Efficacy of Early Optimization of Infliximab Guided by Therapeutic Drug Monitoring during Induction—A Prospective Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Population
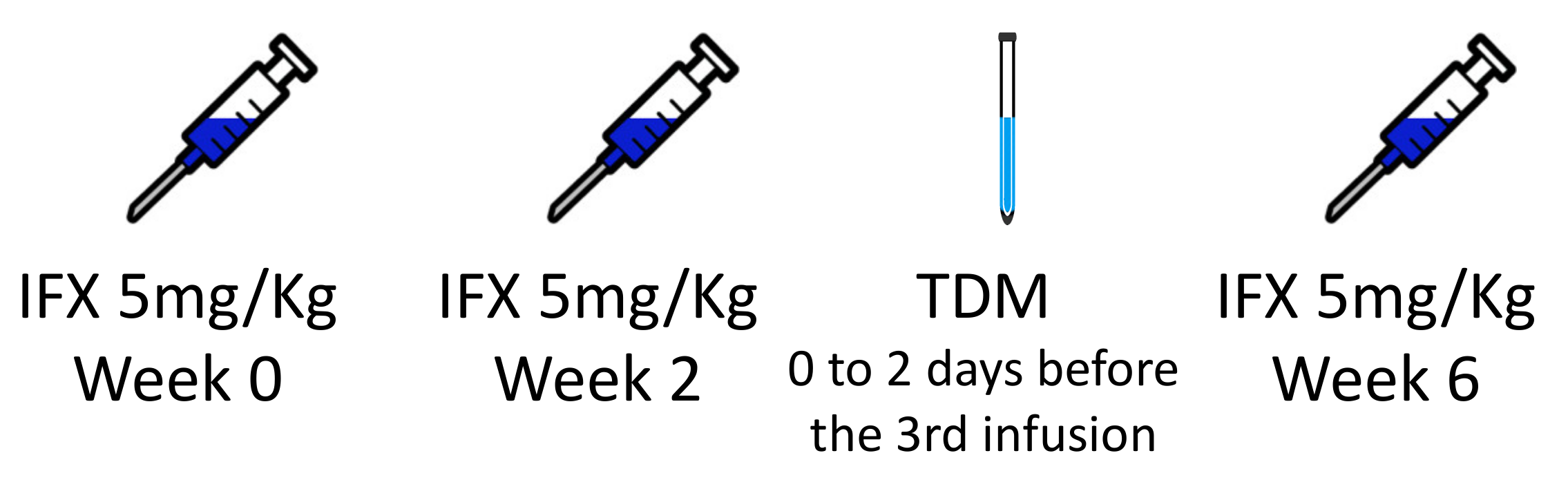
2.2. Infliximab Therapeutic Drug Monitoring
2.3. Data Collection
2.4. Outcomes Measures
2.5. Statistical Analysis
2.6. Ethical Considerations
3. Results
3.1. Study Population Characteristics
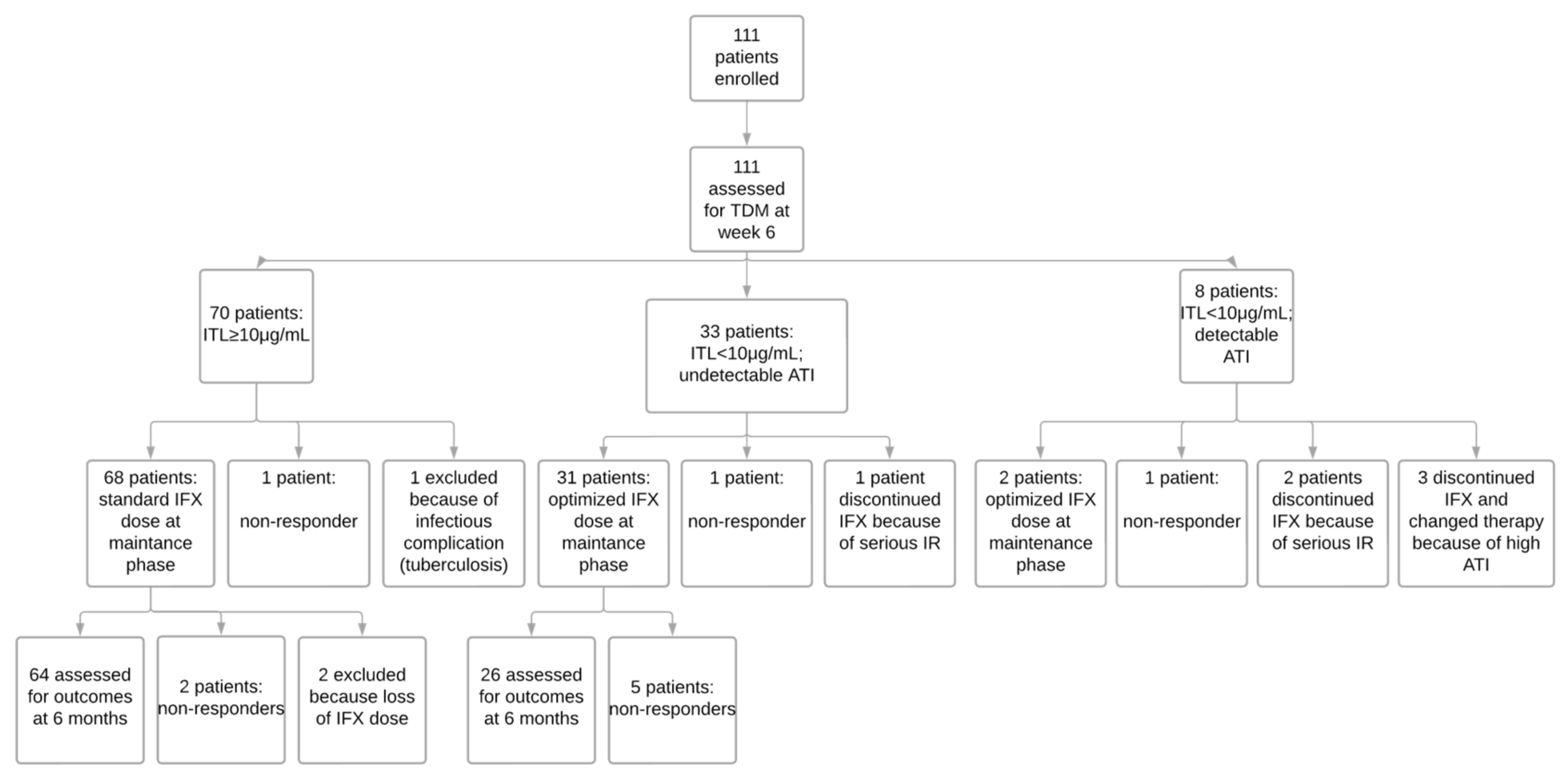
3.2. Post-Induction Strategy According to the Algorithm
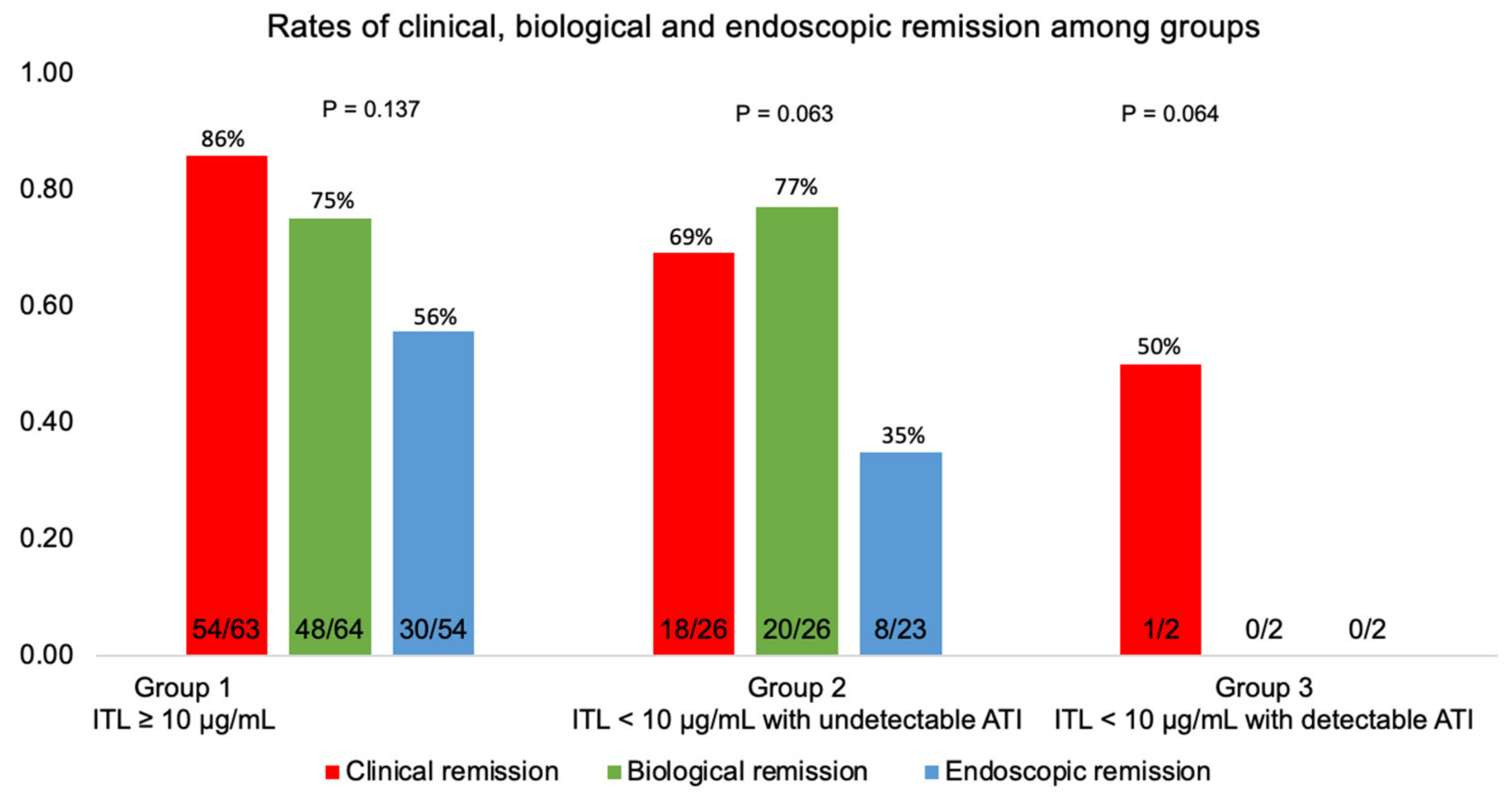
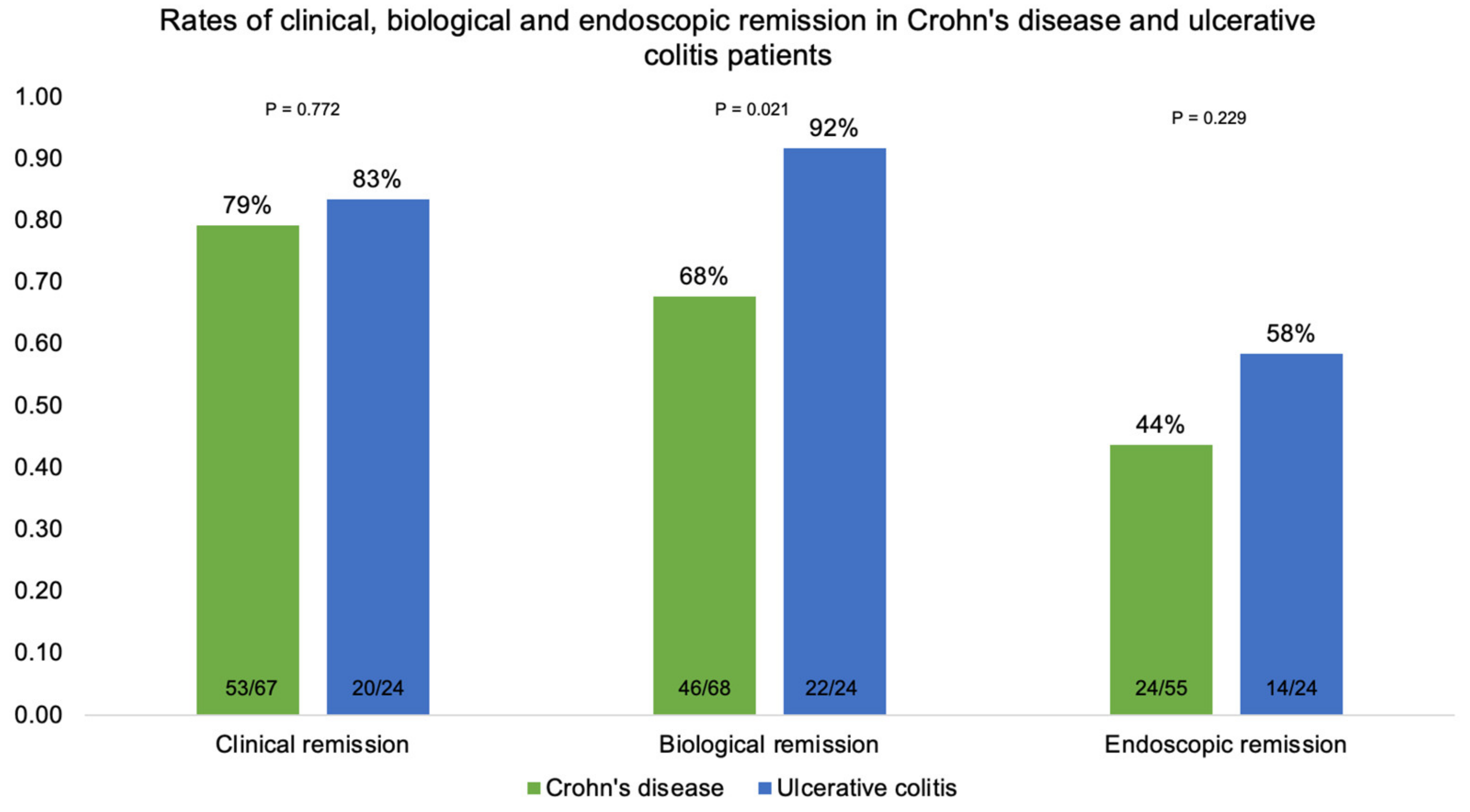
3.3. Clinical Remission at Six Months
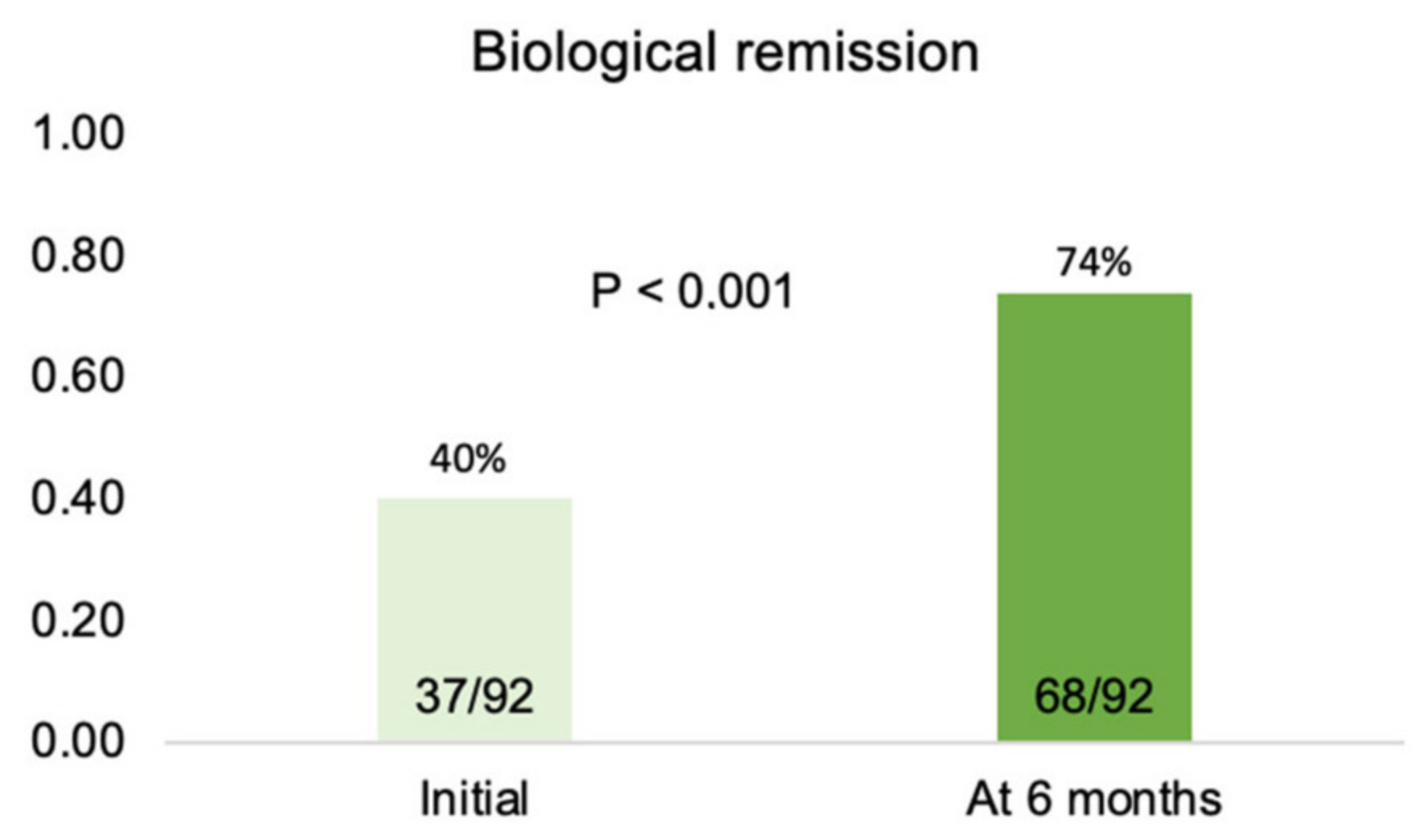
3.4. Biological Remission at Six Months
3.5. Endoscopic Remission at Six Months
3.6. Predictors of Outcomes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hanauer, S.B.; Feagan, B.G.; Lichtenstein, G.R.; Mayer, L.F.; Schreiber, S.; Colombel, J.F.; Rachmilewitz, D.; Wolf, D.C.; Olson, A.; Bao, W.; et al. Maintenance infliximab for Crohn’s disease: The ACCENT I randomised trial. Lancet 2002, 359, 1541–1549. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Fumery, M.; Sandborn, W.J.; Murad, M.H. Systematic review with network meta-analysis: First- and second-line pharmacotherapy for moderate-severe ulcerative colitis. Aliment. Pharmacol. Ther. 2018, 47, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Rutgeerts, P.; Sandborn, W.J.; Feagan, B.G.; Reinisch, W.; Olson, A.; Johanns, J.; Travers, S.; Rachmilewitz, D.; Hanauer, S.B.; Lichtenstein, G.R.; et al. Infliximab for Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2005, 353, 2462–2476. [Google Scholar] [CrossRef] [PubMed]
- Ben-Horin, S.; Chowers, Y. Review article: Loss of response to anti-TNF treatments in Crohn’s disease. Aliment. Pharmacol. Ther. 2011, 33, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Papamichael, K.; Gils, A.; Rutgeerts, P.; Levesque, B.G.; Vermeire, S.; Sandborn, W.J.; Vande Casteele, N. Role for therapeutic drug monitoring during induction therapy with TNF antagonists in IBD: Evolution in the definition and management of primary nonresponse. Inflamm. Bowel Dis. 2015, 21, 182–197. [Google Scholar] [CrossRef]
- Allez, M.; Karmiris, K.; Louis, E.; van Assche, G.; Ben-Horin, S.; Klein, A.; Van der Woude, J.; Baert, F.; Eliakim, R.; Katsanos, K.; et al. Report of the ECCO pathogenesis workshop on anti-TNF therapy failures in inflammatory bowel diseases: Definitions, frequency and pharmacological aspects. J. Crohn’s Colitis 2010, 4, 355–366. [Google Scholar] [CrossRef]
