The Maastricht Acquisition Platform for Studying Mechanisms of Cell–Matrix Crosstalk (MAPEX): An Interdisciplinary and Systems Approach towards Understanding Thoracic Aortic Disease
Abstract
1. Introduction
2. Scientific Underpinning and Research Question
2.1. Loss of Mechanical Homeostasis
2.2. Pathophysiological Framework
2.2.1. Wall Shear Stress Homeostasis
2.2.2. Wall Stress Homeostasis
2.3. Focus of MAPEX Platform Infrastructure
- Preoperative 4D-flow MRI, to capture wall shear stress patterns in relation to local vessel geometry;
- Strain imaging during open-chest surgery, to capture local vessel wall deformations;
- Tissue sampling co-localised with local tissue strain and wall shear stress measurements;
- To capture tissue and cell-specific biomarkers, as well as ultrastructural properties;
- Inclusion of a large cohort (minimal target of 400) of aneurysm patients undergoing cardiac surgery, complemented by patients with normal aortic diameter as controls (with limited tissue sampling);
- Data structuring ready for (i) computational biomechanical modelling, (ii) omics, and (iii) artificial intelligence approaches, for data integration and interpretation, as well as new hypothesis generation.
3. Methodological Setup
3.1. Acquisition Platform Management
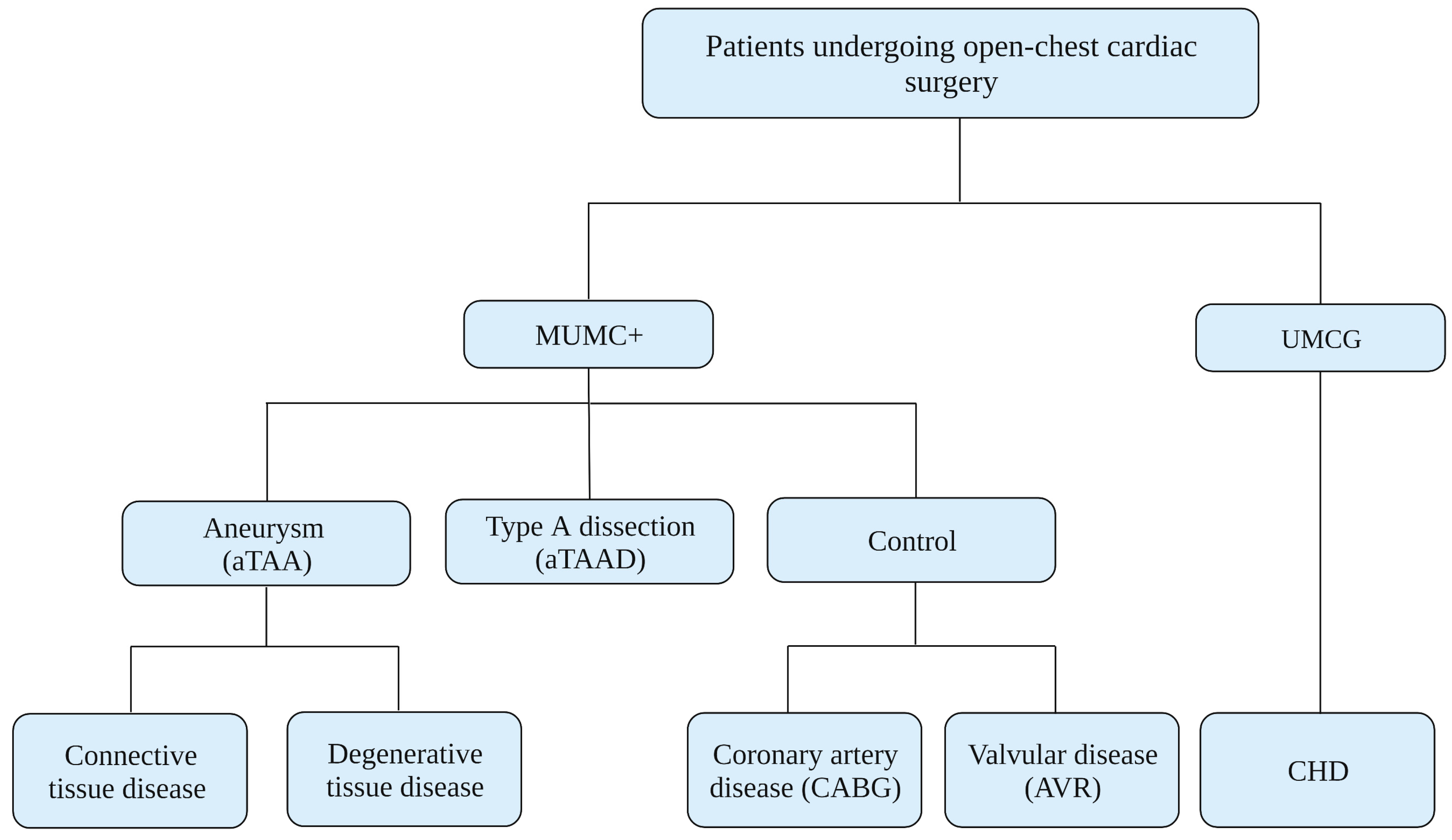
3.2. Study Population
3.3. Recruitment Procedure
3.4. Pre- and Peri-Operative Procedures
4. Materials and Data Processing
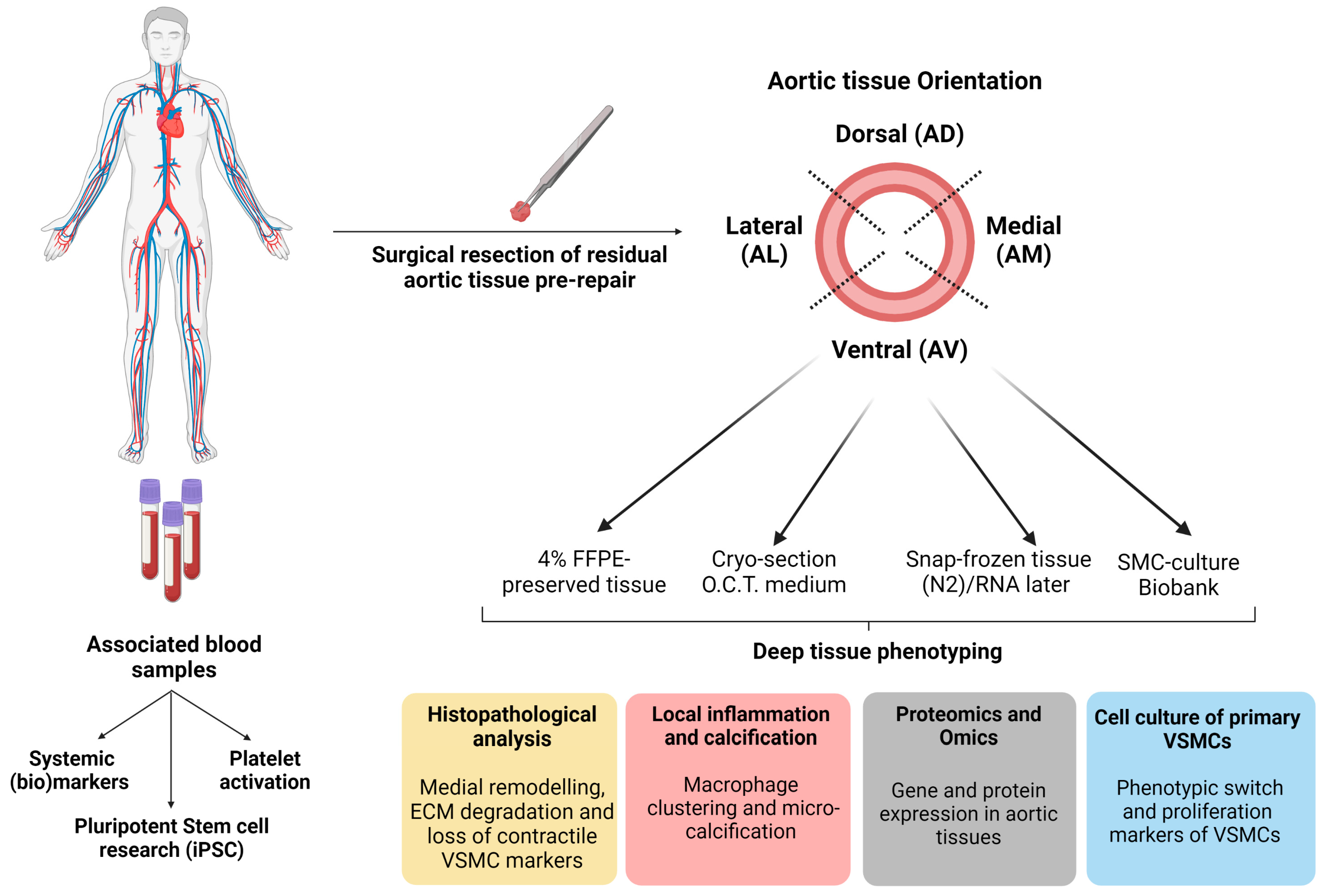
4.1. Location-Specific Characterisation Methods of Aortic Tissue
4.1.1. Aortic Tissue Samples
4.1.2. Histopathological Analysis
4.1.3. Genotyping and Omics-Approach
4.2. Circulating Markers and iPSC-Oriented Research
4.2.1. Circulating Blood Markers
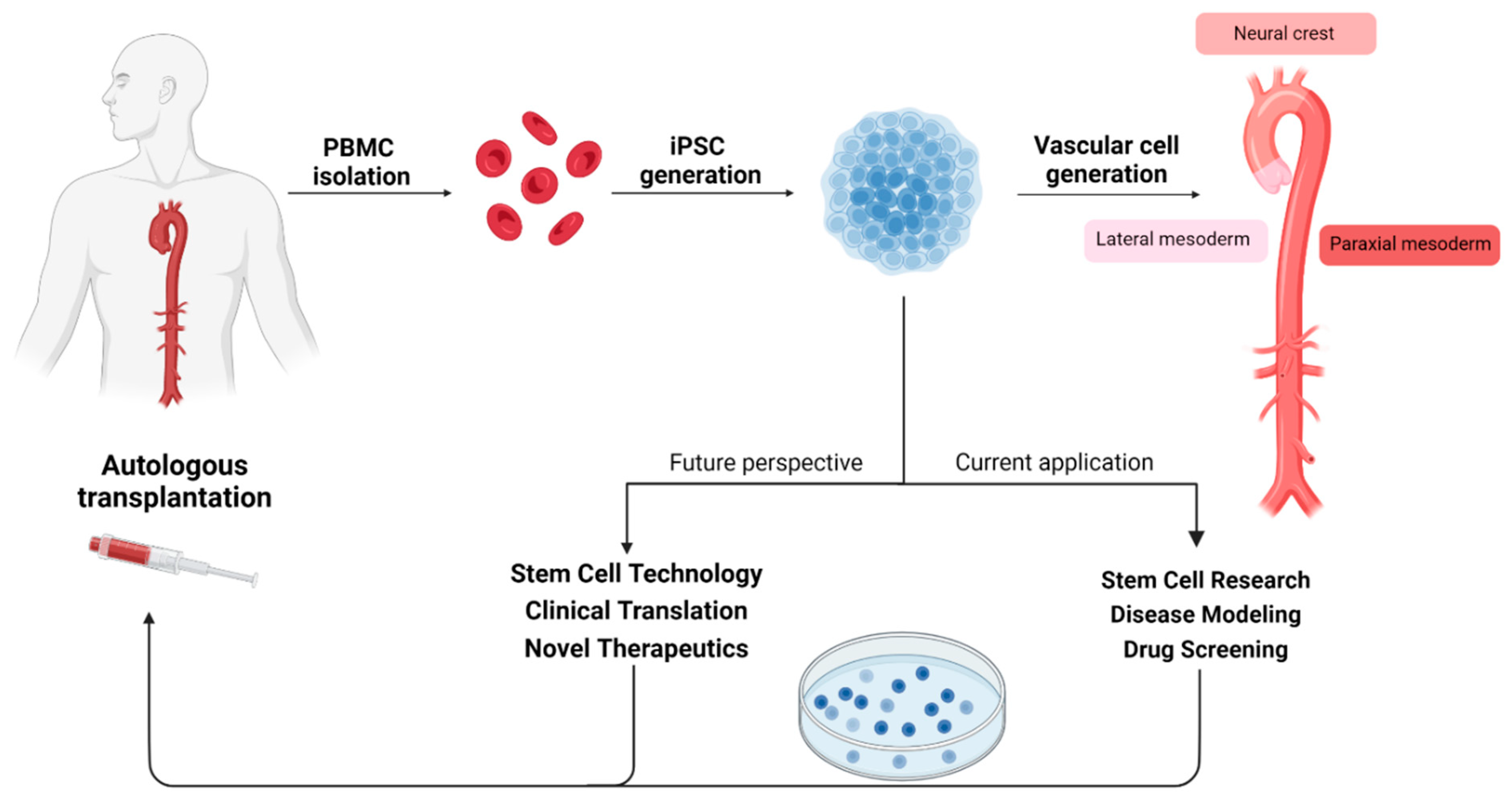
4.2.2. Pluripotent Stem Cell Research
5. Statistics and Methodology
6. Research Scope
7. Clinical Application
8. Future Directions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuzmik, G.A.; Sang, A.X.; Elefteriades, J.A. Natural history of thoracic aortic aneurysms. J. Vasc. Surg. 2012, 56, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Elefteriades, J.A.; Farkas, E.A. Thoracic Aortic Aneurysm. J. Am. Coll. Cardiol. 2010, 55, 841–857. [Google Scholar] [CrossRef]
- Paruchuri, V.; Salhab, K.F.; Kuzmik, G.; Gubernikoff, G.; Fang, H.; Rizzo, J.A.; Ziganshin, B.A.; Elefteriades, J.A. Aortic Size Distribution in the General Population: Explaining the Size Paradox in Aortic Dissection. Cardiology 2015, 131, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Davies, R.R.; Goldstein, L.J.; Coady, M.A.; Tittle, S.L.; Rizzo, J.A.; Kopf, G.S.; Elefteriades, J.A. Yearly rupture or dissection rates for thoracic aortic aneurysms: Simple prediction based on size. Ann. Thorac. Surg. 2002, 73, 17–27; discussion 27–18. [Google Scholar] [CrossRef] [PubMed]
