Phenotypic Modulation of Adipose-Derived Stem Cells and Fibroblasts Treated with Povidone–Iodine and Chlorhexidine in Mono and Coculture Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Antiseptics
2.3. 2D Migration Assay—Cell Exclusion Zone Assay
2.4. 3D Collagen Sprouting Assay
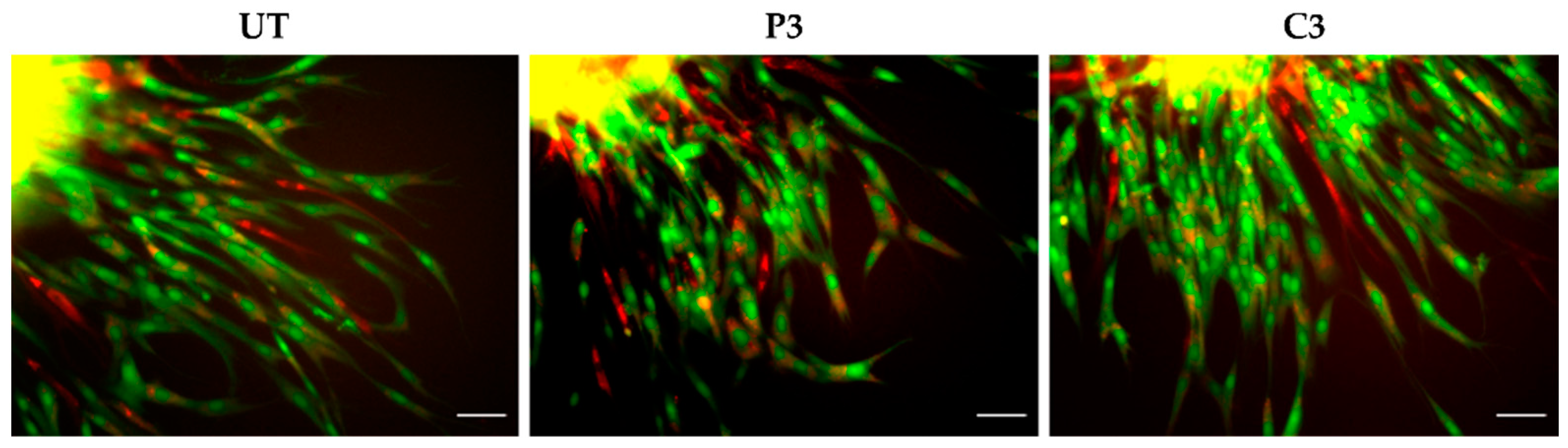
2.5. Immunofluorescence for α-SMA and Ki67 Markers
2.6. Imaging and Statistical Analysis
3. Results
3.1. Cytotoxicity of Antiseptics
3.2. The 2D Cell Exclusion Zone Assay for Migration Assessment
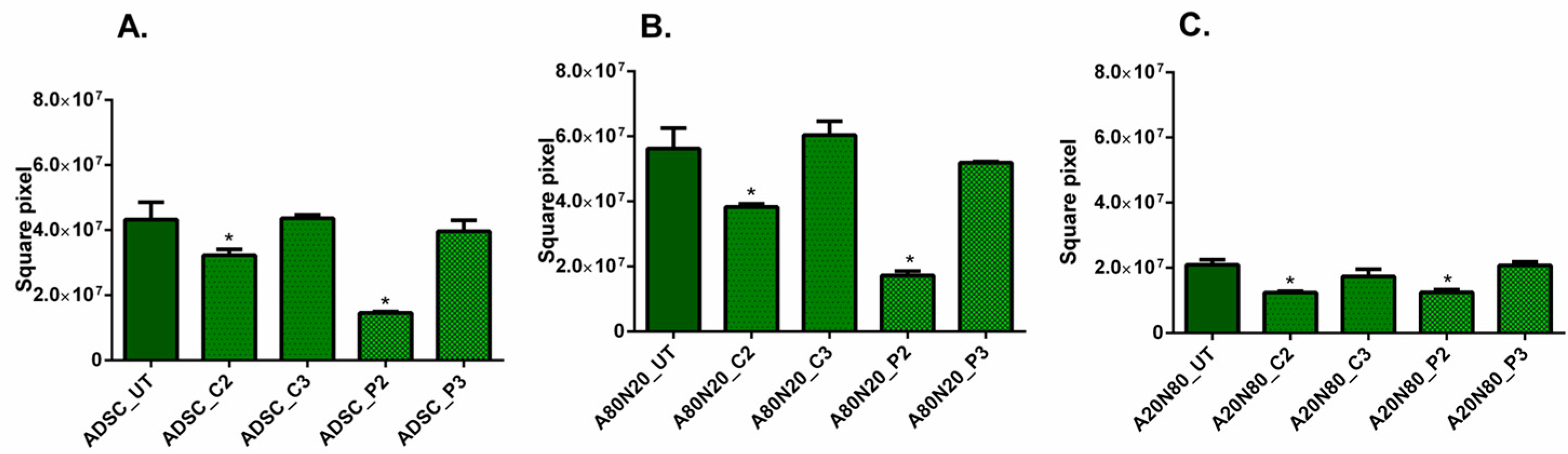
3.3. The 3D Collagen Sprouting Assay
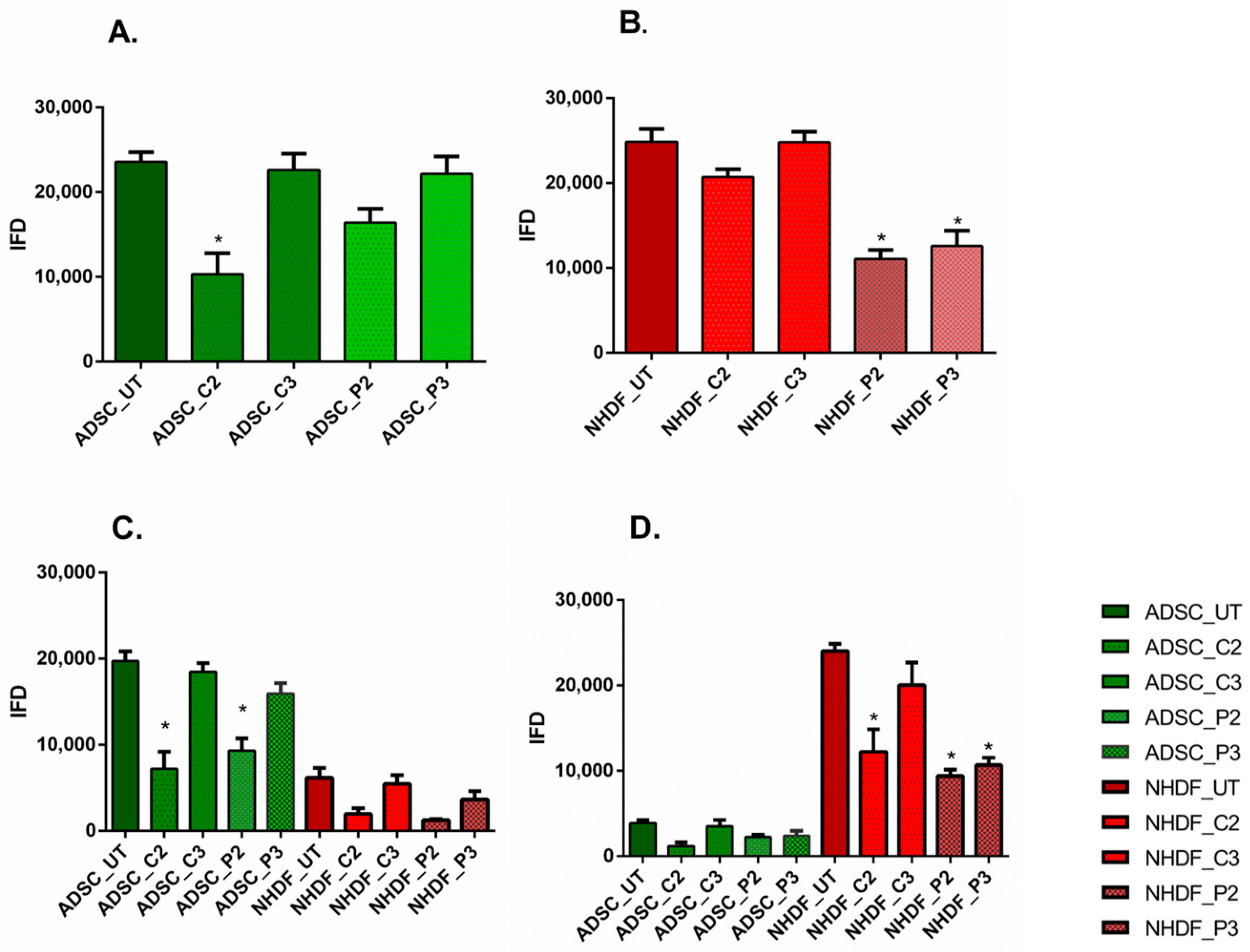
3.4. α-SMA and Ki67 Markers Evaluation for 2D Constructs
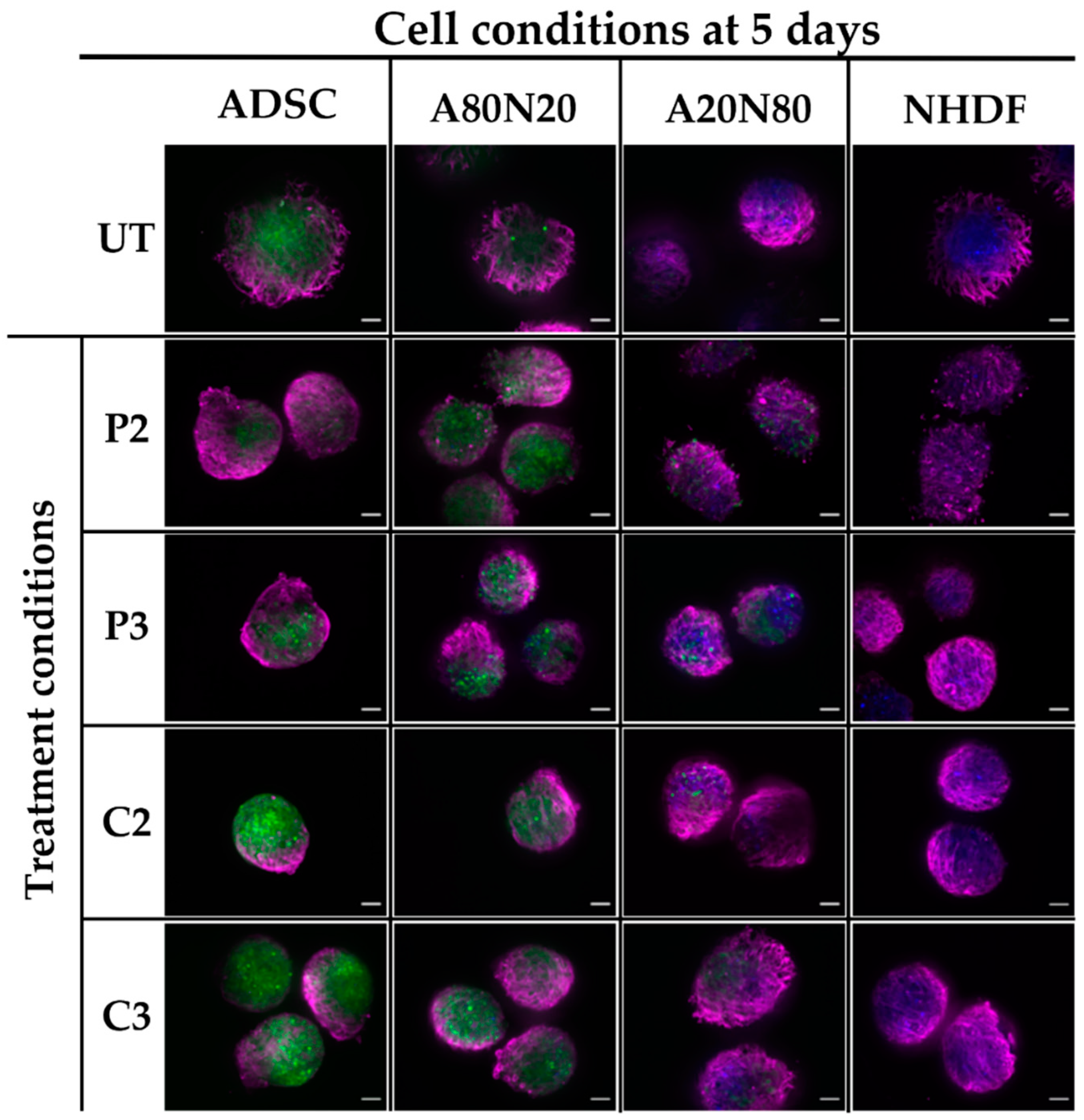
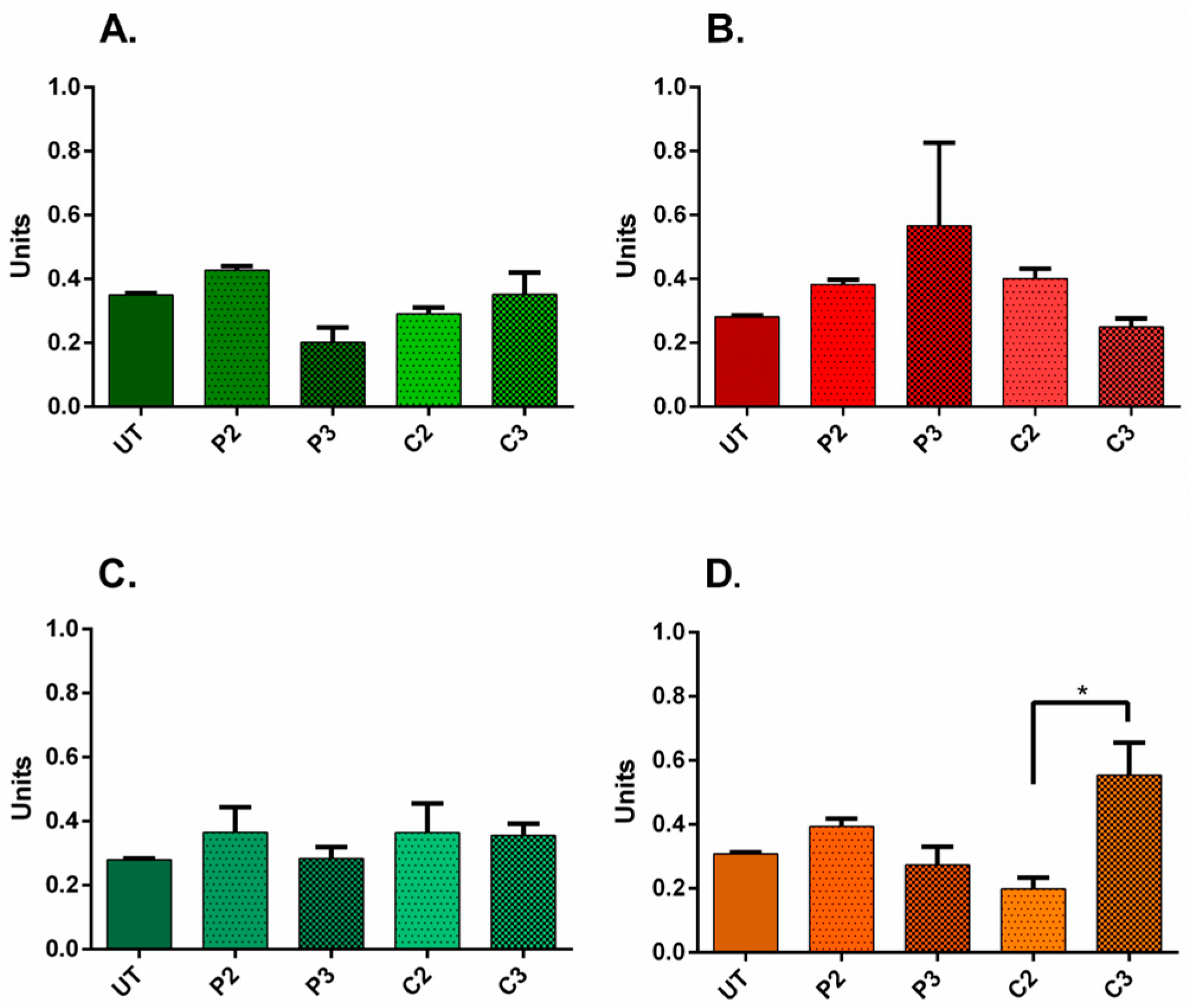
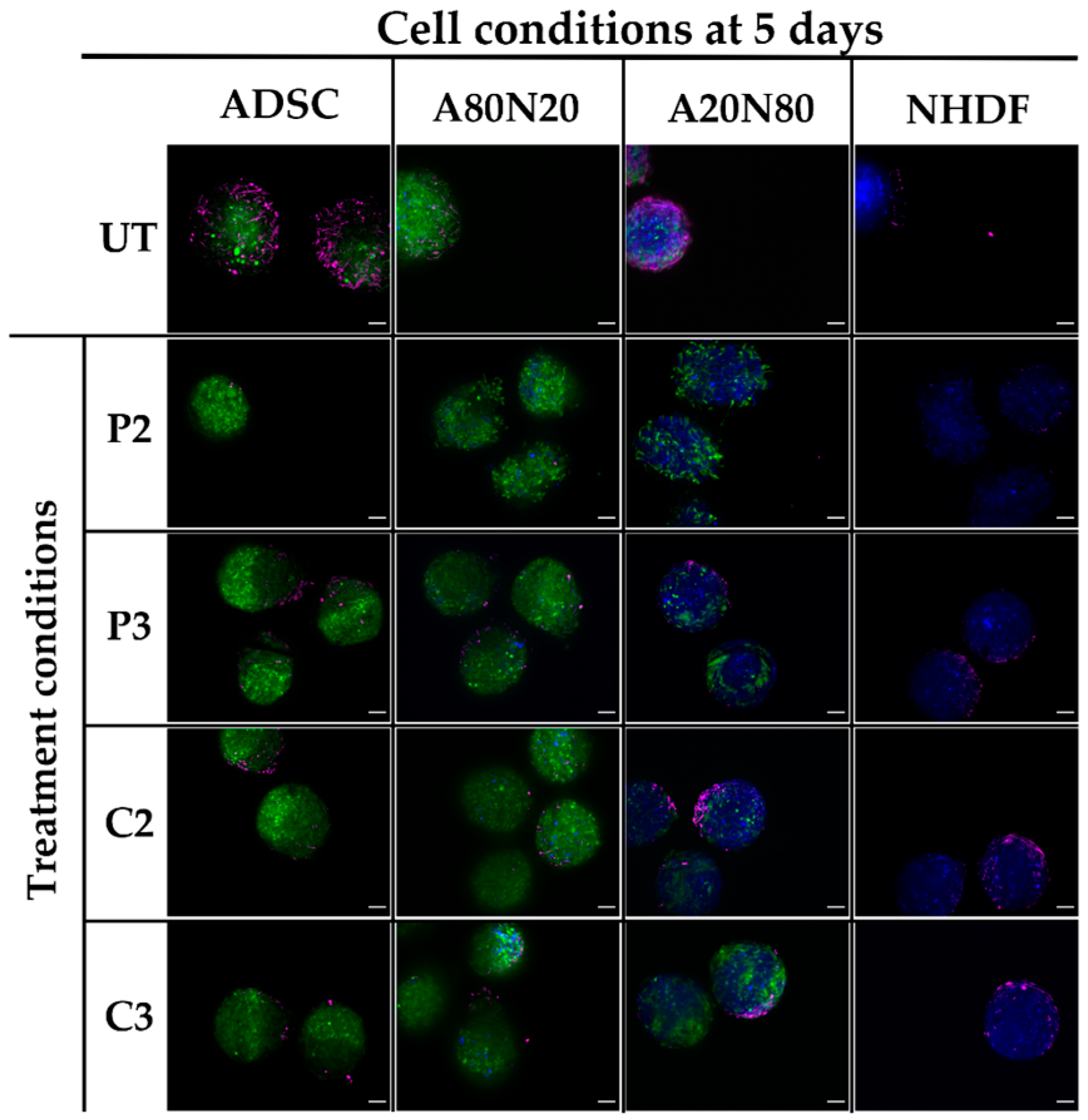
3.5. α-SMA and Ki67 Markers Evaluation for 3D Spheroid Cultures
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| α-SMA | α-smooth muscle actin |
| A20N80 | Coculture with 20% adipose-derived stem cells and 80% fibroblasts |
| A80N20 | Coculture with 80% adipose-derived stem cells and 20% fibroblasts |
| ADSC | Adipose-derived stem cells |
| CHX | Chlorhexidine |
| IFD | Integrated fluorescent density |
| NHDF | Normal human dermal fibroblasts |
| PBS | Dulbecco’s phosphate-buffered saline |
| PVP-I | Povidone–iodine |
| UT | Untreated |
References
- Babalska, Z.Ł.; Korbecka-Paczkowska, M.; Karpiński, T.M. Wound Antiseptics and European Guidelines for Antiseptic Application in Wound Treatment. Pharmaceuticals 2021, 14, 1253. [Google Scholar] [CrossRef]
