Immunohistochemical Analysis of Single-Stranded DNA Binding Protein 2 in Non-Melanoma Skin Cancers
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Clinical Data Collection
2.2. Pathological Evaluation
2.3. SSBP2 Immunohistochemistry
2.4. Assessment of SSBP2 Expression
2.5. Statistical Analyses
2.6. Functional Analysis
3. Results
3.1. Clinicopathological Characteristics
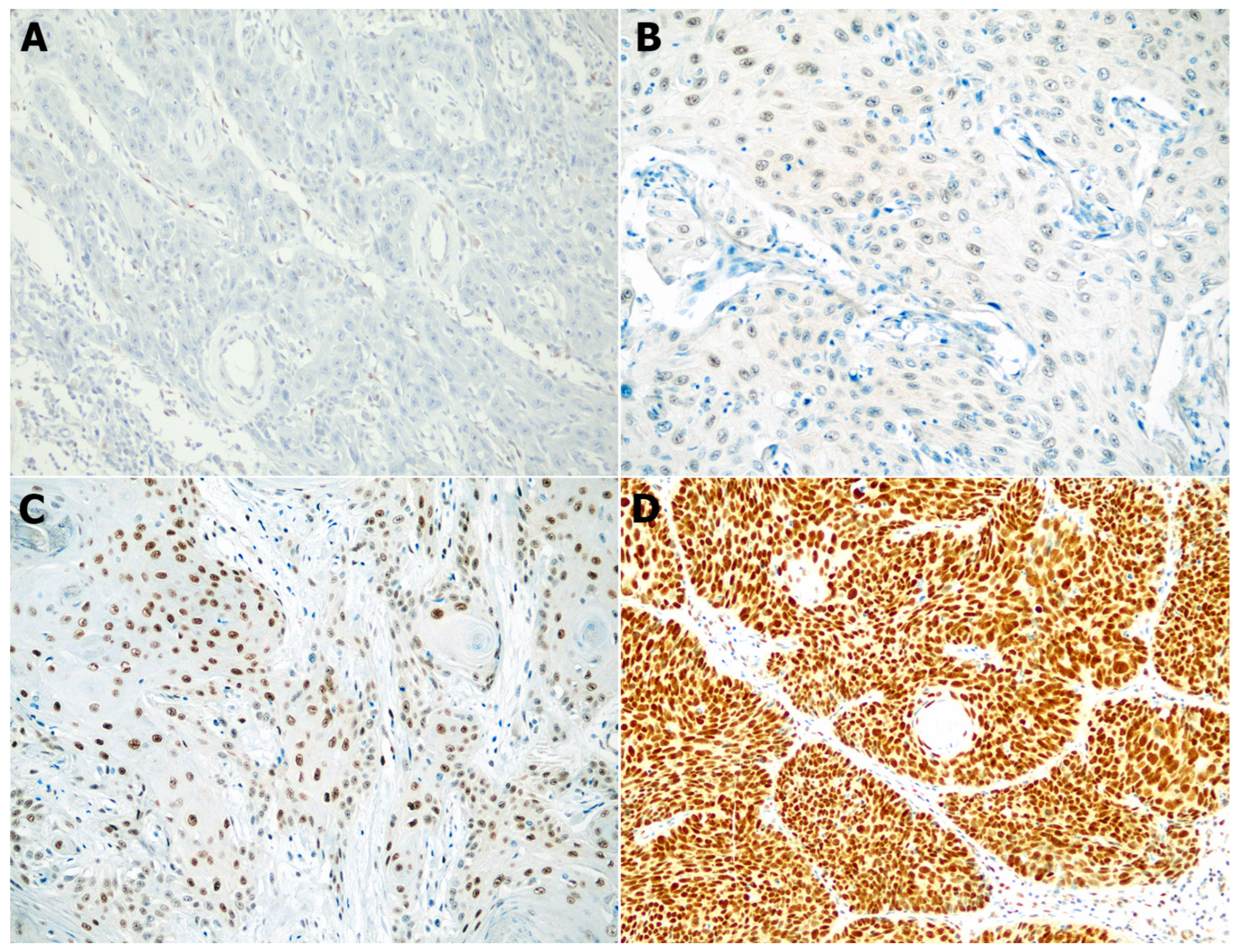
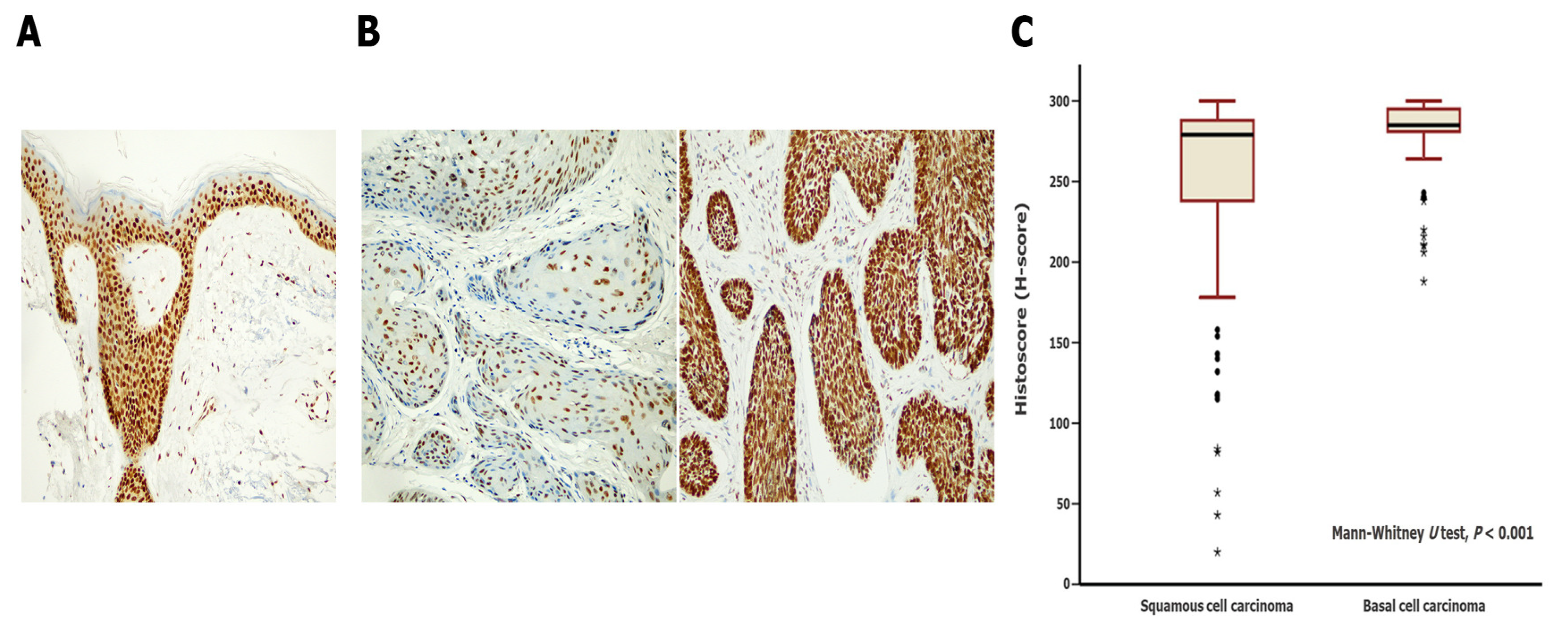
3.2. Differences in SSBP2 Expression between SCC and BCC
3.3. Clinicopathological Implications of Low SSBP2 Expression in SCC and BCC
3.4. Enrichment Analysis on SSBP2-Related Gene Sets in SCC
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Simoes, M.C.F.; Sousa, J.J.S.; Pais, A. Skin cancer and new treatment perspectives: A review. Cancer Lett. 2015, 357, 8–42. [Google Scholar] [CrossRef] [PubMed]
- Barton, V.; Armeson, K.; Hampras, S.; Ferris, L.K.; Visvanathan, K.; Rollison, D.; Alberg, A.J. Nonmelanoma skin cancer and risk of all-cause and cancer-related mortality: A systematic review. Arch. Dermatol. Res. 2017, 309, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Ciazynska, M.; Kaminska-Winciorek, G.; Lange, D.; Lewandowski, B.; Reich, A.; Slawinska, M.; Pabianek, M.; Szczepaniak, K.; Hankiewicz, A.; Ulanska, M.; et al. The incidence and clinical analysis of non-melanoma skin cancer. Sci. Rep. 2021, 11, 4337. [Google Scholar] [CrossRef]
- Gordon, R. Skin cancer: An overview of epidemiology and risk factors. Semin. Oncol. Nurs. 2013, 29, 160–169. [Google Scholar] [CrossRef]
- Castro, P.; Liang, H.; Liang, J.C.; Nagarajan, L. A novel, evolutionarily conserved gene family with putative sequence-specific single-stranded DNA-binding activity. Genomics 2002, 80, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Samanta, S.; Nagarajan, L. SSBP2, a candidate tumor suppressor gene, induces growth arrest and differentiation of myeloid leukemia cells. Oncogene 2005, 24, 2625–2634. [Google Scholar] [CrossRef]
- Wang, Y.; Klumpp, S.; Amin, H.M.; Liang, H.; Li, J.; Estrov, Z.; Zweidler-McKay, P.; Brandt, S.J.; Agulnick, A.; Nagarajan, L. SSBP2 is an in vivo tumor suppressor and regulator of LDB1 stability. Oncogene 2010, 29, 3044–3053. [Google Scholar] [CrossRef]