- Hemperly, A.; Vande Casteele, N. Clinical Pharmacokinetics and Pharmacodynamics of Infliximab in the Treatment of Inflammatory Bowel Disease. Clin. Pharmacokinet. 2018, 57, 929–942. [Google Scholar] [CrossRef]
- Adedokun, O.J.; Sandborn, W.J.; Feagan, B.G.; Rutgeerts, P.; Xu, Z.; Marano, C.W.; Johanns, J.; Zhou, H.; Davis, H.M.; Cornillie, F.; et al. Association between serum concentration of infliximab and efficacy in adult patients with ulcerative colitis. Gastroenterology 2014, 147, 1296–1307.e5. [Google Scholar] [CrossRef]
- Papamichael, K.; Rakowsky, S.; Rivera, C.; Cheifetz, A.S.; Osterman, M.T. Infliximab trough concentrations during maintenance therapy are associated with endoscopic and histologic healing in ulcerative colitis. Aliment. Pharmacol. Ther. 2018, 47, 478–484. [Google Scholar] [CrossRef]
- Sánchez-Hernández, J.G.; Rebollo, N.; Martin-Suarez, A.; Calvo, M.V.; Muñoz, F. A 3-year prospective study of a multidisciplinary early proactive therapeutic drug monitoring programme of infliximab treatments in inflammatory bowel disease. Br. J. Clin. Pharmacol. 2020, 86, 1165–1175. [Google Scholar] [CrossRef]
- Sparrow, M.P.; Papamichael, K.; Ward, M.G.; Riviere, P.; Laharie, D.; Paul, S.; Roblin, X. Therapeutic Drug Monitoring of Biologics during Induction to Prevent Primary Non-Response. J. Crohn’s Colitis 2020, 14, 543–556. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, B.P.; Martinez-Vazquez, M.; Patwardhan, V.R.; Moss, A.C.; Sandborn, W.J.; Cheifetz, A.S. Proactive Therapeutic Concentration Monitoring of Infliximab May Improve Outcomes for Patients with Inflammatory Bowel Disease: Results from a Pilot Observational Study Byron. Inflamm. Bowel Dis. 2014, 20, 1996–2003. [Google Scholar] [CrossRef]
- Vande Casteele, N.; Ferrante, M.; Van Assche, G.; Ballet, V.; Compernolle, G.; Van Steen, K.; Simoens, S.; Rutgeerts, P.; Gils, A.; Vermeire, S. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology 2015, 148, 1320–1329.e3. [Google Scholar] [CrossRef] [PubMed]
