Treatment with Myo-Inositol Does Not Improve the Clinical Features in All PCOS Phenotypes
Abstract
:1. Introduction
2. Materials and Methods
3. Results
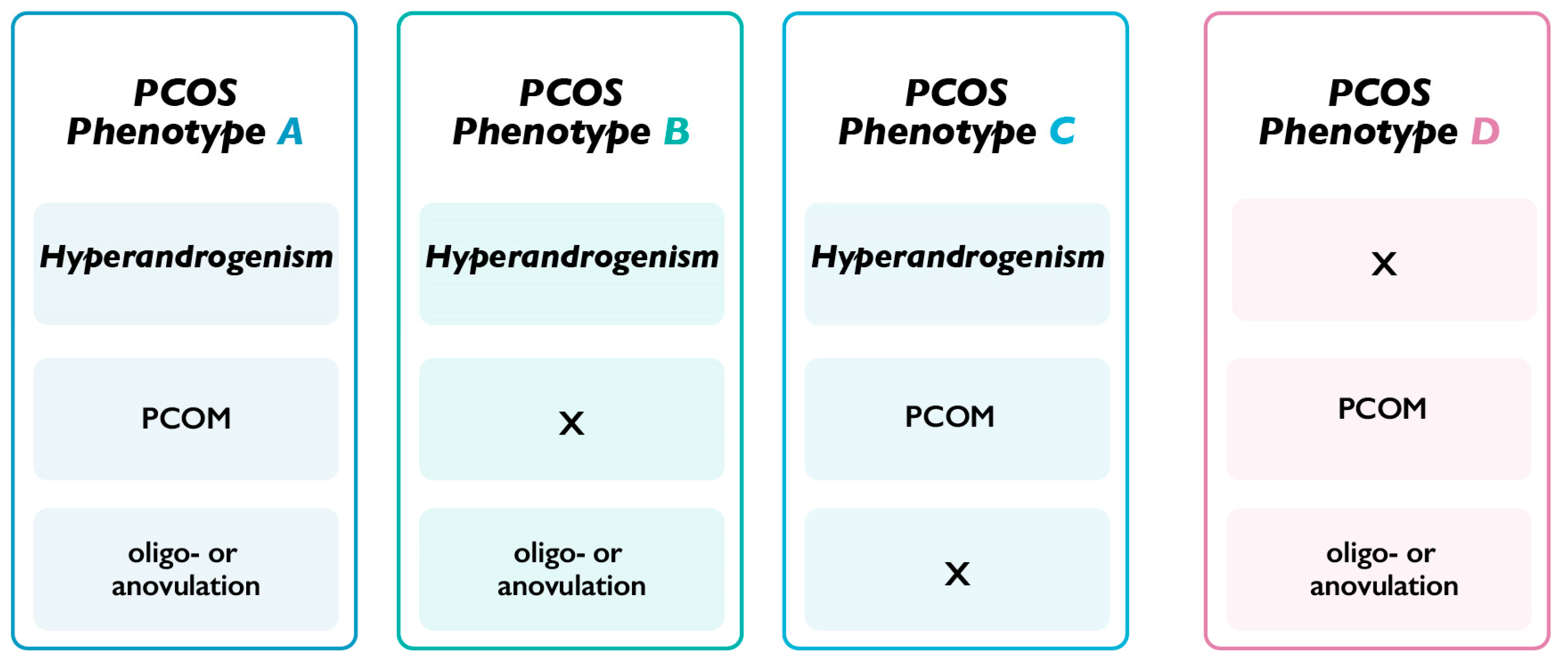
3.1. Patients with PCOS Phenotype A-B-C (H-PCOS)
3.2. Patients with PCOS Phenotype D (NH-PCOS)
3.3. H-PCOS Patients vs. NH-PCOS Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rosenfield, R.L.; Ehrmann, D.A. The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The Hypothesis of PCOS as Functional Ovarian Hyperandrogenism Revisited. Endocr. Rev. 2016, 37, 467–520. [Google Scholar] [CrossRef]
- The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum. Reprod. 2004, 19, 41–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azziz, R. Polycystic Ovary Syndrome. Obstet. Gynecol. 2018, 132, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Gleicher, N.; Darmon, S.; Patrizio, P.; Barad, D.H. Reconsidering the Polycystic Ovary Syndrome (PCOS). Biomedicines 2022, 10, 1505. [Google Scholar] [CrossRef]
- Greff, D.; Juhász, A.E.; Váncsa, S.; Váradi, A.; Sipos, Z.; Szinte, J.; Park, S.; Hegyi, P.; Nyirády, P.; Ács, N.; et al. Inositol is an effective and safe treatment in polycystic ovary syndrome: A systematic review and meta-analysis of randomized controlled trials. Reprod. Biol. Endocrinol. 2023, 21, 10. [Google Scholar] [CrossRef] [PubMed]
- Azizi Kutenaei, M.; Hosseini Teshnizi, S.; Ghaemmaghami, P.; Eini, F.; Roozbeh, N. The effects of myo-inositol vs. metformin on the ovarian function in the polycystic ovary syndrome: A systematic review and meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 3105–3115. [Google Scholar] [CrossRef]
- Siracusa, L.; Napoli, E.; Ruberto, G. Novel Chemical and Biological Insights of Inositol Derivatives in Mediterranean Plants. Molecules 2022, 27, 1525. [Google Scholar] [CrossRef]
- Laganà, A.S.; Forte, G.; Bizzarri, M.; Kamenov, Z.A.; Bianco, B.; Kaya, C.; Gitas, G.; Alkatout, I.; Terzic, M.; Unfer, V. Inositols in the ovaries: Activities and potential therapeutic applications. Expert Opin. Drug Metab. Toxicol. 2022, 18, 123–133. [Google Scholar] [CrossRef]
- Russo, M.; Forte, G.; Montanino Oliva, M.; Laganà, A.S.; Unfer, V. Melatonin and Myo-Inositol: Supporting Reproduction from the Oocyte to Birth. Int. J. Mol. Sci. 2021, 22, 8433. [Google Scholar] [CrossRef]
- Kamenov, Z.; Gateva, A. Inositols in PCOS. Molecules 2020, 25, 5566. [Google Scholar] [CrossRef]