- Liu, J.W.; Nagpal, J.K.; Sun, W.; Lee, J.; Kim, M.S.; Ostrow, K.L.; Zhou, S.; Jeronimo, C.; Henrique, R.; Van Criekinge, W.; et al. ssDNA-binding protein 2 is frequently hypermethylated and suppresses cell growth in human prostate cancer. Clin. Cancer Res. 2008, 14, 3754–3760. [Google Scholar] [CrossRef]
- Huang, Y.; Chang, X.; Lee, J.; Cho, Y.G.; Zhong, X.; Park, I.S.; Liu, J.W.; Califano, J.A.; Ratovitski, E.A.; Sidransky, D.; et al. Cigarette smoke induces promoter methylation of single-stranded DNA-binding protein 2 in human esophageal squamous cell carcinoma. Int. J. Cancer 2011, 128, 2261–2273. [Google Scholar] [CrossRef]
- Kim, H.; Kim, Y.; Bang, S.; Park, S.; Jee, S.; Sim, J.; Shin, S.J.; Paik, S.S.; Jang, K. Low Expression of Single-stranded DNA Binding Protein 2 (SSBP2) Predicts Unfavourable Postoperative Outcomes in Patients With Clear Cell Renal Cell Carcinoma. In Vivo 2020, 34, 101–107. [Google Scholar] [CrossRef]
- Bang, S.; Kim, H.; Jang, K.; Paik, S.S.; Shin, S.J. The loss of nuclear expression of single-stranded DNA binding protein 2 of gastric adenocarcinoma and its prognostic role: Analysis of molecular subtype. PLoS ONE 2020, 15, e0236896. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.; Kim, H.; Bang, S.; Jang, K.; Paik, S.S.; Shin, S.J. Nuclear Expression Loss of SSBP2 Is Associated with Poor Prognostic Factors in Colorectal Adenocarcinoma. Diagnostics 2020, 10, 1097. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Jee, S.; Son, H.; Cha, H.; Bang, S.; Kim, H.; Shin, S.J.; Cha, C.; Chung, M.S.; Myung, J.; et al. Loss of Single-Stranded DNA Binding Protein 2 Expression Is Associated with Aggressiveness and Poor Overall Survival in Patients with Invasive Breast Carcinoma. Diagnostics 2022, 12, 487. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Decker, P.A.; Rice, T.; McCoy, L.S.; Smirnov, I.; Patoka, J.S.; Hansen, H.M.; Wiemels, J.L.; Tihan, T.; Prados, M.D.; et al. SSBP2 variants are associated with survival in glioblastoma patients. Clin. Cancer Res. 2012, 18, 3154–3162. [Google Scholar] [CrossRef]
- Kim, H.; Kim, Y.; Chung, Y.; Abdul, R.; Sim, J.; Ahn, H.; Shin, S.J.; Paik, S.S.; Kim, H.J.; Jang, K.; et al. Single-stranded DNA binding protein 2 expression is associated with patient survival in hepatocellular carcinoma. BMC Cancer 2018, 18, 1244. [Google Scholar] [CrossRef]