- Isselbacher, E.M.; Preventza, O.; Black, I.J.H.; Augoustides, J.G.; Beck, A.W.; Bolen, M.A.; Braverman, A.C.; Bray, B.E.; Brown-Zimmerman, M.M.; Chen, E.P.; et al. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2022, 146, e334–e482. [Google Scholar] [CrossRef]
- Barker, A.J.; Markl, M.; Bürk, J.; Lorenz, R.; Bock, J.; Bauer, S.; Schulz-Menger, J.; von Knobelsdorff-Brenkenhoff, F. Bicuspid aortic valve is associated with altered wall shear stress in the ascending aorta. Circ. Cardiovasc. Imaging 2012, 5, 457–466. [Google Scholar] [CrossRef]
- Brownstein, A.J.; Ziganshin, B.A.; Kuivaniemi, H.; Body, S.C.; Bale, A.E.; Elefteriades, J.A. Genes Associated with Thoracic Aortic Aneurysm and Dissection: An Update and Clinical Implications. Aorta 2017, 5, 11–20. [Google Scholar] [CrossRef]
- Ostberg, N.P.; Zafar, M.A.; Ziganshin, B.A.; Elefteriades, J.A. The Genetics of Thoracic Aortic Aneurysms and Dissection: A Clinical Perspective. Biomolecules 2020, 10, 182. [Google Scholar] [CrossRef]
- Heuts, S.; Adriaans, B.P.; Rylski, B.; Mihl, C.; Bekkers, S.; Olsthoorn, J.R.; Natour, E.; Bouman, H.; Berezowski, M.; Kosiorowska, K.; et al. Evaluating the diagnostic accuracy of maximal aortic diameter, length and volume for prediction of aortic dissection. Heart 2020, 106, 892–897. [Google Scholar] [CrossRef]
- Adriaans, B.P.; Ramaekers, M.; Heuts, S.; Crijns, H.; Bekkers, S.; Westenberg, J.J.M.; Lamb, H.J.; Wildberger, J.E.; Schalla, S. Determining the optimal interval for imaging surveillance of ascending aortic aneurysms. Neth. Heart J. 2021, 29, 623–631. [Google Scholar] [CrossRef]
- Booher, A.M.; Eagle, K.A. Diagnosis and management issues in thoracic aortic aneurysm. Am. Heart J. 2011, 162, 38–46.e31. [Google Scholar] [CrossRef] [PubMed]
- Cebull, H.L.; Rayz, V.L.; Goergen, C.J. Recent Advances in Biomechanical Characterization of Thoracic Aortic Aneurysms. Front. Cardiovasc. Med. 2020, 7, 75. [Google Scholar] [CrossRef] [PubMed]
- Umeda, H.; Aikawa, M.; Libby, P. Liberation of desmosine and isodesmosine as amino acids from insoluble elastin by elastolytic proteases. Biochem. Biophys. Res. Commun. 2011, 411, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Karatolios, K.; Wittek, A.; Nwe, T.H.; Bihari, P.; Shelke, A.; Josef, D.; Schmitz-Rixen, T.; Geks, J.; Maisch, B.; Blase, C.; et al. Method for aortic wall strain measurement with three-dimensional ultrasound speckle tracking and fitted finite element analysis. Ann. Thorac. Surg. 2013, 96, 1664–1671. [Google Scholar] [CrossRef] [PubMed]
- Pasta, S.; Agnese, V.; Di Giuseppe, M.; Gentile, G.; Raffa, G.M.; Bellavia, D.; Pilato, M. In Vivo Strain Analysis of Dilated Ascending Thoracic Aorta by ECG-Gated CT Angiographic Imaging. Ann. Biomed. Eng. 2017, 45, 2911–2920. [Google Scholar] [CrossRef]
