Role of Macrophage lncRNAs in Mediating Inflammatory Processes in Atherosclerosis and Sepsis
Abstract
1. Introduction
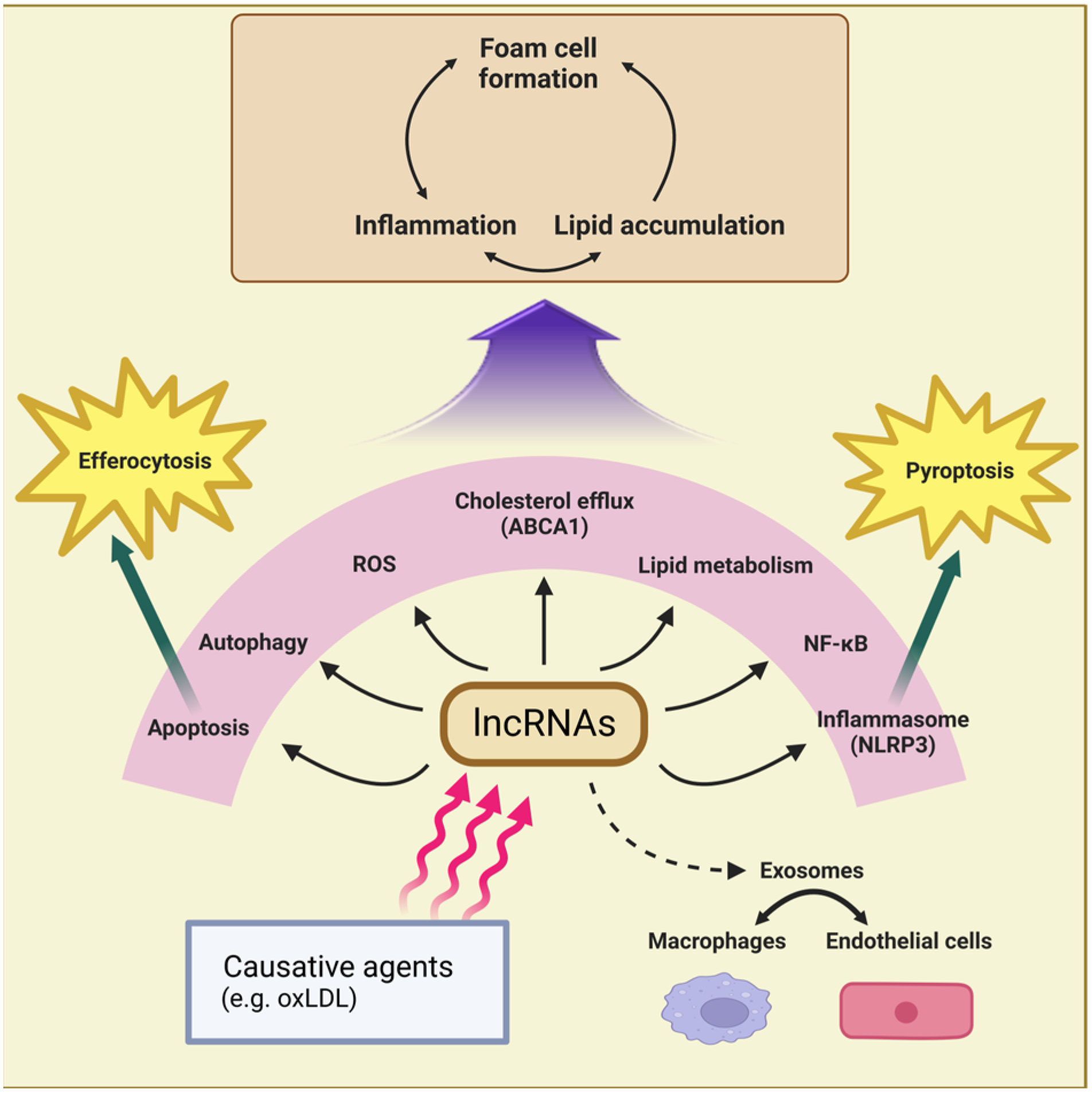
2. Atherosclerosis
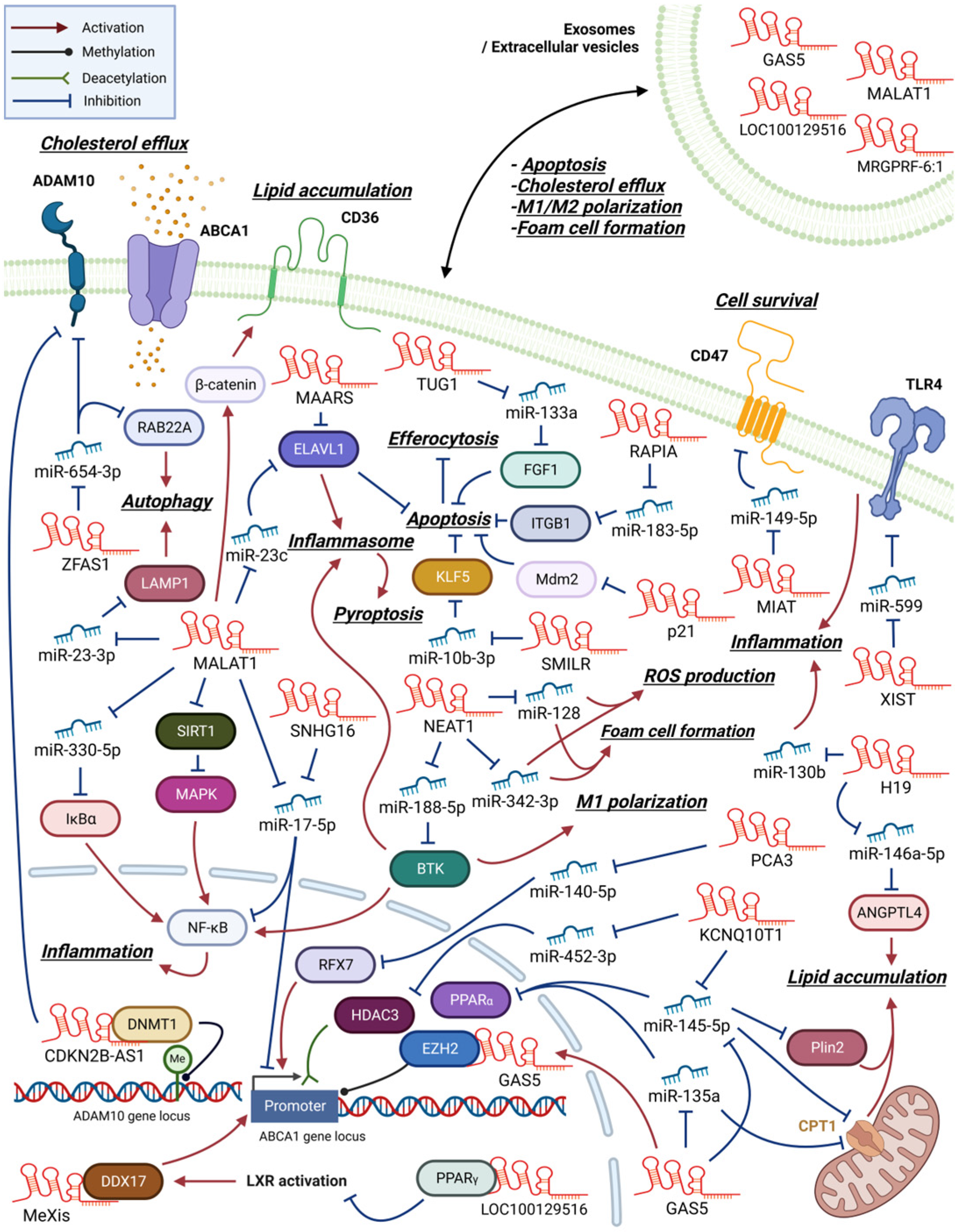
2.1. LncRNAs That Promote Inflammation and Foam Cell Formation
2.2. LncRNAs That Regulate Cholesterol Efflux and Foam Cell Formation
2.3. LncRNAs That Regulate Macrophage Apoptosis, Pyroptosis, or Autophagy in Atherosclerosis
| LncRNA | Cell Type | Target | Affected Function | Ref. |
|---|---|---|---|---|
| Group 1. LncRNAs that regulate inflammation and foam cell formation | ||||
| H19 | - | miR-130b | Modulates cell survival, lipid accumulation, and inflammatory response | [24] |
| H19 | Foam cell | miR-146a-5p/ANGPTL4 | ox-LDL-induced, regulates lipid accumulation, accelerates foam cell formation | [23] |
| Dnm3os | - | miR-27b-3p/SLAMF7 | Streptosotocin-induced, intermedin-repressed, participates in ox-LDL absorption | [33] |
| KCNQ10T1 | - | miR-145-5p/PPARα | ox-LDL-induced, participates in lipid metabolism and inflammation | [42] |
| MALAT1 | Foam cell | miR-330-5p/IκBα | Activates NF-κB pathway, enhances lipid accumulation and inflammation | [43] |
| MALAT1 | - | - | MALAT1-deficient apoE−/− mice exhibit enhanced inflammation and atherosclerosis | [44] |
| MALAT1 | Foam cell | β-catenin/CD36 | ox-LDL-induced, induces lipid uptake | [45] |
| MALAT1 | Foam cell | SIRT1/MAPK/NF-κB | ox-LDL-induced, inhibits activation of NF-κB pathway | [46] |
| NEAT1 | - | NONO and p65 | ox-LDL-induced, mediates paraspeckle formation, regulates lipid uptake | [27] |
| NEAT1 | M1 | miR-188-5p or KLF4/BTK | Activates NLRP3 inflammasome and NF-κB pathway, induces M1 polarization | [30] |
| NEAT1 | Foam cell | miR-128 | Facilitates inflammation, oxidative stress responses and foam cell formation | [28] |
| NEAT1 | - | miR-342-3p | ox-LDL-induced, regulates inflammation and lipid uptake | [29] |
| NEXN-AS1 | - | BAZ1A/NEXN | Attenuates adhesion activity of macrophage, suppresses inflammatory gene expression | [47] |
| SNHG16 | Foam cell | miR-17-5p/NF-κB | ox-LDL-induced, enhances cell proliferation and inflammatory responses | [48] |
| UCA1 | Foam cell | miR-206 | ox-LDL-induced, facilitates foam cell formation, regulates ROS levels | [32] |
| Group 2. LncRNAs that regulate cholesterol efflux and foam cell formation | ||||
| AI662270 | Foam cell | ABCA1 | ox-LDL-induced, suppresses cholesterol efflux, enhances lipid accumulation | [41] |
| CDKN2B-AS1 | Foam cell | DNMT1/ADAM10 | Promotes cholesterol efflux, reduces inflammatory responses | [49] |
| GAS5 | Foam cell | EZH2/ABCA1 | Inhibits cholesterol efflux, enhances lipid accumulation and atherogenesis | [38] |
| HAND2-AS1 | Foam cell | miR-1208/SIRT1/ABCA1 | Inhibits foam cell formation, accelerates cholesterol efflux, attenuates atherosclerosis | [37] |
| KCNQ1OT1 | - | miR-452-3p/HDAC3/ABCA1 | ox-LDL-induced, inhibits cholesterol efflux, facilitates foam cell formation | [40] |
| MALAT1 | Foam cell | miR-17-5p/ABCA1 | Decreases in ox-LDL induced THP-1 derived macrophage, inhibits cholesterol efflux | [50] |
| MeXis | Foam cell | DDX17/ABCA1 | LXR-induced, remodels chromatin at the target locus, induces cholesterol efflux | [35] |
| PCA3 | Foam cell | miR-140-5p/RFX7ABCA1 | promotes cholesterol efflux, inhibits atherosclerosis progression | [36] |
| TUG1 | Foam cell | miR-92a/FXR1 | Regulates apolipoprotein M, downregulates cholesterol efflux, aggravates atherosclerosis | [51] |
| ZFAS1 | Foam cell | miR-654-3p/ADAM10 miR-654-3p/RAB22A | ox-LDL-induced, activates inflammation, inhibits cholesterol efflux | [52] |