- Kramer, A.; Dissemond, J.; Kim, S.; Willy, C.; Mayer, D.; Papke, R.; Tuchmann, F.; Assadian, O. Consensus on Wound Antisepsis: Update 2018. Skin Pharmacol. Physiol. 2018, 31, 28–58. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.W.; Rael, L.T.; Bar-Or, R.; Shimonkevitz, R.; Mains, C.W.; Slone, D.S.; Craun, M.L.; Bar-Or, D. Mechanisms of delayed wound healing by commonly used antiseptics. J. Trauma 2009, 66, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Bigliardi, P.L.; Alsagoff, S.A.L.; El-Kafrawi, H.Y.; Pyon, J.K.; Wa, C.T.C.; Villa, M.A. Povidone iodine in wound healing: A review of current concepts and practices. Int. J. Surg. 2017, 44, 260–268. [Google Scholar] [CrossRef]
- Khan, M.N.; Naqvi, A.H. Antiseptics, iodine, povidone iodine and traumatic wound cleansing. J. Tissue Viability 2006, 16, 6–10. [Google Scholar] [CrossRef]
- Abdel-Sayed, P.; Tornay, D.; Hirt-Burri, N.; de Buys Roessingh, A.; Raffoul, W.; Applegate, L.A. Implications of chlorhexidine use in burn units for wound healing. Burns 2020, 46, 1150–1156. [Google Scholar] [CrossRef]
- Slaviero, L.; Avruscio, G.; Vindigni, V.; Tocco-Tussardi, I. Antiseptics for burns: A review of the evidence. Ann. Burns Fire Disasters 2018, 31, 198–203. [Google Scholar] [PubMed]
- Lefebvre, E.; Vighetto, C.; Di Martino, P.; Larreta Garde, V.; Seyer, D. Synergistic antibiofilm efficacy of various commercial antiseptics, enzymes and EDTA: A study of Pseudomonas aeruginosa and Staphylococcus aureus biofilms. Int. J. Antimicrob. Agents 2016, 48, 181–188. [Google Scholar] [CrossRef]
