Role of Angiopoietic Coronary Endothelial Dysfunction in the Pathogenesis of Ischemic Cardiomyopathy
Abstract
1. Introduction
2. Methods
2.1. Clinical Characteristics of CHD Patients
2.2. Research Material
2.3. Immunophenotyping of EPCs and EDCs Using Flow Cytometry
2.4. Measurement of the Concentration of Mediators in the Blood
2.5. Statistical Analysis of Data
3. Results
3.1. Evaluation of the Balance of Destructive and Reparative Processes in the Coronary Vessels and in the Systemic Circulation in CHD Patients with and without ICMP
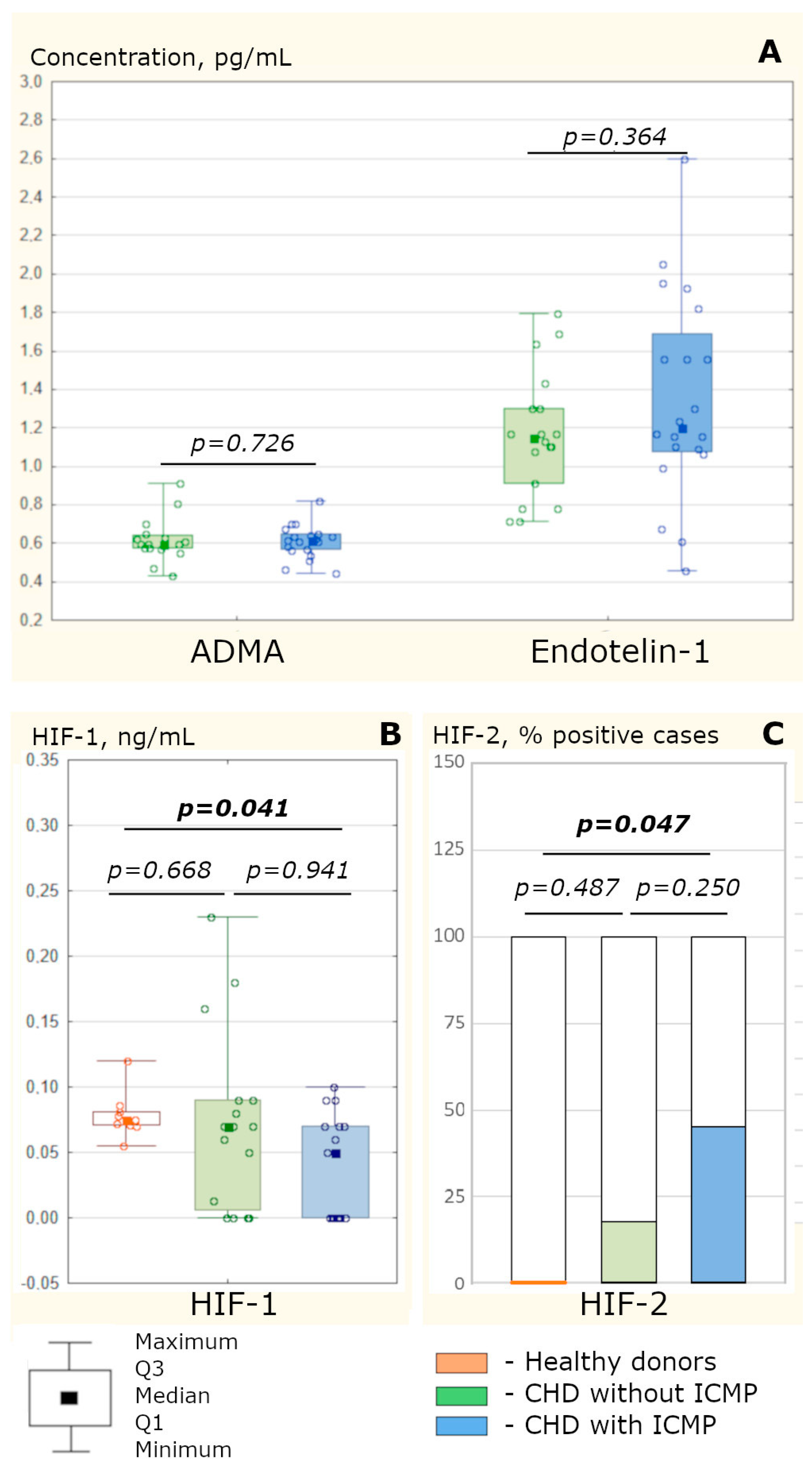
3.2. The Content of Vasomotor Endothelial Dysfunction Mediators in the Heart and the HIF-Dependent Tissue Response Imbalance to Hypoxia in CHD Patients, Suffering and Not Suffering from ICMP
3.3. Balance of the Mediators of Angiopoietic Endothelial Dysfunction in the Heart and Systemic Circulation of Patients with Coronary Heart Disease, Suffering and Not Suffering from ICMP
3.4. Interrelationship in the Balance between the Reparative and Destructive Processes in the Endothelium and HIFs-Dependent Mediator Response to Hypoxia with Factors of Angiopoietic and Vasomotor Forms of Endothelial Dysfunction in the Coronary and Systemic Circulation of CHD Patients, Suffering and Not Suffering from ICMP
4. Discussion
4.1. The Development of ICMP Is Not Accompanied by a More Pronounced Vasomotor Endothelial Dysfunction of the Coronary Vessels Compared to CHD without Cardiomyopathy
4.2. The Coronary Vessels’ Endothelium Desquamation in ICMP Is Increased, Which Is Not Accompanied by the EPC Mobilization from the Bone Marrow Due to the Absence of the Excess of SDF-1 and MCP-1 Chemoattractants in the Blood
4.3. HIF-1 and HIF-2 Imbalance in ICMP Does Not Affect VEGF-A Production but Reduces PDGF Production and VEGF-B Consumption in the Myocardium, Disrupting Vessels’ Early Stabilization
4.4. In ICMP, a High Content of Ang-2 and MMP-9 without an Adequate Increase in VEGF-A in the Myocardium Has a Destructive Effect on the Vessels Associated with Galectin-3 Hyperproduction in the Heart
4.5. The ICMP Pathogenesis Is Associated with Angiopoietic Endothelial Dysfunction, which Involves an Uncoordinated Mediator Response to Ischemia and Atherogenesis
5. Conclusions