| Group 3. LncRNAs that regulate apoptosis, pyroptosis, and autophagy | ||||
| ANRIL | - | Alu repeats etc. | Increases proliferation, enhances metabolic activity, decreases apoptosis | [53] |
| GAS5 | Foam cell | miR-145-5p/Plin2 | Facilitates oxLDL uptake and autophagy, accelerates foam cell formation | [54] |
| MIAT | - | miR-149-5p/CD47 | ox-LDL-induced, inhibits efferocytosis of macrophage, accelerates atherogenic process | [55] |
| MALAT1 | - | miR-23c/ELAVL1 | ox-LDL-induced, activates NLRP3 inflammasome mediate pyroptosis | [56] |
| MAARS | - | ELAVL1(HuR) | Activates HuR target gene expression, promotes apoptosis, decreases efferocytosis | [57] |
| MALAT1 | - | miR-23-3p/LAMP1 | Rapa induced, facilitates autophagy activity | [58] |
| p21 | - | Mdm2 | Inhibits cell proliferation, induces apoptosis, regulates p300/p53 interaction | [59] |
| TUG1 | - | miR-133a/FGF1 | Facilitates cell proliferation, activates inflammation, inhibits apoptosis | [60] |
| XIST | - | miR-599/TLR4 | ox-LDL-induced, inhibits apoptosis, aggravates atherosclerosis progression | [61] |
| RAPIA | - | miR-183-5p/ITGB1 | Induced by the action of FoxO1, activates cell proliferation, inhibits apoptosis | [62] |
| SMILR | - | miR-10b-3p/KLF5 | ox-LDL-induced, activates cell proliferation, inhibits apoptosis | [63] |
| Group 4. LncRNAs that acts through exosomes | ||||
| GAS5 | - | p53 | Produced by macrophages, induces apoptosis in macrophages and vascular endothelial cells | [64] |
| MALAT1 | M2 | - | Exported via ox-LDL-induced HUVEC-derived exosome, induces M2 polarization | [65] |
| MALAT1 | M1 | miR-25-3p/CDC42 | Affects endothelial cells. Inhibits angiogenesis and myocardial regeneration, aggravates MI | [66] |
| MRGPRF-6:1 | M1 | TLR4/MyD88/MAPK | Enhances foam cell formation and M1 polarization. Detected in the plasma exosomes | [67] |
| LOC100129516 | Foam cell | PPARγ/LXRα/ABCA1 | MSC-derived exosomes, inhibits foam cell cholesterol efflux, aggravates atherosclerosis | [68] |
2.4. LncRNAs Functioning via Exosomes in Atherogenesis
2.5. Multiple Function of MALAT1 in Atherogenesis
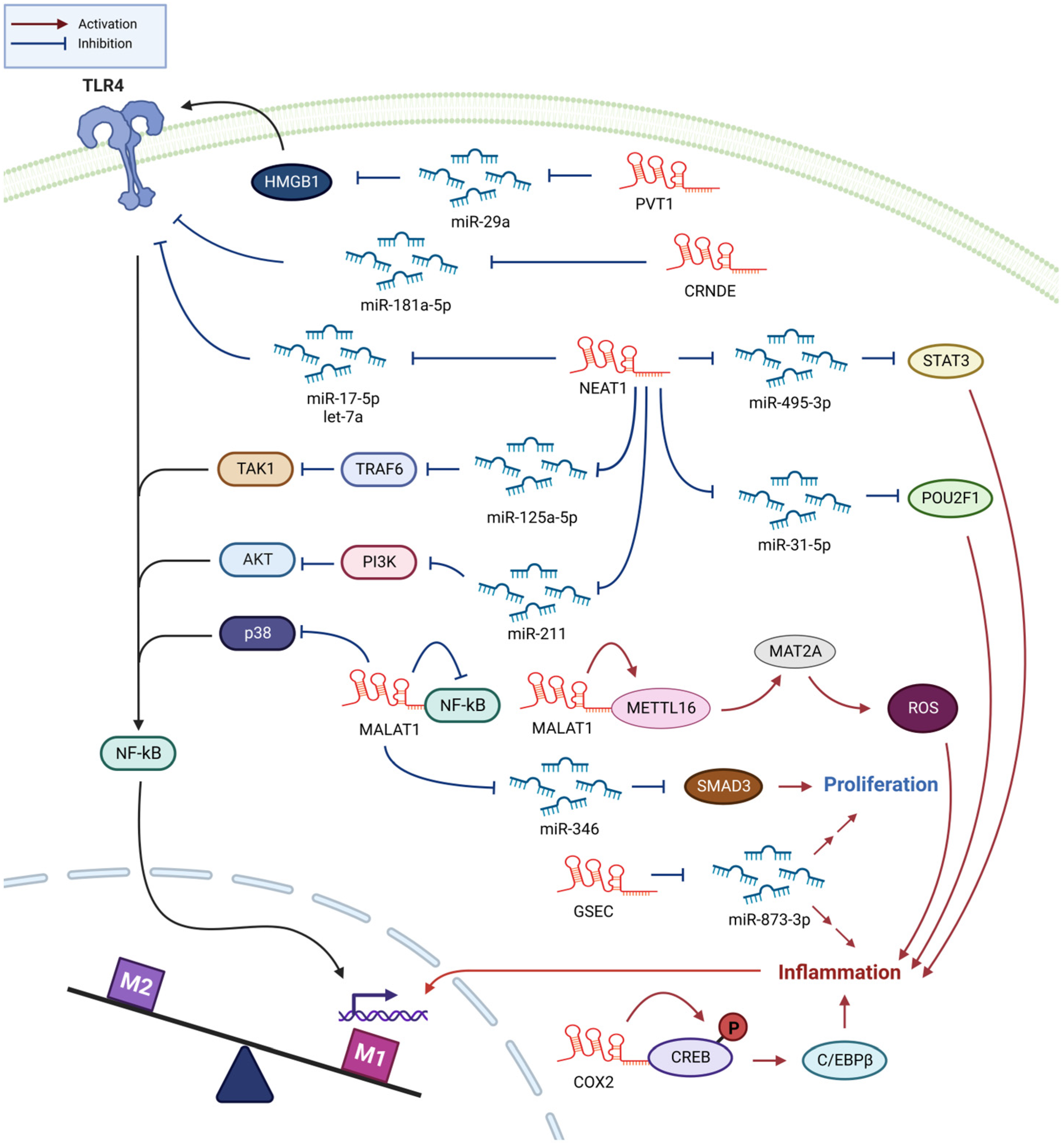
3. Sepsis
3.1. NEAT1 Enhances Sepsis Progression through Promoting Inflammation
3.2. MALAT1 Promotes M1 Polarization and Inflammation in Sepsis
3.3. Other lncRNAs Involved in Sepsis Development
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Winkle, M.; El-Daly, S.M.; Fabbri, M.; Calin, G.A. Noncoding RNA therapeutics—Challenges and potential solutions. Nat. Rev. Drug Discov. 2021, 20, 629–651. [Google Scholar] [CrossRef] [PubMed]
- Herman, A.B.; Tsitsipatis, D.; Gorospe, M. Integrated lncRNA function upon genomic and epigenomic regulation. Mol. Cell 2022, 82, 2252–2266. [Google Scholar] [CrossRef]
- Huang, W.; Li, H.; Yu, Q.; Xiao, W.; Wang, D.O. LncRNA-mediated DNA methylation: An emerging mechanism in cancer and beyond. J. Exp. Clin. Cancer Res. 2022, 41, 100. [Google Scholar] [CrossRef]
- Senmatsu, S.; Hirota, K. Roles of lncRNA transcription as a novel regulator of chromosomal function. Genes Genet. Syst. 2021, 95, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, L.; Ding, Y.; Lu, X.; Zhang, G.; Yang, J.; Zheng, H.; Wang, H.; Jiang, Y.; Xu, L. LncRNA Structural Characteristics in Epigenetic Regulation. Int. J. Mol. Sci. 2017, 18, 2659. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Sun, W.; Guo, Z.; Zhang, J.; Yu, H.; Liu, B. Mechanisms of lncRNA/microRNA interactions in angiogenesis. Life Sci. 2020, 254, 116900. [Google Scholar] [CrossRef] [PubMed]
