Abstract
Peptides mediate cancer progression favoring the mitogenesis, migration, and invasion of tumor cells, promoting metastasis and anti-apoptotic mechanisms, and facilitating angiogenesis/lymphangiogenesis. Tumor cells overexpress peptide receptors, crucial targets for developing specific treatments against cancer cells using peptide receptor antagonists and promoting apoptosis in tumor cells. Opioids exert an antitumoral effect, whereas others promote tumor growth and metastasis. This review updates the findings regarding the involvement of opioid peptides (enkephalins, endorphins, and dynorphins) in cancer development. Anticancer therapeutic strategies targeting the opioid peptidergic system and the main research lines to be developed regarding the topic reviewed are suggested. There is much to investigate about opioid peptides and cancer: basic information is scarce, incomplete, or absent in many tumors. This knowledge is crucial since promising anticancer strategies could be developed alone or in combination therapies with chemotherapy/radiotherapy.
Keywords:
enkephalin; endorphin; dynorphin; opioid receptor; apoptosis; metastasis; cancer progression; tumor cell; angiogenesis 1. Introduction
New molecular targets and compounds that specifically destroy tumor cells must be investigated to reach higher cure rates and a better quality of life for cancer patients. The peptidergic systems are one of the promising targets that open up new research lines and possibilities to explore new antitumor therapies and improve cancer diagnosis. These systems have attracted increasing interest because many peptides mediate cancer progression [1,2,3,4,5,6,7]. Peptides, through autocrine, paracrine, and endocrine mechanisms, favor the mitogenesis, migration, and invasion of tumor cells, promoting metastasis; exert an anti-apoptotic mechanism in these cells, and facilitate angiogenesis/lymphangiogenesis [1,5,6,7]. Tumor cells overexpress peptide receptors, which represent crucial targets for developing specific treatments against cancer cells using peptide receptor antagonists, which promote apoptosis in tumor cells, block the migration of cancer cells, and inhibit angiogenesis [1,5,6,7]. In combination therapy with chemotherapy, these antagonists exert a synergic effect and decrease the side effects promoted by cytostatics [8]. A common specific anticancer strategy using peptide receptor antagonists seems possible, irrespective of the tumor type [1,5,6,7]. Moreover, some peptide receptors are involved in the viability of tumor cells [9]. The overexpression of the peptidergic systems has been associated with tumor size, higher tumor aggressiveness, increased relapse risk, worse sensitivity to chemotherapy agents, and poor prognosis [1,5,6,7].
The involvement of opioid peptides in cancer has been widely demonstrated [10,11,12,13]; some studies have shown that opioids exert an antitumoral effect, whereas others have shown that opioid peptides promote tumor growth, metastasis, and vascularization [14,15,16,17,18]. Accordingly, this review aims to update the findings regarding the involvement of opioid peptides (enkephalins, endorphins, and dynorphins) in cancer development (e.g., mitogenesis, metastasis, angiogenesis); to suggest anticancer therapeutic strategies targeting the opioid peptidergic system (e.g., using opioid-receptor antagonists) and to highlight the main research lines to be developed in the future focused on the involvement of enkephalins, endorphins, and dynorphins in tumor development and progression.
2. The Endogenous Opioid System: Peptides and Receptors
Three genes, namely PDYN, PENK, and POMC, encode multiple endogenous peptides forming three distinct families of classical endogenous opioid peptides. A fourth gene, PNOC, encodes a group of non-classical opioid peptides more recently discovered to be closely related to opioid physiological action [19,20] (Figure 1). Endogenous opioid peptides may be classified into different families according to their preferential affinity for the four opioid receptors (OR), the so-called classical μ, δ, κ (MOP, DOP, KOP), and the opioid-related nociceptin/orphanin (NOP), respectively, into endomorphins, β-endorphins, enkephalins, dynorphins, and nociceptins [19,21]. A fifth opioid-like receptor type with a role in cell proliferation, activated by methionine-enkephalin (MET), acts at the nucleus membrane and is named the opioid growth factor receptor, or receptor zeta (ζ) (OGP/ZOP) [19,22,23]. The receptor has no sequence homology or structural resemblance with the opioid receptors, but naltrexone antagonizes methionine-enkephalin’s specific binding and action on ZOP [22].
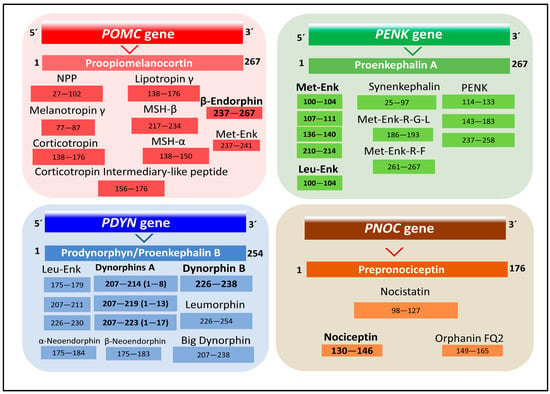
Figure 1.
Representation of the four families of the endogenous opioid peptides. The numbers indicate amino acid positions in the respective peptide precursor (UniProt codes P01189, Proopiomelanocortin; P01210, Proenkephalin A; P01213, Prodynorphin/Proenkephalin B; Q13519, Prepronociceptin, [19]).
Endogenous opioids participate in numerous actions in the central nervous system and peripheral tissues, modulating analgesia, stress, memory, cardiovascular and respiratory control, gland secretion, development, and angiogenesis (reviewed in [24,25]).
2.1. Endomorphins
Endogenous tetrapeptides, endomorphins 1 (H2N-YPWF-amide) and 2 (H2N-YPFF-amide) (Figure 2) were isolated from the mammalian brain. These tetrapeptides exhibit high affinity and selectivity for the μ opioid receptor and may be considered the natural ligands of MOP [26]. The origin of endomorphins has not been determined [27]. Mexneurin, a 91-amino-acids protein encoded by gene Trnp1 (mouse) (Uniprot code I6L9U2, [19]), has been proposed as a possible precursor from which the peptides may originate after posttranslational proteolysis and amidation of the C-terminal Phe residue [28].
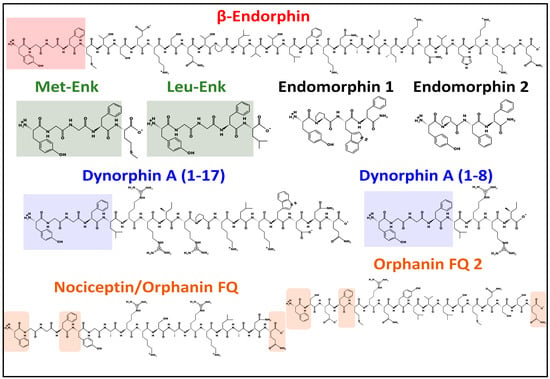
Figure 2.
Structure of representative endogenous opioids. Colored squares highlight the shared N-terminal sequence, YGGF, in β-endorphin, enkephalins, dynorphin A (1–17), and dynorphin A (1–8). Colored squares in nociceptin and orphanin depict common amino acid positions. All peptides shown have a Phe residue at the fourth position. All peptides shown, except heptadecapeptides, nociceptin, and orphanin FQ2 (sharing a Phe residue), share a Tyr residue at the N-terminus. All peptide structures were drawn at pH = 7 with PepDraw [29], the free web-based software from Tulane University (https://pepdraw.com/ (accessed on 17 May 2023)).
2.2. β-Endorphins
The human POMC gene has the 2p23.3 chromosomal localization. It encodes the preprotein proopiomelanocortin that bears tissue-specific posttranslational proteolysis catalyzed by prohormone convertases PC1 and PC2 [30]. The preprotein may yield ten different bioactive peptides, including adrenocorticotropin (ACTH), lipotropin, melanotropins, and β-endorphins (Figure 1) [19,31]. Although multiple forms of β-endorphin exist, the most common form has 31 amino acids (Figure 2). β-endorphins are preferential endogenous agonists of μ opioid receptors (MOR) but bind and activate other opioid receptors (DOP and KOP). They display multiple effects, including pain relief, stress control, brain metabolism homeostasis, eating and drinking behavior, and memory modulation, to name a few [24,32,33].
2.3. Enkephalins
The PENK gene, located at chromosome 8q12.1, encodes a preprotein, proenkephalin A, posttranslationally processed by proteolysis to give several peptide products, including mainly the pentapeptides leucine-enkephalin (LEU) and MET [19,31]. Both peptides may also originate from PDYN and POMC genes (Figure 1).
Soon after demonstrating the opioid receptor sites in nervous tissue [34], two pentapeptides, LEU and MET, were first isolated from the pig brain and found to inhibit in vitro electrically stimulated smooth muscle contraction of isolated guinea pig ileum preparations. Their effect was found to be blocked by the opioid antagonist naloxone [35]. The pentapeptides share the N-terminal sequence YGGF with β-endorphin and dynorphin A (1–17) (Figure 2). Both enkephalins and endorphins bind to μ and δ receptors with similar affinity, even though enkephalins are considered delta opioid ligands [36]. MET is also considered an inhibitory growth regulator. It specifically binds to OGR/ZOR, a protein at the nuclear membrane. After binding to MET, it penetrates the nucleus interior, affecting cell proliferation [22,37,38]. However, many aspects of this receptor’s function and three-dimensional structure remain unsolved and further studies are needed to establish its role.
2.4. Dynorphins
The human PDYN gene, with 20p13 chromosomal localization, encodes a preprotein, prodynorphin, from which different bioactive peptides with preferential selectivity for KOP originate after posttranslational proteolysis, including β-neoendorphin, dynorphin, LEU, and leumorphin (Figure 1) [19]. A representative of this family of peptides, the octapeptide dynorphin A (1–8), is considered a natural ligand of KOP. However, it also activates MOP, DOP, and NMDA (N-methyl-D-aspartate) receptors [39,40]. Dynorphin A (1–8) has a role in the modulation of analgesia, stress, emotional states, and memory, to mention a few [24]. It also has a role in neuroprotection by inhibiting apoptosis and oxidative stress mechanisms [40].
2.5. Nociceptins
The human PNOC gene, localized at chromosome 8p21.1, encodes prepronociceptin, which after proteolytic processing, generates several peptides, including the triacontapeptide nocistatin and two heptadecapeptides, nociceptin and orphanin FQ2 (Figure 1 and Figure 2) [19]. The representative peptide of this family, nociceptin, binds to the protein opioid-related receptor NOP [23]. Nociceptin was first isolated and found to induce a decrease of latencies in hot plate and tail flick tests after intracerebroventricular injection in mice [41]. Later, it was discovered that nociceptin mediated the blocking of opioid-receptors-induced analgesia [42]. The nociceptins family have a Phe residue in the N-terminus, compared with the N-terminal Tyr in the other families of endogenous peptides (Figure 2), and participate in numerous biological activities, such as substance abuse, analgesia control, memory, posttraumatic stress disorder, neuronal differentiation, and cell proliferation [43,44,45].
2.6. The Structure and Dynamics of the Opioid Receptors (OP)
Opioid receptors MOP, DOP, KOP, and NOP belong to the GPCR (G-protein-coupled receptors) family of proteins. The ζ Receptor (ZOP) does not belong to the GPCR family of proteins, and its 3D architecture remains undefined.
Two principal methods, X-ray diffraction and cryo-electron microscopy, are potent methods to define receptor conformations that facilitate structure-based drug design (SBDD) [46]. In this study, we highlight these proteins’ structural and functional features and their impact on intracellular signaling pathways related to possible disturbances of cell proliferation and cell life control leading to cancer.
The resolution of the architecture and ligand binding pockets of the OR fits well with the pharmacological concept of “message address,” which establishes that ligands have a module responsible for the message (recognition and efficacy) and another governing the address (additional recognition and selectivity) when contacting their binding site [47]. Together with structural analysis, it is paramount to use in vivo models to grasp their physiological role. In this respect, the current availability of mice knock-out models is valuable [48].
2.6.1. The μ Receptor (MOP)
The human MOP is encoded by the gene OPRM1 (chromosomal location 6q25.2). It has 400 amino acids. Posttranslational processing events of MOP include a disulfide bridge and N-glycosylation, phosphorylation, and lipidation sites (Figure 3A) [49,50].
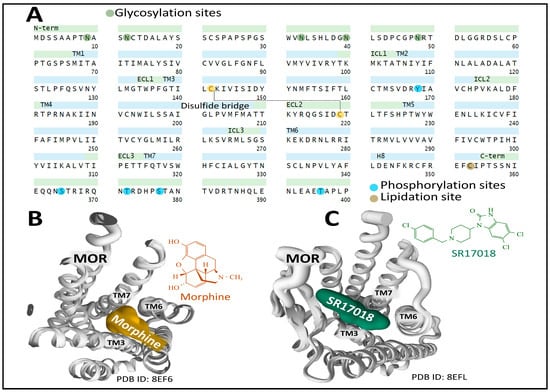
Figure 3.
Primary structure and helices organization of the MOP receptor, depicting posttranslational modifications (A) [19,49,50]. An external view from the extracellular site of the structure of human MOP bound to the agonists morphine (B) and SR17018 (5,6-dichloro-3-[1-[(4-chlorophenyl)methyl]piperidin-4-yl]-1H-benzimidazol-2-one) (C) shows the interaction of both agonists with transmembrane domains 3, 6, and 7. Figures were obtained from the Protein Data Bank [51] and correspond to PDB ID 8EF6 and 8EFL [52] and drawn with the free web-based open-source toolkit Molstar (https://molstar.org/ (accessed on 17 May 2023) [53]. Two-dimensional structures of morphine and SR17018 were drawn with KingDraw software [54] (version 1.1.0) (https://www.kingdraw.cn/en/ (accessed on 17 May 2023)).
The existence of multiple spliced and truncated variants of the receptor with functional significance and tissue-specific expression has added complexity and plasticity to the signaling panorama and intracellular events triggered by MOP [55,56].
The first X-ray diffraction study on the three-dimensional structure of murine MOP covalently bound to the opioid antagonist β-FNA (beta-funaltrexamine) through K2335.39 (Ballesteros and Weinstein numbering for CPCRs [57]) revealed the existence of a ligand pocket significantly exposed to the extracellular space and defined by both hydrophobic and polar interactions with amino acid residues positioned on transmembrane helices 3, 6, and 7. The exposure of opioid ligands to the receptor’s extracellular surface may explain the rapid half-lives of dissociation constants of opioid drugs binding to this receptor [58]. Huang et al. [59] determined the active state structure of murine MOP (stabilized with a G-protein mimetic nanobody) bound to the potent morphinan agonist BU72 (a bridged pyrrolidine morphinan). The binding pocket for BU72 is similar to the one described for β-FNA and secures its position within the orthostatic pocket through hydrophobic, aromatic, and polar interactions.
Several analyses have provided relevant information on the structural dynamics of MOP. Recent cryo-electron microscopy studies on MOP architecture have unveiled valuable information regarding the functional states of the receptor leading to the broad plasticity and pliability of MOP signaling. Zhuang et al. [52] determined the structure of the human MOP bound to several agonists, including morphine (Figure 3B), fentanyl, PZM2, SR17018, and TRV130. They applied mutagenesis analysis of the transmembrane domain 6 and 7 interfaces to define the binding pocket occupied by the agonists analyzed. Interestingly, morphine and fentanyl bind to a pocket delimited by transmembrane region 3 (TM3) and the interface between TM6/7. In contrast, other agonists, including SR17018, preferentially bind to TM3 and interact more loosely with the interface TM6/7 (Figure 3C). The consequences of this different coupling mode may have biological significance related to biased signaling through preferential β-arrestin activation over Gi when the binding to the TM6/7 is not tight enough [52].
Cryo-electron microscopy of MOP bound to the synthetic selective peptide agonist DAMGO (H-Tyr-d-Ala-Gly-N(Me)Phe-Gly-OH) and a heterotrimeric Gi protein with not bound nucleotide unveiled a specific pocket where the peptide sits with its N-terminus amino group occupying a similar site compared with BU72, establishing a salt bride with D1473.32 and a hydrogen bond with Y3267.43. However, the C-terminus continues towards the extracellular loops of MOP [60].
The elucidation of binding sites for agonists and antagonists is paramount to defining receptor activation and inactivation states that may determine biased signaling through Gi or β-arrestin transducers. Docking and molecular dynamics simulation analysis of MOP1 provided relevant clues on the specific interaction of receptor-biased agonist PZM21 that establishes strong interactions with TM residue Y7.43 and TM3 residues (D3.32 and Y3.33), leading to an intracellular opening of the receptor protein that facilitates G-protein binding [61]. Determination of cryo-EM structures of MOP-Gi bound to biased agonists PZM21 and its naphthyl-substituted acryl amide analog derivative, FH210, that exhibits a more pronounced G-protein bias, compared with the parent compound PZM21 [62], revealed the existence of an extended receptor pocket where PZM21 interacts with polar groups D3.32 and Y7.43, and its phenol group connects with H6.52 by a water-mediated interaction. The PZM21 thiophene group sits in a hydrophobic indentation of MOP delimited by I3.29, V3.28, and Q2.60 residues (Figure 4).
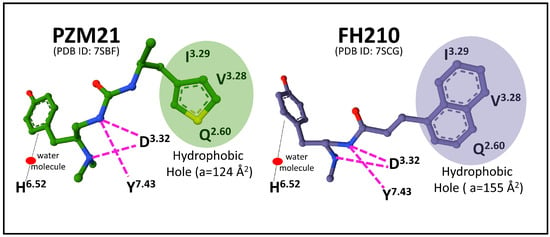
Figure 4.
Representation of the main interactions of MOP-biased agonists PZM21 (1-[(2S)-2-(dimethylamino)-3-(4-hydroxyphenyl)propyl]-3-[(2S)-1-thiophen-3-ylpropan-2-yl]urea) and FH210 (a naphthyl-substituted acryl amide derivative of PZM21) with MOP-Gi complex (see text for details). The structures were obtained from the Protein Data Bank (PDB ID 7SBF and 7SCG, [62]) and drawn with the free web-based open-source toolkit Molstar (https://molstar.org/ (accessed on 17 May 2023)) [53]). Amino acid positions are numbered according to Ballesteros and Weinstein [57].
The agonist FH210 fits its naphthyl group in the hydrophobic indentation delimited by residues of the transmembrane domains 3 and 2 better than the thiophene group of PZM21 does (Figure 4). This binding mode may explain a stable conformation that favors the receptor activation of Gi protein more successfully than PZM21, avoiding β-arrestin signaling [62]. These observations open new perspectives concerning drug design targeting specific receptor transducers and possible biased intracellular responses [63].
2.6.2. The δ Receptor (DOP.)
The human DOP protein is encoded by gene OPRD1 (chromosomal location 1p35.3). Its primary structure consists of 372 amino acids. Posttranslational processing events of DOP include a disulfide bridge and N-glycosylation, phosphorylation, and lipidation sites (Figure 5A) (GPCR database).
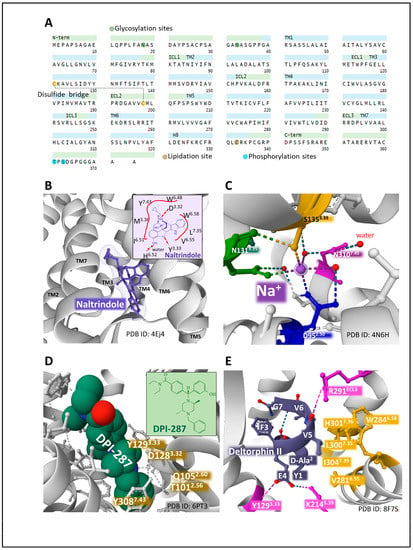
Figure 5.
The primary structure and domains of the DOP receptor with the indication of posttranslational modifications (A) [19,49,50]. The structure of murine DOP bound to the antagonist naltrindole (B) and the 2D structure of naltrindole depicting close interactions with DOP amino acid residues (B, inset). (C) represents the allosteric sodium site of human DOP and the contacts established with residues of the receptor. (D) shows the pocket of human DOP for the agonist DPI-287 and the interactions with DOP residues (inset). E highlights the interaction of peptide agonist deltorphin II with human MOP. The interactions of the amino acid residues of the peptide with the receptors illustrate the orthosteric binding cavity (E). Figures obtained from the Protein Data Bank [51] correspond to PDB ID 4EJ4 [64], 4N6H [65], 6PT3 [66], and 8F7S [67] drawn with the free web-based open-source toolkit Molstar (https://molstar.org/ (accessed on 17 May 2023)) [53]. Two-dimensional structures of naltrindole and DPI-287 were drawn with KingDraw software (version 1.1.0) (https://www.kingdraw.cn/en/ (accessed on 17 May 2023)). Amino acid residues are numbered according to Ballesteros and Weinstein’s nomenclature [57].
Two types of DOP have been pharmacologically defined: type 1, activated by DPDPE [(D-Pen 2, D-PenS)enkephalin] and antagonized by BNTX (7-benzylidene naltrexone), and type 2, sensitive to DELT II [(D-Ala2) deltorphin II], antagonized by Naltribe, (a benzofuran derivative of naltrindole) [68]. The functioning of DOP receptor types may have physiological implications, as a coordinated role in pain and anxiety modulation has been reported [69].
X-ray diffraction analysis of murine DOP bound to antagonist naltrindole reported the hole where the ligand establishes contacts with amino acid residues situated in transmembrane domains 3, 6, and 7 (for example, D3.32, Y3.33, I6.51, H6.52, V6.55, W6.58, L7.35, Y7.43) (Figure 5B) [64]. This space accommodates the structure of naltrindole. Leucine in position 3007.33 keeps contact with the naltrindole indole ring and determines naltrindole selectivity. Interestingly, positions W7.35 in MOP and Y7.35 in KOP offer a significant steric hindrance hampering stable connection with the naltrindole indole group [64].
The resolution of human DOP in its inactivated state bound to naltrindole [65] provided a similar structure to mouse DOP. It contributed new information regarding the molecular bases for receptor activation, including the definition of the allosteric sodium site, acting as a molecular switch (negative cooperativity), situated amid a plexus of polar interactions, in close contact with neighboring residues S3.39, N3.35, N7.45, and D2.50 [65] (Figure 5C).
Claff et al. (2019) reported an analysis of the structure of activated DOP bound to the agonist DPI-287 (4-[(4-benzyl-2,5-dimethylpiperazin-1-yl)-(3-hydroxyphenyl)methyl]-N, N-diethyl benzamide) [66]. The authors contributed structural details explaining agonist binding, selectivity, MOP activation, and the role of the allosteric sodium site, which appears disintegrated upon agonist binding. DPI-287 sits on a cavity of DOP where certain amino acid positions establish close contacts (for example, T2.56, Q2.60, D3.32, Y3.33, Y7.43) (Figure 5D).
A recent structural dynamics and pharmacological analysis on human DOP bound to peptide deltorphin II (Y[D-Ala2]FEVVG [67] establishes the accommodation of the peptide within the receptor protein and indicates differences that explain distinct selectivities of ligands. The deltorphin N-terminus region Y[D-Ala2]F sits at the bottom of the orthosteric cavity, whereas the C-terminus end, EVVG, interacts with the extracellular regions of TM2, TM6, TM7, and ECL3. The contact of deltorphin glutamic acid in position 4 through a salt bridge with K2145.39 and a hydrogen bond with Y1293.33 determines binding selectivity. Additionally, valines in positions 5 and 6 adapted to a hydrophobic pocket defined by V2816.55, W2846.58, L3007.35, H3017.36, and I3047.39. Additionally, the carbonyl group of valine 5 of the peptide makes an ionic contact with R29ECL3, which forces an inward movement of TM6, TM7, and ECL3 toward the orthostatic binding site that contributes to ligand selectivity (Figure 5E) and differs from the one established by agonist DPI-287 with DOP [66].
Unveiling the atomic interactions of DOP with ligands and determining interactome maps [70] are excellent tools to speed drug design to obtain new compounds with defined properties to fine-modulate receptor activity.
2.6.3. The κ Receptor (KOP)
The human KOP is encoded by the gene OPRK1 (chromosomal location 8q11.23). It has 380 amino acids. Posttranslational processing events of KOP include a disulfide bridge, N-glycosylation, and lipidation sites (Figure 6A) [49,50].
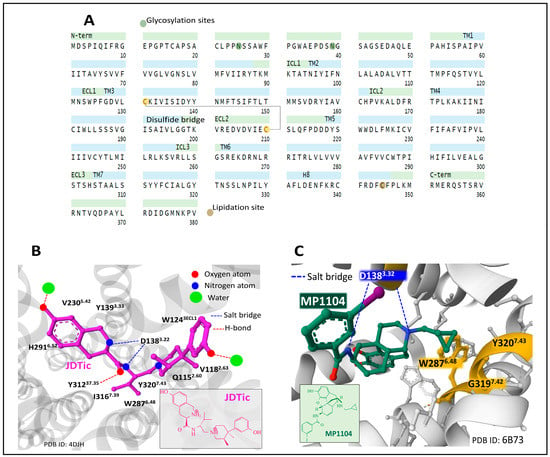
Figure 6.