- Matos, L.L.; Trufelli, D.C.; de Matos, M.G.; da Silva Pinhal, M.A. Immunohistochemistry as an important tool in biomarkers detection and clinical practice. Biomark. Insights 2010, 5, 9–20. [Google Scholar] [CrossRef]
- Sanders, D.; Carr, R. The use of immunohistochemistry in the differential diagnosis of common epithelial tumours of the skin. Curr. Diagn. Pathol. 2007, 13, 237–251. [Google Scholar] [CrossRef]
- Balasescu, E.; Gheorghe, A.C.; Moroianu, A.; Turcu, G.; Brinzea, A.; Antohe, M.; Hodorogea, A.; Manea, L.; Balaban, M.; Andrei, R.; et al. Role of immunohistochemistry in the diagnosis and staging of cutaneous squamous-cell carcinomas (Review). Exp. Ther. Med. 2022, 23, 383. [Google Scholar] [CrossRef]
- Inman, G.J.; Wang, J.; Nagano, A.; Alexandrov, L.B.; Purdie, K.J.; Taylor, R.G.; Sherwood, V.; Thomson, J.; Hogan, S.; Spender, L.C.; et al. The genomic landscape of cutaneous SCC reveals drivers and a novel azathioprine associated mutational signature. Nat. Commun. 2018, 9, 3667. [Google Scholar] [CrossRef]
- Moon, D.; Randall, G.; Higgins, S.; Sutton, A.V.; Wysong, A. Misclassification of Aggressive Basal Cell Carcinoma Subtypes and Implications for Management. Dermatol. Surg. 2021, 47, 593–598. [Google Scholar] [CrossRef]
- Camela, E.; Ilut Anca, P.; Lallas, K.; Papageorgiou, C.; Manoli, S.M.; Gkentsidi, T.; Eftychidou, P.; Liopyris, K.; Sgouros, D.; Apalla, Z.; et al. Dermoscopic Clues of Histopathologically Aggressive Basal Cell Carcinoma Subtypes. Medicina 2023, 59, 349. [Google Scholar] [CrossRef] [PubMed]
- Stratigos, A.; Garbe, C.; Lebbe, C.; Malvehy, J.; del Marmol, V.; Pehamberger, H.; Peris, K.; Becker, J.C.; Zalaudek, I.; Saiag, P.; et al. Diagnosis and treatment of invasive squamous cell carcinoma of the skin: European consensus-based interdisciplinary guideline. Eur. J. Cancer 2015, 51, 1989–2007. [Google Scholar] [CrossRef]