- Laganà, A.S.; Garzon, S.; Casarin, J.; Franchi, M.; Ghezzi, F. Inositol in Polycystic Ovary Syndrome: Restoring Fertility through a Pathophysiology-Based Approach. Trends Endocrinol. Metab. 2018, 29, 768–780. [Google Scholar] [CrossRef] [PubMed]
- Facchinetti, F.; Bizzarri, M.; Benvenga, S.; D’Anna, R.; Lanzone, A.; Soulage, C.; Di Renzo, G.C.; Hod, M.; Cavalli, P.; Chiu, T.T.; et al. Results from the International Consensus Conference on Myo-inositol and d-chiro-inositol in Obstetrics and Gynecology: The link between metabolic syndrome and PCOS. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 195, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Balen, A.H.; Laven, J.S.; Tan, S.L.; Dewailly, D. Ultrasound assessment of the polycystic ovary: International consensus definitions. Hum. Reprod. Update 2003, 9, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Nordio, M.; Basciani, S.; Camajani, E. The 40:1 myo-inositol/D-chiro-inositol plasma ratio is able to restore ovulation in PCOS patients: Comparison with other ratios. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 5512–5521. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowska, A.; Osowski, A.; Jóźwik, M.; Górecki, R.; Rynkiewicz, A.; Wojtkiewicz, J. Inositols’ Importance in the Improvement of the Endocrine-Metabolic Profile in PCOS. Int. J. Mol. Sci. 2019, 20, 5787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Facchinetti, F.; Unfer, V.; Dewailly, D.; Kamenov, Z.A.; Diamanti-Kandarakis, E.; Laganà, A.S.; Nestler, J.E.; Soulage, C.O. Inositols in Polycystic Ovary Syndrome: An Overview on the Advances. Trends Endocrinol. Metab. 2020, 31, 435–447. [Google Scholar] [CrossRef]
- Hernandez Marin, I.; Picconi, O.; Laganà, A.S.; Costabile, L.; Unfer, V. A multicenter clinical study with myo-inositol and alpha-lactalbumin in Mexican and Italian PCOS patients. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 3316–3324. [Google Scholar] [CrossRef]
- Pkhaladze, L.; Russo, M.; Unfer, V.; Nordio, M.; Basciani, S.; Khomasuridze, A. Treatment of lean PCOS teenagers: A follow-up comparison between Myo-Inositol and oral contraceptives. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 7476–7485. [Google Scholar] [CrossRef]
- Merviel, P.; James, P.; Bouée, S.; Le Guillou, M.; Rince, C.; Nachtergaele, C.; Kerlan, V. Impact of myo-inositol treatment in women with polycystic ovary syndrome in assisted reproductive technologies. Reprod. Health 2021, 18, 13. [Google Scholar] [CrossRef]
