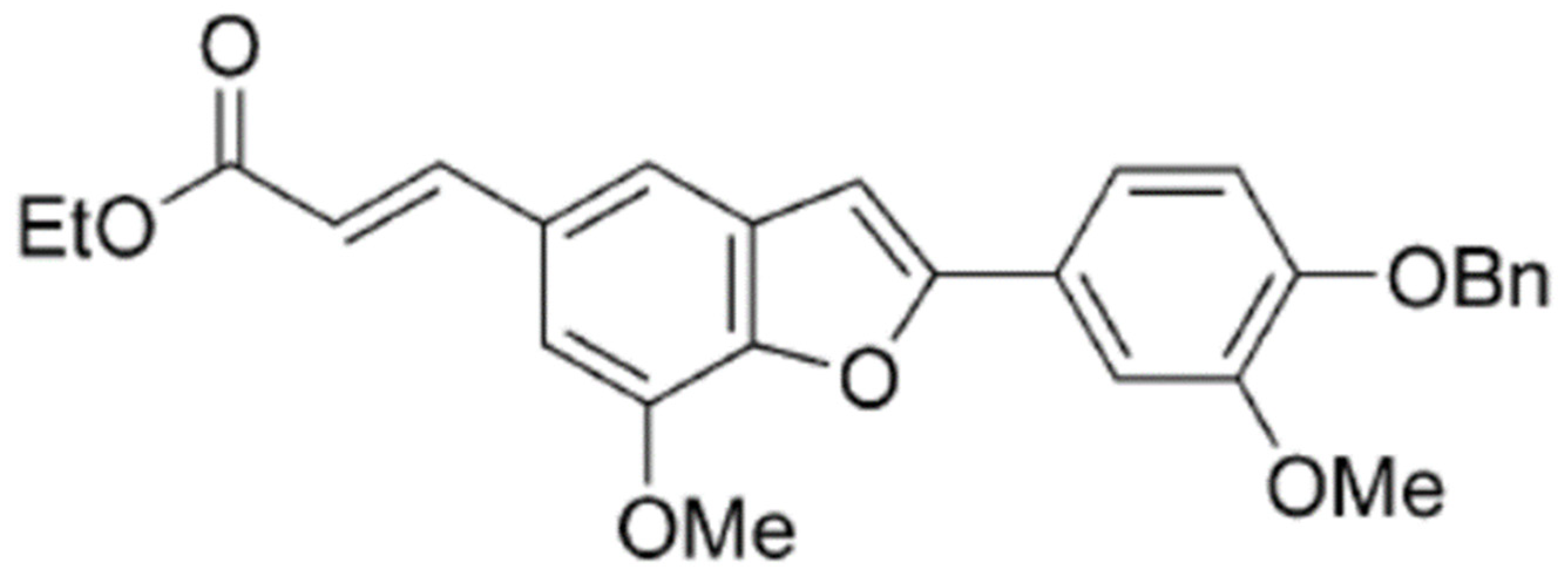
2-(4-Benzyloxy-3-methoxyphenyl)-5-(carbethoxyethylene)-7-methoxy-benzofuran, a Benzofuran Derivative, Suppresses Metastasis Effects in P53-Mutant Hepatocellular Carcinoma Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture and Cell Viability Assay
2.3. Microscopic Examination
2.4. Scratch Motility Assay
2.5. Cell Migration and Invasion Assay
2.6. Preparation of Total Cell Extracts and Immunoblot Analysis
2.7. Transfection of p53siRNA
2.8. Statistical Analysis
3. Results
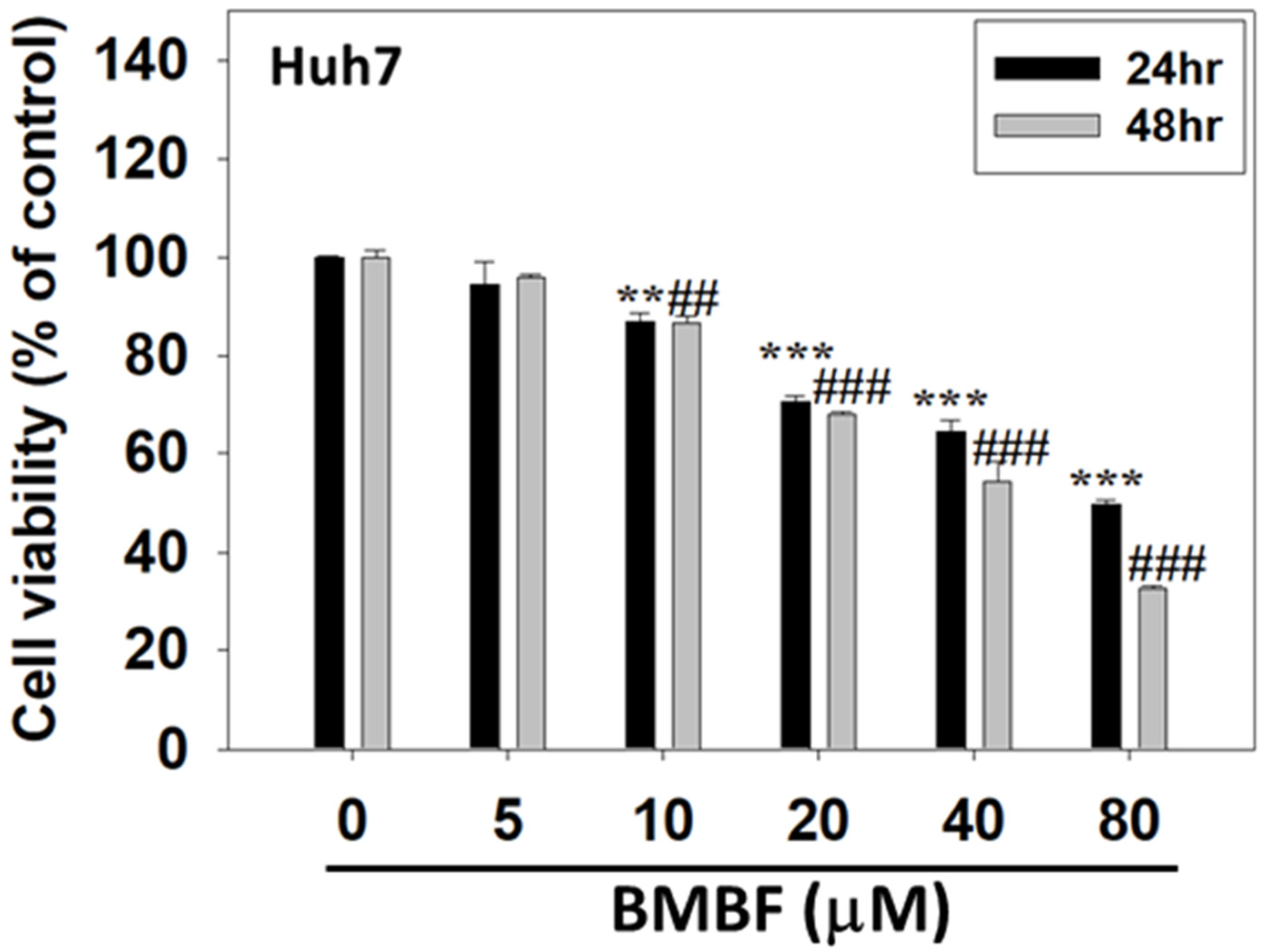
3.1. BMBF Suppressed the Viability of Huh7 Cells
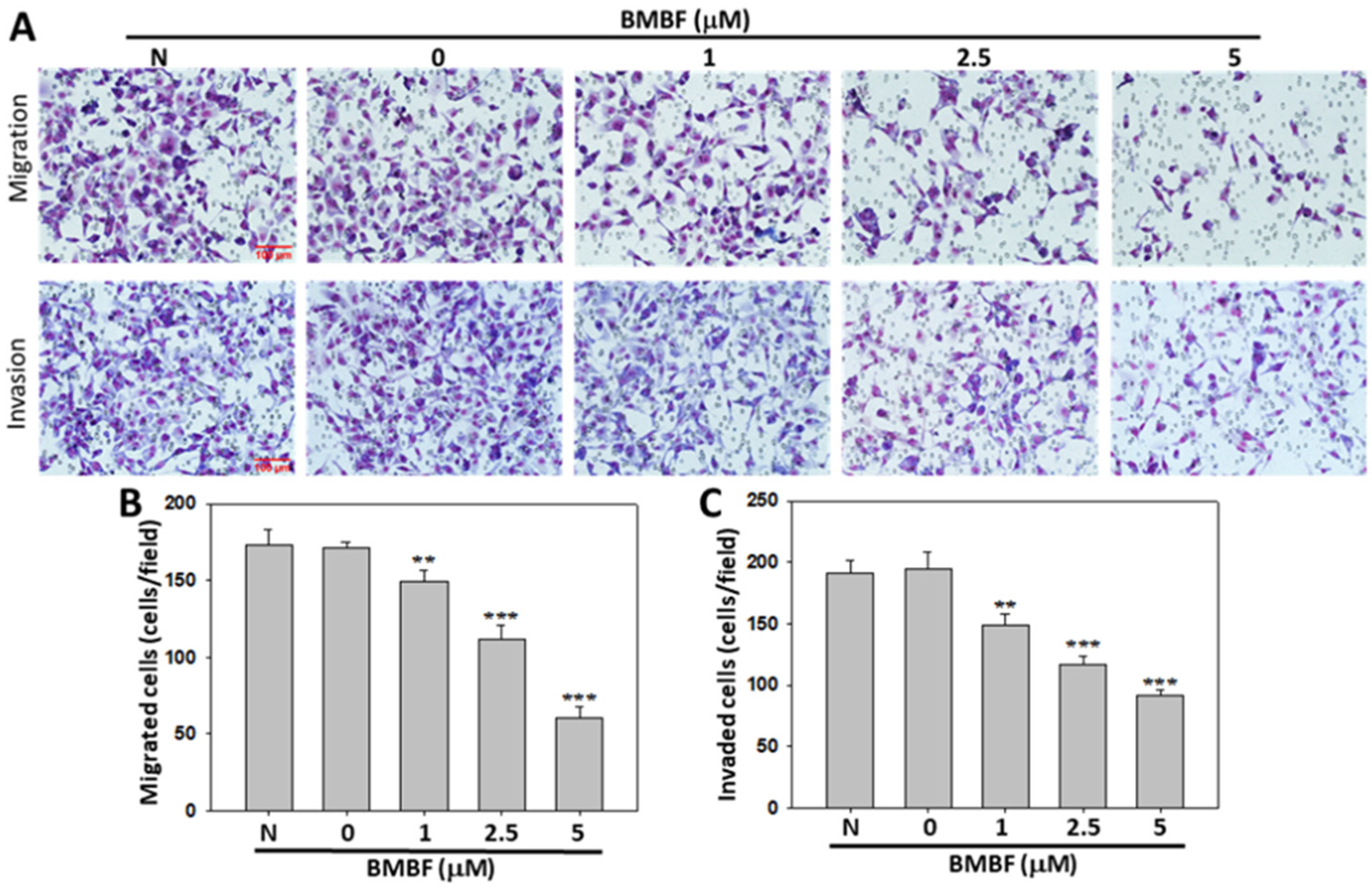
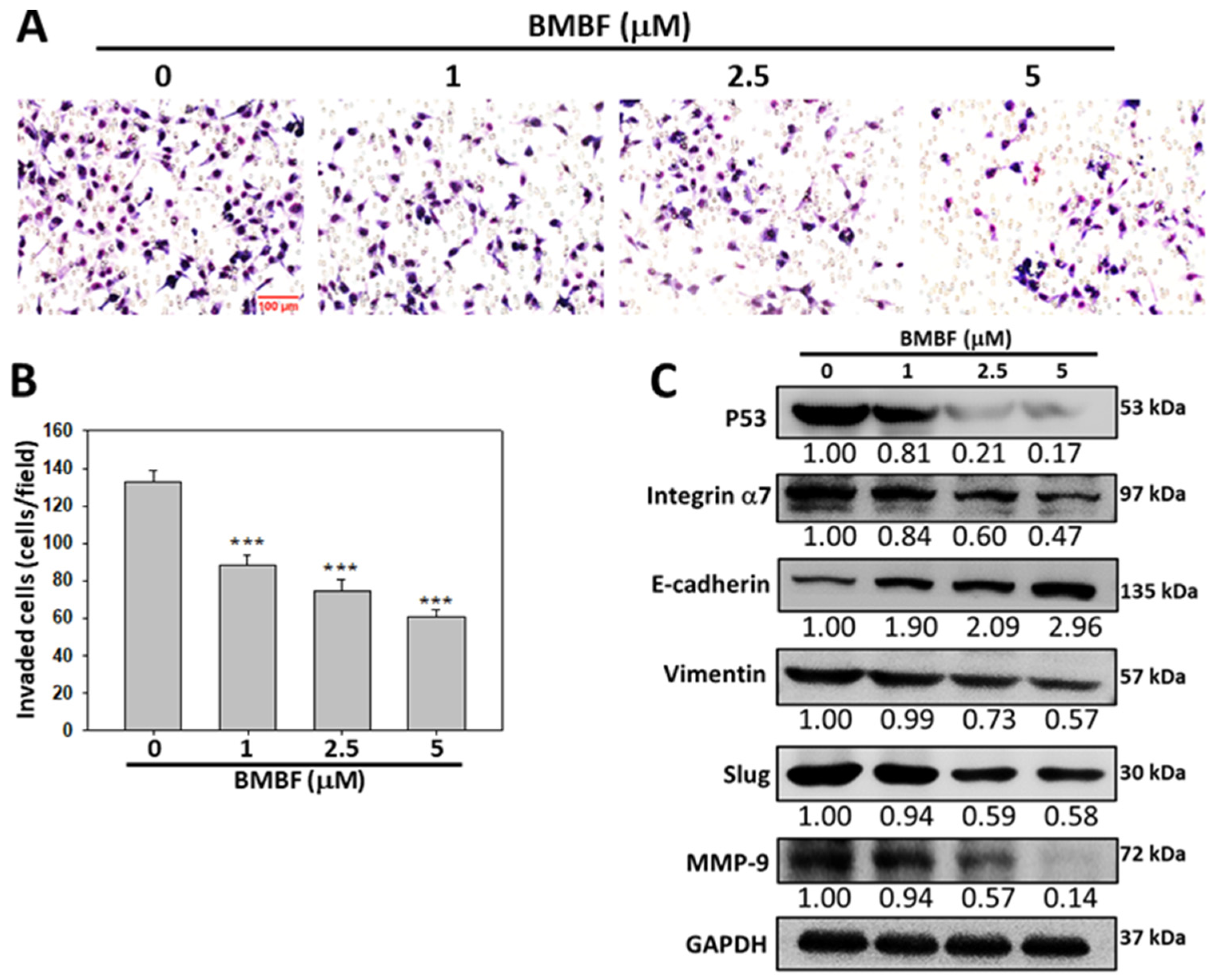
3.2. BMBF Reduced the Cytoskeletal Changes and Inhibited Motility, Migration, and Invasion in Huh7 Cells
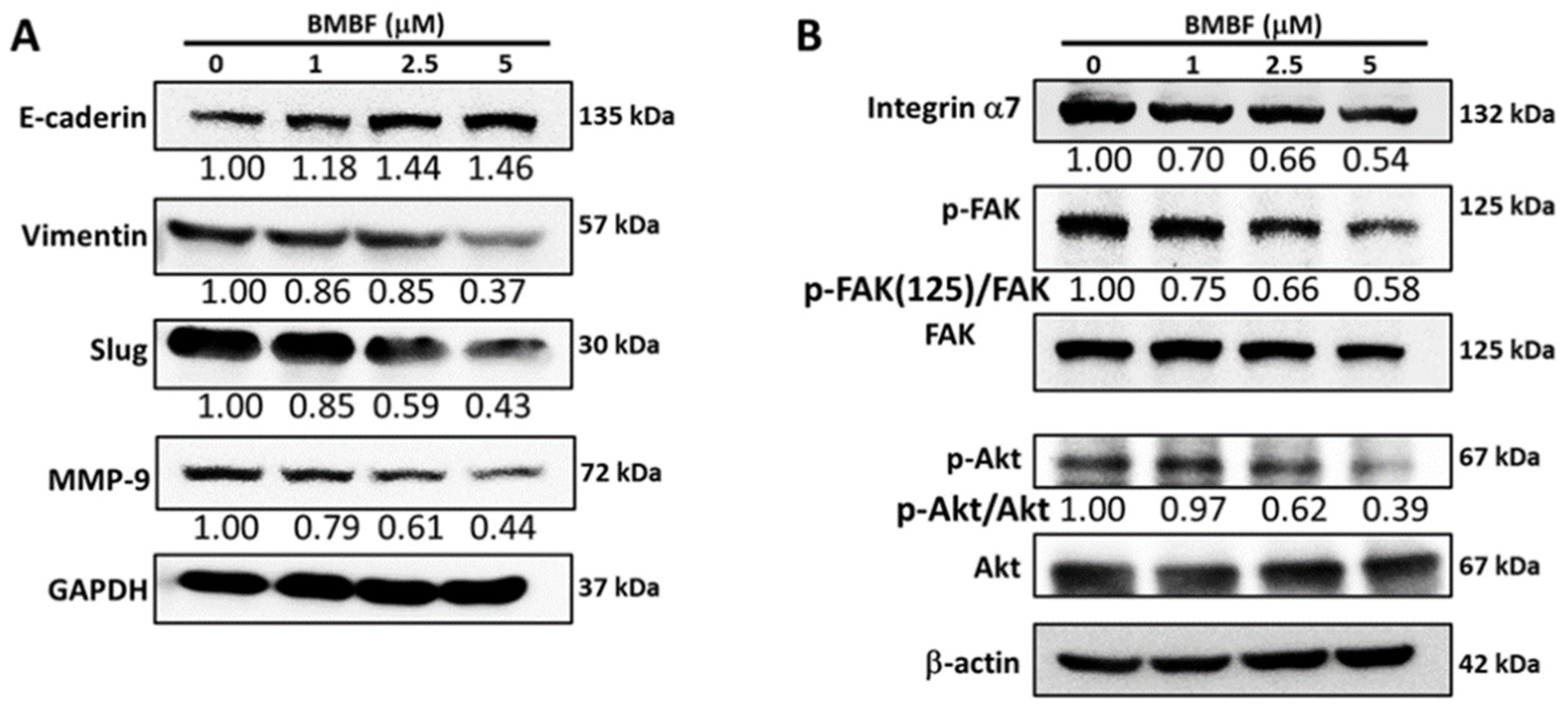
3.3. Inhibitory Effects of BMBF on EMT-Related Proteins and Integrin α7 in Huh7 Cells
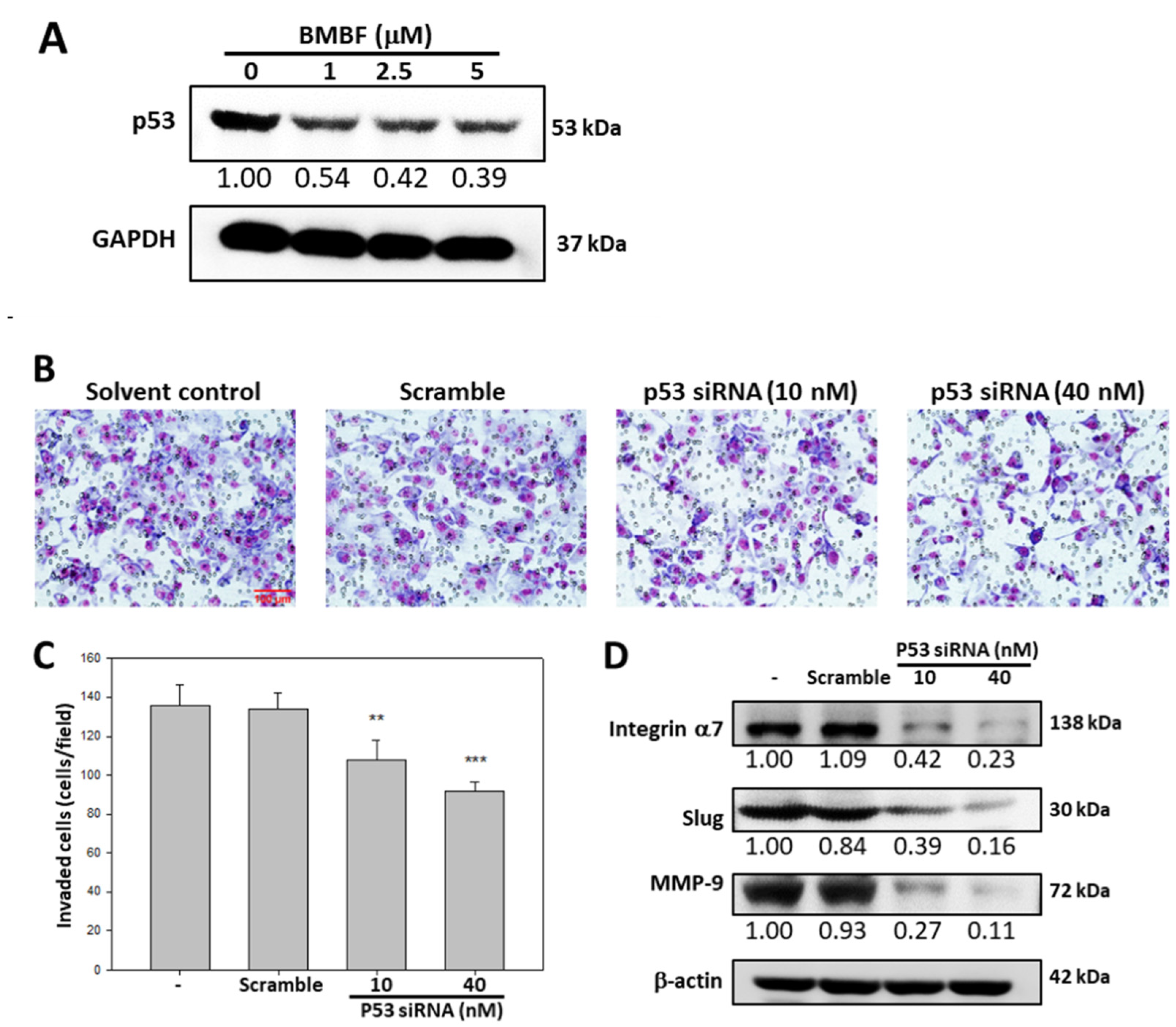
3.4. BMBF Suppressed the Invasion in Huh7 Cells with p53 Knockdown
3.5. Anti-Invasion of BMBF in PLC/PRF/5 cells
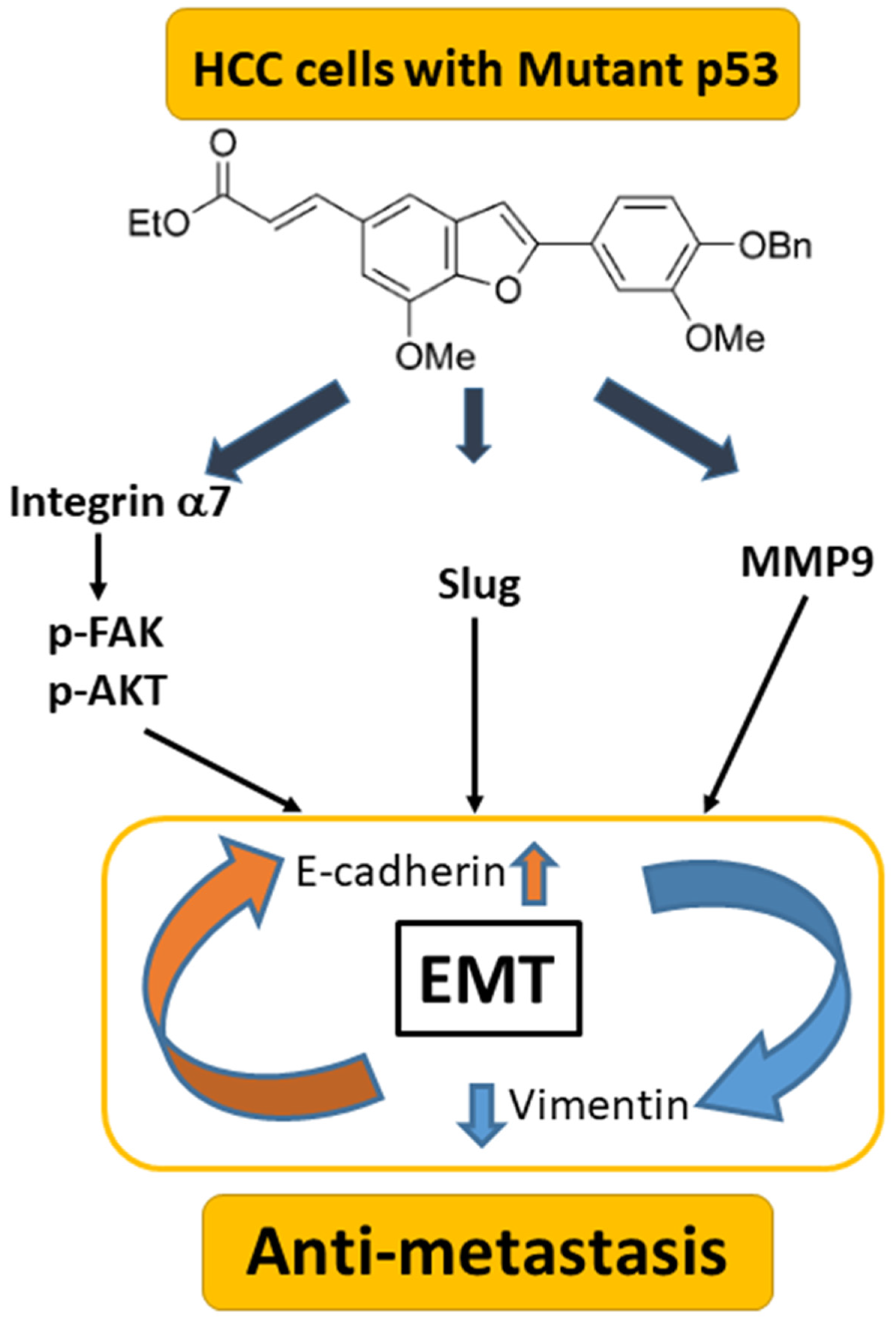
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef] [PubMed]
- Pastushenko, I.; Blanpain, C. EMT transition states during tumor progression and metastasis. Trends Cell Biol. 2019, 29, 212–226. [Google Scholar] [CrossRef]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Shu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef]