- Ferrè, F.; Colantoni, A.; Helmer-Citterich, M. Revealing protein-lncRNA interaction. Brief. Bioinform. 2016, 17, 106–116. [Google Scholar] [CrossRef]
- Khorkova, O.; Hsiao, J.; Wahlestedt, C. Basic biology and therapeutic implications of lncRNA. Adv. Drug Deliv. Rev. 2015, 87, 15–24. [Google Scholar] [CrossRef]
- Mathieu, E.L.; Belhocine, M.; Dao, L.T.; Puthier, D.; Spicuglia, S. Functions of lncRNA in development and diseases. Med. Sci. 2014, 30, 790–796. [Google Scholar] [CrossRef]
- Peng, W.X.; Koirala, P.; Mo, Y.Y. LncRNA-mediated regulation of cell signaling in cancer. Oncogene 2017, 36, 5661–5667. [Google Scholar] [CrossRef]
- Orecchioni, M.; Ghosheh, Y.; Pramod, A.B.; Ley, K. Macrophage Polarization: Different Gene Signatures in M1(LPS+) vs. Classically and M2(LPS-) vs. Alternatively Activated Macrophages. Front. Immunol. 2019, 10, 1084. [Google Scholar] [CrossRef]
- Tardito, S.; Martinelli, G.; Soldano, S.; Paolino, S.; Pacini, G.; Patane, M.; Alessandri, E.; Smith, V.; Cutolo, M. Macrophage M1/M2 polarization and rheumatoid arthritis: A systematic review. Autoimmun. Rev. 2019, 18, 102397. [Google Scholar] [CrossRef]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef]
- Bashir, S.; Sharma, Y.; Elahi, A.; Khan, F. Macrophage polarization: The link between inflammation and related diseases. Inflamm. Res. 2016, 65, 1–11. [Google Scholar] [CrossRef]
- Funes, S.C.; Rios, M.; Escobar-Vera, J.; Kalergis, A.M. Implications of macrophage polarization in autoimmunity. Immunology 2018, 154, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Li, X. Regulatory Mechanism of lncRNAs in M1/M2 Macrophages Polarization in the Diseases of Different Etiology. Front. Immunol. 2022, 13, 835932. [Google Scholar] [CrossRef] [PubMed]
- Liao, K.; Xu, J.; Yang, W.; You, X.; Zhong, Q.; Wang, X. The research progress of LncRNA involved in the regulation of inflammatory diseases. Mol. Immunol. 2018, 101, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Tabas, I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat. Rev. Immunol. 2010, 10, 36–46. [Google Scholar] [CrossRef]
- Gibson, M.S.; Domingues, N.; Vieira, O.V. Lipid and Non-lipid Factors Affecting Macrophage Dysfunction and Inflammation in Atherosclerosis. Front. Physiol. 2018, 9, 654. [Google Scholar] [CrossRef]
- Momtazi-Borojeni, A.A.; Abdollahi, E.; Nikfar, B.; Chaichian, S.; Ekhlasi-Hundrieser, M. Curcumin as a potential modulator of M1 and M2 macrophages: New insights in atherosclerosis therapy. Heart Fail. Rev. 2019, 24, 399–409. [Google Scholar] [CrossRef]
- Oh, J.; Riek, A.E.; Weng, S.; Petty, M.; Kim, D.; Colonna, M.; Cella, M.; Bernal-Mizrachi, C. Endoplasmic reticulum stress controls M2 macrophage differentiation and foam cell formation. J. Biol. Chem. 2012, 287, 11629–11641. [Google Scholar] [CrossRef] [PubMed]
- Bitarafan, S.; Yari, M.; Broumand, M.A.; Ghaderian, S.M.H.; Rahimi, M.; Mirfakhraie, R.; Azizi, F.; Omrani, M.D. Association of Increased Levels of lncRNA H19 in PBMCs with Risk of Coronary Artery Disease. Cell J. 2019, 20, 564–568. [Google Scholar] [CrossRef]
- Huang, S.F.; Zhao, G.; Peng, X.F.; Ye, W.C. The Pathogenic Role of Long Non-coding RNA H19 in Atherosclerosis via the miR-146a-5p/ANGPTL4 Pathway. Front. Cardiovasc. Med. 2021, 8, 770163. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Ma, J.; Wang, J.; Wang, L. Silencing of H19 inhibits the adipogenesis and inflammation response in ox-LDL-treated Raw264.7 cells by up-regulating miR-130b. Mol. Immunol. 2018, 93, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.; Qamar, A.; Qu, L.; Qasim, A.N.; Mehta, N.N.; Reilly, M.P.; Rader, D.J. Differential association of plasma angiopoietin-like proteins 3 and 4 with lipid and metabolic traits. Arter. Thromb. Vasc. Biol. 2014, 34, 1057–1063. [Google Scholar] [CrossRef]