- Ghafouri, H.B.; Zavareh, M.; Jalili, F.; Cheraghi, S. Is 1% povidone-iodine solution superior to normal saline for simple traumatic wound irrigation? Wound Med. 2016, 15, 1–5. [Google Scholar] [CrossRef]
- Ben-Yehuda Greenwald, M.; Frušić-Zlotkin, M.; Soroka, Y.; Ben-Sasson, S.; Bianco-Peled, H.; Kohen, R. A novel role of topical iodine in skin: Activation of the Nrf2 pathway. Free Radic. Biol. Med. 2017, 104, 238–248. [Google Scholar] [CrossRef]
- Kramer, S.A. Effect of povidone-iodine on wound healing: A review. J. Vasc. Nurs. 1999, 17, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Alves, P.J.; Barreto, R.T.; Barrois, B.M.; Gryson, L.G.; Meaume, S.; Monstrey, S.J. Update on the role of antiseptics in the management of chronic wounds with critical colonisation and/or biofilm. Int. Wound J. 2021, 18, 342–358. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.X.; Werner, J.A.; Buza, J.A., III; Kirsch, T.; Zuckerman, J.D.; Virk, M.S. Povidone-iodine Solutions Inhibit Cell Migration and Survival of Osteoblasts, Fibroblasts, and Myoblasts. Spine 2017, 42, 1757–1762. [Google Scholar] [CrossRef] [PubMed]
- Faria, G.; Cardoso, C.R.; Larson, R.E.; Silva, J.S.; Rossi, M.A. Chlorhexidine-induced apoptosis or necrosis in L929 fibroblasts: A role for endoplasmic reticulum stress. Toxicol. Appl. Pharmacol. 2009, 234, 256–265. [Google Scholar] [CrossRef]
- Salami, A.A.; Imosemi, I.O.; Owoeye, O.O. A Comparison of the Effect of Chlorhexidine, Tap Water and Normal Saline on Healing Wounds. Int. J. Morphol. 2006, 24, 673–676. [Google Scholar] [CrossRef]
