Spontaneous Epiretinal Membrane Resolution and Angiotensin Receptor Blockers: Case Observation, Literature Review and Perspectives
Abstract
1. Introduction
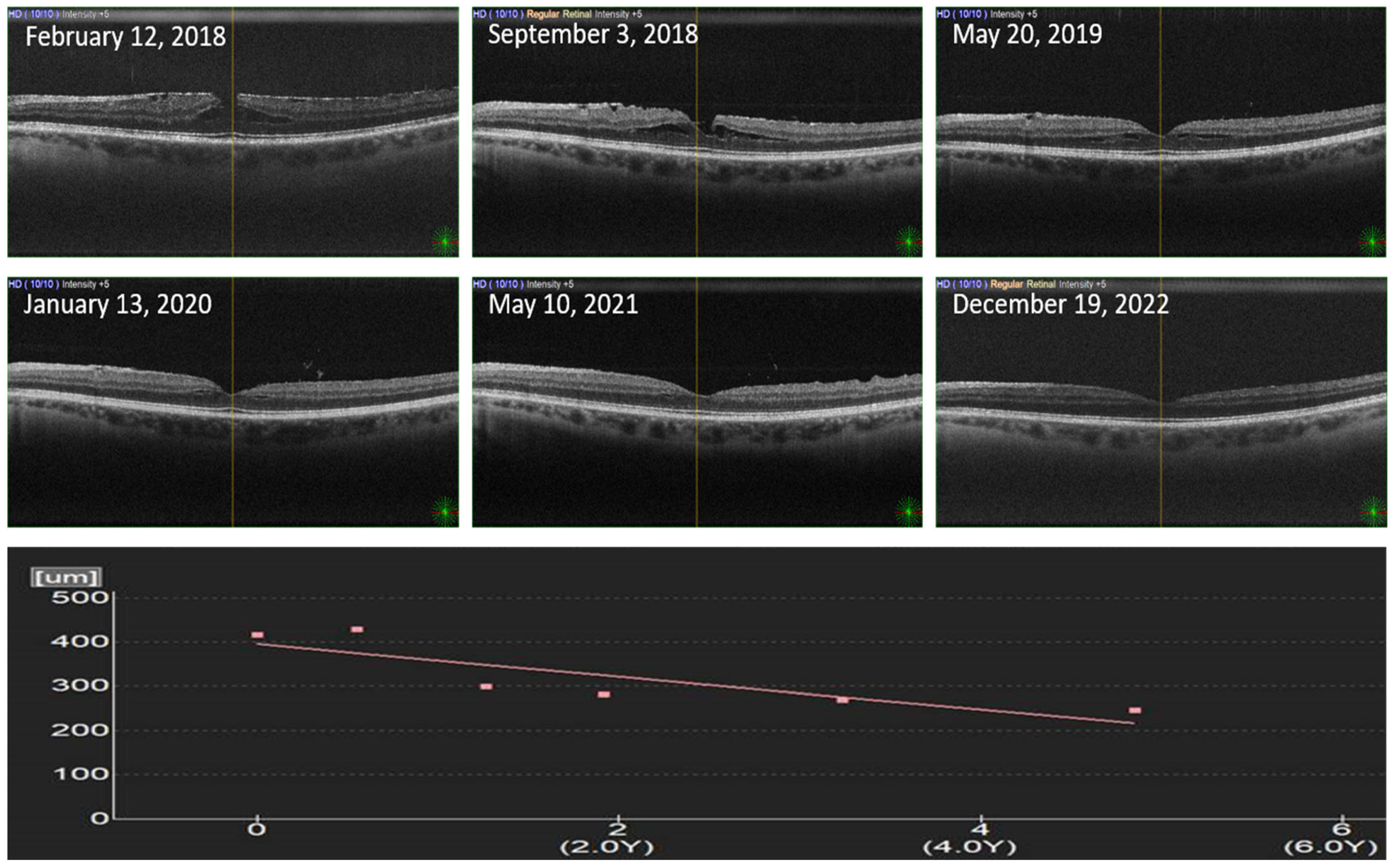
2. Case Description and Materials and Methods
3. Results
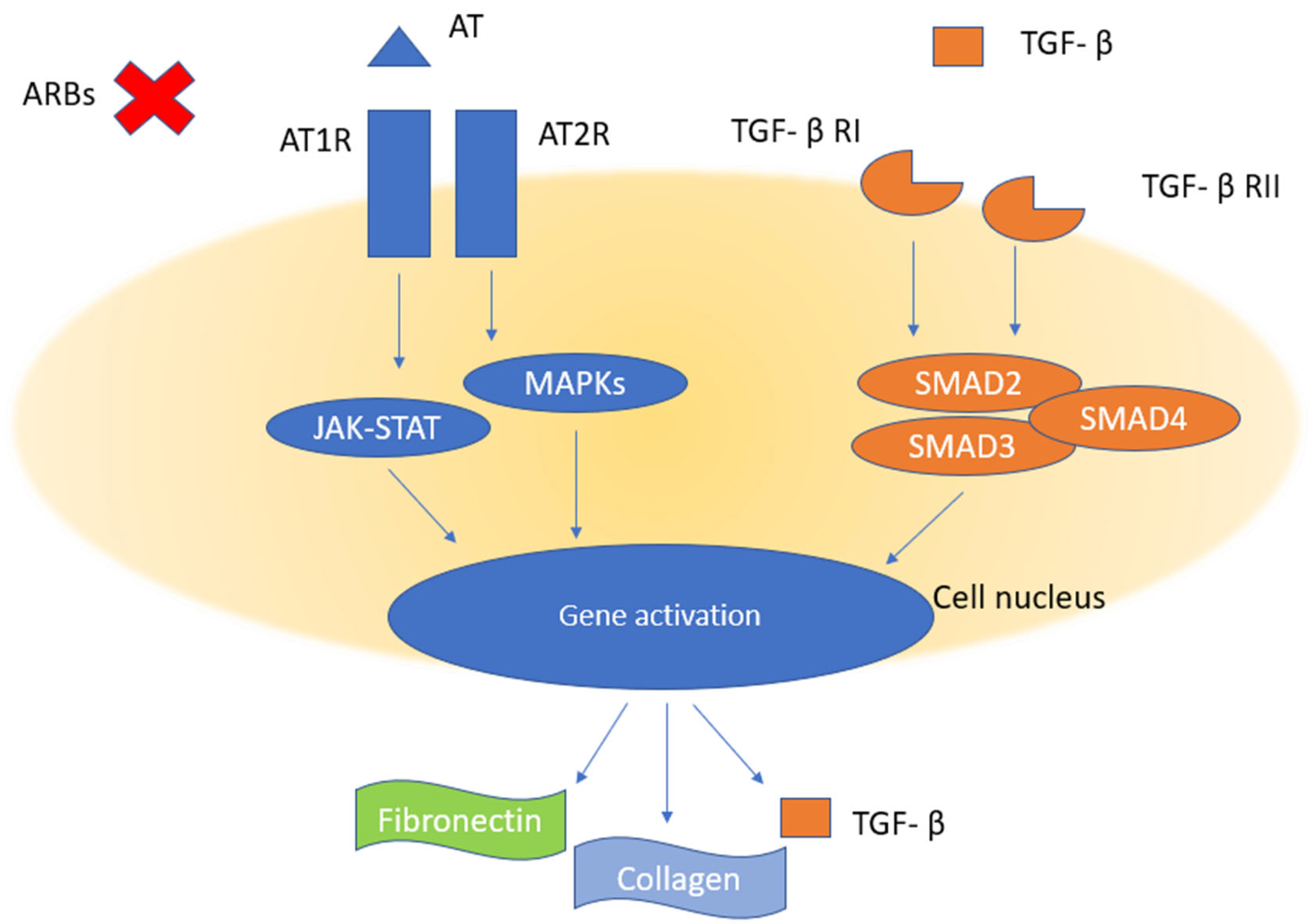
3.1. ARBs and Fibrosis
3.2. ACE-Is and Fibrosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ng, C.H.; Cheung, N.; Wang, J.J.; Islam, A.F.; Kawasaki, R.; Meuer, S.M.; Cotch, M.F.; Klein, B.E.; Klein, R.; Wong, T.Y. Prevalence and Risk Factors for Epiretinal Membranes in a Multi-Ethnic United States Population. Ophthalmology 2011, 118, 694–699. [Google Scholar] [CrossRef]
- Klein, R.; Klein, B.E.; Wang, Q.; Moss, S.E. The Epidemiology of Epiretinal Membranes. Trans. Am. Ophthalmol. Soc. 1994, 92, 403–425; discussion 425–430. [Google Scholar]
- Fraser-Bell, S.; Guzowski, M.; Rochtchina, E.; Wang, J.J.; Mitchell, P. Five-Year Cumulative Incidence and Progression of Epiretinal Membranes: The Blue Mountains Eye Study. Ophthalmology 2003, 110, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Fraser-Bell, S.; Ying-Lai, M.; Klein, R.; Varma, R. Los Angeles Latino Eye Study Prevalence and Associations of Epiretinal Membranes in Latinos: The Los Angeles Latino Eye Study. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1732–1736. [Google Scholar] [CrossRef]
- McCarty, D.J.; Mukesh, B.N.; Chikani, V.; Wang, J.J.; Mitchell, P.; Taylor, H.R.; McCarty, C.A. Prevalence and Associations of Epiretinal Membranes in the Visual Impairment Project. Am. J. Ophthalmol. 2005, 140, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, M.; Nakamura, H.; Kubo, M.; Kiyohara, Y.; Iida, M.; Ishibashi, T.; Nose, Y. Prevalence and Risk Factors for Epiretinal Membranes in a Japanese Population: The Hisayama Study. Graefes Arch. Clin. Exp. Ophthalmol. Albrecht Graefes Arch. Klin. Exp. Ophthalmol. 2003, 241, 642–646. [Google Scholar] [CrossRef] [PubMed]
- You, Q.; Xu, L.; Jonas, J.B. Prevalence and Associations of Epiretinal Membranes in Adult Chinese: The Beijing Eye Study. Eye 2008, 22, 874–879. [Google Scholar] [CrossRef]
- Kawasaki, R.; Wang, J.J.; Mitchell, P.; Aung, T.; Saw, S.-M.; Wong, T.Y. Singapore Malay Eye Study Group Racial Difference in the Prevalence of Epiretinal Membrane between Caucasians and Asians. Br. J. Ophthalmol. 2008, 92, 1320–1324. [Google Scholar] [CrossRef]
- Kawasaki, R.; Wang, J.J.; Sato, H.; Mitchell, P.; Kato, T.; Kawata, S.; Kayama, T.; Yamashita, H.; Wong, T.Y. Prevalence and Associations of Epiretinal Membranes in an Adult Japanese Population: The Funagata Study. Eye 2009, 23, 1045–1051. [Google Scholar] [CrossRef]
- Xiao, W.; Chen, X.; Yan, W.; Zhu, Z.; He, M. Prevalence and Risk Factors of Epiretinal Membranes: A Systematic Review and Meta-Analysis of Population-Based Studies. BMJ Open 2017, 7, e014644. [Google Scholar] [CrossRef]
- Mitchell, P.; Smith, W.; Chey, T.; Wang, J.J.; Chang, A. Prevalence and Associations of Epiretinal Membranes: The Blue Mountains Eye Study, Australia. Ophthalmology 1997, 104, 1033–1040. [Google Scholar] [CrossRef]
