A Clinical Scale for Rating the Severity of Bulbar Lower Motor Neuron Dysfunction in Amyotrophic Lateral Sclerosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Scale Development
2.2. Statistical Analysis
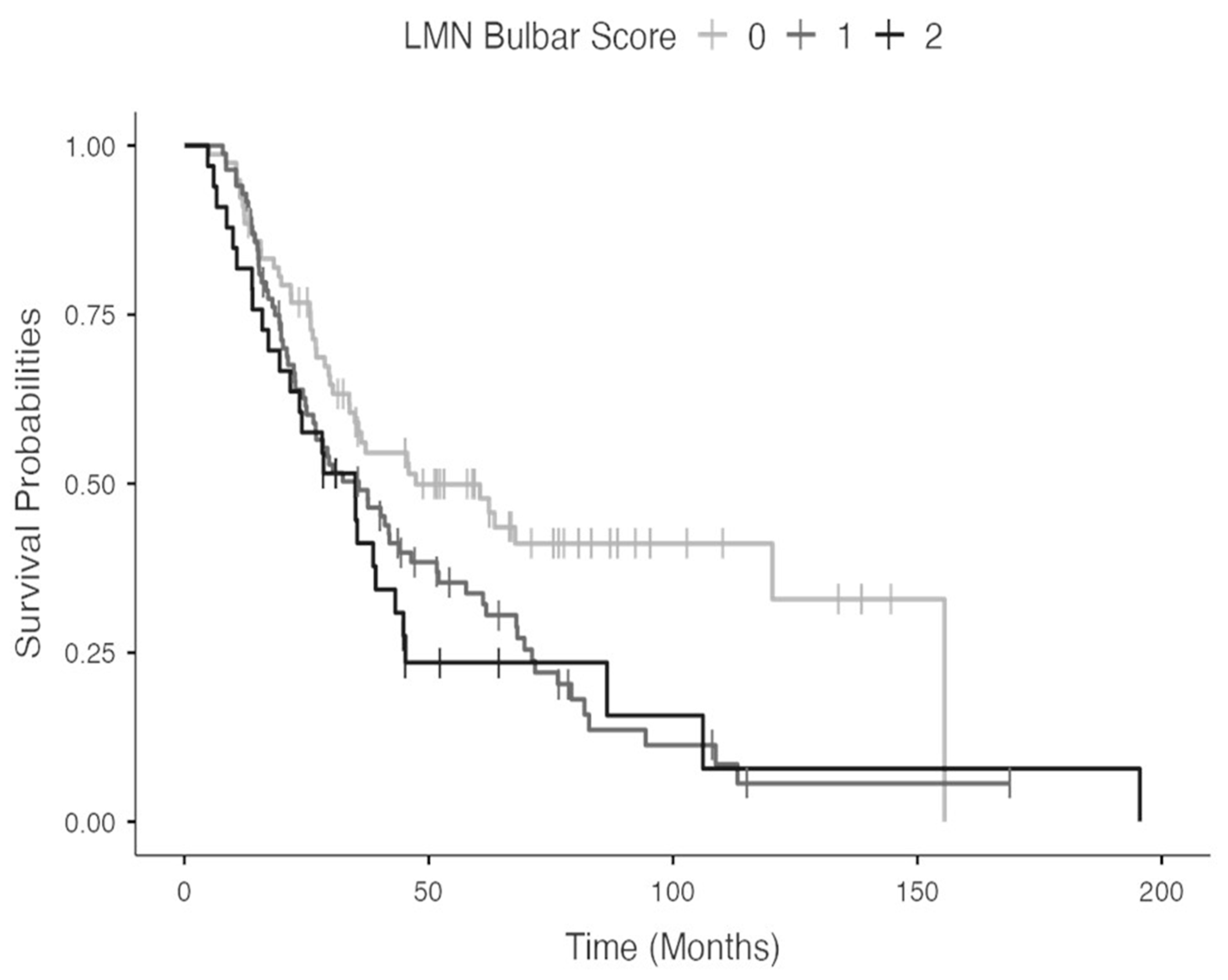
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kiernan, M.C.; Vucic, S.; Cheah, B.C.; Turner, M.R.; Eisen, A.; Hardiman, O.; Burrell, J.R.; Zoing, M.C. Amyotrophic lateral sclerosis. Lancet 2011, 377, 942–955. [Google Scholar] [CrossRef] [PubMed]
- Zoccolella, S.; Beghi, E.; Palagano, G.; Fraddosio, A.; Guerra, V.; Samarelli, V.; Lepore, V.; Simone, I.L.; Lamberti, P.; Serlenga, L.; et al. Predictors of long survival in amyotrophic lateral sclerosis: A population-based study. J. Neurol. Sci. 2008, 268, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Maranzano, A.; Verde, F.; Colombo, E.; Poletti, B.; Doretti, A.; Bonetti, R.; Gagliardi, D.; Meneri, M.; Maderna, L.; Messina, S.; et al. Regional spreading pattern is associated with clinical phenotype in amyotrophic lateral sclerosis. Brain 2023, awad129. [Google Scholar] [CrossRef] [PubMed]
- Milella, G.; Zoccolella, S.; Urso, D.; Nigro, S.; Tamburrino, L.; Gnoni, V.; Filardi, M.; Logroscino, G. Different patterns of spreading direction and motor neurons involvement in a cohort of limb-onset amyotrophic lateral sclerosis patients from Southern Italy: Potential implication on disease course or progression? Brain Behav. 2023, 13, e2899. [Google Scholar] [CrossRef] [PubMed]
- Chiò, A.; Logroscino, G.; Hardiman, O.; Swingler, R.; Mitchell, D.; Beghi, E.; Traynor, B.G.; Eurals Consortium. Prognostic factors in ALS: A critical review. Amyotroph. Lateral Scler. 2009, 10, 310–323. [Google Scholar] [CrossRef] [PubMed]
- Milella, G.; Zoccolella, S.; Giugno, A.; Filardi, M.; Urso, D.; Nigro, S.; Tafuri, B.; Tamburrino, L.; Gnoni, V.; Logroscino, G. The impact of upper and lower motor neuron burden on diagnostic certainty, and clinical course of spinal-onset amyotrophic lateral sclerosis: A cluster-based approach. J. Neurol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Sabatelli, M.; Zollino, M.; Luigetti, M.; Grande, A.D.; Lattante, S.; Marangi, G.; Monaco, M.L.; Madia, F.; Meleo, E.; Bisogni, G.; et al. Uncovering amyotrophic lateral sclerosis phenotypes: Clinical features and long-term follow-up of upper motor neuron-dominant ALS. Amyotroph. Lateral Scler. 