Age, Dose, and Locomotion: Decoding Vulnerability to Ketamine in C57BL/6J and BALB/c Mice
Abstract
:1. Introduction
2. Materials and Methods
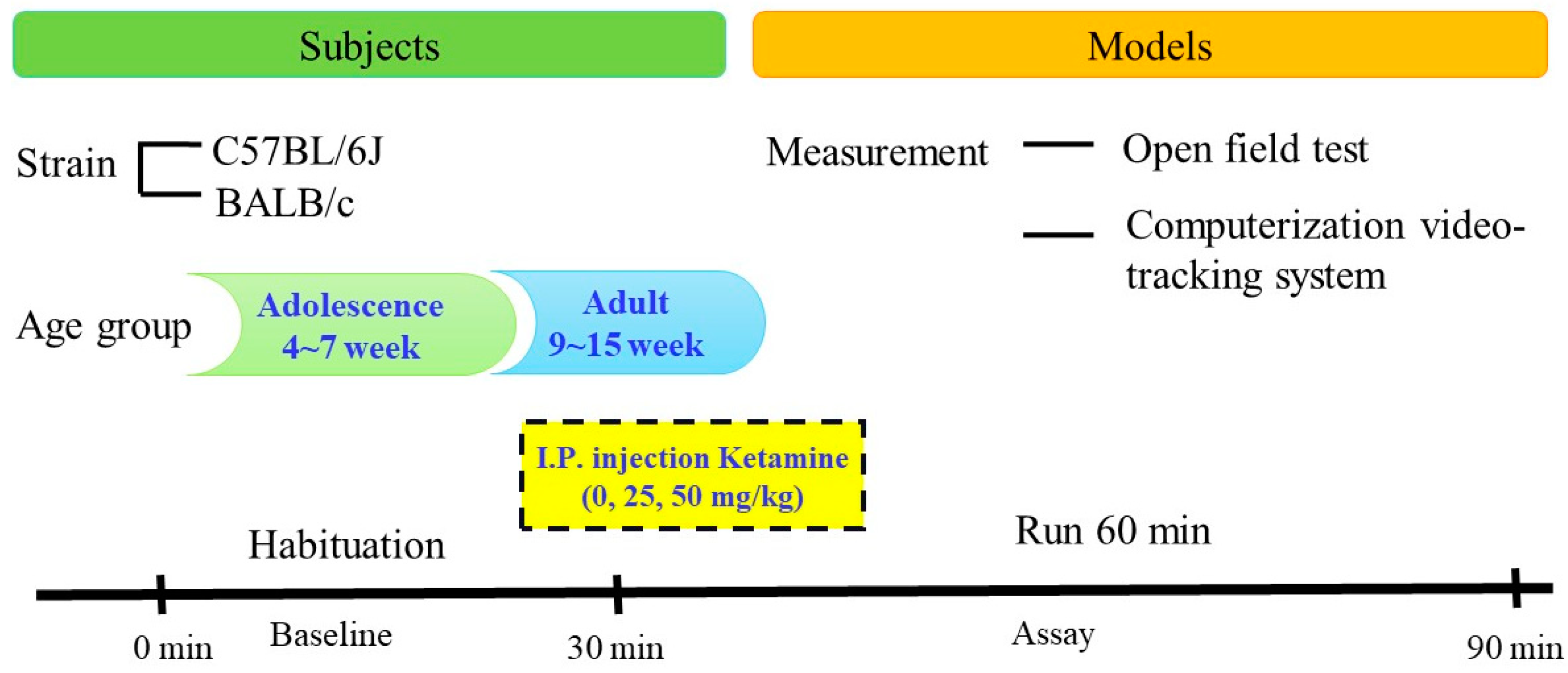
2.1. Subjects
2.2. Drug Preparation
2.3. Study Procedure
2.4. Statistical Analysis
3. Results
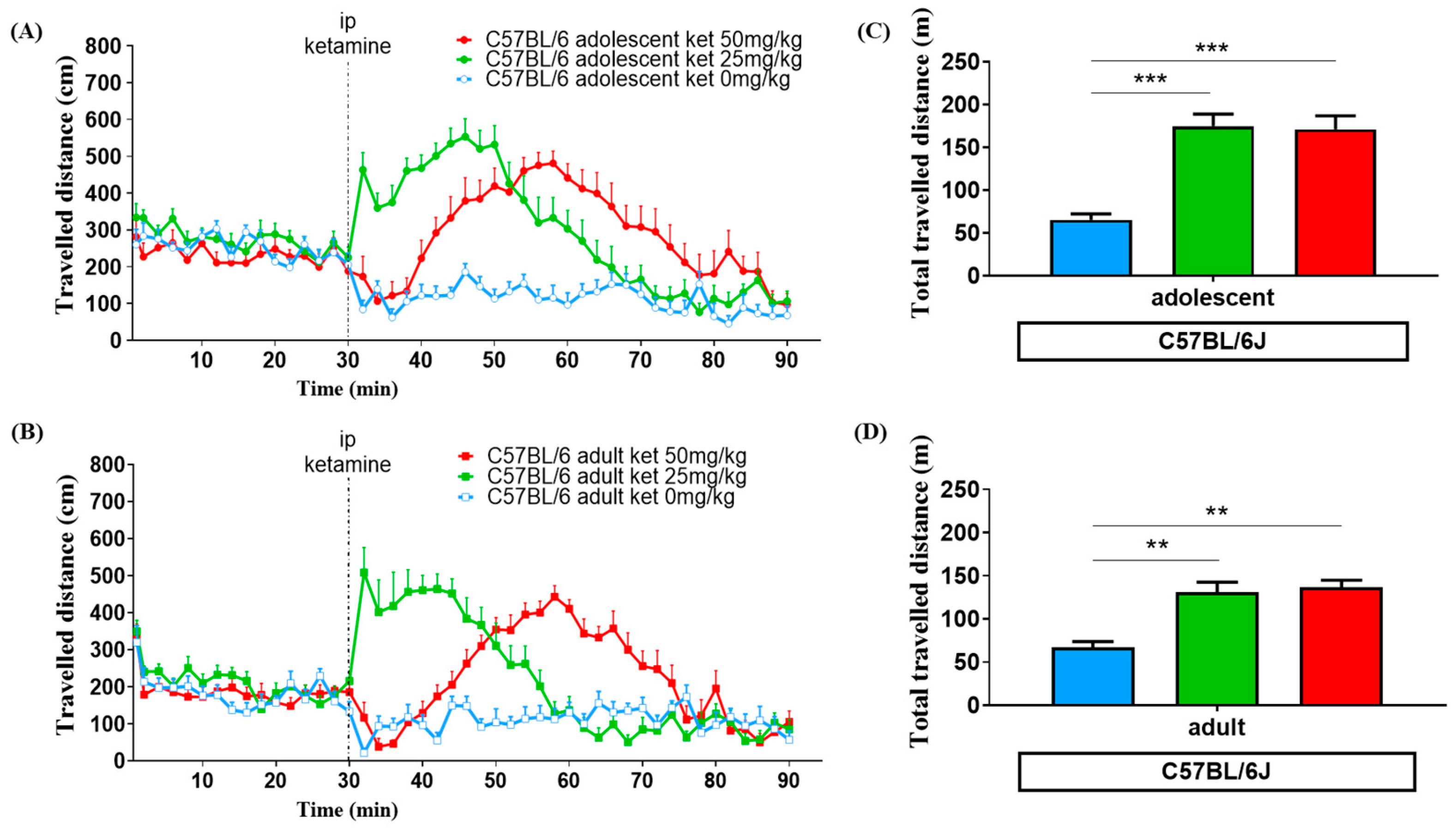
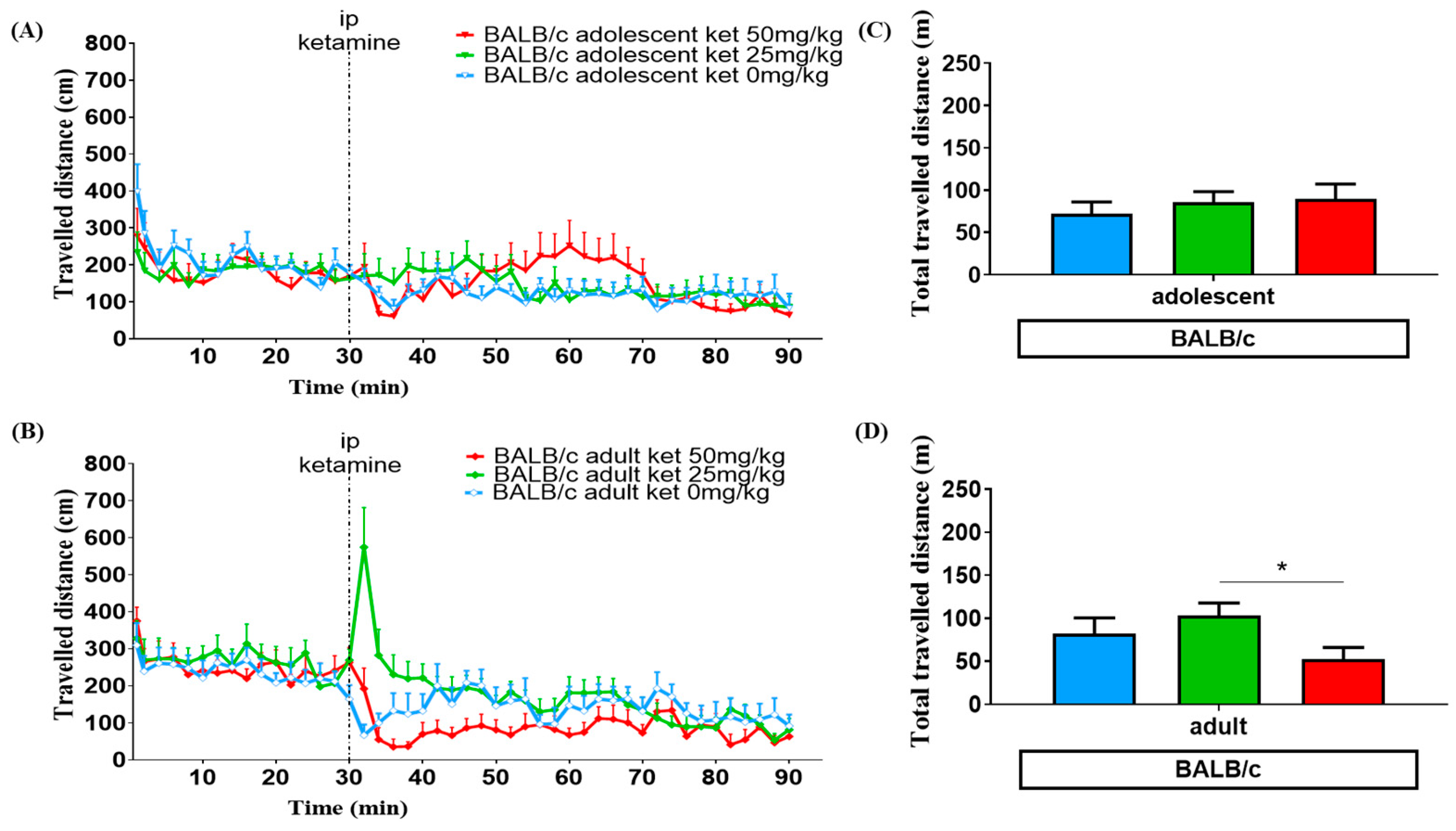
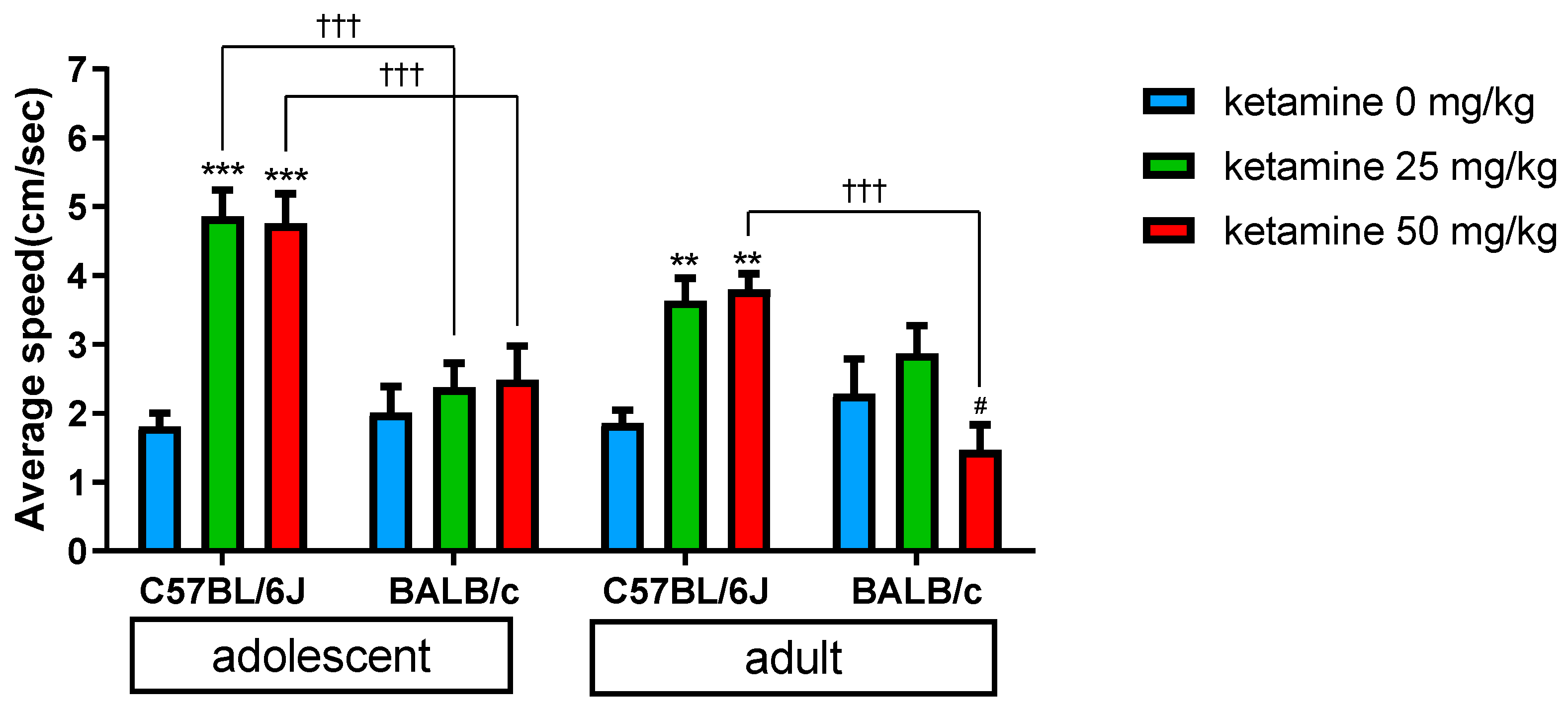
3.1. Age Effect
3.2. Ketamine Dose Effect
3.3. Strain Variation
4. Discussion
4.1. Ketamine Induced Locomotor Change
4.2. Interactions between Strain, Age, and Dosage Effects
4.3. Adolescent Vulnerability to Ketamine-Related Psychotomimetic Effects
4.4. Clinical Implications of the Study
4.5. Limitation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Eldufani, J.; Nekoui, A.; Blaise, G. Nonanesthetic Effects of Ketamine: A Review Article. Am. J. Med. 2018, 131, 1418–1424. [Google Scholar] [CrossRef]
- Zanos, P.; Gould, T.D. Mechanisms of ketamine action as an antidepressant. Mol. Psychiatry 2018, 23, 801–811. [Google Scholar] [CrossRef]
- Ionescu, D.F.; Fu, D.J.; Qiu, X.; Lane, R.; Lim, P.; Kasper, S.; Hough, D.; Drevets, W.C.; Manji, H.; Canuso, C.M. Esketamine Nasal Spray for Rapid Reduction of Depressive Symptoms in Patients with Major Depressive Disorder Who Have Active Suicide Ideation with Intent: Results of a Phase 3, Double-Blind, Randomized Study (ASPIRE II). Int. J. Neuropsychopharmacol. 2021, 24, 22–31. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, D.; Wu, B.; Zhou, W. Ketamine abuse potential and use disorder. Brain Res. Bull. 2016, 126, 68–73. [Google Scholar] [CrossRef] [Green Version]
- Middela, S.; Pearce, I. Ketamine-induced vesicopathy: A literature review. Int. J. Clin. Pract. 2011, 65, 27–30. [Google Scholar] [CrossRef] [Green Version]
- Li, J.H.; Vicknasingam, B.; Cheung, Y.W.; Zhou, W.; Nurhidayat, A.W.; Jarlais, D.C.; Schottenfeld, R. To use or not to use: An update on licit and illicit ketamine use. Subst. Abuse Rehabil. 2011, 2, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Stahl, S.M. Mechanism of action of ketamine. CNS Spectr. 2013, 18, 171–174. [Google Scholar] [CrossRef]
