Abstract
Differential diagnosis of hypoglycemia in the non-diabetic adult patient is complex and comprises various diseases, including endogenous hyperinsulinism caused by functional β-cell disorders. The latter is also designated as nesidioblastosis or non-insulinoma pancreatogenous hypoglycemia syndrome (NIPHS). Clinically, this rare disease presents with unspecific adrenergic and neuroglycopenic symptoms and is, therefore, often overlooked. A combination of careful clinical assessment, oral glucose tolerance testing, 72 h fasting, sectional and functional imaging, and invasive insulin measurements can lead to the correct diagnosis. Due to a lack of a pathophysiological understanding of the condition, conservative treatment options are limited and mostly ineffective. Therefore, nearly all patients currently undergo surgical resection of parts or the entire pancreas. Consequently, apart from faster diagnosis, more elaborate and less invasive treatment options are needed to relieve the patients from the dangerous and devastating symptoms. Based on a case of a 23-year-old man presenting with this disease in our department, we performed an extensive review of the medical literature dealing with this condition and herein presented a comprehensive discussion of this interesting disease, including all aspects from epidemiology to therapy.
1. Introduction
Hypoglycemia in an adult patient can have diverse causes. Apart from drugs such as insulin or insulin secretagogues, alcohol ingestion, critical illness (including sepsis, hepatic, renal, and cardiac failure), hormone deficiencies (e.g., adrenal or pituitary insufficiency) or endogenous overproduction of insulin and insulin-like growth factors are important to consider [1]. When evaluating apparently healthy patients, it is important to distinguish reactive/functional hypoglycemia from endogenous hyperinsulinism/hyperinsulinemic hypoglycemia [2]. The latter is defined by low blood glucose with simultaneous, inadequately high insulin/proinsulin and C-peptide levels [3].
In an adult patient, endogenous hyperinsulinism is mainly caused by insulinoma, functional β-cell disorders, insulin autoimmune hypoglycemia (Hirata’s disease), or insulin secretagogues [1]. Insulinomas are tumors arising from the β-cells of the islets of Langerhans and are usually benign. They account for approximately 90% of cases of adult-onset hyperinsulinemic hypoglycemia [4]. Hirata’s disease is caused by antibodies against insulin or the insulin receptor. The most accepted current pathophysiological hypothesis is the double-phase mechanism: in the postprandial phase, the insulin autoantibodies bind to insulin or the insulin receptor and prevent the interaction of insulin and its receptor, potentially resulting in hyperglycemia. Later, the antibodies dissociate from insulin or the receptor, enabling the proper receptor–substrate interaction, which lowers the blood glucose irrespective of the current blood glucose concentration [5,6].
Functional β-cell disorders are a group of less well-defined entities, which are also characterized by excess endogenous secretion of insulin but whose morphological features can vary considerably between individuals. Since 2005, functional β-cell disorders have frequently been reported in association with bariatric surgery, especially in patients who underwent Roux-en-Y bypass operations [7]. These disorders are often subsumed under the term “nesidioblastosis”, which originally describes the neoformation of nesidioblasts, i.e., the cells building the islets of Langerhans [8]. The scientist to first describe this condition, George F. Laidlaw, wrote in 1938:
“…there is some evidence pointing to a diffuse or disseminated proliferation of islet cells as a possible cause of hypoglycemia. Such a diffuse proliferation of nesidioblasts would be a nesidioblastosis.”
This description aimed at separating the findings of diffuse islet cell proliferation from localized adenomas (= nesidioblastoma/insulinoma) of the endocrine pancreas [9]. As nesidioblastosis is a morphological/histopathological term, the diagnosis can be established only in terms of the pathological evaluation of a biopsy or a surgical specimen. Clinical and biochemical findings are often described as non-insulinoma pancreatogenous hypoglycemia syndrome (NIPHS). The adult-onset form is considerably less frequent than the findings associated with congenital hyperinsulinism (CHI)/persistent hyperinsulinemic hypoglycemia of infancy and childhood (PHH). CHI/PHH is usually caused by well-known mutations in various genes associated with insulin secretion, and clinical symptoms appear within the first weeks or months after birth [10]. To separate the pathological findings in CHI/PHH from adult-onset nesidioblastosis, Anlauf and colleagues defined major and minor criteria for the latter´s diagnosis in 2005. The major criteria include microscopic, macroscopic, and immunohistochemical exclusion of an insulinoma, β-cells with hyperchromatic and enlarged nuclei and abundant clear cytoplasm, normal distribution of the various cell types (i.e., A, B, D, and PP cells) within the islets of Langerhans, as well as endocrine cells without (significant) proliferative activity. These criteria should be present in each case. Minor criteria are, however, not fulfilled by every patient and comprise enlarged islets (islet hypertrophy), a lobulated structure of the islets, an increase in the number of islets (islet hyperplasia), and macronucleoli in β-cells [11,12]. The presence of so-called ductulo–insular complexes, which describes the proximity or even budding of islets or islet cells from exocrine pancreatic ducts, is no hallmark for the diagnosis of the disease since autopsy studies have shown that at least mild forms of this finding frequently occur in healthy/asymptomatic adults [13]. This is important to know, as the term nesidioblastosis is sometimes used synonymic to ductulo–insular complexes or the scattering of single islet cells throughout the exocrine pancreatic tissue [14,15,16,17]. Confusion additionally arises from the many synonyms that are used to describe this disease. The terms endocrine cell dysplasia, nesidiodysplasia, multifocal ductulo–insular proliferation, islet cell adenomatosis, islet hyperplasia, and islet cell hypertrophy are frequently found in the literature [14,18,19,20].
In the following review, we describe and discuss all relevant aspects of adult-onset nesidioblastosis/NIPHS and related morphological findings, including epidemiology, pathophysiology, clinical findings, and principles of therapy. Additionally, we recently presented a case of a 23-year-old man with this extremely rare disease in a separate Case Report, which beautifully illustrates many facets of the diagnostic process and potential pitfalls (see Dieterle et al. 2023, accepted for publication in Biomedicines).
2. Methodology of the Literature Research and Limitations
We performed a literature research to find out more about this intriguing disease and to describe interesting associations of islet cell hyperplasia/nesidioblastosis with other diseases. Our semi-systematic research strategy included searches for “nesidioblastosis”, “islet [cell] hyperplasia”, “endocrine cell dysplasia”, “islet [cell] hypertrophy”, “β OR B OR beta cell hyperplasia”, “hyperinsulinemic hypoglycemia”, “islet [cell] adenomatosis”, “nesidiodysplasia”, “multifocal ductulo-insular [ductuloinsular] complexes”, “α OR alpha cell hyperplasia”, “D OR somatostatin cell hyperplasia”, and “PP OR pancreatic polypeptide cell hyperplasia”, in Medline until May 2022. The expressions in the next step also combined with “adult” to exclude the pediatric population, where much more literature is available. A literature research with the corresponding MeSH terms was also performed. Additionally, the reference sections of the retrieved papers were systematically screened for further publications. In total, 460 publications of interest were found. Unfortunately, in one-third of the publications, full texts were not available. Nonetheless, we provide a comprehensive overview of the main findings of these research papers in Supplementary Table S1. This is, to the best of our knowledge, the most complete presentation of such cases. Where available, we documented the year of publication, the number of cases, special features of the case (including age and sex of the patients), pathological features of the pancreas, clinical symptoms/laboratory findings, therapy, and a (personal) evaluation of the case. Missing full texts are indicated by an asterisk * in Supplementary Table S1. When the literature was found only as a reference in another original paper, we describe this in the “literature” section of Supplementary Table S1. The references for Supplementary Table S1 can be found in the reference section [3,5,7,11,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224,225,226,227,228,229,230,231,232,233,234,235,236,237,238,239,240,241,242,243,244,245,246,247,248,249,250,251,252,253,254,255,256,257,258,259,260,261,262,263,264,265,266,267,268,269,270,271,272,273,274,275,276,277,278,279,280,281,282,283,284,285,286,287,288,289,290,291,292,293,294,295,296,297,298,299,300,301,302,303,304,305,306,307,308,309,310,311,312,313,314,315,316,317,318]. In total, we collected information from approximately 330 research papers, which may contribute to a deeper understanding of the disease spectrum of adult-onset nesidioblastosis and islet cell hyperplasia.
However, there are some potential limitations of our literature research. Controversies about post-gastric bypass surgery hyperinsulinemic hypoglycemia/nesidioblastosis make it difficult to classify these patients and distinguish them from late dumping syndrome/reactive (hyperinsulinemic) hypoglycemia (see below). Additionally, in the last years, pancreatectomies to treat these patients have become more and more uncommon due to the considerable morbidity and mortality, which reduces the number of cases with histopathological diagnoses.
Only cases with definitive histopathology and some illustrative cases with clinical presentations highly suspicious of underlying nesidioblastosis/islet cell hyperplasia were included in Supplementary Table S1. Patients diagnosed with multiple endocrine neoplasia type 1 (MEN1), Zollinger–Ellison syndrome, Verner–Morrison syndrome, or islet cell adenomatosis were not studied separately since islet cell hyperplasia is a relatively common finding in this collective but is not always reported separately. Therefore, only cases where islet cell hyperplasia/nesidioblastosis is explicitly mentioned in the publications were included. The differentiation between pancreatic microadenoma/microadenomatosis (e.g., in the context of MEN1) and nesidioblastosis is sometimes arbitrary in the literature (adenoma is mostly defined as consisting of only one cell type and/or to be encapsulated). Again, only cases where islet cell hyperplasia/nesidioblastosis is explicitly mentioned in the publications were included. Islet cell hyperplasia is also sometimes found in chronic pancreatitis, areas of pancreatic fibrosis (e.g., in Cystic fibrosis), anabolic-androgenic steroid use, development of type II diabetes (at least in animal models), or while taking several drugs [65,319,320,321,322,323]. Cases comprising these conditions were only included in Supplementary Table S1 when islet cell hyperplasia/nesidioblastosis was a major characterizing feature of the patient, if hyperinsulinemic hypoglycemia or symptoms associated with hypoglycemia were present, or if the histological changes could also be attributed to other findings in the patients. Imaging studies for insulinoma detection, especially emerging methods in the field of nuclear medicine, often incidentally detect some cases of adult-onset nesidioblastosis [324,325]. Although many imaging studies are included in Supplementary Table S1, we cannot exclude that some of these studies have been overlooked since the nuclear medicine literature has not been explicitly searched for (random) detection of islet cell hyperplasia/nesidioblastosis. Some cases of adult-onset nesidioblastosis/NIPHS might also have been reported in the context of families with defined mutations (“familial hyperinsulinism”) since sometimes hyperinsulinism in adults is incidentally detected when an offspring presents with PHH/CHI. The separating line between PHH/CHI with a defined mutation and adult-onset nesidioblastosis/NIPHS with the same mutation and a clinical presentation at higher age is thus not always clear, and our literature research might have missed such cases. Some full texts and some citations found in the reference section of other publications were not available. Therefore, sometimes it was not possible to decide whether it was really a case of adult nesidioblastosis. The age at which to classify the cases as an adult is also ambiguous in the literature (see discussion below) [247]. Other papers did not fully report all demographic data to adequately classify the case (e.g., no age or gender was reported). Equivocal cases are described as such in the “Evaluation” column of Supplementary Table S1. The literature search via Medline might also be biased towards more recent literature since older publications are systematically underrepresented in the database. Whenever possible, however, original publications (dating back to 1925) were included.
3. History, Histopathological, and Clinical Definition of Hyperinsulinemic Hypoglycemia and Nesidioblastosis/Islet Cell Hyperplasia
Idiopathic/sporadic adult-onset nesidioblastosis with hyperinsulinemic hypoglycemia is a rare disease that occurs much less frequently than insulinoma in the adult population (see Section 6 below). Seale Harris first proposed hyperinsulinism as a clinical syndrome in 1924 [326]. The first cases of a (malignant) insulinoma were described not much later [327]. However, it is not entirely clear when the first unequivocal case of adult-onset nesidioblastosis was described in the medical literature. Some authors claim that a report by Bradley and colleagues in 1976 might be the first case [74]. Our literature research suggests that hyperplasia of the islets of Langerhans has been recognized long before. The clinical correlation to hyperinsulinemic hypoglycemia is, however, not that clear. In 1925, Lang reported nodular hyperplasia of islets (also described as adenomatosis), which could be a description of what is known today as diffuse islet cell hyperplasia [31]. As we could not retrieve the original manuscript by Lang, it is, unfortunately, unclear if there were any symptoms related to this finding and if the patient was an adult. Six years later, John reported a patient with insulin-dependent diabetes, hyperthyroidism, cirrhosis of the liver, liver/gallbladder carcinoma, and interstitial pancreatitis [31]. Insulin had to be discontinued in this patient due to hypoglycemia. The observed coexistence of hypertrophy and atrophy of the islets of Langerhans could, however, also be a consequence of pancreatitis. Hypertrophic and hyperplastic islets have been repeatedly described in this context [328]. The hypoglycemia could thus result from hepatic insufficiency and is not necessarily a consequence of endogenous hyperinsulinism.