The primary structure and domains of the KOP receptor with the indication of posttranslational modifications (A) [19,49,50]. The structure of human KOP is bound to the selective antagonist JDTic ((3R)-7-hydroxy-N-[(2S)-1-[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethylpiperidin-1-yl]-3-methylbutan-2-yl]-1,2,3,4-tetrahydroisoquinoline-3-carboxamide) (2D structure in the inset), indicating some amino acid contacts which secure its position within the orthosteric cave (B). The binding of non-selective agonist MP1104 (N-[(4R,7R,7aR,12bS)-3-(cyclopropylmethyl)-9-hydroxy-2,4,4a,7,7a,13-hexahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinolin-7-yl]-3-iodobenzamide) (2D structure in the inset) to KOP reveals the ionic interaction of the ligand with D1383.32 and the hydrophobic hole delimited by W2876.48, G3197.42, and Y3207.43 (C). Figures obtained from the Protein Data Bank [51] correspond to PDB ID 4DJH [71] and 6B73 [72], drawn with the free web-based open-source toolkit Molstar (https://molstar.org/ (accessed on 17 May 2023)) [53]. Two-dimensional structures of JDTic and MP1104 were drawn with KingDraw software (version 1.1.0) (https://www.kingdraw.cn/en/ (accessed on 17 May 2023)). Amino acid residues are numbered according to Ballesteros and Weinstein’s nomenclature [57].
Early NMR structure and dynamics studies on the interaction of dynorphin (1–13) peptide with human KOP [73] shed light on the contacts and conformations adopted by dynorphin when accommodated by the receptor. The peptide N and C-termini adopt flexible and disordered conformations, and the central part forms a helical turn. The observed disordered and flexible conformations of the peptide may reflect a process of binding and activation where ligand and receptor adapt to each other through intermediary-bound states with functional meaning.
The crystal structure of KOP bound to selective antagonist JDTic ((3R)-7-hydroxy-N-[(2S)-1-[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethylpiperidin-1-yl]-3-methylbutan-2-yl]-1,2,3,4-tetrahydroisoquinoline-3-carboxamide) helps to understand the fitting of the ligand and its affinity, selectivity, and potency [74]. The ligand interacts with amino acid residues through polar and hydrophobic contacts, delimiting the binding pocket (Figure 6B).
Determination of the crystal structure of KOP bound to non-selective agonist MP1104, stabilized by nanobody Nb39, and comparison of active and inactive states of the receptor gave insights into the conformations explaining the pharmacology and biased signaling of KOP [75]. Additionally, the use of nanobodies to regulate KOP activity has been further developed, and an inactive state of KOP has been resolved with nanobody Nb6 [72].
By contrasting the inactive state ensemble KOP-JDTic [74] with the active complex KOP-MP1104, the authors communicated that in the active state, ECL2 and TM domains 4–6 move inwardly toward the receptor complex. Consequently, the orthosteric cavity contracts and its volume diminishes by around 10% compared with the cavity in the inactive structure, a similar finding observed for MOP [59]. A common feature between both complexes is the interaction with D1383.22 through an ionic interaction and the contact of the phenolic moiety with TM5. The cyclopropyl methyl group of MP1104 sits in a hydrophobic hole built by residues W2876.48, G3197.42, and Y3207.43 (Figure 6C). Mutagenesis analysis of these positions pointed out that mutations G3197.42L and Y3207.43L showed a reduced G protein and β-arrestin activation, whereas, with mutation W2876.48L, a selective reduction of β-arrestin recruitment was observed [75].
In a recent cryo-electron microscopy study, different active states of KOP were elucidated by analyzing complexes of KOP and multiple G-protein heterotrimers, which may help better understand ligand selectivity and receptor–G-protein interaction [76].
Resolved structure of KOP bound to peptide dynorphin (1–13), a few contacts differ from the site occupied by MP1104. The amine group of Y1 at the N-terminus sits at the bottom of the orthosteric binding pocket, similarly to MP1104 contacts through its tertiary amine group (Figure 6C). However, the peptide exhibits additional contacts towards the top region of the binding site, with R6 and R7 interacting with E209ECL2 and E2976.58 through salt bridges [67].
Structural analysis at the atomic level provides essential knowledge to ascertain the functionality of the receptor. Additionally, in vitro and in vivo pharmacological assessment completes the panorama and offers the necessary insight to develop drugs that offer specific traits with putative application in human therapy. Experiments with recombinant KOP expressed in a human embryonic cell line (HEK-293), and naltrexone competition, receptor internalization, G-protein activation, β-arrestin recruitment, and docking prediction analysis, Ji et al. [77] reported that butorphanol, with a 20-fold higher affinity for KOP, compared with MOP, exhibited a dual role on the KOP activation. It behaved as a partial agonist activating G-protein and a full agonist recruiting β-arrestin and inducing receptor internalization. The butorphanol binding site is very similar to the orthosteric binding site occupied by MP1104 (see Figure 6C). The analysis of the opioid binding sites in MOP and KOP has given clues regarding the importance of the region defined by ECL2 and TM5 for controlling β-arrestin recruitment [78]. The docking manner of the natural compound salvinorin, a diterpenoid furanelactone, to KOP has also generated valuable structural details for designing and evaluating KOP selective and effective drugs [79].
2.6.4. The Nociceptin/Orphanin FQ Receptor (NOP.)
The human NOP is encoded by the gene OPRL1 (chromosomal location 20q13.33). It has 370 amino acids. Posttranslational processing events of NOP include a disulfide bridge, N-glycosylation, phosphorylation, and lipidation sites (Figure 7A) [49,50].
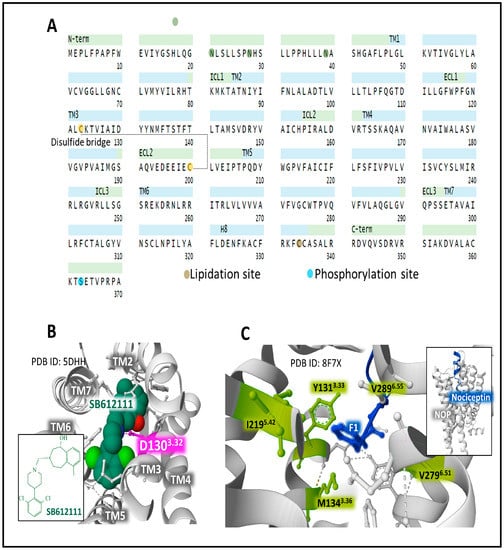
Figure 7.
The primary structure and domains of the NOP receptor with the indication of posttranslational modifications (A) [19,49,50]. The structure of human NOP is bound to the selective antagonist SB612111 ((5S,7S)-7-[[4-(2,6-Dichlorophenyl)piperidin-1-yl]methyl]-1-methyl-6,7,8,9-tetrahydro-5H-benzo [7]annulen-5-ol)) (2D structure in the inset), indicating amino acid contacts in the orthosteric binding pocket (B). The binding nociceptin to NOP (C) reveals the hydrophobic contacts of the ligand with the inferior domain of the binding site delimited by Y1313.33, M1343.36, I2195.42, V2796.51, and V2836.55. The inset figure in C illustrates the position of nociceptin within the orthosteric site of NOP. Figures are from the Protein Data Bank [51] formatting and correspond to PDB ID 5DDH [80] and 8F7X [67], drawn with the free web-based open-source toolkit Molstar (https://molstar.org/ (accessed on 17 May 2023)) [53]. The two-dimensional structure of SB612111 was drawn with KingDraw software (version 1.1.0) (https://www.kingdraw.cn/en/ (accessed on 17 May 2023)). Amino acid residues are numbered according to Ballesteros and Weinstein’s nomenclature [57].
Nociceptin, a heptadecapeptide, binds and activates NOP, exhibiting a unique functional profile that relates to and distinguishes the receptor system from the considered classical opioid receptors (MOP, DOP, and KOP) [42,81,82]. Unlike the endogenous opioid peptides, a Phe residue, instead of Tyr, appears at its N-terminal end (Figure 2). This feature has consequences regarding binding affinity and receptor selectivity. The architectural traits of NOP and its conformational states support the development of ligands with selected properties [82,83,84].
X-ray diffraction analysis of the complex NOP-SB612111 places the selective NOP antagonist in a cavity where residue D3.32 establishes a salt interaction with the ligand [80] (Figure 7B), as it has been observed for other orthosteric opioid-binding pockets DOP and KOP (see Figure 5D and Figure 6D) and for the interaction of MOP D1473.32 with the selective inverse agonist alvimopan [85] or with fentanyl [86]. A recent assessment [67] of the structure of the complex NOP-nociceptin shows how the peptide ligand directs its N-terminus toward the bottom of the binding site. Residue F1 connects with Y1313.33 through π-π interactions and adapts to a hydrophobic environment shaped by Y1313.33, M1343.36, I2195.42, V2796.51, and V2836.55 (Figure 7C).
The human ZOP (OGF) protein has 677 amino acids and is encoded by the gene OGFR (chromosomal location 20q13.33). Posttranslational processing events of NOP include Ser phosphorylation (Figure 8A) [49,50].
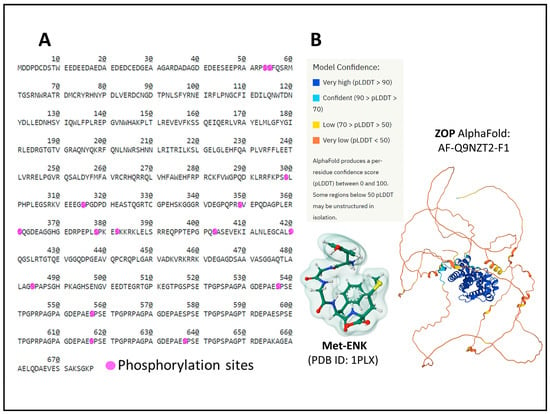
Figure 8.
The primary structure of the ZOP receptor with the indication of posttranslational phosphorylation sites (A) [19]. Panel (B) represents the AlphaFold prediction [87,88] of the human ZOP structure and the structure (Gaussian volume) of its ligand Met-ENK. The figure of Met-ENK is from the Protein Data Bank [51] and corresponds to PDB ID 1PLX [89], drawn with the free web-based open-source toolkit Molstar (https://molstar.org/ (accessed on 17 May 2023) [53]). The AlphaFold representation is from the AlphaFold Protein Structure Database (https://alphafold.ebi.ac.uk/entry/Q9NZT2, accessed on 12 June 2023)).
The structure of ZOP is different from the structure of OR. Additionally, the receptor protein localizes in the outer nuclear membrane and internalizes, bound to its natural ligand, [Met5]-ENK (MET) (Figure 8B), to the interior of the cell nucleus through the nuclear pores [22,37,38].
We do not have an accurate three-dimensional atomic-level structure of ZOP. The AlphaFold prediction [87,88] calculates the organization of the protein’s tertiary structure (Figure 8B). However, further analysis is needed to ascertain its role in analgesia, cell growth control, metabolic and immune responses [90,91,92,93], and the mechanism of internalization upon ligand activation and regulation by other proteins, including opioid receptors [70].
2.7. The Distribution of OR
The endogenous opioid system, including opioid peptides and receptors, is widely distributed in the central and peripheral nervous system and other tissues (UniProt references P35372 for MOP; P41143 for DOP; P41145 for KOP; P41146 for NOP and Q9NZT2 for ZOP) [19]. Hence, its modulatory role in numerous biological activities, from analgesia to intermediary metabolism or cell growth control. Recent research focuses on OR distribution and capacities related to their function and dysfunction in different pathologies, including cancer (see, for example, [42,94,95,96,97,98,99]).
2.8. Signaling Pathways of OR
Figure 9 illustrates representative signaling pathways activated by OR and associated with cancer development and progression (Figure 9).
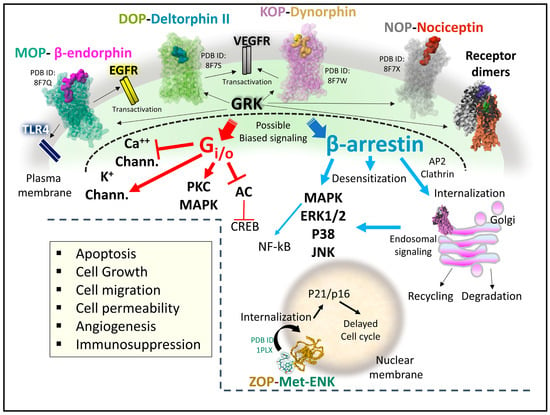
Figure 9.
Representative signaling pathways relating opioid receptors activity and cancer development. OR structures 87FQ (MOP-beta-endorphin), 87FS (DOP-deltorphin II, 87FW (KOP-dynorphin, 87FX (NOP-nociceptin) [67] and 1PLX (Met-ENK structure, Gaussian volume, [89], are from the Protein Data Bank [51]. The AlphaFold representation of ZOP is from the AlphaFold Protein Structure Database (https://alphafold.ebi.ac.uk/entry/Q9NZT2, accessed on June 12, 2023)). All receptor structures were drawn with the free web-based open-source toolkit Molstar (https://molstar.org/ (accessed on 17 May 2023) [53]). Abbreviations: AC, adenylyl cyclase; CREB, cAMP response element-binding protein; EGFR, epidermal growth factor receptor; ERK, extracellular receptor kinase; GRK, G-protein-coupled receptor kinase; JNK, c-Jun N-terminal kinase; MAPK, mitogen-activated protein kinase; NF-kB, Nuclear factor kappa-light-chain-enhancer of activated B cells; PKC, protein kinase C; VEGFR, vascular endothelial growth factor receptor; TLR4, toll-like receptor 4.
When opioid ligands bind to the classical OR, conformational changes determine the recruitment of signaling transducers Gi/o heterotrimeric protein or β-arrestin [20,40,74,100,101,102] (Figure 9). Upon G-protein activation, the heterotrimer dissociates. The beta–gamma complex controls ionic potassium and calcium currents through plasma membrane channels, and the alpha subunit bound to GTP inhibits adenylyl cyclase. Activation of β-arrestin may have different outcomes depending on the phosphorylation state of the receptor protein through the kinase activity of GRKs (G-protein coupled receptor kinases) on specific receptor residues [103]. It could lead to receptor internalization, inactivation, recycling, or effective subcellular signaling [104,105,106]. Additionally, receptor internalization of opioid receptors after ubiquitination and independent of Gi/o or phosphorylation mechanisms contributes to the signaling display and deserves exploration in terms of physiological meaning [107].
Activation of ZOP, the opioid growth receptor, by its endogenous ligand, MET, occurs at the cell’s nuclear membrane. The complex receptor-ligand internalizes to the nucleus and interferes with proteins responsible for the operation of the cell cycle [108].
Opioid receptors, adopting multiple structural active states that regulate intracellular action by biased signaling [109] through different transducers, may generate various downstream molecular events achieved with selective ligands. In this line, much basic research supports the pharmacological profile of biased ligands and allosteric modulators for MOP [61,110], DOP [111,112], and KOP [84,113,114]. However, careful revision of biased signaling impact is needed to establish whether some effects observed after ligand-activated receptors have functionally biased significance or may be attributable to the low efficacy of some drugs [115].
As it has been shown for MOP, the up-regulation of specific splice variants may be responsible for the switch of Gs activation instead of Gi/o, amplifying and diversifying the cellular responses dependent on ligand-activated MOP [56]. The diversification of opioid signaling may also source from the transactivation of receptor tyrosine kinases (RTK) that signal through different routes [116,117]. Also, the formation of heteromer structures between OR and OR with other GPCRs may be physiologically relevant and serve as molecular targets of designed drugs [118].
The occurrence of subcellular signaling events after opioid receptor internalization also contributes to the intricate net of OR cellular signaling. This process effectively creates a reduced influence space with distinctive signaling properties [119]. Opioid receptors at the Golgi apparatus activate Gi/o but do not recruit β-arrestin. Also, the receptors signal selectively depending on the lipid composition where OR are immersed [106].
The signaling landscape of OR through direct or interconnected biochemical pathways is complex and malleable. When disrupted, it may provoke alteration of metabolic and gene expression signatures leading to uncontrolled cell growth and migration and causing cancer development or progression [120,121]. Consequently, the analysis of OR signaling in cancer scenarios is paramount to clarify their participation and design new pharmacological strategies that disrupt biochemical cancer pathways.
3. Involvement of Opioid Peptides in Cancer
Many studies have demonstrated the involvement of opioid peptides in cancer [10,11,12,13]; these peptides have enhanced the tumor growth induced by stress [122]. MET and dynorphin (DYN) A are released from immune cells under inflammatory conditions [123], and the level of DYN in the cerebrospinal fluid increased in patients with cancer pain [124]. The re-expression of the mu-opioid receptor gene in tumor cells increased the release of beta-endorphin (END) from these cells [125]. Moreover, skin-derived beta-END mediates the fatigue induced by radiation therapy in cancer patients; plasma beta-END level augmented in rats receiving radiation but was reversed with naloxone [126]. The level of plasma beta-END decreased in patients with malignant tumors treated with oxycontin; this treatment also relieved pain and improved clinical symptoms [127]. HSC-3 cells (human tongue squamous cell carcinoma) transfected with the mu-opioid receptor gene released more beta-END than control HSC-3 cells, and this virus-mediate gene delivery also attenuated cancer-induced pain [128]. Another study has demonstrated that the administration of flurbiprofen increased the analgesic effects of opioids by increasing plasma beta-END levels in patients showing refractory cancer pain [129].
Moreover, MET, through the opioid growth factor receptor, exerts an antitumoral effect against colon cancer, neuroblastoma, ovarian cancer, head and neck squamous cell carcinoma, and pancreatic cancer [14,15,16,17]. However, other studies have reported that opioids promote tumor growth, metastasis, and vascularization. Because opioids are used to treat pain in cancer patients, the mechanisms involving both dual and opposite actions must be studied in-depth [18].
3.1. Bone Cancer
Endorphin
Cinobufagin, used for the treatment of cancer pain, promoted beta-END mRNA and protein expressions, but not DYN A, in the microglia of the spinal cord in a rat bone cancer model; this effect was mediated by the alpha7-nicotinic acetylcholine receptor and induced mechanical anti allodynia [130]. Moreover, cinobufagin relieved cancer pain by upregulating the expressions of both mu-opioid receptors and beta-END in the hind paw tumor and tissues placed close to the tumor in an experimental animal model of paw cancer pain [131]. Compared with the sham group, the concentration of beta-END decreased in the rostral ventromedial medulla and spinal cord in an experimental model of cancer-induced bone pain; electroacupuncture and wrist-ankle acupuncture did not affect beta-END concentrations in these regions [132]. Beta-END increased body weight gain, NK (natural killer) cell cytotoxicity, T cell proliferation, and the relative quantities of T cell subtypes but did not affect T cell release in an experimental rat model of bone cancer pain [133].
3.2. Brain Tumors
3.2.1. Enkephalin
Pro-enkephalin and MET have been observed in human brain tumors (e.g., ganglioglioma, glioma, meningioma) and associated cyst fluids [134]. Moreover, an inverse correlation between MET and brain tumor malignancy degree has been reported: higher MET level, lower tumor degree [134]. MET promotes apoptosis in rat C6 glioma cells, increases the activity of caspases 3, 8, and 9 and the expressions of Bax, FasL, and Fas, decreases Bcl-2 expression, and increases Ca++ influx into the cytoplasm and NFAT1 accumulation into the cell nucleus [135]. No effect on tumor cell viability and FasL upregulation was observed when NFAT1 was knocked down [135]. The results suggest that NFAT1 regulates downstream genes (e.g., FasL) and promotes apoptosis in tumor cells. MET-binding sites decrease with increasing malignancy of gliomas, and a shift from mu-opioid receptors in low-grade gliomas to delta-opioid receptors in high-grade gliomas has been reported [136]. These results suggest an inactivation of the MET/opioid receptor system in glioma, which blocks the inhibitory action exerted by MET on average astrocyte growth, promoting tumor progression. Moreover, pro-enkephalin expression was favored by norepinephrine and downregulated by endothelin-1 in C6 rat glioma cells [137,138], and MET favored the growth of human U-373 MG astrocytoma cells [139].
3.2.2. Endorphin
Beta-END-binding sites were reported in the human glioblastoma SF126 cell line [140], and beta-END was observed in human brain tumor cyst fluids [141].
3.2.3. Dynorphin
Glutamate augmented the water content in C6 glioma cells, whereas DYN A1-13, via kappa receptors, decreased it [142].
3.3. Breast Cancer
A polymorphism in the mu-opioid receptor gene has been associated with a reduced response to opioids in breast cancer; patients showing this polymorphism had better survival [143]. MET, LEU, and beta-END expressions have been reported in cells and stroma of human breast cancer samples [144]. The activation of hypoxia-inducible factor 1alpha by delta-opioid receptors promoted cyclooxygenase 2 expression, through phosphatidylinositol 3 kinase (PI3k)/protein kinase B (Akt) stimulation, in breast cancer cells (MCF-7, T47D) causing a paracrine activation of the vascular endothelial cells by prostaglandin E2 receptors [18].
3.3.1. Enkephalin
MET, but not LEU stimulated the migration of MDA-MB-468 human breast carcinoma cells [145], and END did not affect the migratory capacity of these cells [145]. A study has reported that the low-fasting plasma level of pro-enkephalin is correlated with an increased risk of breast cancer development in postmenopausal/middle-aged women [146]. The opioid growth factor (MET)/opioid growth factor receptor (receptor zeta) system blocked the proliferation of triple-negative breast cancer cell lines (BT-20, MDA-MD-231), and this effect was mediated by p21 cyclin-dependent inhibitory kinase pathways [147].
3.3.2. Endorphin
High plasma beta-END concentrations have been observed in healthy women, which were even higher in healthy postmenopausal women; however, lower levels were found in women with breast cancer, and no difference was observed between premenopausal and postmenopausal women who have breast cancer [148]. Moreover, chemotherapy only improved beta-END levels in postmenopausal women but without reaching the levels observed in healthy women [148]. Beta-END activates the survival/mitogenic signaling pathways (Akt, signal transducer and activator of transcription 3 (STAT3) and mitogen-activated protein kinases (MAPK)/extracellular signal-regulated kinase (ERK)) in human MDA-MB-231 breast cancer cells. Increasing plasma beta-END levels have been correlated with increasing tumor burden in a mouse model of breast cancer [149]. This observation means that the peptide is involved in cancer progression, and, significantly, the high levels of plasma beta-END did not decrease pain in mice with breast tumors; quite the opposite, the pain increased in these animals [149]. Patients with breast carcinoma treated with a galactose-specific lectin standardized mistletoe extract showed an increase in the level of plasma beta-END and an enhancement of T lymphocytes and NK cells [150]. A correlation between plasma beta-END level and the activity of the last two cells was also reported [150] and between an increased plasma beta-END level and an improved quality of life in patients with breast cancer [151].
In female rats in which a mammary tumor was developed, the level of beta-END was higher in the midbrain, striatum, and pituitary; the level of MET was lower in the striatum, and that of DYN decreased in the hypothalamus and pituitary when compared with the levels observed in control animals [152]. Fetal alcohol exposure reduces beta-END levels, causing a hyper-stress response and inhibiting the immune action against tumors [153]. Fetal alcohol exposure and control rats treated with N-nitroso-N-methylurea to promote mammary cancer growth were studied, and neurons expressing beta-END were transplanted into the hypothalamus after tumor development to augment beta-END production [153]. This strategy blocked tumor development in fetal alcohol-exposed and control animals and reversed the effects mediated by fetal alcohol exposure regarding the susceptibility to breast cancer. In this sense, it has been suggested that beta-END regulates the stress response and promotes innate immunity preventing breast cancer development [154].
Moreover, it seems that the cancer-preventive effect mediated by beta-END was due to an inhibition of the sympathetic neuronal action, which increased the activities of NK cells and macrophages and the production of anti-inflammatory cytokines [155]. Thus, beta-END counteracts breast cancer development by favoring immune-mediated anticancer defenses [156,157]. In addition, beta-END alters the tumor microenvironment by inhibiting the production of catecholamines and inflammatory cytokines, which alter cell-matrix attachment, DNA repair, epithelial-mesenchymal transition, and angiogenesis [155].
3.3.3. Dynorphin
The presence of DYN A1-17 and DYN A1-8 has been reported in Walker 256 tumors, a carcinosarcoma originated from the mammary gland of rats, but no opioid binding site was observed [158].