- Javitt, D.C. Glutamate as a therapeutic target in psychiatric disorders. Mol. Psychiatry 2004, 9, 984–997, 979. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, K. Molecular mechanisms of the rapid-acting and long-lasting antidepressant actions of (R)-ketamine. Biochem. Pharmacol. 2020, 177, 113935. [Google Scholar] [CrossRef]
- Kokane, S.S.; Armant, R.J.; Bolaños-Guzmán, C.A.; Perrotti, L.I. Overlap in the neural circuitry and molecular mechanisms underlying ketamine abuse and its use as an antidepressant. Behav. Brain Res. 2020, 384, 112548. [Google Scholar] [CrossRef]
- Ionescu, D.F.; Felicione, J.M.; Gosai, A.; Cusin, C.; Shin, P.; Shapero, B.G.; Deckersbach, T. Ketamine-Associated Brain Changes: A Review of the Neuroimaging Literature. Harv. Rev. Psychiatry 2018, 26, 320–339. [Google Scholar] [CrossRef]
- Bonaventura, J.; Lam, S.; Carlton, M.; Boehm, M.A.; Gomez, J.L.; Solís, O.; Sánchez-Soto, M.; Morris, P.J.; Fredriksson, I.; Thomas, C.J.; et al. Pharmacological and behavioral divergence of ketamine enantiomers: Implications for abuse liability. Mol. Psychiatry 2021, 26, 6704–6722. [Google Scholar] [CrossRef]
- Yang, Y.; Ju, W.; Zhang, H.; Sun, L. Effect of Ketamine on LTP and NMDAR EPSC in Hippocampus of the Chronic Social Defeat Stress Mice Model of Depression. Front. Behav. Neurosci. 2018, 12, 229. [Google Scholar] [CrossRef] [Green Version]
- Guo, D.; Gan, J.; Tan, T.; Tian, X.; Wang, G.; Ng, K.T. Neonatal exposure of ketamine inhibited the induction of hippocampal long-term potentiation without impairing the spatial memory of adult rats. Cogn. Neurodyn. 2018, 12, 377–383. [Google Scholar] [CrossRef]
- Chatterjee, M.; Verma, R.; Ganguly, S.; Palit, G. Neurochemical and molecular characterization of ketamine-induced experimental psychosis model in mice. Neuropharmacology 2012, 63, 1161–1171. [Google Scholar] [CrossRef]