- Wilson, J.S.; Taylor, W.R.; Oshinski, J. Assessment of the regional distribution of normalized circumferential strain in the thoracic and abdominal aorta using DENSE cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2019, 21, 59. [Google Scholar] [CrossRef]
- Guala, A.; Teixidó-Tura, G.; Rodríguez-Palomares, J.; Ruiz-Muñoz, A.; Dux-Santoy, L.; Villalva, N.; Granato, C.; Galian, L.; Gutiérrez, L.; González-Alujas, T.; et al. Proximal aorta longitudinal strain predicts aortic root dilation rate and aortic events in Marfan syndrome. Eur. Heart J. 2019, 40, 2047–2055. [Google Scholar] [CrossRef]
- Chiu, P.; Lee, H.P.; Dalal, A.R.; Koyano, T.; Nguyen, M.; Connolly, A.J.; Chaudhuri, O.; Fischbein, M.P. Relative strain is a novel predictor of aneurysmal degeneration of the thoracic aorta: An ex vivo mechanical study. JVS Vasc. Sci. 2021, 2, 235–246. [Google Scholar] [CrossRef]
- Pasta, S.; Agnese, V.; Gallo, A.; Cosentino, F.; Di Giuseppe, M.; Gentile, G.; Raffa, G.M.; Maalouf, J.F.; Michelena, H.I.; Bellavia, D.; et al. Shear Stress and Aortic Strain Associations with Biomarkers of Ascending Thoracic Aortic Aneurysm. Ann. Thorac. Surg. 2020, 110, 1595–1604. [Google Scholar] [CrossRef]
- Humphrey, J.D.; Dufresne, E.R.; Schwartz, M.A. Mechanotransduction and extracellular matrix homeostasis. Nat. Rev. Mol. Cell Biol. 2014, 15, 802–812. [Google Scholar] [CrossRef]
- Tsamis, A.; Krawiec, J.T.; Vorp, D.A. Elastin and collagen fibre microstructure of the human aorta in ageing and disease: A review. J. R. Soc. Interface 2013, 10, 20121004. [Google Scholar] [CrossRef] [PubMed]
- Van Hemelrijk, C.; Renard, M.; Loeys, B. The Loeys-Dietz syndrome: An update for the clinician. Curr. Opin. Cardiol. 2010, 25, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Jaminon, A.; Reesink, K.; Kroon, A.; Schurgers, L. The Role of Vascular Smooth Muscle Cells in Arterial Remodeling: Focus on Calcification-Related Processes. Int. J. Mol. Sci. 2019, 20, 5694. [Google Scholar] [CrossRef] [PubMed]
- Kapustin, A.N.; Chatrou, M.L.; Drozdov, I.; Zheng, Y.; Davidson, S.M.; Soong, D.; Furmanik, M.; Sanchis, P.; De Rosales, R.T.; Alvarez-Hernandez, D.; et al. Vascular smooth muscle cell calcification is mediated by regulated exosome secretion. Circ. Res. 2015, 116, 1312–1323. [Google Scholar] [CrossRef]
- Furmanik, M.; Chatrou, M.; van Gorp, R.; Akbulut, A.; Willems, B.; Schmidt, H.; van Eys, G.; Bochaton-Piallat, M.L.; Proudfoot, D.; Biessen, E.; et al. Reactive Oxygen-Forming Nox5 Links Vascular Smooth Muscle Cell Phenotypic Switching and Extracellular Vesicle-Mediated Vascular Calcification. Circ. Res. 2020, 127, 911–927. [Google Scholar] [CrossRef]
- Humphrey, J.D.; Schwartz, M.A. Vascular Mechanobiology: Homeostasis, Adaptation and Disease. Annu. Rev. Biomed. Eng. 2021, 23, 1–27. [Google Scholar] [CrossRef]
