Disturbed Antioxidant Capacity in Patients with Systemic Sclerosis Associates with Lung and Gastrointestinal Symptoms
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Patients, and Controls
2.2. Measurement of Oxidative Stress Markers in the Systemic Circulation
2.3. COMET Assay
2.4. Statistical Analysis and Clinical Significance
3. Results
3.1. Characteristics of SSc Patients
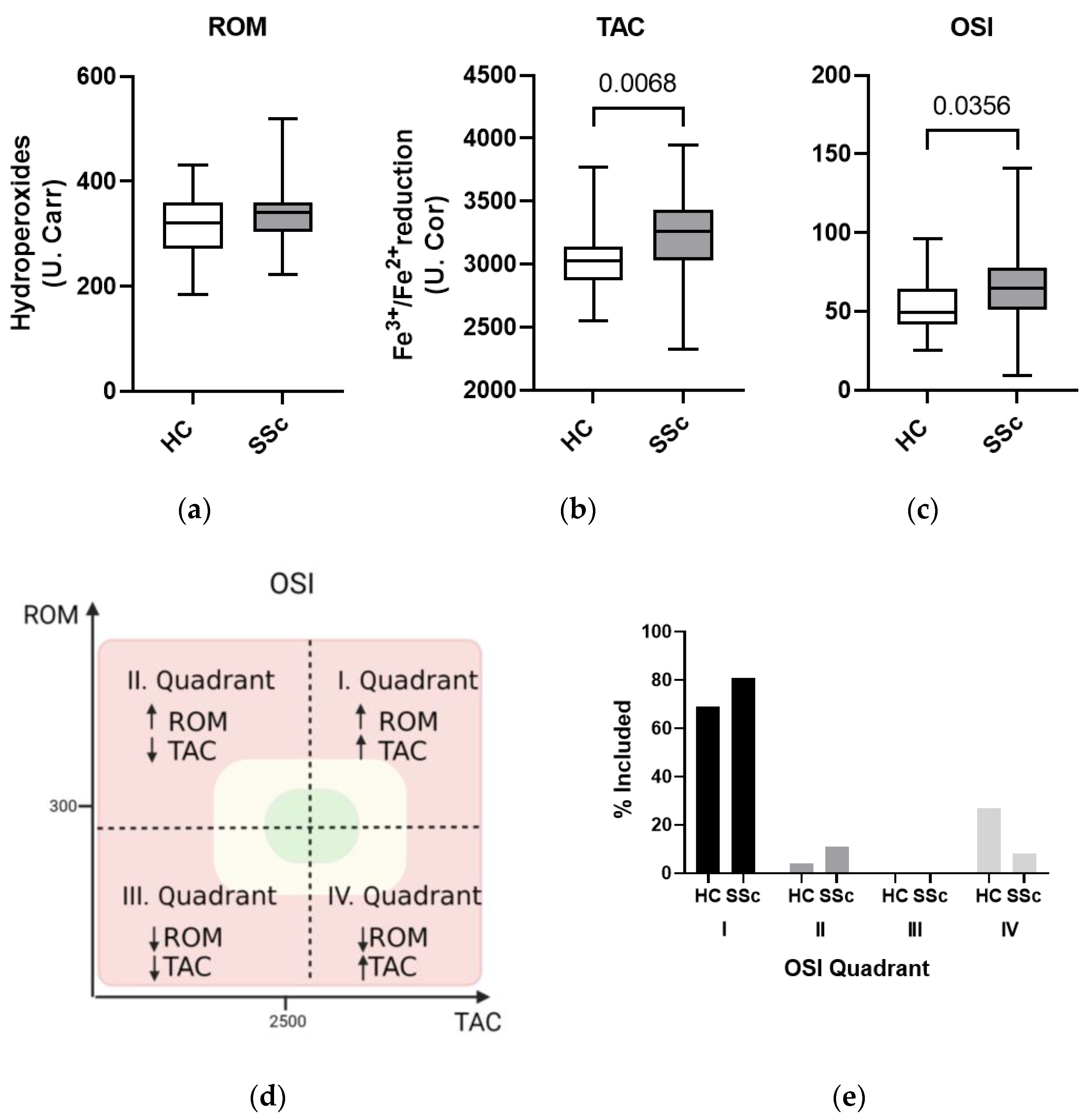
3.2. Oxidative Imbalance in the Systemic Circulation of Systemic Sclerosis Patients
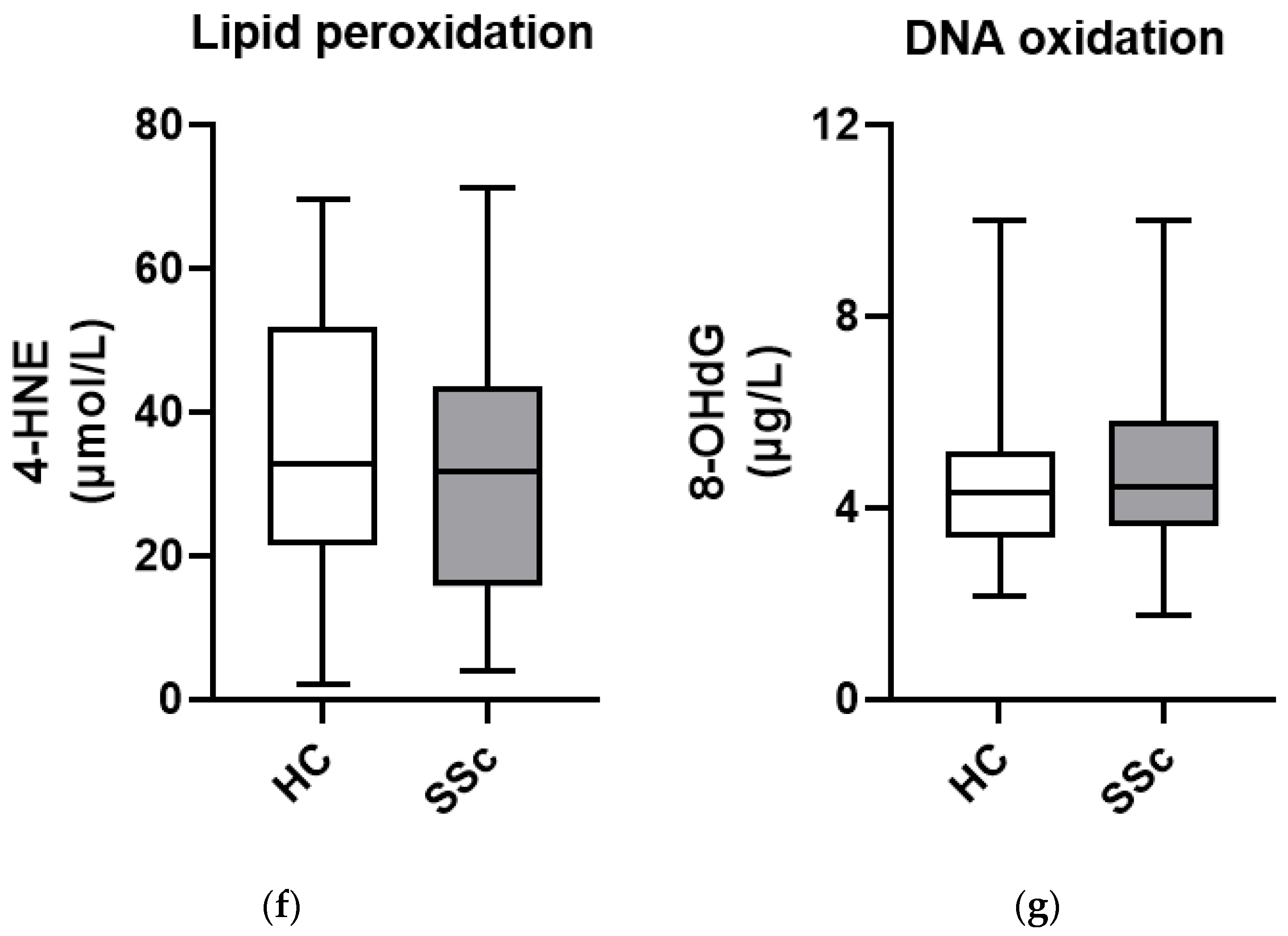
3.3. Markers of Oxidative Damage to Lipids and DNA Show Similar Levels in Serum from Patients with Systemic Sclerosis and Healthy Controls
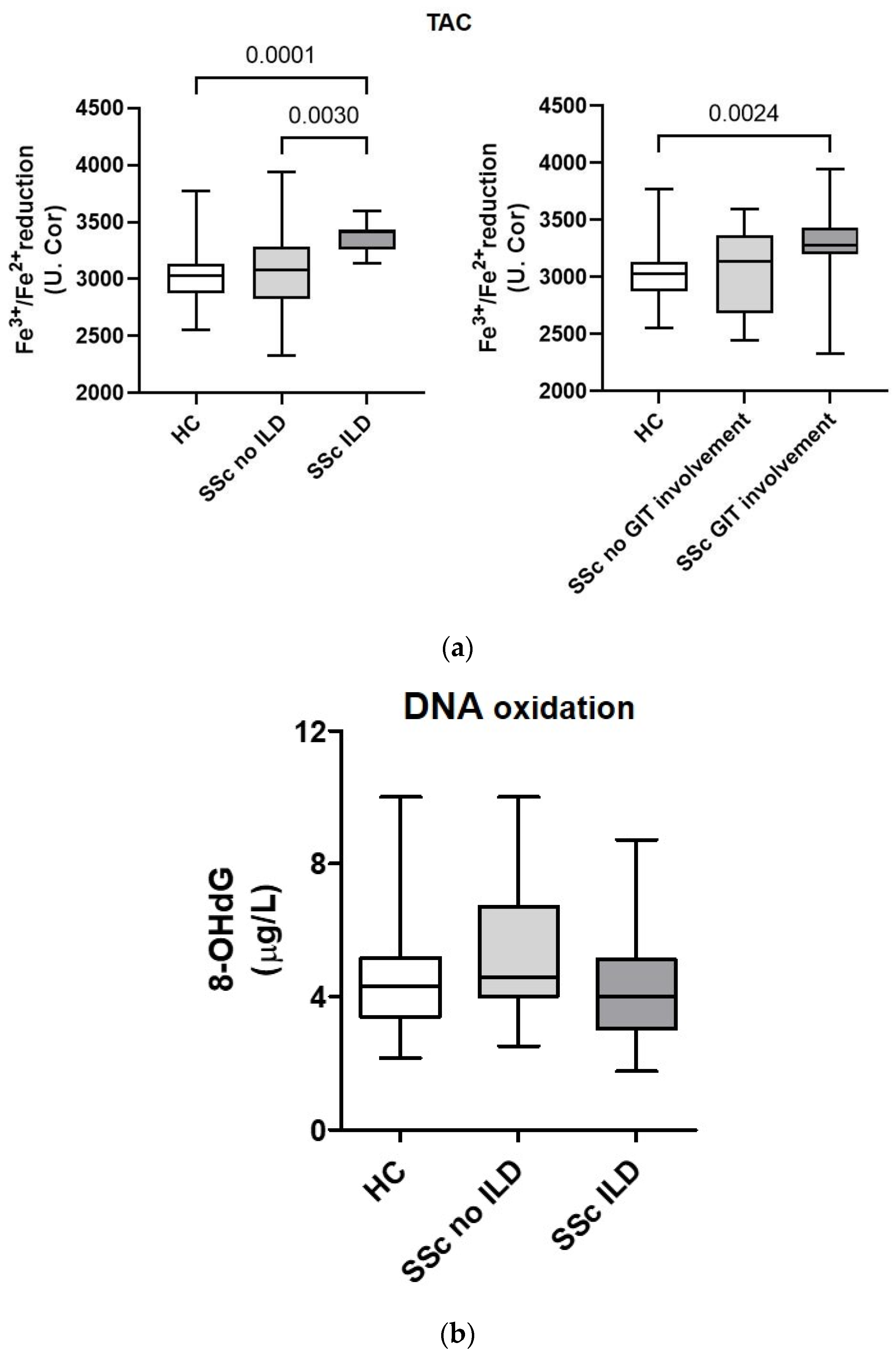
3.4. Total Antioxidant Capacity and DNA Oxidation Marker 8-Hydroxy-2-Deoxyguanosine in Serum from Patients with Systemic Sclerosis Are Associated with Lung and Gastrointestinal Complications
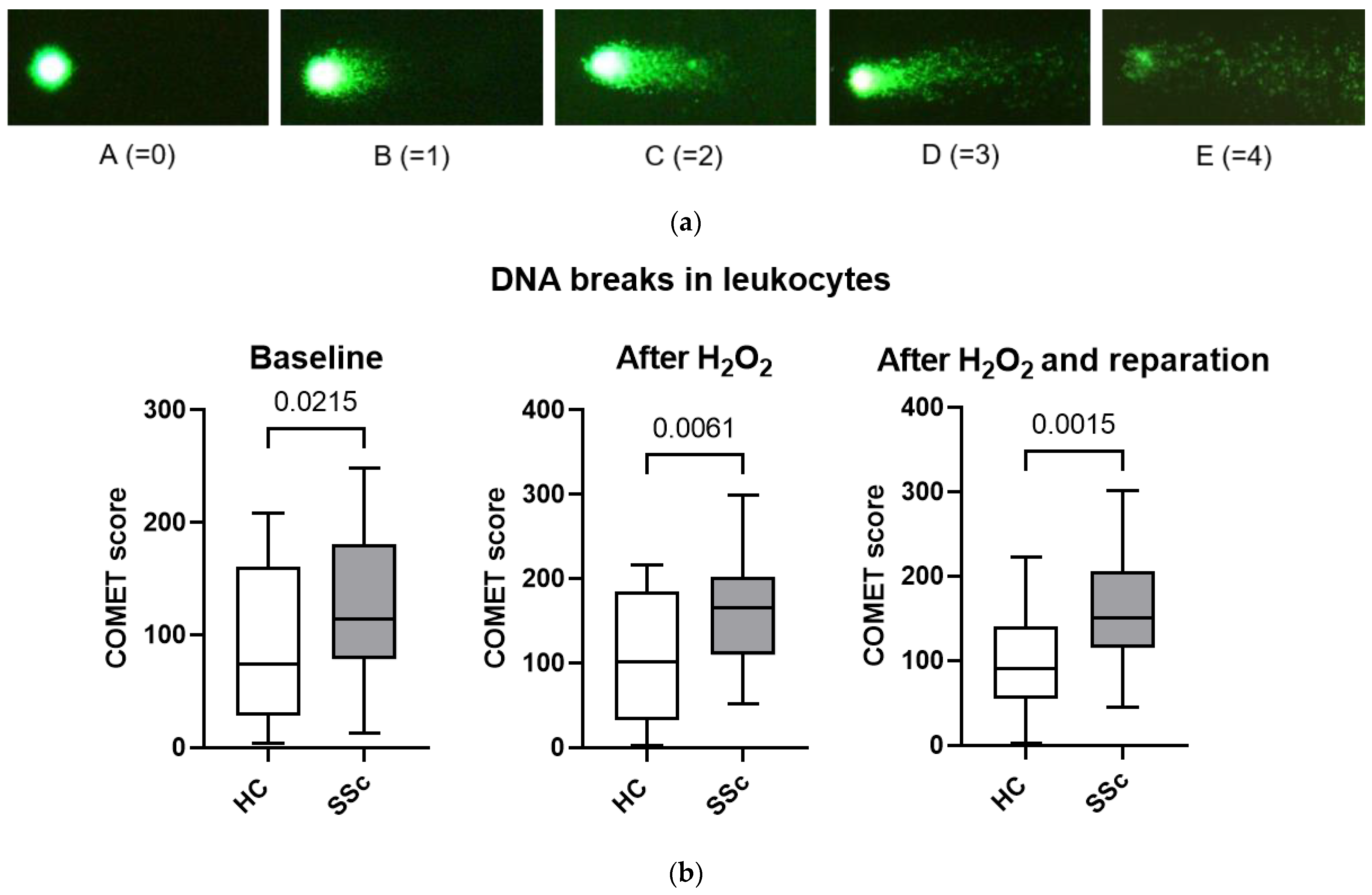
3.5. Leukocytes from Patients with Systemic Sclerosis Are Characterized by Increased DNA Breaks and Poorer DNA Repair Mechanisms
3.6. Total Antioxidant Capacity and DNA Breaks in Leukocytes from SSc Patients Are Associated with the Presence of Autoantibodies
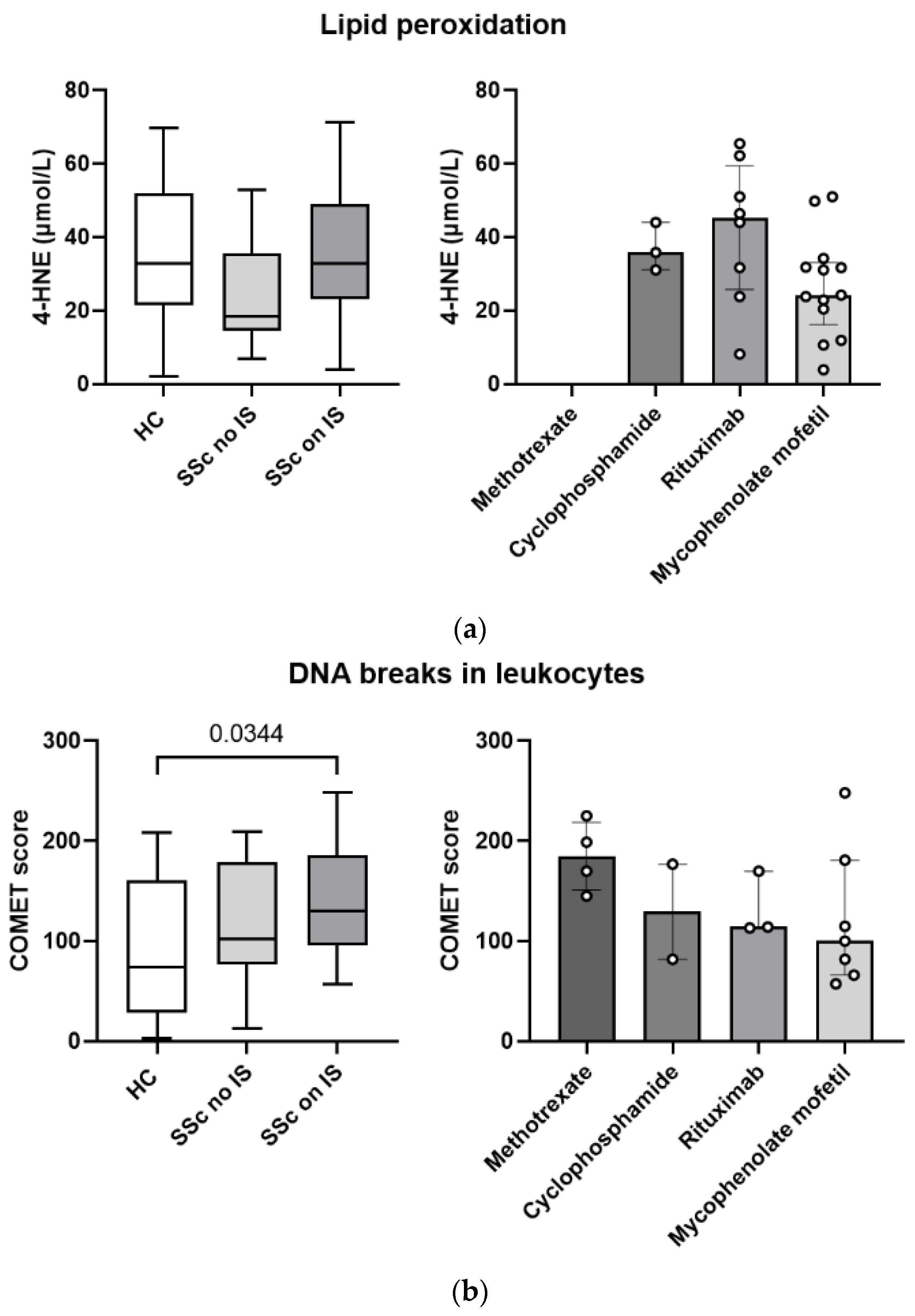
3.7. Pharmacotherapy with Immunosuppressants Is Associated with Greater Systemic Lipid Peroxidation and More DNA Breaks in Leukocytes in Patients with Systemic Sclerosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vona, R.; Giovannetti, A.; Gambardella, L.; Malorni, W.; Pietraforte, D.; Straface, E. Oxidative stress in the pathogenesis of systemic scleroderma: An overview. J. Cell. Mol. Med. 2018, 22, 3308–3314. [Google Scholar] [CrossRef] [PubMed]