3.4. Cervical Cancer
3.4.1. Enkephalin
MET blocked cervical carcinoma progression in vivo; decreased myeloid-derived suppressor cell-infiltrated both tumor and circulation; induced apoptosis, and increased the expressions of caspase 3 and 8, Fas, and the signaling pathway mediator Bax [159]. This result suggests that MET is a promising antitumor research line in cervical cancer.
3.4.2. Endorphin
Electroacupuncture increased plasma beta-END levels in cervical cancer patients [160].
3.5. Colorectal Cancer
A low-dose of naltrexone (an opioid antagonist) blocked colorectal cancer progression in vivo and in vitro [161]. This treatment augmented the expressions of macrophage markers (CD68, F4/80), M1 macrophage phenotypic markers (CD80), and the level of cytokines (tumor necrosis factor-alpha) [161]. Moreover, a low-dose of naltrexone upregulated the expressions of the opioid growth factor receptor and apoptotic factors (PARP, caspases 3 and 9, Bax) and downregulated the expressions of Ki67 and Bcl-2, causing apoptosis in tumor cells [161].
3.5.1. Enkephalin
The presence of enkephalin has been reported in rectal carcinoids [162]. MET enhanced in vivo colon carcinogenesis induced by azoxymethane [163]. However, MET exerted an antitumor action in vivo against colorectal tumors (MC38 cell line) by acting on the tumor microenvironment and the immune system [164]. MET augmented both immunogenicity and recognition of tumor cells; downregulated Kras (oncogene), Bcl2, and Bclxl (anti-apoptotic proteins); blocked the synthesis of inflammatory cytokines; reduced immune checkpoints (2b4, Flgl1, Lag3, Pd-11, Pd-1) in tumor cells, and increased CD4+T, CD8+T, and macrophages (M1) infiltration [164]. The antitumor effect exerted by MET was also mediated by effector T cells; the peptide upregulated the opioid growth factor receptor, and the specific inhibitor of this receptor, naltrexone, blocked the antitumor action induced by MET [164]. The data support that MET is a promising therapeutic agent against colorectal cancer by improving immunotherapy efficacy. Moreover, CD10 (a marker for liver metastasis in colorectal cancer) increased colorectal cancer cell metastasis by abrogating the antitumor action mediated by MET since the peptide blocked the growth, invasion, and survival of tumor cells after thiorphan (inhibitor of the enzyme that degrades enkephalins)-induced CD10 inactivation [165]. MET, via delta opioid receptors, decreased the phosphorylation of ERK/epidermal growth factor receptors and increased p38-dependent apoptosis [165]. LEU decreased the invasive capacity of murine colon 26-L5 adenocarcinoma cells [166].
3.5.2. Endorphin
Beta-END has been reported in adenocarcinomas derived from the colon mucosa; its expression was higher in adenocarcinomas than in the mucosal layer of normal colons [167], and the peptide has also been observed in rectal carcinoids [162]. The effects of ultraviolet A eye irradiation on colon carcinoma induced by dextran sodium sulfate and azoxymethane have been studied in an experimental mouse model [168]. Mu-opioid receptors, MET, and beta-END expressions increased in treated animals, and these expressions increased more when these animals received ultraviolet A eye irradiation [168]. Colon carcinoma symptoms were decreased after this irradiation, but these beneficial effects were reduced when beta-END inhibitors were administered and disappeared with naltrexone [168]. This fact suggests that the beneficial effects observed in colon carcinoma after the ultraviolet A eye irradiation were mediated by beta-END and MET. Hypothalamic neurons containing beta-END inhibited the development of preneoplastic/neoplastic lesions in an experimental colon cancer model induced by 1, 2-dimethylhydrazine [169]. Animals with hypothalamic beta-END neuronal transplants (70%) failed to develop tumors, and animals with transplanted beta-END neurons showed a lesser adenoma development in the colon and tissue lesions (e.g., aberrant crypt foci) and decreased expressions of Ki-67, tumor necrosis factor-alpha, and NF-κB nuclear translocation in colonic tissues [169].
Moreover, decreased levels of transcription factors linked to epithelial-mesenchymal transition (e.g., Twist, Snail, N-cadherin) and increased levels of E-cadherin were observed in the colon tissue of transplanted animals [169]. The data suggest that beta-END neuron transplants inhibited colon cancer progression by reducing the epithelial-mesenchymal transition and the inflammatory mechanisms. However, the administration of beta-END into the nucleus of the raphe magnus induced analgesia. It facilitated the metastasis, which was inhibited with naloxone, and when this nucleus was electrically stimulated and analgesia induced, the metastasis was considerably attenuated [170].
3.5.3. Dynorphin
MET, DYN A1-8, beta-END, and LEU (10−4 M; 10−6 M) did not affect the migration, chemotaxis, or invasion of colorectal tumor cells (HCT-116, HT-29) [171]. Moreover, MET, DYN A1-8, and beta-END (10−6 M) did not alter the viability of the HT-29 tumor cell line [172].
3.6. Cutaneous Squamous Cell Carcinoma
Enkephalin
MET blocked the cell growth of cutaneous squamous cell carcinomas by inducing the G0/G1 cell cycle arrest and by promoting apoptotic mechanisms through the caspase 3/Bax/Bcl-2 signaling pathway [173]. MET, through the opioid growth factor receptor, blocked the proliferation of A431 cells by inducing apoptosis, promoting autophagy in cutaneous squamous cell carcinoma cells, and activating dendritic cells [174]. MET also decreased immunosuppression by reducing the number of myeloid-derived suppressor cells, controlling the polarization of tumor-associated macrophages from M2 to M1, and inhibiting the JAK2/STAT3 tumor-promotion/immunosuppression signaling pathway, which is involved in macrophage polarization and myeloid-derived suppressor cell expansion [173]. Thus, MET exerts an antitumor effect against cutaneous squamous cell carcinoma.
3.7. Gastric Cancer
3.7.1. Enkephalin
MET blocked the growth of human gastric cancer HGC27 and SGC7901 cell lines [175]. The peptide arrested the cell cycle in the G0/G1 phase, reducing Ki67, cyclin D1, and c-myc mRNA and promoting apoptosis by upregulating the Bax expression through downregulating Bcl-2/surviving expressions and by activating PARP and caspase 3 [175]. MET also upregulated the expression of the opioid growth factor receptor. Another study has demonstrated that MET-induced apoptosis in human gastric tumor cells (HGC27, SGC7901) by inhibiting the PI3k/Akt/mammalian target of rapamycin (mTOR) signal pathway, reduced the number of M2-type macrophages and increased the M1-type [176]. Tumor cell apoptosis was blocked when the opioid receptor expression was knocked down [176]. The data suggest that MET is a promising antitumor agent against gastric cancer.
3.7.2. Endorphin
Plasma beta-END levels decreased in gastric cancer patients after transcutaneous electrical acupoint stimulation [177], and beta-END was observed in adenocarcinomas derived from the antral mucosa [178].
3.8. Head and Neck Cancer
The activation of the mu-opioid receptor promoted head and neck squamous cell carcinoma growth in vitro and in vivo experiments, increasing the proliferation and migration of tumor cells (FaDu, MDA6868Tu) [179]. Thus, the mu-opioid receptor is a promising antitumor target to treat head and neck squamous cell carcinomas.
3.8.1. Enkephalin
MET expression has been reported in human head and neck squamous cell carcinomas [180]. Reduced DNA synthesis and tumor weight/volume have been reported in the head and neck squamous cell carcinomas after treatment with MET, imiquimod, and naltrexone (low dose) [181]. The inhibitory action exerted by the MET/opioid growth factor receptor system is mediated through the p16 pathway; MET increases the expression of the cyclin-dependent kinase inhibitor p16 protein [182]. Downregulation of the opioid growth factor receptor favored the progression of head and neck squamous cell carcinoma [183], and LEU has been detected in head and neck paragangliomas [184].
3.8.2. Endorphin
Beta-END increased the production of the leukocyte migration inhibitory factor, reaching almost normal levels, in patients with squamous carcinoma of the head and neck; this means that the peptide regulates the immune system [185].
3.8.3. Dynorphin
MET, DYN A1-8, beta-END, and LEU (10−4 M; 10−6 M) did not affect the migration, chemotaxis, or invasion of squamous carcinoma cells (CAL-27, SCC-1) [186]. Moreover, MET, DYN A1-8, and beta-END (10−6 M) did not affect either the differentiation of SCC-1 and CAL-27 tumor cells [187] or the viability of the latter cells [172].
3.9. Larynx Cancer
3.9.1. Enkephalin
MET has been observed in neuroendocrine tumors (paragangliomas) of the larynx [188].
3.9.2. Endorphin
Beta-END has been reported in tumor cells in an oat cell carcinoma of the larynx [189].
3.10. Leukemia
3.10.1. Enkephalin
The presence of pro-enkephalin A has been reported in leukocytes from patients with chronic lymphoblastic leukemia [190], and MET increased CD10 expression and inhibited the metabolic activity of the leukemic NALM-1 cell line [191]. MET promoted apoptosis in K562 human erythroid leukemia cells [192], and the peptide favored pre-B acute lymphoblastoid cell migration (LAZ 221, NALM 6) and increased the CD9 surface expression (a leukemia cell marker) in the latter cells [193]. This migration, induced by MET, was considerably decreased when pre-B acute lymphoblastoid cells were incubated with antibodies against CD9 [193].
3.10.2. Endorphin
Beta-END has been reported in the cerebrospinal fluid of children with acute lymphoblastic leukemia; the highest level was observed at the end of the intensification chemotherapy, whereas treatment with glucocorticoids decreased beta-END levels [194]. However, plasma beta-END levels decreased in patients with solid tumors after treatment with the chemotherapeutic drug CDDP [195]. Plasma beta-END levels were higher in patients with acute leukemia than in healthy individuals, and stress factors (e.g., high temperature, anemia, hypoxic conditions, pain, cancer) increased the synthesis of beta-END, particularly in the white blood cells of patients with acute leukemia during chemotherapy treatment [196]. Beta-END promoted the growth of T-lymphoblastoid cells; however, this was not observed in promyelocyte and B-lymphoblastoid cells [197]. Finally, naloxone-resistant receptors for beta-END are downregulated after activation of the protein kinase C in the U937 cell line (isolated from a histiocytic lymphoma) [198].
3.11. Liver Cancer
3.11.1. Enkephalin
The proliferation of hepatocellular carcinoma cells (Hep 3B, Hep G2, SK-HEP-1) was inhibited after treatment with MET due to the blockade of the DNA synthesis and not to necrotic/apoptotic mechanisms [199]. Moreover, silencing the opioid growth factor receptor promoted the proliferation of these cells [199]. MET concentration was higher in metastasis-positive human livers than in normal ones [165]. This finding is important since it suggests that the increase in MET could be an endogenous antitumor mechanism to counteract tumor progression. Two patients with hepatoblastoma were cured after surgical resection and treatment with naltrexone (low dose) and MET [200]. It seems that this treatment is a promising antitumor therapeutic strategy.
3.11.2. Endorphin
In an experimental animal model of liver cancer, neurons expressing beta-END transplanted into the hypothalamus prevented hepatocellular carcinoma formation and hepatocellular injuries [201]. This strategy inhibited carcinogen-induced liver histopathologies (e.g., fibrosis, collagen deposition, inflammatory infiltration) and augmented the concentration of NK cell cytotoxic agents in the liver [201].
3.12. Lung Cancer
3.12.1. Enkephalin
Pro-enkephalin and MET have been reported in human lung cancer cells [202], and serum LEU level was higher in patients with bronchial carcinoma than in control individuals [203]. Morphine, via the opioid growth factor receptor, suppressed lung cancer cell proliferation (H1975); this suppression occurred in the cell cycle S phase [204]. By controlling the Wnt/beta-catenin pathway, MET exerted an antitumor effect against lung cancer in vitro and in vivo experiments, leading to cell cycle arrest at the G0/G1 phase [205]. Moreover, the antitumor action of the peptide was abolished in the knockdown of growth factor receptor, and MET augmented the infiltration of dendritic cells, CD4+ T and CD8+ T cells, macrophages (M1) and NK cells, and reduced the number of macrophages (M2) and myeloid inhibitory cells [205]. MET also upregulated the expression of interleukin-15, interleukin-21, interferon-gamma and downregulated interleukin-10, and tumor necrosis-beta 1 expression in the tumor microenvironment [205]. MET increased the expression of the opioid growth factor receptor and, by activating the caspase 3/Bax/Bcl-1 signaling pathway, promoted apoptosis in lung cancer cells [206]. These effects disappeared when the previous receptor was blocked. MET also increased the immunogenicity of lung cancer cells, NK cell activity, and the expression of NK cell-related cytokines (e.g., interferon-gamma, granzyme B) [206]. Previous findings suggest that MET is a promising antitumor agent against lung cancer. However, another study has demonstrated that methylnaltrexone (an opioid antagonist) counteracted Lewis lung carcinoma growth (cancer cells express mu-receptors) and decreased lung metastasis and that morphine (a mu-receptor agonist) favored tumor growth [207]. It is important to note that nicotine partially or reversed opioid-induced growth suppression in 9/14 lung cancer cell lines studied [208]. Moreover, MET blocked pulmonary metastasis and enhanced the activity of NK cells [209].
3.12.2. Endorphin
Beta-END has been reported in the bronchoalveolar lavage fluid of patients with lung cancer [210] and in the plasma of patients with this disease [211]. Beta-END has been reported in lung small-cell carcinomas and carcinoid tumors [212]. Human small-cell lung carcinoma cell lines (NCI-N417, NCI-H345, NCI-H69) express naloxone-insensitive endorphin binding sites that were insensitive to naloxone and other mu-, delta- or kappa-opioid receptor ligands [213]. The U1,690 cell line (small-cell lung carcinoma) expresses beta-END, and the peptide promotes the proliferation of this cell line through non-opioid binding sites; moreover, beta-END binding did not affect the synthesis of cAMP [214]. Beta-END also acts as a chemoattractant for small-cell lung carcinoma cells favoring migration and metastasis [215].
3.12.3. Dynorphin
Pre-proDYN mRNA has been reported in small-cell lung carcinomas [216], and serum DYN A/B and MET levels increased in a non-small-cell lung cancer cell xenograft stress reduction mouse model [217]. Moreover, via Gαi, DYN B blocked cAMP formation in non-small-cell lung cancer cells [217]. Most lung tumor cells co-expressed pro-DYN and DYN, prohormone convertase 1, prohormone convertase 2, or carboxypeptidase E. In contrast, a few lung cancer cells only expressed one of the last markers [218]. DYN was observed in cancer cells infiltrating human lung tissues and nerve fibers in the bronchial submucosa [218]. Lung cancer cells express mu, kappa, and delta opioid receptors and contain several combinations of opioid peptides (DYN, ENK, beta-END). After opioid administration, cAMP concentration was decreased in these cells [208]. In addition, agonists of the previous three receptors (1-100 nM) blocked the growth of tumor cells in vitro, whereas nicotine (100-200 nM) totally or partially counteracted the growth blockade mediated by opioids [208].
Pro-opiomelanocortin gene delivery blocked the growth of alpha-melanocyte-stimulating hormone/melanocortin 1 receptor (MC1R)-deficient Lewis lung carcinoma cells as well as the growth of these cells in mice; apoptotic mechanisms mediated these effects through an MC1R-independent pathway [219]. The authors also demonstrated that, in this case, via an MC1R-dependent pathway, this delivery blocked tumor progression and metastasis of B16-F10 melanoma cells [219]. Pro-opiomelanocortin gene delivery attenuated the beta-catenin signaling pathway by decreasing protein concentrations of beta-catenin and its downstream proto-oncogenes (e.g., c-myc, cyclin D1) and blocked tumor vasculature [219].
3.13. Melanoma
3.13.1. Enkephalin
Compared to normal skin, the expression of MET and LEU was decreased in melanocytic tumors [220]. MET and LEU have been detected in six of seven secondary neuroendocrine carcinomas of the skin, whereas both peptides were not found in skin primary neuroendocrine carcinomas (Merkel cell carcinoma) [221]. MET exerted an antitumor effect in mice xenografted with melanoma B16-BL6 cells which was inhibited with naloxone; the antitumor action was due to the immune system’s modulation and a cytotoxic effect on melanoma cells [222]. In the same experimental model, MET promoted cell cycle arrest. It increased the plasma levels of interferon-γ, tumor necrosis factor-alpha, and interleukin-2 [223]. MET promotes cell cycle arrest in the G0/G1 phase, decreases the number of cells in the S and G2/M phases, and increases the expression of the opioid growth factor receptor in B16 melanoma cells [223]. Tumor growth and tumor cell dissemination were counteracted with MET, and the peptide blocked A375 melanoma cell proliferation through apoptotic mechanisms [223,224]. MET also promoted cell cycle arrest in the G0/G1 phase, decreased the cell number in S and G2/M phases, and favored apoptosis in human A375 melanoma cells [224]. Imiquimod also upregulates the expression of the opioid growth factor receptor, increasing MET’s antitumor effect [225]. This synthetic immune response modifier has been successfully applied in melanoma treatment [226,227,228,229,230]. MET did not affect adenylate cyclase activity in AB16 melanoma cells [231]. Moreover, MET decreased tumor weight and volume in vivo and increased the ratio of CD4+ to CD8+ T cells [223].
3.13.2. Endorphin
Beta-END was observed in 30 of 42 melanoma samples [232]. Beta-END was found in six of seven secondary neuroendocrine carcinomas of the skin but was absent in primary neuroendocrine carcinomas (Merkel cell carcinoma) [221]. B16 melanoma cells synthesize and release beta-END [233]. Tumor growth was studied in mu-opioid receptor-deficient and wild-type mice administered with B16 melanoma cells producing beta-END [233]. B16 cells decreased tumor growth and increased the infiltration of immune cells into the tumor in mu-opioid receptor-deficient animals. Opioids in the B16 cell supernatant reduced the proliferation of normal leukocytes but not those from mu-opioid receptor-deficient animals [233]. A correlation was observed between beta-END levels and tumor progression in melanoma tissues [233]. In this sense, beta-END immunoreactivity was lower in benign melanocytic naevi than in metastatic and advanced melanomas [232]. Moreover, a low-dose ultraviolet exposure promoted beta-END synthesis in epidermal keratinocytes and increased the plasma level of the peptide [234]. Thus, mu-opioid peptides modulate the immune response and control the development of tumors. Moreover, beta-END did not affect adenylate cyclase activity in AB16 melanoma cells [231].
3.14. Myeloma
Dynorphin
U50,488, a kappa-opioid receptor agonist, favored Fas-induced apoptosis without Fas receptor expression increase and decreased cell proliferation in human multiple myeloma LP-1 cells expressing mu- and kappa-opioid receptors [235]. However, this study demonstrated that the antiproliferative effect mediated by U50,488 was independent of the kappa receptor. This effect was due to a G0/G1 phase blockade, and cell cycle inhibitors (e.g., p53, p27Kip1, p21Cip1) were not upregulated [235]. DYN or morphine did not regulate apoptosis or cell proliferation in LP-1 cells [235].
3.15. Neuroblastoma
3.15.1. Enkephalin
MET has been located in mouse neuroblastoma Neuro2a cells, and the peptide was released from these cells with a high K+ stimulation [236]. MET arrested the growth of human SK-N-SH neuroblastoma cells [237].
3.15.2. Endorphin
Neuroblastoma Kelly, NMB, and IMR-32 cell lines express Beta-END-binding sites [238]. Tumors of transplanted neuroblastoma S20Y cells in mice and treated with complete or intermittent opioid receptor blockade with naltrexone showed an upregulation of beta-END and MET levels and MET-binding sites [239]. In this study, MET decreased the tumor mitotic index, which was counteracted with naltrexone. Thus, a complete blockade with naltrexone increased tumor cell proliferation, whereas an intermittent blockade inhibited cancer cell proliferation [239].
3.15.3. Dynorphin
Pre-pro-DYN mRNA and pre-pro-ENK have been reported in neuroblastoma SK-N-MC and SCLC H69 cell lines [216] and opioid binding sites in the neuroblastoma S20Y cell line [240]. A cysteine protease-degrading DYN A1-13 and DYN A1-17 has been reported in the membrane of mouse neuroblastoma N18 cells [241]. DYN exerted a cytotoxic action, promoted apoptosis, and downregulated the expression of the anti-apoptotic protein Bcl-2 in SH-SY5Y neuroblastoma cells; previous effects were inhibited with the anesthetic isoflurane [242]. MET, DYN A1-8, and beta-END did not affect the differentiation of SK-N-SH neuroblastoma cells at the concentration administered (10−6 M) [187]. DYN A, at high concentration, binds to neuropeptide Y receptors (Y1 and Y2) in SK-N-MC and SMS-MSN neuroblastoma cell lines, and it has been suggested that DYN A could exert an antagonistic effect on these cells [243]. Moreover, this binding was not mediated either by changes in receptor-G protein interaction or by receptor phosphorylation.
3.16. Ovarian Cancer
3.16.1. Enkephalin
Enkephalin has been reported in tumor cells in ovarian carcinoids [244] and MET and the opioid growth factor receptor in human ovarian cancer cells [245]. MET, in a dose-dependent manner, exerted an inhibitory proliferative effect on ovarian tumor cells (HEY, CAOV-3, SW626), whereas the neutralization of MET promoted cell proliferation and the silencing of the opioid growth factor receptor favored tumor cell replication; MET, via the opioid growth factor receptor, delayed cells moving by upregulating the cyclin-dependent inhibitory kinase pathways [16,245]. A low dose of naltrexone inhibited ovarian tumor progression and, in combination with cisplatin, exerted an enhanced inhibitory effect [246].
3.16.2. Endorphin
Beta-END has been observed in ovarian sex cord-stromal tumors [247] and ovarian carcinoids [244]. A positive correlation has been reported between survival time/disease-free time and plasma beta-END level in patients with ovarian cancer [248]. Lower beta-END concentrations were observed in patients with recurrence than those without recurrence [248]. Moreover, beta-END and MET blocked the proliferation of human ovarian KF cancer cells; this effect was counteracted with naloxone, and it seems that both peptides blocked protein/RNA synthesis but not DNA synthesis [249].
3.17. Pancreatic Cancer
3.17.1. Enkephalin
A lipid conjugation of MET increased the tumor-suppression activity of the peptide against human pancreatic adenocarcinomas [250], and the MET/opioid growth factor receptor system increased the cyclin-dependent kinase inhibitor p21 protein expression to attenuate the progression of human pancreatic cancer [251]. Moreover, the administration of MET ameliorated clinical symptoms and survival in patients with advanced pancreatic cancer [252], and a high plasma level of MET has been observed in patients with pancreatic cancer [253].
3.17.2. Dynorphin
DYN A1-8, MET, beta-END, and LEU (10−4 M; 10−6 M) did not affect the migration, chemotaxis, or invasion of pancreatic tumor cells (MIA PaCa-2, PANC-1, BxPC-3) [186] and the first three peptides mentioned did not alter the viability of the MIA PaCa-2 tumor cell line at the concentration administered (10−6 M) [172].
Mouse insulinoma beta TC3 cells show a high expression of pro-DYN mRNA and its derived peptides (DYN A1-8, DYN B1-13, alpha-neo-END) [254]. These cells also expressed prohormone convertase 1 and 2 mRNAs but not convertase 5 mRNA, and cells administered with 8-bromo-cAMP increased pro-DYN levels and the release of opioid peptides [254].
3.18. Pheochromocytoma
3.18.1. Enkephalin
Proprotein convertase 2 and pro-enkephalin have been reported in human pheochromocytomas [255]. MET has been observed in human pheochromocytomas, and nicotine promoted the release of the peptide from cultured pheochromocytoma cells [256,257]. LEU was also observed in pheochromocytomas [257]. MET and LEU levels differed in extramedullary and medullary tumors: the MET to LEU ratio was higher in extramedullary than in medullary pheochromocytomas [258].
3.18.2. Endorphin
Beta-END has been reported in pheochromocytomas [257,259], and the release of the peptide has also been demonstrated [260].
3.18.3. Dynorphin
The rat pheochromocytoma PC12 cell line expresses the pro-DYN gene and releases DYN [171]. The presence of DYN, but not alpha-neo-END, has been reported in human pheochromocytomas [257,261]; however, another study has shown the presence of both DYN and alpha-neo-END in these tumors and, in addition, it was demonstrated that nicotine favored the release of both peptides from pheochromocytomas [262]. In another study, MET, LEU, beta-END, and DYN were reported in all the pheochromocytomas studied in which the concentration of enkephalins was higher than that of DYN, and the DYN level was higher than that reported for beta-END [257]. DYN A1-17 was the major component observed in pheochromocytomas, whereas DYN A1-13 and DYN A1-12 were minor components in these tumors [263,264].
3.19. Pituitary Cancer
3.19.1. Enkephalin
MET level was increased in prolactin-releasing human pituitary adenomas [265].