- Bertuccio, P.; Turati, F.; Carioli, G.; Rodriguez, T.; Vecchia, C.L.; Malvezzi, M.; Negri, E. Global trends and predictions in hepatocellular carcinoma mortality. J. Hepatol. 2017, 67, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Giannini, E.G.; Bucci, L.; Garuti, F.; Brunacci, M.; Lenzi, B.; Valente, M.; Caturelli, E.; Cabibbo, G.; Piscaglia, F.; Virdone, R.; et al. Patients with advanced hepatocellular carcinoma need a personalized management: A lesson from clinical practice. Hepatology 2018, 67, 1784–1796. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Wang, A.; Bai, Y.; Lin, J.; Yang, X.; Wang, D.; Yang, X.; Jiang, Y.; Zhao, H. Development and validation of a TP53-associated immune prognostic model for hepatocellular carcinoma. EBioMedicine 2019, 42, 363–374. [Google Scholar] [CrossRef]
- Brosh, R.; Rotter, V. When mutants gain new powers: News from the mutant P53 field. Nat. Rev. Cancer 2009, 9, 701–713. [Google Scholar] [CrossRef]
- Roszkowska, K.A.; Piecuch, A.; Sady, M.; Gajewski, Z.; Olszewski, M.B. Gain-of-function mutations in p53 in cancer invasiveness and metastasis. Int. J. Mol. Sci. 2020, 21, 1334. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, Y.; Guan, D.; Li, J.; Yin, H.; Pan, Y.; Xie, D.; Chen, Y. Critical roles of p53 in epithelial-mesenchymal transition and metastasis of hepatocellular carcinoma cells. PLoS ONE 2013, 8, e72846. [Google Scholar] [CrossRef]
- Ohashi, S.; Natsuizaka, M.; Wong, G.S.; Michaylira, C.Z.; Grugan, K.D.; Stairs, D.B.; Kalabis, J.; Vega, M.E.; Kalman, R.A.; Nakagama, M.; et al. Epidermal growth factor receptor and mutant p53 expand an esophageal cellular subpopulation capable of epithelial-to-mesenchymal transition through ZEB transcription factors. Cancer Res. 2010, 70, 4174–4184. [Google Scholar] [CrossRef]