- Kallioniemi, O.P.; Wagner, U.; Kononen, J.; Sauter, G. Tissue microarray technology for high-throughput molecular profiling of cancer. Hum. Mol. Genet. 2001, 10, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Birkbak, N.J.; Gyorffy, B.; Szallasi, Z.; Eklund, A.C. Jetset: Selecting the optimal microarray probe set to represent a gene. BMC Bioinform. 2011, 12, 474. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Tan, Q.; Kir, J.; Liu, D.; Bryant, D.; Guo, Y.; Stephens, R.; Baseler, M.W.; Lane, H.C.; et al. DAVID Bioinformatics Resources: Expanded annotation database and novel algorithms to better extract biology from large gene lists. Nucleic Acids Res. 2007, 35, W169–W175. [Google Scholar] [CrossRef] [PubMed]
- Criscione, V.D.; Weinstock, M.A.; Naylor, M.F.; Luque, C.; Eide, M.J.; Bingham, S.F.; Department of Veteran Affairs Topical Tretinoin Chemoprevention Trial Group. Actinic keratoses: Natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer 2009, 115, 2523–2530. [Google Scholar] [CrossRef]
- Smoller, B.R. Squamous cell carcinoma: From precursor lesions to high-risk variants. Mod. Pathol. 2006, 19 (Suppl. 2), S88–S92. [Google Scholar] [CrossRef]
- Kim, Y.S.; Shin, S.; Jung, S.H.; Park, Y.M.; Park, G.S.; Lee, S.H.; Chung, Y.J. Genomic Progression of Precancerous Actinic Keratosis to Squamous Cell Carcinoma. J. Investig. Dermatol. 2022, 142, 528–538. [Google Scholar] [CrossRef]
- Thomson, J.; Bewicke-Copley, F.; Anene, C.A.; Gulati, A.; Nagano, A.; Purdie, K.; Inman, G.J.; Proby, C.M.; Leigh, I.M.; Harwood, C.A.; et al. The Genomic Landscape of Actinic Keratosis. J. Investig. Dermatol. 2021, 141, 1664–1674. [Google Scholar] [CrossRef]
- Hedberg, M.L.; Berry, C.T.; Moshiri, A.S.; Xiang, Y.; Yeh, C.J.; Attilasoy, C.; Capell, B.C.; Seykora, J.T. Molecular Mechanisms of Cutaneous Squamous Cell Carcinoma. Int. J. Mol. Sci. 2022, 23, 3478. [Google Scholar] [CrossRef]
- Tilli, C.M.; Van Steensel, M.A.; Krekels, G.A.; Neumann, H.A.; Ramaekers, F.C. Molecular aetiology and pathogenesis of basal cell carcinoma. Br. J. Dermatol. 2005, 152, 1108–1124. [Google Scholar] [CrossRef] [PubMed]
- Goppner, D.; Leverkus, M. Basal cell carcinoma: From the molecular understanding of the pathogenesis to targeted therapy of progressive disease. J. Skin Cancer 2011, 2011, 650258. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, Y.S.; Lee, C.; Shin, M.S.; Kim, J.W.; Jang, B.G. Expression profile of sonic hedgehog signaling-related molecules in basal cell carcinoma. PLoS ONE 2019, 14, e0225511. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, C.; Maturo, M.G.; Di Nardo, L.; Ciciarelli, V.; Gutierrez Garcia-Rodrigo, C.; Fargnoli, M.C. Understanding the Molecular Genetics of Basal Cell Carcinoma. Int. J. Mol. Sci. 2017, 18, 2485. [Google Scholar] [CrossRef]