In 1944, Frantz described different cases of insulin-producing neoplasms, adenomas, and adenomatosis. Among this cohort, there were 11 cases of hypertrophy and/or hyperplasia of the islets of Langerhans without any evidence of coexisting neoplasia [31]. Hypoglycemia was reported for all patients, and three of them underwent partial pancreatectomy. The symptoms were not relieved by the surgical interventions. From our point of view, these cases might be the first genuine descriptions of adult-onset, diffuse nesidioblastosis with clinically detected hypoglycemia. In the subsequent years between 1944 and the case presented by Bradley in 1976, other authors, such as Vance et al., reported patients with hypoglycemia symptoms and concomitant islet cell hyperplasia. The latter cases were found in a family suffering from MEN1 [172]. Of interest, Stefanini and colleagues reviewed approximately 130 cases of adult patients who presented with hypoglycemia and showed islet cell hyperplasia upon surgery or autopsy [216]. They reported that symptom onset preceded diagnosis by 4.5 years on average. A total of 122 of the 148 cases reported (among them 16 children) underwent surgery, and symptom control was achieved in approximately 70%. These cases are especially interesting since, at that time, hyperplasia of the islets of Langerhans as a cause of CHI was widely accepted, whereas adult patients with organic hyperinsulinism were thought to suffer exclusively from insulinomas. To the best of our knowledge, the publication by Stefanini and colleagues represents the oldest and largest series of patients with islet cell hyperplasia, which also contains otherwise unpublished cases from personal communications with physicians from all around the world. It can be assumed that these cases would today be diagnosed as having NIPHS or diffuse, adult-onset nesidioblastosis.
The definition of adult-onset nesidioblastosis still leads to confusion among physicians today. Some assume that hyperinsulinemic hypoglycemia is always related to β-cell neoplasia, namely insulinoma, and that cases diagnosed as nesidioblastosis or islet cell hyperplasia represent overlooked, small tumors/insulinomas [329,330]. However, thorough histopathological work-up of many pancreata from patients diagnosed with nesidioblastosis did not lead to the detection of occult insulinomas [11].
As described above, the terms nesidioblastosis, endocrine cell dysplasia, nesidiodysplasia, multifocal ductulo–insular proliferation, islet cell adenomatosis, islet hyperplasia, and islet cell hypertrophy are frequently used as synonyms to describe roughly the same findings, which sometimes complicates communication among experts. From a histopathological point of view, nesidioblastosis is a questionable denomination since the initial definition by Laidlaw does not adequately fit the current diagnostic criteria for adult-onset nesidioblastosis as proposed by Anlauf et al., which rely on the findings in a relatively big number of patients with hyperinsulinemic hypoglycemia [11]. While Laidlaw underscores the diffuse or disseminated proliferation of islet cells, the criteria by Anlauf and colleagues stress the existence of endocrine cells without significant proliferative activity, e.g., represented by a low Ki-67 index. Yakovac proposed the following definition in 1971 (albeit for pediatric patients): “…continuous or continual differentiation of insulin-producing β-cells from any or all divisions of the ductular system of the exocrine pancreas. […] However, it is in the additional differentiation solely of abundant, tiny clusters of β-cells and their commingling, in diffuse and random fashion, about or within acinar parenchymal elements, that the term “beta cell nesidioblastosis” signifies a condition of pathophysiologic importance.” [331]. However, nesidioblastosis according to these definitions, i.e., proliferation of endocrine cells within the adult pancreas, as represented by the neo-formation of islets from pancreatic ducts (“ductulo-insular complexes”), as well as scattered proliferation/distribution of small groups of endocrine cells within the exocrine parenchyma of the pancreas or focal islet cell hyperplasia, are also found in autopsy studies of asymptomatic adults [13,236,332]. Since one major diagnostic criterion is the presence of enlarged, hyperchromatic nuclei in β-cells, the terms “nesidiodysplasia”, ” islet dysplasia”, or “islet cell atypia” would fit better because these properties are also found in atypic cells, and the islets themselves possess histoarchitecture disturbances [333]. As β-cells are explicitly mentioned, this eliminates the possibility of classifying similar morphological findings of other pancreatic endocrine cell types under the term nesidioblastosis and would also limit its clinical application to patients suffering from hyperinsulinemic hypoglycemia.
Regular patterning and spatial distribution of the different endocrine cell types (A/alpha/α-cells (glucagon-secreting), B/beta/β-cells (insulin-secreting), D-cells (somatostatin-secreting), PP-cells (pancreatic polypeptide secreting)) additionally excludes findings, where one cell type within abnormal endocrine pancreata dominates (e.g., in genuine insulinomatosis with a clear predominance of β-cells or the various cases of pancreatic polypeptide (PP) or alpha/A/glucagon cell hyperplasia) [32,39,74,245]. The latter findings can, however, produce clinically detectable symptoms associated with excess hormone synthesis and release, although not necessarily related to insulin. These morphologies are sometimes reported as α-cell- or PP-cell-nesidioblastosis or -hyperplasia (see below), which further complicates the differentiation of these findings.
Exclusion of an insulinoma by means of macroscopic, microscopic, and immunohistochemical evaluation of the tissue is the last major criterion to diagnose diffuse, adult-onset nesidioblastosis. From our point of view, this is problematic because many patients have been reported that suffered from pathologically detectable insulinoma and concomitant islet cell hyperplasia/nesidioblastosis (also termed “background nesidioblastosis”) [16,58,75,109,231,235,283,285,286,316,317]. One patient was even reported to have an insulinoma relapse, which was possibly related to the coexistence of islet cell hyperplasia/nesidioblastosis [231]. The finding of coexisting insulinoma and changes in the morphology and probably also a function of the remaining islets of Langerhans is thus no rarity and not necessarily mutually exclusive.
Another important point is the differentiation of diffuse and focal adult nesidioblastosis. While focal forms of hyperinsulinemic hypoglycemia account for roughly 40% of cases in children/infants, focal adult nesidioblastosis is extremely rare, and only 10 cases have been described in the medical literature [12,55,111,151,159,203,210,225,250]. All other forms are described as diffuse, adult-onset nesidioblastosis since the morphological findings are distributed throughout (nearly) the entire pancreas.
Concerning the minor diagnostic criteria, islet hyperplasia is most often (and sometimes the only feature) reported in the literature, meaning an increase in the number of islets per area. Winstock and co-workers even suggested replacing the term nesidioblastosis with islet cell hyperplasia to create a terminology that includes nesidioblastosis, islet hypertrophy, septal islets, islet dysplasia, and adenomatosis. However, especially in patients suffering from post-bariatric surgery hyperinsulinemic hypoglycemia, the presence of islet hyperplasia has been questioned [95]. Moreover, Goudswaard and colleagues convincingly showed that the total endocrine area could not distinguish nesidioblastosis-hyperinsulinism from normal adult pancreata [329]. They proposed that the impression of islet hyperplasia arose when immunohistochemical staining was introduced in the evaluation of pancreata from patients with neuroendocrine disorders, and endocrine cells were suddenly better recognized than by standard hematoxylin and eosin (HE) staining. Therefore, they also questioned the existence of the pathologic entity “nesidioblastosis” in non-bariatric patients.
Islet hypertrophy, the enlargement of islets, is also frequently reported, while islets’ diameters > 300 µm are regarded as pathologic [12]. Islet hyperplasia and hypertrophy, as well as a lobulated islet structure or macronucleoli of β-cells, however, vary from patient to patient, rendering a standardized evaluation difficult [281]. Klöppel et al. concluded that approximately 2/3 of patients exhibit pancreata fulfilling all diagnostic criteria, whereas 1/3 of the specimens could also be classified as “normal” [12].
Some authors also reported peliosis, i.e., vascular ectasia, within the islets of Langerhans in patients (up to 50% in nesidioblastotic pancreata vs. 12% in normal controls) suffering from hyperinsulinemic hypoglycemia [137]. The significance of this finding is, however, not entirely clear.
From a clinical point of view, morphological findings declared as nesidioblastosis or islet cell hyperplasia are found in different diseases (see also next section). As in the case recently presented by the authors of this review (see Dieterle et al. 2023; accepted for publication in Biomedicines), there sometimes exists a good correlation of clinical findings, i.e., endogenous hyperinsulinism, with pathological findings. Then, how should these symptoms be named clinically? Non-insulinoma pancreatogenous hyperinsulinemic hypoglycemia syndrome (NIPHS) was introduced by Service and colleagues in 1999 [45], which is a good description of the etiology and clinics of hyperinsulinemic hypoglycemia associated with a functional β-cell disorder. However, the original definition of NIPHS relies on several criteria, including postprandial neuroglycopenic symptoms with concomitant detectable hypoglycemia, a negative 72 h fasting test, negative preoperative imaging studies, and a positive selective arterial calcium stimulation test with hepatic venous sampling (SACS). These criteria are not fulfilled by every patient, as some (such as the case presented by the authors of this review) have a positive fasting test without insulinoma, show exercise-induced hypoglycemia, or other additional features [71]. The histological appearance of these patients is, however, not different from NIPHS patients, strictly fulfilling the diagnostic criteria. Some authors also diagnose a nesidioblastosis clinically, which is impossible by definition [117].
To overcome the shortcomings in the current reporting of such cases, we propose a standardized work-up of the clinical (see below) and, where available, histopathological features (according to Anlauf et al., 2005, Raffel et al. 2007, or Klöppel et al. 2008 [11,12,93]) that include the following points (also see Figure 3 of our Case Report; Dieterle et al. 2023; accepted for publication in Biomedicines):
- (1)
- Evaluation of general hypoglycemia symptoms and their situational occurrence (i.e., postprandial, fasting, spontaneous, exercise-induced);
- (2)
- Results of a 4 h (–6 h) oral glucose tolerance test (OGTT);
- (3)
- Results of a 72 h fasting test;
- (4)
- Results of conventional imaging studies (CT, MRI);
- (5)
- Optional (might replace the next point in the future): results of functional imaging studies (where available, e.g., 68Ga-DOTA-Exendin-4 PET/CT or Somatostatin-receptor scintigraphy);
- (6)
- SACS with proof of an insulin gradient (might also be useful to define the extent of pancreatic resection if surgery is planned);
- (7)
- Exclusion of other conditions (e.g., Hirata´s disease, insulin secretagogues, etc.).
If the clinical findings prove hyperinsulinemic hypoglycemia and conventional imaging studies are negative; SACS shows an increased, non-localized insulin response; and/or functional imaging studies suggest diffuse tracer enrichment compatible with a functional β-cell disorder, we think that the clinical diagnosis of a NIPHS is justified, even if the fasting test is positive or hypoglycemia occurs independently of food intake. Subsequently, the criteria of Anlauf et al., Raffel et al., and Klöppel et al. should be applied to establish a histopathological diagnosis. According to our discussion above, we are of the opinion that “islet dysplasia” or “islet cell atypia” in a general sense, i.e., meaning an abnormal histologic appearance of the islets (and not necessarily a neoplastic precursor), would best describe the morphological findings. This would abolish the ill-defined term “nesidioblastosis” and avoid the reporting of inconsistent findings such as “islet cell hyperplasia”.
4. Nesidioblastosis and Islet Cell Hyperplasia in Other Adult Diseases
Not all patients diagnosed with nesidioblastosis or islet cell hyperplasia of the pancreas simultaneously exhibit symptoms of hyperinsulinemic hypoglycemia. This is both a consequence of adaptive changes in the endocrine pancreas in response to other diseases and of the inconsistent use of these denominations. In the more recent medical literature, the term nesidioblastosis is, however, more or less restricted to disorders related to endogenous hyperinsulinism (see above).
In infants, hypertrophy or hyperplasia of the islets of Langerhans was reported in association with many clinical conditions, including infants of diabetic mothers, Beckwith–Wiedemann syndrome, erythroblastosis fetalis, Zellweger spectrum disorder, α-Thalassemia, Donohue syndrome, Tyrosinemia, cyanotic congenital heart disease, long-term parenteral nutrition, MEN2 syndrome, Smith–Magenis syndrome and, of course, PHH/CHI/focal adenomatosis [334].