- Papamichael, K.; Vande Casteele, N.; Ferrante, M.; Gils, A.; Cheifetz, A.S. Therapeutic Drug Monitoring during Induction of Anti-Tumor Necrosis Factor Therapy in Inflammatory Bowel Disease: Defining a Therapeutic Drug Window. Inflamm. Bowel Dis. 2017, 23, 1510–1515. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.; Ricciuto, A.; Lewis, A.; D’Amico, F.; Dhaliwal, J.; Griffiths, A.M.; Bettenworth, D.; Sandborn, W.J.; Sands, B.E.; Reinisch, W.; et al. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology 2021, 160, 1570–1583. [Google Scholar] [CrossRef]
- Colombel, J.F.; Adedokun, O.J.; Gasink, C.; Gao, L.L.; Cornillie, F.J.; D’Haens, G.R.; Rutgeerts, P.J.; Reinisch, W.; Sandborn, W.J.; Hanauer, S.B. Combination Therapy with Infliximab and Azathioprine Improves Infliximab Pharmacokinetic Features and Efficacy: A Post Hoc Analysis. Clin. Gastroenterol. Hepatol. 2019, 17, 1525–1532.e1. [Google Scholar] [CrossRef]
- Papamichael, K.; Afif, W.; Drobne, D.; Dubinsky, M.C.; Ferrante, M.; Irving, P.M.; Kamperidis, N.; Kobayashi, T.; Kotze, P.G.; Lambert, J.; et al. Therapeutic drug monitoring of biologics in inflammatory bowel disease: Unmet needs and future perspectives. Lancet Gastroenterol. Hepatol. 2022, 7, 171–185. [Google Scholar] [CrossRef]
- Melmed, G.Y.; Irving, P.M.; Jones, J.; Kaplan, G.G.; Kozuch, P.L.; Velayos, F.S.; Baidoo, L.; Sparrow, M.P.; Bressler, B.; Cheifetz, A.S.; et al. Appropriateness of Testing for Anti–Tumor Necrosis Factor Agent and Antibody Concentrations, and Interpretation of Results. Clin. Gastroenterol. Hepatol. 2016, 14, 1302–1309. [Google Scholar] [CrossRef]
- Cheifetz, A.S.; Abreu, M.T.; Afif, W.; Cross, R.K.; Dubinsky, M.C.; Loftus, E.V.; Osterman, M.T.; Saroufim, A.; Siegel, C.A.; Yarur, A.J.; et al. A Comprehensive Literature Review and Expert Consensus Statement on Therapeutic Drug Monitoring of Biologics in Inflammatory Bowel Disease. Am. J. Gastroenterol. 2021, 116, 2014–2025. [Google Scholar] [CrossRef]
- Feuerstein, J.D.; Nguyen, G.C.; Kupfer, S.S.; Falck-Ytter, Y.; Singh, S.; American Gastroenterological Association Institute Clinical Guidelines Committee. American Gastroenterological Association Institute Guideline on Therapeutic Drug Monitoring in Inflammatory Bowel Disease. Gastroenterology 2017, 153, 827–834. [Google Scholar] [CrossRef]
- Bossuyt, P.; Pouillon, L.; Claeys, S.; D’Haens, S.; Hoefkens, E.; Strubbe, B.; Marichal, D.; Peeters, H. Ultra-proactive Therapeutic Drug Monitoring of Infliximab Based on Point of Care Testing in Inflammatory Bowel Disease: Results of a Pragmatic Trial. J. Crohn’s Colitis 2022, 16, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Brandse, J.F.; van den Brink, G.R.; Wildenberg, M.E.; van der Kleij, D.; Rispens, T.; Jansen, J.M.; Mathôt, R.A.; Ponsioen, C.Y.; Löwenberg, M.; D’Haens, G.R. Loss of infliximab into feces is associated with lack of response to therapy in patients with severe ulcerative colitis. Gastroenterology 2015, 149, 350–355.e2. [Google Scholar] [CrossRef] [PubMed]