- Wound Healing Management Node Group. Evidence Summary: Wound management–Chlorhexidine. Wound Pract. Res. 2017, 25, 49–51. [Google Scholar]
- Touzel, R.E.; Sutton, J.M.; Wand, M.E. Establishment of a multi-species biofilm model to evaluate chlorhexidine efficacy. J. Hosp. Infect. 2016, 92, 154–160. [Google Scholar] [CrossRef]
- Chawner, J.A.; Gilbert, P. Adsorption of alexidine and chlorhexidine to Escherichia coli and membrane components. Int. J. Pharm. 1989, 55, 209–215. [Google Scholar] [CrossRef]
- Pakyari, M.; Farrokhi, A.; Maharlooei, M.K.; Ghahary, A. Critical Role of Transforming Growth Factor Beta in Different Phases of Wound Healing. Adv. Wound Care 2013, 2, 215–224. [Google Scholar] [CrossRef]
- Caldwell, M.D. Bacteria and Antibiotics in Wound Healing. Surg. Clin. N. Am. 2020, 100, 757–776. [Google Scholar] [CrossRef]
- Öhnstedt, E.; Lofton Tomenius, H.; Vågesjö, E.; Phillipson, M. The discovery and development of topical medicines for wound healing. Expert Opin. Drug Discov. 2019, 14, 485–497. [Google Scholar] [CrossRef]
- Pilloni, A.; Ceccarelli, S.; Bosco, D.; Gerini, G.; Marchese, C.; Marini, L.; Rojas, M.A. Effect of Chlorhexidine Digluconate in Early Wound Healing of Human Gingival Tissues. A Histological, Immunohistochemical and Biomolecular Analysis. Antibiotics 2021, 10, 1192. [Google Scholar] [CrossRef]
- Karppinen, S.M.; Heljasvaara, R.; Gullberg, D.; Tasanen, K.; Pihlajaniemi, T. Toward understanding scarless skin wound healing and pathological scarring. F1000Research 2019, 8, 787. [Google Scholar] [CrossRef]