- Doridot, L.; Jeljeli, M.; Chêne, C.; Batteux, F. Implication of oxidative stress in the pathogenesis of systemic sclerosis via inflammation, autoimmunity and fibrosis. Redox Biol. 2019, 25, 101122. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.-Y.; Liu, X.; Jiang, M.; Zhao, H.-P.; Zhao, J.-J. Oxidative stress markers in blood in systemic sclerosis: A meta-analysis. Mod. Rheumatol. 2017, 27, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.Y.; Lu, C.H.; Wu, C.H.; Li, K.J.; Kuo, Y.M.; Hsieh, S.C.; Yu, C.L. Molecular Basis of Accelerated Aging with Immune Dysfunction-Mediated Inflammation (Inflamm-Aging) in Patients with Systemic Sclerosis. Cells 2021, 10, 3402. [Google Scholar] [CrossRef] [PubMed]
- Kavian, N.; Mehlal, S.; Jeljeli, M.; Saidu, N.E.B.; Nicco, C.; Cerles, O.; Chouzenoux, S.; Cauvet, A.; Camus, C.; Ait-Djoudi, M.; et al. The Nrf2-Antioxidant Response Element Signaling Pathway Controls Fibrosis and Autoimmunity in Scleroderma. Front. Immunol. 2018, 9, 1896. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug. Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef] [PubMed]
- Palomino, G.M.; Bassi, C.L.; Wastowski, I.J.; Xavier, D.J.; Lucisano-Valim, Y.M.; Crispim, J.C.O.; Rassi, D.M.; Marques-Neto, J.F.; Sakamoto-Hojo, E.T.; Moreau, P.; et al. Patients with Systemic Sclerosis Present Increased DNA Damage Differentially Associated with DNA Repair Gene Polymorphisms. J. Rheumatol. 2014, 41, 458–465. [Google Scholar] [CrossRef]
- Vlachogiannis, N.I.; Pappa, M.; Ntouros, P.A.; Nezos, A.; Mavragani, C.P.; Souliotis, V.L.; Sfikakis, P.P. Association Between DNA Damage Response, Fibrosis and Type I Interferon Signature in Systemic Sclerosis. Front. Immunol. 2020, 11, 582401. [Google Scholar] [CrossRef]
- Paludan, S.R.; Bowie, A.G. Immune Sensing of DNA. Immunity 2013, 38, 870–880. [Google Scholar] [CrossRef]
- Wu, M.; Assassi, S. The Role of Type 1 Interferon in Systemic Sclerosis. Front. Immunol. 2013, 4, 266. [Google Scholar] [CrossRef]