- Lenfert, E.; Maenz, C.; Heinlein, C.; Jannasch, K.; Schumacher, U.; Pantel, K.; Tolstonog, G.V.; Deppert, W.; Wegwitz, F. Mutant p53 promotes epithelial-mesenchymal plasticity and enhances metastasis in mammary carcinomas of WAP-T mice. Int. J. Cancer 2015, 136, E521–E533. [Google Scholar] [CrossRef]
- Semenov, O.; Daks, A.; Fedorova, O.; Shuvalov, O.; Barlev, N.A. Opposing roles of wild-type and mutant p53 in the process of epithelial to mesenchymal transition. Front. Mol. Biosci. 2022, 9, 928399. [Google Scholar] [CrossRef] [PubMed]
- Nevagi, R.J.; Dighe, S.N.; Dighe, S.N. Biological and medicinal significance of benzofuran. Eur. J. Med. Chem. 2015, 97, 561–581. [Google Scholar] [CrossRef] [PubMed]
- Khanam, H.; Shamsuzzaman. Bioactive benzofuran derivatives: A review. Eur. J. Med. Chem. 2015, 97, 483–504. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.Y.; Chen, C.L.; Lee, Y.J. Total synthesis of ailanthoidol and precursor XH14 by Stille coupling. J. Org. Chem. 2003, 68, 2968–2971. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kao, E.S.; Chu, C.Y.; Lin, W.L.; Chiou, Y.H.; Tseng, T.H. Inhibitory effect of ailanthoidol on 12-O-tetradecanoyl-phorbol-13-acetate-induced tumor promotion in mouse skin. Oncol. Rep. 2006, 16, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Tseng, T.H.; Lee, H.J.; Lee, Y.J.; Lee, K.C.; Shen, C.H.; Kuo, H.C. Ailanthoidol, a neolignan, suppresses TGF-β1-induced HepG2 heptoblastoma cell progression. Biomedicines 2021, 9, 1110. [Google Scholar] [CrossRef]