- Kokkinou, M.; Ashok, A.H.; Howes, O.D. The effects of ketamine on dopaminergic function: Meta-analysis and review of the implications for neuropsychiatric disorders. Mol. Psychiatry 2018, 23, 59–69. [Google Scholar] [CrossRef] [Green Version]
- Lahti, A.C.; Koffel, B.; LaPorte, D.; Tamminga, C.A. Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology 1995, 13, 9–19. [Google Scholar] [CrossRef]
- Forrest, A.D.; Coto, C.A.; Siegel, S.J. Animal Models of Psychosis: Current State and Future Directions. Curr. Behav. Neurosci. Rep. 2014, 1, 100–116. [Google Scholar] [CrossRef] [Green Version]
- Moghaddam, B.; Krystal, J.H. Capturing the angel in “angel dust”: Twenty years of translational neuroscience studies of NMDA receptor antagonists in animals and humans. Schizophr. Bull. 2012, 38, 942–949. [Google Scholar] [CrossRef] [Green Version]
- Crews, F.; He, J.; Hodge, C. Adolescent cortical development: A critical period of vulnerability for addiction. Pharmacol. Biochem. Behav. 2007, 86, 189–199. [Google Scholar] [CrossRef]
- Casey, B.J.; Jones, R.M. Neurobiology of the adolescent brain and behavior: Implications for substance use disorders. J. Am. Acad. Child Adolesc. Psychiatry 2010, 49, 1189–1201. [Google Scholar] [CrossRef]
- Owen, M.J.; Sawa, A.; Mortensen, P.B. Schizophrenia. Lancet 2016, 388, 86–97. [Google Scholar] [CrossRef] [Green Version]
- Morgan, C.J.; Curran, H.V. Acute and chronic effects of ketamine upon human memory: A review. Psychopharmacology 2006, 188, 408–424. [Google Scholar] [CrossRef]
- Leuchter, A.F.; Hunter, A.M.; Krantz, D.E.; Cook, I.A. Rhythms and blues: Modulation of oscillatory synchrony and the mechanism of action of antidepressant treatments. Ann. N. Y. Acad. Sci. 2015, 1344, 78–91. [Google Scholar] [CrossRef] [Green Version]
- Ippolito, G.; Bertaccini, R.; Tarasi, L.; Di Gregorio, F.; Trajkovic, J.; Battaglia, S.; Romei, V. The Role of Alpha Oscillations among the Main Neuropsychiatric Disorders in the Adult and Developing Human Brain: Evidence from the Last 10 Years of Research. Biomedicines 2022, 10, 3189. [Google Scholar] [CrossRef]