- Benfaremo, D.; Svegliati, S.; Paolini, C.; Agarbati, S.; Moroncini, G. Systemic Sclerosis: From Pathophysiology to Novel Therapeutic Approaches. Biomedicines 2022, 10, 163. [Google Scholar] [CrossRef]
- van den Hoogen, F.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-Cerinic, M.; Naden, R.P.; Medsger, T.A., Jr.; Carreira, P.E.; et al. 2013 classification criteria for systemic sclerosis: An American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum. 2013, 65, 2737–2747. [Google Scholar] [CrossRef]
- LeRoy, E.C.; Black, C.; Fleischmajer, R.; Jablonska, S.; Krieg, T.; Medsger, T.A., Jr.; Rowell, N.; Wollheim, F. Scleroderma (systemic sclerosis): Classification, subsets and pathogenesis. J. Rheumatol. 1988, 15, 202–205. [Google Scholar] [PubMed]
- Bunn, C.; Kveder, T. Counterimmunoelectrophoresis and immunodiffusion for the detection of antibodies to soluble cellular antigens. In Manual of Biological Markers of Disease; Van Venrooij, W.J., Maini, R.N., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 1996; pp. 33–44. [Google Scholar] [CrossRef]
- Cabarkapa, A.; Zivković, L.; Zukovec, D.; Djelić, N.; Bajić, V.; Dekanski, D.; Spremo-Potparević, B. Protective effect of dry olive leaf extract in adrenaline induced DNA damage evaluated using in vitro comet assay with human peripheral leukocytes. Toxicol. In Vitro 2014, 28, 451–456. [Google Scholar] [CrossRef]
- Piera-Velazquez, S.; Jimenez, S.A. Oxidative Stress Induced by Reactive Oxygen Species (ROS) and NADPH Oxidase 4 (NOX4) in the Pathogenesis of the Fibrotic Process in Systemic Sclerosis: A Promising Therapeutic Target. J. Clin. Med. 2021, 10, 4791. [Google Scholar] [CrossRef] [PubMed]
- Bourji, K.; Meyer, A.; Chatelus, E.; Pincemail, J.; Pigatto, E.; Defraigne, J.O.; Singh, F.; Charlier, C.; Geny, B.; Gottenberg, J.E.; et al. High reactive oxygen species in fibrotic and nonfibrotic skin of patients with diffuse cutaneous systemic sclerosis. Free Radic. Biol. Med. 2015, 87, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Gorni, D.; Finco, A. Oxidative stress in elderly population: A prevention screening study. Aging Med. 2020, 3, 205–213. [Google Scholar] [CrossRef]