3.19.2. Endorphin
Beta-END was observed in a pituitary adenoma [266], and the peptide was released from human pituitary cancer cells [267]. The presence of beta-END has been reported in clinically silent pituitary corticotroph adenomas [268], and beta-END and beta-END1-27 have been found in extracts of pituitary melanotroph tumors transplanted subcutaneously in mice [269]. W7, a calmodulin inhibitor, potentiated beta-END release promoted by 8-BcrAMP from the mouse anterior pituitary AtT-20 cancer cell line [270]. Cerebrospinal fluid beta-END levels increased after resectioning an adrenocorticotropic hormone-secreting pituitary adenoma; however, the MET level was not altered [271].
3.20. Prostate Cancer
The expression of opioid receptors has been described in prostate cancer cells and tissues [272]. Zeta-opioid receptor mRNA was expressed in all the prostate cancer cell lines studied, kappa-opioid receptors in only two cell lines (VCaP, LNCaP), and no expression was observed for mu- and delta-opioid mRNA receptors [272]. Compared with other prostate cancer cell lines, LNCaP (an androgen-sensitive cell) showed a higher expression of kappa- and zeta-opioid receptors and a synthetic androgen (R1881) repressed mRNA of both receptors [272]. Moreover, zeta-opioid receptors showed a higher expression in prostate cancer tissues than in normal ones, and this expression was elevated in aggressive and undifferentiated prostate cancer tissues [272]. A high mu-opioid receptor expression has been associated with poorer survival in patients with prostate cancer [273].
3.20.1. Enkephalin
DAGO ([D-Ala2, N-Me-Phe4-Gly-ol] enkephalin), DADLE ([D-Ala2, D-Leu5] enkephalin), and DSLET ([D-Ser2, Leu5] enkephalin) blocked the proliferation on human prostate cancer cell lines (PC3, DU145, LNCaP); this effect was counteracted with the opioid antagonist diprenorphine [274].
3.20.2. Endorphin
Beta-END and LEU have been reported in prostatic carcinomas [275]. Rats with transplanted neurons expressing beta-END into the hypothalamic paraventricular nucleus showed a protective effect against prostate cancer development; an increased NK cell cytolytic action in the spleen and peripheral blood mononuclear cells; a decreased level of inflammatory cytokines (tumor necrosis factor-alpha) in plasma, and a higher level of anti-inflammatory cytokines (interferon-gamma) in plasma [276]. This observation indicates that the release of beta-END from the hypothalamic transplanted neurons counteracts the inflammatory mechanisms and increases the immune system’s response.
3.20.3. Dynorphin
DYN A, DYN A1-13, and DYN A1-7 promote the growth of the DU145 prostatic carcinoma cell line [277]. Naloxone blocked the effect mediated by DYN A but increased the growth of tumor cells at a high concentration (10−7 M). Electroacupuncture counteracted bone-cancer-promoted hyperalgesia in a rat model that received AT-3.1 prostate cancer cells into the tibia [278]. Electroacupuncture blocked DYN and pre-pro-DYN mRNA upregulation, and the administration of anti-DYN A1-17 antisera also blocked hyperalgesia [278]. In this sense, an upregulation of DYN A has been reported in the spinal dorsal horn, and the release of the peptide has been related to spontaneous pain in a mouse model of neuropathic cancer pain [279]. Moreover, in a murine model of cancer-pain, the number of immunoreactive neurons containing DYN was increased in the spinal cord (ipsilateral to the limb containing the tumor) [280].
3.21. Renal Cancer
Enkephalin
MET blocked the proliferation of human renal cancer cells (Caki-2) [281].
3.22. Retinoblastoma
3.22.1. Enkephalin
MET has been reported in human retinoblastoma [282].
3.22.2. Endorphin
Beta-END-binding sites have been reported in the human retinoblastoma McA and Y79 cell lines [283].
3.23. Testicular Cancer
Dynorphin
Pro-DYN mRNA and its derived peptides have been observed in the R2C rat Leydig tumor cell line [284].
3.24. Thymic Cancer
3.24.1. Enkephalin
MET has been reported in a thymic carcinoid [285].
3.24.2. Endorphin
Beta-END binds to non-opioid binding sites expressed in thymoma cells [286], and beta-END has been observed in an oncocytic carcinoid tumor of the thymus [287].
3.24.3. Dynorphin
A released dibasic cleaving peptidase that converts DYNs (e.g., DYN A1-12, DYN A1-9, proDYN B) into LEU-Arg6 has been obtained from the medium of EL-4 mouse thymoma cells [288].
3.25. Thyroid Cancer
3.25.1. Enkephalin
Human anaplastic thyroid cancer cells (KAT-18) express MET and the opioid growth factor receptor; MET blocked cell replication, the opioid antagonist naltrexone promoted cell growth, and anti-MET antibodies counteracted the inhibitory action mediated by MET [289].
3.25.2. Endorphin
Opioid peptides (e.g., beta-END, alpha-neo-END) derived from the three opioid precursors have been reported in human thyroid medullary carcinomas [11], and the release of beta-END has been demonstrated from cultured medullary thyroid carcinoma cells [290].
Table 1, Table 2 and Table 3 and Figure 10, Figure 11 and Figure 12 show the involvement of MET, LEU, beta-END, and DYN in cancer.

Table 1.
Involvement of MET/LEU in cancer.

Table 2.
Involvement of beta-END in cancer.

Table 3.
Involvement of DYN in cancer.
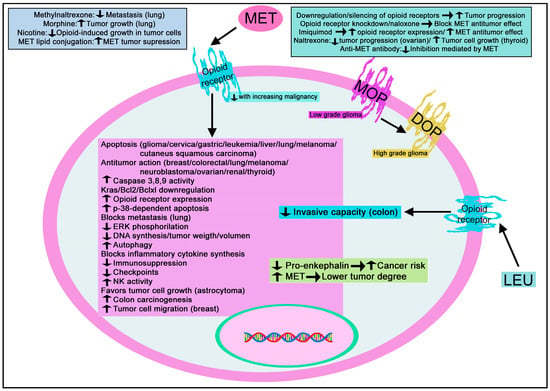
Figure 10.
Summary of the mechanisms mediated by MET/LEU in cancer. DOP: delta-opioid receptor; ERK: extracellular signal-regulated kinase; MOP: mu-opioid receptor; NK: natural killer cells. ↑: increase; ↓: decrease.
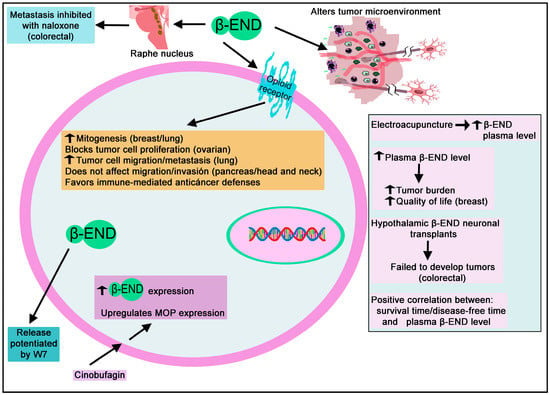
Figure 11.
Summary of the mechanisms mediated by beta-END in cancer. ↑: increase; ↓: decrease.
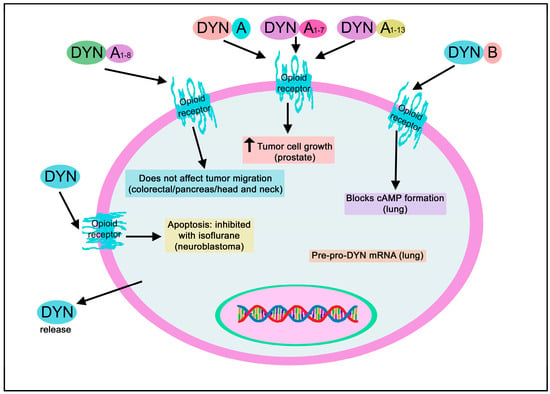
Figure 12.
Summary of the mechanisms mediated by DYN in cancer. cAMP: cyclic adenosine 3′, 5′-monophosphate.
4. Antitumor Therapeutic Strategies
The use of opioid peptides as antitumor drugs must be developed; much data support this potential therapeutic strategy. MET blocks the proliferation of triple-negative breast cancer cells through the p21 cyclin-dependent inhibitory kinase pathway [147], and the peptide induces apoptosis in tumor cells and inhibits cervical carcinoma progression [159]. MET also exerts an antitumor action in vivo against colorectal tumors by acting on the tumor microenvironment and improving immunotherapy efficacy [164]; MET inhibits the cell growth of cutaneous squamous cell carcinoma by promoting apoptotic mechanisms and decreasing immunosuppression [173], and MET blocks the growth of human gastric cancer cell lines by promoting apoptosis [175]. Moreover, morphine and MET exert an antitumor effect against lung cancer cells [204,205], and the antitumor action mediated by MET was abolished in the knockdown of growth factor receptors [205]. MET promotes apoptosis in lung cancer cells and increases the expression of the opioid growth factor receptor, but these effects disappear when the receptor is blocked [206]. MET inhibits pulmonary metastasis and increases NK cell activity [209], exerts an antiproliferative action against ovarian tumor cells (HEY, CAOV-3, SW626), delays cell moving [16,245], and blocks the proliferation of human renal cancer cells (Caki-2) [281]. Human anaplastic thyroid cancer cells (KAT-18) express MET; the peptide blocked cell replication, naltrexone promoted cell growth, and anti-MET antibodies counteracted the inhibitory action mediated by MET [289]. MET neutralization favored cell proliferation, and silencing the opioid growth factor receptor promoted tumor cell replication. Beta-END and MET blocked the expansion of human ovarian KF cancer cells; this was counteracted with naloxone, and it seems that both peptides blocked protein/RNA synthesis but not DNA synthesis [249]. MET exerts an antitumor effect, through apoptotic mechanisms, against melanoma cells which were inhibited with naloxone [222,223,224]. MET and imiquimod upregulate the expression of the opioid growth factor receptor, increasing MET’s antitumor effect [223,225].
Moreover, DAGO, DADLE, and DSLET inhibited the proliferation of human prostate cancer cell lines (PC3, DU145, LNCaP), which was counteracted with the opioid antagonist diprenorphine [274]. Thus, MET exerts an antitumor action against cervical carcinoma, colorectal tumor, cutaneous squamous cell carcinoma, and breast, gastric, lung, ovarian, renal, and thyroid cancers. The observation implies that MET is a promising broad-spectrum antitumor agent; this potential and good antitumor strategy must be developed.
By contrast, opioids promoted the growth of MCF-7 breast cancer cells, which was blocked with the mu-opioid receptor antagonist naloxone, and naltrexone promoted apoptosis in a mouse model of MCF-7 cells and decreased the growth of the triple-negative breast cancer MDA-MB-231 cell line [147,291,292]. Naltrexone also inhibited ovarian tumor progression and, with cisplatin, exerted an enhanced inhibitory effect [246]. A low dose of naltrexone blocked colorectal cancer progression in vivo and in vitro by inducing the apoptosis of tumor cells [161]. Methylnaltrexone (mu-opioid receptor antagonist) increased the activity of the antitumor drug docetaxel by inhibiting gastric cancer cell growth-suppressive pathways in vivo [293]. Thus, it seems that the blockade of the pathways that inhibit cell growth increases the actions of the antitumor agents. Methylnaltrexone had antimetastatic and antiproliferative effects against lung carcinoma [207]. Methylnaltrexone also inhibited the angiogenesis mediated by opioids. It blocked the proliferation/migration of the endothelial cells mediated by opioids by inhibiting the vascular endothelial growth factor (VEGF) receptor phosphorylation/transactivation, leading to the blockade of RhoA activation [294]. Methylnaltrexone counteracted Lewis lung carcinoma growth and decreased lung metastasis, and the mu-receptor agonist morphine favored tumor growth [207]. MET declined the tumor mitotic index, which was counteracted with naltrexone: a complete blockade with naltrexone increased neuroblastoma cell proliferation, whereas an intermittent blockade inhibited cancer cell proliferation [239]. Beta-END favors small-cell lung carcinoma cells’ proliferation, migration, and metastasis [215]. Opioid peptides (e.g., MET) facilitate the migration of tumor cells [145]; this means that opioid peptide-receptor antagonists could exert an antimetastatic action. This antitumor strategy must be fully exploited.
Thus, opioids exert a dual action on tumor cells: a proliferative or antiproliferative activity. This observation illustrates that opioid peptides (e.g., MET) and opioid-receptor antagonists (e.g., naloxone) could be used as antitumor drugs. Thus, it is vital to ascertain the opioid receptors/signaling pathways involved in the proliferative/antiproliferative effects mediated by opioids on cancer cells. More in vitro and in vivo studies must be developed to confirm the promising antitumor-mediated impacts of opioid peptides and opioid-peptide antagonists. This research line must be urgently developed and potentiated.
5. Future Research
According to previous sections, there is much to do regarding the involvement of the opioid system in cancer. Some of the research lines that would be developed/improved are suggested in this section.
5.1. Basic Research
In general, the involvement of MET in cancer has been studied more than the involvement of LEU, beta-END, and DYN. Much information is currently lacking regarding the roles played by the last three peptides in tumor progression. For example, in some tumors (e.g., brain, breast, cervical, cutaneous squamous cell carcinoma, head and neck, larynx, renal, retinoblastoma, testicular, thymic), it is currently unknown whether specific opioid peptides exert or not proliferative/antiproliferative actions and even in some tumors the expression of peptides belonging to the opioid-peptide family is unknown. Opioid peptides (e.g., beta-END) have been reported in tumors, but it is currently unknown whether the peptide favor or not a proliferative/antiproliferative action on tumor cells [140]. MET has been reported in human retinoblastoma [282] and Beta-END-binding sites in the human retinoblastoma McA and Y79 cell lines [283]. However, the physiological significance of these findings is currently unknown. Thus, basic information is lacking; this crucial knowledge must be urgently tackled to develop antitumor strategies.
5.2. MET/LEU Research
Most of the studies on the involvement of enkephalins in cancer have been focused on MET; thus, the role played by LEU in cancer progression is practically unknown, meaning that the work that remains to be done is enormous. A higher MET level has been related to a lower brain tumor degree [134], and MET-binding sites decrease with increasing malignancy of gliomas [136]. MET favors apoptotic mechanisms in rat C6 glioma cells [135], and it seems that the inactivation of the MET/opioid receptor system inhibits the blockade action exerted by MET on the growth of astrocytes, promoting glioma development. Previous data open the door to a promising anti-glioma strategy using MET as an antitumor agent; however, this must be studied in-depth since MET also favored the growth of human U-373 MG astrocytoma cells [139]. This note highlights the importance of identifying the opioid receptors/signaling pathways involved in tumor proliferative and antiproliferative mechanisms. An important finding is known in this sense: a shift from mu-opioid receptors in low-grade gliomas to delta-opioid receptors in high-grade gliomas occurs [136].
MET blocked the proliferation of hepatocellular carcinoma cells, and silencing the opioid growth factor receptor favored their proliferation [199]. In metastasis-positive human livers, the level of MET was higher than that found in normal livers [165]. Therefore, MET increase could be an endogenous mechanism to counteract tumor progression. This hypothesis must be confirmed. Moreover, two patients with hepatoblastoma were cured after surgical resection and treatment with MET and naltrexone (low dose) [200]. This promising finding must be confirmed in a higher number of patients.
Other findings must be studied in-depth and confirmed. For example, a lipid conjugation of MET increased tumor suppression activity against human pancreatic adenocarcinomas [250]. This result must be confirmed in other tumors. Mu-opioid receptors mediate head and neck squamous cell carcinoma growth by activating the proliferation/migration of cancer cells [179]. Downregulation of the opioid growth factor receptor favored the progression of head and neck squamous cell carcinoma [183]. These effects must be investigated in-depth. The MET level was increased in prolactin-releasing human pituitary adenoma [265]; however, its physiological significance is unknown. Finally, LEU reduced the invasive capacity of murine colon 26-L5 adenocarcinoma cells [166]; this must be investigated in human colorectal cancer cells.
5.3. Beta-END Research
Although essential data are known (see Table 2 and Figure 11), much remains to be done to understand the role of beta-END in cancer fully. This peptide activates in vitro the survival/mitogenic signaling pathways in human breast cancer cells (MDA-MB-231) [149]; however, a correlation between an increased plasma beta-END level and an improved quality of life in patients with breast cancer has been reported [151]. Beta-END seems to counteract breast cancer development by favoring the immune-mediated anticancer defenses in vivo [156,157]. Another experiment has shown that ultraviolet A eye irradiation reduced colon carcinoma symptoms; however, these beneficial effects were reduced when beta-END inhibitors were administered and totally disappeared after the administration of naltrexone [168]. However, the mechanisms by which beta-END exerts these beneficial effects are currently unknown.
Moreover, data suggest that hypothalamic beta-END neuronal transplants blocked colon cancer progression by decreasing inflammatory mechanisms and the epithelial-mesenchymal transition [169]. This research line must be developed because animals with transplanted beta-END neurons showed a lesser adenoma development in the colon, and beta-END-transplanted neurons also prevented hepatocellular carcinoma formation [169,201]. Rats with transplanted neurons expressing beta-END into the hypothalamic paraventricular nucleus showed a protective effect against prostate cancer development, increased immune response, and counteracted inflammatory mechanisms [276]. Other experiments showed that beta-END administration into the raphe magnus nucleus facilitated metastasis inhibited with naloxone; however, metastasis was significantly attenuated when the nucleus was electrically stimulated [170]. The molecular mechanisms involved in these processes must be elucidated.
5.4. DYN Research
After LEU, DYN is the less-known reviewed peptide concerning its implication in cancer progression. Like LEU, the work that remains for DYN is enormous. U50,488, a kappa-opioid receptor agonist, favored apoptosis in human multiple-myeloma cells expressing mu- and kappa-opioid receptors; but the antiproliferative effect was not mediated by kappa receptors [235]. This result demands further investigation. DYN A1-17 is the major component observed in pheochromocytomas, and DYN A1-13 and DYN A1-12 are minor components, but it is currently unknown what the physiological significance of these findings is [263,264]. The DYN A2-13 fragment failed to increase tumor cell growth, but DYN A, DYN A1-13, and DYN A1-7 favored the growth of the prostatic carcinoma cell line (DU145); naloxone inhibited the effect mediated by DYN A but increased the growth of tumor cells [277]. This study is vital because fragments of the opioid peptides mediate different results; this issue must be fully developed using opioid fragments in other tumors to know their proliferative/antiproliferative actions. These actions could be mediated by different proteins G/receptors but await in-depth elucidation.
Regarding DYN A, other research lines must be investigated in-depth. For example, DYN favored apoptosis in neuroblastoma cells, but the anesthetic isoflurane inhibited this effect [242]. At high concentrations, DYN A binds to neuropeptide Y receptors (Y1 and Y2) in neuroblastoma cells; however, the physiological significance of this observation is unknown [243].
5.5. Signaling Pathways
Some data regarding the opioid-mediated signaling pathways involved in cancer (see Figure 9) should be analyzed. For example, the MET/opioid growth factor receptor system augmented the cyclin-dependent kinase inhibitor p21 protein expression, decreasing the progression of human pancreatic cancer [251]. However, the signaling pathways that mediate the proliferative or antiproliferative mechanisms controlled by opioids in tumor cells deserve more investigation. Determining the signaling pathways leading to cancer triggered by opioids is a promising research line that must be fully developed.
5.6. Tumor Cell Migration
Tumor cell migration is a crucial issue that must be studied in-depth to develop future antitumor strategies. The use of opioid peptide receptor antagonists as antimetastatic agents must be exploited since opioid peptides (e.g., MET) promote the migration of cancer cells (e.g., breast cancer cells) [145]. In contrast, using opioid peptides (e.g., LEU) is also a promising antimetastatic strategy because they reduce cancer cells’ invasive capacity [166]. Currently, it is not known whether opioid peptides favor or not tumor cell migration in many cancer types.
5.7. Angiogenesis
Pro-opiomelanocortin gene delivery inhibited the vasculature of tumors and blocked the growth of lung carcinoma cells by apoptotic mechanisms and melanoma progression and metastasis [219]. It is an important finding, but much information is lacking regarding the involvement of opioid peptides in tumor angiogenesis. This information is essential to develop anti-angiogenic strategies.
5.8. Polymorphisms
Another topic that must be studied in-depth is the expression of polymorphisms in patients with cancer. In this sense, a study has reported that patients with a polymorphism in the mu-opioid receptor gene showed better survival [143]. This research must be fully developed.
5.9. Substances Regulating the Opioid System
MET upregulates the expression of opioid receptors [175]; imiquimod also upregulates this expression, increasing the antitumor effect mediated by MET [225], and the downregulation of opioid receptors favors the progression of tumors [183]. Nicotine partially or totally reversed opioid-induced growth suppression in lung cancer cells [208]. These cells express mu, kappa, and delta opioid receptors, and their agonists block the growth of tumor cells [208]. Nicotine favored the release of DYN and alpha-neo-END peptides from pheochromocytomas [262]. Cinobufagin, used for the treatment of cancer pain, relieved cancer pain by upregulating the expressions of both mu-opioid receptors and beta-END in the hind paw tumor and tissues placed close to the tumor in a rat model of paw bone cancer pain [131]. The knowledge of the different compounds that regulate the release of opioid peptides is essential since, for example, DYN favors apoptosis in neuroblastoma cells but also promotes the growth of prostate cancer cells (see Table 3). Knowing how endogenous or exogenous substances regulate the release of opioid peptides from nerve terminals or from normal/tumor cells is crucial to establishing anticancer strategies.
5.10. Endopeptidases
A released dibasic cleaving peptidase that converts DYNs (e.g., DYN A1-12, DYN A1-9, proDYN B) into LEU-Arg6 has been obtained from the medium of EL-4 mouse thymoma cells [288]. Much information is lacking regarding the involvement of opioid endopeptidases in tumor progression.
5.11. The Opioid System as a Cancer-Predictive Factor
This topic must also be developed, and the published promising data confirmed: an increased risk for developing breast cancer has been associated with a low plasma level of pro-enkephalin [146]; beta-END immunoreactivity was lower in benign melanocytic naevi than in metastatic and advanced melanomas [232], and a positive correlation between plasma beta-END levels and survival time/disease-free time has been suggested in patients with ovarian cancer: lower beta-END concentrations were observed in patients with recurrence than in those without recurrence [248]. MET improved survival/clinical symptoms in patients with advanced pancreatic cancer [252]; high MET plasma levels in patients with pancreatic cancer have been reported [253]; zeta-opioid receptors are more expressed in prostate cancer tissues than in normal ones, and this expression was high in aggressive and undifferentiated prostate cancer tissues [272]. An association has been reported between high mu-opioid receptor expression and poorer survival in patients with prostate cancer [273] and a correlation between increased plasma beta-END levels and improved quality of life in patients with breast cancer [151].
5.12. Structural Dynamics of OR
The analysis of the structural dynamics, conformational states, and functional splice variants of OR related to cancer development and progression deserves further investigation to ascertain better their influence on molecular events that initiate abnormal cell proliferation in different tissues [295]. One aspect calling for specific attention is the definition of OR biased and cross-talk signaling events that may force the activity of OR toward activating metabolic routes firing cancer appearance and progression [113,296,297]. The study of structural and biochemical mechanisms should be accompanied by population observations relating to the influence of opioid systems in different cancers, considering putative-associated or confounding factors [298,299].
5.13. Other Research Lines
Some studies have suggested microbiota involvement in cancer, especially gastrointestinal cancer [300]. In this sense, it has been stated that a dysregulation of the expression of non-coding RNA through the gut microbiome could be the origin of gastrointestinal cancer (see [300] for review). This is an exciting line of research that must be investigated and developed.
Although it is known that peptides derived from proopiomelanocortin, including ACTH, regulate cancer cell proliferation [301], the effect of ACTH on opioid peptides in cancer development is currently unknown.
Opioid receptors might control apoptotic mechanisms in cancer cells by modulating apoptosis-related proteins, including tumor-suppressor protein p53. For example, an endomorphin 2 analog reduced the expression of Bcl-2 and increased the expression of Bax, p53, and pro-caspases 3 and 9 in human DLD-1 and RKO colon cancer cells [302].
