Novel Iron Chelator SK4 Drives Cytotoxicity through Inhibiting Mitochondrial Metabolism in Ovarian and Triple Negative Breast Cancer Cell Lines
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatments
2.2. Proteomics
2.3. Metabolomics and Lipidomics Sample Preparation
2.4. Mass Spectrometry Acquisition Metabolomics
2.5. Mass Spectrometry Acquisition Lipidomics
2.6. Seahorse Real-Time Analysis
3. Results
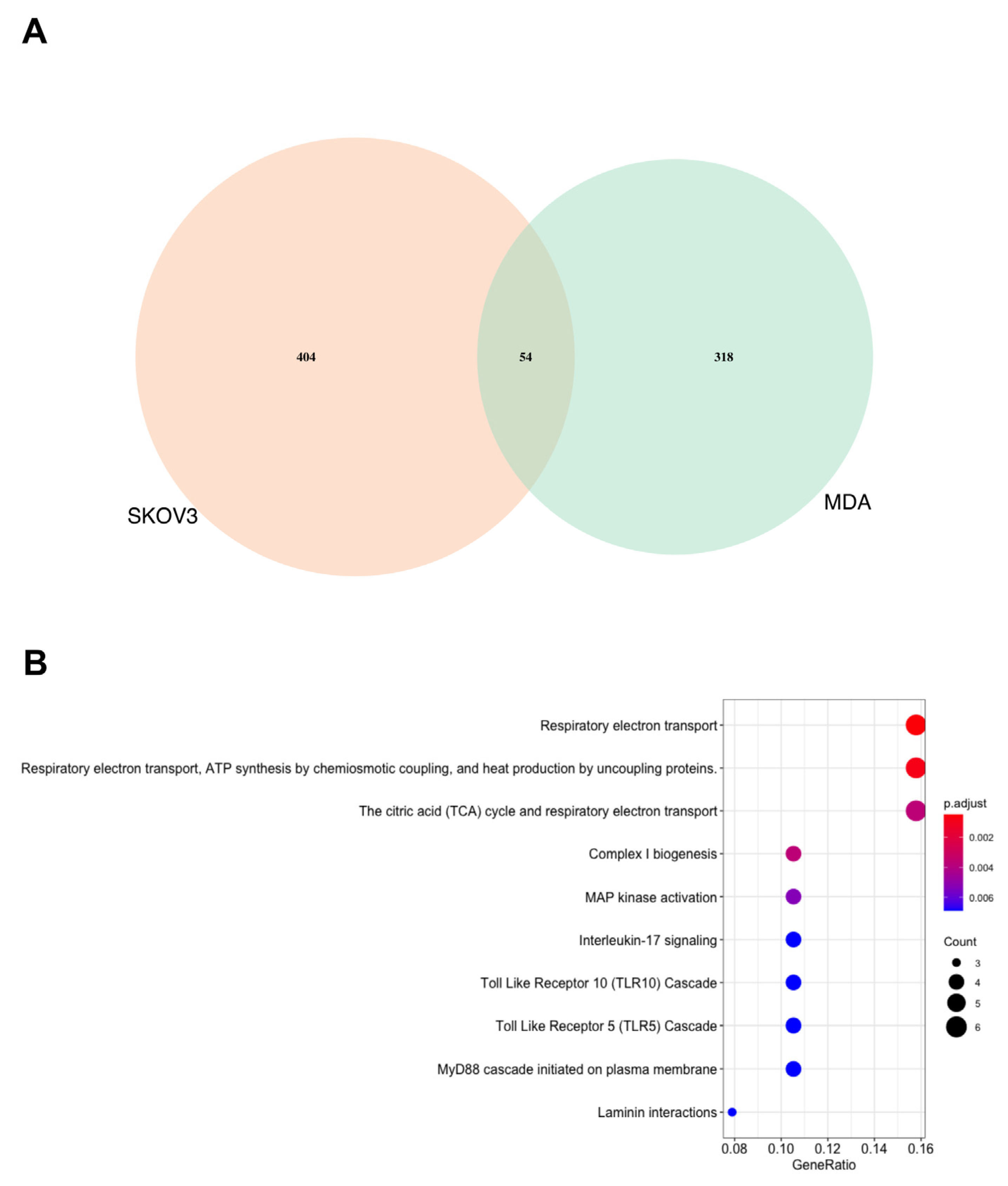
3.1. Proteomics Analysis Demonstrates That the MDA MB 231 Cells Are More Significantly Affected by SK4 Treatment Than SKOV3 Cells
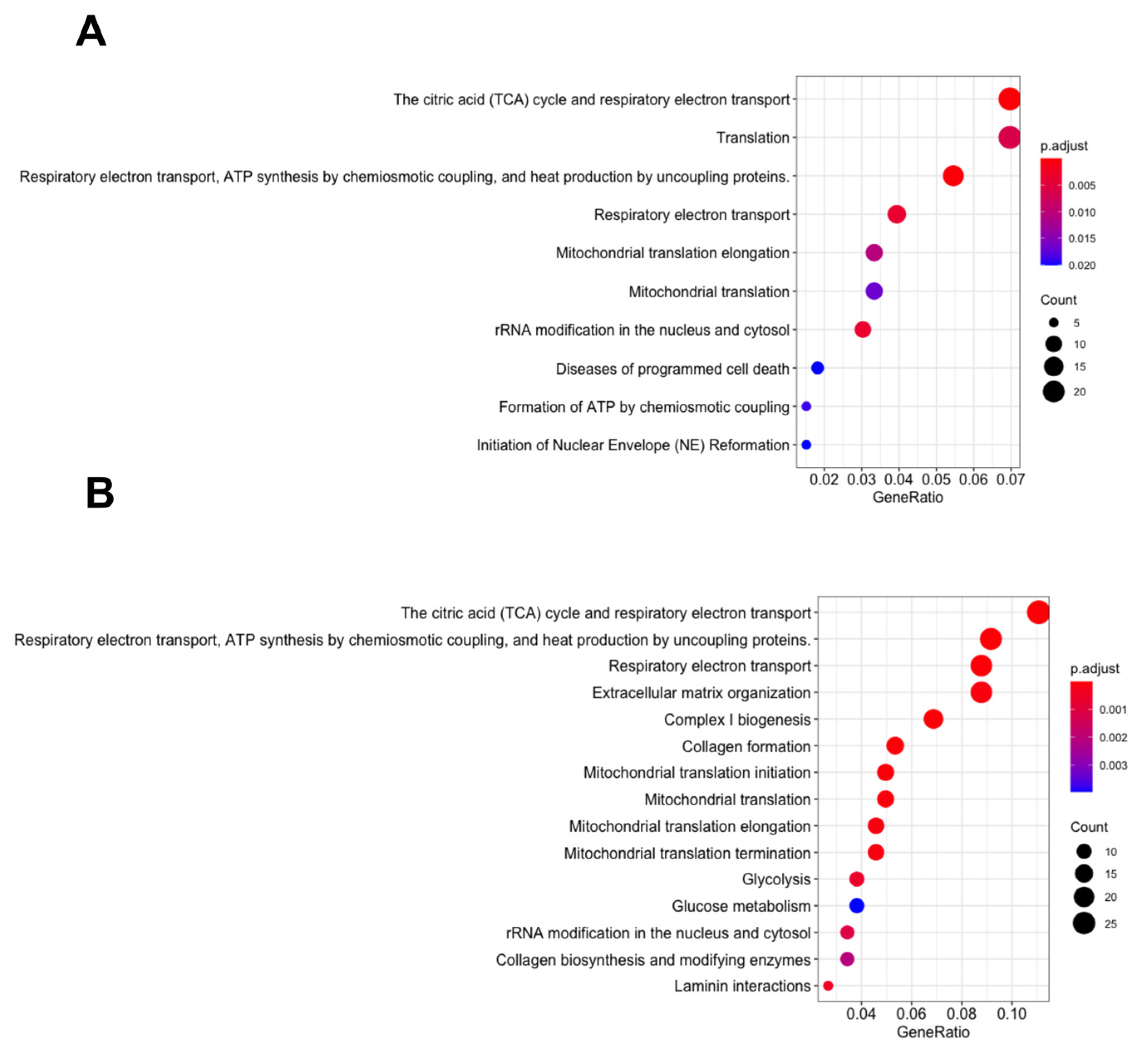
3.2. Energy Metabolism Related Pathways Were Enriched in the MDA MB 231 and SKOV3 Cells
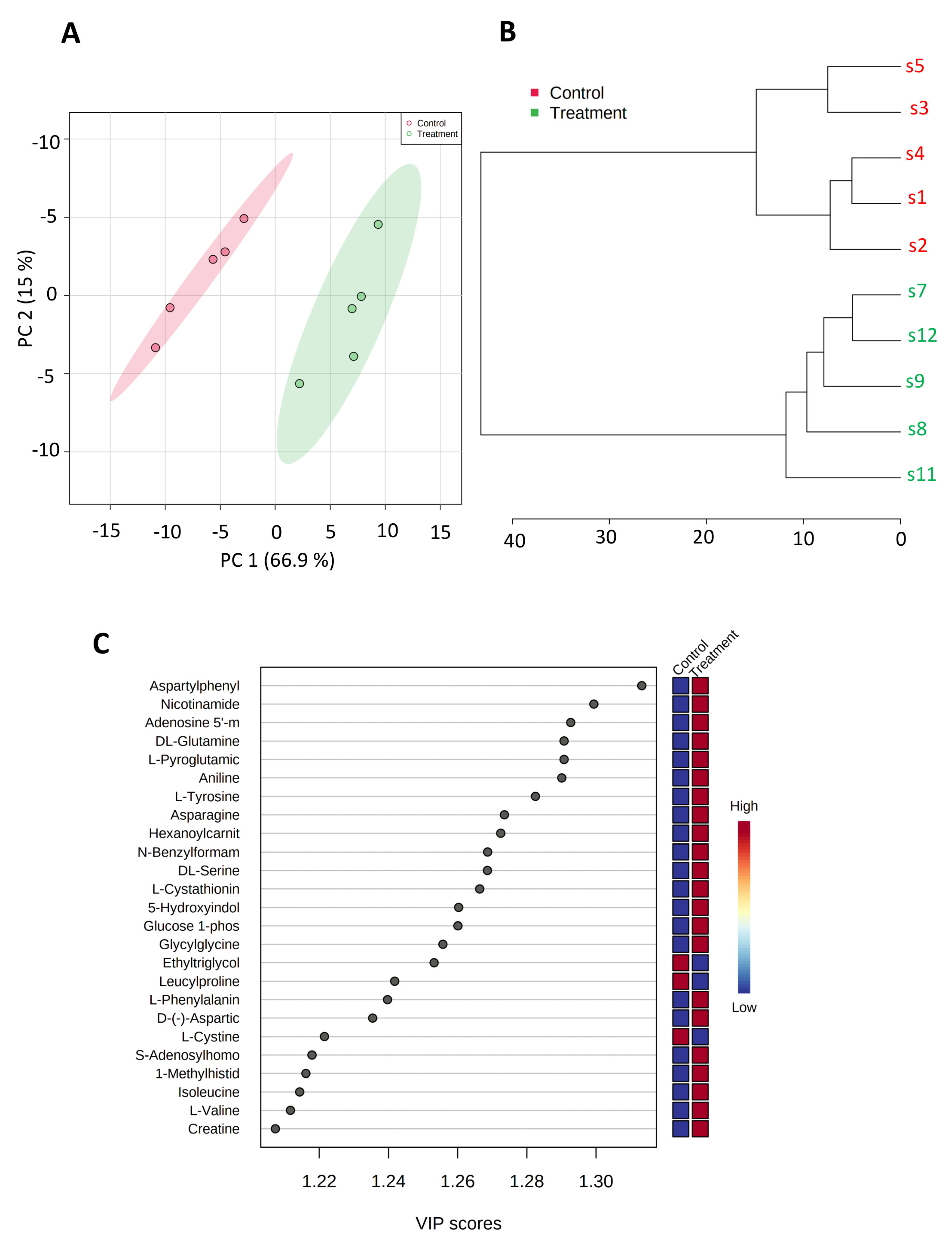
3.3. SK4 Upregulates AMP and Glucose-1-Phosphate in SKOV3 Cells
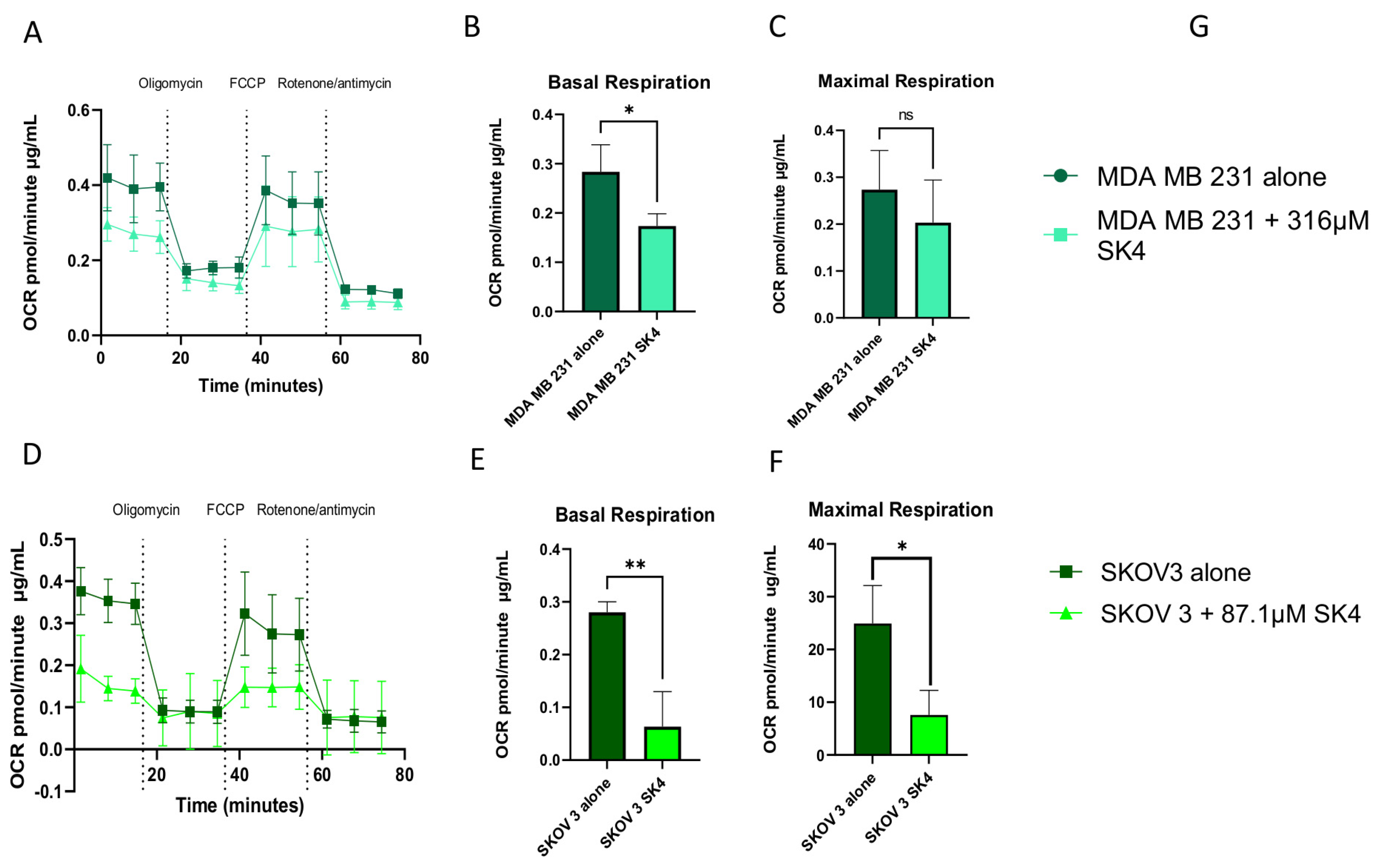
3.4. SK4 Reduces Maximal Mitochondrial Respiration and ATP Linked Respiration in SKOV3 and MDA MB 231 Cells
3.5. SK4 Downregulates Phospholipids in the SKOV3 Cell Lines
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gutierrez, E.; Richardson, D.R.; Jansson, P.J. The anticancer agent di-2-pyridylketone 4,4-dimethyl-3-thiosemicarbazone (Dp44mT) overcomes prosurvival autophagy by two mechanisms: Persistent induction of autophagosome synthesis and impairment of lysosomal integrity. J. Biol. Chem. 2014, 289, 33568–33589. [Google Scholar] [CrossRef]