- Di Gregorio, F.; La Porta, F.; Petrone, V.; Battaglia, S.; Orlandi, S.; Ippolito, G.; Romei, V.; Piperno, R.; Lullini, G. Accuracy of EEG Biomarkers in the Detection of Clinical Outcome in Disorders of Consciousness after Severe Acquired Brain Injury: Preliminary Results of a Pilot Study Using a Machine Learning Approach. Biomedicines 2022, 10, 1897. [Google Scholar] [CrossRef]
- Sankoorikal, G.M.; Kaercher, K.A.; Boon, C.J.; Lee, J.K.; Brodkin, E.S. A mouse model system for genetic analysis of sociability: C57BL/6J versus BALB/cJ inbred mouse strains. Biol. Psychiatry 2006, 59, 415–423. [Google Scholar] [CrossRef]
- Akillioglu, K.; Melik, E.B.; Melik, E.; Boga, A. Effect of ketamine on exploratory behaviour in BALB/C and C57BL/6 mice. Pharmacol. Biochem. Behav. 2012, 100, 513–517. [Google Scholar] [CrossRef]
- Brinks, V.; van der Mark, M.; de Kloet, R.; Oitzl, M. Emotion and cognition in high and low stress sensitive mouse strains: A combined neuroendocrine and behavioral study in BALB/c and C57BL/6J mice. Front. Behav. Neurosci. 2007, 1, 8. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.C.; Chan, M.H.; Lee, M.Y.; Chen, Y.C.; Chen, H.H. N,N-dimethylglycine differentially modulates psychotomimetic and antidepressant-like effects of ketamine in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 71, 7–13. [Google Scholar] [CrossRef]
- Lin, J.C.; Lee, M.Y.; Chan, M.H.; Chen, Y.C.; Chen, H.H. Betaine enhances antidepressant-like, but blocks psychotomimetic effects of ketamine in mice. Psychopharmacology 2016, 233, 3223–3235. [Google Scholar] [CrossRef]
- Irifune, M.; Shimizu, T.; Nomoto, M. Ketamine-induced hyperlocomotion associated with alteration of presynaptic components of dopamine neurons in the nucleus accumbens of mice. Pharmacol. Biochem. Behav. 1991, 40, 399–407. [Google Scholar] [CrossRef]