- Hayashi, K.; Naiki, T. Adaptation and remodeling of vascular wall: Biomechanical response to hypertension. J. Mech. Behav. Biomed. Mater. 2009, 2, 3–19. [Google Scholar] [CrossRef]
- Roux, E.; Bougaran, P.; Dufourcq, P.; Couffinhal, T. Fluid Shear Stress Sensing by the Endothelial Layer. Front. Physiol. 2020, 11, 861. [Google Scholar] [CrossRef]
- Callaghan, F.M.; Grieve, S.M. Normal patterns of thoracic aortic wall shear stress measured using four-dimensional flow MRI in a large population. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1174–H1181. [Google Scholar] [CrossRef]
- Guala, A.; Dux-Santoy, L.; Teixido-Tura, G.; Ruiz-Muñoz, A.; Galian-Gay, L.; Servato, M.L.; Valente, F.; Gutiérrez, L.; González-Alujas, T.; Johnson, K.M.; et al. Wall Shear Stress Predicts Aortic Dilation in Patients With Bicuspid Aortic Valve. JACC Cardiovasc. Imaging 2022, 15, 46–56. [Google Scholar] [CrossRef]
- Clark, J.M.; Glagov, S. Transmural organization of the arterial media. The lamellar unit revisited. Arteriosclerosis 1985, 5, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Dajnowiec, D.; Langille, B.L. Arterial adaptations to chronic changes in haemodynamic function: Coupling vasomotor tone to structural remodelling. Clin. Sci. 2007, 113, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Jaminon, A.M.G.; Akbulut, A.C.; Rapp, N.; Kramann, R.; Biessen, E.A.L.; Temmerman, L.; Mees, B.; Brandenburg, V.; Dzhanaev, R.; Jahnen-Dechent, W.; et al. Development of the BioHybrid Assay: Combining Primary Human Vascular Smooth Muscle Cells and Blood to Measure Vascular Calcification Propensity. Cells 2021, 10, 2097. [Google Scholar] [CrossRef]
- Spronck, B.; Heusinkveld, M.H.G.; Donders, W.P.; de Lepper, A.G.W.; Op’t Roodt, J.; Kroon, A.A.; Delhaas, T.; Reesink, K.D. A constitutive modeling interpretation of the relationship among carotid artery stiffness, blood pressure, and age in hypertensive subjects. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H568–H582. [Google Scholar] [CrossRef]
- Humphrey, J.D.; Milewicz, D.M.; Tellides, G.; Schwartz, M.A. Dysfunctional Mechanosensing in Aneurysms. Science 2014, 344, 477–479. [Google Scholar] [CrossRef]
- Davis, M.J.; Hill, M.A. Signaling mechanisms underlying the vascular myogenic response. Physiol. Rev. 1999, 79, 387–423. [Google Scholar] [CrossRef]
- Leloup, A.J.A.; Van Hove, C.E.; De Moudt, S.; De Meyer, G.R.Y.; De Keulenaer, G.W.; Fransen, P. Vascular smooth muscle cell contraction and relaxation in the isolated aorta: A critical regulator of large artery compliance. Physiol. Rep. 2019, 7, e13934. [Google Scholar] [CrossRef]