6. Conclusions
MET is the opioid peptide more studied in cancer, followed by beta-END. Most of the studies performed on opioid peptides are descriptive since they were mainly focused on the expression or not of these peptides and their receptors in tumor cells. In many cancer types, fragmentary, scarce, or absent data are currently available regarding the involvement of these peptides in cancer development. This fact applies to DYN and LEU. Thus, a systematic study on the expression/role (antiproliferative/proliferative effects, metastasis, angiogenesis) of opioid peptides and their receptors in many types of cancers is urgently needed. This study must include the involvement of peptide fragments in tumor progression. It is also important to know whether the expression of opioid receptors is essential for the viability of tumor cells and whether tumor cells express more opioid receptors than normal cells. Secondly, more studies must focus on using opioid peptides (e.g., MET) and opioid-receptor antagonists/agonists as antitumor agents. In particular, an anticancer effect is generally mediated by MET against different tumors, although the peptide also promotes the growth of some tumors. Thus, depending on the type of cancer, opioid-peptide agonists or antagonists could be used as antitumor agents. It is crucial to know in-depth which opioid receptors and signaling pathways are involved in cancer progression to establish better antitumor strategies. In the same way, the use of opioid-receptor antagonists must be fully developed since only a few studies have been focused on the anticancer actions mediated by these antagonists; in addition, the antitumor effect mediated by other antagonists than those currently studied must be tested in pre-clinical studies. Developing specific opioid-receptor antagonists exerting an antitumor action is crucial without affecting other receptors. Consequently, the structure-function relationships between opioid peptides and their receptors must be well known to design new broad-spectrum antitumor drugs and for drug-design studies.
More studies must be focused on the tumor microenvironment. For example, the cross-talk between cancer development and the release of opioid peptides from nerve fibers must be studied in-depth because these peptides control the progression of tumors. The knowledge about the agents that control the expression of opioid peptides and their receptors must be increased to develop new antitumor strategies. Peptides regulate the immune response in cancer; this is important since, for example, beta-END favors the immune-mediated anticancer defenses [156,157], MET increases immunogenicity and recognition of cancer cells, blocks the formation of inflammatory cytokines and decreases immune checkpoints in colorectal cancer cells, and MET reduced immunosuppression in cutaneous squamous cell carcinoma. It must also be investigated how DYN A interacts with other surface receptors since this peptide binds to neuropeptide Y receptors (Y1 and Y2) in neuroblastoma cells and the effects mediated by opioid peptides regulating the glycolysis in tumor cells because this process is essential for tumor cells to obtain ATP. This knowledge will lead to developing future new anticancer strategies. Combined antitumor methods using peptides/opioid-receptor antagonists and chemotherapy must be performed, and treatments increasing the chemosensitivity of cancer cells must be tested.
Moreover, new tracers such as radiopharmaceuticals (e.g., opioid peptide-drug conjugates) for cancer imaging and treatment is a promising and attractive research field that must be developed. Moreover, opioid peptides/receptors as prognostic factors or tumor biomarkers must be studied in-depth (e.g., higher MET level: lower tumor degree; increasing glioma malignancy: MET-binding sites decrease; beta-END level and tumor progression). Also, the confirmation of their role in several tumors and studies focused on single nucleotide polymorphisms.
Although the involvement of peptides in cancer is a promising line of research, there is much to investigate regarding the participation of opioid peptides in cancer progression: basic information is scarce, incomplete, or absent in many tumors, and, in fact, no clinical study regarding the antitumor action of opioid peptides/opioid-peptide receptor antagonists has been developed to date. According to the current and future knowledge acquired, promising anticancer strategies could be developed alone or in combination therapy with chemotherapy/radiotherapy.
Author Contributions
Conceptualization, M.L.S., F.D.R. and R.C.; sources, M.L.S., F.D.R. and R.C.; writing—original draft preparation, M.L.S., F.D.R. and R.C.; writing—review and editing, M.L.S., F.D.R. and R.C.; supervision, M.L.S., F.D.R. and R.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
No patient consent or ethics approval was required for this review.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Support from Programa XIII, Universidad de Salamanca, and the GIR group BMD (Bases Moleculares del Desarrollo) is acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Coveñas, R.; Muñoz, M. Involvement of the Substance P/Neurokinin-1 Receptor System in Cancer. Cancers 2022, 14, 3539. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, E.; Pei, G.; Kim, S.T.; German, A.; Robinson, P. Substance P Antagonism as a Novel Therapeutic Option to Enhance Efficacy of Cisplatin in Triple Negative Breast Cancer and Protect PC12 Cells against Cisplatin-Induced Oxidative Stress and Apoptosis. Cancers 2021, 13, 3871. [Google Scholar] [CrossRef] [PubMed]
- García-Aranda, M.; Téllez, T.; McKenna, L.; Redondo, M. Neurokinin-1 Receptor (NK-1R) Antagonists as a New Strategy to Overcome Cancer Resistance. Cancers 2022, 14, 2255. [Google Scholar] [CrossRef]
- Beirith, I.; Renz, B.W.; Mudusetti, S.; Ring, N.S.; Kolorz, J.; Koch, D.; Bazhin, A.V.; Berger, M.; Wang, J.; Angele, M.K.; et al. Identification of the Neurokinin-1 Receptor as Targetable Stratification Factor for Drug Repurposing in Pancreatic Cancer. Cancers 2021, 13, 2703. [Google Scholar] [CrossRef]
- Sánchez, M.L.; Coveñas, R. The Galaninergic System: A Target for Cancer Treatment. Cancers 2022, 14, 3755. [Google Scholar] [CrossRef]
- Sánchez, M.L.; Rodríguez, F.D.; Coveñas, R. Peptidergic Systems and Cancer: Focus on Tachykinin and Calcitonin/Calcitonin Gene-Related Peptide Families. Cancers 2023, 15, 1694. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.L.; Coveñas, R. The Neurotensinergic System: A Target for Cancer Treatment. Curr. Med. Chem. 2022, 29, 3231–3260. [Google Scholar] [CrossRef]
- Robinson, P.; Coveñas, R.; Muñoz, M. Combination Therapy of Chemotherapy or Radiotherapy and the Neurokinin-1 Receptor Antagonist Aprepitant: A New Antitumor Strategy? Curr. Med. Chem. 2023, 29, 1798–1812. [Google Scholar] [CrossRef]
- Muñoz, M.F.; Argüelles, S.; Rosso, M.; Medina, R.; Coveñas, R.; Ayala, A.; Muñoz, M. The Neurokinin-1 Receptor Is Essential for the Viability of Human Glioma Cells: A Possible Target for Treating Glioblastoma. BioMed Res. Int. 2022, 2022, 6291504. [Google Scholar] [CrossRef]
- Bostwick, D.G.; Null, W.E.; Holmes, D.; Weber, E.; Barchas, J.D.; Bensch, K. Expression of Opioid Peptides in Tumors. N. Engl. J. Med. 1987, 317, 1439–1443. [Google Scholar] [CrossRef]
- Roth, K.A.; Bensch, K.G.; Hoffman, A.R. Characterization of Opioid Peptides in Human Thyroid Medullary Carcinoma. Cancer 1987, 59, 1594–1598. [Google Scholar] [CrossRef]
- Cronin-Fenton, D. Opioids, and Breast Cancer Recurrence. Curr. Opin. Support. Palliat. Care 2019, 13, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Zagon, I.S.; McLaughlin, P.J.; Goodman, S.R.; Rhodes, R.E. Opioid Receptors and Endogenous Opioids in Diverse Human and Animal Cancers23. JNCI J. Natl. Cancer Inst. 1987, 79, 1059–1065. [Google Scholar] [CrossRef]
- Zagon, I.S.; Smith, J.P.; McLaughlin, P.J. Human Pancreatic Cancer Cell Proliferation in Tissue Culture Is Tonically Inhibited by Opioid Growth Factor. Int. J. Oncol. 1999, 14, 577–584. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, P.J.; Levin, R.J.; Zagon, I.S. Regulation of Human Head and Neck Squamous Cell Carcinoma Growth in Tissue Culture by Opioid Growth Factor. Int. J. Oncol. 1999, 14, 991–998. [Google Scholar] [CrossRef]
- Donahue, R.N.; McLaughlin, P.J.; Zagon, I.S. Cell Proliferation of Human Ovarian Cancer Is Regulated by the Opioid Growth Factor-Opioid Growth Factor Receptor Axis. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2009, 296, R1716–R1725. [Google Scholar] [CrossRef]
- Zagon, I.S.; Donahue, R.N.; McLaughlin, P.J. Opioid Growth Factor-Opioid Growth Factor Receptor Axis Is a Physiological Determinant of Cell Proliferation in Diverse Human Cancers. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2009, 297, R1154–R1161. [Google Scholar] [CrossRef] [PubMed]
- Schoos, A.; Gabriel, C.; Knab, V.M.; Fux, D.A. Activation of HIF-1 Alpha by Gamma-Opioid Receptors Induces COX-2 Expression in Breast Cancer Cells and Leads to Paracrine Activation of Vascular Endothelial Cells. J. Pharmacol. Exp. Ther. 2019, 370, 480–489. [Google Scholar] [CrossRef]
- UniProt Database. Available online: https://www.uniprot.org/ (accessed on 10 June 2023).
- Parker, K.E.; Pedersen, C.E.; Gomez, A.M.; Spangler, S.M.; Walicki, M.C.; Feng, S.Y.; Stewart, S.L.; Otis, J.M.; Al-Hasani, R.; McCall, J.G.; et al. A Paranigral VTA Nociceptin Circuit That Constrains Motivation for Reward. Cell 2019, 178, 653–671.e19. [Google Scholar] [CrossRef] [PubMed]
- Guide to Pharmacology IUPAHR. Available online: https://www.guidetopharmacology.org/ (accessed on 15 May 2023).
- Zagon, I.S.; Verderame, M.F.; Zimmer, W.E.; Mclaughlin, P.J. Molecular Characterization and Distribution of the Opioid Growth Factor Receptor (OGFr) in Mouse. Mol. Brain Res. 2000, 84, 106. [Google Scholar] [CrossRef]
- Borsodi, A.; Bruchas, M.; Caló, G.; Chavkin, C.; Christie, M.J.; Civelli, O.; Connor, M.; Cox, B.M.; Devi, L.A.; Evans, C.; et al. Opioid Receptors in GtoPdb v.2021.3. IUPHARBPS Guide Pharmacol. CITE 2021, 2021. [Google Scholar] [CrossRef]
- Bodnar, R.J. Endogenous Opiates and Behavior: 2021. Peptides 2023, 164, 171004. [Google Scholar] [CrossRef]
- Petrocelli, G.; Pampanella, L.; Abruzzo, P.M.; Ventura, C.; Canaider, S.; Facchin, F. Endogenous Opioids and Their Role in Stem Cell Biology and Tissue Rescue. Int. J. Mol. Sci. 2022, 23, 3819. [Google Scholar] [CrossRef]
- Zadina, J.E.; Hackler, L.; Ge, L.-J.; Kastin, A.J. A Potent and Selective Endogenous Agonist for the Μ-Opiate Receptor. Nature 1997, 386, 499–502. [Google Scholar] [CrossRef]
- Terskiy, A.; Wannemacher, K.M.; Yadav, P.N.; Tsai, M.; Tian, B.; Howells, R.D. Search of the Human Proteome for Endomorphin-1 and Endomorphin-2 Precursor Proteins. Life Sci. 2007, 81, 1593–1601. [Google Scholar] [CrossRef] [PubMed]
- Kaiya, H. Chapter 21—Mexneurin. In Handbook of Hormones; Elsevier Inc.: Amsterdam, The Netherlands, 2021; pp. 173–174. [Google Scholar]
- PepDraw. Available online: https://pepdraw.com/ (accessed on 17 May 2023).
- Tanaka, S. Comparative Aspects of Intracellular Proteolytic Processing of Peptide Hormone Precursors: Studies of Proopiomelanocortin Processing. Zoolog. Sci. 2003, 20, 1183–1198. [Google Scholar] [CrossRef] [PubMed]
- Gene NCBI Database. Available online: https://www.ncbi.nlm.nih.gov/gene/ (accessed on 12 June 2023).
- Pilozzi, A.; Carro, C.; Huang, X. Roles of β-Endorphin in Stress, Behavior, Neuroinflammation, and Brain Energy Metabolism. Int. J. Mol. Sci. 2020, 22, 338. [Google Scholar] [CrossRef] [PubMed]
- Netto, C.A. Role of Brain Β-Endorphin in Memory Modulation Revisited. Neuroscience 2022, 497, 30–38. [Google Scholar] [CrossRef]
- Pert, C.B.; Snyder, S.H. Opiate Receptor: Demonstration in Nervous Tissue. Science 1973, 179, 1011–1014. [Google Scholar] [CrossRef]
- Hughes, J.; Smith, T.W.; Kosterlitz, H.W.; Fothergill, L.A.; Morgan, B.A.; Morris, H.R. Identification of Two Related Pentapeptides from the Brain with Potent Opiate Agonist Activity. Nature 1975, 258, 577–580. [Google Scholar] [CrossRef]
- Ndong, D.B.; Blais, V.; Holleran, B.; Proteau-Gagné, A.; Cantin-Savoie, I.; Robert, W.; Nadon, J.-F.; Beauchemin, S.; Leduc, R.; Pineyro, G.; et al. Exploration of the Fifth Position of Leu-Enkephalin and Its Role in Binding and Activating Delta (DOP) and Mu (MOP) Opioid Receptors. Pept. Sci. 2018, 111, e24070. [Google Scholar] [CrossRef]
- Zagon, I.S.; McLaughlin, P.J. Opioid Growth Factor and the Treatment of Human Pancreatic Cancer: A Review. World J. Gastroenterol. WJG 2014, 20, 2218–2223. [Google Scholar] [CrossRef] [PubMed]
- Zagon, I.S.; Verderame, M.F.; Mclaughlin, P.J. The Biology of the Opioid Growth Factor Receptor (OGFr). Brain Res. Rev. 2002, 38, 351. [Google Scholar] [CrossRef]
- Schwarzer, C. 30 Years of Dynorphins—New Insights on Their Functions in Neuropsychiatric Diseases. Pharmacol. Ther. 2009, 123, 353–370. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, X.; Fan, J.; Sun, H.; Yao, Q.; Shi, J.; Qu, H.; Du, S.; Cheng, Y.; Ma, S.; et al. Dynorphin A (1–8) Inhibits Oxidative Stress and Apoptosis in MCAO Rats, Affording Neuroprotection through NMDA Receptor and κ-Opioid Receptor Channels. Neuropept. Edinb. 2021, 89, 102182. [Google Scholar] [CrossRef]
- Meunier, J.C.; Mollereau, C.; Toll, L.; Suaudeau, C.; Moisand, C.; Alvinerie, P.; Butour, J.L.; Guillemot, J.C.; Ferrara, P.; Monsarrat, B. Isolation and Structure of the Endogenous Agonist of Opioid Receptor-like ORL1 Receptor. Nature 1995, 377, 532–535. [Google Scholar] [CrossRef]
- Toll, L.; Cippitelli, A.; Ozawa, A. The NOP Receptor System in Neurological and Psychiatric Disorders: Discrepancies, Peculiarities and Clinical Progress in Developing Targeted Therapies. CNS Drugs 2021, 35, 591–607. [Google Scholar] [CrossRef]
- Tariq, S.; Nurulain, S.M.; Tekes, K.; Adeghate, E. Deciphering Intracellular Localization and Physiological Role of Nociceptin and Nocistatin. Peptides 2013, 43, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Yacoub, O.N.A.; Awwad, H.O.; Zhang, Y.; Standifer, K.M. Therapeutic Potential of Nociceptin/Orphanin FQ Peptide (NOP) Receptor Modulators for Treatment of Traumatic Brain Injury, Traumatic Stress, and Their Co-Morbidities. Pharmacol. Ther. 2022, 231, 107982. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Chen, D.; Yan, Y.; Li, Q.; Xing, W.; Liu, Y.; Chen, Y.; Wang, D.; Yuan, Y.; Xie, J.; et al. The Nociceptin Receptor Promotes Autophagy through NF-KB Signaling and Is Transcriptionally Regulated by E2F1 in HCC. Cell Death Discov. 2022, 8, 165. [Google Scholar] [CrossRef]
- García-Nafría, J.; Tate, C.G. Structure Determination of GPCRs: Cryo-EM Compared with X-ray Crystallography. Biochem. Soc. Trans. 2021, 49, 2345–2355. [Google Scholar] [CrossRef]
- Cheng, J.-X.; Cheng, T.; Li, W.-H.; Liu, G.-X.; Zhu, W.-L.; Tang, Y. Computational Insights into the Subtype Selectivity and “Message-Address-Efficacy” Mechanisms of Opioid Receptors through JDTic Binding and Unbinding. Acta Pharmacol. Sin. 2018, 39, 482. [Google Scholar] [CrossRef] [PubMed]
- Degrandmaison, J.; Rochon-Haché, S.; Parent, J.-L.; Gendron, L. Knock-In Mouse Models to Investigate the Functions of Opioid Receptors in Vivo. Front. Cell. Neurosci. 2022, 16, 807549. [Google Scholar] [CrossRef] [PubMed]
- GPCR Database. Available online: https://gpcrdb.org/ (accessed on 17 May 2023).
- Kooistra, A.J.; Mordalski, S.; Pándy-Szekeres, G.; Esguerra, M.; Mamyrbekov, A.; Munk, C.; Keserű, G.M.; Gloriam, D.E. GPCRdb in 2021: Integrating GPCR Sequence, Structure and Function. Nucleic Acids Res. 2021, 49, D335–D343. [Google Scholar] [CrossRef] [PubMed]
- RCSB PDB. Available online: https://www.rcsb.org/ (accessed on 30 May 2023).
- Zhuang, Y.; Wang, Y.; He, B.; He, X.; Zhou, X.E.; Guo, S.; Rao, Q.; Yang, J.; Liu, J.; Zhou, Q.; et al. Molecular Recognition of Morphine and Fentanyl by the Human μ-Opioid Receptor. Cell 2022, 185, 4361–4375.e19. [Google Scholar] [CrossRef]
- Sehnal, D.; Bittrich, S.; Deshpande, M.; Svobodová, R.; Berka, K.; Bazgier, V.; Velankar, S.; Burley, S.K.; Koča, J.; Rose, A.S. Mol* Viewer: Modern Web App for 3D Visualization and Analysis of Large Biomolecular Structures. Nucleic Acids Res. 2021, 49, W431–W437. [Google Scholar] [CrossRef]
- KingDraw-Free Chemical Structure Editor. Available online: http://www.kingdraw.cn/en/index.html (accessed on 17 May 2023).