6. Research Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Del Buono, M.G.; Moroni, F.; Montone, R.A.; Azzalini, L.; Sanna, T.; Abbate, A. Ischemic cardiomyopathy and heart failure after acute myocardial infarction. Curr. Cardiol. Rep. 2022, 24, 1505–1515. [Google Scholar] [CrossRef]
- Shipulin, V.M.; Pryakhin, A.S.; Andreev, S.L.; Chumakova, S.P.; Ryabova, T.R.; Stelmashenko, A.I.; Belyaeva, S.A.; Lelik, E.V. Modern clinical and fundamental aspects in the diagnosis and treatment of patients with ischemic cardiomyopathy (Review). Sib. J. Clin. Exp. Med. 2021, 36, 20–29. [Google Scholar] [CrossRef]
- Gyöngyösi, M.; Winkler, J.; Ramos, I.; Do, Q.; Firat, H.; McDonald, K.; González, A.; Thum, T.; Díez, J.; Jaisser, F.; et al. Myocardial fibrosis: Biomedical research from bench to bedside. Eur. J. Heart Fail. 2017, 19, 177–191. [Google Scholar] [CrossRef]
- Dang, H.; Ye, Y.; Zhao, X.; Zeng, Y. Identification of candidate genes in ischemic cardiomyopathy by gene expression omnibus database. BMC Cardiovasc. Disord. 2020, 20, 320. [Google Scholar] [CrossRef]
- Argunova, Y.; Belik, E.; Gruzdeva, O.; Ivanov, S.; Pomeshkina, S.; Barbarash, O. Effects of Physical Prehabilitation on the Dynamics of the Markers of Endothelial Function in Patients Undergoing Elective Coronary Bypass Surgery. J. Pers. Med. 2022, 12, 471. [Google Scholar] [CrossRef] [PubMed]
- Poston, R.N. Atherosclerosis: Integration of its pathogenesis as a self-perpetuating propagating inflammation: A review. Cardiovasc. Endocrinol. Metab. 2019, 8, 51–61. [Google Scholar] [CrossRef]
- Zhang, J. Biomarkers of endothelial activation and dysfunction in cardiovascular diseases. Rev. Cardiovasc. Med. 2022, 23, 73. [Google Scholar] [CrossRef] [PubMed]
- Melnikova, Y.S.; Makarova, T.P. Endothelial dysfunction as a central link in the pathogenesis of chronic diseases. Kazan Med. J. 2015, 96, 659–665. [Google Scholar] [CrossRef]
- Eligini, S.; Cosentino, N.; Fiorelli, S.; Fabbiocchi, F.; Niccoli, G.; Refaat, H.; Camera, M.; Calligaris, G.; De Martini, S.; Bonomi, A.; et al. Biological profile of monocyte-derived macrophages in coronary heart disease patients: Implications for plaque morphology. Sci. Rep. 2019, 9, 8680. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Jiang, J.; Chen, W.; Li, W.; Chen, Z. Vascular Macrophages in Atherosclerosis. J. Immunol. Res. 2019, 2019, 4354786. [Google Scholar] [CrossRef]