Adult patients with nesidioblastosis/islet cell hyperplasia can also exhibit a huge variety of comorbidities.
The relationship between insulinoma, nesidioblastosis/islet cell hyperplasia, and clinical symptoms of hypoglycemia in the adult population was established early. This is sometimes called “background nesidioblastosis” [231]. The first case was reported by Knight et al. in 1967 [75]. Our literature research identified at least 54 cases of insulinoma with coexisting, significant changes in the morphology in the remaining islets of Langerhans [16,40,58,68,71,75,109,121,130,170,177,182,215,218,231,266,276,283,285,286,316,318]. Two of the patients were found to have metastatic disease (a 40-year-old woman and a 34-year-old man [109,266]). The case presented by Campbell et al. in 1985 additionally showed serological evidence of autoantibodies to islet cells [285]. If these antibodies are related to the islet cell hyperplasia, duct-associated endocrine proliferation, or budding of islets remains unclear. A 58-year-old woman with insulinoma, chronic pancreatitis, villous adenomatosis of the pancreatic duct, and islet cell hyperplasia of A, B, and somatostatin-producing D cells was described in 1993 [318]. When applying the above-discussed strict definition of adult-onset nesidioblastosis, this case could not be classified as nesidioblastosis. A large populational study from Japan reported 1085 cases of organic hyperinsulinism in 1998, of whom 44 were diagnosed with nesidioblastosis. Sixty percent of these patients had simultaneous insulinoma [40]. Multiple insulinomas and nesidioblastosis are also a feature of MEN1 (see below). Dauriz and colleagues reported an especially interesting case of a 63-year-old woman who underwent distal pancreatectomy for insulinoma 4 years before the onset of recurrent hypoglycemia symptoms [231]. The patient experienced an insulinoma relapse due to background nesidioblastosis and was treated with a subtotal pancreatectomy since conservative treatment with diazoxide and pasireotide was not successful. This case illustrates a potential pathogenetic relationship between islet cell hyperplasia/nesidioblastosis and the emergence of insulinomas. It is still a matter of debate if, at least in some cases, the changes in the islets precede the formation of the actual tumor and if nesidioblastosis should therefore be regarded as a (facultative) pre-neoplasia (see below) [180,335].
In adults, findings of islet cell hyperplasia or nesidioblastosis were also reported independently of hyperinsulinism.
Glucagon is produced in the A/α/alpha cells of the islets of Langerhans. Tumors arising from these cells are called glucagonomas. Hyperglucagonemia can also arise from diffuse hyperplasia of the α-cells. A clinicopathological classification of related disorders accompanied by α-cell hyperplasia, as well as a collection of cases, was presented by Ouyang et al. in 2011 and Yu in 2014 [96,336]. We identified 18 cases of α-cell hyperplasia and/or glucagonoma associated with nesidioblastosis [38,74,88,96,115,141,162,163,164,179,189,213,298,312,336]. To the best of our knowledge, the first report of nesidioblastosis with scattered α-cells throughout the acinar tissue was presented by Balas et al. in 1988 [298]. A 35-year-old woman was diagnosed with a tumor in the head of the pancreas and was shown to have an additional silent glucagonoma as well as α-cell nesidioblastosis. Two cases of malignant glucagonoma with nesidioblastosis were reported in 1991. While one presented with β-cell hyperplasia, the other presented with α-cell hyperplasia [312] (the latter cited in [96]). Furthermore, two cases described by Brown et al. became clinically apparent with diabetes mellitus, as would be expected from an excess of glucagon [38]. A necrolytic migratory erythema was also reported in association with diffuse α-cell hyperplasia, which was diagnosed as “diffuse glucagonoma” since no actual tumor mass was found [88]. An unusual case of hyperinsulinemic hypoglycemia was reported by Roberts et al. in 2012 [164], where a 56-year-old male presented with glucagonoma and a glucagon-like peptide 1 (GLP-1) secreting metastatic mass (“GLPoma”). First, the patient presented with diabetes (most likely due to the predominance of the glucagonoma) and later developed hyperinsulinemic hypoglycemia, which is probably the consequence of the GLP-1 excess, which acts as a potent insulin secretagogue. A coexistence of α-cell hyperplasia with renal cell carcinoma and a cystic lesion of the pancreas in a 56-year-old man was also described [179]. Another notable case was a 35-year-old woman who suffered from recurrent acute pancreatitis and glucagonoma with α-cell nesidioblastosis [189]. A middle-aged woman was diagnosed with a microadenoma showing glucagon expression. Of interest, this patient reported by Schwetz et al. was diagnosed with hyperinsulinemic hypoglycemia and required distal pancreatectomy and pasireotide postoperatively [213]. The cases of α-cell nesidioblastosis and/or glucagonoma thus show various clinical presentations ranging from diabetes mellitus to severe hypoglycemia. As proposed by Yu, these diseases can be classified as reactive, functional, and non-functional. Accordingly, reactive α-cell hyperplasia arises from defects in glucagon receptor signaling and is associated with nonspecific symptoms such as abdominal pain. The pancreatic mass is often an incidental finding, as even extreme hyperglucagonemia might not be associated with a clinical glucagonoma syndrome. If the mutation (e.g., P86S homozygous inactivating mutation of the human glucagon receptor) is clearly defined, this clinical picture is also known as Mahvash disease [337]. Functional glucagonoma syndromes show a characteristic clinical appearance with elevated glucagon levels but exhibit no gross pancreatic neuroendocrine tumor (PNET). Nonfunctional cases have no specific symptoms, do not have elevated glucagon levels, have no glucagonoma syndrome, and might or might not have a gross PNET. Mutations in the latter cases are not known [336]. From the 12 cases reported by Yu (all included in our summary), 5 were classified as reactive, 1–2 as functional, 3 as nonfunctional, and 2 could not be classified.
An association of islet cell hyperplasia without symptoms of hypoglycemia was reported in a 41-year-old woman suffering from von Hippel–Lindau disease [83]. The patient also had bilateral pheochromocytoma, renal cell carcinoma, and multiple lesions in the pancreas (including an endocrine carcinoma).
Cases of MEN1 with concomitant pancreatic nesidioblastosis or islet cell hyperplasia have been repeatedly reported (according to our literature research criteria described above, we found a total of 28 patients [20,28,42,80,172,245,277,288,294]). In some of them, coexisting insulinomas were found, as in the two cases reported by Proye et al. [80]. However, not all patients with MEN1 and islet cell changes exhibited clinical symptoms of hypoglycemia. The patient reported by Franksson et al. in 1960 is presumably the first patient with MEN1 reported to have islet cell hyperplasia. Additionally, chief-cell hyperplasia of the parathyroid, adenomata of the pancreas, and gastro-duodenal ulcers were found [42]. It is likely that this case is actually an overlap syndrome of MEN1 and Zollinger–Ellison syndrome (MEN1 with gastrinoma) [338]. Members from a MEN1-family described in 1972 presented with excessive insulin secretion, and the individuals who underwent pancreatectomy also had islet cell hyperplasia [172]. A 30-year-old man with MEN1, who was also diagnosed with nesidioblastosis and hypoglycemia, had an additional growth hormone-releasing hormone (GHRH) producing tumor, hyperparathyroidism, hyperprolactinemia, and multiple endocrine pancreatic tumors [294]. A suspected MEN1/MEN2 overlap syndrome became clinically apparent through a medullary thyroid carcinoma, watery diarrhea, and flushing. Pancreatic nesidioblastosis of the pancreatic polypeptide (PP) expressing cells were recognized (see below) [292].
Nesidioblastosis or islet cell hyperplasia in association with Zollinger–Ellison syndrome is also a relatively common finding (according to our literature research criteria described above, we identified a total of 64 patients with (Pseudo) Zollinger–Ellison syndrome [32,42,64,86,107,139,183,194,262,271,284,303,339]). The study by Ellison et al. is especially interesting since a large number of patients was studied, of whom 10% showed islet cell hyperplasia [64]. In most cases, pancreatic islet hyperplasia was associated with a morphologically detectable gastro-pancreaticoduodenal gastrinoma (e.g., Larsson et al. 1973 [183]). Of interest, increased β-cell replication was reported in islets directly adjacent to the gastrinoma but not in islets further away from the tumor [339]. This suggests that there might be a paracrine, a secretory factor that contributes to the morphological changes seen in those pancreata. Above, we could identify a possible case of Pseudo Zollinger–Ellison syndrome with islet cell hyperplasia [262]. This means that there is no macroscopic or microscopic tumor detectable, but hypergastrinemia arises from hyperplasia of gastrin-producing cells. The case described by Varas Lorenzo et al. might be an example of hypergastrinemia related to islet cell hyperplasia causing a Pseudo Zollinger–Ellison syndrome.
Verner–Morrison syndrome is defined by secretory diarrhea, hypokalemia, and hypochlorhydria. Islet cell hyperplasia has been reported in some cases (according to our literature research criteria described above, we identified a total of 19 patients with definite or suspected (Pseudo) Verner–Morrison syndrome/VIPoma [44,118,138,161,205,258,261,264,282,310]). While Sircues et al. described an overrepresentation of β-cells in their patients, Jacobs et al. reported non-β-islet hyperplasia [118,161]. Verner et al. agree with the finding by Jacobs and colleagues [205]. As Verner–Morrison syndrome is mostly caused by tumors, which induce an overproduction of vasoactive intestinal peptide (VIP), also called VIPomas, the report of increased VIP blood levels with concomitant islet cell hyperplasia by Schwarz et al. was important for establishing the causal relationship [258]. Tomita et al. were the first to report that the cell population responsible for the observed islet hyperplasia in clinically suspected Verner–Morrison syndrome are, in some instances, the PP-producing cells [264]. They proposed that, in the absence of a VIPoma, PP excess due to PP-cell hyperplasia can cause a similar clinical picture, which is consequently termed Pseudo Verner–Morrison syndrome. PP-cell hyperplasia in association with A-cell hyperplasia was later reported by the same group [282]. A concise review of A- and PP-cell hyperplasia of the pancreas and their clinical implications were presented by Ouyang and colleagues in 2011 [96]. From a diagnostic point of view, the finding by Francesconi et al. is interesting [138]. A 60-year-old man presented with fecal urgency and diarrhea and was found to have a focal pancreatic uptake in 111In-Pentetreotide scintigraphy. Clinically, a VIPoma was suspected. The lesion was found to be a (focal?) nesidioblastosis without any signs of hyperinsulinism. The symptoms persisted after surgery, and chromogranin A levels remained within the normal range. Although clinically highly suggestive of Verner–Morrison syndrome, no neuroendocrine tumor was found, and the nesidioblastosis seemed to be unrelated to the symptoms. Careful differential diagnosis, therefore, remains highly important. Additionally, PP cell hyperplasia without diarrhea or suspected VIPoma was reported in two elderly males [100,113]. One of them presented with pseudo-obstruction of bowels and had additional microadenomas of the pancreas. None of the patients had symptoms of hypoglycemia.
In areas of pancreatic fibrosis and during chronic pancreatitis, islet cell hyperplasia is commonly found and is most likely a reactive adaptation to the chronic inflammatory stimuli and a sign of regenerative processes [17,96,129,291,328]. Pancreatic duct obstruction/ligation was shown both experimentally and clinically to induce nesidioblastosis [291,340,341,342]. In rare instances, pancreatitis/pancreatic fibrosis was associated with hyperinsulinemic hypoglycemia. Such a case of a 20-year-old man suffering from recurrent abdominal pain, vomiting, chronic familial pancreatitis, and hyperinsulinemic hypoglycemia with histologically proven nesidioblastosis was reported by Wig et al. in 2008 [129]. In another case of a 67-year-old man, calcified pancreatitis was associated with hyperparathyroidism and hyperplasia of α-cells of the pancreas (Paloyan et al., 1967; cited in [96]).
Morphological studies with pancreata from cystic fibrosis patients have been conducted by different authors [322,343,344]. Nesidioblastosis, in this case, meaning the neo-formation of islets, might be a compensatory mechanism in this disease and protract the onset of diabetes mellitus. Brown et al. described patterns of nesidioblastosis in cystic fibrosis patients in 1971 (cited in [128]).