- Riccieri, V.; Spadaro, A.; Fuksa, L.; Firuzi, O.; Saso, L.; Valesini, G. Specific oxidative stress parameters differently correlate with nailfold capillaroscopy changes and organ involvement in systemic sclerosis. Clin. Rheumatol. 2008, 27, 225–230. [Google Scholar] [CrossRef]
- Firuzi, O.; Fuksa, L.; Spadaro, C.; Boušovà, I.; Riccieri, V.; Spadaro, A.; Petrucci, R.; Marrosu, G.; Saso, L. Oxidative stress parameters in different systemic rheumatic diseases. J. Pharm. Pharmacol. 2010, 58, 951–957. [Google Scholar] [CrossRef]
- Murphy, M.P.; Bayir, H.; Belousov, V.; Chang, C.J.; Davies, K.J.A.; Davies, M.J.; Dick, T.P.; Finkel, T.; Forman, H.J.; Janssen-Heininger, Y.; et al. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat. Metab. 2022, 4, 651–662. [Google Scholar] [CrossRef]
- Prior, R.L.; Cao, G. In vivo total antioxidant capacity: Comparison of different analytical methods. Free Radic. Biol. Med. 1999, 27, 1173–1181. [Google Scholar] [CrossRef]
- Poljsak, B.; Šuput, D.; Milisav, I. Achieving the Balance between ROS and Antioxidants: When to Use the Synthetic Antioxidants. Oxidative Med. Cell. Longev. 2013, 2013, 956792. [Google Scholar] [CrossRef]
- Ogawa, F.; Shimizu, K.; Muroi, E.; Hara, T.; Sato, S. Increasing levels of serum antioxidant status, total antioxidant power, in systemic sclerosis. Clin. Rheumatol. 2011, 30, 921–925. [Google Scholar] [CrossRef]
- Bozkurt, M.; Evin, D.; Oktayoğlu, P.; Serda, E.; Yüksel, H.K.; Mehmet, C.; Layan, M.; Akif Sariyildiz, M.; Batmaz, B.; Ucar, D.; et al. Serum Prolidase Enzyme Activity and Oxidative Status in Patients With Scleroderma. Acta Medica Mediterr. 2014, 30, 127. [Google Scholar]