- Pasternak, G.W.; Pan, Y.-X. Mu Opioids and Their Receptors: Evolution of a Concept. Pharmacol. Rev. 2013, 65, 1257–1317. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Liu, N.-J.; Gintzler, A.R. Relevance of Mu-Opioid Receptor Splice Variants and Plasticity of Their Signaling Sequelae to Opioid Analgesic Tolerance. Cell. Mol. Neurobiol. 2021, 41, 855–862. [Google Scholar] [CrossRef]
- Ballesteros, J.A.; Weinstein, H. Integrated Methods for the Construction of Three-Dimensional Models and Computational Probing of Structure-Function Relations in G Protein-Coupled Receptors. Methods Neurosci. 1995, 25, 366–428. [Google Scholar] [CrossRef]
- Manglik, A.; Kruse, A.C.; Kobilka, T.S.; Thian, F.S.; Mathiesen, J.M.; Sunahara, R.K.; Pardo, L.; Weis, W.I.; Kobilka, B.K.; Granier, S. Crystal Structure of the Μ-Opioid Receptor Bound to a Morphinan Antagonist. Nature 2012, 485, 321–326. [Google Scholar] [CrossRef]
- Huang, W.; Manglik, A.; Venkatakrishnan, A.J.; Laeremans, T.; Feinberg, E.N.; Sanborn, A.L.; Kato, H.E.; Livingston, K.E.; Thorsen, T.S.; Kling, R.C.; et al. Structural Insights into µ-opioid Receptor Activation. Nature 2015, 524, 315. [Google Scholar] [CrossRef] [PubMed]
- Koehl, A.; Hu, H.; Maeda, S.; Zhang, Y.; Qu, Q.; Paggi, J.M.; Latorraca, N.R.; Hilger, D.; Dawson, R.; Matile, H.; et al. Structure of the Μ-Opioid Receptor–Gi Protein Complex. Nature 2018, 558, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Tan, K.; Floyd, C.; Bong, D.; Pino, M.J.; Wu, C. Probing Biased Activation of Mu-Opioid Receptor by the Biased Agonist PZM21 Using All Atom Molecular Dynamics Simulation. Life Sci. 2021, 269, 119026. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hetzer, F.; Huang, W.; Qu, Q.; Meyerowitz, J.; Kaindl, J.; Hübner, H.; Skiniotis, G.; Kobilka, B.K.; Gmeiner, P. Structure-Based Evolution of G Protein-Biased μ-Opioid Receptor Agonists. Angew. Chem. Int. Ed. 2022, 61, e202200269. [Google Scholar] [CrossRef]
- Azzam, A.A.H.; McDonald, J.; Lambert, D.G. Hot Topics in Opioid Pharmacology: Mixed and Biased Opioids. Br. J. Anaesth. BJA 2019, 122, e136–e145. [Google Scholar] [CrossRef]
- Granier, S.; Manglik, A.; Kruse, A.C.; Tong, S.K.; Foon, S.T.; Weis, W.I.; Kobilka, B.K. Structure of the δ-Opioid Receptor Bound to Naltrindole. Nature 2012, 485, 400–404. [Google Scholar] [CrossRef]
- Fenalti, G.; Giguere, P.M.; Katritch, V.; Huang, X.-P.; Thompson, A.A.; Cherezov, V.; Roth, B.L.; Stevens, R.C. Molecular Control of δ-Opioid Receptor Signalling. Nature 2014, 506, 191–196. [Google Scholar] [CrossRef]
- Claff, T.; Yu, J.; Blais, V.; Patel, N.; Martin, C.; Wu, L.; Han, G.W.; Holleran, B.J.; Poorten, O.V.D.; White, K.L.; et al. Elucidating the Active δ-Opioid Receptor Crystal Structure with Peptide and Small-Molecule Agonists. Sci. Adv. 2019, 5, eaax9115. [Google Scholar] [CrossRef]
- Wang, Y.; Zhuang, Y.; DiBerto, J.F.; Zhou, X.E.; Schmitz, G.P.; Yuan, Q.; Jain, M.K.; Liu, W.; Melcher, K.; Jiang, Y.; et al. Structures of the Entire Human Opioid Receptor Family. Cell 2023, 186, 413–427.e17. [Google Scholar] [CrossRef]
- Sofuoglu, M.; Portoghese, P.S.; Takemori, A.E. 7-Benzylidenenaltrexone (BNTX): A Selective Δ1 Opioid Receptor Antagonist in the Mouse Spinal Cord. Life Sci. 1993, 52, 769. [Google Scholar] [CrossRef]
- Zhou, W.; Li, Y.; Meng, X.; Liu, A.; Mao, Y.; Zhu, X.; Meng, Q.; Jin, Y.; Zhang, Z.; Tao, W. Switching of Delta Opioid Receptor Subtypes in Central Amygdala Microcircuits Is Associated with Anxiety States in Pain. J. Biol. Chem. 2021, 296, 100277. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Elladki, R.; Jiang, J.; Wei, G.-W. Machine-Learning Analysis of Opioid Use Disorder Informed by MOR, DOR, KOR, NOR and ZOR-Based Interactome Networks. Comput. Biol. Med. 2023, 157, 106745. [Google Scholar] [CrossRef] [PubMed]
- Huixian, W.U.; Wacker, D.; Mascarella, S.W.; Westkaemper, R.B.; Mosier, P.D.; Roth, B.L.; Cherezov, V.; Stevens, R.C.; Mileni, M.; Katritch, V.; et al. Structure of the Human κ-Opioid Receptor in Complex with JDTic. Nature 2012, 485, 327–332. [Google Scholar] [CrossRef]
- Che, T.; English, J.; Krumm, B.E.; Kim, K.; Pardon, E.; Olsen, R.H.J.; Wang, S.; Zhang, S.; Diberto, J.F.; Sciaky, N.; et al. Nanobody-Enabled Monitoring of Kappa Opioid Receptor States. Nat. Commun. 2020, 11, 1145. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, C.; White, K.L.; Doncescu, N.; Didenko, T.; Roth, B.L.; Czaplicki, G.; Stevens, R.C.; Wüthrich, K.; Milon, A. NMR Structure and Dynamics of the Agonist Dynorphin Peptide Bound to the Human Kappa Opioid Receptor. Proc. Natl. Acad. Sci. USA 2015, 112, 11852. [Google Scholar] [CrossRef]
- Wu, Q.; Yang, H.; Zheng, Q.; Chen, Q.; Li, X.; Guo, J. κ-Opioid Receptors Improve Vascular Endothelial Dysfunction in Salt-Sensitive Hypertension via PI3K/Akt/ENOS Signaling Pathway. Oxid. Med. Cell. Longev. 2023, 2023, 5352959-13. [Google Scholar] [CrossRef]
- Che, T.; Majumdar, S.; Zaidi, S.A.; Ondachi, P.; McCorvy, J.D.; Wang, S.; Mosier, P.D.; Uprety, R.; Vardy, E.; Krumm, B.E.; et al. Structure of the Nanobody-Stabilized Active State of the Kappa Opioid Receptor. Cell 2018, 172, 55–67.e15. [Google Scholar] [CrossRef]
- Han, J.; Zhang, J.; Nazarova, A.L.; Bernhard, S.M.; Krumm, B.E.; Zhao, L.; Lam, J.H.; Rangari, V.A.; Majumdar, S.; Nichols, D.E.; et al. Ligand and G-Protein Selectivity in the κ-Opioid Receptor. Nature 2023, 617, 417. [Google Scholar] [CrossRef]
- Ji, J.; Lin, W.; Vrudhula, A.; Xi, J.; Yeliseev, A.; Grothusen, J.; Bu, W.; Liu, R. Molecular Interaction Between Butorphanol and κ-Opioid Receptor. Obstet. Anesthesia Dig. 2020, 131, 935–942. [Google Scholar] [CrossRef]
- Uprety, R.; Che, T.; Zaidi, S.A.; Grinnell, S.G.; Varga, B.R.; Faouzi, A.; Slocum, S.T.; Allaoa, A.; Varadi, A.; Nelson, M.; et al. Controlling Opioid Receptor Functional Selectivity by Targeting Distinct Subpockets of the Orthosteric Site. eLife 2021, 10, e56519. [Google Scholar] [CrossRef]
- Puls, K.; Wolber, G. Solving an Old Puzzle: Elucidation and Evaluation of the Binding Mode of Salvinorin A at the Kappa Opioid Receptor. Molecules 2023, 28, 718. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.L.; Thompson, A.A.; Trapella, C.; Guerrini, R.; Malfacini, D.; Patel, N.; Han, G.W.; Cherezov, V.; Caló, G.; Katritch, V.; et al. The Importance of Ligand-Receptor Conformational Pairs in Stabilization: Spotlight on the N/OFQ G Protein-Coupled Receptor. Structure 2015, 23, 2291–2299. [Google Scholar] [CrossRef] [PubMed]
- Pacifico, S.; Albanese, V.; Illuminati, D.; Marzola, E.; Fabbri, M.; Ferrari, F.; Holanda, V.A.D.; Sturaro, C.; Malfacini, D.; Ruzza, C.; et al. Novel Mixed NOP/Opioid Receptor Peptide Agonists. J. Med. Chem. 2021, 64, 6656. [Google Scholar] [CrossRef] [PubMed]
- Kiguchi, N.; Ding, H.; Ko, M. Therapeutic Potentials of NOP and MOP Receptor Coactivation for the Treatment of Pain and Opioid Abuse. J. Neurosci. Res. 2022, 100, 191. [Google Scholar] [CrossRef] [PubMed]
- Kamakolanu, U.G.; Meyer, M.E.; Yasuda, D.; Polgar, W.E.; Marti, M.; Mercatelli, D.; Pisanò, C.A.; Brugnoli, A.; Morari, M.; Zaveri, N.T. Discovery and Structure–Activity Relationships of Nociceptin Receptor Partial Agonists That Afford Symptom Ablation in Parkinson’s Disease Models. J. Med. Chem. 2020, 63, 2688–2704. [Google Scholar] [CrossRef] [PubMed]
- Daibani, A.E.; Paggi, J.M.; Kim, K.; Laloudakis, Y.D.; Popov, P.; Bernhard, S.M.; Krumm, B.E.; Olsen, R.H.J.; Diberto, J.; Carroll, F.I.; et al. Molecular Mechanism of Biased Signaling at the Kappa Opioid Receptor. Nat. Commun. 2023, 14, 1338. [Google Scholar] [CrossRef] [PubMed]
- Robertson, M.J.; Papasergi-Scott, M.; He, F.; Seven, A.B.; Meyerowitz, J.G.; Panova, O.; Peroto, M.C.; Che, T.; Skiniotis, G. Structure Determination of Inactive-State GPCRs with a Universal Nanobody. Nat. Struct. Mol. Biol. 2022, 29, 1188. [Google Scholar] [CrossRef]
- Vo, Q.N.; Mahinthichaichan, P.; Shen, J.; Ellis, C.R. How μ-Opioid Receptor Recognizes Fentanyl. Nat. Commun. 2021, 12, 984. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively Expanding the Structural Coverage of Protein-Sequence Space with High-Accuracy Models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef]
- Marcotte, I.; Separovic, F.; Auger, M.; Gagné, S.M. A Multidimensional 1H NMR Investigation of the Conformation of Methionine-Enkephalin in Fast-Tumbling Bicelles. Biophys. J. 2004, 86, 1587. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Geng, J.; Shan, F.; Shan, Y.; Griffin, N.; Wu, B.; Wang, X. Methionine Enkephalin Suppresses Lung Cancer Metastasis by Regulating the Polarization of Tumor-Associated Macrophages and the Distribution of Myeloid-Derived Suppressor Cells in the Tumor Microenvironment and Inhibiting Epithelial-Mesenchymal Transition. Int. Immunopharmacol. 2023, 118, 110064. [Google Scholar] [CrossRef] [PubMed]
- Budka, J.; Kowalski, S.; Chylinska, M.; Dzierzbicka, K.; Inkielewicz-Stepniak, I. Opioid Growth Factor and Its Derivatives as Potential Non-Toxic Multifunctional Anticancer and Analgesic Compounds. Curr. Med. Chem. 2021, 28, 673–686. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, P.J.; Sassani, J.W.; Diaz, D.; Zagon, I.S. Elevated Opioid Growth Factor Alters the Limbus in Type 1 Diabetic Rats. J. Diabetes Clin. Res. 2023, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, P.J.; Sassani, J.W.; Zagon, I.S. Dysregulation of the OGF-OGFr Pathway and Associated Diabetic Complications. J. Diabetes Clin. Res. 2021, 3, 64–67. [Google Scholar] [CrossRef]
- Bodnar, R.J. Endogenous Opiates and Behavior: 2020. Peptides 2022, 151, 170752. [Google Scholar] [CrossRef]
- Quirion, B.; Beaulieu, C.; Cote, L.; Parent, J.-L.; Gendron, L. Distribution of Delta and Mu Opioid Receptor mRNA in Rodent Dorsal Root Ganglia Neurons. Eur. J. Neurosci. 2022, 56, 4031–4044. [Google Scholar] [CrossRef]
- Tollefson, S.; Stoughton, C.; Himes, M.L.; McKinney, K.E.; Mason, S.; Ciccocioppo, R.; Narendran, R. Imaging Nociceptin Opioid Peptide Receptors in Alcohol Use Disorder with [11C]NOP-1A and Positron Emission Tomography: Findings from a Second Cohort. Biol. Psychiatry 2023. [Google Scholar] [CrossRef]
- Zamfir, M.; Sharif, B.; Locke, S.; Ehrlich, A.T.; Ochandarena, N.E.; Scherrer, G.; Ribeiro-Da-Silva, A.; Kieffer, B.L.; Séguéla, P. Distinct and Sex-Specific Expression of Mu Opioid Receptors in Anterior Cingulate and Somatosensory S1 Cortical Areas. Pain 2022, 164, 703. [Google Scholar] [CrossRef]
- Kareem, Z.Y.; McLaughlin, P.J.; Kumari, R. Opioid Growth Factor Receptor: Anatomical Distribution and Receptor Colocalization in Neurons of the Adult Mouse Brain. Neuropept. Edinb. 2023, 99, 102325. [Google Scholar] [CrossRef]
- Olabarrieta, E.; Totorikaguena, L.; Matorras, R.; Agirregoitia, E.; Agirregoitia, N. Delta and Kappa Opioid Receptors in Human Endometrium during the Menstrual Cycle: Expression and Localization. Eur. J. Obstet. Amp Gynecol. Reprod. Biol. 2023, 283, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.K.; Smith, C.M.; Rahmatullah, M.; Nissapatorn, V.; Wilairatana, P.; Spetea, M.; Gueven, N.; Dietis, N. Opioid Analgesia and Opioid-Induced Adverse Effects: A Review. Pharmaceuticals 2021, 14, 1091. [Google Scholar] [CrossRef] [PubMed]
- Lešnik, S.; Bertalan, É.; Bren, U.; Bondar, A.-N. Opioid Receptors and Protonation-Coupled Binding of Opioid Drugs. Int. J. Mol. Sci. 2021, 22, 13353. [Google Scholar] [CrossRef]
- Gopalakrishnan, L.; Chatterjee, O.; Ravishankar, N.; Suresh, S.; Raju, R.; Mahadevan, A.; Prasad, T.S.K. Opioid Receptors Signaling Network. J. Cell Commun. Signal. 2022, 16, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Miess, E.; Gondin, A.B.; Yousuf, A.; Steinborn, R.; Mösslein, N.; Yang, Y.; Göldner, M.; Ruland, J.G.; Bünemann, M.; Krasel, C.; et al. Multisite Phosphorylation Is Required for Sustained Interaction with GRKs and Arrestins during Rapid μ-Opioid Receptor Desensitization. Sci. Signal. 2018, 11, eaas9609. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Vargas, N.N.; Gong, J.; Wisdom, M.J.; Jensen, D.D.; Latorre, R.; Hegron, A.; Teng, S.; DiCello, J.J.; Rajasekhar, P.; Veldhuis, N.A.; et al. Endosomal Signaling of Delta Opioid Receptors Is an Endogenous Mechanism and Therapeutic Target for Relief from Inflammatory Pain. Proc. Natl. Acad. Sci. USA 2020, 117, 15281–15292. [Google Scholar] [CrossRef]
- Shiraki, A.; Shimizu, S. The Molecular Associations in Clathrin-Coated Pit Regulate β-Arrestin-Mediated MAPK Signaling Downstream of μ-Opioid Receptor. Biochem. Biophys. Res. Commun. 2023, 640, 64–72. [Google Scholar] [CrossRef]
- Radoux-Mergault, A.; Oberhauser, L.; Aureli, S.; Gervasio, F.L.; Stoeber, M. Subcellular Location Defines GPCR Signal Transduction. Sci. Adv. 2023, 9, eadf6059. [Google Scholar] [CrossRef]
- Miyoshi, K.; Shimizu, S.; Shiraki, A.; Egi, M. Ubiquitination of the μ-Opioid Receptor Regulates Receptor Internalization without Affecting Gi/o-Mediated Intracellular Signaling or Receptor Phosphorylation. Biochem. Biophys. Res. Commun. 2023, 643, 96–104. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, Y.; Shan, F. Interaction of Opioid Growth Factor (OGF) and Opioid Antagonist and Their Significance in Cancer Therapy. Int. Immunopharmacol. 2019, 75, 105785. [Google Scholar] [CrossRef]
- Faouzi, A.; Varga, B.R.; Majumdar, S. Biased Opioid Ligands. Molecules 2020, 25, 4257. [Google Scholar] [CrossRef] [PubMed]
- Groom, S.; Blum, N.K.; Conibear, A.E.; Disney, A.A.; Hill, R.R.; Husbands, S.M.; Li, Y.; Toll, L.; Kliewer, A.; Schulz, S.; et al. A Novel G Protein-biased Agonist at the μ Opioid Receptor Induces Substantial Receptor Desensitisation through G Protein-coupled Receptor Kinase. Br. J. Pharmacol. 2020, 180, 943. [Google Scholar] [CrossRef] [PubMed]
- Meqbil, Y.; Rijn, R. van Opportunities and Challenges for In Silico Drug Discovery at Delta Opioid Receptors. Pharmaceuticals 2022, 15, 873. [Google Scholar] [CrossRef]
- Deo, O.; Alvi, S.; Pham, V.; Christopoulos, A.; Thal, D.M.; Jörg, M.; Capuano, B.; Valant, C.; Scammells, P.J. The Design, Synthesis, and Evaluation of Novel 9-Arylxanthenedione-Based Allosteric Modulators for the δ-Opioid Receptor. J. Med. Chem. 2022, 65, 12367. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Miyano, K.; Hirayama, S.; Karasawa, Y.; Ohshima, K.; Uezono, E.; Komatsu, A.; Nonaka, M.; Fujii, H.; Yamaguchi, K.; et al. Evaluation of the Intracellular Signaling Activities of κ-Opioid Receptor Agonists, Nalfurafine Analogs; Focusing on the Selectivity of G-Protein- and β-Arrestin-Mediated Pathways. Molecules 2022, 27, 7065. [Google Scholar] [CrossRef]
- Brust, T.F. Biased Ligands at the Kappa Opioid Receptor: Fine-Tuning Receptor Pharmacology. In The Kappa Opioid Receptor; Springer: Cham, Switzerland, 2020; pp. 115–135. [Google Scholar]
- Kelly, E.; Conibear, A.; Henderson, G. Biased Agonism: Lessons from Studies of Opioid Receptor Agonists. Annu. Rev. Pharmacol. Toxicol. 2023, 63, 491–515. [Google Scholar] [CrossRef]
- Puig, S.; Barker, K.E.; Szott, S.R.; Kann, P.T.; Morris, J.S.; Gutstein, H.B. Spinal Opioid Tolerance Depends upon Platelet-Derived Growth Factor Receptor-β Signaling, Not μ-Opioid Receptor Internalization. Mol. Pharmacol. 2020, 98, 487–496. [Google Scholar] [CrossRef]
- Gamble, M.C.; Williams, B.R.; Singh, N.; Posa, L.; Freyberg, Z.; Logan, R.W.; Puig, S. Mu-Opioid Receptor and Receptor Tyrosine Kinase Crosstalk: Implications in Mechanisms of Opioid Tolerance, Reduced Analgesia to Neuropathic Pain, Dependence, and Reward. Front. Syst. Neurosci. 2022, 16, 1059089. [Google Scholar] [CrossRef]
- Gaborit, M.; Massotte, D. Therapeutic Potential of Opioid Receptor Heteromers in Chronic Pain and Associated Comorbidities. Br. J. Pharmacol. 2023, 180, 994–1013. [Google Scholar] [CrossRef] [PubMed]
- Kunselman, J.M.; Gupta, A.; Gomes, I.; Devi, L.A.; Puthenveedu, M.A. Compartment-Specific Opioid Receptor Signaling Is Selectively Modulated by Different Dynorphin Peptides. eLife 2021, 10, e60270. [Google Scholar] [CrossRef]
- Lec, P.M.; Lenis, A.T.; Golla, V.; Brisbane, W.; Shuch, B.; Garraway, I.P.; Reiter, R.E.; Chamie, K. The Role of Opioids and Their Receptors in Urological Malignancy: A Review. J. Urol. 2020, 204, 1150–1159. [Google Scholar] [CrossRef]
- Carli, M.; Donnini, S.; Pellegrini, C.; Coppi, E.; Bocci, G. Opioid Receptors beyond Pain Control: The Role in Cancer Pathology and the Debated Importance of Their Pharmacological Modulation. Pharmacol. Res. 2020, 159, 104938. [Google Scholar] [CrossRef]
- Lewis, J.W.; Shavit, Y.; Terman, G.W.; Nelson, L.R.; Gale, R.P.; Liebeskind, J.C. Apparent Involvement of Opioid Peptides in Stress-Induced Enhancement of Tumor Growth. Peptides 1983, 4, 635–638. [Google Scholar] [CrossRef] [PubMed]
- Cabot, P.J.; Carter, L.; Schäfer, M.; Stein, C. Methionine-Enkephalin-and Dynorphin A-Release from Immune Cells and Control of Inflammatory Pain. Pain 2001, 93, 207–212. [Google Scholar] [CrossRef]
- Samuelsson, H.; Ekman, R.; Hedner, T. CSF Neuropeptides in Cancer Pain: Effects of Spinal Opioid Therapy. Acta Anaesthesiol. Scand. 1993, 37, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Viet, C.T.; Dang, D.; Ye, Y.; Ono, K.; Campbell, R.R.; Schmidt, B.L. Demethylating Drugs as Novel Analgesics for Cancer Pain. Clin. Cancer Res. 2014, 20, 4882–4893. [Google Scholar] [CrossRef] [PubMed]
- Hermann, A.L.; Fell, G.L.; Kemény, L.V.; Fung, C.Y.; Held, K.D.; Biggs, P.J.; Rivera, P.D.; Bilbo, S.D.; Igras, V.; Willers, H.; et al. β-Endorphin Mediates Radiation Therapy Fatigue. Sci. Adv. 2022, 8, eabn6025. [Google Scholar] [CrossRef] [PubMed]
- Chong, D.; Shao, L.; Yang, Y.; Wang, R.; Yang, C.; Zhang, B. Correlations of Cancer Pain Degree with Levels of β-EP, CGRP, and PGE2 and the Effects of Oxycontin on Them. J BUON 2018, 23, 1552–1557. [Google Scholar]
- Yamano, S.; Viet, C.T.; Dang, D.; Dai, J.; Hanatani, S.; Takayama, T.; Kasai, H.; Imamura, K.; Campbell, R.; Ye, Y.; et al. Ex Vivo Nonviral Gene Delivery of μ-Opioid Receptor to Attenuate Cancer-Induced Pain. Pain 2017, 158, 240–251. [Google Scholar] [CrossRef]
- Wu, T.-T.; Wang, Z.-G.; Ou, W.-L.; Wang, J.; Yao, G.-Q.; Yang, B.; Rao, Z.-G.; Gao, J.-F.; Zhang, B.-C. Intravenous Flurbiprofen Axetil Enhances Analgesic Effect of Opioids in Patients with Refractory Cancer Pain by Increasing Plasma β-Endorphin. Asian Pac. J. Cancer Prev. 2015, 15, 10855–10860. [Google Scholar] [CrossRef]
- Apryani, E.; Ali, U.; Wang, Z.-Y.; Wu, H.-Y.; Mao, X.-F.; Ahmad, K.A.; Li, X.-Y.; Wang, Y.-X. The Spinal Microglial IL-10/β-Endorphin Pathway Accounts for Cinobufagin-Induced Mechanical Antiallodynia in Bone Cancer Pain Following Activation of A7-Nicotinic Acetylcholine Receptors. J. Neuroinflamm. 2020, 17, 75. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Hu, W.; He, H.; Gong, Z.; Wang, J.; Yu, X.; Ai, T.; Zhan, L. A Study on the Mechanism of Cinobufagin in the Treatment of Paw Cancer Pain by Modulating Local β -Endorphin Expression In Vivo. Evid. Based Complement. Alternat. Med. 2013, 2013, 851256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xia, C.; Zhang, X.; Li, W.; Miao, X.; Zhou, Q. Wrist–Ankle Acupuncture Attenuates Cancer-Induced Bone Pain by Regulating Descending Pain-Modulating System in a Rat Model. Chin. Med. 2020, 15, 13. [Google Scholar] [CrossRef] [PubMed]
- Du, J.-Y.; Liang, Y.; Fang, J.-F.; Jiang, Y.-L.; Shao, X.-M.; He, X.-F.; Fang, J.-Q. Effect of Systemic Injection of Heterogenous and Homogenous Opioids on Peripheral Cellular Immune Response in Rats with Bone Cancer Pain: A Comparative Study. Exp. Ther. Med. 2016, 12, 2568–2576. [Google Scholar] [CrossRef]
- Gustin, T.; Bachelot, T.; Verna, J.M.; Molin, L.F.; Brunet, J.F.; Berger, F.R.; Benabid, A.L. Immunodetection of Endogenous Opioid Peptides in Human Brain Tumors and Associated Cyst Fluids. Cancer Res. 1993, 53, 4715–4719. [Google Scholar]
- Lu, W.; Xie, H.; Tie, X.; Wang, R.; Wu, A.; Shan, F. NFAT-1 Hyper-Activation by Methionine Enkephalin (MENK) Significantly Induces Cell Apoptosis of Rats C6 Glioma in Vivo and in Vitro. Int. Immunopharmacol. 2018, 56, 1–8. [Google Scholar] [CrossRef]
- Molin, L.; Verna, J.-M.; Nissou, M.-F.; Benabid, A.L. Met-Enkephalin Receptors in Human Gliomas. NeuroReport 1994, 5, 2474–2476. [Google Scholar] [CrossRef]
- Mar, E.-C.; Suh, H.H.; Hong, J.-S. Regulation of Proenkephalin Expression in C6 Rat Glioma Cells. Mol. Cell. Neurosci. 1992, 3, 518–528. [Google Scholar] [CrossRef]