- Hocking, A.M. The Role of Chemokines in Mesenchymal Stem Cell Homing to Wounds. Adv. Wound Care 2015, 4, 623–630. [Google Scholar] [CrossRef]
- Fathke, C.; Wilson, L.; Hutter, J.; Kapoor, V.; Smith, A.; Hocking, A.; Isik, F. Contribution of bone marrow-derived cells to skin: Collagen deposition and wound repair. Stem Cells 2004, 22, 812–822. [Google Scholar] [CrossRef]
- Katz, A.J.; Mericli, A.F. Stem Cells Derived from Fat–Un Update. In Handbook of Stem Cells, 2nd ed.; Atala, A., Lanza, R., Eds.; Elsevier: New York, NY, USA, 2013; Volume 2, pp. 529–530. [Google Scholar] [CrossRef]
- Kim, B.S.; Ott, V.; Boecker, A.H.; Stromps, J.P.; Paul, N.E.; Alharbi, Z.; Cakmak, E.; Bernhagen, J.; Bucala, R.; Pallua, N. The Effect of Antiseptics on Adipose-Derived Stem Cells. Plast. Reconstr. Surg. 2017, 139, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Ivascu, A.; Kubbies, M. Rapid generation of single-tumor spheroids for high-throughput cell function and toxicity analysis. J. Biomol. Screen 2006, 11, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2021, 9, 676–682. [Google Scholar] [CrossRef]
- Rueda-Fernández, M.; Melguizo-Rodríguez, L.; Costela-Ruiz, V.J.; de Luna-Bertos, E.; Ruiz, C.; Ramos-Torrecillas, J.; Illescas-Montes, R. Effect of the most common wound antiseptics on human skin fibroblasts. Clin. Exp. Dermatol. 2022, 47, 1543–1549. [Google Scholar] [CrossRef] [PubMed]