- Unfer, V.; Facchinetti, F.; Orrù, B.; Giordani, B.; Nestler, J. Myo-inositol effects in women with PCOS: A meta-analysis of randomized controlled trials. Endocr. Connect. 2017, 6, 647–658. [Google Scholar] [CrossRef] [Green Version]
- Papadakis, G.; Kandaraki, E.A.; Garidou, A.; Koutsaki, M.; Papalou, O.; Diamanti-Kandarakis, E.; Peppa, M. Tailoring treatment for PCOS phenotypes. Expert Rev. Endocrinol. Metab. 2021, 16, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Zacchè, M.M.; Caputo, L.; Filippis, S.; Zacchè, G.; Dindelli, M.; Ferrari, A. Efficacy of myo-inositol in the treatment of cutaneous disorders in young women with polycystic ovary syndrome. Gynecol. Endocrinol. 2009, 25, 508–513. [Google Scholar] [CrossRef]
- Pkhaladze, L.; Barbakadze, L.; Kvashilava, N. Myo-Inositol in the Treatment of Teenagers Affected by PCOS. Int. J. Endocrinol. 2016, 2016, 1473612. [Google Scholar] [CrossRef] [Green Version]
- Croze, M.L.; Soulage, C.O. Potential role and therapeutic interests of myo-inositol in metabolic diseases. Biochimie 2013, 95, 1811–1827. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, M.A.; Deshmukh, H.; Atkin, S.; Sathyapalan, T. A review of therapeutic options for managing the metabolic aspects of polycystic ovary syndrome. Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018820938305. [Google Scholar] [CrossRef]
- Dinicola, S.; Unfer, V.; Facchinetti, F.; Soulage, C.O.; Greene, N.D.; Bizzarri, M.; Laganà, A.S.; Chan, S.Y.; Bevilacqua, A.; Pkhaladze, L.; et al. Inositols: From Established Knowledge to Novel Approaches. Int. J. Mol. Sci. 2021, 22, 10575. [Google Scholar] [CrossRef]
- Unfer, V.; Dinicola, S.; Russo, M. A PCOS Paradox: Does Inositol Therapy Find a Rationale in All the Different Phenotypes? Int. J. Mol. Sci. 2023, 24, 6213. [Google Scholar] [CrossRef] [PubMed]
- Tosi, F.; Villani, M.; Migazzi, M.; Faccin, G.; Garofalo, S.; Fiers, T.; Kaufman, J.M.; Bonora, E.; Moghetti, P. Insulin-Mediated Substrate Use in Women with Different Phenotypes of PCOS: The Role of Androgens. J. Clin. Endocrinol. Metab. 2021, 106, e3414–e3425. [Google Scholar] [CrossRef]
- Tosi, F.; Bonora, E.; Moghetti, P. Insulin resistance in a large cohort of women with polycystic ovary syndrome: A comparison between euglycaemic-hyperinsulinaemic clamp and surrogate indexes. Hum. Reprod. 2017, 32, 2515–2521. [Google Scholar] [CrossRef] [Green Version]
- Cassar, S.; Misso, M.L.; Hopkins, W.G.; Shaw, C.S.; Teede, H.J.; Stepto, N.K. Insulin resistance in polycystic ovary syndrome: A systematic review and meta-analysis of euglycaemic-hyperinsulinaemic clamp studies. Hum. Reprod. 2016, 31, 2619–2631. [Google Scholar] [CrossRef] [Green Version]