- Kilgour, J.M.; Jia, J.L.; Sarin, K.Y. Review of the Molecular Genetics of Basal Cell Carcinoma; Inherited Susceptibility, Somatic Mutations, and Targeted Therapeutics. Cancers 2021, 13, 3870. [Google Scholar] [CrossRef]
- van Meyel, D.J.; Thomas, J.B.; Agulnick, A.D. Ssdp proteins bind to LIM-interacting co-factors and regulate the activity of LIM-homeodomain protein complexes in vivo. Development 2003, 130, 1915–1925. [Google Scholar] [CrossRef]
- Xu, Z.; Meng, X.; Cai, Y.; Liang, H.; Nagarajan, L.; Brandt, S.J. Single-stranded DNA-binding proteins regulate the abundance of LIM domain and LIM domain-binding proteins. Genes. Dev. 2007, 21, 942–955. [Google Scholar] [CrossRef]
- Li, J.; Kurasawa, Y.; Wang, Y.; Clise-Dwyer, K.; Klumpp, S.A.; Liang, H.; Tailor, R.C.; Raymond, A.C.; Estrov, Z.; Brandt, S.J.; et al. Requirement for ssbp2 in hematopoietic stem cell maintenance and stress response. J. Immunol. 2014, 193, 4654–4662. [Google Scholar] [CrossRef]
- Wang, H.; Kim, J.; Wang, Z.; Yan, X.X.; Dean, A.; Xu, W. Crystal structure of human LDB1 in complex with SSBP2. Proc. Natl. Acad. Sci. USA 2020, 117, 1042–1048. [Google Scholar] [CrossRef]
- Zhu, M.; Jiang, B.; Zuo, H.; Wang, X.; Ge, H.; Huang, Z. LIM-Domain-Binding Protein 1 Mediates Cell Proliferation and Drug Resistance in Colorectal Cancer. Front. Surg. 2021, 8, 790380. [Google Scholar] [CrossRef]
- Matthews, J.M.; Lester, K.; Joseph, S.; Curtis, D.J. LIM-domain-only proteins in cancer. Nat. Rev. Cancer 2013, 13, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Garcia, S.A.; Swiersy, A.; Radhakrishnan, P.; Branchi, V.; Kanth Nanduri, L.; Gyorffy, B.; Betzler, A.M.; Bork, U.; Kahlert, C.; Reissfelder, C.; et al. LDB1 overexpression is a negative prognostic factor in colorectal cancer. Oncotarget 2016, 7, 84258–84270. [Google Scholar] [CrossRef] [PubMed]