- Tseng, T.H.; Wang, C.J.; Lee, Y.J.; Shao, Y.C.; Shen, C.H.; Lee, K.C.; Tung, S.Y.; Kuo, H.C. Suppression of the proliferation of Huh7 hepatoma cells involving the downregulation of mutant p53 protein and inactivation of the STAT 3 pathway with ailanthoidol. Int. J. Mol. Sci. 2022, 23, 5102. [Google Scholar] [CrossRef]
- Fife, C.M.; McCarroll, J.A.; Kavallaris, M. Movers and shakers: Cell cytoskeleton in cancer metastasis. Br. J. Pharmacol. 2014, 171, 5507–5523. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef]
- van Zijl, F.; Zulehner, G.; Petz, M.; Schneller, D.; Kornauth, C.; Hau, M.; Machat, G.; Grubinger, M.; Huber, H.; Mikulits, W. Epithelial-mesenchymal transition in hepatocellular carcinoma. Future Oncol 2009, 5, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Scheau, C.; Badarau, I.A.; Costache, R.; Caruntu, C.; Mihai, G.L.; Didilescu, A.C.; Constantin, C.; Neagu, M. The role of matrix metalloproteinases in the epithelial-mesenchymal transition of hepatocellular carcinoma. Anal. Cell. Pathol. 2019, 2019, 9423907. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, H.; Ivaska, J. Every step of the way: Integrins in cancer progression and metastasis. Nat. Rev. Cancer 2018, 18, 533–548. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Wang, Y.; Chen, Z.; Chen, D. Integrin alpha 7 correlates with poor clinical outcomes, and it regulates cell proliferation, apoptosis and stemness via PTK2-PI3K-Akt signaling pathway in hepatocellular carcinoma. Cell. Signal. 2020, 66, 109465. [Google Scholar] [CrossRef]