- Moroni, F.; Ammirati, E.; Norata, G.D.; Magnoni, M.; Camici, P.G. The Role of Monocytes and Macrophages in Human Atherosclerosis, Plaque Neoangiogenesis, and Atherothrombosis. Mediat. Inflamm. 2019, 2019, e7434376. [Google Scholar] [CrossRef] [PubMed]
- Chopra, H.; Hung, M.K.; Kwong, D.L.; Zhang, C.F.; Pow, E.H.N. Insights into endothelial progenitor cells: Origin, classification, potentials, and prospects. Stem Cells Int. 2018, 2018, 9847015. [Google Scholar] [CrossRef] [PubMed]
- Denisenko, O.A.; Chumakova, S.P.; Urazova, O.I. Endothelial progenitor cells: Origin and role in angiogenesis in cardiovascular pathology. Sib. J. Clin. Exp. Med. 2021, 36, 23–29. [Google Scholar] [CrossRef]
- Felker, G.M.; Shaw, G.M.; O’Connor, C.M. A standardized definition of ischemic cardiomyopathy for use in clinical research. J. Am. Coll. Cardiol. 2002, 39, 208–210. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, J.; Khondkar, W.; Morelli, M.B.; Wang, X.; Santulli, G.; Trimarco, V. Arginine and Endothelial Function. Biomedicines 2020, 8, 277. [Google Scholar] [CrossRef]
- Crea, F.; Montone, R.A.; Rinaldi, R. Pathophysiology of Coronary Microvascular Dysfunction. Circ J. 2022, 86, 1319–1328. [Google Scholar] [CrossRef]
- Dimova, I.; Karthik, S.; Makanya, A.; Hlushchuk, R.; Semela, D.; Volarevic, V.; Djonov, V. SDF-1/CXCR4 signalling is involved in blood vessel growth and remodelling by intussusception. J. Cell. Mol. Med. 2019, 23, 3916–3926. [Google Scholar] [CrossRef]
- Chertok, V.M.; Chertok, A.G.; Zenkina, V.G. Endothelium-dependent regulation of angiogenesis. Cytology 2017, 59, 243–258. [Google Scholar]
- Keshavarz, S.; Nassiri, S.M.; Siavashi, V.; Alimi, N.S. Regulation of plasticity and biological features of endothelial progenitor cells by MSC-derived SDF-1. Biochim. Biophys. Acta (BBA) Bioenerg. 2019, 1866, 296–304. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, H.; Guo, L.; Wang, S.; Cheng, W.; Wan, L.; Zhang, Z.; Xing, L.; Zhou, Q.; Yang, X.; et al. SDF-1 secreted by mesenchymal stem cells promotes the migration of endothelial progenitor cells via CXCR4/PI3K/AKT pathway. J. Mol. Histol. 2021, 52, 1155–1164. [Google Scholar] [CrossRef]
- Fujiyama, S.; Amano, K.; Uehira, K.; Yoshida, M.; Nishiwaki, Y.; Nozawa, Y.; Jin, D.; Takai, S.; Miyazaki, M.; Egashira, K.; et al. Bone Marrow Monocyte Lineage Cells Adhere on Injured Endothelium in a Monocyte Chemoattractant Protein-1–Dependent Manner and Accelerate Reendothelialization as Endothelial Progenitor Cells. Circ. Res. 2003, 93, 980–989. [Google Scholar] [CrossRef] [PubMed]