- Herrera, A.M.; McParland, B.E.; Bienkowska, A.; Tait, R.; Paré, P.D.; Seow, C.Y. ‘Sarcomeres’ of smooth muscle: Functional characteristics and ultrastructural evidence. J. Cell Sci. 2005, 118, 2381–2392. [Google Scholar] [CrossRef] [PubMed]
- Lacolley, P.; Regnault, V.; Segers, P.; Laurent, S. Vascular Smooth Muscle Cells and Arterial Stiffening: Relevance in Development, Aging and Disease. Physiol. Rev. 2017, 97, 1555–1617. [Google Scholar] [CrossRef]
- Ramaekers, M.; Adriaans, B.P.; Juffermans, J.F.; van Assen, H.C.; Bekkers, S.; Scholte, A.; Kenjeres, S.; Lamb, H.J.; Wildberger, J.E.; Westenberg, J.J.M.; et al. Characterization of Ascending Aortic Flow in Patients with Degenerative Aneurysms: A 4D Flow Magnetic Resonance Study. Investig. Radiol. 2021, 56, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Parikh, S.; Ganizada, B.; Debeij, G.; Natour, E.; Maessen, J.; Spronck, B.; Schurgers, L.; Delhaas, T.; Huberts, W.; Bidar, E.; et al. Intra-Operative Video-Based Measurement of Biaxial Strains of the Ascending Thoracic Aorta. Biomedicines 2021, 9, 670. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Cheung, C.; Bernardo, A.S.; Pedersen, R.A.; Sinha, S. Directed differentiation of embryonic origin-specific vascular smooth muscle subtypes from human pluripotent stem cells. Nat. Protoc. 2014, 9, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Granata, A.; Serrano, F.; Bernard, W.G.; McNamara, M.; Low, L.; Sastry, P.; Sinha, S. An iPSC-derived vascular model of Marfan syndrome identifies key mediators of smooth muscle cell death. Nat. Genet. 2017, 49, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Maguire, E.M.; Xiao, Q.; Xu, Q. Differentiation and Application of Induced Pluripotent Stem Cell-Derived Vascular Smooth Muscle Cells. Arter. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 2026–2037. [Google Scholar] [CrossRef]
- Davaapil, H.; Shetty, D.K.; Sinha, S. Aortic “Disease-in-a-Dish”: Mechanistic Insights and Drug Development Using iPSC-Based Disease Modeling. Front. Cell Dev. Biol. 2020, 8, 550504. [Google Scholar] [CrossRef]
- Li, T.; Jing, J.J.; Sun, L.P.; Gong, Y.H.; Dong, N.N.; Yang, J.; Yuan, Y. Serum Toll-like receptor 4: A novel and promising biomarker for identification of aortic aneurysmal diseases. Clin. Chim. Acta 2018, 483, 69–75. [Google Scholar] [CrossRef]
- Liao, M.; Zou, S.; Bao, Y.; Jin, J.; Yang, J.; Liu, Y.; Green, M.; Yang, F.; Qu, L. Matrix metalloproteinases are regulated by MicroRNA 320 in macrophages and are associated with aortic dissection. Exp. Cell Res. 2018, 370, 98–102. [Google Scholar] [CrossRef]
- Marshall, L.M.; Carlson, E.J.; O’Malley, J.; Snyder, C.K.; Charbonneau, N.L.; Hayflick, S.J.; Coselli, J.S.; Lemaire, S.A.; Sakai, L.Y. Thoracic aortic aneurysm frequency and dissection are associated with fibrillin-1 fragment concentrations in circulation. Circ. Res. 2013, 113, 1159–1168. [Google Scholar] [CrossRef]