- Wu, Z.; Kong, X.; Wang, Z. Integrin α7 knockdown suppresses cell proliferation, migration, invasion and EMT in hepatocellular carcinoma. Exp. Ther. Med. 2021, 21, 309. [Google Scholar] [CrossRef]
- Guttilla, R.I. Mechanism and regulation of epithelial–mesenchymal transition in cancer. Cell Health Cytoskelet. 2015, 7, 155–166. [Google Scholar] [CrossRef]
- Vijayakumaran, R.; Tan, K.H.; Miranda, P.J.; Haupt, S.; Haupt, Y. Regulation of mutant p53 protein expression. Front. Oncol. 2015, 5, 284. [Google Scholar] [CrossRef]
- Ming, X.Y.; Li, F.; Zhang, L.Y.; Qin, Y.R.; Cao, T.T.; Chan, K.W.; Ma, S.; Xie, D.; Guan, X.Y. Integrin α7 is a functional cancer stem cell surface marker in oesophageal squamous cell carcinoma. Nat. Commun. 2016, 7, 13568. [Google Scholar] [CrossRef]
- Nunes, A.M.; Barraza-Flores, P.; Smith, C.R.; Burkin, D.J. Integrin α7: A major driver and therapeutic target for glioblastoma malignancy. Stem Cell Investig. 2017, 4, 97. [Google Scholar] [CrossRef]
- Burkin, D.J.; Fontelonga, T.M. Mesothelioma cells breaking bad: Loss of integrin α7 promotes cell motility and poor clinical outcomes in patients. J. Pathol. 2015, 237, 282–284. [Google Scholar] [CrossRef]
- Haas, T.L.; Sciuto, M.R.; Brunetto, L.; Valvo, C.; Signore, M.; Fiori, M.E.; di Martino, S.; Giannetti, S.; Morgante, L.; Boe, A.; et al. Integrin α7 Is a Functional Marker and Potential Therapeutic Target in Glioblastoma. Cell Stem Cell 2017, 21, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.R.; Wei, J.; Mo, X.; Yuan, Z.; Wang, J.; Zhang, C.; Xie, Y.; You, Q.; Sun, H. Discovery and optimization of new benzofuran derivatives against p53-independent malignant cancer cells through inhibition of HIF-1 pathway. Bioorg. Med. Chem. Lett. 2016, 26, 2713–2718. [Google Scholar] [CrossRef]
