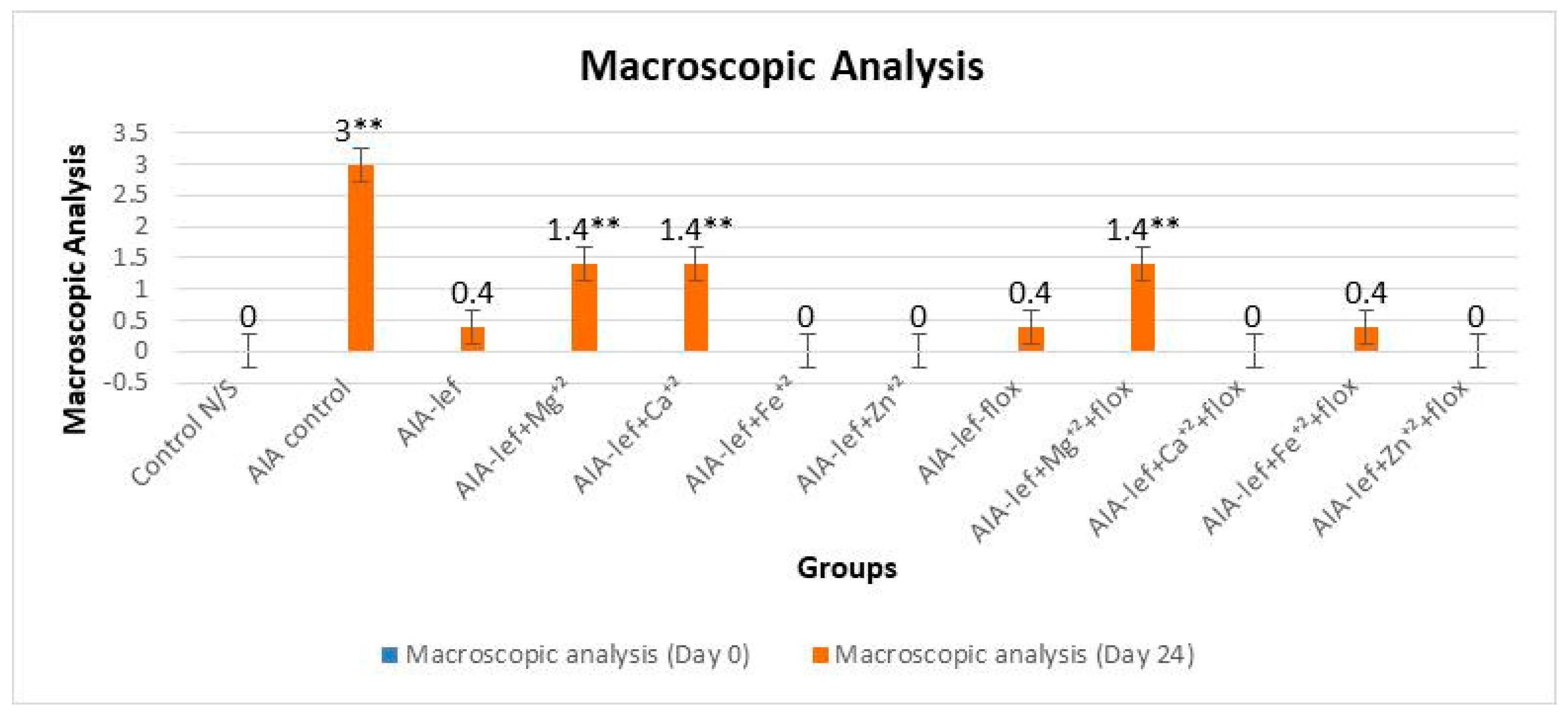
2.4. Anti-Inflammatory Activity
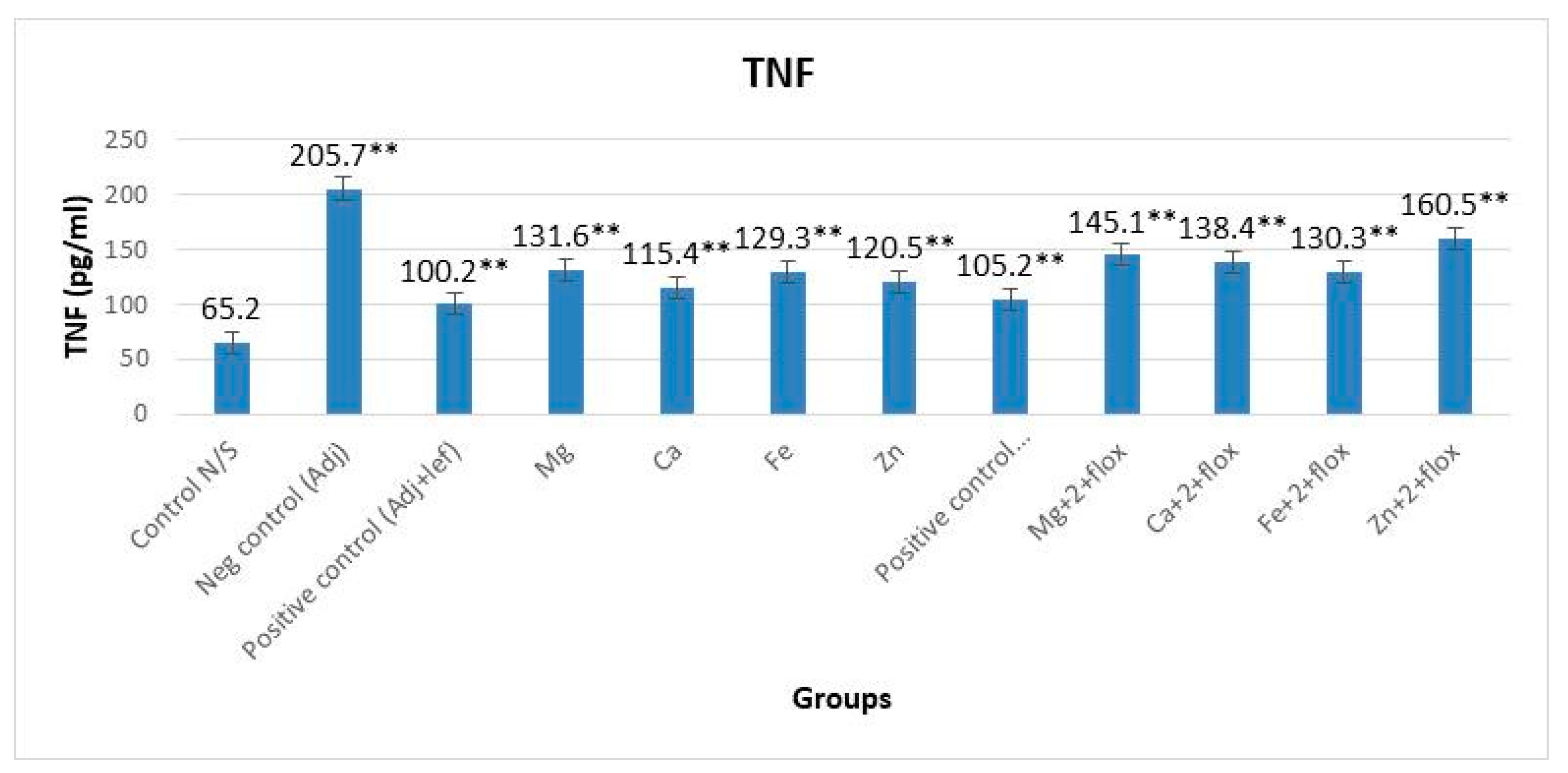
2.5. Analysis of TNF-α and ROS
2.6. Brain Dissection Technique
2.7. Extraction of Indolamines from the Brain
2.8. Estimation of Brain Indolamines
2.9. Statistical Analysis
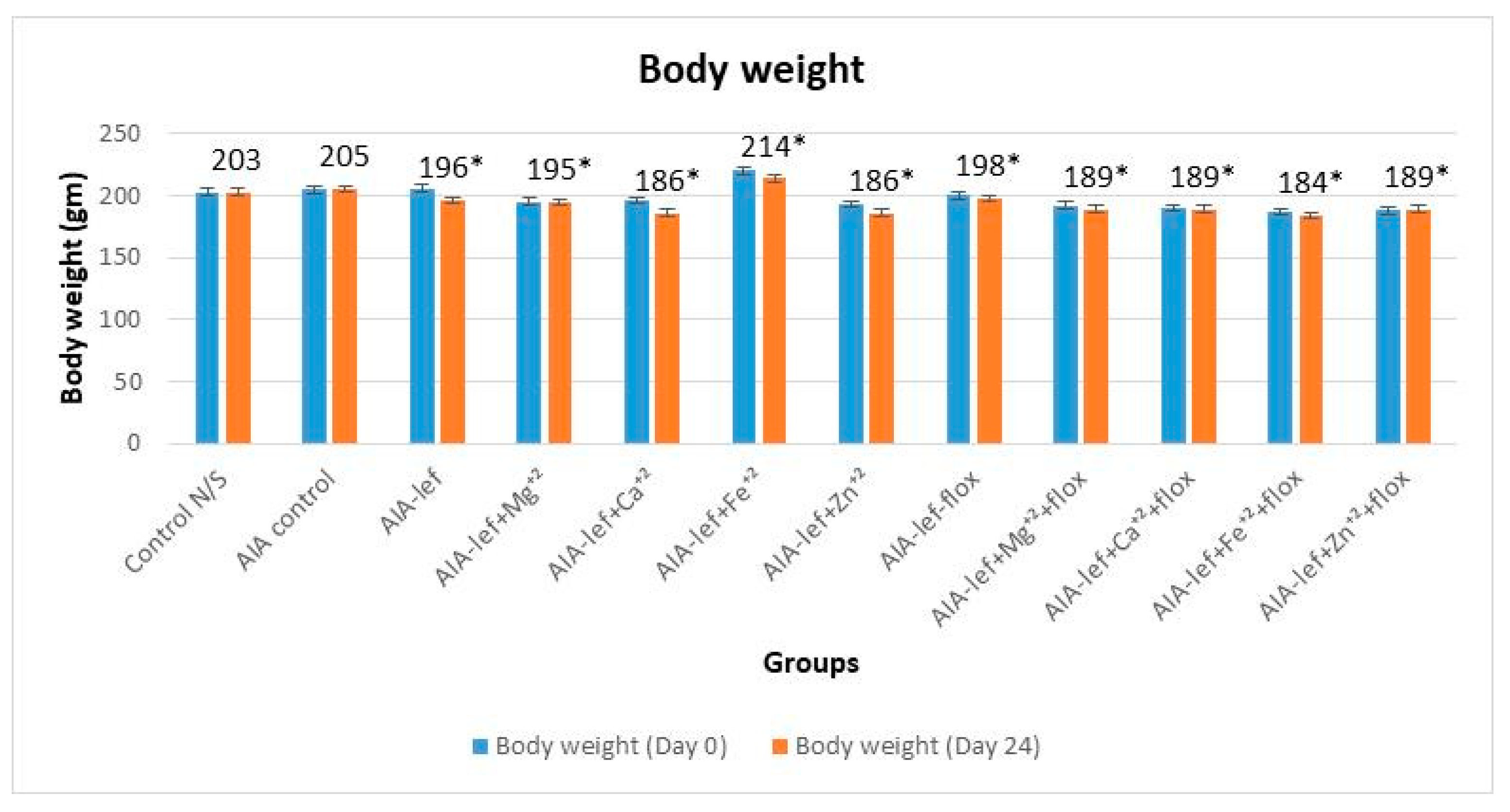
3. Results
3.1. TNF-α alongside and without Fluoxetine
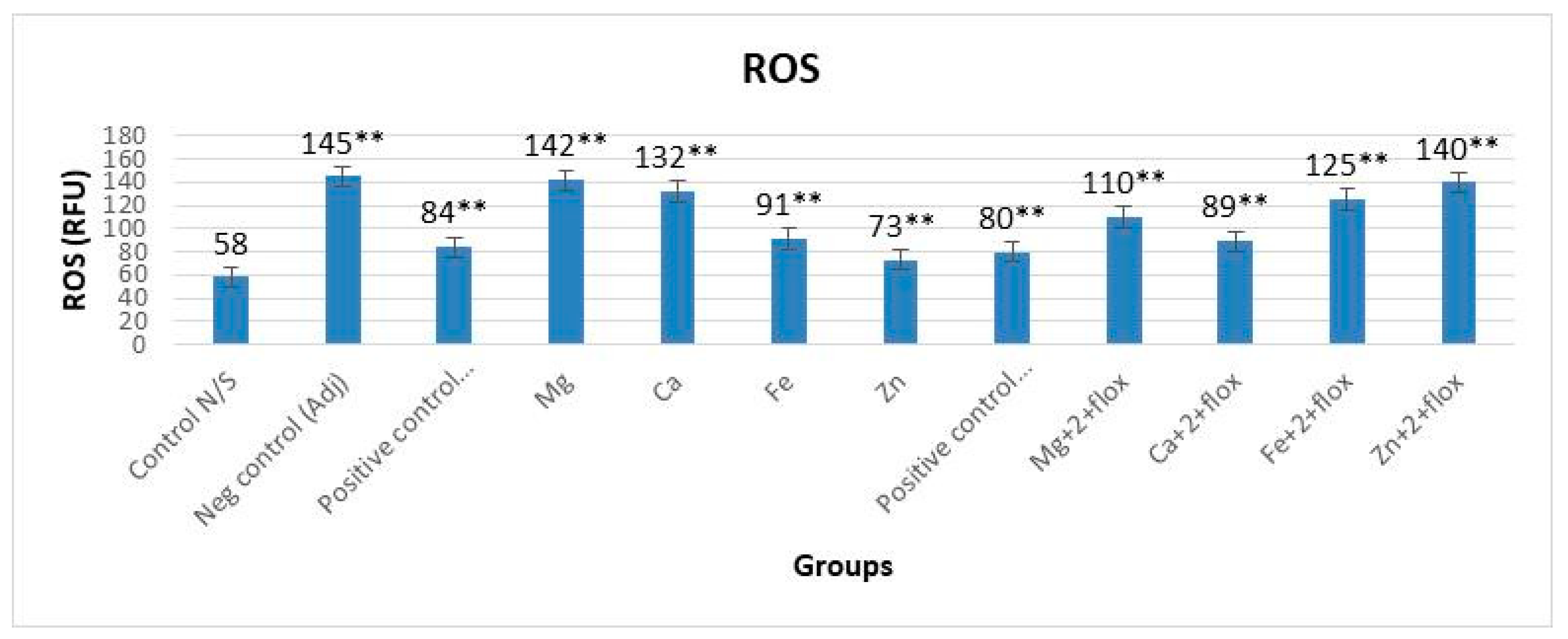
3.2. ROS with and without Fluoxetine
3.3. Indolamines Determination
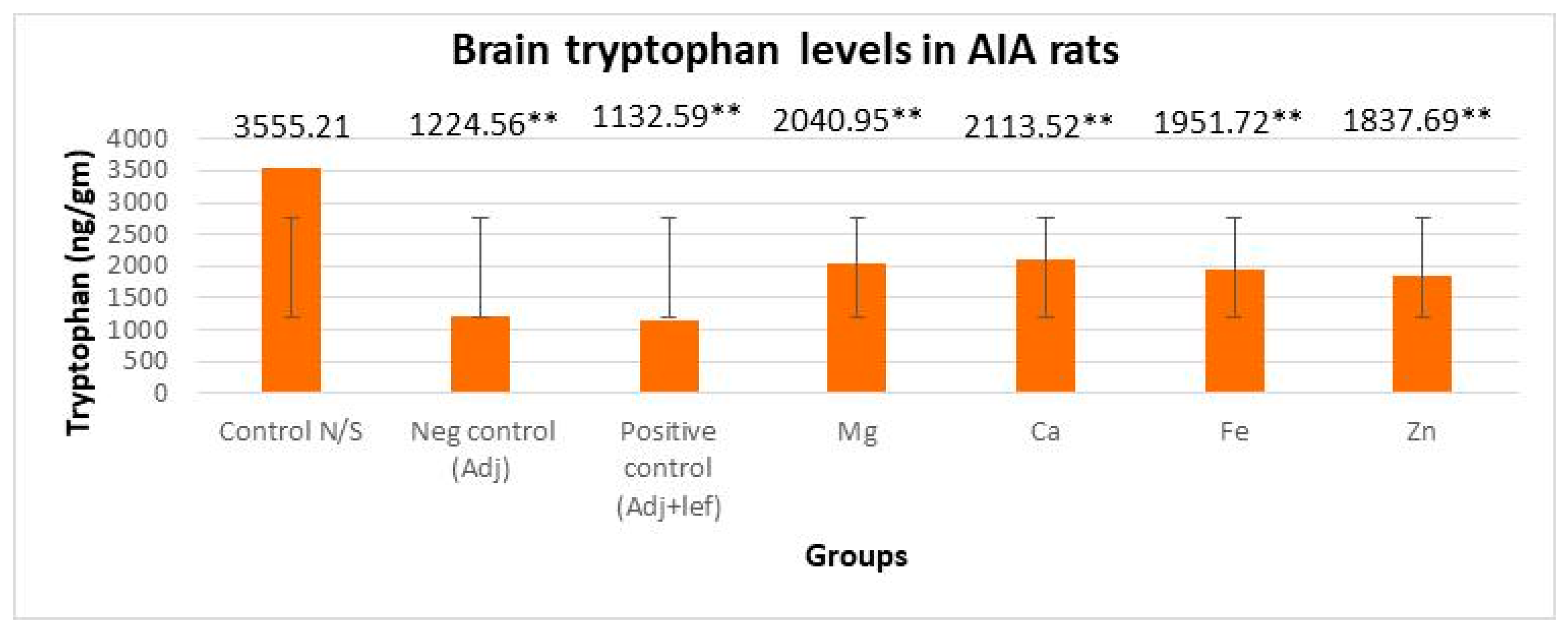
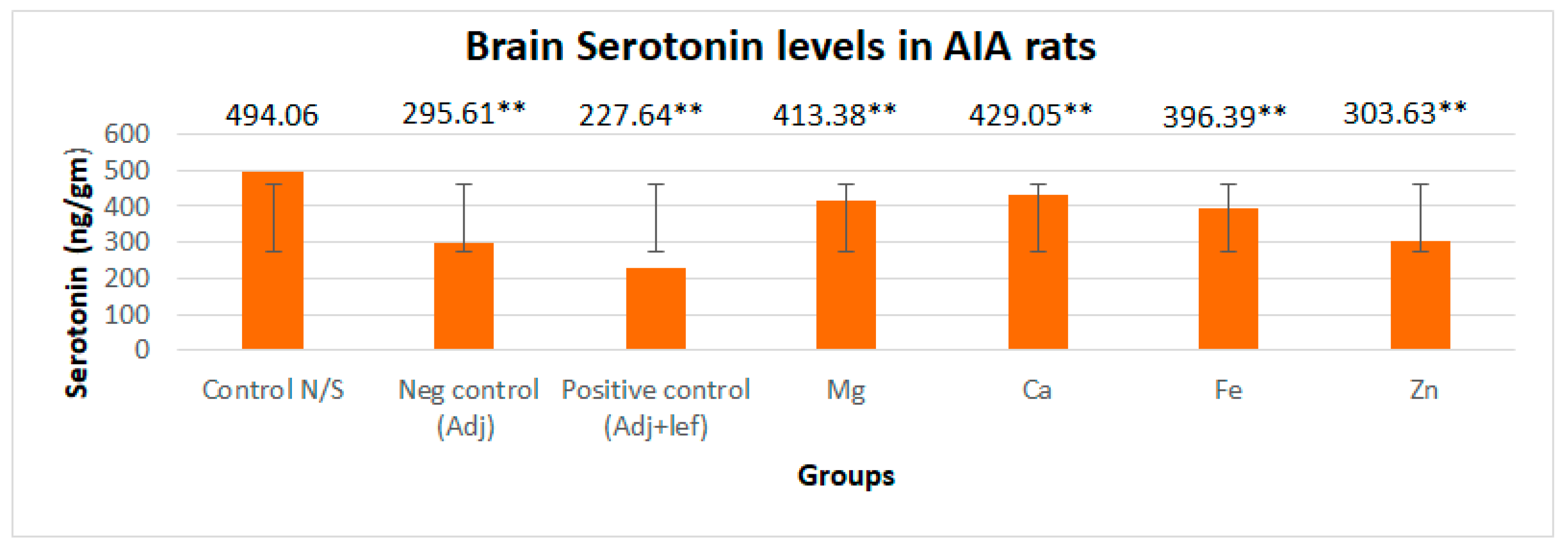
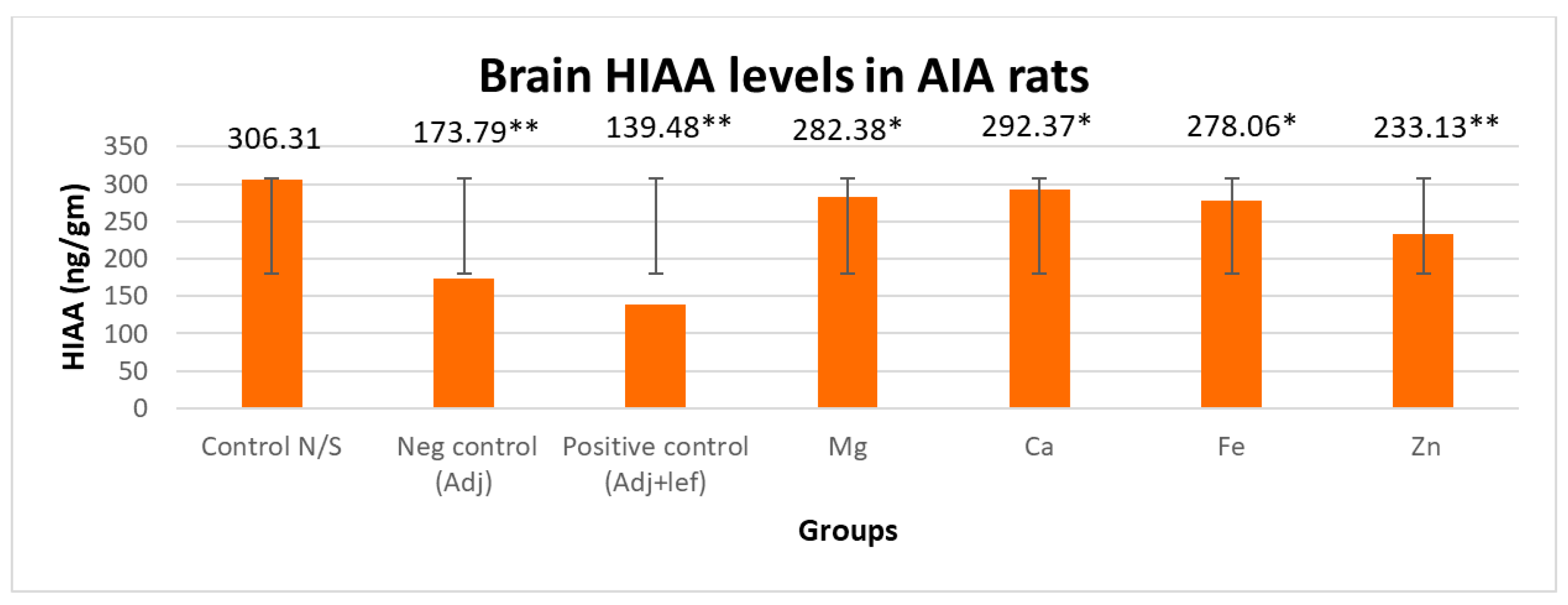
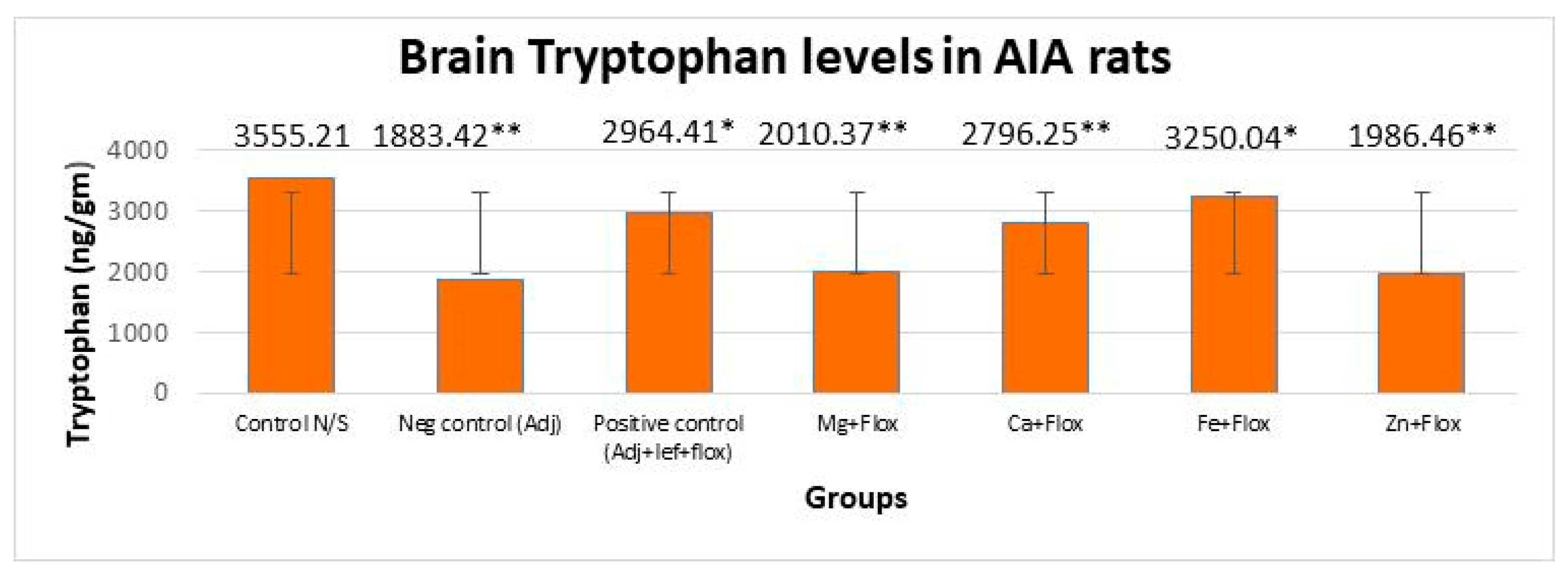
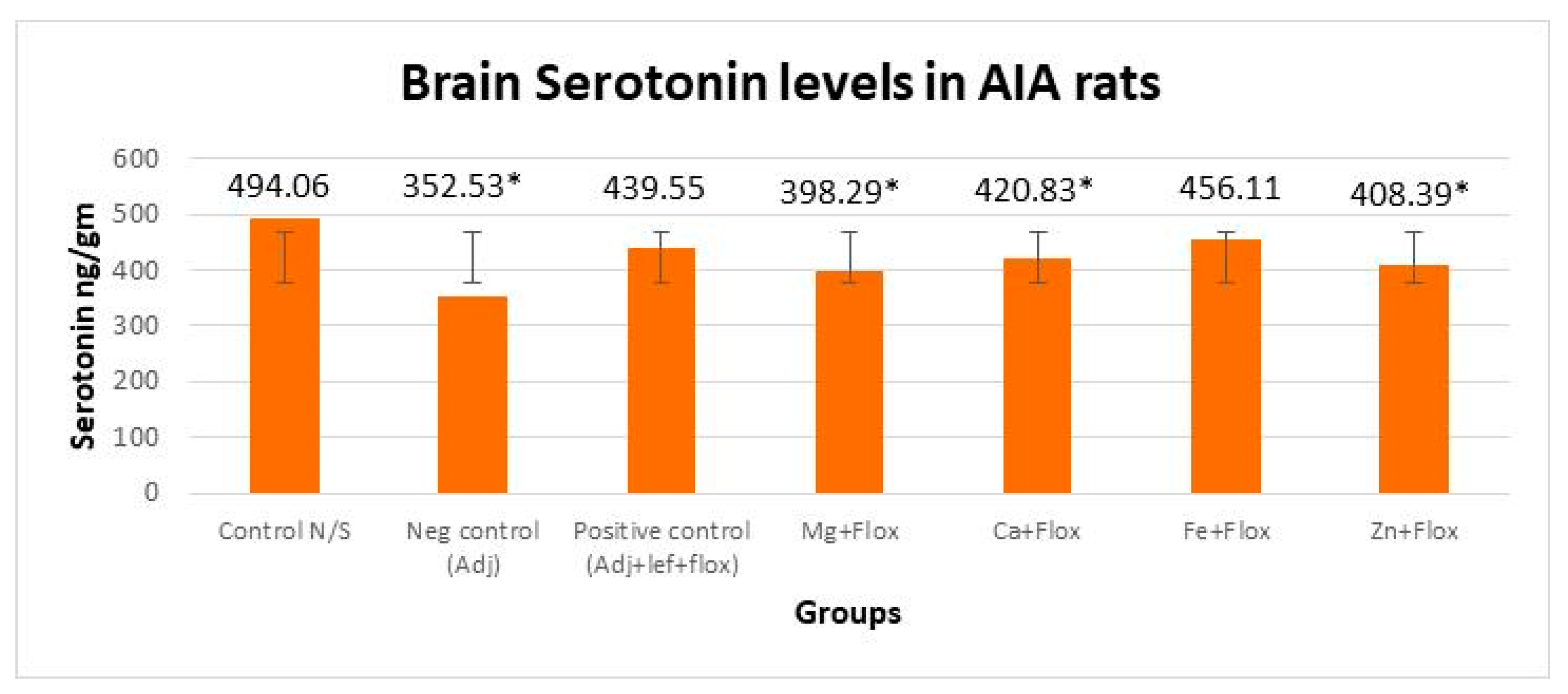
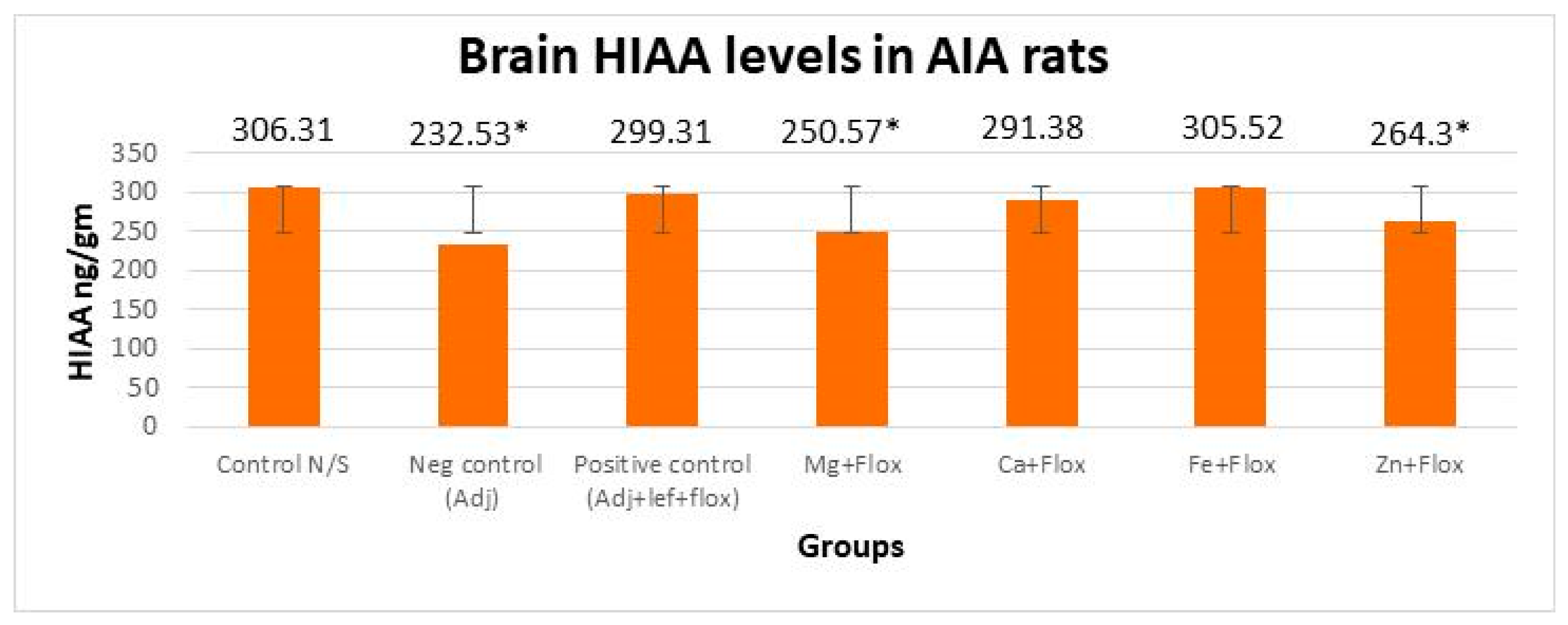
3.3.1. Drugs’ Effect on Brain Indolamines (Tryptophan, Serotonin, and Hydroxyindole Acetic Acid) without the Fluoxetine Group
3.3.2. Drugs’ Effect on Brain Indolamines (Tryptophan, Serotonin, and Hydroxyindole Acetic Acid) alongside the Fluoxetine Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gabriel, S.E.; Michaud, K. Epidemiological studies in incidence, prevalence, mortality, and comorbidity of the rheumatic diseases. Arthritis Res. Ther. 2009, 11, 229. [Google Scholar] [CrossRef]