- Naganuma, T.; Nakagawa, S.; Tanigawa, A.; Sasaki, Y.F.; Goshima, N.; Hirose, T. Alternative 3′-end processing of long noncoding RNA initiates construction of nuclear paraspeckles. EMBO J. 2012, 31, 4020–4034. [Google Scholar] [CrossRef]
- Huang-Fu, N.; Cheng, J.S.; Wang, Y.; Li, Z.W.; Wang, S.H. Neat1 regulates oxidized low-density lipoprotein-induced inflammation and lipid uptake in macrophages via paraspeckle formation. Mol. Med. Rep. 2018, 17, 3092–3098. [Google Scholar] [CrossRef]
- Chen, D.D.; Hui, L.L.; Zhang, X.C.; Chang, Q. NEAT1 contributes to ox-LDL-induced inflammation and oxidative stress in macrophages through inhibiting miR-128. J. Cell. Biochem. 2018, 120, 2493–2501. [Google Scholar] [CrossRef]
- Wang, L.; Xia, J.W.; Ke, Z.P.; Zhang, B.H. Blockade of NEAT1 represses inflammation response and lipid uptake via modulating miR-342-3p in human macrophages THP-1 cells. J. Cell. Physiol. 2019, 234, 5319–5326. [Google Scholar] [CrossRef]
- Lin, S.; Wen, Z.; Li, S.; Chen, Z.; Li, C.; Ouyang, Z.; Lin, C.; Kuang, M.; Xue, C.; Ding, Y. LncRNA Neat1 promotes the macrophage inflammatory response and acts as a therapeutic target in titanium particle-induced osteolysis. Acta Biomater. 2022, 142, 345–360. [Google Scholar] [CrossRef]
- Gast, M.; Rauch, B.H.; Haghikia, A.; Nakagawa, S.; Haas, J.; Stroux, A.; Schmidt, D.; Schumann, P.; Weiss, S.; Jensen, L.; et al. Long noncoding RNA NEAT1 modulates immune cell functions and is suppressed in early onset myocardial infarction patients. Cardiovasc. Res. 2019, 115, 1886–1906. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Ma, R.; Fu, W.; Zhang, C.; Du, X. LncRNA UCA1 sponges miR-206 to exacerbate oxidative stress and apoptosis induced by ox-LDL in human macrophages. J. Cell. Physiol. 2019, 234, 14154–14160. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Guan, P.; Li, D.; Hang, Y.; Ye, X.; Han, L.; Lu, Y.; Bai, X.; Zhang, P.; Hu, W. Intermedin attenuates macrophage phagocytosis via regulation of the long noncoding RNA Dnm3os/miR-27b-3p/SLAMF7 axis in a mouse model of atherosclerosis in diabetes. Biochem. Biophys. Res. Commun. 2021, 583, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Gu, M.; Jia, X.; Wang, X.; Wu, C.; Guo, J.; Zhang, L.; Du, Y.; Wang, J. Integrated DNA methylation and gene expression analysis identifies SLAMF7 as a key regulator of atherosclerosis. Aging 2018, 10, 1324–1337. [Google Scholar] [CrossRef] [PubMed]
- Sallam, T.; Jones, M.; Thomas, B.J.; Wu, X.; Gilliland, T.; Qian, K.; Eskin, A.; Casero, D.; Zhang, Z.; Sandhu, J.; et al. Transcriptional regulation of macrophage cholesterol efflux and atherogenesis by a long noncoding RNA. Nat. Med. 2018, 24, 304–312. [Google Scholar] [CrossRef]
- Zhao, Z.W.; Zhang, M.; Liao, L.X.; Zou, J.; Wang, G.; Wan, X.J.; Zhou, L.; Li, H.; Qin, Y.S.; Yu, X.H.; et al. Long non-coding RNA PCA3 inhibits lipid accumulation and atherosclerosis through the miR-140-5p/RFX7/ABCA1 axis. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 158904. [Google Scholar] [CrossRef]
- Ma, L.; He, S.; Li, H.; Zhang, S.; Yin, Y. HAND2-AS1 targeting miR-1208/SIRT1 axis alleviates foam cell formation in atherosclerosis. Int. J. Cardiol. 2022, 346, 53–61. [Google Scholar] [CrossRef]