- Vermeire, S.; Dreesen, E.; Papamichael, K.; Dubinsky, M.C. How, When, and for Whom Should We Perform Therapeutic Drug Monitoring? Clin. Gastroenterol. Hepatol. 2020, 18, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- Papamichael, K.; Van Stappen, T.; Vande Casteele, N.; Gils, A.; Billiet, T.; Tops, S.; Claes, K.; Van Assche, G.; Rutgeerts, P.; Vermeire, S.; et al. Infliximab Concentration Thresholds During Induction Therapy Are Associated with Short-term Mucosal Healing in Patients with Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2016, 14, 543–549. [Google Scholar] [CrossRef]
- Vande Casteele, N.; Jeyarajah, J.; Jairath, V.; Feagan, B.G.; Sandborn, W.J. Infliximab Exposure-Response Relationship and Thresholds Associated with Endoscopic Healing in Patients with Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2019, 17, 1814–1821.e1. [Google Scholar] [CrossRef]
- Davidov, Y.; Ungar, B.; Bar-Yoseph, H.; Carter, D.; Haj-Natour, O.; Yavzori, M.; Chowers, Y.; Eliakim, R.; Ben-Horin, S.; Kopylov, U. Association of Induction Infliximab Levels with Clinical Response in Perianal Crohn’s Disease. J. Crohn’s Colitis 2017, 11, 549–555. [Google Scholar] [CrossRef]
- Dreesen, E.; D’haens, G.; Baert, F.; Pariente, B.; Bouhnik, Y.; Van der Woude, J.; Moreau, J.; Laharie, D.; Vermeire, S.; Gils, A. Infliximab exposure predicts superior endoscopic outcomes in patients with active Crohn’s disease: Pharmacokinetic–pharmacodynamic analysis of TAILORIX. J. Crohn’s Colitis 2018, 12, S063–S064. [Google Scholar] [CrossRef]
- Bar-Yoseph, H.; Levhar, N.; Selinger, L.; Manor, U.; Yavzori, M.; Picard, O.; Fudim, E.; Kopylov, U.; Eliakim, R.; Ben-Horin, S.; et al. Early drug and anti-infliximab antibody levels for prediction of primary nonresponse to infliximab therapy. Aliment. Pharmacol. Ther. 2018, 47, 212–218. [Google Scholar] [CrossRef]
- Kennedy, N.A.; Heap, G.A.; Green, H.D.; Hamilton, B.; Bewshea, C.; Walker, G.J.; Thomas, A.; Nice, R.; Perry, M.H.; Bouri, S.; et al. Predictors of anti-TNF treatment failure in anti-TNF-naive patients with active luminal Crohn’s disease: A prospective, multicentre, cohort study. Lancet Gastroenterol. Hepatol. 2019, 4, 341–353. [Google Scholar] [CrossRef]