- Yu, Y.; Suryo Rahmanto, Y.; Hawkins, C.L.; Richardson, D.R. The potent and novel thiosemicarbazone chelators di-2-pyridylketone-4,4-dimethyl-3-thiosemicarbazone and 2-benzoylpyridine-4,4-dimethyl-3-thiosemicarbazone affect crucial thiol systems required for ribonucleotide reductase activity. Mol. Pharmacol. 2011, 79, 921–931. [Google Scholar] [CrossRef]
- Harima, H.; Kaino, S.; Takami, T.; Shinoda, S.; Matsumoto, T.; Fujisawa, K.; Yamamoto, N.; Yamasaki, T.; Sakaida, I. Deferasirox, a novel oral iron chelator, shows antiproliferative activity against pancreatic cancer in vitro and in vivo. BMC Cancer 2016, 16, 702. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, D.; Yue, F.; Zheng, M.; Kovacevic, Z.; Richardson, D.R. The iron chelators Dp44mT and DFO inhibit TGF-beta-induced epithelial-mesenchymal transition via up-regulation of N-Myc downstream-regulated gene 1 (NDRG1). J. Biol. Chem. 2012, 287, 17016–17028. [Google Scholar] [CrossRef]
- Lui, G.Y.; Kovacevic, Z.; S, V.M.; Kalinowski, D.S.; Merlot, A.M.; Sahni, S.; Richardson, D.R. Novel thiosemicarbazones regulate the signal transducer and activator of transcription 3 (STAT3) pathway: Inhibition of constitutive and interleukin 6-induced activation by iron depletion. Mol. Pharmacol. 2015, 87, 543–560. [Google Scholar] [CrossRef]
- Kovacevic, Z.; Chikhani, S.; Lui, G.Y.; Sivagurunathan, S.; Richardson, D.R. The iron-regulated metastasis suppressor NDRG1 targets NEDD4L, PTEN, and SMAD4 and inhibits the PI3K and Ras signaling pathways. Antioxid. Redox Signal 2013, 18, 874–887. [Google Scholar] [CrossRef]