- Gupta, G.P.; Massagué, J. Review cancer metastasis: Building a framework. Cell 2006, 17, 679–695. [Google Scholar] [CrossRef]
- Roger, L.; Jullien, L.; Gire, V.; Roux, P. Gain of oncogenic function of p53 mutants regulates E-cadherin expression uncoupled from cell invasion in colon cancer cells. J. Cell Sci. 2010, 123, 1295–1305. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Wang, Z.; Fu, J.; Ji, L.; Liu, J.; Li, L.; Wang, H.; Chen, J.; Caulin, C.; Myers, J.N.; et al. Differential regulation of the REGγ-proteasome pathway by p53/TGF-β signalling and mutant p53 in cancer cells. Nat. Commun. 2013, 4, 2667. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Xu, J.; Liu, J.; Amjad, A.; Zhang, K.; Liu, Q.; Zhou, L.; Xiao, J.; Li, X. Mutant p53 promotes tumor cell malignancy by both positive and negative regulation of the transforming growth factor β (TGF-β) pathway. J. Biol. Chem. 2015, 290, 11729–11740. [Google Scholar] [CrossRef] [PubMed]
- Muller, P.; Caswell, P.; Doyle, B.; Iwanicki, M.; Tan, E.; Karim, S.; Lukashchuk, N.; Gillespie, D.; Ludwig, R.; Gosselin, P.; et al. Mutant p53 drives invasion by promoting integrin recycling. Cell 2009, 139, 1327–1341. [Google Scholar] [CrossRef]
- Vogiatzi, F.; Brandt, D.T.; Schneikert, J.; Fuchs, J.; Grikscheit, K.; Wanzel, M.; Pavlakis, E.; Charles, J.P.; Timofeev, O.; Nist, A.; et al. Mutant p53 promotes tumor progression and metastasis by the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA 2016, 53, E8433–E8442. [Google Scholar] [CrossRef]