- Meng, X.D.; Yao, H.H.; Wang, L.M.; Yu, M.; Shi, S.; Yuan, Z.X.; Liu, J. Knockdown of GAS5 Inhibits Atherosclerosis Progression via Reducing EZH2-Mediated ABCA1 Transcription in ApoE−/− Mice. Mol. Ther. Nucleic Acids 2020, 19, 84–96. [Google Scholar] [CrossRef]
- Jiang, Y.; Du, T. Relation of circulating lncRNA GAS5 and miR-21 with biochemical indexes, stenosis severity, and inflammatory cytokines in coronary heart disease patients. J. Clin. Lab. Anal. 2022, 36, e24202. [Google Scholar] [CrossRef]
- Yu, X.H.; Deng, W.Y.; Chen, J.J.; Xu, X.D.; Liu, X.X.; Chen, L.; Shi, M.W.; Liu, Q.X.; Tao, M.; Ren, K. LncRNA kcnq1ot1 promotes lipid accumulation and accelerates atherosclerosis via functioning as a ceRNA through the miR-452-3p/HDAC3/ABCA1 axis. Cell Death Dis. 2020, 11, 1043. [Google Scholar] [CrossRef]
- Hong, Y.; Zhang, Y.; Chen, H.; Tang, X.; Zhao, H.; Meng, Z.; Jia, X.; Liu, W.; Li, X.; Wang, L.; et al. Genetic dissection of the impact of lncRNA AI662270 during the development of atherosclerosis. J. Transl. Med. 2023, 21, 97. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Li, J. LncRNA KCNQ1OT1 depletion inhibits the malignant development of atherosclerosis by miR-145-5p. Microvasc. Res. 2022, 139, 104236. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Zheng, Z.; Lin, X.; Ma, H. Long Noncoding RNA MALAT1 Regulates the Progression of Atherosclerosis by miR-330-5p/NF-kappaB Signal Pathway. J. Cardiovasc. Pharmacol. 2021, 78, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Gast, M.; Rauch, B.H.; Nakagawa, S.; Haghikia, A.; Jasina, A.; Haas, J.; Nath, N.; Jensen, L.; Stroux, A.; Bohm, A.; et al. Immune system-mediated atherosclerosis caused by deficiency of long non-coding RNA MALAT1 in ApoE−/− mice. Cardiovasc. Res. 2019, 115, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Huangfu, N.; Xu, Z.; Zheng, W.; Wang, Y.; Cheng, J.; Chen, X. LncRNA MALAT1 regulates oxLDL-induced CD36 expression via activating beta-catenin. Biochem. Biophys. Res. Commun. 2018, 495, 2111–2117. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lin, X.; Wang, L.; Sun, T.; Zhao, Q.; Ma, Q.; Zhou, Y. LncRNA MALAT1 Enhances ox-LDL-Induced Autophagy through the SIRT1/MAPK/NF-kappaB Pathway in Macrophages. Curr. Vasc. Pharmacol. 2020, 18, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.W.; Guo, F.X.; Xu, Y.J.; Li, P.; Lu, Z.F.; McVey, D.G.; Zheng, L.; Wang, Q.; Ye, J.H.; Kang, C.M.; et al. Long noncoding RNA NEXN-AS1 mitigates atherosclerosis by regulating the actin-binding protein NEXN. J. Clin. Investig. 2019, 129, 1115–1128. [Google Scholar] [CrossRef]
- An, J.H.; Chen, Z.Y.; Ma, Q.L.; Wang, H.J.; Zhang, J.Q.; Shi, F.W. LncRNA SNHG16 promoted proliferation and inflammatory response of macrophages through miR-17-5p/NF-kappaB signaling pathway in patients with atherosclerosis. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8665–8677. [Google Scholar] [CrossRef]
- Li, H.; Han, S.; Sun, Q.; Yao, Y.; Li, S.; Yuan, C.; Zhang, B.; Jing, B.; Wu, J.; Song, Y.; et al. Long non-coding RNA CDKN2B-AS1 reduces inflammatory response and promotes cholesterol efflux in atherosclerosis by inhibiting ADAM10 expression. Aging 2019, 11, 1695–1715. [Google Scholar] [CrossRef]
- Liu, L.; Tan, L.; Yao, J.; Yang, L. Long non-coding RNA MALAT1 regulates cholesterol accumulation in ox-LDL-induced macrophages via the microRNA-17-5p/ABCA1 axis. Mol. Med. Rep. 2020, 21, 1761–1770. [Google Scholar] [CrossRef]