- Akhter, E.; Bilal, S.; Haque, U. Prevalence of arthritis in India and Pakistan: A review. Rheumatol. Int. 2011, 31, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Ortona, E.; Pierdominici, M.; Maselli, A.; Veroni, C.; Aloisi, F.; Shoenfeld, Y. Sex-based differences in autoimmune diseases. Ann. Dell’istituto Super. Di Sanita 2016, 52, 205–212. [Google Scholar]
- Khan, M.M.; Sultana, N.; Arayne, M.S.; Saleem, M.D. Decreased Serotonin Level in Adjuvant Induced Arthritic rats. Int. J Toxicol. Pharmacol. Res. 2011, 3, 9–13. [Google Scholar]
- Simjee, S.U.; Jawed, H.; Quadri, J.; Saeed, S.A. Quantitative gait analysis as a method to assess mechanical hyperalgesia modulated by disease-modifying antirheumatoid drugs in the adjuvant-induced arthritic rat. Arthritis Res. Ther. 2007, 9, R91. [Google Scholar] [CrossRef]
- Yu, D.H.; Yi, J.K.; Yuh, H.S.; Park, S.; Kim, H.J.; Bae, K.B.; Ji, Y.R.; Kim, N.R.; Park, S.J.; Kim, D.H.; et al. Over-expression of extracellular superoxide dismutase in mouse synovial tissue attenuates the inflammatory arthritis. Exp. Mol. Med. 2012, 44, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Margaretten, M.; Julian, L.; Katz, P.; Yelin, E. Depression in patients with rheumatoid arthritis: Description, causes and mechanisms. Int. J. Clin. Rheumatol. 2011, 6, 617–623. [Google Scholar] [CrossRef]
- Malemud, C.J.; Miller, A.H. Pro-inflammatory cytokine-induced SAPK/MAPK and JAK/STAT in rheumatoid arthritis and the new anti-depression drugs. Expert Opin. Ther. Targets 2008, 12, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Dickens, C.; McGowan, L.; Clark-Carter, D.; Creed, F. Depression in rheumatoid arthritis: A systematic review of the literature with meta-analysis. Psychosom. Med. 2002, 64, 52–60. [Google Scholar] [CrossRef]