- Chang, W.-C.; Lin, C.; Lee, C.-H.; Sung, T.-L.; Tung, T.-H.; Liu, J.-H. Vitrectomy with or without Internal Limiting Membrane Peeling for Idiopathic Epiretinal Membrane: A Meta-Analysis. PLoS ONE 2017, 12, e0179105. [Google Scholar] [CrossRef]
- Kanukollu, V.M.; Agarwal, P. Epiretinal Membrane; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Wynn, T. Cellular and Molecular Mechanisms of Fibrosis. J. Pathol. 2008, 214, 199–210. [Google Scholar] [CrossRef]
- Okada, M.; Ogino, N.; Matsumura, M.; Honda, Y.; Nagai, Y. Histological and Immunohistochemical Study of Idiopathic Epiretinal Membrane. Ophthalmic Res. 1995, 27, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Herman, L.L.; Padala, S.A.; Ahmed, I.; Bashir, K. Angiotensin Converting Enzyme Inhibitors (ACEI); StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Hill, R.D.; Vaidya, P.N. Angiotensin II Receptor Blockers (ARB); StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Fang, Q.-Q.; Wang, X.-F.; Zhao, W.-Y.; Ding, S.-L.; Shi, B.-H.; Xia, Y.; Yang, H.; Wu, L.-H.; Li, C.-Y.; Tan, W.-Q. Angiotensin-Converting Enzyme Inhibitor Reduces Scar Formation by Inhibiting Both Canonical and Noncanonical TGF-Β1 Pathways. Sci. Rep. 2018, 8, 3332. [Google Scholar] [CrossRef]
- Murphy, A.M.; Wong, A.L.; Bezuhly, M. Modulation of Angiotensin II Signaling in the Prevention of Fibrosis. Fibrogen. Tissue Repair 2015, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Khalifa, M.O.; Li, P.; Huang, Y.; Gu, W.; Li, T.-S. Angiotensin Receptor Blocker Alleviates Liver Fibrosis by Altering the Mechanotransduction Properties of Hepatic Stellate Cells. Am. J. Physiol.-Gastrointest. Liver Physiol. 2022, 322, G446–G456. [Google Scholar] [CrossRef]
- Tokuda, K.; Kai, H.; Kuwahara, F.; Imaizumi, T. Sub-Depressor Dose of Angiotensin Type-1 Receptor Blocker Inhibits Transforming Growth Factor-β-Mediated Perivascular Fibrosis in Hypertensive Rat Hearts. J. Cardiovasc. Pharmacol. 2003, 42 (Suppl. 1), S61–S65. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Kuno, A.; Masuda, K.; Ogawa, K.; Sogawa, M.; Nakamura, S.; Ando, T.; Sano, H.; Nakazawa, T.; Ohara, H.; et al. Candesartan, an Angiotensin II Receptor Antagonist, Suppresses Pancreatic Inflammation and Fibrosis in Rats. J. Pharmacol. Exp. Ther. 2003, 307, 17–23. [Google Scholar] [CrossRef]
- Ueki, M.; Koda, M.; Yamamoto, S.; Matsunaga, Y.; Murawaki, Y. Preventive and Therapeutic Effects of Angiotensin II Type 1 Receptor Blocker on Hepatic Fibrosis Induced by Bile Duct Ligation in Rats. J. Gastroenterol. 2006, 41, 996–1004. [Google Scholar] [CrossRef]
- Ueki, M.; Koda, M.; Shimizu, T.; Mitsuta, A.; Yamamoto, T.; Murawaki, Y. Effect of an Angiotensin-II Type-1 Receptor Blocker, Candesartan on Hepatic Fibrosis in Chronic Hepatitis C: A Prospective Study. Hepatogastroenterology. 2009, 56, 1100–1104. [Google Scholar]
- Kato, T.; Yamashita, T.; Sekiguchi, A.; Tsuneda, T.; Sagara, K.; Takamura, M.; Kaneko, S.; Aizawa, T.; Fu, L.-T. Angiotensin II Type 1 Receptor Blocker Attenuates Diabetes-Induced Atrial Structural Remodeling. J. Cardiol. 