- Yang, L.; Li, T. LncRNA TUG1 regulates ApoM to promote atherosclerosis progression through miR-92a/FXR1 axis. J. Cell. Mol. Med. 2020, 24, 8836–8848. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Yin, R.; Shi, H.; Wang, X.; Shen, D.; Wang, X.; Pan, C. LncRNA ZFAS1 confers inflammatory responses and reduces cholesterol efflux in atherosclerosis through regulating miR-654-3p-ADAM10/RAB22A axis. Int. J. Cardiol. 2020, 315, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Gareev, I.; Kudriashov, V.; Sufianov, A.; Begliarzade, S.; Ilyasova, T.; Liang, Y.; Beylerli, O. The role of long non-coding RNA ANRIL in the development of atherosclerosis. Non-Coding RNA Res. 2022, 7, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Qin, Q.; Xu, J.; Li, X.; Cong, H. Phthalate promotes atherosclerosis through interacting with long-non coding RNA and induces macrophage foam cell formation and vascular smooth muscle damage. Chemosphere 2022, 308, 136383. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.M.; Yang, S.; Xia, Y.P.; Hu, R.T.; Chen, S.; Li, B.W.; Chen, S.L.; Luo, X.Y.; Mao, L.; Li, Y.; et al. LncRNA MIAT sponges miR-149-5p to inhibit efferocytosis in advanced atherosclerosis through CD47 upregulation. Cell Death Dis. 2019, 10, 138. [Google Scholar] [CrossRef]
- Han, Y.; Qiu, H.; Pei, X.; Fan, Y.; Tian, H.; Geng, J. Low-dose Sinapic Acid Abates the Pyroptosis of Macrophages by Downregulation of lncRNA-MALAT1 in Rats With Diabetic Atherosclerosis. J. Cardiovasc. Pharmacol. 2018, 71, 104–112. [Google Scholar] [CrossRef]
- Simion, V.; Zhou, H.; Haemmig, S.; Pierce, J.B.; Mendes, S.; Tesmenitsky, Y.; Perez-Cremades, D.; Lee, J.F.; Chen, A.F.; Ronda, N.; et al. A macrophage-specific lncRNA regulates apoptosis and atherosclerosis by tethering HuR in the nucleus. Nat. Commun. 2020, 11, 6135. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, J.; Xu, X.; Qu, Y.; Dong, H.; Dang, J.; Huo, Z.; Xu, G. LncRNA expression profile during autophagy and Malat1 function in macrophages. PLoS ONE 2019, 14, e0221104. [Google Scholar] [CrossRef]
- Wu, G.; Cai, J.; Han, Y.; Chen, J.; Huang, Z.P.; Chen, C.; Cai, Y.; Huang, H.; Yang, Y.; Liu, Y.; et al. LincRNA-p21 regulates neointima formation, vascular smooth muscle cell proliferation, apoptosis, and atherosclerosis by enhancing p53 activity. Circulation 2014, 130, 1452–1465. [Google Scholar] [CrossRef]
- Zhang, L.; Cheng, H.; Yue, Y.; Li, S.; Zhang, D.; He, R. TUG1 knockdown ameliorates atherosclerosis via up-regulating the expression of miR-133a target gene FGF1. Cardiovasc. Pathol. 2018, 33, 6–15. [Google Scholar] [CrossRef]
- Yang, K.; Xue, Y.; Gao, X. LncRNA XIST Promotes Atherosclerosis by Regulating miR-599/TLR4 Axis. Inflammation 2021, 44, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Fu, Y.; Gu, X.; Xi, X.; Peng, X.; Wang, C.; Sun, Q.; Wang, X.; Qian, F.; Qin, Z.; et al. Macrophage-Enriched lncRNA RAPIA: A Novel Therapeutic Target for Atherosclerosis. Arter. Thromb. Vasc. Biol. 2020, 40, 1464–1478. [Google Scholar] [CrossRef]
- Li, H.; Pan, Z.; Chen, Q.; Yang, Z.; Zhang, D. SMILR Aggravates the Progression of Atherosclerosis by Sponging miR-10b-3p to Regulate KLF5 Expression. Inflammation 2020, 43, 1620–1633. [Google Scholar] [CrossRef]
- Chen, L.; Yang, W.; Guo, Y.; Chen, W.; Zheng, P.; Zeng, J.; Tong, W. Exosomal lncRNA GAS5 regulates the apoptosis of macrophages and vascular endothelial cells in atherosclerosis. PLoS ONE 2017, 12, e0185406. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Han, J.; Wu, Y.; Li, S.; Wang, Q.; Lin, W.; Zhu, J. Exosomal MALAT1 derived from oxidized low-density lipoprotein-treated endothelial cells promotes M2 macrophage polarization. Mol. Med. Rep. 2018, 18, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Luo, L.; Wei, X.; Gong, D.; Li, Z.; Li, S.; Tang, W.; Jin, L. M1 Bone Marrow-Derived Macrophage-Derived Extracellular Vesicles Inhibit Angiogenesis and Myocardial Regeneration Following Myocardial Infarction via the MALAT1/MicroRNA-25-3p/CDC42 Axis. Oxidative Med. Cell. Longev. 2021, 2021, 9959746. [Google Scholar] [CrossRef]