- Weissmueller, S.; Manchado, E.; Saborowski, M.; Morris, J.P., IV; Wagenblast, E.; Davis, C.A.; Moon, S.-H.; Pfister, N.T.; Tschaharganeh, D.F.; Kitzing, T.; et al. Mutant p53 drives pancreatic cancer metastasis through cell-autonomous PDGF receptor β signaling. Cell 2014, 157, 382–394. [Google Scholar] [CrossRef]
- Grugan, K.D.; Vega, M.E.; Wong, G.S.; Diehl, J.A.; Bass, A.J.; Wong, K.K.; Nakagawa, H.; Rustgi, A.K. A common p53 mutation (R175H) activates c-Met receptor tyrosine kinase to enhance tumor cell invasion. Cancer Biol. Ther. 2013, 14, 835–839. [Google Scholar] [CrossRef]
- Novo, D.; Heath, N.; Mitchell, L.; Caligiuri, G.; MacFarlane, A.; Reijmer, D.; Charlton, L.; Knight, J.; Calka, M.; McGhee, E.; et al. Mutant p53s generate pro-invasive niches by influencing exosome podocalyxin levels. Nat. Commun. 2018, 9, 5069. [Google Scholar] [CrossRef] [PubMed]
- Cooks, T.; Pateras, I.S.; Jenkins, L.M.; Patel, K.M.; Robles, A.I.; Morris, J.; Forshew, T.; Appella, E.; Gorgoulis, V.G.; Harris, C.C. Mutant p53 cancers reprogram macrophages to tumor supporting macrophages via exosomal miR-1246. Nat. Commun. 2018, 9, 771. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Hao, Q.; Lu, H. Mutant p53 in cancer therapy—The barrier or the path. J. Mol. Cell Biol. 2019, 11, 293–305. [Google Scholar] [CrossRef]