- Syversen, S.W.; Jørgensen, K.K.; Goll, G.L.; Brun, M.K.; Sandanger, Ø.; Bjørlykke, K.H.; Sexton, J.; Olsen, I.C.; Gehin, J.E.; Warren, D.J.; et al. Effect of Therapeutic Drug Monitoring vs. Standard Therapy during Infliximab Induction on Disease Remission in Patients with Chronic Immune-Mediated Inflammatory Diseases: A Randomized Clinical Trial. JAMA J. Am. Med. Assoc. 2021, 325, 1744–1754. [Google Scholar] [CrossRef]
- Lega, S.; Phan, B.L.; Rosenthal, C.J.; Gordon, J.; Haddad, N.; Pittman, N.; Benkov, K.; Dubinsky, M. Proactively Optimized Infliximab Monotherapy Is as Effective as Combination Therapy in IBD. Inflamm. Bowel Dis. 2019, 25, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Papamichael, K.; Cheifetz, A.S.; Melmed, G.Y.; Irving, P.M.; van de Casteele, N.; Kozuch, P.L.; Raffals, L.; Baidoo, L.; Bressler, B.; Devlin, S.; et al. Appropriate Therapeutic Drug Monitoring of Biologic Agents for Patients with Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2019, 17, 1655–1668.e3. [Google Scholar] [CrossRef] [PubMed]
- Papamichael, K.; Jairath, V.; Zou, G.; Cohen, B.; Ritter, T.; Sands, B.; Siegel, C.; Valentine, J.; Smith, M.; Vande Casteele, N.; et al. Proactive infliximab optimisation using a pharmacokinetic dashboard versus standard of care in patients with Crohn’s disease: Study protocol for a randomised, controlled, multicentre, open-label study (the OPTIMIZE trial). BMJ Open 2022, 12, e057656. [Google Scholar] [CrossRef] [PubMed]


| Characteristc | Description |
|---|---|
| Gender, n (%) | |
| Female | 65 (58.6) |
| Male | 46 (41.4) |
| Age (years), median (IQR) | 37 (25; 50) |
| Body mass index (kg m−2), median (IQR) | 23.5 (20.5; 26.9) |
| Current smoker, n (%) | 10 (9) |
| IBD type, n (%) | |
| CD | 76 (68.5) |
| UC | 35 (31.5) |
| CD Location, n (%) | |
| L1 (ileal) | 26 (34.2) |
| L2 (colonic) | 20 (26.3) |
| L3 (ileocolonic) | 29 (38.2) |
| L4 (upper GI disease) | 1 (1.3) |
| CD behaviour, n (%) | |
| B1 (nonstricturing, nonpenetrating) | 39 (51.3) |
| B2 (stricturing) | 25 (32.9) |
| B3 (penetrating), | 12 (15.8) |
| Perianal fistulizing disease, n (%) | 27 (35.5) |
| UC extent, n (%) | |
| E2 (left-side colitis) | 8 (22.9) |
| E3 (pancolitis) | 27 (77.1) |
| Previous segmental ressection, n (%) | 16 (14.4) |
| Right ileocolectomy | 8 (7.2) |