An association of intraductal papillary mucinous neoplasm (IPMN) or pseudopapillary neoplasm of the pancreas has been reported at least four times [145,158,220,345]. Two of these cases had additional neuroendocrine tumors, and one exhibited α-cell hyperplasia [220,345].
Islet cell hyperplasia/nesidioblastosis is also reported in response to drug intake. An early post-mortem study by Bloodworth conducted in 1963 describes the neo-formation of islets, islet hyperplasia, an increase in α-cells, and a decrease in β-cells of adults treated with the sulfonylurea compound tolbutamide [53]. If these patients had any symptoms related to hypoglycemia, it was not clear. In 1984, a case of a 25-year-old woman with hyperinsulinemic hypoglycemia with pathohistological signs of islet cell hyperplasia and nesidioblastosis after subtotal pancreatectomy was also reported in response to chlorpropamide treatment (sulfonylurea). Moreover, three cases of narcotic addicts suffering from nesidioblastosis are documented [97,125]. All three women presented with hyperinsulinemic hypoglycemia, and two were HIV positive but without retroviral therapy.
The association of nesidioblastosis or islet cell hyperplasia with pancreatitis, pancreatic fibrosis, cystic fibrosis, the tumor syndromes MEN1, and von Hippel–Lindau syndrome, as well as with Zollinger–Ellison syndrome, Verner–Morrison syndrome, glucagonoma, insulinoma, and sulfonylurea intake are well-established and, although not experimentally proven, thought to be related to the pathophysiological bases of the diseases. The associations between nesidioblastosis or islet cell hyperplasia and other diseases, which are described next, are mostly based on single/a few reports and might, therefore, rather present simple comorbidities that are not related causally. For reasons of completeness, they are, however, presented here.
An autopsy evaluation of the pancreas of a 54-year-old man who suffered from multiple malignancies and who was a kidney transplant recipient revealed islet cell hyperplasia [252]. Clinical symptoms were, however, not reported. Yeh et al. presented the case of an autopsied 72-year-old woman with myelodysplastic syndrome and nodular glomerulosclerosis, who was, however, never diagnosed with diabetes [49]. She had a history of symptomatic hyperinsulinemic hypoglycemia, and the pancreas showed signs of nesidioblastosis/islet cell hyperplasia. It is plausible to assume that hyperinsulinism might have reversed a preexisting type II diabetes. A 36-year-old man with end-stage chronic kidney disease presented with hyperinsulinemic hypoglycemia [230]. Since insulin clearance is impaired in chronic kidney disease, SACS was performed to observe whether there was an inadequate insulin response to calcium stimulation. The test was positive and subtotal pancreatectomy was performed, revealing islet cell hyperplasia/nesidioblastosis. A 73-year-old man, who depended on dialysis for 18 years, also suffered from hyperinsulinemic hypoglycemia [244]. Duodenopancreatectomy was not sufficient for symptom control. The patient is now treated with continuous subcutaneous octreotide infusion and corticosteroids. The histopathological evaluation confirmed adult-onset nesidioblastosis. These four cases are all related to kidney diseases, but we are not aware of any common pathophysiological mechanism.
Larsson et al. reported a case of pernicious anemia and atrophic gastritis with concomitant nesidioblastosis of the pancreas [253]. Chronic laxative abuse was also shown to be associated with an increased number of islets and prominent ductulo–insular complexes in a 39-year-old man [255]. A 59-year-old woman with hyperinsulinism, hypoglycemia, and hyperglucagonemia was shown to have islet hyperplasia [267]. She additionally suffered from biliary cirrhosis and had a portocaval anastomosis and a gastrojejunostomy. This case of islet cell hyperplasia might either be related to some undetected neuroendocrine neoplasia or represent an early case of gastric surgery-associated nesidioblastosis (see below).
Autoimmune phenomena in the context of nesidioblastosis were reported in at least three instances, including one of the above-discussed cases with coexisting insulinoma [195,274,285]. One patient with a history of scleroderma suffered from anti-insulin receptor antibody-induced diabetes mellitus [274]. Islet cell hyperplasia was detected in the pancreas, and the authors concluded that the neo-formation of endocrine cells might represent a compensatory reaction to antibody-induced diabetes. A woman described by Woo et al. exhibited simultaneous nesidioblastosis and insulin autoantibodies [195]. Clinically, she presented with hyperinsulinemic hypoglycemia. Such rare instances might represent overlap syndromes of Hirata´s disease and adult-onset nesidioblastosis, as the insulin–autoantibody syndrome can provoke both episodes of hypoglycemia and hyperglycemia. If the latter phenomenon dominates, islet hyperplasia could indeed be a compensatory mechanism for developing hyperglycemia. Alternatively, the antibodies could exert a stimulatory function on the islet cells and thereby favor proliferation or hypertrophy. The exact mechanism, however, remains to be determined.
Five adults with genetic forms of α1-antitrypsin deficiency were shown to have islet cell hyperplasia and/or nesidioblastosis [289]. Symptoms of hypoglycemia were not reported.
Nesidioblastosis in heterotopic pancreata was found twice [110,239]. A 24-year-old woman had an enteric intussusception due to a jejunal heterotopic pancreas with nesidioblastosis but no hypoglycemia [110]. A 32-year-old man had pancreatic nesidioblastosis and heterotopic pancreatic tissue with nesidioblastosis, accompanied by hyperinsulinemic hypoglycemia [239].
Of interest, nesidioblastosis has been reported as coexisting disease in diabetic patients. Thus far, 13 cases have been described in the literature [17,27,57,65,66,67,75,101,132,180,233]. In some of the patients, the nesidioblastosis-related hyperinsulinemia led to a reversal of the diabetic syndrome, and insulin therapy could be discontinued. The others still needed diabetic treatment.
Nesidioblastosis in neuroendocrine tumors other than insulinoma, glucagonoma, and gastrinoma has been described [197,200,229,236]. A 59-year-old woman was diagnosed with a non-functioning PNET and hyperinsulinemic hypoglycemia due to islet cell hyperplasia [197]. Although D-cell hyperplasia has probably been only reported once [318], a case of metastasizing somatostatinoma has been described by Wiesli et al. [229]. Although the patient had episodes of hyperglycemia (somatostatin can inhibit both glucagon and insulin secretion), postprandial hyperinsulinemic hypoglycemia was also detected and related to postmortem findings of islet cell hyperplasia. This is interesting since it indicates different functions of somatostatin during different nutritional phases.
Single case reports documenting islet cell hyperplasia/nesidioblastosis with the following diseases can also be found in the medical literature: Choristoma (no reported hyperinsulinism) [304]; fibrocystic pancreatic atrophy with concomitant pancreatic carcinoma (no reported hyperinsulinism) [321]; familial adenomatous polyposis (FAP, with hyperinsulinemic hypoglycemia) [66]; after pancreas transplantation for diabetes mellitus type I (with hyperinsulinemic hypoglycemia) [67]; orbital lymphoma, hypopituitarism, and secondary adrenal insufficiency (with hyperinsulinemic hypoglycemia) [70]; Sheehan syndrome (with hyperinsulinemic hypoglycemia) [198]; Hashimoto thyroiditis with chronic renal failure, incidental adrenal mass, and ovarian thecal metaplasia (no reported hyperinsulinism) [76]; and a patient with short bowel syndrome with GLP1-agonist treatment for diabetes mellitus type II (with hyperinsulinemic hypoglycemia) [233].
As can be seen from this extensive discussion, there is a huge spectrum of diseases associated with nesidioblastosis/islet cell hyperplasia. While some findings have been reported repeatedly and might thus be connected pathophysiologically, other coexistences might just be incidental comorbidities. Since there are cases of A, B, and PP cell hyperplasia with different clinical symptoms, it can be concluded that these represent different clinical and pathophysiological entities, albeit with some shared histopathological characteristics. We, however, think that the nomenclature should be adjusted accordingly and that histopathological findings should be more strictly correlated with the clinical picture to avoid inaccurate reporting as “nesidioblastosis” or “islet cell hyperplasia”.
5. Etiology and Pathophysiology of NIPHS/Nesidioblastosis with Hyperinsulinism
The etiology of NIPHS/nesidioblastosis with hyperinsulinism in the adult is largely unknown. For PHH/CHI, defined disease-causing mutations in various genes are known [346]. To date, ten genes have been identified that are involved in different, non-syndromic clinical forms of the disease: ABCC8 (ATP binding cassette subfamily C member 8; modulates ATP sensitive potassium channels and therefore interacts with KCNJ11), KCNJ11 (ATP-sensitive potassium channel = Kir6.2/KATP; interacts with the sulfonylurea receptor), GCK (Glucokinase), HK (hexokinase), GLUD-1 (Glutamate Dehydrogenase type 1), HADH1 (Hydroxyacyl-coenzyme A Dehydrogenase = SCHAD = short-chain-3-hydroxyacyl-coenzyme A Dehydrogenase), SLC16A1 (solute carrier family 16, member 1 = MCP1 = monocarboxylate transporter 1), HNF1A (hepatic nuclear factor 1A), HNF4A (hepatic nuclear factor 4A), and UCP2 (uncoupling protein 2). Due to different pathophysiological mechanisms, the mutations in these genes also determine the patients´ responses to specific treatments such as diazoxide administration. A concise discussion of the genetics of CHI and CHI associated with other syndromic diseases is provided by Rosenfeld et al. and by Gilis-Januszewska et al. [347,348].
An important question that arises when discussing the differences between CHI/PHH and adult-onset nesidioblastosis is, which age of symptom onset can be regarded as an adult? The case presented in our recent Case Report (see Dieterle et al., 2023; submitted to Biomedicines) was clinically apparent for the first time at the age of 15. In this context, a study by Dahms et al. from 1980 contributes important information [263]. They analyzed cases of hyperinsulinemic hypoglycemia with age-matched autopsy controls. A certain degree of “nesidioblastosis” (in this case, meaning the neo-formation of islets and their association with the exocrine ducts) was found to be normal in newborns and early infancy. Quantitatively, this feature significantly decreases until the age of approximately 3 years. CHI/PHH usually becomes clinically apparent within the first days, weeks, or months of life [331]. According to the data obtained from the literature, we, therefore, think that new-onset hyperinsulinemic hypoglycemia above the age of 3 years without one of the characteristic mutations might be seen as a form of late/”adult”-onset hyperinsulinism. This should be regarded as pathophysiologically different from the genetic forms described above. Late-onset NIPHS/nesidioblastosis/islet hyperplasia/islet dysplasia might therefore be a better characterization than adult-onset and would thus include older children, adolescents, and adults. It would also eliminate the uncertainty of the exact onset of symptoms as in our presented case, where the patient was an adult at diagnosis but not at the expected beginning of the disease. A recent article by Castillo-López and colleagues also discussed this issue. They reported a 15-year-old male with hyperinsulinemic hypoglycemia and histological findings typical of nesidioblastosis [247]. This case was classified as “adolescent” according to their definition (children: aged <15 years; adolescents: aged 15–21 years; adults: aged >21 years). The authors performed a literature research aiming at the adolescent population of nesidioblastosis patients and found 41 cases, 24 of which were not retrieved by our search strategy but would be classified as adult when using our definition [349,350,351,352,353,354,355,356]. This again underscores the problem associated with non-standardized definitions of disease entities and the overlap of pediatric, adolescent, and adult patient collectives.