- Severing, A.L.; Borkovic, M.; Stuermer, E.K.; Rembe, J.D. Composition of Challenge Substance in Standardized Antimicrobial Efficacy Testing of Wound Antimicrobials Is Essential to Correctly Simulate Efficacy in the Human Wound Micro-Environment. Biomedicines 2022, 10, 2751. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yan, Y.; Li, Y.; Shen, C.; Zhang, Y. Topical effect of benzalkonium bromide on wound healing and potential cellular and molecular mechanisms. Int. Wound J. 2021, 18, 566–576. [Google Scholar] [CrossRef]
- Wang, L.; Qin, W.; Zhou, Y.; Chen, B.; Zhao, X.; Zhao, H.; Mi, E.; Mi, E.; Wang, Q.; Ning, J. Transforming growth factor β plays an important role in enhancing wound healing by topical application of Povidone-iodine. Sci. Rep. 2017, 7, 991. [Google Scholar] [CrossRef]
- Fumal, I.; Braham, C.; Paquet, P.; Piérard-Franchimont, C.; Piérard, G.E. The beneficial toxicity paradox of antimicrobials in leg ulcer healing impaired by a polymicrobial flora: A proof-of-concept study. Dermatology 2002, 204, 70–74. [Google Scholar] [CrossRef]
- Archer, H.G.; Barnett, S.; Irving, S.; Middleton, K.R.; Seal, D.V. A controlled model of moist wound healing: Comparison between semi-permeable film, antiseptics and sugar paste. J. Exp. Pathol. 1990, 71, 155–170. [Google Scholar]
- Desmoulière, A.; Chaponnier, C.; Gabbiani, G. Tissue repair, contraction, and the myofibroblast. Wound Repair. Regen. 2005, 13, 7–12. [Google Scholar] [CrossRef]
- Hosseini, M.; Brown, J.; Khosrotehrani, K.; Bayat, A.; Shafiee, A. Skin biomechanics: A potential therapeutic intervention target to reduce scarring. Burns Trauma 2022, 10, tkac036. [Google Scholar] [CrossRef]