- Shinohara, T.; Suzuki, K.; Okada, M.; Shiigai, M.; Shimizu, M.; Maehara, T.; Ohsuzu, F. Soluble elastin fragments in serum are elevated in acute aortic dissection. Arter. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1839–1844. [Google Scholar] [CrossRef]
- Rueda-Martínez, C.; Lamas, O.; Carrasco-Chinchilla, F.; Robledo-Carmona, J.; Porras, C.; Sánchez-Espín, G.; Navarro, M.J.; Fernández, B. Increased blood levels of transforming growth factor β in patients with aortic dilatation. Interact. Cardiovasc. Thorac. Surg. 2017, 25, 571–574. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; Liu, S.; Li, D.; Liu, Z.; Yang, H.; Sun, B.; Liu, H. Expression of platelet-derived growth factor B is upregulated in patients with thoracic aortic dissection. J. Vasc. Surg. 2018, 68, 3s–13s. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hu, J.; Qian, H.; Gu, J.; Meng, W.; Zhang, E.Y. Novel findings: Expression of angiotensin-converting enzyme and angiotensin-converting enzyme 2 in thoracic aortic dissection and aneurysm. J. Renin Angiotensin Aldosterone Syst. 2015, 16, 1130–1134. [Google Scholar] [CrossRef]
- Kazamia, R.; Keravnou, A.; Moushi, A.; Sokratous, K.; Michailidou, K.; Yiangou, K.; Soteriou, M.; Xenophontos, S.; Cariolou, M.A.; Bashiardes, E. Tissue and plasma proteomic profiling indicates AHSG as a potential biomarker for ascending thoracic aortic aneurysms. BMC Cardiovasc. Disord. 2023, 23, 138. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.D.; Schurgers, L.J.; Brandenburg, V.M.; Christenson, R.H.; Vermeer, C.; Ketteler, M.; Shlipak, M.G.; Whooley, M.A.; Ix, J.H. The associations of fibroblast growth factor 23 and uncarboxylated matrix Gla protein with mortality in coronary artery disease: The Heart and Soul Study. Ann. Intern. Med. 2010, 152, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Parikh, S.; Moerman, K.M.; Ramaekers, M.J.F.G.; Schalla, S.; Bidar, E.; Delhaas, T.; Reesink, K.; Huberts, W. Biomechanical Characterisation of Thoracic Ascending Aorta with Preserved Pre-Stresses. Bioengineering 2023, 10, 846. [Google Scholar] [CrossRef]



| aTAA | HTAA | Controls | aTAAD | |
|---|---|---|---|---|
| Type of index event | Aneurysm | Heritable aneurysm | CABG/AVR | Type-A dissection |
| Number of subjects | 86 | 9 | 30 | 20 |
| Gender (male %) | 70 | 67 | 90 | 65 |
| Age (years) | 60 ± 14 | 44 ± 14 | 63 ± 12 | 60 ± 14 |
| BMI (kg/m3) | 26 ± 5 | 23 ± 4 | 26 ± 4 | 28 ± 7 |
| Weight (kg) | 81 ± 17 | 67 ± 12 | 83 ± 16 | 85 ± 24 |
| Aortic diameter (mm) | 53 [47;59] | 48 [40;60] | 41 [36;44] | 55 [52;52] |
| Hypertension (%) | 73 | 33 | 77 | 95 |
| Diabetes mellitus (%) | 5 | 11 | 7 | - |
| Hypercholesterolemia (%) | 53 | - | 70 | 60 |
| COPD (%) | 7 | 22 | 10 | 20 |
| Myocardial infarction (%) | 9 | - | 23 | 20 |