In older children, adolescents, or adults, sporadic or idiopathic cases that are not associated with a genetically defined disease, such as MEN1 or bariatric surgery, are not well understood [12]. Idiopathic cases with MENIN mutations, the gene related to MEN1, were never found [11]. Mutations in subunits of the sulfonylurea receptor (SUR) were also not described in the literature [45]. The same is true for MODY2/3 (maturity-onset diabetes of the young type 2 and 3) genes [101]. A definite genetic cause of adult-onset hyperinsulinemic hypoglycemia with islet cell hyperplasia/nesidioblastosis has only been found in a limited number of adults or cases with symptom onset at school age [187,212]. All the respective mutations were described as activating glucokinase (GCK) variants. To the best of our knowledge, the first of these cases was published in 1998 by Glaser and colleagues, who described a p.Val455Met mutation in a family presenting with hyperinsulinemic hypoglycemia (listed as rs104894012 in the NCBI SNP database) [357]. Euglycemia was achieved via diazoxide treatment in all patients. Christesen et al. reported a second activating GCK variant, namely p.Ala456Val, which also affected a 42-year-old woman (listed as rs104894014 in the NCBI SNP database) [358]. Due to a lack of clinical symptoms (the only finding was fasting hypoglycemia, classified as “relative hyperinsulinemia”), the patient never needed treatment. Activating mutations of GCK in adults were also found at positions p.Thr65Ile and p.Trp99Arg, as reported in [359]. Only the woman reported in this study needed treatment with pancreatic head resection and diazoxide. Five adult cases (all from one family) were shown to have activating p.Val389Leu mutations (listed as rs1350717554 in the NCBI SNP database) [187]. All patients suffered from hypoglycemia, but only three were symptomatic. Histopathological evaluation of the pancreata was, however, not available since the patients were treated with diazoxide. Lu et al. found two patients with activating GCK mutations who had hyperinsulinemic hypoglycemia at school age. These cases illustrate that simple age-dependent clinical evaluation of patients is sometimes unsuccessful in properly distinguishing adult/late-onset hyperinsulinemic hypoglycemia/NIPHS from inherited forms. Therefore, thorough genetic work-up, especially in patients presenting at a relatively young age but not directly in the newborn/early infancy period, should always be considered. An intriguing case of an obese (BMI 49.1 kg/m2) 22-year-old was recently reported by Koneshamoorthy and co-workers [248]. The patient presented with hyperinsulinemic hypoglycemia and received a distal pancreatectomy, which, however, did not lead to symptom relief. A review of the medical history revealed that neonatal hypoglycemia was diagnosed in this patient (macrosomic newborn) and treated with diazoxide until the age of 3. Genetic testing revealed a novel activating GCK variant (c.269A>C; p.Lys90Thr), which was also found in the mother (partial pancreatectomy at age 6 for hypoglycemic seizes), his sister (hyperinsulinemic hypoglycemia discovered upon monitoring for gestational diabetes), and the nephew (neonatal hypoglycemia treated with diazoxide). A postoperative treatment attempt with diazoxide was unsuccessful, while pasireotide proved suitable to control hypoglycemia. Functional studies of islet cells from this patient are reported below. This case underscores the clinical heterogeneity (asymptomatic cases are possible; the degree of symptoms might vary with hepatic regulation of GCK) and different ages of onset of GCK-related nesidioblastosis, which needs to be considered when evaluating patients suffering from hyperinsulinemic hypoglycemia at any age.
Although not exclusively studied in the context of adult-onset nesidioblastosis/NIPHS, the molecular mechanism of hyperinsulinism related to GCK mutations might be similar for pediatric CHI/PHH and adult NIPHS cases. Structurally, some of the known GCK mutations were shown to map to the allosteric activator site of the enzyme. Mechanistically, there are two groups of activating GCK mutations leading to hyperinsulinemic hypoglycemia: one group leads to a “left shift” of glucose binding, i.e., an increased glucose affinity of the enzyme (e.g., p.Thr65Ile and p.Ala456Val). The other group, e.g., p.Val455Met and p.Trp99Arg, favor the isomerization of the enzyme into its active form [360,361]. The common final path of both mutation groups is the same: the glucose metabolizing activity of the pancreatic β-cells increases, resulting in a rise in the glycolytic and citric acid cycle/respiratory chain activity, which is accompanied by an increase in the concentration ratio of ATP and ADP (. ATP inhibits the Kir6.2/KATP activity, which depolarizes the cells and enhances the open-state probability of voltage-gated calcium channels. Calcium influx enables the fusion of preformed insulin-containing vesicles with the plasma membrane. In summary, the glucose-stimulated insulin secretion of the β-cells increases, giving rise to hyperinsulinemic hypoglycemia (see Figure 1) [347,362].
Apart from genetics, environmental causes have been proposed as triggers for late-onset nesidioblastosis [12]. However, the impact of, e.g., nutritional factors such as carbohydrate intake or exposure to nutritional or environmental toxins has never been investigated systematically.
Experimental findings on late-onset nesidioblastosis are scarce. Campbell and colleagues were able to establish cell cultures of islet cells from a patient with islet hyperplasia. Of interest, the islets could be grown in culture for up to 60 days, whereas normal islet cells usually die after 14 days. Overgrowth of the islets was also reported in the culture system [285]. Similar experiments were performed by Roncari and co-workers. They observed faster growth of adult hyperplasia-derived islet cells in culture in comparison with control conditions [296]. Functionally, the in vitro studies also confirmed that nesidioblastosis-derived islet cells have a higher basal insulin secretion, an overall higher insulin content, and express more insulin mRNA [37]. The insulin secretion persisted throughout the culture period of an infant-derived nesidioblastosis cell line, which is not the case upon a long-term passage in normal islet cells [363]. Insulin secretion and peripherally detectable C-peptide levels in comparison with insulinoma are nonetheless lower [112]. Islet neogenesis-associated protein (INGAP) expression in some adult-onset nesidioblastosis patients was increased, which points in the direction of increased neo-formation of endocrine cells in the pancreas. However, the key pancreatic transcription factors NK6 homeobox 1(Nkx6.1) and pancreatic and duodenal homeobox 1 (PDX1), as well as GLP-1, showed no abnormalities in nesidioblastosis patients [91,106]. The expression pattern of several growth factors and their receptors was also studied in nesidioblastosis. An increase in insulin-like growth factor 2 (IGF2), IGF 1 receptor subtype alpha (IGF1Rα), and transforming growth factor β receptor 3 (TGFβR3) expression was detected, whereas no difference was apparent in epidermal growth factor receptor (EGFR), TGFβ1, or TGFβ2 levels [137]. Of interest, the IGF2 overexpression was only present in idiopathic nesidioblastosis but not in post-bariatric surgery cases. In the above-discussed case by Koneshamoorthy and co-workers, single-cell transcriptomic studies uncovered an elevated insulin (INS) and creatine kinase B (CKB) expression, whereas protein delta homolog 1 (DLK1) and neuropeptide Y (NPY) expression was downregulated [248]. DLK1 and NPY inhibit the switch of islet cells from proliferation to differentiation. Thus, their downregulation indicates that the mutation, in this case, does not lead to a hyperproliferation or genuine neoplasia of islet cells. Genes associated with cell cycle progression, β-cell differentiation, or glycolysis did not differ between patient-derived cells and control conditions. The same was true for the insulin receptor, insulin receptor substrate 1 (IRS1), insulin receptor substrate 2 (IRS2), and KIAA1324.
Electrophysiological experiments were also conducted with nesidioblastosis-derived cells [149]. Even at low glucose concentrations (3 mmol/L), depolarization, as well as action potentials, were registered at the β-cell membrane. While the resting membrane potential at 3 mmol/L in normal islet cells is around −60 to −70 mV, nesidioblastosis-derived cells were depolarized to −30 to −5 mV. This is unusual since low glucose values normally suppress endogenous insulin secretion. The function of the Kir6.2 channel was, however, inconspicuous. These observations, although only reported in a single study, propose a molecular mechanism for the clinically observed functional β-cell disorder that not necessarily depends on an increase in β-cell mass and makes the diagnosis of nesidioblastosis independent of actual islet cell hyperplasia. The cells of the patient from the Koneshamoorthy study were also subjected to electrophysiological analyses [248]. Cytoplasmic Ca2+ concentrations of nesidioblastosis-derived and control cells were not significantly different at baseline. However, upon glucose administration, there was a remarkable increase in the calcium response in nesidioblastosis-derived cells, which consecutively led to higher insulin secretion. Although this case cannot be classified as genuinely adult, it might nonetheless contribute to a pathophysiological understanding of the disease.
Since the nuclei of the β-cells in nesidioblastosis were shown to be enlarged, which is one major histopathological diagnostic criterion, some authors assumed that the DNA content of these islet cells might be increased [333]. Flow cytometric testing of this hypothesis revealed that, in contrast to β-cell derived adenomas and carcinomas, which are often aneuploid, nesidioblastosis-derived cells are diploid [23].
Concerning the formal pathogenesis, a hyperplasia–dysplasia–adenoma (carcinoma) sequence was also proposed in the context of islet cell hyperplasia [335]. According to this model, an external or internal stimulus would induce the proliferation and/or hypertrophy of islet cells. Genetic and morphological changes in the respective cells would represent the next step, called dysplasia. A true adenoma, i.e., a neoplastic entity, would arise subsequently. Malignant transformation of an insulinoma would finally lead to metastatic, cancerous disease. This model could explain the frequently observed coexistence of islet hyperplasia and insulinoma. However, the opposite, that insulinomas lead to a paracrine stimulation of islet growth and/or proliferation of the islets of Langerhans, is also possible. Hyperplasia of ghrelin and obestatin immunoreactive cells at the periphery of islets has been reported and might contribute to such phenomena [186]. Both possibilities have never been adequately challenged experimentally [316]. There is only one study reporting the progression from nesidioblastosis/islet cell hyperplasia to a pancreatic tumor in SV40 large T transgenic mice [364]. The significance of this experimental finding to the human disease is, however, questionable since SV40 large T exhibits transforming activity in many cell types.
Other authors suggested that a lack of somatostatin, which inhibits β-cell function, leads to an unrestrained proliferation of islets. As discussed in the context of autoimmune phenomena and nesidioblastosis, circulating autoantibodies against islet cell epitopes could also transmit pro-proliferative stimuli and lead to hyperplasia [316].
The functional outcome of the β-cell disorder has also been intensively discussed in the literature [33]. Since a definitive increase in β-cell mass has not been convincingly shown, a quantitative increase in the number of islet cells is most likely not the cause of hypoglycemia. A relative increase in β-cells, i.e., an overrepresentation in comparison with A, D, and PP cells, also does not seem to be the main mechanism of hyperinsulinemic hypoglycemia since many authors did not find such a shift [11]. Changes in islet architecture, which could be a consequence of or result in aberrant patterns of paracrine signaling, could, however, contribute to inadequate insulin secretion. Additional changes in functional regulation, whose morphological correlate might be the changes in the β-cell nuclei, have also been proposed.
Of interest, nesidioblastosis with or without hyperinsulinemic hypoglycemia was reported in animals. Cases of a 2-day-old Simmental calf (no hyperinsulinism) [365], young Beagle dogs (no hyperinsulinism) [366], a 6-year-old castrated male Australian Shepherd (hyperinsulinemic hypoglycemia) [367], old dogs (some with hypoglycemia and/or hyperinsulinism) [368], two squirrel monkeys (hyperglycemia with glucagon-reactive nesidioblastosis) [369], a cat (hyperinsulinemic hypoglycemia) [370], and several aged horses (no documented hyperinsulinism) [371] were found in the literature. If the pathophysiology and etiology of these morphological findings are similar to the ones in humans remains to be established.
Animal models (e.g., hamster, mouse, monkey) or distinct cell culture models of nesidioblastosis/islet cell hyperplasia/proliferation have mainly been developed in the context of regenerative treatments for diabetes mellitus, experimental pancreatitis, or in the study of pancreatic malignancies [340,341,342,364,372,373,374,375,376,377,378,379,380,381,382,383,384,385,386,387]. Due to a lack of significant contribution to the understanding of the pathophysiology of human nesidioblastosis, this is not further discussed here.
Although not the direct topic of this review, some important findings concerning nesidioblastosis/islet cell hyperplasia after gastrointestinal surgery are discussed. For post-gastric bypass hyperinsulinemic hypoglycemia (PGBHH), the etiology and pathophysiology of hyperinsulinemic hypoglycemia seem to be different from idiopathic cases. The reports by Patti et al. and Service et al. in 2005 are generally thought to be the first cases of PGBHH. A prominent feature of the disease is postprandial hyperinsulinemic hypoglycemia [7,84]. However, we suppose that the case by Brennan et al. from 1980 might also be related to a gastroenterostomy [267]. Most patients reported with this syndrome underwent Roux-en-Y bypass surgery. According to our literature research, there are just single cases after vertical banded gastroplasty [84], duodenal switch [143], fundoplication [156], esophagectomy [127], or sleeve gastrectomy [234]. The existence of PGBHH with pancreatic nesidioblastosis is, however, under debate. Some authors deny its overall existence, whereas histopathological findings were consistent with the above-discussed criteria for adult-onset nesidioblastosis in many cases. Sometimes, the diagnosis is also established only clinically. An overlap with other (functional) hyperinsulinemic hypoglycemia syndromes after gastric bypass surgery, such as late dumping syndrome, is also likely. Supplementary Table S1 mostly contains cases with histopathologically confirmed nesidioblastosis/islet cell hyperplasia or a highly suggestive clinical presentation. We counted a total of 201 cases plus additional 135 cases discussed at a conference in Harvard [140] (it is not known if some of them were reported before or overlap otherwise) [5,7,84,99,102,103,105,114,114,116,119,120,122,124,127,133,137,140,142,143,144,145,153,154,155,156,157,165,167,168,169,171,174,177,188,190,191,193,201,204,207,209,217,223,224,234,235,237,243,267]. Where demographic data were available, a female:male ratio of approximately 4:1 was calculated, which might represent the fact that more women undergo bariatric surgery for morbid obesity or might be related to sex hormone-dependent mechanisms (see below) [388]. The post-bariatric population thus represents a considerable fraction of the patients treated for non-insulinoma hyperinsulinemic hypoglycemia in the adult population.