- Olczyk, K.; Kucharz, E.J.; Winsz, K.; Bogdanowski, T.; Brzezińska-Wcisło, L.; Haj-Ali, Y. Free radical activity and antioxidative systems in patients with systemic sclerosis. Eur. J. Intern. Med. 1999, 10, 206–208. [Google Scholar] [CrossRef]
- Pellegrini, N.; Vitaglione, P.; Granato, D.; Fogliano, V. Twenty-five years of total antioxidant capacity measurement of foods and biological fluids: Merits and limitations. J. Sci. Food Agric. 2020, 100, 5064–5078. [Google Scholar] [CrossRef]
- Michel, P.; Eggert, W.; Albrecht-Nebe, H.; Grune, T. Increased lipid peroxidation in children with autoimmune diseases. Acta Paediatr. 1997, 86, 609–612. [Google Scholar] [CrossRef] [PubMed]
- Avouac, J.; Borderie, D.; Ekindjian, O.G.; Kahan, A.; Allanore, Y. High DNA Oxidative Damage in Systemic Sclerosis. J. Rheumatol. 2010, 37, 2540–2547. [Google Scholar] [CrossRef]
- Wang, C.C.; Chen, W.L.; Lin, C.M.; Lai, C.H.; Loh, C.H.; Chen, H.I.; Liou, S.H. The relationship between plasma and urinary 8-hydroxy-2-deoxyguanosine biomarkers measured by liquid chromatography tandem mass spectrometry. Environ. Sci. Pollut. Res. Int. 2016, 23, 17496–17502. [Google Scholar] [CrossRef]
- Guo, C.; Li, X.; Wang, R.; Yu, J.; Ye, M.; Mao, L.; Zhang, S.; Zheng, S. Association between Oxidative DNA Damage and Risk of Colorectal Cancer: Sensitive Determination of Urinary 8-Hydroxy-2′-deoxyguanosine by UPLC-MS/MS Analysis. Sci. Rep. 2016, 6, 32581. [Google Scholar] [CrossRef]
- Denton, C.P. Advances in pathogenesis and treatment of systemic sclerosis. Clin. Med. 2015, 15, s58–s63. [Google Scholar] [CrossRef] [PubMed]
- Servettaz, A.; Goulvestre, C.; Kavian, N.; Nicco, C.; Guilpain, P.; Chéreau, C.; Vuiblet, V.; Guillevin, L.; Mouthon, L.; Weill, B.; et al. Selective Oxidation of DNA Topoisomerase 1 Induces Systemic Sclerosis in the Mouse. J. Immunol. 2009, 182, 5855–5864. [Google Scholar] [CrossRef] [PubMed]