2011, 58, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, M.; Fushida, S.; Harada, S.; Tsukada, T.; Kinoshita, J.; Oyama, K.; Tajima, H.; Ninomiya, I.; Fujimura, T.; Ohta, T. The Angiotensin II Type 1 Receptor Blocker Candesartan Suppresses Proliferation and Fibrosis in Gastric Cancer. Cancer Lett. 2014, 355, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Loging, J.A.; New, R.B.; Baicu, S.C.; King, M.K.; Hendrick, J.W.; Crawford, F.A.; de Gasparo, M.; Spinale, F.G. Effects of Angiotensin Type-I Receptor Blockade on Pericardial Fibrosis. J. Surg. Res. 1999, 87, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Molteni, A.; Moulder, J.E.; Cohen, E.F.; Ward, W.F.; Fish, B.L.; Taylor, J.M.; Wolfe, L.F.; Brizio-Molteni, L.; Veno, P. Control of Radiation-Induced Pneumopathy and Lung Fibrosis by Angiotensin-Converting Enzyme Inhibitors and an Angiotensin II Type 1 Receptor Blocker. Int. J. Radiat. Biol. 2000, 76, 523–532. [Google Scholar] [CrossRef]
- Molteni, A.; Moulder, J.E.; Cohen, E.P.; Fish, B.L.; Taylor, J.M.; Veno, P.A.; Wolfe, L.F.; Ward, W.F. Prevention of Radiation-Induced Nephropathy and Fibrosis in a Model of Bone Marrow Transplant by an Angiotensin II Receptor Blocker. Exp. Biol. Med. 2001, 226, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Mano, T.; Yoshida, J.; Sakata, Y.; Nishikawa, N.; Nishio, M.; Ohtani, T.; Hori, M.; Miwa, T.; Masuyama, T. ACE Inhibitor and Angiotensin II Type 1 Receptor Blocker Differently Regulate Ventricular Fibrosis in Hypertensive Diastolic Heart Failure. J. Hypertens. 2005, 23, 393–400. [Google Scholar] [CrossRef]
- De Carvalho Frimm, C.; Sun, Y.; Weber, K.T. Angiotensin II Receptor Blockade and Myocardial Fibrosis of the Infarcted Rat Heart. J. Lab. Clin. Med. 1997, 129, 439–446. [Google Scholar] [CrossRef]
- Kellner, D.; Chen, J.; Richardson, I.; Seshan, S.V.; El Chaar, M.; Vaughan, E.D.; Poppas, D.; Felsen, D. Angiotensin Receptor Blockade Decreases Fibrosis and Fibroblast Expression in a Rat Model of Unilateral Ureteral Obstruction. J. Urol. 2006, 176, 806–812. [Google Scholar] [CrossRef]
- Bedair, H.S.; Karthikeyan, T.; Quintero, A.; Li, Y.; Huard, J. Angiotensin II Receptor Blockade Administered after Injury Improves Muscle Regeneration and Decreases Fibrosis in Normal Skeletal Muscle. Am. J. Sports Med. 2008, 36, 1548–1554. [Google Scholar] [CrossRef]
- Chou, H.-C.; Lang, Y.-D.; Wang, L.-F.; Wu, T.-Y.; Hsieh, Y.-F.; Chen, C.-M. Angiotensin II Type 1 Receptor Antagonist Attenuates Lung Fibrosis in Hyperoxia-Exposed Newborn Rats. J. Pharmacol. Exp. Ther. 2012, 340, 169–175. [Google Scholar] [CrossRef]
- Feng, R.; Wan, J.; He, Y.; Gong, H.; Xu, Z.; Feng, J. Angiotensin-Receptor Blocker Losartan Alleviates Atrial Fibrillation in Rats by Downregulating Frizzled 8 and Inhibiting the Activation of WNT-5A Pathway. Clin. Exp. Pharmacol. Physiol. 2023, 50, 19–27. [Google Scholar] [CrossRef]
- Molteni, A.; Wolfe, L.F.; Ward, W.F.; Ts’ao, C.H.; Molteni, L.B.; Veno, P.; Fish, B.L.; Taylor, J.M.; Quintanilla, N.; Herndon, B.; et al. Effect of an Angiotensin II Receptor Blocker and Two Angiotensin Converting Enzyme Inhibitors on Transforming Growth Factor-β (TGF-β) and α-Actomyosin (α SMA), Important Mediators of Radiation-Induced Pneumopathy and Lung Fibrosis. Curr. Pharm. Des. 2007, 13, 1307–1316. [Google Scholar] [CrossRef] [PubMed]