- Hu, D.; Wang, Y.; You, Z.; Lu, Y.; Liang, C. lnc-MRGPRF-6:1 Promotes M1 Polarization of Macrophage and Inflammatory Response through the TLR4-MyD88-MAPK Pathway. Mediat. Inflamm. 2022, 2022, 6979117. [Google Scholar] [CrossRef]
- Sun, L.; He, X.; Zhang, T.; Han, Y.; Tao, G. Knockdown of mesenchymal stem cell-derived exosomal LOC100129516 suppresses the symptoms of atherosclerosis via upregulation of the PPARgamma/LXRalpha/ABCA1 signaling pathway. Int. J. Mol. Med. 2021, 48, 1–11. [Google Scholar] [CrossRef]
- Huang, P.; Wang, L.; Li, Q.; Tian, X.; Xu, J.; Xu, J.; Xiong, Y.; Chen, G.; Qian, H.; Jin, C.; et al. Atorvastatin enhances the therapeutic efficacy of mesenchymal stem cells-derived exosomes in acute myocardial infarction via up-regulating long non-coding RNA H19. Cardiovasc. Res. 2020, 116, 353–367. [Google Scholar] [CrossRef]
- Pravda, J. Sepsis: Evidence-based pathogenesis and treatment. World J. Crit. Care Med. 2021, 10, 66–80. [Google Scholar] [CrossRef]
- Chousterman, B.G.; Swirski, F.K.; Weber, G.F. Cytokine storm and sepsis disease pathogenesis. Semin. Immunopathol. 2017, 39, 517–528. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Y.; Gao, Y.; Shou, S.; Chai, Y. The roles of macrophage polarization in the host immune response to sepsis. Int. Immunopharmacol. 2021, 96, 107791. [Google Scholar] [CrossRef]
- Zhang, C.C.; Niu, F. LncRNA NEAT1 promotes inflammatory response in sepsis-induced liver injury via the Let-7a/TLR4 axis. Int. Immunopharmacol. 2019, 75, 105731. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Yao, R.; Zhou, P.; Wang, C.; Xia, Y.; Xu, S. LncRNA NEAT1 reversed the hindering effects of miR-495-3p/STAT3 axis and miR-211/PI3K/AKT axis on sepsis-relevant inflammation. Mol. Immunol. 2020, 117, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.Y.; Fang, Y.; Zheng, F.X.; Zhang, Y.Z.; Li, Q.L. LncRNA NEAT1 facilitates the progression of sepsis through up-regulating TSP-1 via sponging miR-370-3p. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 333–344. [Google Scholar] [CrossRef]
- Li, Y.; Guo, W.; Cai, Y. NEAT1 Promotes LPS-induced Inflammatory Injury in Macrophages by Regulating MiR-17-5p/TLR4. Open Med. 2020, 15, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xue, J.; Qin, L.; Zhang, J.; Liu, J.; Yu, J. LncRNA NEAT1 Promotes Inflammatory Response in Sepsis via the miR-31-5p/POU2F1 Axis. Inflammation 2021, 44, 1518–1528. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Guo, Z.H. Downregulation of lncRNA NEAT1 Ameliorates LPS-Induced Inflammatory Responses by Promoting Macrophage M2 Polarization via miR-125a-5p/TRAF6/TAK1 Axis. Inflammation 2020, 43, 1548–1560. [Google Scholar] [CrossRef]
- Chen, J.; Tang, S.; Ke, S.; Cai, J.J.; Osorio, D.; Golovko, A.; Morpurgo, B.; Guo, S.; Sun, Y.; Winkle, M.; et al. Ablation of long noncoding RNA MALAT1 activates antioxidant pathway and alleviates sepsis in mice. Redox Biol. 2022, 54, 102377. [Google Scholar] [CrossRef]
- Bottiglieri, T. S-Adenosyl-L-methionine (SAMe): From the bench to the bedside--molecular basis of a pleiotrophic molecule. Am. J. Clin. Nutr. 2002, 76, 1151S–1157S. [Google Scholar] [CrossRef]
- Lin, L.P.; Niu, G.H.; Zhang, X.Q. Influence of lncRNA MALAT1 on septic lung injury in mice through p38 MAPK/p65 NF-kappaB pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1296–1304. [Google Scholar] [CrossRef]