- Chumakova, S.; Urazova, O.; Vins, M.; Kolobovnikova, Y.; Churina, E.; Novitskiy, V.; Shipulin, V.; Pryakhin, A.; Sukhodolo, I.; Stelmashenko, A.; et al. Galectin 3 and non-classical monocytes of blood as myocardial remodeling factors at ischemic cardiomyopathy. IJC Heart Vasc. 2021, 33, 100766. [Google Scholar] [CrossRef]
- Zimna, A.; Kurpisz, M. Hypoxia-Inducible Factor-1 in Physiological and Pathophysiological Angiogenesis: Applications and Therapies. BioMed Res. Int. 2015, 2015, 549412. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Shen, H.; Shao, L.; Teng, X.; Chen, Y.; Liu, X.; Yang, Z.; Shen, Z. HIF-1α overexpression in mesenchymal stem cell-derived exosomes mediates cardioprotection in myocardial infarction by enhanced angiogenesis. Stem Cell Res. Ther. 2020, 11, 373. [Google Scholar] [CrossRef] [PubMed]
- Korbecki, J.; Simińska, D.; Gąssowska-Dobrowolska, M.; Listos, J.; Gutowska, I.; Chlubek, D.; Baranowska-Bosiacka, I. Chronic and Cycling Hypoxia: Drivers of Cancer Chronic Inflammation through HIF-1 and NF-κB Activation: A Review of the Molecular Mechanisms. Int. J. Mol. Sci. 2021, 22, 10701. [Google Scholar] [CrossRef]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef]
- Laakkonen, J.P.; Lähteenvuo, J.; Jauhiainen, S.; Heikura, T.; Ylä-Herttuala, S. Beyond endothelial cells: Vascular endothelial growth factors in heart, vascular anomalies and placenta. Vas. Pharmacol. 2019, 112, 91–101. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, X.; Cui, H.; Shi, J.; Yuan, G.; Shi, S.; Hu, Y. The Role of the VEGF Family in Coronary Heart Disease. Front. Cardiovasc. Med. 2021, 24, 738325. [Google Scholar] [CrossRef]
- Bowler, E.; Oltean, S. Alternative Splicing in Angiogenesis. Int. J. Mol. Sci. 2019, 20, 2067. [Google Scholar] [CrossRef]
- Sil, S.; Periyasamy, P.; Thangaraj, A.; Chivero, E.T.; Buch, S. PDGF/PDGFR axis in the neural systems. Mol. Aspects Med. 2018, 62, 63–74. [Google Scholar] [CrossRef]
- Marushima, A.; Nieminen, M.; Kremenetskaia, I.; Gianni-Barrera, R.; Woitzik, J.; von Degenfeld, G.; Banfi, A.; Vajkoczy, P.; Hecht, N. Balanced single-vector co-delivery of VEGF/PDGF-BB improves functional collateralization in chronic cerebral ischemia. J. Cereb. Blood Flow Metab. 2020, 40, 404–419. [Google Scholar] [CrossRef]