- Hirose, A.; Ono, M.; Saibara, T.; Nozaki, Y.; Masuda, K.; Yoshioka, A.; Takahashi, M.; Akisawa, N.; Iwasaki, S.; Oben, J.A.; et al. Angiotensin II Type 1 Receptor Blocker Inhibits Fibrosis in Rat Nonalcoholic Steatohepatitis. Hepatology 2007, 45, 1375–1381. [Google Scholar] [CrossRef] [PubMed]
- Waseda, Y.; Yasui, M.; Nishizawa, Y.; Inuzuka, K.; Takato, H.; Ichikawa, Y.; Tagami, A.; Fujimura, M.; Nakao, S. Angiotensin II Type 2 Receptor Antagonist Reduces Bleomycin-Induced Pulmonary Fibrosis in Mice. Respir. Res. 2008, 9, 43. [Google Scholar] [CrossRef]
- Burke, R.M.; Lighthouse, J.K.; Mickelsen, D.M.; Small, E.M. Sacubitril/Valsartan Decreases Cardiac Fibrosis in Left Ventricle Pressure Overload by Restoring PKG Signaling in Cardiac Fibroblasts. Circ. Heart Fail. 2019, 12, e005565. [Google Scholar] [CrossRef]
- Clements, R.T.; Vang, A.; Fernandez-Nicolas, A.; Kue, N.R.; Mancini, T.J.; Morrison, A.R.; Mallem, K.; McCullough, D.J.; Choudhary, G. Treatment of Pulmonary Hypertension with Angiotensin II Receptor Blocker and Neprilysin Inhibitor Sacubitril/Valsartan. Circ. Heart Fail. 2019, 12, e005819. [Google Scholar] [CrossRef]
- von Lueder, T.G.; Wang, B.H.; Kompa, A.R.; Huang, L.; Webb, R.; Jordaan, P.; Atar, D.; Krum, H. Angiotensin Receptor Neprilysin Inhibitor LCZ696 Attenuates Cardiac Remodeling and Dysfunction after Myocardial Infarction by Reducing Cardiac Fibrosis and Hypertrophy. Circ. Heart Fail. 2015, 8, 71–78. [Google Scholar] [CrossRef]
- Wang, J.; Duan, L.; Gao, Y.; Zhou, S.; Liu, Y.; Wei, S.; An, S.; Liu, J.; Tian, L.; Wang, S. Angiotensin II Receptor Blocker Valsartan Ameliorates Cardiac Fibrosis Partly by Inhibiting MiR-21 Expression in Diabetic Nephropathy Mice. Mol. Cell. Endocrinol. 2018, 472, 149–158. [Google Scholar] [CrossRef]
- Miyoshi, T.; Nakamura, K.; Miura, D.; Yoshida, M.; Saito, Y.; Akagi, S.; Ohno, Y.; Kondo, M.; Ito, H. Effect of LCZ696, a Dual Angiotensin Receptor Neprilysin Inhibitor, on Isoproterenol-Induced Cardiac Hypertrophy, Fibrosis, and Hemodynamic Change in Rats. Cardiol. J. 2019, 26, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Han, J.-Y.; Kim, Y.-J.; Kim, L.; Choi, S.-J.; Park, I.-S.; Kim, J.-M.; Chu, Y.C.; Cha, D.-R. PPARgamma Agonist and Angiotensin II Receptor Antagonist Ameliorate Renal Tubulointerstitial Fibrosis. J. Korean Med. Sci. 2010, 25, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Côté, N.; Mahmut, A.; Fournier, D.; Boulanger, M.-C.; Couture, C.; Després, J.-P.; Trahan, S.; Bossé, Y.; Pagé, S.; Pibarot, P.; et al. Angiotensin Receptor Blockers Are Associated with Reduced Fibrosis and Interleukin-6 Expression in Calcific Aortic Valve Disease. Pathobiol. J. Immunopathol. Mol. Cell. Biol. 2014, 81, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Goh, G.B.; Pagadala, M.R.; Dasarathy, J.; Unalp-Arida, A.; Sargent, R.; Hawkins, C.; Sourianarayanane, A.; Khiyami, A.; Yerian, L.; Pai, R.; et al. Renin-Angiotensin System and Fibrosis in Non-Alcoholic Fatty Liver Disease. Liver Int. Off. J. Int. Assoc. Study Liver 2015, 35, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, J.R.; Clouston, A.D.; Ando, Y.; Kelemen, L.I.; Horn, M.J.; Adamson, M.D.; Purdie, D.M.; Powell, E.E. Angiotensin-Converting Enzyme Inhibition Attenuates the Progression of Rat Hepatic Fibrosis. Gastroenterology 2001, 121, 148–155. [Google Scholar] [CrossRef]