| Family history for CVD (%) | 20 | 56 | 53 | 20 |
| Current smoker (%) | 9 | 22 | 27 | 25 |
| Alcohol use (%) | 65 | 56 | 57 | 45 |
| Aortic insufficiency (%) | 77 | 67 | 40 | 35 |
| Aortic stenosis (%) | 20 | - | 60 | 5 |
| Valve morphology (% BAV) | 31 | 44 | 27 | 10 |
| LVEF (%) | 53 [46;59] | 55 [52;57] | 55 [45;60] | - |
| Biomaterials | Storage | Analysis Material | Storage Temperature |
|---|---|---|---|
| Blood | EDTA BD Vacutainer | Plasma | <−80 °C |
| PBMCs | <−80 °C | ||
| Sodium Citrated BD Vacutainer | Serum | <−80 °C | |
| Tissue | Serum free M199/DMEM medium | Tissue sections for smooth muscle cell isolation | 4 °C |
| 4% paraformaldehyde-fixed | Paraffin-embedded tissue sections | 20–22 °C | |
| Fresh frozen in OCT | Fresh-frozen tissue sections | <−80 °C | |
| RNA later | RNA stabilised tissue for gene expression | <−80 °C | |
| Snap frozen in N2 | Snap-frozen tissue for gene expression | <−80 °C |
| Category | Phenotypes | Marker of Target | Type of Analysis | Name of Stain/Assay | References |
|---|---|---|---|---|---|
| Tissue | Cell nuclei, extracellular matrix, cytoplasm, inflammatory infiltration, adipose tissue | VSMC nuclei | Histological | Haematoxylin and eosin | SOP 13LU-0406 |
| Extracellular matrix | Collagen I and III | Histological | Picrosirius red (Direct red 80) | SOP 16CM-421 | |
| Extracellular matrix | Elastin | Histological | Elastica van Gieson | SOP 12PL-0403 | |
| Contractile VSMC | Calponin-1 (CNN1) | Immunohistological (IHC) | CNN1 stain | SOP 14LU-0412 | |
| Contractile VSMC | Alpha-smooth muscle actin (α-SMA) | Immunohistological (IHC) | α-SMA stain | SOP 14LU-0404 | |
| Vascular vitamin K status and vascular calcification | Uncarboxylated matrix gamma carboxyglutamate protein (ucMGP) | Immunohistological (IHC) | ucMGP stain | SOP 14LU-0413 | |
| Proteinase inhibitor | Alpha-1 Antitrypsin (A1AT, SERPINA1) | Immunohistological (IHC) | SERPINA1 strain | SOP 22RK BG-439 | |
| Blood serum | Breakdown products of elastin | Desmosine (DES) | Enzyme-linked immunosorbent assay (ELISA) | DES ELISA kit | n.a. |
| Breakdown products of elastin | Isodesmosine (IDES) | Enzyme-linked immunosorbent assay (ELISA) | IDES ELISA kit | n.a. |
| Gene | Transcript Reference (Ensembl) | Alternative Exon | Transcript Reference for Alternative Exon (Ensembl) |
|---|---|---|---|
| ABL1 | ENST00000372348 | ||
| ACTA2 | ENST00000458208 | ||
| ARIH1 | ENST00000379887 | ||
| BGN | ENST00000331595 | ||
| COL3A1 | ENST00000304636 | ||
| EFEMP2/FBLN4 | ENST00000307998 | ||
| ELN | ENST00000358929 | ||
| EMILIN1 | ENST00000380320 | ||
| FBN1 | ENST00000316623 | ||
| FBN2 | ENST00000262464 | ||
| FLNA | ENST00000369850 | ||
| FOXE3 a | ENST00000335071 | ||
| HCN4 | ENST00000261917 | ||
| IPO8 | ENST00000256079 | ||