- Zhou, J.; Shao, L.; Yu, J.; Huang, J. Fengcorresponding Q PDGF-BB promotes vascular smooth muscle cell migration by enhancing Pim-1 expression via inhibiting miR-214. Ann. Transl. Med. 2021, 9, 1728. [Google Scholar] [CrossRef]
- Ha, J.M.; Jin, S.Y.; Lee, H.S.; Kum, H.J.; Vafaeinik, F.; Ha, H.K.; Song, S.H.; Kim, C.D.; Bae, S.S. Akt1-dependent expression of angiopoietin 1 and 2 in vascular smooth muscle cells leads to vascular stabilization. Exp. Mol. Med. 2022, 54, 1133–1145. [Google Scholar] [CrossRef]
- Lee, S.-J.; Lee, C.-K.; Kang, S.; Park, I.; Kim, Y.H.; Kim, S.K.; Hong, S.P.; Bae, H.; He, Y.; Kubota, Y.; et al. Angiopoietin-2 exacerbates cardiac hypoxia and inflammation after myocardial infarction. J. Clin. Investig. 2018, 128, 5018–5033. [Google Scholar] [CrossRef]
- Jaipersad, A.S.; Lip, G.Y.H.; Silverman, S.; Shantsila, E. The Role of Monocytes in Angiogenesis and Atherosclerosis. J. Am. Coll. Cardiol. 2014, 63, A22. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, C.T.; Bhargava, M.; Torzilli, P.A. A Comparative Study of Fibronectin Cleavage by MMP-1, -3, -13, and -14. Cartilage 2012, 3, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, H.; Ivaska, J. Vascular Morphogenesis: An Integrin and Fibronectin Highway. Curr Biol. 2017, 27, R158–R161. [Google Scholar] [CrossRef] [PubMed]
- Blanda, V.; Bracale, U.M.; Di Taranto, M.D.; Fortunato, G. Galectin-3 in Cardiovascular Diseases. Int. J. Mol. Sci. 2020, 21, 9232. [Google Scholar] [CrossRef] [PubMed]
- Calvier, L.; Miana, M.; Reboul, P.; Cachofeiro, V.; Martinez-Martinez, E.; de Boer, R.A.; Poirier, F.; Lacolley, P.; Zannad, F.; Rossignol, P.; et al. Galectin-3 mediates aldosterone-induced vascular fibrosis. Atheroscler. Thromb. Vasc. Biol. 2013, 33, 67–75. [Google Scholar] [CrossRef]
- Zhao, H.-Y.; Zhu, Y.-P.; Wen, Y.; Ding, X.-Y.; Sun, J.; Ji, R.-P.; Han, Q.-J.; Li, L.-Y. MCP-1 facilitates VEGF production by removing miR-374b-5p blocking of VEGF mRNA translation. Biochem. Pharmacol. 2022, 206, 115334. [Google Scholar] [CrossRef]


| Clinical Parameters | CHD Patients without ICMP | CHD Patients with ICMP | p |
|---|---|---|---|
| Number of patients: | 40 | 47 | - |
| male | 33 (82.50%) | 42(89.36%) | 0.540 |
| female | 7 (17.50%) | 5(10.64%) | 0.540 |
| Age, years | 64.5 [58.5; 68.0] | 61.0 [57.0; 64.5] | 0.123 |
| CHD duration, years | 5.00 [2.50; 9.00] | 3.50 [1.00; 6.50] | 0.264 |