- Peng, H.; Carretero, O.A.; Vuljaj, N.; Liao, T.-D.; Motivala, A.; Peterson, E.L.; Rhaleb, N.-E. Angiotensin-Converting Enzyme Inhibitors: A New Mechanism of Action. Circulation 2005, 112, 2436–2445. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Fang, L.; Ma, P. The Angiotensin-Converting Enzyme Inhibitor, Captopril, Suppressed Hepatic Stellate Cell Activation via NF-KappaB or Wnt3α/β-Catenin Pathway. Bioengineered 2021, 12, 8370–8377. [Google Scholar] [CrossRef]
- Rha, E.Y.; Kim, J.W.; Kim, J.H.; Yoo, G. Angiotensin-Converting Enzyme Inhibitor, Captopril, Improves Scar Healing in Hypertensive Rats. Int. J. Med. Sci. 2021, 18, 975–983. [Google Scholar] [CrossRef]
- Koga, H.; Yang, H.; Adler, J.; Zimmermann, E.M.; Teitelbaum, D.H. Transanal Delivery of Angiotensin Converting Enzyme Inhibitor Prevents Colonic Fibrosis in a Mouse Colitis Model: Development of a Unique Mode of Treatment. Surgery 2008, 144, 259–268. [Google Scholar] [CrossRef]
- Uzun, H.; Bitik, O.; Hekimoğlu, R.; Atilla, P.; Kaykçoğlu, A.U. Angiotensin-Converting Enzyme Inhibitor Enalapril Reduces Formation of Hypertrophic Scars in a Rabbit Ear Wounding Model. Plast. Reconstr. Surg. 2013, 132, 361e–371e. [Google Scholar] [CrossRef]
- Sun, N.; Zhai, L.; Li, H.; Shi, L.-H.; Yao, Z.; Zhang, B. Angiotensin-Converting Enzyme Inhibitor (ACEI)-Mediated Amelioration in Renal Fibrosis Involves Suppression of Mast Cell Degranulation. Kidney Blood Press. Res. 2016, 41, 108–118. [Google Scholar] [CrossRef]
- Garvin, A.M.; De Both, M.D.; Talboom, J.S.; Lindsey, M.L.; Huentelman, M.J.; Hale, T.M. Transient ACE (Angiotensin-Converting Enzyme) Inhibition Suppresses Future Fibrogenic Capacity and Heterogeneity of Cardiac Fibroblast Subpopulations. Hypertension 2021, 77, 904–918. [Google Scholar] [CrossRef]
- Attia, Y.M.; Elalkamy, E.F.; Hammam, O.A.; Mahmoud, S.S.; El-Khatib, A.S. Telmisartan, an AT1 Receptor Blocker and a PPAR Gamma Activator, Alleviates Liver Fibrosis Induced Experimentally by Schistosoma Mansoni Infection. Parasit. Vectors 2013, 6, 199. [Google Scholar] [CrossRef]
- Kuno, A.; Yamada, T.; Masuda, K.; Ogawa, K.; Sogawa, M.; Nakamura, S.; Nakazawa, T.; Ohara, H.; Nomura, T.; Joh, T.; et al. Angiotensin-Converting Enzyme Inhibitor Attenuates Pancreatic Inflammation and Fibrosis in Male Wistar Bonn/Kobori Rats. Gastroenterology 2003, 124, 1010–1019. [Google Scholar] [CrossRef] [PubMed]
- Kakutani, N.; Takada, S.; Nambu, H.; Maekawa, S.; Hagiwara, H.; Yamanashi, K.; Obata, Y.; Nakano, I.; Fumoto, Y.; Hata, S.; et al. Angiotensin-Converting Enzyme Inhibitor Prevents Skeletal Muscle Fibrosis in Diabetic Mice. Exp. Physiol. 2021, 106, 1785–1793. [Google Scholar] [CrossRef]
- Reza, H.M.; Tabassum, N.; Sagor, M.A.T.; Chowdhury, M.R.H.; Rahman, M.; Jain, P.; Alam, M.A. Angiotensin-Converting Enzyme Inhibitor Prevents Oxidative Stress, Inflammation, and Fibrosis in Carbon Tetrachloride-Treated Rat Liver. Toxicol. Mech. Methods 2016, 26, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Erpolat, O.P.; Senturk, E.; Saribas, S.; Pasinlioglu, B.; Gulbahar, O.; Tuncer, S.; Demircan, V.; Catli Dinc, S.; Polat, O.; Elmas, C. Angiotensin-Converting Enzyme Inhibitor Reduces Radiation-Induced Periprosthetic Capsular Fibrosis. J. Surg. Res. 2021, 263, 167–175. [Google Scholar] [CrossRef]