- Stochmal, A.; Czuwara, J.; Trojanowska, M.; Rudnicka, L. Antinuclear Antibodies in Systemic Sclerosis: An Update. Clin. Rev. Allergy Immunol. 2020, 58, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Guillen-Del Castillo, A.; Pilar Simeón-Aznar, C.; Fonollosa-Pla, V.; Alonso-Vila, S.; Reverte-Vinaixa, M.M.; Muñoz, X.; Pallisa, E.; Selva-O’allaghan, A.; Fernández-Codina, A.; Vilardell-Tarrés, M. Good outcome of interstitial lung disease in patients with scleroderma associated to anti-PM/Scl antibody. Semin. Arthritis Rheum. 2014, 44, 331–337. [Google Scholar] [CrossRef]
- Goudarzi, M.; Kalantar, M.; Sadeghi, E.; Karamallah, M.H.; Kalantar, H. Protective effects of apigenin on altered lipid peroxidation, inflammation, and antioxidant factors in methotrexate-induced hepatotoxicity. Naunyn-Schmiedebergs Arch. Pharmacol. 2021, 394, 523–531. [Google Scholar] [CrossRef]
- Uraz, S.; Tahan, V.; Aygun, C.; Eren, F.; Unluguzel, G.; Yuksel, M.; Senturk, O.; Avsar, E.; Haklar, G.; Celikel, C.; et al. Role of Ursodeoxycholic Acid in Prevention of Methotrexate-induced Liver Toxicity. Dig. Dis. Sci. 2008, 53, 1071–1077. [Google Scholar] [CrossRef]
- Herman-Edelstein, M.; Rozen-Zvi, B.; Zingerman, B.; Lichtenberg, S.; Malachi, T.; Gafter, U.; Ori, Y. Effect of immunosuppressive drugs on DNA repair in human peripheral blood mononuclear cells. Biomed. Pharmacother. 2012, 66, 111–115. [Google Scholar] [CrossRef]
- Nelkine, L.; Vrolijk, M.F.; Drent, M.; Bast, A. Role of antioxidants in the treatment of gastroesophageal reflux disease-associated idiopathic pulmonary fibrosis. Curr. Opin. Pulm. Med. 2020, 26, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Day, B.J. Antioxidants as potential therapeutics for lung fibrosis. Antioxid. Redox Signal. 2008, 10, 355–370. [Google Scholar] [CrossRef]

| Systemic Markers of Oxidative Stress | DNA Breaks in Leukocytes | |||
|---|---|---|---|---|
| SSc | HC | SSc | HC | |