- Tschaharganeh, D.F.; Xue, W.; Calvisi, D.F.; Evert, M.; Michurina, T.V.; Dow, L.E.; Banito, A.; Katz, S.F.; Kastenhuber, E.R.; Weissmueller, S.; et al. p53-Dependent nestin regulation links tumor suppression to cellular plasticity in liver cancer. Cell 2014, 158, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Hu, Y.; Yang, J.; Liu, T.; Sun, J.; Wang, X. Natural source, bioactivity and synthesis of benzofuran derivatives. RSC Adv. 2019, 9, 27510–27540. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tseng, T.-H.; Shao, Y.-C.; Lee, Y.-J.; Lee, H.-J. 2-(4-Benzyloxy-3-methoxyphenyl)-5-(carbethoxyethylene)-7-methoxy-benzofuran, a Benzofuran Derivative, Suppresses Metastasis Effects in P53-Mutant Hepatocellular Carcinoma Cells. Biomedicines 2023, 11, 2027. https://doi.org/10.3390/biomedicines11072027
Tseng T-H, Shao Y-C, Lee Y-J, Lee H-J. 2-(4-Benzyloxy-3-methoxyphenyl)-5-(carbethoxyethylene)-7-methoxy-benzofuran, a Benzofuran Derivative, Suppresses Metastasis Effects in P53-Mutant Hepatocellular Carcinoma Cells. Biomedicines. 2023; 11(7):2027. https://doi.org/10.3390/biomedicines11072027
Chicago/Turabian StyleTseng, Tsui-Hwa, Yi-Chia Shao, Yean-Jang Lee, and Huei-Jane Lee. 2023. "2-(4-Benzyloxy-3-methoxyphenyl)-5-(carbethoxyethylene)-7-methoxy-benzofuran, a Benzofuran Derivative, Suppresses Metastasis Effects in P53-Mutant Hepatocellular Carcinoma Cells" Biomedicines 11, no. 7: 2027. https://doi.org/10.3390/biomedicines11072027
APA StyleTseng, T.-H., Shao, Y.-C., Lee, Y.-J., & Lee, H.-J. (2023). 2-(4-Benzyloxy-3-methoxyphenyl)-5-(carbethoxyethylene)-7-methoxy-benzofuran, a Benzofuran Derivative, Suppresses Metastasis Effects in P53-Mutant Hepatocellular Carcinoma Cells. Biomedicines, 11(7), 2027. https://doi.org/10.3390/biomedicines11072027