One characteristic of PGBHH is that it occurs with a delay of months to years after the surgical intervention (late dumping syndrome is mostly recognized much earlier). Generally, hypoglycemic symptoms after this type of surgery were reported to occur in about 2.6% postoperatively, with a high spontaneous recovery rate [389]. Patients after gastric bypass show elevated plasma levels of GLP-1 since nutrients have rapid contact with the distal ileum, where the GLP-1-producing L cells reside [84]. As GLP-1 induces β-cell proliferation in rodents, it was suspected that this mechanism might contribute to the observed syndrome. A concomitant decrease in plasma ghrelin, which is an inhibitor of pancreatic insulin secretion, was also recognized [390]. According to Meier et al., histological studies of pancreata from PGBHH patients did not reveal an increase in the β-cell area or neogenesis of islets. However, the nuclear diameter of the insulin-secreting cells was increased and correlated to the approximate body mass index (BMI) before surgery. The authors concluded that the observed hyperinsulinemia is thus just a maladaptation to the decrease in weight, and the apparent islet hyperplasia/hypertrophy represents an artifact of the preoperative insulin excess due to peripheral insulin resistance [95]. One group, however, described unrestrained β-cell proliferation from ductal progenitors in PGBHH samples [224]. According to the available literature, PGBHH is most likely a spectrum or overlap of related disorders. Therefore, different gastrointestinal anatomy; concomitant changes in incretin responses; and pancreatic changes due to preoperative obesity, which is very often associated with peripheral insulin resistance, and an overlap phase with late dumping syndrome, might best explain this syndrome. Due to the controversy about islet cell hyperplasia, surgical strategies (gastric bypass reversal or (partial) pancreatectomy) should be applied only in cases refractory to conservative treatment [389].
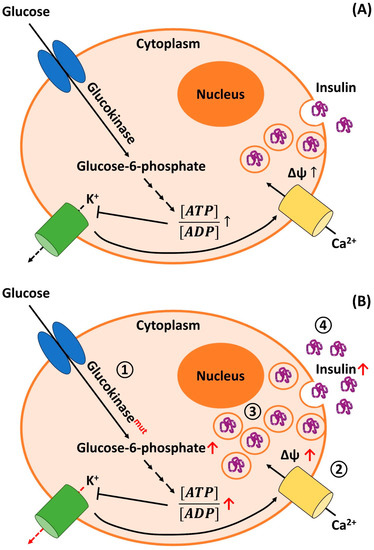

Figure 1.
Physiological mechanism of insulin secretion in human pancreatic β-cells (A) and pathophysiological aspects of adult-onset nesidioblastosis/NIPHS: (A) Glucose enters the pancreatic β-cells through glucose transporting proteins (GLUT) subtypes 1 and 3 [391]. Glucose is phosphorylated via Glucokinase (GCK; also termed hexokinase IV), leading to the production of Glucose-6-phosphate, which is trapped intracellularly. Subsequent chemical reactions (small arrows) are part of the glycolysis, the citric acid/Krebs cycle, and the respiratory chain (oxidative phosphorylation). Finally, complete oxidative glucose metabolism leads to the production of adenosine triphosphate (ATP). A relative increase in intracellular ATP concentrations upon glucose intake leads to a rise in the ATP:ADP (adenosine diphosphate) ratio. ATP inhibits the activity of the Kir6.2/KATP channel (green barrel). A reduced efflux of potassium (K+) ions results in a rise in the membrane potential (Δψ). When reaching the threshold potential, voltage-gated calcium (Ca2+) channels (yellow barrel) open. The influx of calcium stimulates the fusion of preformed insulin-containing vesicles with the plasma membrane, i.e., insulin release is triggered. (B) In patients suffering from adult-onset nesidioblastosis/NIPHS, different pathophysiological mechanisms are discussed (also see main text; main metabolic changes as well as mutated enzymes are indicated in red). (1) Activating mutations of the GCK (Glucokinasemut) gene lead to a higher activity of the corresponding enzyme. This increases the metabolic flux through the oxidative metabolic pathways, which raises the ATP:ADP ratio. This, in turn, leads to an ATP-dependent inhibition of K+ channels, which reduces the potassium efflux from the cell (red dashed arrow). (2) β-cells from adult-onset nesidioblastosis/NIPHS patients were shown to have a higher resting membrane potential Δψ. This results in a higher open-state probability of voltage-gated calcium channels. (3) β-cells from patients may possess an overall higher amount of intracellular insulin synthesis and intracellularly stored insulin. (4) A higher basal insulin secretion rate was also reported for adult-onset nesidioblastosis/NIPHS cells. To summarize, all mechanisms (1)–(4) increase insulin secretion from the islets of Langerhans, resulting in hyperinsulinemic hypoglycemia and the associated clinical symptoms.
6. Epidemiology
The epidemiology of late-/adult-onset nesidioblastosis/NIPHS with hyperinsulinemic hypoglycemia is unknown. However, according to the available literature, the disease is rare. When excluding post-bariatric surgery patients and patients with diagnosed syndromes (such as MEN1), we could identify approximately 535 cases (approximately 560 when counting the “adolescent” cases reported by Castillo-López et al. [247]), although some of these cases, especially early ones and where the full text is not available/is written in another language than English/German, or demographic data are missing, are equivocal [3,5,7,11,14,15,16,17,18,19,22,23,25,26,27,29,30,31,33,34,35,39,40,41,43,45,46,47,48,49,50,52,54,55,56,57,58,59,61,62,63,66,67,68,69,70,71,73,74,77,81,82,85,87,89,91,92,94,97,98,101,104,106,108,109,110,111,112,117,121,123,125,126,129,130,131,132,134,135,136,146,147,148,149,151,152,158,159,160,164,166,170,173,175,176,178,180,181,182,184,185,186,187,192,195,196,197,198,200,203,206,208,210,211,212,214,215,216,218,219,220,221,222,225,227,228,229,230,231,232,233,237,239,240,241,242,244,247,251,256,257,259,263,266,267,269,270,272,273,275,276,277,278,279,280,281,283,285,286,293,295,296,302,305,306,307,308,314,315,316,317,318]. Many of the cases counted here overlap with the ones presented in the “Nesidioblastosis and Islet Cell Hyperplasia in other Adult Diseases” sections (e.g., the cases with concomitant insulinoma and diseases with unknown correlations to nesidioblastosis). The reporting period ranges from 1944 to 2021, and the details for every case can be seen in Supplementary Table S1. We are aware that other authors may classify the cases slightly differently. Nonetheless, no such detailed list has been provided in the literature so far. Our main criteria for counting the cases as idiopathic, late-onset nesidioblastosis were symptom onset at ≥ 3 years, hyperinsulinemic hypoglycemia, histological proof of islet hyperplasia/nesidioblastosis, a highly suggestive clinical presentation, and/or typical response to certain drugs (e.g., diazoxide/calcium channel inhibitors/somatostatin analogs). The age of the reported patients ranges from 3 to 89 years. The female:male ratio in the patients, where demographic data were available, is approximately 1.7:1, which supports the findings of Kim et al., who estimated a ratio of 1.5:1 in 2000 [54]. Unfortunately, there is, to the best of our knowledge, no definite scientific explanation for why more women are affected by the disease. It is tempting to speculate that differences in sex hormones could explain this observation. Estrogen renders cells more sensitive to insulin and reduces gluconeogenesis. This mechanism is protective concerning the onset of type 2 diabetes in premenopausal women [392,393]. However, even a mild overproduction of insulin may consequently become clinically apparent more easily in women, i.e., the likelihood increases that the blood glucose drops below values where vegetative or neuroglycopenic symptoms appear. According to our literature research, NIPHS/late-onset nesidioblastosis is thus more common than initially thought and reported by other authors. Different authors reviewed a collection of cases in the past. Stefanini et al. reported approximately 129 cases in 1974 [216]. The next largest collection was presented by Albers et al. in 1989 with a review of 20 cases [306]. Further studies with reviews of a substantial amount of cases were published later [11,46,63,74,81,128,197]. In 1995, Walmsley and colleagues suggested that nesidioblastosis accounts for approximately 0.5–5% of adult cases with hyperinsulinemic hypoglycemia [26]. Since insulinoma is already a rare disease with a yearly incidence of approximately 4 cases per 1 million persons, this would mean that nesidioblastosis occurs with a frequency of 2 to 20 cases per 100 million persons per year [394]. Service et al. found one NIPHS case among 216 insulinomas, which corresponds to approximately 0.5%. Larger populational studies were reported in 1998, 2015, and 2020. Soga et al. reviewed 1085 patients with organic hyperinsulinism from Japan over a 25-year period, of which 4.1% were diagnosed with nesidioblastosis. Approximately 2/3 of the cases had a coexisting insulinoma [40]. A Korean population was reviewed by Woo et al. in 2015 [195]. Of 84 patients with endogenous hyperinsulinism, 5 were diagnosed with nesidioblastosis. The study by Yamada et al. presented the results of a nationwide survey on endogenous hyperinsulinism in Japan in 2017–2018 [5]. Despite the relatively low response rate of adult clinics (38.2%), 205 insulinomas and 111 cases of NIPHS were reported. Among their definition of NIPHS cases, 33 were post-bariatric, 57 were classified as postprandial hyperinsulinemic hypoglycemia, 10 as nesidioblastosis, and 11 as unknown subtypes. The overall incidence of NIPHS was estimated to be 0.09/100.000 and year. Thus, with approximately 10% “genuine” nesidioblastoses, the incidence would be around 9/100.000.000 and year. This is in the range of the estimation by Walmsley and colleagues. The differentiation of postprandial hyperinsulinemic hypoglycemia and nesidioblastosis was, however, not convincingly reported. Since the large population studies are all from East Asia, ethnic differences in the incidence of the disease might be overlooked. Since there are, to the best of our knowledge, no registers counting the cases of NIPHS/nesidioblastosis, the true incidence remains unknown. The list presented in Supplementary Table S1 also only documents reported cases and is biased towards unpublished cases, and includes foremostly cases from Western countries and East Asia. We are additionally convinced that the diagnosis might be overlooked in many patients presenting with unspecific symptoms, as in our recently published case (see Dieterle et al., 2023, accepted for publication in Biomedicines), or might be missed at autopsy due to autolytic pancreatic tissue [395].
7. Symptoms
The symptoms of NIPHS/adult-onset nesidioblastosis with hyperinsulinemic hypoglycemia can be highly diverse and range from predominant cardiological to neurological or even psychiatric manifestations [15,216,226]. Our recently published case (see Dieterle et al., 2023, accepted for publication in Biomedicines) clearly illustrates that the clinical findings might be relatively unspecific, and only the correlation between exercising or food intake was suggestive of a metabolic disorder. Autonomic signs due to the adrenergic counterregulatory response include trembling, sweating, palpitation, tachycardia, hunger, and pallor. Neuroglycopenic symptoms can mimic many neurologic and psychiatric disorders and comprise dizziness, aphasia, visual impairment, atypical behavior, paresthesia, transient hemiplegia, psychosis, convulsion, loss of consciousness, and coma [1]. Additionally, some extraordinary manifestations of nesidioblastosis are described in the literature. Galizia et al. reported a case presenting with abdominal pain, vomiting, blurred vision, lethargy, and dehydration. Bradycardia with intermittent atrioventricular block and ST segment depression, as well as hypotension, was recognized. The patient died from sudden cardiac death due to hypoglycemia [29]. QTc changes in response to hypoglycemia are also reported in the literature. Of interest, reversible forms of hypertrophic cardiomyopathy were reported in children with CHI/PHH. The symptoms resolved after treatment of hyperinsulinism [396,397].
Weight gain was also reported in adult-onset nesidioblastosis, although it is more frequent in insulinoma [30].
Similar to our presented case, a 22-year-old patient had episodes of severe tachycardia during hypoglycemic events and reported a recurrent loss of consciousness [176].
Martin-Grace et al. described a patient who suffered from clinical symptoms of hypoglycemia 20 min after exercise onset. This is also comparable to our case [196].