- Lin, Z.P.; Zhu, Y.L.; Lo, Y.C.; Moscarelli, J.; Xiong, A.; Korayem, Y.; Huang, P.H.; Giri, S.; LoRusso, P.; Ratner, E.S. Combination of triapine, olaparib, and cediranib suppresses progression of BRCA-wild type and PARP inhibitor-resistant epithelial ovarian cancer. PLoS ONE 2018, 13, e0207399. [Google Scholar] [CrossRef]
- Finch, R.A.; Liu, M.; Grill, S.P.; Rose, W.C.; Loomis, R.; Vasquez, K.M.; Cheng, Y.; Sartorelli, A.C. Triapine (3-aminopyridine-2-carboxaldehyde- thiosemicarbazone): A potent inhibitor of ribonucleotide reductase activity with broad spectrum antitumor activity. Biochem. Pharmacol. 2000, 59, 983–991. [Google Scholar] [CrossRef]
- Attia, S.; Kolesar, J.; Mahoney, M.R.; Pitot, H.C.; Laheru, D.; Heun, J.; Huang, W.; Eickhoff, J.; Erlichman, C.; Holen, K.D. A phase 2 consortium (P2C) trial of 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP) for advanced adenocarcinoma of the pancreas. Investig. New Drugs 2008, 26, 369–379. [Google Scholar] [CrossRef]
- Kyriakou, S.; Mitsiogianni, M.; Mantso, T.; Cheung, W.; Todryk, S.; Veuger, S.; Pappa, A.; Tetard, D.; Panayiotidis, M.I. Anticancer activity of a novel methylated analogue of L-mimosine against an in vitro model of human malignant melanoma. Investig. New Drugs 2020, 38, 621–633. [Google Scholar] [CrossRef]