2011, 12, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Devine, M.S.; Ballard, E.; O’Rourke, P.; Kiernan, M.C.; Mccombe, P.A.; Henderson, R.D. Targeted assessment of lower motor neuron burden is associated with survival in amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Front. Degener. 2016, 17, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Quinn, C.; Edmundson, C.; Dahodwala, N.; Elman, L. Reliable and efficient scale to assess upper motor neuron disease burden in amyotrophic lateral sclerosis. Muscle Nerve 2020, 61, 508–511. [Google Scholar] [CrossRef] [PubMed]
- Brooks, B.R. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. Subcommittee on Motor Neuron Diseases/Amyotrophic Lateral Sclerosis of the World Federation of Neurology Research Group on Neuromuscular Diseases and the El Escorial “Clinical limits of amyotrophic lateral sclerosis” workshop contributors. J. Neurol. Sci. 1994, 124, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Cedarbaum, J.M.; Stambler, N.; Malta, E.; Fuller, C.; Hilt, D.; Thurmond, B.; Nakanishi, A.; BDNF ALS Study Group (Phase III). The ALSFRS-R: A revised ALS functional rating scale that incorporates assessments of respiratory function. J. Neurol. Sci. 1999, 169, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Roche, J.C.; Rojas-Garcia, R.; Scott, K.M.; Scotton, W.; Ellis, C.E.; Burman, R.; Wijesekera, L.; Turner, M.R.; Leigh, P.N.; Shaw, C.E.; et al. A proposed staging system for amyotrophic lateral sclerosis. Brain 2012, 135, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Tramacere, I.; Dalla Bella, E.; Chiò, A.; Mora, G.; Filippini, G.; Lauria, G.; EPOS Trial Study Group. The MITOS system predicts long-term survival in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 2015, 86, 1180–1185. [Google Scholar] [CrossRef] [PubMed]
- Armon, C.; Moses, D. Linear estimates of rates of disease progression as predictors of survival in patients with ALS entering clinical trials. J. Neurol. Sci. 1998, 160 (Suppl. S1), S37–S41. [Google Scholar] [CrossRef] [PubMed]
- Colombo, E.; Doretti, A.; Scheveger, F.; Maranzano, A.; Pata, G.; Gagliardi, D.; Meneri, M.; Messina, S.; Verde, F.; Morelli, C.; et al. Correlation between clinical phenotype and electromyographic parameters in amyotrophic lateral sclerosis. J. Neurol. 2023, 270, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Yunusova, Y.; Plowman, E.K.; Green, J.R.; Barnett, C.; Bede, P. Clinical Measures of Bulbar Dysfunction in ALS. Front. Neurol. 2019, 10, 106. [Google Scholar] [CrossRef] [PubMed]