- Kim, S.; Kim, S.J.; Yoon, H.E.; Chung, S.; Choi, B.S.; Park, C.W.; Shin, S.J. Fimasartan, a Novel Angiotensin-Receptor Blocker, Protects against Renal Inflammation and Fibrosis in Mice with Unilateral Ureteral Obstruction: The Possible Role of Nrf2. Int. J. Med. Sci. 2015, 12, 891–904. [Google Scholar] [CrossRef]
- Czechowska, G.; Celinski, K.; Korolczuk, A.; Wojcicka, G.; Dudka, J.; Bojarska, A.; Madro, A.; Brzozowski, T. The Effect of the Angiotensin II Receptor, Type 1 Receptor Antagonists, Losartan and Telmisartan, on Thioacetamide-Induced Liver Fibrosis in Rats. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2016, 67, 575–586. [Google Scholar]
- Moreno, M.; Gonzalo, T.; Kok, R.J.; Sancho-Bru, P.; Van Beuge, M.; Swart, J.; Prakash, J.; Temming, K.; Fondevila, C.; Beljaars, L.; et al. Reduction of Advanced Liver Fibrosis by Short-Term Targeted Delivery of an Angiotensin Receptor Blocker to Hepatic Stellate Cells in Rats. Hepatology 2010, 51, 942–952. [Google Scholar] [CrossRef]
- Song, L.-J.; Xiang, F.; Ye, H.; Huang, H.; Yang, J.; Yu, F.; Xiong, L.; Xu, J.-J.; Greer, P.A.; Shi, H.-Z.; et al. Inhibition of Angiotensin II and Calpain Attenuates Pleural Fibrosis. Pulm. Pharmacol. Ther. 2018, 48, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.Y.; Baik, S.K.; Park, D.H.; Jang, Y.O.; Suk, K.T.; Yea, C.J.; Lee, I.Y.; Kim, J.W.; Kim, H.S.; Kwon, S.O.; et al. Angiotensin Receptor Blockers Are Superior to Angiotensin-Converting Enzyme Inhibitors in the Suppression of Hepatic Fibrosis in a Bile Duct-Ligated Rat Model. J. Gastroenterol. 2008, 43, 889–896. [Google Scholar] [CrossRef]
- Kreuter, M.; Lederer, D.J.; Molina-Molina, M.; Noth, I.; Valenzuela, C.; Frankenstein, L.; Weycker, D.; Atwood, M.; Kirchgaessler, K.-U.; Cottin, V. Association of Angiotensin Modulators with the Course of Idiopathic Pulmonary Fibrosis. Chest 2019, 156, 706–714. [Google Scholar] [CrossRef]
- Fujinaga, Y.; Kawaratani, H.; Kaya, D.; Tsuji, Y.; Ozutsumi, T.; Furukawa, M.; Kitagawa, K.; Sato, S.; Nishimura, N.; Sawada, Y.; et al. Effective Combination Therapy of Angiotensin-II Receptor Blocker and Rifaximin for Hepatic Fibrosis in Rat Model of Nonalcoholic Steatohepatitis. Int. J. Mol. Sci. 2020, 21, 5589. [Google Scholar] [CrossRef]
- Hu, Y.-Y.; Fang, Q.-Q.; Wang, X.-F.; Zhao, W.-Y.; Zheng, B.; Zhang, D.-D.; Tan, W.-Q. Angiotensin-Converting Enzyme Inhibitor and Angiotensin II Type 1 Receptor Blocker: Potential Agents to Reduce Post-Surgical Scar Formation in Humans. Basic Clin. Pharmacol. Toxicol. 2020, 127, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Namisaki, T.; Kaji, K.; Shimozato, N.; Kaya, D.; Ozutsumi, T.; Tsuji, Y.; Fujinaga, Y.; Kitagawa, K.; Furukawa, M.; Sato, S.; et al. Effect of Combined Farnesoid X Receptor Agonist and Angiotensin II Type 1 Receptor Blocker on Ongoing Hepatic Fibrosis. Indian J. Gastroenterol. Off. J. Indian Soc. Gastroenterol. 2022, 41, 169–180. [Google Scholar] [CrossRef]
- Angiotensin Converting Enzyme Inhibition Modulates Cardiac Fibroblast Growth. Available online: https://oce-ovid-com.ezproxy.uio.no/article/00004872-199816030-00015/PDF (accessed on 1 January 2023).