- Abdelaal, G.; Carter, A.; Panayiotides, M.I.; Tetard, D.; Veuger, S. Novel iron chelator SK4 demonstrates cytotoxicity in a range of tumour derived cell lines. Front. Mol. Biosci. 2022, 9, 1005092. [Google Scholar] [CrossRef] [PubMed]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 2016, 13, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; He, Q.Y. ReactomePA: An R/Bioconductor package for reactome pathway analysis and visualization. Mol. Biosyst. 2016, 12, 477–479. [Google Scholar] [CrossRef] [PubMed]
- Kyriakou, S.; Cheung, W.; Mantso, T.; Mitsiogianni, M.; Anestopoulos, I.; Veuger, S.; Trafalis, D.T.; Franco, R.; Pappa, A.; Tetard, D.; et al. A novel methylated analogue of L-Mimosine exerts its therapeutic potency through ROS production and ceramide-induced apoptosis in malignant melanoma. Investig. New Drugs 2021, 39, 971–986. [Google Scholar] [CrossRef]
- Masini, A.; Salvioli, G.; Cremonesi, P.; Botti, B.; Gallesi, D.; Ceccarelli, D. Dietary iron deficiency in the rat. I. Abnormalities in energy metabolism of the hepatic tissue. Biochim. Biophys. Acta 1994, 1188, 46–52. [Google Scholar] [CrossRef]
- Jarvis, J.H.; Jacobs, A. Morphological abnormalities in lymphocyte mitochondria associated with iron-deficiency anaemia. J. Clin. Pathol. 1974, 27, 973–979. [Google Scholar] [CrossRef]
- Wu, H.; Wei, H.; Zhang, D.; Sehgal, S.A.; Zhang, D.; Wang, X.; Qin, Y.; Liu, L.; Chen, Q. Defective mitochondrial ISCs biogenesis switches on IRP1 to fine tune selective mitophagy. Redox Biol. 2020, 36, 101661. [Google Scholar] [CrossRef]