- Karwacki, Z.; Kowiański, P.; Moryś, J. General anaesthesia in rats undergoing experiments on the central nervous system. Folia Morphol. 2001, 60, 235–242. [Google Scholar]
- Becker, A.; Peters, B.; Schroeder, H.; Mann, T.; Huether, G.; Grecksch, G. Ketamine-induced changes in rat behaviour: A possible animal model of schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2003, 27, 687–700. [Google Scholar] [CrossRef]
- Trujillo, K.A.; Smith, M.L.; Sullivan, B.; Heller, C.Y.; Garcia, C.; Bates, M. The neurobehavioral pharmacology of ketamine: Implications for drug abuse, addiction, and psychiatric disorders. ILAR J. 2011, 52, 366–378. [Google Scholar] [CrossRef] [Green Version]
- Hefner, K.; Holmes, A. Ontogeny of fear-, anxiety- and depression-related behavior across adolescence in C57BL/6J mice. Behav. Brain Res. 2007, 176, 210–215. [Google Scholar] [CrossRef] [Green Version]
- Krystal, J.H.; Karper, L.P.; Seibyl, J.P.; Freeman, G.K.; Delaney, R.; Bremner, J.D.; Heninger, G.R.; Bowers, M.B., Jr.; Charney, D.S. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch. Gen. Psychiatry 1994, 51, 199–214. [Google Scholar] [CrossRef]
- Xu, K.; Krystal, J.H.; Ning, Y.; Chen, D.C.; He, H.; Wang, D.; Ke, X.; Zhang, X.; Ding, Y.; Liu, Y.; et al. Preliminary analysis of positive and negative syndrome scale in ketamine-associated psychosis in comparison with schizophrenia. J. Psychiatr. Res. 2015, 61, 64–72. [Google Scholar] [CrossRef] [Green Version]
- Schramm-Sapyta, N.L.; Walker, Q.D.; Caster, J.M.; Levin, E.D.; Kuhn, C.M. Are adolescents more vulnerable to drug addiction than adults? Evidence from animal models. Psychopharmacology 2009, 206, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Rocha, A.; Hart, N.; Trujillo, K.A. Differences between adolescents and adults in the acute effects of PCP and ketamine and in sensitization following intermittent administration. Pharmacol. Biochem. Behav. 2017, 157, 24–34. [Google Scholar] [CrossRef]
- Bates, M.L.S.; Trujillo, K.A. Long-lasting effects of repeated ketamine administration in adult and adolescent rats. Behav. Brain Res. 2019, 369, 111928. [Google Scholar] [CrossRef]