- Yoshiji, H.; Kuriyama, S.; Noguchi, R.; Yoshii, J.; Ikenaka, Y.; Yanase, K.; Namisaki, T.; Kitade, M.; Yamazaki, M.; Tsujinoue, H.; et al. Combination of Interferon-β and Angiotensin-Converting Enzyme Inhibitor, Perindopril, Attenuates the Murine Liver Fibrosis Development. Liver Int. Off. J. Int. Assoc. Study Liver 2005, 25, 153–161. [Google Scholar] [CrossRef]
- Tan, W.; Fang, Q.; Shen, X.Z.; Giani, J.F.; Zhao, T.V.; Shi, P.; Zhang, L.; Khan, Z.; Li, Y.; Li, L.; et al. Angiotensin-converting Enzyme Inhibitor Works as a Scar Formation Inhibitor by Down-regulating Smad and TGF-β-activated Kinase 1 (TAK1) Pathways in Mice. Br. J. Pharmacol. 2018, 175, 4239–4252. [Google Scholar] [CrossRef] [PubMed]
- Cabello-Verrugio, C.; Morales, M.G.; Cabrera, D.; Vio, C.P.; Brandan, E. Angiotensin II Receptor Type 1 Blockade Decreases CTGF/CCN2-Mediated Damage and Fibrosis in Normal and Dystrophic Skeletal Muscles. J. Cell. Mol. Med. 2012, 16, 752–764. [Google Scholar] [CrossRef]
- Bianchi, L.; Altera, A.; Barone, V.; Bonente, D.; Bacci, T.; De Benedetto, E.; Bini, L.; Tosi, G.M.; Galvagni, F.; Bertelli, E. Untangling the Extracellular Matrix of Idiopathic Epiretinal Membrane: A Path Winding among Structure, Interactomics and Translational Medicine. Cells 2022, 11, 2531. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Nikolic-Paterson, D.J.; Lan, H.Y. TGF-β: The Master Regulator of Fibrosis. Nat. Rev. Nephrol. 2016, 12, 325–338. [Google Scholar] [CrossRef]
- Tsotridou, E.; Loukovitis, E.; Zapsalis, K.; Pentara, I.; Asteriadis, S.; Tranos, P.; Zachariadis, Z.; Anogeianakis, G. A Review of Last Decade Developments on Epiretinal Membrane Pathogenesis. Med. Hypothesis Discov. Innov. Ophthalmol. 2020, 9, 91–110. [Google Scholar]
- Shin, G.T.; Kim, S.J.; Ma, K.A.; Kim, H.S.; Kim, D. ACE Inhibitors Attenuate Expression of Renal Transforming Growth Factor-β1 in Humans. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2000, 36, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.S.; Yim, H.E.; Bae, I.S.; Choi, J.H.; Choi, B.M.; Yoo, K.H.; Hong, Y.S.; Lee, J.W.; Kim, S.K. ACE Inhibition Modulates Transforming Growth Factor-β Receptors in the Young Rat. Pediatr. Nephrol. 2003, 18, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Yoo, K.H.; Wolstenholme, J.T.; Chevalier, R.L. Angiotensin-Converting Enzyme Inhibition Decreases Growth Factor Expression in the Neonatal Rat Kidney. Pediatr. Res. 1997, 42, 588–592. [Google Scholar] [CrossRef] [PubMed]
- AlQudah, M.; Hale, T.M.; Czubryt, M.P. Targeting the Renin-Angiotensin-Aldosterone System in Fibrosis. Matrix Biol. J. Int. Soc. Matrix Biol. 2020, 91–92, 92–108. [Google Scholar] [CrossRef]
- Chung, S.E.; Lee, J.-H.; Kang, S.W.; Kim, Y.T.; Lee, S.W. Characteristics of Epiretinal Membranes according to the Presence or Absence of Posterior Vitreous Detachment. Eye 2011, 25, 1341–1346. [Google Scholar] [CrossRef]