An intriguing case of sensorineural deafness and recurrent facial paralysis in response to hypoglycemia is also well documented [227].
The slight hypokalemia in our presented case was retrospectively attributed to episodes of severe hyperinsulinemia. These examples demonstrate that a broad spectrum of clinical symptoms can be provoked by nesidioblastosis, despite (nearly) normal laboratory examination and a seemingly healthy patient upon office evaluation.
8. Clinical Diagnosis and Differential Diagnosis
The core clinical diagnostic criteria for NIPHS/adult-onset nesidioblastosis with hyperinsulinemic hypoglycemia was already cursorily discussed in Section 3. The variability in the correlation of the symptoms with food intake was also described. Fasting hypoglycemia does not exclude NIPHS, nor does postprandial hypoglycemia rule out an insulinoma [200,398]. Nonetheless, OGTT and a 72 h fasting test are the first important steps to define endogenous/organic hyperinsulinism and to document Whipple´s triad, which is characterized by a low plasma glucose (usually < 45–55 mg/dL) and clinical adrenergic and/or neuroglycopenic symptoms, which rapidly resolve upon (intravenous) glucose intake [399]. Tolbutamid stimulation of β-cells and the C-peptide suppression test were also described in the literature to demonstrate endogenous hyperinsulinism [52,216]. Subsequently, imaging studies (CT, MRI) should be performed to exclude a macroscopically detectable insulinoma. SACS of the splenic, gastroduodenal, superior mesenteric, and proper hepatic arteries can then help to detect an inadequate insulin response to calcium stimulation [34]. Additionally, SACS can support the decision on the extent of surgical intervention if planned. Coeliac angiography and percutaneous transhepatic portal venous sampling have also been applied in the past to exclude (small) insulinomas and prove the excess secretion of insulin, respectively [30]. Intraoperative insulin assays were proposed by Carneiro in 2002 to prove the successful resection of the source of insulin overproduction [69].
SACS was not performed in our present case (see Dieterle et al., 2023, accepted for publication in Biomedicines) since functional imaging with 68Ga-DOTA-Exendin-4 PET/CT showed diffuse tracer uptake in the entire pancreas, which made the diagnosis of NIPHS/nesidioblastosis highly likely. Since SACS is an invasive procedure, functional imaging techniques with high sensitivity and specificity are urgently needed to avoid the former procedure.
Some years ago, somatostatin receptor scintigraphy (Octreoscan/111In-pentetreotide scintigraphy; mainly targets Somatostatin receptor type 2 [SSTR2]) and 18F-DOPA PET were successfully used to detect focal nesidioblastosis [55,111]. Targeting of the GLP-1 receptor was first introduced in 2008 [175]. [Lys40(Ahx-DTPA-111In)NH2]exendin-4 was then used as a ligand. Subsequently, other Exendin-derived peptide tracers were developed, such as 99mTc-GLP-1 or 111In-DOTA-Exendin-4 for planar scintigraphy/SPECT/CT, and 68Ga-DOTA-Exendin-4 for PET/CT [206,218,400]. The success of nesidioblastosis detection with these ligands, however, varies between studies. The GLP-1 receptor was reported not to be overexpressed in PGBHH [154,175]. Kallf et al. reported that SUVmax could not distinguish between NIPHS and PGBHH [237]. In idiopathic nesidioblastosis, a threefold overexpression of the GLP-1 receptor was shown histologically. Christ et al. reported a SUVmax of 6.9 in a patient, which is slightly lower than in our presented case (see Dieterle et al., 2023, accepted for publication in Biomedicines) and much lower than in typical insulinomas [206]. An important pitfall in GLP-1 imaging is the physiological uptake in Brunner´s gland in the proximal duodenum. Brunner gland hyperplasia (e.g., in association with Helicobacter pylori infection) was reported to cause false positive imaging (SUVmax of 10.0) due to the proximity to the pancreatic head [401].
Important differential diagnoses of hypoglycemia in adult patients have been briefly mentioned in the introduction section. These include drugs such as insulin, insulin secretagogues, and alcohol. In the critically ill patient, hepatic, renal, or cardiac failure, systemic inflammation/sepsis, and inanition must be considered. Hormone deficiencies (pituitary/adrenal insufficiency, glucagon deficiency) must be ruled out. Tumors producing insulin or IGFs (e.g., Doege–Potter syndrome) are another rare cause of adult-onset hypoglycemia [402]. An interesting case reported by Schröder and co-workers in 2009 describes a 54-year-old female patient with hypoglycemia and elevated IGF-1 and growth hormone levels. This constellation was later explained by an increased production of adiponectin, which was most likely due to autoimmune disease [403]. Endogenous hyperinsulinism, including autoimmune insulin hypoglycemia (antibodies against insulin or the insulin receptor; Hirata´s disease), insulinoma (or insulinomatosis with insulin expressing monohormonal endocrine cell clusters (IMECCs); a case with recurrent hypoglycemia even after total pancreatectomy was recently reported [245]), nesidioblastosis/NIPHS, and PGBHH have been discussed. Hypoglycemia factitia sometimes occurs, especially in psychiatric patients (e.g., Munchausen syndrome) [1,404].
Some attention should also be paid to the rare instances of late-onset inborn errors of metabolism presenting with hypoglycemia. Fasting hypoglycemia can occur in glycogenolysis disorders, fatty acid oxidation defects, and gluconeogenesis disorders. Postprandial hypoglycemia is associated with inherited fructose intolerance, a congenital disorder of glycosylation type Id, and late-onset forms of CHI. Exercise-induced hypoglycemia can sometimes be attributed to mutations in MCP1 (see above). A concise review of these extremely rare presentations is provided by Douillard and colleagues [405].
These remarks underscore the diagnostic challenges in adult-onset nesidioblastosis/NIPHS and the complex differential diagnostic process in adults presenting with hypoglycemia. In the corresponding Case Report (see Figure 3 in Dieterle et al., 2023; accepted for publication to Biomedicines), we also present a comprehensive and efficient algorithm for differential diagnosis of the non-diabetic adult patient presenting with hypoglycemia. Further clinical aspects of hypoglycemia in the adult population are discussed in the following references [1,215,402,406,407].
9. Therapy
The therapeutic options for adult-onset nesidioblastosis/NIPHS are limited [5,217,408]. Since the pathophysiology of the disease is still barely understood, interventions that causally address the functional β-cell disorder have not been developed.
The first step is usually to try a low-carbohydrate diet with a low glycemic index to avoid strong insulin responses. This scheme is, however, mostly not successful. Pharmacological treatment relies on α-glucosidase inhibitors (Acarbose, Voglibose) [98], the ATP-sensitive potassium channel agonist diazoxide, calcium channel antagonists (Verapamil, Amlodipine, Nifedipine) [63,116,145], and somatostatin analogs (octreotide, lanreotide, pasireotide) [309]. Except for acarbose, which slows down carbohydrate resorptions, all other drugs aim at indirectly reducing insulin secretion from the islets of Langerhans. Unfortunately, these treatments are often associated with intolerable adverse effects. Apart from fluid retention, diazoxide can rarely cause severe symptoms such as angina pectoris or even myocardial infarction, as seen in our patient (see Dieterle et al., 2023, accepted for publication in Biomedicines). Calcium channel inhibitors lead to hypotension and can significantly impair the quality of life.
The different somatostatin analogs have distinctive affinities for the various somatostatin receptors. While octreotide mainly acts through SSTR2, pasireotide has a high affinity for SST5, which contributes to the explanation of different clinical effects [213]. Of note, somatostatin analogs, especially octreotide, also reduce glucagon secretion, which can lead to an exacerbation of hypoglycemic events [196]. This was also the case with our patient (see Dieterle et al., 2023, accepted for publication in Biomedicines).
In some instances, glucocorticoids, β-blockers such as propranolol, or antipsychotic/antiepileptic drugs such as phenytoin were also used in the treatment of NIPHS/nesidioblastosis since these drugs induce hyperglycemia [15]. Everolimus was also tried as a treatment in one patient but failed to establish euglycemia [213] (Table 1).

Table 1.
Therapeutic options for adult-onset nesidioblastosis/NIPHS.
In PGBHH, feeding via a gastric tube was successful in some patients [410]. Pharmacological blocking of the GLP-1 receptor with exendin derivates could also mitigate disease symptoms in preliminary studies [411,412].
In many patients, partial pancreatectomy or even subtotal/total pancreatectomy remains the only option to control the symptoms adequately. The extent of resection is still a matter of debate. While some prefer limited pancreatic resection (50–60%), others suggest subtotal pancreatectomy (80–95%), as usually performed in children suffering from CHI [89,135]. In a subset of patients, symptoms recur after partial/distal pancreatectomy, making completion pancreatectomy necessary, as seen in our patient (see Dieterle et al., 2023, accepted for publication in Biomedicines) and others [87]. The surgical intervention is, however, associated with considerable postoperative morbidity (diabetes mellitus type 3c, exocrine pancreatic insufficiency) and mortality. Thus, surgery should be performed in high-volume centers only [413,414]. In PGBHH, reversal of the bypass or pancreatic resection has been successfully used [165,174].
Since these treatment options are somewhat unsatisfactory, innovative, and less invasive strategies are needed. Boss et al. presented an experimental study evaluating a receptor-targeted photodynamic therapy for GLP-1 receptor-positive lesions [415]. Exendin-4 was coupled to a photosensitizer, and interstitial irradiation of the molecule led to the selective killing of GLP-1 receptor-positive cells in a hamster model. While endocrine pancreatic cells (derived from a rat insulinoma cell line or hamster lung cells transfected with GLP-1 receptor) underwent apoptosis, exocrine pancreatic cells (human pancreatic tumor cell line) survived, showing the specificity and selectivity of this approach. Such techniques or peptide-receptor radionuclide therapies directed against the GLP-1 receptor could be used in the future to overcome current therapeutic challenges. Thus, promising options for a selective reduction in β-cell mass without the need for surgical intervention are under development.
The gender-specific efficiency of the different drugs currently available for adult-onset nesidioblastosis/NIPHS should be further evaluated [416]. To the best of our knowledge, there is currently no evidence that there are sex differences in the response of NIPHS to the different treatment options. However, such differences were reported for several drugs, such as GLP-1 agonists in diabetes therapy, where therapeutic effects seem to be more favorable in women due to hormonal mechanisms [417]. This is of interest in the context of NIPHS/PGBHH since the GLP-1 signaling system is potentially involved in the pathogenesis of the disease. Sex-specific differences in drug action have also been reported for somatostatin analogs in the in vivo rat models of pituitary tumors. The authors report that the differential expression of somatostatin receptor subtypes may be responsible for these observations and could also be of relevance for clinical application [418]. Therefore, this interesting field of study should be further evaluated in the context of NIPHS/adult-onset nesidioblastosis to avoid unnecessary surgical interventions.
The current therapeutic options for adult-onset nesidioblastosis/NIPHS are summarized in Table 1 and visually illustrated in Figure 2.
Figure 2.
A selection of current pharmacological treatment options for adult-onset nesidioblastosis/NIPHS with the corresponding mechanisms of actions: (A) α-Glucosidase inhibitors such as Acarbose inhibit (red cross) intestinal disaccharidases (blue enzyme) in the cuticular layer of enterocytes. Thus, disaccharides such as maltose (connected green hexagons) cannot be hydrolyzed into monosaccharides. This, in turn, reduces the resorption of carbohydrates and the corresponding insulin response. (B) Other pharmacological compounds directly act on molecular targets on/within the pancreatic β-cells. Diazoxide stimulates (green arrow) Kir6.2/KATP potassium (K+) channels (green barrel), which stabilizes the resting membrane potential (Δψ) of the β-cells. Calcium (Ca2+) channel inhibitors such as Verapamil or Nifedipine block (red arrow) the activity of voltage-gated calcium channels (yellow barrel), which prevents the depolarization of the endocrine cells. Somatostatin (SS) receptors (yellow Y-structure) are G-protein coupled receptors and can be subdivided into different isoforms (see main text). Upon binding of somatostatin or somatostatin analogs such as octreotide, lanreotide, or pasireotide (blue rectangle), which activate (green arrow) SS receptors, intracellular downstream signaling cascades of the SS receptors (details are exemplarily given in [419,420]) result in an inhibition (red arrow) of insulin release. Inhibitors (red arrow) of mammalian targets of rapamycin (mTOR; blue pentagon), such as Everolimus, also lead to a decrease in insulin synthesis and secretion through complex mechanisms (for details, see [421]).