- Timonen, M.; Viilo, K.; Hakko, H.; Särkioja, T.; Ylikulju, M.; Meyer-Rochow, V.B.; Väisänen, E.; Räsänen, P. Suicides in persons suffering from rheumatoid arthritis. Rheumatology 2003, 42, 287–291. [Google Scholar] [CrossRef]
- Ang, D.C.; Choi, H.; Kroenke, K.; Wolfe, F. Comorbid depression is an independent risk factor for mortality in patients with rheumatoid arthritis. J. Rheumatol. 2005, 32, 1013–1019. [Google Scholar]
- Kurz, K.; Winkler, C.; Duftner, C.; Wirleitner, B.; Schirmer, M.; Fuchs, D. Tryptophan degradation increases with stage in patients with rheumatoid arthritis. Clin. Rheumatol. 2006, 25, 334–337. [Google Scholar] [CrossRef]
- Sultana, N.; Arayne, M. Synthesis, Characterization and Anti-inflammatory Activity of Metal Complexes of 5-methyl-N-[4-(trifluoromethyl) phenyl]-isoxazole-4-carboxamide on Carrageenan Induced Arthritic Rats. JSM Chem. 2014, 2, 1006. [Google Scholar]
- Arayne, M.; Sultana, N.; Khan, M.; Su, S. Leflunomide with Meloxicam on Progression of Rheumatoid Arthritis and its Associated Depression in AIA Rats. Clin. Pharmacol. Biopharm. 2014, 3, 1000114. [Google Scholar] [CrossRef]