- Yin, J.; Lee, J.A.; Howells, R.D. Stimulation of C-Fos and c-Jun Gene Expression and Down-Regulation of Proenkephalin Gene Expression in C6 Glioma Cells by Endothelin-1. Mol. Brain Res. 1992, 14, 213–220. [Google Scholar] [CrossRef]
- Lee, Y.S.; Wurster, R.D. Differential Effects of Methionine Enkephalin on the Growth of Brain Tumor Cells. J. Neurooncol. 1994, 19, 11–15. [Google Scholar] [CrossRef]
- Westphal, M.; Li, C.H. Beta-Endorphin: Characterization of Binding Sites Specific for the Human Hormone in Human Glioblastoma SF126 Cells. Proc. Natl. Acad. Sci. USA 1984, 81, 2921–2923. [Google Scholar] [CrossRef] [PubMed]
- Vivekanandan, S.; Rao, A.P.K.; Sampathkumar, M.M.; Kanaka, T.S. Presence of Immunoreactive Beta-Endorphin in Human Brain Tumor Cyst Fluids. J. Neurol. Sci. 1983, 59, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.J.; Wang, X.Q.; Wang, C.H.; Lu, C.L. Effect of Dynorphin A1-13 on C6 Glioma Cells Swelling Induced by Glutamate. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2001, 17, 76–78. [Google Scholar] [PubMed]
- Bortsov, A.V.; Millikan, R.C.; Belfer, I.; Boortz-Marx, R.L.; Arora, H.; McLean, S.A. μ-Opioid Receptor Gene A118G Polymorphism Predicts Survival in Patients with Breast Cancer. Anesthesiology 2012, 116, 896–902. [Google Scholar] [CrossRef]
- Chatikhine, V.A.; Chevrier, A.; Chauzy, C.; Duval, C.; d’Anjou, J.; Girard, N.; Delpech, B. Expression of Opioid Peptides in Cells and Stroma of Human Breast Cancer and Adenofibromas. Cancer Lett. 1994, 77, 51–56. [Google Scholar] [CrossRef]
- Drell, T.L.; Joseph, J.; Lang, K.; Niggemann, B.; Zaenker, K.S.; Entschladen, F. Effects of Neurotransmitters on the Chemokinesis and Chemotaxis of MDA-MB-468 Human Breast Carcinoma Cells. Breast Cancer Res. Treat. 2003, 80, 63–70. [Google Scholar] [CrossRef]
- Melander, O.; Orho-Melander, M.; Manjer, J.; Svensson, T.; Almgren, P.; Nilsson, P.M.; Engström, G.; Hedblad, B.; Borgquist, S.; Hartmann, O.; et al. Stable Peptide of the Endogenous Opioid Enkephalin Precursor and Breast Cancer Risk. J. Clin. Oncol. 2015, 33, 2632–2638. [Google Scholar] [CrossRef]
- Zagon, I.S.; Porterfield, N.K.; McLaughlin, P.J. Opioid Growth Factor—Opioid Growth Factor Receptor Axis Inhibits Proliferation of Triple Negative Breast Cancer. Exp. Biol. Med. 2013, 238, 589–599. [Google Scholar] [CrossRef]
- Ramírez-Expósito, M.J.; Dueñas-Rodríguez, B.; Carrera-González, M.P.; Navarro-Cecilia, J.; Martínez-Martos, J.M. Circulating Levels of β-Endorphin and Cortisol in Breast Cancer. Compr. Psychoneuroendocrinol. 2021, 5, 100028. [Google Scholar] [CrossRef]
- Argueta, D.A.; Aich, A.; Lei, J.; Kiven, S.; Nguyen, A.; Wang, Y.; Gu, J.; Zhao, W.; Gupta, K. β-Endorphin at the Intersection of Pain and Cancer Progression: Preclinical Evidence. Neurosci. Lett. 2021, 744, 135601. [Google Scholar] [CrossRef]
- Heiny, B.M.; Albrecht, V.; Beuth, J. Correlation of Immune Cell Activities and Beta-Endorphin Release in Breast Carcinoma Patients Treated with Galactose-Specific Lectin Standardized Mistletoe Extract. Complement. Ther. Med. 1999, 7, 121. [Google Scholar] [CrossRef]
- Heiny, B.M.; Beuth, J. Mistletoe Extract Standardized for the Galactoside-Specific Lectin (ML-1) Induces Beta-Endorphin Release and Immunopotentiation in Breast Cancer Patients. Anticancer Res. 1994, 14, 1339–1342. [Google Scholar] [PubMed]
- Vaswani, K.K.; Tejwani, G.A.; Abou-Issa, H.M. Effect of 7,12-Dimethylbenz[a]Anthracene-Induced Mammary Carcinogenesis on the Opioid Peptide Levels in the Rat Central Nervous System. Cancer Lett. 1986, 31, 115–122. [Google Scholar] [CrossRef]
- Zhang, C.; Franklin, T.; Sarkar, D.K. Inhibition of Mammary Cancer Progression in Fetal Alcohol Exposed Rats by β -Endorphin Neurons. Alcohol. Clin. Exp. Res. 2016, 40, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.K.; Zhang, C. Beta-Endorphin Neuron Regulates Stress Response and Innate Immunity to Prevent Breast Cancer Growth and Progression. In Vitamins & Hormones; Elsevier: Amsterdam, The Netherlands, 2013; Volume 93, pp. 263–276. ISBN 978-0-12-416673-8. [Google Scholar]
- Sarkar, D.K.; Murugan, S.; Zhang, C.; Boyadjieva, N. Regulation of Cancer Progression by β-Endorphin Neuron. Cancer Res. 2012, 72, 836–840. [Google Scholar] [CrossRef]
- Faith, R.E.; Liang, H.J.; Murgo, A.J.; Plotnikoff, N.P. Neuroimmunomodulation with Enkephalins: Enhancement of Human Natural Killer (NK) Cell Activity in Vitro. Clin. Immunol. Immunopathol. 1984, 31, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Faith, R.E.; Liang, H.J.; Plotnikoff, N.P.; Murgo, A.J.; Nimeh, N.F. Neuroimmunomodulation with Enkephalins: In Vitro Enhancement of Natural Killer Cell Activity in Peripheral Blood Lymphocytes from Cancer Patients. Nat. Immun. Cell Growth Regul. 1987, 6, 88–98. [Google Scholar]
- Bryant, H.U.; Conroy, W.G.; Isom, G.E.; Malven, P.V.; Yim, G.K.W. Presence of Dynorphin-like Immunoreactivity but Not Opiate Binding in Walker-256 Tumors. Life Sci. 1985, 37, 155–160. [Google Scholar] [CrossRef]
- Qu, N.; Wang, R.; Meng, Y.; Liu, N.; Zhai, J.; Shan, F. Methionine Enkephalin Inhibited Cervical Carcinoma via Apoptosis Promotion and Reduction of Myeloid Derived Suppressor Cell Infiltrated in Tumor. Int. Immunopharmacol. 2022, 110, 108933. [Google Scholar] [CrossRef]
- Saraswati, W.; Wardani, R.; Suhatno, S.; Hartono, P.; Imandiri, A. The Effect of Electroacupuncture Therapy on Pain, Plasma β-Endorphin, and Quality of Life of Stage III Cervical Cancer Patients: A Randomized Control Trial. J. Acupunct. Meridian Stud. 2021, 14, 4–12. [Google Scholar] [CrossRef]
- Ma, M.; Wang, X.; Liu, N.; Shan, F.; Feng, Y. Low-Dose Naltrexone Inhibits Colorectal Cancer Progression and Promotes Apoptosis by Increasing M1-Type Macrophages and Activating the Bax/Bcl-2/Caspase-3/PARP Pathway. Int. Immunopharmacol. 2020, 83, 106388. [Google Scholar] [CrossRef] [PubMed]
- Alumets, J.; Sundler, F.; Alm, P.; Falkmer, S.; Ljungberg, O.; Håkanson, R.; Mårtensson, H.; Tibblin, S. Immunohistochemical Evidence of Peptide Hormones in Endocrine Tumors of the Rectum. Cancer 1981, 48, 2409–2415. [Google Scholar] [CrossRef] [PubMed]
- Iishi, H.; Tatsuta, M.; Baba, M.; Okuda, S.; Taniguchi, H. Enhancement by Methionine Enkephalin of Colon Carcinogenesis Induced by Azoxymethane. Cancer Res. 1991, 51, 785–788. [Google Scholar] [PubMed]
- Wang, X.; Li, S.; Yan, S.; Shan, Y.; Wang, X.; Jingbo, Z.; Wang, Y.; Shan, F.; Griffin, N.; Sun, X. Methionine Enkephalin Inhibits Colorectal Cancer by Remodeling the Immune Status of the Tumor Microenvironment. Int. Immunopharmacol. 2022, 111, 109125. [Google Scholar] [CrossRef] [PubMed]
- Kuniyasu, H.; Luo, Y.; Fujii, K.; Sasahira, T.; Moriwaka, Y.; Tatsumoto, N.; Sasaki, T.; Yamashita, Y.; Ohmori, H. CD10 Enhances Metastasis of Colorectal Cancer by Abrogating the Anti-Tumoural Effect of Methionine-Enkephalin in the Liver. Gut 2010, 59, 348–356. [Google Scholar] [CrossRef]
- Ogasawara, M.; Murata, J.; Ayukawa, K.; Saiki, I. Differential Effect of Intestinal Neuropeptides on Invasion and Migration of Colon Carcinoma Cells in Vitro. Cancer Lett. 1997, 119, 125–130. [Google Scholar] [CrossRef]
- Tari, A.; Miyachi, Y.; Sumii, K.; Haruma, K.; Yoshihara, M.; Kajiyama, G.; Miyoshi, A. Beta-Endorphin-like Immunoreactivity in Normal Mucosa, Muscle Layer, Adenocarcinoma, and Polyp of the Colon. Dig. Dis. Sci. 1988, 33, 429–434. [Google Scholar] [CrossRef]
- Hiramoto, K.; Yokoyama, S.; Yamate, Y. Ultraviolet A Eye Irradiation Ameliorates Colon Carcinoma Induced by Azoxymethane and Dextran Sodium Sulfate through β-Endorphin and Methionine-Enkephalin. Photodermatol. Photoimmunol. Photomed. 2017, 33, 84–91. [Google Scholar] [CrossRef]
- Murugan, S.; Dave, Y.; Rakhit, A.; Sarkar, D.K. Hypothalamic Beta-Endorphin Neurons Suppress Preneoplastic and Neoplastic Lesions Development in 1,2-Dimethylhydrazine Induced Rat Colon Cancer Model. J. Cancer 2017, 8, 3105–3113. [Google Scholar] [CrossRef]
- Simon, R.H.; Arbo, T.E.; Lundy, J. β-Endorphin Injected into the Nucleus of the Raphe Magnus Facilitates Metastatic Tumor Growth. Brain Res. Bull. 1984, 12, 487–491. [Google Scholar] [CrossRef]
- Karl, M.; Saviolakis, G.A.; Gravanis, A.; Chrousos, G.P.; Margioris, A.N. The PC12 Rat Pheochromocytoma Cell Line Expresses the Prodynorphin Gene and Secretes the 8 KDa Dynorphin Product. Regul. Pept. 1996, 61, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Zagon, I.S.; McLaughlin, P.J. Opioids and the Apoptotic Pathway in Human Cancer Cells. Neuropeptides 2003, 37, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Shan, F.; Qu, N.; Huang, H.; Handley, M.; Griffin, N.; Zhang, S.; Cao, X. Regulatory Role of Methionine Enkephalin in Myeloid-Derived Suppressor Cells and Macrophages in Human Cutaneous Squamous Cell Carcinoma. Int. Immunopharmacol. 2021, 99, 107996. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Cao, X.; Qu, N.; Huang, H.; Handley, M.; Zhang, S.; Shan, F. Methionine Enkephalin Activates Autophagy and Stimulates Tumour Cell Immunogenicity in Human Cutaneous Squamous Cell Carcinoma. Int. Immunopharmacol. 2021, 96, 107733. [Google Scholar] [CrossRef]
- Wang, X.; Tian, J.; Jiao, X.; Geng, J.; Wang, R.; Liu, N.; Gao, X.; Griffin, N.; Gao, Y.; Shan, F. The Novel Mechanism of Anticancer Effect on Gastric Cancer through Inducing G0/G1 Cell Cycle Arrest and Caspase-Dependent Apoptosis in Vitro and in Vivo by Methionine Enkephalin. Cancer Manag. Res. 2018, 10, 4773–4787. [Google Scholar] [CrossRef]
- Wang, X.; Jiao, X.; Meng, Y.; Chen, H.; Griffin, N.; Gao, X.; Shan, F. Methionine Enkephalin (MENK) Inhibits Human Gastric Cancer through Regulating Tumor Associated Macrophages (TAMs) and PI3K/AKT/mTOR Signaling Pathway inside Cancer Cells. Int. Immunopharmacol. 2018, 65, 312–322. [Google Scholar] [CrossRef]
- Zhou, X.; Cao, S.-G.; Tan, X.-J.; Liu, X.-D.; Li, Z.-Q.; Kong, L.-X.; Tian, Y.-L.; Liu, D.; Shen, S.; Sun, Y.-Q.; et al. Effects of Transcutaneous Electrical Acupoint Stimulation (TEAS) on Postoperative Recovery in Patients with Gastric Cancer: A Randomized Controlled Trial. Cancer Manag. Res. 2021, 13, 1449–1458. [Google Scholar] [CrossRef]
- Tari, A.; Miyachi, Y.; Hide, M.; Sumii, K.; Kajiyama, G.; Tahara, E.; Tanaka, K.; Miyoshi, A. β-Endorphinlike Immunoreactivity and Somatostatinlike Immunoreactivity in Normal Gastric Mucosa, Muscle Layer, and Adenocarcinoma. Gastroenterology 1985, 88, 670–674. [Google Scholar] [CrossRef]
- Gorur, A.; Patiño, M.; Takahashi, H.; Corrales, G.; Pickering, C.R.; Gleber-Netto, F.O.; Myers, J.N.; Cata, J.P. Mu-Opioid Receptor Activation Promotes in Vitro and in Vivo Tumor Growth in Head and Neck Squamous Cell Carcinoma. Life Sci. 2021, 278, 119541. [Google Scholar] [CrossRef]
- Levin, R.J.; Wu, Y.; McLaughlin, P.J.; Zagon, I.S. Expression of the Opioid Growth Factor, [Met5]-Enkephalin, and the Zeta Opioid Receptor in Head and Neck Squamous Cell Carcinoma. Laryngoscope 1997, 107, 335–339. [Google Scholar] [CrossRef]
- McLaughlin, P.J.; Stucki, J.K.; Zagon, I.S. Modulation of the Opioid Growth Factor ([Met5]-Enkephalin)-Opioid Growth Factor Receptor Axis: Novel Therapies for Squamous Cell Carcinoma of the Head and Neck. Head Neck 2012, 34, 513–519. [Google Scholar] [CrossRef]
- Cheng, F.; Zagon, I.S.; Verderame, M.F.; McLaughlin, P.J. The Opioid Growth Factor (OGF)–OGF Receptor Axis Uses the P16 Pathway to Inhibit Head and Neck Cancer. Cancer Res. 2007, 67, 10511–10518. [Google Scholar] [CrossRef]
- McLaughlin, P.; Zagon, I. Progression of Squamous Cell Carcinoma of the Head and Neck Is Associated with Down-Regulation of the Opioid Growth Factor Receptor. Int. J. Oncol. 2006, 28, 1577–1583. [Google Scholar] [CrossRef]
- Warren, W.H.; Lee, I.; Gould, V.E.; Memoli, V.A.; Jao, W. Paragangliomas of the Head and Neck: Ultrastructural and Immunohistochemical Analysis. Ultrastruct. Pathol. 1985, 8, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Wolf, G.T.; Peterson, K.A. Beta Endorphin Enhances in Vitro Lymphokine Production in Patients with Squamous Carcinoma of the Head and Neck. Otolaryngol. Neck Surg. 1986, 94, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Zagon, I.S.; Rahn, K.A.; McLaughlin, P.J. Opioids and Migration, Chemotaxis, Invasion, and Adhesion of Human Cancer Cells. Neuropeptides 2007, 41, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Zagon, I.S.; McLaughlin, P.J. Opioids and Differentiation in Human Cancer Cells. Neuropeptides 2005, 39, 495–505. [Google Scholar] [CrossRef]
- Salim, S.A.; Milroy, C.; Rode, J.; Corrin, B.; Hamid, Q. Immunocytochemical Characterization of Neuroendocrine Tumours of the Larynx. Histopathology 1993, 23, 69–73. [Google Scholar] [CrossRef]
- Bishop, J.W.; Osamura, R.Y.; Tsutsumi, Y. Multiple Hormone Production in an Oat Cell Carcinoma of the Larynx. Pathol. Int. 1985, 35, 915–923. [Google Scholar] [CrossRef]
- Monstein, H.-J.; Folkesson, R.; Terenius, L. Proenkephalin A-like MRNA in Human Leukemia Leukocytes and CNS-Tissues. Life Sci. 1986, 39, 2237–2241. [Google Scholar] [CrossRef]
- Martin-Kleiner, I.; Gabrilovac, J.; Kušec, R.; Boranić, M. Methionine Enkephalin Suppresses Metabolic Activity of a Leukemic Cell Line (NALM-1) and Enhances CD10 Expression. Int. Immunopharmacol. 2003, 3, 707–711. [Google Scholar] [CrossRef] [PubMed]
- Mernenko, O.A.; Blishchenko, E.Y.; Mirkina, I.I.; Karelin, A.A. Met-Enkephalin Induces Cytolytic Processes of Apoptotic Type in K562 Human Erythroid Leukemia Cells. FEBS Lett. 1996, 383, 230–232. [Google Scholar] [CrossRef]
- Heagy, W.; Duca, K.; Finberg, R.W. Enkephalins Stimulate Leukemia Cell Migration and Surface Expression of CD9. J. Clin. Investig. 1995, 96, 1366–1374. [Google Scholar] [CrossRef]
- Iannetti, P.; Fabbri, A.; Meloni, G.; Moleti, M.L.; Ulisse, S.; Mandelli, F.; Isidori, A.; Imperato, C. Immunoreactive Beta-Endorphin Levels in Cerebrospinal Fluid of Children with Acute Lymphoblastic Leukemia: Relationship with Glucocorticoid Therapy and Neurological Complications. J. Endocrinol. Investig. 1989, 12, 623–629. [Google Scholar] [CrossRef]
- Barni, S.; Lissoni, P.; Tancini, G.; Crispino, S.; Paolorossi, F.; Rovelli, F.; Fumagalli, G.; Ferri, L.; Esposti, D.; Esposti, G.; et al. Acute Effects of Various Chemotherapeutic Combinations on Hypophyseal and Pineal Hormone Secretions in Cancer Patients. Tumori J. 1987, 73, 181–185. [Google Scholar] [CrossRef]
- Makeshova, A.B.; Mamukova, L.I.; Levina, A.A.; Sysoeva, E.P.; Eralieva, M.O.; Savchenko, V.G. Dynamic Beta-Endorphin Determination in Hematologic Patients. Ter Arkh 2012, 84, 22–25. [Google Scholar]
- Malkova, N.V.; Krasnova, S.B.; Navolotskaya, E.V.; Zargarova, T.A.; Prasolov, V.S. Effect of Beta-Endorphin and Beta-Endorphin-like Peptide Immunorphin on the Growth of Human Leukemic Cells in Vitro. Russ J Immunol 2002, 7, 239–244. [Google Scholar]
- Shahabi, N.A.; Sharp, B.M. Activation of Protein Kinase C Rapidly Down- Regulates Naloxone-Resistant Receptors for Beta-Endorphin on U937 Cells. J Pharmacol Exp Ther 1993, 64, 276–281. [Google Scholar]
- Avella, D.M.; Kimchi, E.T.; Donahue, R.N.; Tagaram, H.R.S.; McLaughlin, P.J.; Zagon, I.S.; Staveley-O’Carroll, K.F. The Opioid Growth Factor-Opioid Growth Factor Receptor Axis Regulates Cell Proliferation of Human Hepatocellular Cancer. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2010, 298, R459–R466. [Google Scholar] [CrossRef]
- Rogosnitzky, M.; Finegold, M.J.; McLaughlin, P.J.; Zagon, I.S. Opioid Growth Factor (OGF) for Hepatoblastoma: A Novel Non-Toxic Treatment. Investig. New Drugs 2013, 31, 1066–1070. [Google Scholar] [CrossRef] [PubMed]
- Murugan, S.; Boyadjieva, N.; Sarkar, D.K. Protective Effects of Hypothalamic Beta-Endorphin Neurons Against Alcohol-Induced Liver Injuries and Liver Cancers in Rat Animal Models. Alcohol. Clin. Exp. Res. 2014, 38, 2988–2997. [Google Scholar] [CrossRef] [PubMed]
- Krajnik, M.; Schäfer, M.; Sobanski, P.; Kowalewski, J.; Bloch-Boguslawska, E.; Zylicz, Z.; Mousa, S.A. Enkephalin, Its Precursor, Processing Enzymes, and Receptor as Part of a Local Opioid Network throughout the Respiratory System of Lung Cancer Patients. Hum. Pathol. 2010, 41, 632–642. [Google Scholar] [CrossRef]
- Gosney, J.R.; Gosney, M.A.; Lye, M. Serum Leucine-Enkephalin in Bronchial Carcinoma and Its Relation to Tumour Location. Thorax 1990, 45, 9–11. [Google Scholar] [CrossRef]
- Kim, J.Y.; Ahn, H.J.; Kim, J.K.; Kim, J.; Lee, S.H.; Chae, H.B. Morphine Suppresses Lung Cancer Cell Proliferation Through the Interaction with Opioid Growth Factor Receptor: An In Vitro and Human Lung Tissue Study. Anesth. Analg. 2016, 123, 1429–1436. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Huang, H.; Handley, M.; Griffin, N.; Bai, X.; Shan, F. A Novel Mechanism of Lung Cancer Inhibition by Methionine Enkephalin through Remodeling the Immune Status of the Tumor Microenvironment. Int. Immunopharmacol. 2021, 99, 107999. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, N.; Ma, M.; Huang, H.; Handley, M.; Bai, X.; Shan, F. Methionine Enkephalin (MENK) Suppresses Lung Cancer by Regulating the Bcl-2/Bax/Caspase-3 Signaling Pathway and Enhancing Natural Killer Cell-Driven Tumor Immunity. Int. Immunopharmacol. 2021, 98, 107837. [Google Scholar] [CrossRef]
- Mathew, B.; Lennon, F.E.; Siegler, J.; Mirzapoiazova, T.; Mambetsariev, N.; Sammani, S.; Gerhold, L.M.; LaRiviere, P.J.; Chen, C.-T.; Garcia, J.G.N.; et al. The Novel Role of the Mu Opioid Receptor in Lung Cancer Progression: A Laboratory Investigation. Anesth. Analg. 2011, 112, 558–567. [Google Scholar] [CrossRef]
- Maneckjee, R.; Minna, J.D. Opioid and Nicotine Receptors Affect Growth Regulation of Human Lung Cancer Cell Lines. Proc. Natl. Acad. Sci. USA 1990, 87, 3294–3298. [Google Scholar] [CrossRef]
- Faith, R. Inhibition of Pulmonary Metastases and Enhancement of Natural Killer Cell Activity by Methionine-Enkephalin. Brain. Behav. Immun. 1988, 2, 114–122. [Google Scholar] [CrossRef]
- Calogero, A.E.; Polosa, R.; Neville, E.; D’Agata, R. Measurements of Hormonal Peptides in the Bronchoalveolar Fluid as Tumor Markers of Lung Cancer. J. Endocrinol. Investig. 1995, 18, 354–358. [Google Scholar] [CrossRef]
- Calogero, A.E.; Minacapilli, G.; Nicolosi, A.M.G.; Moncada, M.L.; Mistretta, A.; Latteri, S.F.; Polosa, P.; D’Agata, R. Limited Clinical Usefulness of Plasma Corticotropin-Releasing Hormone, Adrenocorticotropin and ß-Endorphin Measurements as Markers of Lung Cancer. J. Endocrinol. Investig. 1992, 15, 581–586. [Google Scholar] [CrossRef]
- Black, M.; Carey, F.A.; Farquharson, M.A.; Murray, G.D.; McNicol, A.M. Expression of the Pro-Opiomelanocortin Gene in Lung Neuroendocrine Tumours: In Situ Hybridization and Immunohistochemical Studies. J. Pathol. 1993, 169, 329–334. [Google Scholar] [CrossRef]
- Taylor, J.E. Human Small Cell Lung Cancer Cells Express High-Affinity Naloxone-Insensitive [125I]β-Endorphin Binding Sites. Life Sci. 1994, 56, PL97–PL102. [Google Scholar] [CrossRef]
- Melzig, M.F.; Nylander, I.; Vlaskovska, M.; Terenius, L. β-Endorphin Stimulates Proliferation of Small Cell Lung Carcinoma Cells in Vitro via Nonopioid Binding Sites. Exp. Cell Res. 1995, 219, 471–476. [Google Scholar] [CrossRef]
- Ruff, M.; Schiffmann, E.; Terranova, V.; Pert, C.B. Neuropeptides Are Chemoattractants for Human Tumor Cells and Monocytes: A Possible Mechanism for Metastasis. Clin. Immunol. Immunopathol. 1985, 37, 387–396. [Google Scholar] [CrossRef]
- Geijer, T.; Bergh, J.; Terenius, L. Expression of Preprodynorphin in Human Small Cell Lung Carcinoma Cell Lines. Regul. Pept. 1991, 34, 181–188. [Google Scholar] [CrossRef]
- Banerjee, J.; Papu John, A.M.S.; Schuller, H.M. Regulation of Nonsmall-Cell Lung Cancer Stem Cell like Cells by Neurotransmitters and Opioid Peptides: Neuronal Regulation of Lung Cancer Stem Cells. Int. J. Cancer 2015, 137, 2815–2824. [Google Scholar] [CrossRef] [PubMed]
- Mousa, S.A.; Krajnik, M.; Sobanski, P.; Kowaleswski, J.; Bloch-Boguslawska, E.; Zylicz, M. Dynorphin Expression, Processing and Receptors in the Alveolar Macrophages, Cancer Cells and Bronchial Epithelium of Lung Cancer Patients. Histol. Histopathol. 2010, 25, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.-E.; Liu, L.-F.; Dusting, G.J.; Weng, W.-T.; Chen, S.-C.; Kung, M.-L.; Tee, R.; Liu, G.-S.; Tai, M.-H. Pro-Opiomelanocortin Gene Delivery Suppresses the Growth of Established Lewis Lung Carcinoma through a Melanocortin-1 Receptor-Independent Pathway: POMC Gene Therapy for Lung Cancer. J. Gene Med. 2012, 14, 44–53. [Google Scholar] [CrossRef]