| Body mass index, kg/m2 | 29.48 [26.33; 32.51] | 28.07 [26.69; 31.18] | 0.650 |
| Functional class of angina | |||
| II | 7 (17.50%) | 11 (23.40%) | 0.680 |
| of effort | |||
| III | 29 (72.50%) | 31 (65.96%) | 0.671 |
| IV | 4 (10.00%) | 5 (10.64%) | 0.798 |
| Heart failure I NYHA | 4 (10.00%) | 3 (6.38%) | 0.824 |
| Heart failure II NYHA | 16 (40.00%) | 28 (59.57%) | 0.109 |
| Heart failure III NYHA | 20 (50.00%) | 16 (34.04%) | 0.198 |
| LV ejection fraction, % | 59.25 [50.00; 67.50] | 31.50 [23.25; 36.50] | <0.001 |
| Eventual systolic index of LV, mL/m2 | 52.90 [50.20; 56.90] | 75.30 [64.30; 82.30] | 0.004 |
| LV myocardium mass, g | 184.0 [140.5; 214.5] | 233.0 [221.7; 266.2] | <0.001 |
| Statin therapy | 34 (85.00%) | 39 (82.98%) | 0.971 |
| Hypertensive disease stage III | 33 (82.50%) | 32 (68.09%) | 0.196 |
| Type 2 diabetes mellitus | 13 (32.50%) | 4 (8.51%) | 0.011 |
| Gastric and/or duodenal ulcer | 9 (22.50%) | 5 (10.64%) | 0.227 |
| Diseases of liver and biliary tract | 5 (12.50%) | 3 (6.38%) | 0.541 |
| Chronic kidney disease | 9 (22.50%) | 15 (31.91%) | 0.460 |
| Pulmonary diseases | 6 (15.00%) | 8 (17.02%) | 0.971 |
| Blood Parameters | Healthy Donors | CHD Patients without ICMP | CHD Patients with ICMP |
|---|---|---|---|
| Blood from the Cubital Vein (Peripheral Blood) | |||
| EDC, ×105/L | 5.12 [3.73; 5.84] | 7.25 [6.80; 7.47] Pc = 0.038 | 7.26 [5.43; 17.94] Pc = 0.037 P2 = 0.597 |
| EPC, % | 4.10 [2.70; 5.00] | 6.63 [4.70; 13.00] Pc = 0.042 | 4.93 [2.20; 7.30] Pc = 0.369 P2 = 0.678 |
| VEGF-A, pg/mL | 3.80 [1.00; 6.50] | 4.50 [3.00; 8.00] Pc = 0.314 | 6.00 [3.00; 9.50] Pc = 0.216 P2 = 0.502 |
| VEGF-B, pg/mL | 1.32 [1.00; 3.10] | 1.60 [1.27; 2.20] Pc = 0.772 | 1.30 [1.00; 1.45] Pc = 1.000 P2 = 0.570 |
| PDGF, pg/mL | 2.68 [1.65; 7.10] | 3.10 [2.10; 7.05] Pc = 1.000 | 4.85 [1.20; 9.10] Pc = 1.000 P2 = 0.870 |
| SDF-1, pg/mL | 30.00 [5.00; 45.00] | 60.00 [50.00; 80.00] Pc = 0.042 | 49.00 [37.00; 56.00] Pc = 0.174 P2 = 0.115 |
| MCP-1, pg/mL | 190.0 [140.0; 240.0] | 210.0 [144.4; 268.0] Pc = 0.612 | 202.5 [164.0; 324.0] Pc = 0.864 P2 = 0.527 |
| Galectin-3, ng/mL | 6.50 [5.60; 7.64] | 6.10 [4.30; 7.48] Pc = 0.928 | 8.20 [7.20; 10.00] Pc = 0.025 P2 = 0.017 |
| Angiopoetin-2, pg/mL | 388.0 [317.0; 460.0] | 445.0 [137.5; 552.5] Pc = 1.000 | 430.0 [380.0; 580.0] Pc = 1.000 P2 = 0.971 |