- Cui, H.; Banerjee, S.; Guo, S.; Xie, N.; Ge, J.; Jiang, D.; Zornig, M.; Thannickal, V.J.; Liu, G. Long noncoding RNA Malat1 regulates differential activation of macrophages and response to lung injury. JCI Insight 2019, 4, e124522. [Google Scholar] [CrossRef]
- Yang, Q.; Cao, K.; Jin, G.; Zhang, J. Hsa-miR-346 plays a role in the development of sepsis by downregulating SMAD3 expression and is negatively regulated by lncRNA MALAT1. Mol. Cell. Probes 2019, 47, 101444. [Google Scholar] [CrossRef]
- Zhao, G.; Su, Z.; Song, D.; Mao, Y.; Mao, X. The long noncoding RNA MALAT1 regulates the lipopolysaccharide-induced inflammatory response through its interaction with NF-kappaB. FEBS Lett. 2016, 590, 2884–2895. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Z.; Yue, D.; Zeng, Z.; Yuan, W.; Xu, K. Linkage of lncRNA CRNDE sponging miR-181a-5p with aggravated inflammation underlying sepsis. Innate Immun. 2020, 26, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Wu, Y.; Paranggan, P.; Gao, W.; Gao, Z.; Liu, J.; Wu, L. Involvement of plasma lncRNA GSEC in sepsis discrimination and prognosis, and its correlation with macrophage cell inflammation and proliferation. Immunobiology 2022, 227, 152264. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xie, Y.; He, Q.; Geng, Y.; Xu, J. LncRNA-Cox2 regulates macrophage polarization and inflammatory response through the CREB-C/EBPbeta signaling pathway in septic mice. Int. Immunopharmacol. 2021, 101, 108347. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.Y.; Yang, Z.Q.; Lin, X.F.; Zhao, F.L.; Tu, H.T.; Wang, L.J.; Wen, M.Y.; Xian, S.X. Knockdown of lncRNA PVT1 attenuated macrophage M1 polarization and relieved sepsis induced myocardial injury via miR-29a/HMGB1 axis. Cytokine 2021, 143, 155509. [Google Scholar] [CrossRef]
- Luo, R.; Li, X.; Wang, D. Reprogramming Macrophage Metabolism and its Effect on NLRP3 Inflammasome Activation in Sepsis. Front. Mol. Biosci. 2022, 9, 917818. [Google Scholar] [CrossRef]
- Qiu, P.; Liu, Y.; Zhang, J. Review: The Role and Mechanisms of Macrophage Autophagy in Sepsis. Inflammation 2019, 42, 6–19. [Google Scholar] [CrossRef]
- Gast, M.; Nageswaran, V.; Kuss, A.W.; Tzvetkova, A.; Wang, X.; Mochmann, L.H.; Rad, P.R.; Weiss, S.; Simm, S.; Zeller, T.; et al. tRNA-like Transcripts from the NEAT1-MALAT1 Genomic Region Critically Influence Human Innate Immunity and Macrophage Functions. Cells 2022, 11, 3970. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, H.-S.; Shin, J.-J.; Park, J.; Arab, I.; Suk, K.; Lee, W.-H. Role of Macrophage lncRNAs in Mediating Inflammatory Processes in Atherosclerosis and Sepsis. Biomedicines 2023, 11, 1905. https://doi.org/10.3390/biomedicines11071905
Shin H-S, Shin J-J, Park J, Arab I, Suk K, Lee W-H. Role of Macrophage lncRNAs in Mediating Inflammatory Processes in Atherosclerosis and Sepsis. Biomedicines. 2023; 11(7):1905. https://doi.org/10.3390/biomedicines11071905
Chicago/Turabian StyleShin, Hyeung-Seob, Jae-Joon Shin, Jeongkwang Park, Imene Arab, Kyoungho Suk, and Won-Ha Lee. 2023. "Role of Macrophage lncRNAs in Mediating Inflammatory Processes in Atherosclerosis and Sepsis" Biomedicines 11, no. 7: 1905. https://doi.org/10.3390/biomedicines11071905
APA StyleShin, H.-S., Shin, J.-J., Park, J., Arab, I., Suk, K., & Lee, W.-H. (2023). Role of Macrophage lncRNAs in Mediating Inflammatory Processes in Atherosclerosis and Sepsis. Biomedicines, 11(7), 1905. https://doi.org/10.3390/biomedicines11071905