- Slominski, A.T.; Zmijewski, M.A.; Zbytek, B.; Brozyna, A.A.; Granese, J.; Pisarchik, A.; Szczesniewski, A.; Tobin, D.J. Regulated Proenkephalin Expression in Human Skin and Cultured Skin Cells. J. Investig. Dermatol. 2011, 131, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Wick, M.R.; Millns, J.L.; Sibley, R.K.; Pittelkow, M.R.; Winkelmann, R.K. Secondary Neuroendocrine Carcinomas of the Skin. J. Am. Acad. Dermatol. 1985, 13, 134–142. [Google Scholar] [CrossRef]
- Murgo, A.J. Inhibition of B16-BL6 Melanoma Growth in Mice by Methionine-Enkephalin2. JNCI J. Natl. Cancer Inst. 1985, 75, 341–344. [Google Scholar] [CrossRef]
- Wang, D.-M.; Jiao, X.; Plotnikoff, N.P.; Griffin, N.; Qi, R.-Q.; Gao, X.-H.; Shan, F.-P. Killing Effect of Methionine Enkephalin on Melanoma in Vivo and in Vitro. Oncol. Rep. 2017, 38, 2132–2140. [Google Scholar] [CrossRef]
- Wang, D.-M.; Wang, G.-C.; Yang, J.; Plotnikoff, N.P.; Griffin, N.; Han, Y.-M.; Qi, R.-Q.; Gao, X.-H.; Shan, F.-P. Inhibition of the Growth of Human Melanoma Cells by Methionine Enkephalin. Mol. Med. Rep. 2016, 14, 5521–5527. [Google Scholar] [CrossRef]
- Zagon, I.S.; Donahue, R.N.; Rogosnitzky, M.; Mclaughlin, P.J. Imiquimod Upregulates the Opioid Growth Factor Receptor to Inhibit Cell Proliferation Independent of Immune Function. Exp. Biol. Med. 2008, 233, 968–979. [Google Scholar] [CrossRef]
- O’Hern, K.; Chambers, M.; Ryan, C.; Chapman, M.S. In Lieu of Penectomy: Complete Resolution of Penile Melanoma in Situ with Topical Imiquimod and Tretinoin. Int. J. Dermatol. 2021, 60, e297–e299. [Google Scholar] [CrossRef]
- Nahm, W.J.; Gwillim, E.C.; Badiavas, E.V.; Nichols, A.J.; Kirsner, R.S.; Boggeln, L.H.; Shen, J.T. Treating Melanoma in Situ During a Pandemic with Telemedicine and a Combination of Imiquimod, 5-Fluorouracil, and Tretinoin. Dermatol. Ther. 2021, 11, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Iznardo, H.; Garcia-Melendo, C.; Yélamos, O. Lentigo Maligna: Clinical Presentation and Appropriate Management. Clin. Cosmet. Investig. Dermatol. 2020, 13, 837–855. [Google Scholar] [CrossRef] [PubMed]
- Lobo, Y.; Templeman, R. Conservative Treatment of Lentigo Maligna with Topical Imiquimod 5% Cream: A Case Report. Dermatol. Online J. 2020, 26. [Google Scholar] [CrossRef]
- Scarfì, F.; Patrizi, A.; Veronesi, G.; Lambertini, M.; Tartari, F.; Mussi, M.; Melotti, B.; Dika, E. The Role of Topical Imiquimod in Melanoma Cutaneous Metastases: A Critical Review of the Literature. Dermatol. Ther. 2020, 33, e14165. [Google Scholar] [CrossRef]
- Neil, S.M.; Johnson, S.K.; Bleehen, S.S.; Brown, B.L.; Tomlinson, S. Stimulation of the Adenylate Cyclase of a B16 Melanoma Cell Line by Pro-Opiocortin-Related Peptides—A Structure-Activity Study. Regul. Pept. 1981, 2, 193–200. [Google Scholar] [CrossRef]
- Nagahama, M.; Funasaka, Y.; Fernandez-Frez, M.L.; Ohashi, A.; Chakraborty, A.K.; Ueda, M.; Ichihashi, M. Immunoreactivity of Alpha-Melanocyte-Stimulating Hormone, Adrenocorticotrophic Hormone and Beta-Endorphin in Cutaneous Malignant Melanoma and Benign Melanocytic Naevi. Br. J. Dermatol. 1998, 138, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Boehncke, S.; Hardt, K.; Schadendorf, D.; Henschler, R. Endogenous μ-Opioid Peptides Modulate Immune Response towards Malignant Melanoma. Exp. Dermatol. 2011, 20, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Fell, G.L.; Robinson, K.C.; Mao, J.; Woolf, C.J.; Fisher, D.E. Skin β-Endorphin Mediates Addiction to UV Light. Cell 2014, 157, 1527–1534. [Google Scholar] [CrossRef]
- Kerros, C.; Brood, I.; Sola, B.; Jauzac, P.; Allouche, S. Reduction of Cell Proliferation and Potentiation of Fas-Induced Apoptosis by the Selective Kappa-Opioid Receptor Agonist U50 488 in the Multiple Myeloma LP-1 Cells. J. Neuroimmunol. 2010, 220, 69–78. [Google Scholar] [CrossRef]
- Bamberger, A.-M.; Pu, L.-P.; Cool, D.R.; Loh, Y.P. The Neuro-2a Neuroblastoma Cell Line Expresses [Met]-Enkephalin and Vasopressin mRNA and Peptide. Mol. Cell. Endocrinol. 1995, 113, 155–163. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, P.J.; Zagon, I.S.; Skitzki, J. Human Neuroblastoma Cell Growth in Tissue Culture Is Regulated by Opioid Growth Factor. Int. J. Oncol. 1999, 14, 373–453. [Google Scholar] [CrossRef] [PubMed]
- Westphal, M.; Li, C.H. β-Endorphin: Demonstration of Binding Sites in Three Human Neuroblastoma Cell Lines Specific for the COOH-Terminal Segment of the Human Hormone. Biochem. Biophys. Res. Commun. 1984, 120, 873–878. [Google Scholar] [CrossRef]
- Zagon, I.S.; McLaughlin, P.J. Opioid Antagonist Modulation of Murine Neuroblastoma: A Profile of Cell Proliferation and Opioid Peptides and Receptors. Brain Res. 1989, 480, 16–28. [Google Scholar] [CrossRef]
- Zagon, I.S.; Goodman, S.R.; McLaughlin, P.J. Characterization of Opioid Binding Sites in Murine Neuroblastoma. Brain Res. 1988, 449, 80–88. [Google Scholar] [CrossRef]
- Satoh, M.; Yokosawa, H.; Ishii, S. Degradation of Dynorphin-(1–13) and Dynorphin-(1–17) by the Neuroblastoma Cell Membrane. Evidence for the Involvement of a Cysteine Protease. Biochem. Biophys. Res. Commun. 1986, 140, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.-J.; Chen, W.-F.; Sung, C.-S.; Jean, Y.-H.; Hung, C.-H.; Chen, F.-A.; Hsieh, M.-H.; Wen, Z.-H. Isoflurane Attenuates Dynorphin-Induced Cytotoxicity and Downregulation of Bcl-2 Expression in Differentiated Neuroblastoma SH-SY5Y Cells: Isoflurane Attenuates Dynorphin Toxicity. Acta Anaesthesiol. Scand. 2009, 53, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Miura, M.; Inui, A.; Sano, K.; Ueno, N.; Teranishi, A.; Hirosue, Y.; Nakajima, M.; Okita, M.; Togami, J.; Koshiya, K.; et al. Dynorphin Binds to Neuropeptide Y and Peptide YY Receptors in Human Neuroblastoma Cell Lines. Am. J. Physiol.-Endocrinol. Metab. 1994, 267, E702–E709. [Google Scholar] [CrossRef]
- Sporrong, B.; Falkmer, S.; Robboy, S.J.; Alumets, J.; Hakanson, R.; Ljungberg, O.; Sundler, F. Neurohormonal Peptides in Ovarian Carcinoids: An Immunohistochemical Study of 81 Primary Carcinoids and of Intraovarian Metastases from Six Mid-gut Carcinoids. Cancer 1982, 49, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Zagon, I.S.; Donahue, R.; McLaughlin, P.J. Targeting the Opioid Growth Factor: Opioid Growth Factor Receptor Axis for Treatment of Human Ovarian Cancer. Exp. Biol. Med. 2013, 238, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Donahue, R.N.; McLaughlin, P.J.; Zagon, I.S. Low-Dose Naltrexone Suppresses Ovarian Cancer and Exhibits Enhanced Inhibition in Combination with Cisplatin. Exp. Biol. Med. 2011, 236, 883–895. [Google Scholar] [CrossRef]
- Omar, R.A.; Tabbakh, G.H. Immunoreactive Beta-Endorphin in Ovarian Sex Cord-Stromal Tumors. Arch. Pathol. Lab. Med. 1987, 111, 436–439. [Google Scholar]
- Schneider-Matyka, D.; Skwirczyńska, E.; Gaur, M.; Hukowska-Szematowicz, B.; Kwiatkowski, S.; Mikla, M.; Grochans, E.; Cymbaluk-Płoska, A. Evaluation of the Influence of Biological Factors during the Course of Treatment in Patients with Ovarian Cancer. Int. J. Environ. Res. Public Health 2022, 19, 10516. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Kita, T.; Miyauchi, M.; Iwano, I.; Kato, K. Inhibition of Human Ovarian Cancer Cell Proliferation in Vitro by Neuroendocrine Hormones. Gynecol. Oncol. 1989, 32, 60–64. [Google Scholar] [CrossRef]
- Gopalakrishnan, G.; Lepetre, S.; Maksimenko, A.; Mura, S.; Desmaële, D.; Couvreur, P. Lipid-Conjugation of Endogenous Neuropeptides: Improved Biotherapy against Human Pancreatic Cancer. Adv. Healthc. Mater. 2015, 4, 1015–1022. [Google Scholar] [CrossRef]
- Cheng, F.; McLaughlin, P.J.; Verderame, M.F.; Zagon, I.S. The OGF-OGFr Axis Utilizes the P21 Pathway to Restrict Progression of Human Pancreatic Cancer. Mol. Cancer 2008, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Zagon, I.S. Opioid Growth Factor Improves Clinical Benefit and Survival in Patients with Advanced Pancreatic Cancer. Open Access J. Clin. Trials 2010, 2, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.P.; Conter, R.L.; Demers, L.M.; McLaughlin, P.J.; Zagon, I.S. Elevated Levels of Opioid Growth Factor in the Plasma of Patients with Pancreatic Cancer. Pancreas 2000, 21, 158–164. [Google Scholar] [CrossRef]
- Vieau, D.; Seidah, N.G.; Day, R. Mouse Insulinoma Beta TC3 Cells Express Prodynorphin Messenger Ribonucleic Acid and Derived Peptides: A Unique Cellular Model for the Study of Prodynorphin Biosynthesis and Processing. Endocrinology 1995, 136, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Konoshita, T.; Gasc, J.M.; Villard, E.; Seidah, N.G.; Corvol, P.; Pinet, F. Co-Expression of PC2 and Proenkephalin in Human Tumoral Adrenal Medullary Tissues. Biochimie 1994, 76, 241–244. [Google Scholar] [CrossRef]
- Yanase, T.; Nawata, H.; Kato, K.; Ibayashi, H. Catecholamines and Opioid Peptides in Human Phaeochromocytomas. Acta Endocrinol. 1986, 113, 378–384. [Google Scholar] [CrossRef]
- Cesselin, F.; Pique, L.; Bertagna, X.; Benlot, C.; Antréassian, J.; Proeschel, M.F.; Girard, F.; Zogbi, F.; Legrand, J.C.; Luton, J.P.; et al. Simultaneous Evaluation of the Catecholamine Pathway and Three Opioid Peptide Producing Systems in Human Pheochromocytomas. Neuropeptides 1984, 4, 175–182. [Google Scholar] [CrossRef]
- Yoshimasa, T.; Nakao, K.; Ohtsuki, H.; Li, S.; Imura, H. Methionine-Enkephalin and Leucine-Enkephalin in Human Sympathoadrenal System and Pheochromocytoma. J. Clin. Investig. 1982, 69, 643–650. [Google Scholar] [CrossRef]
- Schroeder, J.O.; Asa, S.L.; Kovacs, K.; Killinger, D.; Hadley, G.L.; Volpé, R. Report of a Case of Pheochromocytoma Producing Immunoreactive ACTH and Beta-Endorphin. J. Endocrinol. Investig. 1984, 7, 117–122. [Google Scholar] [CrossRef]
- Bertagna, X.; Pique, L.; Ochoa, C.; Luton, J.P.; Bricaire, H.; Serin, D.; Girard, F.; Plouin, P.F.; Corvol, P.; Cesselin, F.; et al. Simultaneous Measurement of β-Endorphin, Lipotrophins, and Met-Enkephalin in Phaeochromocytomas. Acta Endocrinol. 1982, 101, 72–77. [Google Scholar] [CrossRef]
- Howlett, T.A.; Besser, G.M.; Rees, L.H. Characterization of Immunoreactive Dynorphin in Human Phaeochromocytomas. J. Endocrinol. 1988, 117, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Yanase, T.; Nawata, H.; Kato, K.; Ibayashi, H. Preproenkephalin B-Derived Opioid Peptides in Human Phaeochromocytomas. Acta Endocrinol. 1987, 114, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Suda, T.; Tozawa, F.; Tachibana, S.; Demura, H.; Shizume, K.; Sasaki, A.; Mouri, T.; Miura, Y. Multiple Forms of Immunoreactive Dynorphin in Human Pituitary and Pheochromocytoma. Life Sci. 1983, 32, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Yoshimasa, T.; Nakao, K.; Oki, S.; Tanaka, I.; Nakai, Y.; Imura, H. Presence of Dynorphin-Like Immunoreactivity in Pheochromocytomas. J. Clin. Endocrinol. Metab. 1981, 53, 213–214. [Google Scholar] [CrossRef]
- Zhu, X.; Robertson, J.T.; Sacks, H.S.; Dohan, F.C.; Tseng, J.-L.; Desiderio, D.M. Opioid and Tachykinin Neuropeptides in Prolactin-Secreting Human Pituitary Adenomas. Peptides 1995, 16, 1097–1107. [Google Scholar] [CrossRef]
- Trouillas, J.; Girod, C.; Sassolas, G.; Vitte, P.A.; Claustrat, B.; Perrin, G.; Lhéritier, M.; Fischer, C.; Dubois, M.P. A Human β-Endorphin Pituitary Adenoma. J. Clin. Endocrinol. Metab. 1984, 58, 242–249. [Google Scholar] [CrossRef]
- Sharp, B.; Melmed, S.; Goldberg, R.; Carlson, H.E.; Refetoff, S.; Hershman, J.M. Radioimmunoassay Detection of Endorphins from Long-Term Culture of Human Pituitary Tumour Cells. Acta Endocrinol. 1982, 99, 174–178. [Google Scholar] [CrossRef]
- Scheithauer, B.W.; Jaap, A.J.; Horvath, E.; Kovacs, K.; Lloyd, R.V.; Meyer, F.B.; Laws, E.R.; Young, W.F. Clinically Silent Corticotroph Tumors of the Pituitary Gland. Neurosurgery 2000, 47, 723–730. [Google Scholar] [CrossRef]
- Low, M.J.; Liu, B.; Hammer, G.D.; Rubinstein, M.; Allen, R.G. Post-Translational Processing of Proopiomelanocortin (POMC) in Mouse Pituitary Melanotroph Tumors Induced by a POMC-Simian Virus 40 Large T Antigen Transgene. J. Biol. Chem. 1993, 268, 24967–24975. [Google Scholar] [CrossRef]
- Beaubien, B.C.; Herbert, E. Modulation of Beta-Endorphin Secretion from Mouse Pituitary Tumor Cells by Calmodulin Inhibitor W7. NIDA Res. Monogr. 1986, 414–417. [Google Scholar]
- Furui, T.; Kageyama, N.; Kuwayama, A.; Nakao, K.; Fukushima, M. Increase of β-Endorphin in Cerebrospinal Fluid after Removal of ACTH-Secreting Pituitary Adenomas. Pain 1981, 11, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, H.; Shuman, L.; Warrick, J.I.; Raman, J.D.; Degraff, D.J. Androgen Represses Opioid Growth Factor Receptor (OGFR) in Human Prostate Cancer LNCaP Cells and OGFR Expression in Human Prostate Cancer Tissue. Am. J. Clin. Exp. Urol. 2018, 6, 164–171. [Google Scholar] [PubMed]
- Zylla, D.; Gourley, B.L.; Vang, D.; Jackson, S.; Boatman, S.; Lindgren, B.; Kuskowski, M.A.; Le, C.; Gupta, K.; Gupta, P. Opioid Requirement, Opioid Receptor Expression, and Clinical Outcomes in Patients with Advanced Prostate Cancer: Opioids and Prostate Cancer Outcomes. Cancer 2013, 119, 4103–4110. [Google Scholar] [CrossRef]
- Kampa, M.; Bakogeorgou, E.; Hatzoglou, A.; Damianaki, A.; Martin, P.-M.; Castanas, E. Opioid Alkaloids, and Casomorphin Peptides Decrease the Proliferation of Prostatic Cancer Cell Lines (LNCaP, PC3, and DU145) through a Partial Interaction with Opioid Receptors. Eur. J. Pharmacol. 1997, 335, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsson, P.-A.; Wadström, L.B.; Alumets, J.; Falkmer, S.; Grimelius, L. Peptide-Hormone- And Serotonin-Immunoreactive Tumour Cells in Carcinoma of the Prostate. Pathol. Res. Pract. 1987, 182, 298–307. [Google Scholar] [CrossRef]
- Sarkar, D.K.; Boyadjieva, N.I.; Chen, C.P.; Ortigüela, M.; Reuhl, K.; Clement, E.M.; Kuhn, P.; Marano, J. Cyclic Adenosine Monophosphate Differentiated β-Endorphin Neurons Promote Immune Function and Prevent Prostate Cancer Growth. Proc. Natl. Acad. Sci. USA 2008, 105, 9105–9110. [Google Scholar] [CrossRef]
- Moon, T.D. The Effect of Opiates upon Prostatic Carcinoma Cell Growth. Biochem. Biophys. Res. Commun. 1988, 153, 722–727. [Google Scholar] [CrossRef]
- Zhang, R.-X.; Li, A.; Liu, B.; Wang, L.; Xin, J.; Ren, K.; Qiao, J.-T.; Berman, B.M.; Lao, L. Electroacupuncture Attenuates Bone Cancer-Induced Hyperalgesia and Inhibits Spinal Preprodynorphin Expression in a Rat Model. Eur. J. Pain 2008, 12, 870–878. [Google Scholar] [CrossRef]
- Shimoyama, M.; Tatsuoka, H.; Ohtori, S.; Tanaka, K.; Shimoyama, N. Change of Dorsal Horn Neurochemistry in a Mouse Model of Neuropathic Cancer Pain. Pain 2005, 114, 221–230. [Google Scholar] [CrossRef]
- Honore, P.; Rogers, S.D.; Schwei, M.J.; Salak-Johnson, J.L.; Luger, N.M.; Sabino, M.C.; Clohisy, D.R.; Mantyh, P.W. Murine Models of Inflammatory, Neuropathic and Cancer Pain Each Generates a Unique Set of Neurochemical Changes in the Spinal Cord and Sensory Neurons. Neuroscience 2000, 98, 585–598. [Google Scholar] [CrossRef]
- Bisignani, G.J.; Mclaughlin, P.J.; Ordille, S.D.; Beltz, M.S.; Jarowenko, M.V.; Zagon, I.S. Human Renal Cell Cancer Proliferation in Tissue Culture Is Tonically Inhibited by Opioid Growth Factor. J. Urol. 1999, 162, 2186–2191. [Google Scholar] [CrossRef] [PubMed]
- Tarkkanen, A.; Tervo, T.; Tervo, K.; Panula, P. Immunohistochemical Evidence for Preproenkephalin A Synthesis in Human Retinoblastoma. Investig. Ophthalmol. Vis. Sci. 1984, 25, 1210–1212. [Google Scholar]
- Westphal, M.; Li, C.H. Human Retinoblastomas Have Binding Sites for the COOH-Terminal Segment of Human β-Endorphin. Int. J. Pept. Protein Res. 2009, 26, 557–559. [Google Scholar] [CrossRef] [PubMed]
- McMurray, C.T.; Devi, L.; Calavetta, L.; Douglass, J.O. Regulated Expression of the Prodynorphin Gene in the R2C Leydig Tumor Cell Line*. Endocrinology 1989, 124, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.; Holdaway, I.M.; Jagusch, M.; Kerr, A.R.; Donald, R.A.; Pullan, P.T. Ectopic Secretion of ACTH and Met-Enkephalin from a Thymic Carcinoid. J. Endocrinol. Investig. 1982, 5, 33–37. [Google Scholar] [CrossRef]
- Schweigerer, L.; Schmidt, W.; Teschemacher, H.; Gramsch, C. Beta-Endorphin: Surface Binding and Internalization in Thymoma Cells. Proc. Natl. Acad. Sci. USA 1985, 82, 5751–5755. [Google Scholar] [CrossRef]
- Yamaji, I.; Iimura, O.; Mito, T.; Yoshida, S.; Shimamoto, K.; Minase, T. An Ectopic, ACTH Producing, Oncocytic Carcinoid Tumor of the Thymus Report of a Case. Jpn. J. Med. 1984, 23, 62–66. [Google Scholar] [CrossRef]
- Csuhai, E.; Safavi, A.; Hersh, L.B. Purification and Characterization of a Secreted Arginine-Specific Dibasic Cleaving Enzyme from EL-4 Cells. Biochemistry 1995, 34, 12411–12419. [Google Scholar] [CrossRef]
- McLaughlin, P.J.; Zagon, I.S.; Park, S.S.; Conway, A.; Donahue, R.N.; Goldenberg, D. Growth Inhibition of Thyroid Follicular Cell-Derived Cancers by the Opioid Growth Factor (OGF)—Opioid Growth Factor Receptor (OGFr) Axis. BMC Cancer 2009, 9, 369. [Google Scholar] [CrossRef]
- Oosterom, R.; Verleun, T.; Bruining, H.A.; Hackeng, W.H.L.; Lamberts, S.W.J. Secretion of Adrenocorticotropin, β -Endorphin and Calcitonin by Cultured Medullary Thyroid Carcinoma Cells. Effects of Synthetic Corticotropin-Releasing Factor and Lysine Vasopressin. Acta Endocrinol. 1986, 113, 65–72. [Google Scholar] [CrossRef]
- Maneckjee, R.; Biswas, R.; Vonderhaar, B.K. Binding of Opioids to Human MCF-7 Breast Cancer Cells and Their Effects on Growth. Cancer Res. 1990, 50, 2234–2238. [Google Scholar] [PubMed]
- Wang, C.-Z.; Li, X.-L.; Sun, S.; Xie, J.-T.; Aung, H.H.; Tong, R.; McEntee, E.; Yuan, C.-S. Methylnaltrexone, a Peripherally Acting Opioid Receptor Antagonist, Enhances Tumoricidal Effects of 5-Fu on Human Carcinoma Cells. Anticancer Res. 2009, 29, 2927–2932. [Google Scholar] [PubMed]
- Suzuki, M.; Chiwaki, F.; Sawada, Y.; Ashikawa, M.; Aoyagi, K.; Fujita, T.; Yanagihara, K.; Komatsu, M.; Narita, M.; Suzuki, T.; et al. Peripheral Opioid Antagonist Enhances the Effect of Anti-Tumor Drug by Blocking a Cell Growth-Suppressive Pathway In Vivo. PLoS ONE 2015, 10, e0123407. [Google Scholar] [CrossRef]
- Singleton, P.A.; Lingen, M.W.; Fekete, M.J.; Garcia, J.G.N.; Moss, J. Methylnaltrexone Inhibits Opiate and VEGF-Induced Angiogenesis: Role of Receptor Transactivation. Microvasc. Res. 2006, 72, 3–11. [Google Scholar] [CrossRef]
- Szczepaniak, A.; Fichna, J.; Zielińska, M. Opioids in Cancer Development, Progression and Metastasis: Focus on Colorectal Cancer. Curr. Treat. Options Oncol. 2020, 21, 6. [Google Scholar] [CrossRef] [PubMed]
- Narayan, A.; Hunkele, A.; Xu, J.; Bassoni, D.L.; Pasternak, G.W.; Pan, Y.-X. Mu Opioids Induce Biased Signaling at the Full-Length Seven Transmembrane C-Terminal Splice Variants of the Mu Opioid Receptor Gene, Oprm1. Cell. Mol. Neurobiol. 2021, 41, 1059–1074. [Google Scholar] [CrossRef] [PubMed]
- Vadhel, A.; Bashir, S.; Mir, A.H.; Girdhar, M.; Kumar, D.; Kumar, A.; Mohan, A.; Malik, T.; Mohan, A. Opium Alkaloids, Biosynthesis, Pharmacology and Association with Cancer Occurrence. Open Biol. 2023, 13, 220355. [Google Scholar] [CrossRef]
- Rashidian, H.; Hadji, M.; Gholipour, M.; Naghibzadeh-Tahami, A.; Marzban, M.; Mohebbi, E.; Safari-Faramani, R.; Bakhshi, M.; Seyyedsalehi, M.S.; Hosseini, B.; et al. Opium Use and Risk of Lung Cancer: A Multicenter Case-Control Study in Iran. Int. J. Cancer 2023, 152, 203–213. [Google Scholar] [CrossRef]
- Houston, M.G.; McMenamin, Ú.; Johnston, B.; McDowell, R.D.; Hughes, C.M.; Murchie, P.; Cardwell, C.R. Exposure to Weak Opioids and Risk of Gastrointestinal Tract Cancers: A Series of Nested Case-Control Studies. Br. J. Clin. Pharmacol. 2023. [Google Scholar] [CrossRef]
- Ağagündüz, D.; Cocozza, E.; Cemali, Ö.; Bayazıt, A.D.; Nanì, M.F.; Cerqua, I.; Morgillo, F.; Saygılı, S.K.; Berni Canani, R.; Amero, P.; et al. Understanding the Role of the Gut Microbiome in Gastrointestinal Cancer: A Review. Front. Pharmacol. 2023, 14, 1130562. [Google Scholar] [CrossRef]
- Duque-Díaz, E.; Meléndez-Flórez, G.; Hurtado Giraldo, H.; Coveñas, R. Involvement of the Adrenocorticotropic Hormone in Cancer and Other Pathologies. In Advances in Health and Disease; Duncan, L.T., Ed.; Nova Science Publishers: New York, NY, USA, 2023; Volume 68, pp. 185–208. [Google Scholar]
- Li, G.; Li, B.; Song, J.; Wang, N.; Gao, Z. Endomorphin-2 Analog Inhibits the Growth of DLD-1 and RKO Human Colon Cancer Cells by Inducing Cell Apoptosis. Med. Sci. Monit. 2020, 26, e921251. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).