| Disease duration (years), median (IQR) | 4 (2;8) |
| Age at diagnosis (y), median (IQR) | 30 (22; 43) |
| Prior use of advanced therapies for IBD, n (%) | |
| Bio naïve | 87 (78.4) |
| Exposed to 1 biologic | 21 (18.9) |
| Exposed to 2 biologics | 3 (2.7) |
| Exposed to 1 small molecule | 2 (1.8) |
| Concomitant IMM at start of infliximab, n (%) | |
| Aazathioprine | 66 (59.5) |
| 6-mercaptopurine | 1 (0.9) |
| Methotrexate | 6 (5.4) |
| Concomitant use of corticosteroids, n (%) | 25 (22.5) |
| Initial CRP (mg L−1), median (IQR) | 7.6 (5; 19) |
| HBI, median (IQR) | 8 (5;12) |
| PMS, median (IQR) | 7 (6; 8) |
| SES-CD, median (IQR) | 13 (8; 17) |
| Rutgeerts score, median (IQR) | 2 (2; 3) |
| EMS, median (IQR) | 3 (2; 3) |
| Characteristic | Clinical Remission | OR | CI (95%) | p | ||
|---|---|---|---|---|---|---|
| No | Yes | Inferior | Superior | |||
| Gender, n (%) | 0.629 & | |||||
| Male | 7 (17.5) | 33 (82.5) | 1.00 | |||
| Female | 11 (21.6) | 40 (78.4) | 0.77 | 0.27 | 2.21 | |
| Age (years) | 0.99 | 0.96 | 1.03 | 0.631 ** | ||
| Median (IQR) | 36 (26.5; 48.8) | 35 (24; 48) | ||||
| BMI (kg m−2) | 0.96 | 0.86 | 1.06 | 0.370 ** | ||
| Median (IQR) | 24.1 (21; 28.2) | 23.4 (20.5; 26.8) | ||||
| IBD, n (%) | 0.772 * | |||||
| CD | 14 (20.9) | 53 (79.1) | 1.00 | |||
| UC | 4 (16.7) | 20 (83.3) | 1.32 | 0.39 | 4.49 | |
| Previous abdominal surgery, n (%) | 0.166 * | |||||
| No | 13 (17.1) | 63 (82.9) | 1.00 | |||
| Yes | 5 (33.3) | 10 (66.7) | 0.41 | 0.12 | 1.41 | |
| Previous use of adalimumab, n (%) | 0.079 * | |||||
| No | 12 (16) | 63 (84) | 1.00 | |||
| Yes | 6 (37.5) | 10 (62.5) | 0.32 | 0.10 | 1.04 | |
| Corticosteroid - dependence, n (%) | 0.051 & | |||||
| No | 6 (12.2) | 43 (87.8) | 1.00 | |||
| Yes | 12 (28.6) | 30 (71.4) | 0.35 | 0.12 | 1.03 | |
| Duration of disease (years) | 0.97 | 0.90 | 1.03 | 0.406 £ | ||
| Median (IQR) | 4.3 (2.4; 11.8) | 4 (2; 8) | ||||
| Age at diagnosis (years) | 1.00 | 0.96 | 1.05 | 0.940 £ | ||
| Median (IQR) | 28 (23.3; 35.5) | 29 (20.5; 41.5) | ||||
| ITL at week 6 (μg mL−1) | 1.05 | 0.98 | 1.13 | 0.159 £ | ||
| Median (IQR) | 10.1 (6.7; 20) | 18.1 (9; 20) | ||||
| Initial CRP (mg L−1) | 0.99 | 0.96 | 1.02 | 0.427 £ | ||
| Median (IQR) | 9.3 (5; 26.3) | 7.3 (5; 19.5) | ||||
| Characteristic | Endoscopic Remission | OR | CI (95%) | p | ||
|---|---|---|---|---|---|---|
| No | Yes | Inferior | Superior | |||
| Gender, n (%) | 0.607 * | |||||