- Carola, V.; D’Olimpio, F.; Brunamonti, E.; Mangia, F.; Renzi, P. Evaluation of the elevated plus-maze and open-field tests for the assessment of anxiety-related behaviour in inbred mice. Behav. Brain Res. 2002, 134, 49–57. [Google Scholar] [CrossRef]
- Tang, X.; Orchard, S.M.; Sanford, L.D. Home cage activity and behavioral performance in inbred and hybrid mice. Behav. Brain Res. 2002, 136, 555–569. [Google Scholar] [CrossRef]
- Wiley, J.L.; Evans, R.L.; Grainger, D.B.; Nicholson, K.L. Age-dependent differences in sensitivity and sensitization to cannabinoids and ’club drugs’ in male adolescent and adult rats. Addict. Biol. 2008, 13, 277–286. [Google Scholar] [CrossRef]
- Parise, E.M.; Alcantara, L.F.; Warren, B.L.; Wright, K.N.; Hadad, R.; Sial, O.K.; Kroeck, K.G.; Iñiguez, S.D.; Bolaños-Guzmán, C.A. Repeated ketamine exposure induces an enduring resilient phenotype in adolescent and adult rats. Biol. Psychiatry 2013, 74, 750–759. [Google Scholar] [CrossRef] [Green Version]
- Hetzler, B.E.; Wautlet, B.S. Ketamine-induced locomotion in rats in an open-field. Pharmacol. Biochem. Behav. 1985, 22, 653–655. [Google Scholar] [CrossRef]
- Allain, F.; Minogianis, E.A.; Roberts, D.C.; Samaha, A.N. How fast and how often: The pharmacokinetics of drug use are decisive in addiction. Neurosci. Biobehav. Rev. 2015, 56, 166–179. [Google Scholar] [CrossRef] [Green Version]
- McCarthy, L.E.; Mannelli, P.; Niculescu, M.; Gingrich, K.; Unterwald, E.M.; Ehrlich, M.E. The distribution of cocaine in mice differs by age and strain. Neurotoxicol. Teratol. 2004, 26, 839–848. [Google Scholar] [CrossRef]
- Veilleux-Lemieux, D.; Castel, A.; Carrier, D.; Beaudry, F.; Vachon, P. Pharmacokinetics of ketamine and xylazine in young and old Sprague-Dawley rats. J. Am. Assoc. Lab. Anim. Sci. 2013, 52, 567–570. [Google Scholar]