- Beghi, E.; Pupillo, E.; Bianchi, E.; Bonetto, V.; Luotti, S.; Pasetto, L.; Bendotti, C.; Tortarolo, M.; Sironi, F.; Camporeale, L.; et al. Effect of RNS60 in amyotrophic lateral sclerosis: A phase II multicentre, randomized, double-blind, placebo-controlled trial. Eur. J. Neurol. 2023, 30, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Edaravone (MCI-186) ALS 16 Study Group. A post-hoc subgroup analysis of outcomes in the first phase III clinical study of edaravone (MCI-186) in amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Front. Degener. 2017, 18, 11–19. [Google Scholar] [CrossRef] [PubMed]
| LMN Signs | Score |
|---|---|
| Not clinically significant | 0 |
| Tongue atrophy and/or fasciculations | 1 |
| Score (1) + tongue hypomobility | 2 |
| Score (2) + jaw and/or face weakness | 3 |
| Male ALS Patients (n = 106) | Female ALS Patients (n = 89) | p Value | ||
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | |||
| Age, y | 62.7 ± 11.5 | 62.5 ± 11.3 | ns | |
| Disease duration, months | 22.2 ± 22 | 21 ± 18.6 | ns | |
| Diagnostic category * | ||||
| Clinically definite ALS | 35 (33%) | 34 (38.2%) | <0.05 | |
| Clinically probable ALS | 19 (17.9%) | 28 (31.5%) | ||
| Clinically possible ALS | 41 (38.7%) | 20 (22.5%) | ||
| Clinically suspect ALS | 11 (10.4%) | 7 (7.9%) | ||
| Type of onset | ||||
| Bulbar | 29 (27.4%) | 37 (41.6%) | <0.05 | |
| Spinal | 77 (72.6%) | 52 (58.4%) | ||
| ALSFRS-R | 35.9 ± 9 | 35.3 ± 7.6 | ns | |
| ALSFRS-R Bulbar subscore | 9 ± 3.1 | 8.7 ± 3.2 | ns | |
| King’s staging | ||||
| Stage 0 | 1 (0.9%) | 2 (2.2%) | ns | |
| Stage 1 | 12 (11.3%) | 8 (9%) | ||
| Stage 2 | 27 (25.5%) | 14 (15.7%) | ||
| Stage 3 | 35 (33%) | 35 (39.3%) | ||
| Stage 4 | 31 (29.2%) | 30 (33.7) | ||
| Milano–Torino staging (MiToS) | ||||
| Stage 0 | 23 (21.7%) | 5 (5.6%) | <0.05 | |
| Stage 1 | 37 (34.9%) | 48 (53.9%) | ||
| Stage 2 | 11 (10.4%) | 16 (18%) | ||
| Stage 3 | 17 (16%) | 11 (12.4%) | ||
| Stage 4 | 18 (17%) | 9 (10.1%) | ||
| Rater 1 | Rater 2 | Rater 3 | Rater 4 | Rater 5 | Rater 6 | Rater 7 | Rater 8 | Rater 9 | Rater 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Rater 1 | - | 0.943 | 0.959 | 0.967 | 0.902 | 0.975 | 0.853 | 0.894 | 0.901 | 0.859 |