- Jahan, N.K.M.; Naeem, A.; Mehjabben, A.F. Simultaneous effect of leflunomide with imipramine and fluoxetine in carrageenan induced acute arthritic model. Int. J. Biol. Pharm. Allied Sci. 2018, 7, 548–557. [Google Scholar]
- Zimmermann, M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Chauhan, P.; Singh, S. Evaluation of the anti-arthritic activity of Cinnamomum cassia bark extract in experimental models. Integr. Med. Res. 2018, 7, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A.; Qureshi, O.S.; Yu, C.H.; Akram, M.; Kim, H.S.; Kim, M.S.; Kang, J.H.; Majid, A.; Chang, S.Y.; Bae, O.N.; et al. Enhanced anti-rheumatic activity of methotrexate-entrapped ultradeformable liposomal gel in adjuvant-induced arthritis rat model. Int. J. Pharm. 2017, 525, 92–100. [Google Scholar] [CrossRef]
- Rao, K.; Aziz, S.; Roome, T.; Razzak, A.; Sikandar, B.; Jamali, K.S.; Imran, M.; Jabri, T.; Shah, M.R. Gum acacia stabilized silver nanoparticles based nano-cargo for enhanced anti-arthritic potentials of hesperidin in adjuvant induced arthritic rats. Artif. Cells Nanomed. Biotechnol. 2018, 46, 597–607. [Google Scholar] [CrossRef]
- van Eden, W.; Wagenaar-Hilbers, J.P.; Wauben, M.H. Adjuvant arthritis in the rat. Curr. Protoc. Immunol. 2001, 15, 15.14.1–15.14.8. [Google Scholar] [CrossRef]
- Best, T.M.; Fiebig, R.; Corr, D.T.; Brickson, S.; Ji, L. Free radical activity, antioxidant enzyme, and glutathione changes with muscle stretch injury in rabbits. J. Appl. Physiol. 1999, 87, 74–82. [Google Scholar] [CrossRef]