- Olney, J.W.; Farber, N.B. NMDA antagonists as neurotherapeutic drugs, psychotogens, neurotoxins, and research tools for studying schizophrenia. Neuropsychopharmacology 1995, 13, 335–345. [Google Scholar] [CrossRef]
- Farber, N.B.; Olney, J.W. Drugs of abuse that cause developing neurons to commit suicide. Brain Res. Dev. Brain Res. 2003, 147, 37–45. [Google Scholar] [CrossRef]
- Cho, H.K.; Kim, K.W.; Jeong, Y.M.; Lee, H.S.; Lee, Y.J.; Hwang, S.H. Efficacy of ketamine in improving pain after tonsillectomy in children: Meta-analysis. PLoS ONE 2014, 9, e101259. [Google Scholar] [CrossRef] [Green Version]
- Colwell, C.S.; Cepeda, C.; Crawford, C.; Levine, M.S. Postnatal development of glutamate receptor-mediated responses in the neostriatum. Dev. Neurosci. 1998, 20, 154–163. [Google Scholar] [CrossRef]
- Henson, M.A.; Roberts, A.C.; Salimi, K.; Vadlamudi, S.; Hamer, R.M.; Gilmore, J.H.; Jarskog, L.F.; Philpot, B.D. Developmental regulation of the NMDA receptor subunits, NR3A and NR1, in human prefrontal cortex. Cereb. Cortex 2008, 18, 2560–2573. [Google Scholar] [CrossRef] [Green Version]
- Insel, T.R.; Miller, L.P.; Gelhard, R.E. The ontogeny of excitatory amino acid receptors in rat forebrain--I. N-methyl-D-aspartate and quisqualate receptors. Neuroscience 1990, 35, 31–43. [Google Scholar] [CrossRef]
- Luo, J.; Bosy, T.Z.; Wang, Y.; Yasuda, R.P.; Wolfe, B.B. Ontogeny of NMDA R1 subunit protein expression in five regions of rat brain. Brain Res. Dev. Brain Res. 1996, 92, 10–17. [Google Scholar] [CrossRef]
- Nowakowski, R.S. The mode of inheritance of a defect in lamination in the hippocampus of BALB/c mice. J. Neurogenet. 1984, 1, 249–258. [Google Scholar] [CrossRef]
- Akillioglu, K.; Binokay, S.; Kocahan, S. The effect of neonatal N-methyl-D-aspartate receptor blockade on exploratory and anxiety-like behaviors in adult BALB/c and C57BL/6 mice. Behav. Brain Res. 2012, 233, 157–161. [Google Scholar] [CrossRef]
- Jacome, L.F.; Burket, J.A.; Herndon, A.L.; Deutsch, S.I. D-Cycloserine enhances social exploration in the Balb/c mouse. Brain Res. Bull. 2011, 85, 141–144. [Google Scholar] [CrossRef]