- Balin, A.K.; Pratt, L. Dilute povidone-iodine solutions inhibit human skin fibroblast growth. Dermatol. Surg. 2002, 28, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Grambow, E.; Sorg, H.; Sorg, C.G.G.; Strüder, D. Experimental Models to Study Skin Wound Healing with a Focus on Angiogenesis. Med. Sci. 2021, 9, 55. [Google Scholar] [CrossRef]
- Sorice, S.; Rustad, K.C.; Li, A.Y.; Gurtner, G.C. The Role of Stem Cell Therapeutics in Wound Healing: Current Understanding and Future Directions. Plast. Reconstr. Surg. 2016, 138, 31S–41S. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Ravi, M.; Paramesh, V.; Kaviya, S.R.; Anuradha, E.; Solomon, F.D. 3D cell culture systems: Advantages and applications. J. Cell Physiol. 2015, 230, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, T.; Nigusse, T.; Dhanaraju, M.D. Silver nanoparticles as real topical bullets for wound healing. J. Am. Coll. Clin. Wound Spec. 2012, 3, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Barreto, R.; Barrois, B.; Lambert, J.; Malhotra-Kumar, S.; Santos-Fernandes, V.; Monstrey, S. Addressing the challenges in antisepsis: Focus on povidone iodine. Int. J. Antimicrob. Agents 2020, 56, 106064. [Google Scholar] [CrossRef] [PubMed]




| Culture Type | Cells | Abbreviation | Cell Ratio |
|---|---|---|---|
| NHDFs | NHDF | 100% NHDF | |
| Monocultures | |||
| ADSCs | ADSC | 100% ADSC | |
| A20N80 | 20% ADSC with 80% NHDF | ||
| Cocultures | ADSCs:NHDFs | ||