- Xian, Y.F.; Mao, Q.Q.; Ip, S.P.; Lin, Z.X.; Che, C.T. Comparison on the anti-inflammatory effect of Cortex Phellodendri Chinensis and Cortex Phellodendri Amurensis in 12-O-tetradecanoyl-phorbol-13-acetate-induced ear edema in mice. J. Ethnopharmacol. 2011, 137, 1425–1430. [Google Scholar] [CrossRef]
- Haleem, D.J.; Naz, H.; Parveen, T.; Haider, S.; Ahmed, S.P.; Khan, N.H.; Haleem, M.A. Serotonin and serotonin 1-A receptors in the failure of ethanol-treated rats to adapt to a repeated stress schedule. J. Stud. Alcohol 2002, 63, 389–396. [Google Scholar] [CrossRef]
- Abbas, G.; Naqvi, S.; Erum, S.; Ahmed, S.; Rahman, A.-u.; Dar, A. Potential Antidepressant Activity of Areca catechu Nut via Elevation of Serotonin and Noradrenaline in the Hippocampus of Rats. Phytother. Res. PTR 2012, 27, 39–45. [Google Scholar] [CrossRef]
- Vasanthi, P.; Nalini, G.; Rajasekhar, G. Role of tumor necrosis factor-alpha in rheumatoid arthritis: A review. APLAR J. Rheumatol. 2007, 10, 270–274. [Google Scholar] [CrossRef]
- Firestein, G.S. Evolving concepts of rheumatoid arthritis. Nature 2003, 423, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Li, J.; Guo, L.; Yu, X.; Wu, D.; Luo, L.; Zhu, L.; Chen, W.; Chen, C.; Ye, C.; et al. Activation of NALP1 inflammasomes in rats with adjuvant arthritis; a novel therapeutic target of carboxyamidotriazole in a model of rheumatoid arthritis. Br. J. Pharmacol. 