- Jacome, L.F.; Burket, J.A.; Herndon, A.L.; Cannon, W.R.; Deutsch, S.I. D-serine improves dimensions of the sociability deficit of the genetically-inbred Balb/c mouse strain. Brain Res. Bull. 2011, 84, 12–16. [Google Scholar] [CrossRef]
- Burket, J.A.; Herndon, A.L.; Deutsch, S.I. Locomotor activity of the genetically inbred Balb/c mouse strain is suppressed by a socially salient stimulus. Brain Res. Bull. 2010, 83, 255–256. [Google Scholar] [CrossRef]
- Martos, D.; Tuka, B.; Tanaka, M.; Vécsei, L.; Telegdy, G. Memory Enhancement with Kynurenic Acid and Its Mechanisms in Neurotransmission. Biomedicines 2022, 10, 849. [Google Scholar] [CrossRef]
| Ketamine | C57BL/6J | BALB/c | ||
|---|---|---|---|---|
| Adolescent | Adult | Adolescent | Adult | |
| 0 mg/kg | 65.2 ± 7.0 | 66.8 ± 6.8 | 72.3 ± 13.8 | 82.3 ± 18.2 |
| 25 mg/kg | 175.0 ± 13.8 a | 130.9 ± 11.8 b | 85.5 ± 12.8 | 103.4 ± 14.5 |
| 50 mg/kg | 171.2 ± 15.7 a | 136.9 ± 8.1 b | 89.6 ± 17.5 | 52.8 ± 13.3 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.-C.; Wang, T.-S.; Chang, F.-Y.; Chen, P.-A.; Chen, Y.-C. Age, Dose, and Locomotion: Decoding Vulnerability to Ketamine in C57BL/6J and BALB/c Mice. Biomedicines 2023, 11, 1821. https://doi.org/10.3390/biomedicines11071821
Chen W-C, Wang T-S, Chang F-Y, Chen P-A, Chen Y-C. Age, Dose, and Locomotion: Decoding Vulnerability to Ketamine in C57BL/6J and BALB/c Mice. Biomedicines. 2023; 11(7):1821. https://doi.org/10.3390/biomedicines11071821
Chicago/Turabian StyleChen, Wen-Chien, Tzong-Shi Wang, Fang-Yu Chang, Po-An Chen, and Yi-Chyan Chen. 2023. "Age, Dose, and Locomotion: Decoding Vulnerability to Ketamine in C57BL/6J and BALB/c Mice" Biomedicines 11, no. 7: 1821. https://doi.org/10.3390/biomedicines11071821
APA StyleChen, W.-C., Wang, T.-S., Chang, F.-Y., Chen, P.-A., & Chen, Y.-C. (2023). Age, Dose, and Locomotion: Decoding Vulnerability to Ketamine in C57BL/6J and BALB/c Mice. Biomedicines, 11(7), 1821. https://doi.org/10.3390/biomedicines11071821