| A80N20 | 80% ADSC with 20% NHDF |
| Antiseptics | Abbreviation | Concentration | Dilution |
|---|---|---|---|
| P1 | 10% | 100 mg PVP-I in 1 mL PBS | |
| PVP-I | P2 | 1% | 0.5 mL of P1 in 4.5 mL PBS |
| P3 | 0.1% | 1 mL of P2 in 9 mL PBS | |
| C1 | 0.1% | 100 µL of the 20% solution in 19.9 mL PBS | |
| CHX | C2 | 0.05% | 2.5 mL of C1 in 2.5 mL PBS |
| C3 | 0.01% | 0.5 mL of C1 in 5.5 mL PBS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chelmuș-Burlacu, A.; Tang, E.; Pieptu, D. Phenotypic Modulation of Adipose-Derived Stem Cells and Fibroblasts Treated with Povidone–Iodine and Chlorhexidine in Mono and Coculture Models. Biomedicines 2023, 11, 1855. https://doi.org/10.3390/biomedicines11071855
Chelmuș-Burlacu A, Tang E, Pieptu D. Phenotypic Modulation of Adipose-Derived Stem Cells and Fibroblasts Treated with Povidone–Iodine and Chlorhexidine in Mono and Coculture Models. Biomedicines. 2023; 11(7):1855. https://doi.org/10.3390/biomedicines11071855
Chicago/Turabian StyleChelmuș-Burlacu, Alina, Eric Tang, and Dragoș Pieptu. 2023. "Phenotypic Modulation of Adipose-Derived Stem Cells and Fibroblasts Treated with Povidone–Iodine and Chlorhexidine in Mono and Coculture Models" Biomedicines 11, no. 7: 1855. https://doi.org/10.3390/biomedicines11071855
APA StyleChelmuș-Burlacu, A., Tang, E., & Pieptu, D. (2023). Phenotypic Modulation of Adipose-Derived Stem Cells and Fibroblasts Treated with Povidone–Iodine and Chlorhexidine in Mono and Coculture Models. Biomedicines, 11(7), 1855. https://doi.org/10.3390/biomedicines11071855