- Moghetti, P.; Tosi, F.; Bonin, C.; Di Sarra, D.; Fiers, T.; Kaufman, J.M.; Giagulli, V.A.; Signori, C.; Zambotti, F.; Dall’Alda, M.; et al. Divergences in insulin resistance between the different phenotypes of the polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2013, 98, E628–E637. [Google Scholar] [CrossRef] [Green Version]
- Lizneva, D.; Suturina, L.; Walker, W.; Brakta, S.; Gavrilova-Jordan, L.; Azziz, R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil. Steril. 2016, 106, 6–15. [Google Scholar] [CrossRef] [Green Version]
- Ye, W.; Xie, T.; Song, Y.; Zhou, L. The role of androgen and its related signals in PCOS. J. Cell. Mol. Med. 2021, 25, 1825–1837. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Yang, S.; Li, R.; Liu, P.; Qiao, J.; Zhang, Y. Effects of hyperandrogenism on metabolic abnormalities in patients with polycystic ovary syndrome: A meta-analysis. Reprod. Biol. Endocrinol. 2016, 14, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moghetti, P.; Tosi, F. Insulin resistance and PCOS: Chicken or egg? J. Endocrinol. Investig. 2021, 44, 233–244. [Google Scholar] [CrossRef]
- Heimark, D.; McAllister, J.; Larner, J. Decreased myo-inositol to chiro-inositol (M/C) ratios and increased M/C epimerase activity in PCOS theca cells demonstrate increased insulin sensitivity compared to controls. Endocr. J. 2014, 61, 111–117. [Google Scholar] [CrossRef] [Green Version]
- Unfer, V.; Nestler, J.E.; Kamenov, Z.A.; Prapas, N.; Facchinetti, F. Effects of Inositol(s) in Women with PCOS: A Systematic Review of Randomized Controlled Trials. Int. J. Endocrinol. 2016, 2016, 1849162. [Google Scholar] [CrossRef] [Green Version]
- Iervolino, M.; Lepore, E.; Forte, G.; Laganà, A.S.; Buzzaccarini, G.; Unfer, V. Natural Molecules in the Management of Polycystic Ovary Syndrome (PCOS): An Analytical Review. Nutrients 2021, 13, 1677. [Google Scholar] [CrossRef] [PubMed]
- Unfer, V.; Carlomagno, G.; Papaleo, E.; Vailati, S.; Candiani, M.; Baillargeon, J.P. Hyperinsulinemia Alters Myoinositol to d-chiroinositol Ratio in the Follicular Fluid of Patients with PCOS. Reprod. Sci. 2014, 21, 854–858. [Google Scholar] [CrossRef]
- Carlomagno, G.; Nordio, M.; Chiu, T.T.; Unfer, V. Contribution of myo-inositol and melatonin to human reproduction. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 159, 267–272. [Google Scholar] [CrossRef]