| Male | 16 (48.5) | 17 (51.5) | 1.00 | |||
| Female | 25 (54.3) | 21 (45.7) | 0.79 | 0.32 | 1.94 | |
| Age (years) | 1.01 | 0.98 | 1.04 | 0.689 ** | ||
| Median (IQR) | 35 (24.5; 45.5) | 34.5 (24; 49) | ||||
| BMI (kg m−2) | 1.01 | 0.92 | 1.10 | 0.855 ** | ||
| Median (IQR) | 23.3 (20.5; 26) | 24.3 (20.9; 27) | ||||
| IBD, n (%) | 0.229 * | |||||
| CD | 31 (56.4) | 24 (43.6) | 1.00 | |||
| UC | 10 (41.7) | 14 (58.3) | 1.81 | 0.69 | 4.77 | |
| Previous abdominal surgery, n (%) | 0.878 * | |||||
| No | 34 (51.5) | 32 (48.5) | 1.00 | |||
| Yes | 7 (53.8) | 6 (46.2) | 0.91 | 0.28 | 3.00 | |
| Previous use of adalimumab, n (%) | 0.171 * | |||||
| No | 32 (48.5) | 34 (51.5) | 1.00 | |||
| Yes | 9 (69.2) | 4 (30.8) | 0.42 | 0.12 | 1.49 | |
| Corticosteroid-dependence, n (%) | 0.576 * | |||||
| No | 19 (48.7) | 20 (51.3) | 1.00 | |||
| Yes | 22 (55) | 18 (45) | 0.78 | 0.32 | 1.88 | |
| Duration of disease (years) | 0.99 | 0.93 | 1.06 | 0.334 £ | ||
| Median (IQR) | 5 (2; 8) | 3 (1.9; 9) | ||||
| Age at diagnosis (years) | 1.02 | 0.98 | 1.05 | 0.438 £ | ||
| Median (IQR) | 25 (20; 33.5) | 26 (22.5; 41.3) | ||||
| ITL at week 6 (μg mL−1) | 1.06 | 0.99 | 1.13 | 0.110 £ | ||
| Median (IQR) | 13.7 (5.3; 20) | 19.5 (10.9; 20) | ||||
| Initial CRP (mg L−1) | 0.99 | 0.97 | 1.02 | 0.770 £ | ||
| Median (IQR) | 7.5 (5; 26.5) | 9.5 (5; 18.3) | ||||
| Characteristic | Biological Remission | OR | CI (95%) | p | ||
|---|---|---|---|---|---|---|
| No | Yes | Inferior | Superior | |||
| Gender, n (%) | 0.533 & | |||||
| Male | 12 (29.3) | 29 (70.7) | 1.00 | |||
| Female | 12 (23.5) | 39 (76.5) | 1.35 | 0.53 | 3.42 | |
| Age (years) | 1.00 | 0.97 | 1.03 | 0.780 ** | ||
| Median (IQR) | 32.5 (24; 49.3) | 37 (25; 48) | ||||
| BMI (kg m−2) | 0.98 | 0.89 | 1.08 | 0.672 ** | ||
| Median (IQR) | 25 (20.1; 20.6) | 23.2 (20.8; 26.9) | ||||
| IBD, n (%) | 0.021 & | |||||
| CD | 22 (32.4) | 46 (67.6) | 1.00 | |||
| UC | 2 (8.3) | 22 (91.7) | 5.26 | 1.14 | 24.40 | |
| Previous abdominal surgery, n (%) | 0.548 * | |||||
| No | 21 (27.6) | 55 (72.4) | 1.00 | |||
| Yes | 3 (18.8) | 13 (81.3) | 1.66 | 0.43 | 6.40 | |
| Previous use of adalimumab, n (%) | 0.134 * | |||||
| No | 17 (22.7) | 58 (77.3) | 1.00 | |||
| Yes | 7 (41.2) | 10 (58.8) | 0.42 | 0.14 | 1.27 | |
| Corticosteroid - dependence, n (%) | 0.983 & | |||||
| No | 13 (26) | 37 (74) | 1.00 | |||
| Yes | 11 (26.2) | 31 (73.8) | 0.99 | 0.39 | 2.52 | |