| N (%) | 36 (58) | 26 (42) | 35 (52) | 32 (48) |
| Age (years) | ||||
| Median (IQR) | 63 (19) | 59 (13) | 57 (19) | 54 (16) |
| Mean (SD) | 61 (14) | 58 (13) | 56 (13) | 53 (13) |
| Minimum | 31 | 29 | 29 | 27 |
| Maximum | 88 | 86 | 80 | 84 |
| Sex N (%) | ||||
| Male | 4 (11) | 3 (12) | 6 (17) | 4 (13) |
| Female | 32 (89) | 23 (88) | 29 (83) | 28 (87) |
| Systemic Markers of Oxidative Stress N (%) | DNA Breaks in Leukocytes N (%) | |
|---|---|---|
| SSc clinical subset | ||
| Diffuse cutaneous | 20 (56) | 11 (31) |
| Limited cutaneous | 14 (39) | 22 (63) |
| No data | 1 (3) | 2 (6) |
| Clinical manifestations | ||
| Raynaud’s phenomenon | 32 (89) | 31 (89) |
| History of digital ulcers | 12 (33) | 18 (51) |
| Digital ulcers | 3 (8) | 7 (20) |
| Digital pitting scars | 5 (14) | 7 (20) |
| Telangiectasia | 18 (50) | 21 (60) |
| Calcinosis | 2 (6) | 7 (20) |
| GIT involvement | 22 (61) | 18 (51) |
| Interstitial lung disease | 15 (42) | 15 (43) |
| Pulmonary arterial hypertension | 3 (8) | 1 (3) |
| Autoantibodies | ||
| ACA | 10 (28) | 14 (40) |
| ATA | 20 (55) | 15 (43) |
| ACA, ATA neg. | 6 (17) | 6 (17) |
| Nail fold capillaroscopy pattern | ||
| Early | 9 (25) | 3 (9) |
| Active | 14 (39) | 20 (57) |
| Late | 6 (17) | 4 (11) |
| No data | 7 (19) | 8 (31) |
| Comorbidities | ||
| Arterial hypertension | 10 (28) | 12 (34) |
| Hyperlipidemia | 8 (22) | 9 (26) |
| Type 2 diabetes | 4 (11) | 1 (3) |
| Asthma | 1 (3) | 3 (9) |
| Chronic obstructive pulmonary disease | 0 (0) | 2 (6) |
| Cancer | 3 (8) | 3 (9) |
| Coronary artery disease | 2 (6) | 3 (9) |
| Atherosclerosis | 6 (17) | 2 (6) |
| Treatment | ||
| Immunosuppressants | 20 (56) | 16 (46) |
| Glucocorticoids | 3 (8) | 7 (20) |
| Methotrexate | 0 (0) | 4 (11) |
| Cyclophosphamide | 3 (8) | 2 (6) |
| Mycophenolate mofetil | 13 (36) | 7 (20) |
| Rituximab | 8 (22) | 3 (9) |