2015, 172, 3446–3459. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.; Wanchu, A.; Bhatnagar, A. Interaction between oxidative stress and chemokines: Possible pathogenic role in systemic lupus erythematosus and rheumatoid arthritis. Immunobiology 2011, 216, 1010–1017. [Google Scholar] [CrossRef]
- Kamanli, A.; Naziroglu, M.; Aydilek, N.; Hacievliyagil, C. Plasma lipid peroxidation and antioxidant levels in patients with rheumatoid arthritis. Cell Biochem. Funct. 2004, 22, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Seven, A.; Guzel, S.; Aslan, M.; Hamuryudan, V. Lipid, protein, DNA oxidation and antioxidant status in rheumatoid arthritis. Clin. Biochem. 2008, 41, 538–543. [Google Scholar] [CrossRef]
- Freire-Garabal, M.; Nunez, M.J.; Pereiro, D.; Riveiro, P.; Losada, C.; Fernandez-Rial, J.C.; Garcia-Iglesias, E.; Prizmic, J.; Mayan, J.M.; Rey-Mendez, M. Effects of fluoxetine on the development of lung metastases induced by operative stress in rats. Life Sci. 1998, 63, PL31–PL38. [Google Scholar] [CrossRef] [PubMed]
- Nunez, M.J.; Novio, S.; Suarez, J.A.; Balboa, J.; Freire-Garabal, M. Effects of psychological stress and fluoxetine on development of oral candidiasis in rats. Clin. Vaccine Immunol. CVI 2010, 17, 668–673. [Google Scholar] [CrossRef]