- Meyer, C.H.; Rodrigues, E.B.; Mennel, S.; Schmidt, J.C.; Kroll, P. Spontaneous Separation of Epiretinal Membrane in Young Subjects: Personal Observations and Review of the Literature. Graefes Arch. Clin. Exp. Ophthalmol. Albrecht Graefes Arch. Klin. Exp. Ophthalmol. 2004, 242, 977–985. [Google Scholar] [CrossRef]
- Greven, C.M.; Slusher, M.M.; Weaver, R.G. Epiretinal Membrane Release and Posterior Vitreous Detachment. Ophthalmology 1988, 95, 902–905. [Google Scholar] [CrossRef]
- Mulligan, T.G.; Daily, M.J. Spontaneous Peeling of an Idiopathic Epiretinal Membrane in a Young Patient. Arch. Ophthalmol. 1992, 110, 1367–1368. [Google Scholar] [CrossRef]
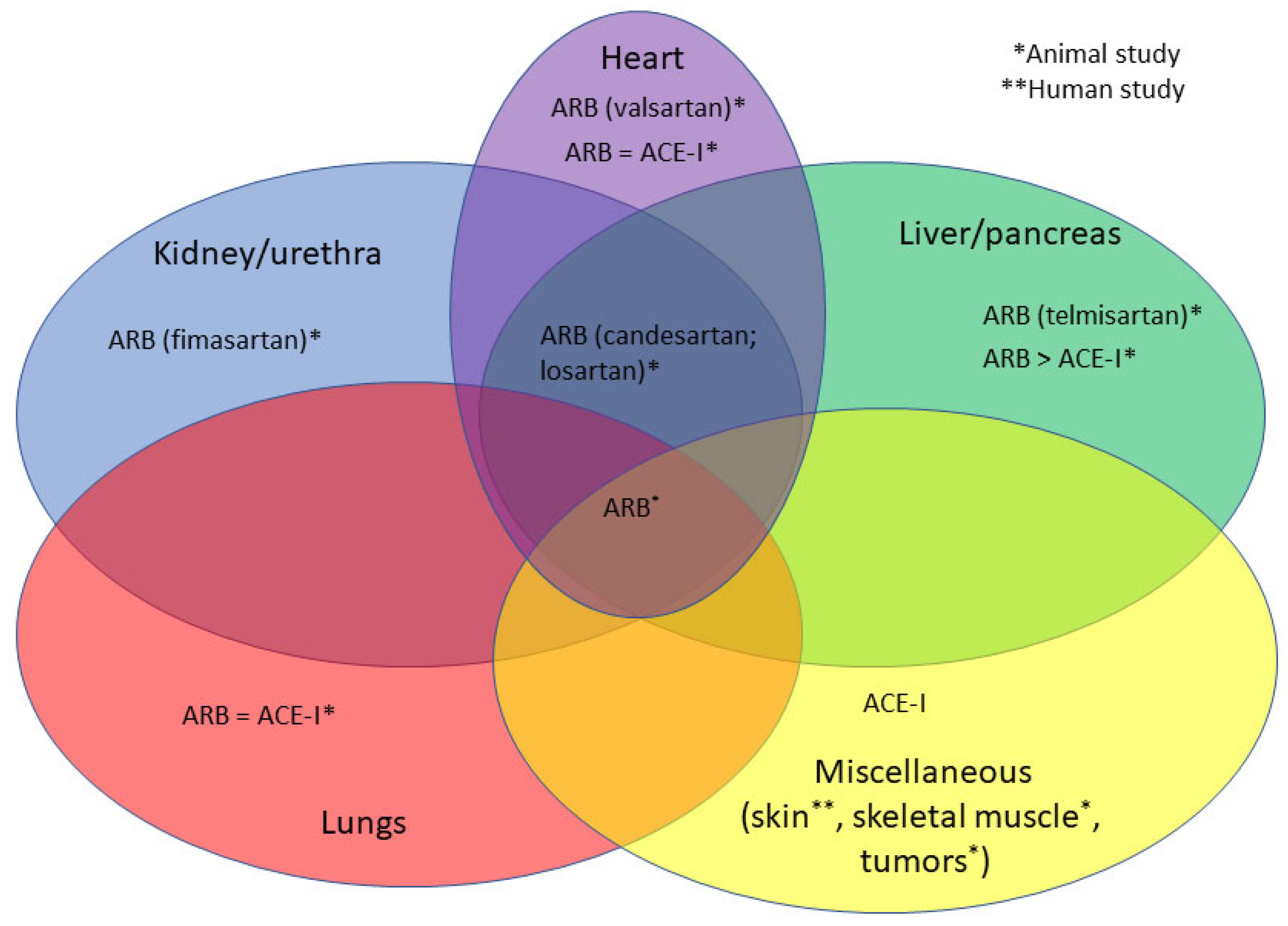
| Drugs Employed | Organs Involved |
|---|---|
| Candesartan [21,22,23,24,25,26] (and Temocapril [27]) | Heart ¥, pancreas ¥, liver ¥,*, stomach tumor § |
| Firmasartan [28] | Kidney §,§ |
| L-158809 (and captopril and enalapril and pioglitazone) [28,29,30] | Lung ¥, kidney ¥ |
| Losartan [20,31,32,33,34,35] | Heart ¥, kidney ¥, liver §, skeletal muscle §, lung ¥ |
| Losartan and irbesartan (and captopril and ramipril) [21]; (and calpain) [22]; (and rifaximin) [30]; (and obeticholic acid) [32]; (and valsartan) [23]; (or r ZD-7155) [36] | Liver ¥, pleura §, skeletal muscle § |
| Losartan-M6PHSA [37] | Liver ¥ |
| Olmesartan [37,38] | Liver ¥, lung § |
| Sacubitril and Valsartan [39,40] | Heart ¥,§ |
| Telmisartan [38] (and Losartan [24]) | Liver §,¥, lung * |
| Valsartan [27,41,42,43] | Pericardium ₤, heart ¥,§ |
| Various [44] | Skin * |
| N/A (ARBs and ACE-Is) [45,46] | Aortic valve * (post mortem), liver * |
| Drugs Employed | Organs Involved |
|---|---|
| Captopril [47,48,49,50] | Heart ¥, liver ¥, skin ¥ |
| Enalapril [51,52,53,54] (and PEG) | Heart ¥, colon §, kidney ¥, skin ‡ |
| Interferon-β and Perindopril [55] | Liver * |
| Lisinopril [56,57] | Pancreas ¥, skeletal muscle § |
| Ramipril [58,59] (and Losartan [18], hydralazine [26]) | Liver ¥, skin ¥,§ capsular tissue around breast implant ¥ |
| Moexiprilat [60] | Heart ¥ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Confalonieri, F.; Lumi, X.; Petrovski, G. Spontaneous Epiretinal Membrane Resolution and Angiotensin Receptor Blockers: Case Observation, Literature Review and Perspectives. Biomedicines 2023, 11, 1976. https://doi.org/10.3390/biomedicines11071976
Confalonieri F, Lumi X, Petrovski G. Spontaneous Epiretinal Membrane Resolution and Angiotensin Receptor Blockers: Case Observation, Literature Review and Perspectives. Biomedicines. 2023; 11(7):1976. https://doi.org/10.3390/biomedicines11071976
Chicago/Turabian StyleConfalonieri, Filippo, Xhevat Lumi, and Goran Petrovski. 2023. "Spontaneous Epiretinal Membrane Resolution and Angiotensin Receptor Blockers: Case Observation, Literature Review and Perspectives" Biomedicines 11, no. 7: 1976. https://doi.org/10.3390/biomedicines11071976
APA StyleConfalonieri, F., Lumi, X., & Petrovski, G. (2023). Spontaneous Epiretinal Membrane Resolution and Angiotensin Receptor Blockers: Case Observation, Literature Review and Perspectives. Biomedicines, 11(7), 1976. https://doi.org/10.3390/biomedicines11071976