- Rensvold, J.W.; Krautkramer, K.A.; Dowell, J.A.; Denu, J.M.; Pagliarini, D.J. Iron Deprivation Induces Transcriptional Regulation of Mitochondrial Biogenesis. J. Biol. Chem. 2016, 291, 20827–20837. [Google Scholar] [CrossRef]
- Oexle, H.; Gnaiger, E.; Weiss, G. Iron-dependent changes in cellular energy metabolism: Influence on citric acid cycle and oxidative phosphorylation. Biochim. Biophys. Acta 1999, 1413, 99–107. [Google Scholar] [CrossRef]
- Torti, S.V.; Torti, F.M. Iron and cancer: More ore to be mined. Nat. Rev. Cancer 2013, 13, 342–355. [Google Scholar] [CrossRef] [PubMed]
- Meier, C.; Ristic, Z.; Klauser, S.; Verrey, F. Activation of system L heterodimeric amino acid exchangers by intracellular substrates. EMBO J. 2002, 21, 580–589. [Google Scholar] [CrossRef]
- Kurozumi, S.; Kaira, K.; Matsumoto, H.; Kurosumi, M.; Yokobori, T.; Kanai, Y.; Sekine, C.; Honda, C.; Katayama, A.; Furuya, M.; et al. Association of L-type amino acid transporter 1 (LAT1) with the immune system and prognosis in invasive breast cancer. Sci. Rep. 2022, 12, 2742. [Google Scholar] [CrossRef]
- Ichinoe, M.; Yanagisawa, N.; Mikami, T.; Hana, K.; Nakada, N.; Endou, H.; Okayasu, I.; Murakumo, Y. L-Type amino acid transporter 1 (LAT1) expression in lymph node metastasis of gastric carcinoma: Its correlation with size of metastatic lesion and Ki-67 labeling. Pathol. Res. Pract. 2015, 211, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Gutbier, S.; Kyriakou, S.; Schildknecht, S.; Uckert, A.K.; Brull, M.; Lewis, F.; Dickens, D.; Pearson, L.; Elson, J.L.; Michel, S.; et al. Design and evaluation of bi-functional iron chelators for protection of dopaminergic neurons from toxicants. Arch. Toxicol. 