- Freire-Garabal, M.; Nunez, M.J.; Riveiro, P.; Balboa, J.; Lopez, P.; Zamorano, B.G.; Rodrigo, E.; Rey-Mendez, M. Effects of fluoxetine on the activity of phagocytosis in stressed mice. Life Sci. 2002, 72, 173–183. [Google Scholar] [CrossRef]
- Nunez, M.J.; Balboa, J.; Rodrigo, E.; Brenlla, J.; Gonzalez-Peteiro, M.; Freire-Garabal, M. Effects of fluoxetine on cellular immune response in stressed mice. Neurosci. Lett. 2006, 396, 247–251. [Google Scholar] [CrossRef]
- Zafir, A.; Banu, N. Antioxidant potential of fluoxetine in comparison to Curcuma longa in restraint-stressed rats. Eur. J. Pharmacol. 2007, 572, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Zafir, A.; Ara, A.; Banu, N. Invivo antioxidant status: A putative target of antidepressant action. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2009, 33, 220–228. [Google Scholar] [CrossRef]
- Galecki, P.; Szemraj, J.; Bienkiewicz, M.; Florkowski, A.; Galecka, E. Lipid peroxidation and antioxidant protection in patients during acute depressive episodes and in remission after fluoxetine treatment. Pharmacol. Rep. PR 2009, 61, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Young, S.N.; Smith, S.E.; Pihl, R.O.; Ervin, F.R. Tryptophan depletion causes a rapid lowering of mood in normal males. Psychopharmacology 1985, 87, 173–177. [Google Scholar] [CrossRef]