| Rater 2 | 0.943 | - | 0.918 | 0.934 | 0.910 | 0.935 | 0.878 | 0.935 | 0.926 | 0.868 |
| Rater 3 | 0.959 | 0.918 | - | 0.942 | 0.910 | 0.934 | 0.861 | 0.869 | 0.892 | 0.850 |
| Rater 4 | 0.967 | 0.934 | 0.942 | - | 0.894 | 0.942 | 0.845 | 0.885 | 0.892 | 0.850 |
| Rater 5 | 0.902 | 0.910 | 0.910 | 0.894 | - | 0.894 | 0.919 | 0.878 | 0.885 | 0.860 |
| Rater 6 | 0.975 | 0.935 | 0.934 | 0.942 | 0.894 | - | 0.861 | 0.886 | 0.893 | 0.834 |
| Rater 7 | 0.853 | 0.878 | 0.861 | 0.845 | 0.919 | 0.861 | - | 0.845 | 0.868 | 0.844 |
| Rater 8 | 0.894 | 0.935 | 0.869 | 0.885 | 0.878 | 0.886 | 0.845 | - | 0.893 | 0.835 |
| Rater 9 | 0.901 | 0.926 | 0.892 | 0.892 | 0.885 | 0.893 | 0.868 | 0.893 | - | 0.866 |
| Rater 10 | 0.859 | 0.868 | 0.850 | 0.850 | 0.860 | 0.834 | 0.844 | 0.835 | 0.866 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zoccolella, S.; Giugno, A.; Milella, G.; Filardi, M.; Introna, A.; Fraddosio, A.; D’Errico, E.; Gnoni, V.; Tamburrino, L.; Urso, D.; et al. A Clinical Scale for Rating the Severity of Bulbar Lower Motor Neuron Dysfunction in Amyotrophic Lateral Sclerosis. Biomedicines 2023, 11, 2039. https://doi.org/10.3390/biomedicines11072039
Zoccolella S, Giugno A, Milella G, Filardi M, Introna A, Fraddosio A, D’Errico E, Gnoni V, Tamburrino L, Urso D, et al. A Clinical Scale for Rating the Severity of Bulbar Lower Motor Neuron Dysfunction in Amyotrophic Lateral Sclerosis. Biomedicines. 2023; 11(7):2039. https://doi.org/10.3390/biomedicines11072039
Chicago/Turabian StyleZoccolella, Stefano, Alessia Giugno, Giammarco Milella, Marco Filardi, Alessandro Introna, Angela Fraddosio, Eustachio D’Errico, Valentina Gnoni, Ludovica Tamburrino, Daniele Urso, and et al. 2023. "A Clinical Scale for Rating the Severity of Bulbar Lower Motor Neuron Dysfunction in Amyotrophic Lateral Sclerosis" Biomedicines 11, no. 7: 2039. https://doi.org/10.3390/biomedicines11072039
APA StyleZoccolella, S., Giugno, A., Milella, G., Filardi, M., Introna, A., Fraddosio, A., D’Errico, E., Gnoni, V., Tamburrino, L., Urso, D., Caputo, F., Misceo, S., & Logroscino, G. (2023). A Clinical Scale for Rating the Severity of Bulbar Lower Motor Neuron Dysfunction in Amyotrophic Lateral Sclerosis. Biomedicines, 11(7), 2039. https://doi.org/10.3390/biomedicines11072039