- Simonik, E.A.; Cai, Y.; Kimmelshue, K.N.; Brantley-Sieders, D.M.; Loomans, H.A.; Andl, C.D.; Westlake, G.M.; Youngblood, V.M.; Chen, J.; Yarbrough, W.G.; et al. LIM-Only Protein 4 (LMO4) and LIM Domain Binding Protein 1 (LDB1) Promote Growth and Metastasis of Human Head and Neck Cancer (LMO4 and LDB1 in Head and Neck Cancer). PLoS ONE 2016, 11, e0164804. [Google Scholar] [CrossRef] [PubMed]
- Kagohara, L.T.; Schussel, J.L.; Subbannayya, T.; Sahasrabuddhe, N.; Lebron, C.; Brait, M.; Maldonado, L.; Valle, B.L.; Pirini, F.; Jahuira, M.; et al. Global and gene-specific DNA methylation pattern discriminates cholecystitis from gallbladder cancer patients in Chile. Future Oncol. 2015, 11, 233–249. [Google Scholar] [CrossRef]
- Brait, M.; Maldonado, L.; Noordhuis, M.G.; Begum, S.; Loyo, M.; Poeta, M.L.; Barbosa, A.; Fazio, V.M.; Angioli, R.; Rabitti, C.; et al. Association of promoter methylation of VGF and PGP9.5 with ovarian cancer progression. PLoS ONE 2013, 8, e70878. [Google Scholar] [CrossRef]

| Parameters | SSBP2 Expression | p Value | |
|---|---|---|---|
| High Expression (%) (n = 58) | Low Expression (%) (n = 12) | ||
| Age | 0.490 * | ||
| <70 years | 14 (77.8%) | 4 (22.2%) | |
| ≥70 years | 44 (84.6%) | 8 (15.4%) | |
| Sex | 0.112 * | ||
| Female | 31 (91.2%) | 3 (8.8%) | |
| Male | 27 (75.0%) | 9 (25.0%) | |
| Tumor size † | 0.148 | ||
| <2.0 cm | 37 (84.1%) | 7 (15.9%) | |
| ≥2.0 cm | 10 (66.7%) | 5 (33.3%) | |
| Location | 0.678 * | ||
| Sun-protected | 8 (80%) | 2 (20%) | |
| Sun-damaged | 50 (83.3%) | 10 (16.7%) | |
| Ulceration | 0.005 * | ||
| Not identified | 45 (91.8%) | 4 (8.2%) | |
| Present | 13 (61.9%) | 8 (38.1%) | |
| Perineural invasion | 1.000 * | ||
| Not identified | 54 (81.8%) | 12 (18.2%) | |
| Present | 4 (100%) | 0 (0%) | |
| Local recurrence | 0.058 * | ||
| No | 55 (85.9%) | 9 (14.1%) | |
| Yes | 3 (50.0%) | 3 (50.0%) | |
| Lymph node metastasis | 0.133 * | ||
| No | 56 (84.8%) | 10 (15.2%) | |
| Yes | 2 (50.0%) | 2 (50.0%) | |
| Histological grade | 0.166 * | ||
| Grade 1 | 41 (78.8%) | 11 (21.2%) | |
| Grade 2 or 3 | 17 (94.4%) | 1 (5.6%) | |
| Level of invasion | 0.012 | ||
| Above subcutis | 45 (90.0%) | 5 (10.0%) | |
| Below subcutis | 13 (65.0%) | 7 (35.0%) | |
| Parameters | SSBP2 Expression | p Value | |
|---|---|---|---|