- Bell, C.; Abrams, J.; Nutt, D. Tryptophan depletion and its implications for psychiatry. Br. J. Psychiatry J. Ment. Sci. 2001, 178, 399–405. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naeem, A.; Jahan, N.; Khan, M.M.; Abbas, G.; Siddiqui, F.; Khalid, M.U.; Farooqui, W.A. Effect of Leflunomide–Metal Complexes on ROS, TNF, and Brain Indolamines in Comparison with Anti-Depressants as Adjunct Therapy in Rheumatoid Arthritic Model. Biomedicines 2023, 11, 2214. https://doi.org/10.3390/biomedicines11082214
Naeem A, Jahan N, Khan MM, Abbas G, Siddiqui F, Khalid MU, Farooqui WA. Effect of Leflunomide–Metal Complexes on ROS, TNF, and Brain Indolamines in Comparison with Anti-Depressants as Adjunct Therapy in Rheumatoid Arthritic Model. Biomedicines. 2023; 11(8):2214. https://doi.org/10.3390/biomedicines11082214
Chicago/Turabian StyleNaeem, Almas, Noor Jahan, Moona Mehboob Khan, Ghulam Abbas, Faheema Siddiqui, Muhammad Usaid Khalid, and Waqas Ahmed Farooqui. 2023. "Effect of Leflunomide–Metal Complexes on ROS, TNF, and Brain Indolamines in Comparison with Anti-Depressants as Adjunct Therapy in Rheumatoid Arthritic Model" Biomedicines 11, no. 8: 2214. https://doi.org/10.3390/biomedicines11082214
APA StyleNaeem, A., Jahan, N., Khan, M. M., Abbas, G., Siddiqui, F., Khalid, M. U., & Farooqui, W. A. (2023). Effect of Leflunomide–Metal Complexes on ROS, TNF, and Brain Indolamines in Comparison with Anti-Depressants as Adjunct Therapy in Rheumatoid Arthritic Model. Biomedicines, 11(8), 2214. https://doi.org/10.3390/biomedicines11082214