- Shahid, R.; Haq, I.-U.; Mahnoor; Awan, K.A.; Iqbal, M.J.; Munir, H.; Saeed, I. Diet and lifestyle modifications for effective management of polycystic ovarian syndrome (PCOS). J. Food Biochem. 2022, 46, e14117. [Google Scholar] [CrossRef]
- Tsuda, H.; Ito, Y.M.; Todo, Y.; Iba, T.; Tasaka, K.; Sutou, Y.; Hirai, K.; Dozono, K.; Dobashi, Y.; Manabe, M.; et al. Measurement of endometrial thickness in premenopausal women in office gynecology. Reprod. Med. Biol. 2018, 17, 29–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bizzarri, M.; Monti, N.; Piombarolo, A.; Angeloni, A.; Verna, R. Myo-Inositol and D-Chiro-Inositol as Modulators of Ovary Steroidogenesis: A Narrative Review. Nutrients 2023, 15, 1875. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Huang, Z.Y.; Xu, X.L.; Li, J.; Fu, X.W.; Deng, S.L. Estrogen Receptor Function: Impact on the Human Endometrium. Front. Endocrinol. 2022, 13, 827724. [Google Scholar] [CrossRef] [PubMed]
| Hyperandrogenic PCOS (Phenotype A-B-C) | Non-Hyperandrogenic PCOS (Phenotype D) | p-Value | |
|---|---|---|---|
| BMI (kg/m2) | 28.3 (25.9–31.6) | 24 (23–25) | 0.00072 |
| Endometrial thickness (mm) | 3 (3–3) | 8 (7–9) | <0.00001 |
| Insulin (mIU/L) | 18.5 (15.6–30) | 12 (11–14) | <0.00001 |
| Glucose (mg/dL) | 89 (85.8–93.3) | 79 (76.5–88) | 0.01174 |
| HOMA | 4.1 (3.6–5.9) | 2.5 (2.1–2.9) | <0.00001 |
| FSH (mIU/mL) | 5.2 (4.2–6.2) | 4 (3–4.5) | 0.00512 |
| LH (mIU/mL) | 11.1 (9.1–12.6) | 3 (3–4.5) | <0.00001 |
| LH/FSH | 2.3 (1.8–2.5) | 1 (0.8–1.2) | <0.00001 |
| Testosterone (ng/dL) | 92.5 (77.5–98.8) | 34 (31.5–36.5) | <0.00001 |
| SHBG (nmol/L) | 44 (39–49.3) | 88 (77–96) | <0.00001 |
| Cholesterol (mg/dL) | 198 (188.3–206.5) | 155 (145.5–163.5) | 0.00008 |
| Triglycerides (mg/dL) | 187.5 (130.3–247) | 76 (66.5–86.5) | <0.00001 |
| Hyperandrogenic PCOS (Phenotype A-B-C) | Non-Hyperandrogenic PCOS (Phenotype D) | |||||
|---|---|---|---|---|---|---|
| T0 | T6 | p-Value | T0 | T6 | p-Value | |
| BMI (kg/m2) | 28.3 (25.9–31.6) | 28 (26.6–31.7) | 0.529 | 24 (23–25) | 23 (23–25) | 1 |
| Endometrial thickness (mm) | 3 (3–3) | 6 (5.5–7) | 0.0004 | 8 (7–9) | 8 (6–8.5) | 0.254 |
| Insulin (mIU/L) | 18.5 (15.6–30) | 19.2 (14–20.9) | 0.056 | 12 (11–14) | 11 (10–12) | 0.009 |
| Glucose (mg/dL) | 89 (85.8–93.3) | 68 (64.5–76.3) | 0.0004 | 79 (76.5–88) | 78 (74.5–80) | 0.099 |
| HOMA | 4.1 (3.6–5.9) | 3 (2.5–3.6) | 0.0004 | 2.5 (2.1–2.9) | 2 (1.8–2.4) | 0.003 |
| FSH (mIU/mL) | 5.2 (4.2–6.2) | 6 (5.5–8.3) | 0.031 | 4 (3–4.5) | 3 (3–4) | 0.084 |
| LH (mIU/mL) | 11.1 (9.1–12.6) | 8.6 (7.9–9.4) | 0.001 | 3 (3–4.5) | 3 (2–4) | 0.294 |