| MMP-9, pg/mL | 13.20 [9.60; 19.00] | 11.95 [7.00; 13.40] Pc = 0.460 | 13.65 [6.50; 19.60] Pc = 0.848 P2 = 0.588 |
| Blood from the coronary sinus (sinus blood) | |||
| EDC, ×105/L | – | 10.17 [6.80; 18.83] P1 = 0.128 | 17.98 [10.27; 22.97] P1 = 0.036 P2 = 0.156 |
| VEGF-A, pg/mL | – | 7.80 [3.25; 9.75] P1 = 0.041 | 6.89 [3.25; 15.60] P1 = 0.007 P2 = 0.918 |
| VEGF-B, pg/mL | – | 1.00 [0.85; 1.36] P1 = 0.011 | 1.02 [0.89; 1.08] P1 = 0.285 P2 = 0.762 |
| PDGF, pg/mL | – | 7.60 [3.70; 9.94] P1 = 0.036 | 7.86 [2.92; 8.77] P1 = 0.674 P2 = 0.736 |
| SDF-1, pg/mL | – | 40.30 [26.00; 62.00] P1 = 0.086 | 46.80 [32.50; 64.00] P1 = 0.286 P2 = 0.623 |
| MCP-1, pg/mL | – | 227.5 [135.2; 331.5] P1 = 0.209 | 242.5 [176.2; 321.8] P1 = 0.585 P2 = 0.638 |
| Galectin-3, ng/mL | – | 13.13 [10.14; 15.86] P1 < 0.001 | 20.15 [14.17; 60.06] P1 < 0.001 P2 = 0.012 |
| Angiopoetin-2, pg/mL | – | 767.0 [494.0; 988.0] P1 = 0.128 | 1111.5 [845.0; 1235.0] P1 < 0.001 P2 = 0.002 |
| MMP-9, pg/mL | – | 5.92 [5.07; 17.42] P1 = 0.972 | 16.64 [6.63; 29.12] P1 = 0.649 P2 = 0.038 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chumakova, S.P.; Urazova, O.I.; Shipulin, V.M.; Andreev, S.L.; Denisenko, O.A.; Gladkovskaya, M.V.; Litvinova, L.S.; Bubenchikov, M.A. Role of Angiopoietic Coronary Endothelial Dysfunction in the Pathogenesis of Ischemic Cardiomyopathy. Biomedicines 2023, 11, 1950. https://doi.org/10.3390/biomedicines11071950
Chumakova SP, Urazova OI, Shipulin VM, Andreev SL, Denisenko OA, Gladkovskaya MV, Litvinova LS, Bubenchikov MA. Role of Angiopoietic Coronary Endothelial Dysfunction in the Pathogenesis of Ischemic Cardiomyopathy. Biomedicines. 2023; 11(7):1950. https://doi.org/10.3390/biomedicines11071950
Chicago/Turabian StyleChumakova, Svetlana P., Olga I. Urazova, Vladimir M. Shipulin, Sergey L. Andreev, Olga A. Denisenko, Margarita V. Gladkovskaya, Larisa S. Litvinova, and Mikhail A. Bubenchikov. 2023. "Role of Angiopoietic Coronary Endothelial Dysfunction in the Pathogenesis of Ischemic Cardiomyopathy" Biomedicines 11, no. 7: 1950. https://doi.org/10.3390/biomedicines11071950
APA StyleChumakova, S. P., Urazova, O. I., Shipulin, V. M., Andreev, S. L., Denisenko, O. A., Gladkovskaya, M. V., Litvinova, L. S., & Bubenchikov, M. A. (2023). Role of Angiopoietic Coronary Endothelial Dysfunction in the Pathogenesis of Ischemic Cardiomyopathy. Biomedicines, 11(7), 1950. https://doi.org/10.3390/biomedicines11071950