2020, 94, 3105–3123. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelaal, G.; Carter, A.; Cheung, W.; Panayiotidis, M.; Racey, S.; Tétard, D.; Veuger, S. Novel Iron Chelator SK4 Drives Cytotoxicity through Inhibiting Mitochondrial Metabolism in Ovarian and Triple Negative Breast Cancer Cell Lines. Biomedicines 2023, 11, 2073. https://doi.org/10.3390/biomedicines11072073
Abdelaal G, Carter A, Cheung W, Panayiotidis M, Racey S, Tétard D, Veuger S. Novel Iron Chelator SK4 Drives Cytotoxicity through Inhibiting Mitochondrial Metabolism in Ovarian and Triple Negative Breast Cancer Cell Lines. Biomedicines. 2023; 11(7):2073. https://doi.org/10.3390/biomedicines11072073
Chicago/Turabian StyleAbdelaal, Gina, Andrew Carter, William Cheung, Mihalis Panayiotidis, Seth Racey, David Tétard, and Stephany Veuger. 2023. "Novel Iron Chelator SK4 Drives Cytotoxicity through Inhibiting Mitochondrial Metabolism in Ovarian and Triple Negative Breast Cancer Cell Lines" Biomedicines 11, no. 7: 2073. https://doi.org/10.3390/biomedicines11072073
APA StyleAbdelaal, G., Carter, A., Cheung, W., Panayiotidis, M., Racey, S., Tétard, D., & Veuger, S. (2023). Novel Iron Chelator SK4 Drives Cytotoxicity through Inhibiting Mitochondrial Metabolism in Ovarian and Triple Negative Breast Cancer Cell Lines. Biomedicines, 11(7), 2073. https://doi.org/10.3390/biomedicines11072073