| LMOD1 | ENST00000367288 | ||
| LOX | ENST00000231004 | ||
| LTBP3 | ENST00000301873 | ||
| MAT2A | ENST00000306434 | ||
| MFAP5 | ENST00000359478 | ||
| MYH11 | ENST00000452625 | Exon 42B | ENST00000396324 |
| MYLK | ENST00000360304 | ||
| NOTCH1 | ENST00000277541 | ||
| NPR3 | ENST00000265074 | ||
| PLOD1 | ENST00000196061 | Exon 2A | ENST00000449038 |
| PMEPA1/TMEPAI | ENST00000341744 | ||
| PRKG1 b | ENST00000401604 | ||
| ROBO4 | ENST00000306534 | ||
| SKI | ENST00000378536 | ||
| SLC2A10 | ENST00000359271 | ||
| SMAD2 | ENST00000402690 | ||
| SMAD3 | ENST00000327367 | Exon 1A | ENST00000439724 |
| SMAD4 | ENST00000342988 | ||
| SMAD6 | ENST00000288840 | ||
| TGFB2 | ENST00000366929 | ||
| TGFB3 | ENST00000238682 | ||
| TGFBR | ENST00000374994 | ||
| TGFBR2 | ENST00000359013 | ||
| THSD4 | ENST00000261862 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganizada, B.H.; Reesink, K.D.; Parikh, S.; Ramaekers, M.J.F.G.; Akbulut, A.C.; Saraber, P.J.M.H.; Debeij, G.P.; MUMC-TAA Student Team; Jaminon, A.M.; Natour, E.; et al. The Maastricht Acquisition Platform for Studying Mechanisms of Cell–Matrix Crosstalk (MAPEX): An Interdisciplinary and Systems Approach towards Understanding Thoracic Aortic Disease. Biomedicines 2023, 11, 2095. https://doi.org/10.3390/biomedicines11082095
Ganizada BH, Reesink KD, Parikh S, Ramaekers MJFG, Akbulut AC, Saraber PJMH, Debeij GP, MUMC-TAA Student Team, Jaminon AM, Natour E, et al. The Maastricht Acquisition Platform for Studying Mechanisms of Cell–Matrix Crosstalk (MAPEX): An Interdisciplinary and Systems Approach towards Understanding Thoracic Aortic Disease. Biomedicines. 2023; 11(8):2095. https://doi.org/10.3390/biomedicines11082095
Chicago/Turabian StyleGanizada, Berta H., Koen D. Reesink, Shaiv Parikh, Mitch J. F. G. Ramaekers, Asim C. Akbulut, Pepijn J. M. H. Saraber, Gijs P. Debeij, MUMC-TAA Student Team, Armand M. Jaminon, Ehsan Natour, and et al. 2023. "The Maastricht Acquisition Platform for Studying Mechanisms of Cell–Matrix Crosstalk (MAPEX): An Interdisciplinary and Systems Approach towards Understanding Thoracic Aortic Disease" Biomedicines 11, no. 8: 2095. https://doi.org/10.3390/biomedicines11082095
APA StyleGanizada, B. H., Reesink, K. D., Parikh, S., Ramaekers, M. J. F. G., Akbulut, A. C., Saraber, P. J. M. H., Debeij, G. P., MUMC-TAA Student Team, Jaminon, A. M., Natour, E., Lorusso, R., Wildberger, J. E., Mees, B., Schurink, G. W., Jacobs, M. J., Cleutjens, J., Krapels, I., Gombert, A., Maessen, J. G., ... Bidar, E. (2023). The Maastricht Acquisition Platform for Studying Mechanisms of Cell–Matrix Crosstalk (MAPEX): An Interdisciplinary and Systems Approach towards Understanding Thoracic Aortic Disease. Biomedicines, 11(8), 2095. https://doi.org/10.3390/biomedicines11082095