| LH/FSH | 2.3 (1.8–2.5) | 1.4 (1.1–1.6) | 0.002 | 1 (0.8–1.2) | 1 (0.7–1.3) | 0.569 |
| Testosterone (ng/dL) | 92.5 (77.5–98.8) | 56.5 (45–62.8) | 0.001 | 34 (31.5–36.5) | 35 (32–37) | 0.795 |
| SHBG (nmol/L) | 44 (39–49.3) | 113 (94.3–123.3) | 0.0004 | 88 (77–96) | 100 (90–100) | 0.005 |
| Cholesterol (mg/dL) | 198 (188.3–206.5) | 175.5 (157–198) | 0.147 | 155 (145.5–163.5) | 156 (154–169.5) | 0.134 |
| Triglycerides (mg/dL) | 187.5 (130.3–247) | 149 (137.3–171) | 0.047 | 76 (66.5–86.5) | 95 (89.5–100) | 0.0003 |
| Hyperandrogenic PCOS (Phenotype A-B-C) | Non-Hyperandrogenic PCOS (Phenotype D) | p-Value | |
|---|---|---|---|
| BMI (kg/m2) | −0.2 (−0.8–0.1) | 0 (0–0) | 0.4009 |
| Endometrial thickness (mm) | 3 (2–4) | −1 (−2–0.5) | <0.00001 |
| Insulin (mIU/L) | −3.4 (−9.0–1.3) | −1 (−4–0) | 0.5157 |
| Glucose (mg/dL) | −18.5 (−29–−12.3) | −6 (−11.5–3.0) | 0.00048 |
| HOMA | −1.2 (−3.1–−0.7) | −0.3 (−0.8–-0.1) | 0.00758 |
| FSH (mIU/mL) | 1.1 (−0.2–3.6) | 0 (−1.5–0.5) | 0.007 |
| LH (mIU/mL) | −1.7 (−3.5–−1) | 0 (−2–1) | 0.01778 |
| LH/FSH | −0.9 (−1.4–−0.4) | 0 (−0.6–0.6) | 0.00244 |
| Testosterone (ng/dL) | −33.5 (−45.3–−21.8) | 1 (−3–3.5) | <0.00001 |
| SHBG (nmol/L) | 60.5 (57.5–79.5) | 6 (1–23.5) | <0.00001 |
| Cholesterol (mg/dL) | −23.5 (−46.8–11.3) | 6 (0–12) | 0.05486 |
| Triglycerides (mg/dL) | −18 (−91.8–1.5) | 22 (9.5–28.5) | 0.00194 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Unfer, V.; Russo, M.; Aragona, C.; Bilotta, G.; Montanino Oliva, M.; Bizzarri, M. Treatment with Myo-Inositol Does Not Improve the Clinical Features in All PCOS Phenotypes. Biomedicines 2023, 11, 1759. https://doi.org/10.3390/biomedicines11061759
Unfer V, Russo M, Aragona C, Bilotta G, Montanino Oliva M, Bizzarri M. Treatment with Myo-Inositol Does Not Improve the Clinical Features in All PCOS Phenotypes. Biomedicines. 2023; 11(6):1759. https://doi.org/10.3390/biomedicines11061759
Chicago/Turabian StyleUnfer, Vittorio, Michele Russo, Cesare Aragona, Gabriele Bilotta, Mario Montanino Oliva, and Mariano Bizzarri. 2023. "Treatment with Myo-Inositol Does Not Improve the Clinical Features in All PCOS Phenotypes" Biomedicines 11, no. 6: 1759. https://doi.org/10.3390/biomedicines11061759
APA StyleUnfer, V., Russo, M., Aragona, C., Bilotta, G., Montanino Oliva, M., & Bizzarri, M. (2023). Treatment with Myo-Inositol Does Not Improve the Clinical Features in All PCOS Phenotypes. Biomedicines, 11(6), 1759. https://doi.org/10.3390/biomedicines11061759