| Duration of disease (years) | 0.95 | 0.89 | 1.01 | 0.079 £ | ||
| Median (IQR) | 6 (3; 10.5) | 3 (2; 8) | ||||
| Age at diagnosis (years) | 1.01 | 0.97 | 1.05 | 0.465 £ | ||
| Median (IQR) | 27 (20; 39.3) | 29 (23; 41) | ||||
| ITL at week 6 (μg mL−1) | 1.04 | 0.97 | 1.11 | 0.112 £ | ||
| Median (IQR) | 14.1 (9; 19.5) | 18.7 (8.8; 20) | ||||
| Initial CRP (mg L−1) | 0.98 | 0.96 | 1.00 | 0.002 £ | ||
| Median (IQR) | 12.5 (7.4; 28.2) | 5 (5; 18) | ||||
| Outcome | Variables | OR | CI (95%) | p | |
|---|---|---|---|---|---|
| Inferior | Superior | ||||
| Clinical remission at 6 months | Previous use of adalimumab | 0.24 | 0.06 | 0.97 | 0.045 |
| ITL at week 6 (μg mL−1) | 1.03 | 0.94 | 1.12 | 0.556 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia, K.S.; de Azevedo, M.F.C.; Carlos, A.d.S.; Barros, L.L.; Oba, J.; Sobrado Junior, C.W.; Sipahi, A.M.; Alves, O.D.d.C.; Navarro-Rodriguez, T.; Parra, R.S.; et al. Efficacy of Early Optimization of Infliximab Guided by Therapeutic Drug Monitoring during Induction—A Prospective Trial. Biomedicines 2023, 11, 1757. https://doi.org/10.3390/biomedicines11061757
Garcia KS, de Azevedo MFC, Carlos AdS, Barros LL, Oba J, Sobrado Junior CW, Sipahi AM, Alves ODdC, Navarro-Rodriguez T, Parra RS, et al. Efficacy of Early Optimization of Infliximab Guided by Therapeutic Drug Monitoring during Induction—A Prospective Trial. Biomedicines. 2023; 11(6):1757. https://doi.org/10.3390/biomedicines11061757
Chicago/Turabian StyleGarcia, Karoline Soares, Matheus Freitas Cardoso de Azevedo, Alexandre de Sousa Carlos, Luísa Leite Barros, Jane Oba, Carlos Walter Sobrado Junior, Aytan Miranda Sipahi, Olívia Duarte de Castro Alves, Tomás Navarro-Rodriguez, Rogério Serafim Parra, and et al. 2023. "Efficacy of Early Optimization of Infliximab Guided by Therapeutic Drug Monitoring during Induction—A Prospective Trial" Biomedicines 11, no. 6: 1757. https://doi.org/10.3390/biomedicines11061757
APA StyleGarcia, K. S., de Azevedo, M. F. C., Carlos, A. d. S., Barros, L. L., Oba, J., Sobrado Junior, C. W., Sipahi, A. M., Alves, O. D. d. C., Navarro-Rodriguez, T., Parra, R. S., Chebli, J. M. F., Chebli, L. A., Flores, C., Vieira, A., do Ceará, C. D. A., Queiroz, N. S. F., & Damião, A. O. M. C. (2023). Efficacy of Early Optimization of Infliximab Guided by Therapeutic Drug Monitoring during Induction—A Prospective Trial. Biomedicines, 11(6), 1757. https://doi.org/10.3390/biomedicines11061757