| High Expression (%) (n = 116) | Low Expression (%) (n = 30) | ||
| Age | 0.498 | ||
| <70 years | 50 (76.9%) | 15 (23.1%) | |
| ≥70 years | 66 (81.5%) | 15 (18.5%) | |
| Sex | 0.266 | ||
| Female | 71 (82.6%) | 15 (17.4%) | |
| Male | 45 (75.0%) | 15 (25.0%) | |
| Tumor size † | 1.000 * | ||
| <2.0 cm | 81 (80.2%) | 20 (19.8%) | |
| ≥2.0 cm | 10 (83.3%) | 2 (16.7%) | |
| Location | 0.765 * | ||
| Sun-protected | 16 (84.2%) | 3 (15.8%) | |
| Sun-damaged | 100 (78.7%) | 27 (21.3%) | |
| Ulceration | 1.000 * | ||
| Not identified | 101 (78.9%) | 27 (21.1%) | |
| Present | 15 (83.3%) | 3 (16.7%) | |
| Perineural invasion | 0.187 * | ||
| Not identified | 114 (80.3%) | 28 (19.7%) | |
| Present | 2 (50.0%) | 2 (50.0%) | |
| Local recurrence | 0.581 * | ||
| No | 112 (78.9%) | 30 (21.1%) | |
| Yes | 4 (100%) | 0 (0%) | |
| Histological subtypes ‡ | 0.413 | ||
| Less aggressive and others | 86 (81.1%) | 20 (18.9%) | |
| Aggressive | 30 (75.0%) | 10 (25.0%) | |
| Level of invasion | 0.224 * | ||
| Above subcutis | 87 (77.0%) | 26 (23.0%) | |
| Below subcutis | 29 (87.9%) | 4 (12.1%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bang, S.; Son, H.; Cha, H.; Song, K.; Park, H.; Kim, H.; Ko, J.Y.; Myung, J.; Paik, S. Immunohistochemical Analysis of Single-Stranded DNA Binding Protein 2 in Non-Melanoma Skin Cancers. Biomedicines 2023, 11, 1818. https://doi.org/10.3390/biomedicines11071818
Bang S, Son H, Cha H, Song K, Park H, Kim H, Ko JY, Myung J, Paik S. Immunohistochemical Analysis of Single-Stranded DNA Binding Protein 2 in Non-Melanoma Skin Cancers. Biomedicines. 2023; 11(7):1818. https://doi.org/10.3390/biomedicines11071818
Chicago/Turabian StyleBang, Seongsik, Hwangkyu Son, Hyebin Cha, Kihyuk Song, Hosub Park, Hyunsung Kim, Joo Yeon Ko, Jaekyung Myung, and Seungsam Paik. 2023. "Immunohistochemical Analysis of Single-Stranded DNA Binding Protein 2 in Non-Melanoma Skin Cancers" Biomedicines 11, no. 7: 1818. https://doi.org/10.3390/biomedicines11071818
APA StyleBang, S., Son, H., Cha, H., Song, K., Park, H., Kim, H., Ko, J. Y., Myung, J., & Paik, S. (2023). Immunohistochemical Analysis of Single-Stranded DNA Binding Protein 2 in Non-Melanoma Skin Cancers. Biomedicines, 11(7), 1818. https://doi.org/10.3390/biomedicines11071818