| Analgesics and anti-inflammatory drugs | 14 (39) | 11 (31) |
| Calcium channel blockers | 20 (56) | 28 (51) |
| Prostacyclins | 4 (11) | 2 (6) |
| PDE5 inhibitors | 6 (17) | 5 (14) |
| Laboratory parameters | Median (IQR) | Median (IQR) |
| Leukocytes (×109/L) | 66 (49) | 64 (28) |
| Thrombocytes (×109/L) | 251 (99) | 232 (87) |
| Hemoglobin (g/L) | 134 (16) | 131 (17) |
| SR (mm/h) | 18 (19) | 20 (26) |
| IL-6 (ng/L) | 4 (6) | 2 (3) |
| Pulmonary function | Median (IQR) | Median (IQR) |
| DLCO (%) | 73 (28) | 70 (30) |
| FVC (%) | 98 (22) | 91 (27) |
| FEV1 (%) | 95 (21) | 92 (21) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brezovec, N.; Perdan-Pirkmajer, K.; Burja, B.; Rotar, Ž.; Osredkar, J.; Sodin-Šemrl, S.; Lakota, K.; Čučnik, S. Disturbed Antioxidant Capacity in Patients with Systemic Sclerosis Associates with Lung and Gastrointestinal Symptoms. Biomedicines 2023, 11, 2110. https://doi.org/10.3390/biomedicines11082110
Brezovec N, Perdan-Pirkmajer K, Burja B, Rotar Ž, Osredkar J, Sodin-Šemrl S, Lakota K, Čučnik S. Disturbed Antioxidant Capacity in Patients with Systemic Sclerosis Associates with Lung and Gastrointestinal Symptoms. Biomedicines. 2023; 11(8):2110. https://doi.org/10.3390/biomedicines11082110
Chicago/Turabian StyleBrezovec, Neža, Katja Perdan-Pirkmajer, Blaž Burja, Žiga Rotar, Joško Osredkar, Snežna Sodin-Šemrl, Katja Lakota, and Saša Čučnik. 2023. "Disturbed Antioxidant Capacity in Patients with Systemic Sclerosis Associates with Lung and Gastrointestinal Symptoms" Biomedicines 11, no. 8: 2110. https://doi.org/10.3390/biomedicines11082110
APA StyleBrezovec, N., Perdan-Pirkmajer, K., Burja, B., Rotar, Ž., Osredkar, J., Sodin-Šemrl, S., Lakota, K., & Čučnik, S. (2023). Disturbed Antioxidant Capacity in Patients with Systemic Sclerosis Associates with Lung and Gastrointestinal Symptoms. Biomedicines, 11(8), 2110. https://doi.org/10.3390/biomedicines11082110