10. Conclusions and Future Perspectives
In this review article, we presented a comprehensive and in-depth discussion of diffuse adult-onset nesidioblastosis/NIPHS, a rare cause of hyperinsulinemic hypoglycemia. Due to clinical, epidemiological, and histopathological features, we propose that “late-onset islet dysplasia/islet cell atypia with NIPHS” would be an adequate description of the disease. When applying strict criteria, only some hundred cases of this rare disease have been described in the international medical literature within the last 100 years. However, it can be assumed that this entity is often overlooked. Patients repeatedly report a long history of seeking medical help and suffer from unbelievable constraints in their daily activities. A complex clinical presentation, in combination with the different ages of onset, makes it difficult to diagnose the condition clearly. Since conservative treatment options are limited, many patients undergo partial or total resection of the pancreas, which results in considerable morbidity and impairment in the quality of life. Thus, innovative and less invasive treatment options are needed in the future, which aim at preserving the exocrine and endocrine pancreatic function while selectively reducing or inhibiting the insulin-secreting β-cells. This, however, depends on a clear pathophysiological understanding of the disease. While there is some convincing experimental evidence for functional dysregulation of β-cells, differences in clinical and histopathological appearances of the disease cannot be explained currently. Clear differentiation from PGBHH is also necessary since different pathogenesis is suspected.
We recently also submitted an illustrative Case report of a 23-year-old man presenting with this exciting disease, and the interested reader is directed to this article (Dieterle et al. 2023, accepted for publication in Biomedicines).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biomedicines11061732/s1, Supplementary Table S1. Overview of cases with adult-onset Nesidioblastosis/NIPHS.
Author Contributions
Conceptualization, M.P.D., A.H., S.N.P., W.R.K., U.P., H.E., T.S., and P.T.; methodology, M.P.D. and A.H.; data acquisition, H.W., A.S., and M.R., data curation, M.P.D., H.S., P.T., and T.S.; writing—original draft preparation, M.P.D., A.H., W.R.K., and H.E.; writing—review and editing, H.S., S.K., and J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable due to singular case report (see below).
Informed Consent Statement
Informed consent was obtained from the subject involved in the study. Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
The data presented in this study are available in Supplementary Table S1.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cryer, P.E.; Axelrod, L.; Grossman, A.B.; Heller, S.R.; Montori, V.M.; Seaquist, E.R.; Service, F.J. Evaluation and Management of Adult Hypoglycemic Disorders: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2009, 94, 709–728. [Google Scholar] [CrossRef] [PubMed]
- Nadeau, A. Functional Hypoglycemia: Facts and Fancies. Can. Fam. Physician 1984, 30, 1333–1335. [Google Scholar] [PubMed]
- Gouta, E.L.; Jerraya, H.; Dougaz, W.; Chaouech, M.A.; Bouasker, I.; Nouira, R.; Dziri, C. Endogenous Hyperinsulinism: Diagnostic and Therapeutic Difficulties. Pan Afr. Med. J. 2019, 33, 57. [Google Scholar] [CrossRef] [PubMed]
- Okabayashi, T.; Shima, Y.; Sumiyoshi, T.; Kozuki, A.; Ito, S.; Ogawa, Y.; Kobayashi, M.; Hanazaki, K. Diagnosis and Management of Insulinoma. World J. Gastroenterol. 2013, 19, 829–837. [Google Scholar] [CrossRef]
- Yamada, Y.; Kitayama, K.; Oyachi, M.; Higuchi, S.; Kawakita, R.; Kanamori, Y.; Yorifuji, T. Nationwide Survey of Endogenous Hyperinsulinemic Hypoglycemia in Japan (2017–2018): Congenital Hyperinsulinism, Insulinoma, Non-Insulinoma Pancreatogenous Hypoglycemia Syndrome and Insulin Autoimmune Syndrome (Hirata’s Disease). J. Diabetes Investig. 2020, 11, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Cappellani, D.; Macchia, E.; Falorni, A.; Marchetti, P. Insulin Autoimmune Syndrome (Hirata Disease): A Comprehensive Review Fifty Years after Its First Description. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 963–978. [Google Scholar] [CrossRef]
- Service, G.J.; Thompson, G.B.; Service, F.J.; Andrews, J.C.; Collazo-Clavell, M.L.; Lloyd, R.V. Hyperinsulinemic Hypoglycemia with Nesidioblastosis after Gastric-Bypass Surgery. N. Engl. J. Med. 2005, 353, 249–254. [Google Scholar] [CrossRef]
- Dravecka, I.; Lazurova, I. Nesidioblastosis in Adults. Neoplasma 2014, 61, 252–256. [Google Scholar] [CrossRef]
- Laidlaw, G.F. Nesidioblastoma, the Islet Tumor of the Pancreas. Am. J. Pathol. 1938, 14, 125–134. [Google Scholar]
- Stanley, C.A. Perspective on the Genetics and Diagnosis of Congenital Hyperinsulinism Disorders. J. Clin. Endocrinol. Metab. 2016, 101, 815–826. [Google Scholar] [CrossRef]
- Anlauf, M.; Wieben, D.; Perren, A.; Sipos, B.; Komminoth, P.; Raffel, A.; Kruse, M.L.; Fottner, C.; Knoefel, W.T.; Mönig, H.; et al. Persistent Hyperinsulinemic Hypoglycemia in 15 Adults with Diffuse Nesidioblastosis: Diagnostic Criteria, Incidence, and Characterization of β-Cell Changes. Am. J. Surg. Pathol. 2005, 29, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Klöppel, G.; Anlauf, M.; Raffel, A.; Perren, A.; Knoefel, W.T. Adult Diffuse Nesidioblastosis: Genetically or Environmentally Induced? Hum. Pathol. 2008, 39, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Karnauchow, P.N. Nesidioblastosis in Adults without Insular Hyperfunction. Am. J. Clin. Pathol. 1982, 78, 511–513. [Google Scholar] [CrossRef] [PubMed]
- Weidenheim, K.M.; Hinchey, W.W.; Campbell, W.G. Hyperinsulinemic Hypoglycemia in Adults with Islet-Cell Hyperplasia and Degranulation of Exocrine Cells of the Pancreas. Am. J. Clin. Pathol. 1983, 79, 14–24. [Google Scholar] [CrossRef]
- Fuller, P.J.; Ehrlich, A.R.; Susil, B.; Zeimer, H. Insulin Gene Expression in Adult-Onset Nesidioblastosis. Clin. Endocrinol. 1997, 47, 245–250. [Google Scholar] [CrossRef]
- Chen, Y.L.; Chu, J.S.; Chang, T.C.; Tai, T.Y. Nesidiodysplasia: An Unusual Cause of Hyperinsulinemic Hypoglycemia in Adults. J. Formos. Med. Assoc. 1993, 92, 1099–1103. [Google Scholar]
- Sandler, R.; Horwitz, D.L.; Rubenstein, A.H.; Kuzuya, H. Hypoglycemia and Endogenous Hyperinsulinism Complicating Diabetes Mellitus. Application of the C-Peptide Assay to Diagnosis and Therapy. Am. J. Med. 1975, 59, 730–736. [Google Scholar] [CrossRef]
- Weinstock, G.; Margulies, P.; Kahn, E.; Susin, M.; Abrams, G. Islet Cell Hyperplasia: An Unusual Cause of Hypoglycemia in an Adult. Metabolism 1986, 35, 110–117. [Google Scholar] [CrossRef]
- Gould, V.; Chejfec, G.; Shah, K.; Paloyan, E.; Lawrence, A.M. Adult Nesidiodysplasia. Semin. Diagn. Pathol. 1984, 1, 43–53. [Google Scholar]
- Vance, J.E.; Stoll, R.W.; Kitabchi, A.E.; Williams, R.H.; Wood, F.C. Nesidioblastosis in Familial Endocrine Adenomatosis. JAMA 1969, 207, 1679–1682. [Google Scholar] [CrossRef]
- Tavcar, I.; Andelković, Z.; Dragojević, R.; Duknić, M.; Ilić, S.; Novaković, O.; Tasić, G.; Lazić, R. Nesidioblastosis of the Pancreas as a Cause of Hyperinsulinemic Hypoglycemia in Adults. Vojnosanit. Pregl. 1994, 51, 73–81. [Google Scholar]
- Geoghegan, J.G.; Jackson, J.E.; Lewis, M.P.; Owen, E.R.; Bloom, S.R.; Lynn, J.A.; Williamson, R.C. Localization and Surgical Management of Insulinoma. Br. J. Surg. 1994, 81, 1025–1028. [Google Scholar] [CrossRef] [PubMed]
- Losada, J.; Sarria, R.; Fernandez Val, J.; Lopez Ariztegui, M. DNA Ploidy and PCNA Index in Pancreatic Lesions Producing Hyperinsulinemic Hypoglycemia. J. Surg. Oncol. 1995, 59, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Farley, D.R.; van Heerden, J.A.; Myers, J.L. Adult Pancreatic Nesidioblastosis: Unusual Presentations of a Rare Entity. Arch. Surg. 1994, 129, 329–332. [Google Scholar] [CrossRef]
- Lechleitner, M.; Hoppichler, F.; Dzien, A.; Feichtinger, H.; Bodner, E.; Patsch, J. Nesidioblastosis in a Young Woman and the Impact of Hyperinsulinaemia on Triglyceride Metabolism. J. Intern. Med. 1995, 237, 117–118. [Google Scholar] [CrossRef]
- Walmsley, D.; Matheson, N.A.; Ewen, S.; Himsworth, R.L.; Bevan, J.S. Nesidioblastosis in an Elderly Patient. Diabet. Med. 1995, 12, 542–545. [Google Scholar] [CrossRef]
- Bell, D.S.; Grizzle, W.E.; Dunlap, E.D. Nesidioblastosis Causing Reversal of Insulin-Dependent Diabetes and Development of Hyperinsulinemic Hypoglycemia. Diabetes Care 1995, 18, 1379–1380. [Google Scholar] [CrossRef]
- Le Bodic, M.F.; Heymann, M.F.; Lecomte, M.; Berger, N.; Berger, F.; Louvel, A.; De Micco, C.; Patey, M.; De Mascarel, A.; Burtin, F.; et al. Immunohistochemical Study of 100 Pancreatic Tumors in 28 Patients with Multiple Endocrine Neoplasia, Type I. Am. J. Surg. Pathol. 1996, 20, 1378–1384. [Google Scholar] [CrossRef]
- Galizia, A.C.; Fava, S.; Foale, R. Nesidioblastosis-Associated Hypoglycaemia Presenting with Prominent Cardiac Manifestations. Postgrad. Med. J. 1996, 72, 231–232. [Google Scholar] [CrossRef]
- Kim, H.K.; Shong, Y.K.; Han, D.J.; Cho, Y.; Lee, I.C.; Kim, G.S. Nesidioblastosis in an Adult with Hyperinsulinemic Hypoglycemia. Endocr. J. 1996, 43, 163–167. [Google Scholar] [CrossRef]
- Frantz, V.K. Adenomatosis of Islet Cells, with Hyperinsulinism. Ann. Surg. 1944, 119, 824–844. [Google Scholar] [CrossRef]
- Martella, E.M.; Ferraro, G.; Azzoni, C.; Marignani, M.; Bordi, C. Pancreatic-Polypeptide Cell Hyperplasia Associated with Pancreatic or Duodenal Gastrinomas. Hum. Pathol. 1997, 28, 149–153. [Google Scholar] [CrossRef]
- Garcia, J.P.; Franca, T.; Pedroso, C.; Cardoso, C.; Cid, M.O. Nesidioblastosis in the Adult Surgical Management. HPB Surg. 1997, 10, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.L.; Won, J.G.; Chiang, J.H.; Hwang, J.I.; Lee, C.H.; Tsay, S.H. Selective Intra-Arterial Calcium Injection in the Investigation of Adult Nesidioblastosis: A Case Report. Diabet. Med. 1997, 14, 985–988. [Google Scholar] [CrossRef]
- Röher, H.D.; Simon, D.; Starke, A.; Goretzki, P.E. Spezielle Diagnostische Und Therapeutische Aspekte Beim Insulinom. Chirurg 1997, 68, 116–121. [Google Scholar] [CrossRef]
- Sanjuán Portugal, F.; Trincado Aznar, P.; Acha Pérez, J.; Velilla Marco, J. Diagnosis of Nesidioblastosis in the Adult. An. Med. Interna 1997, 14, 101. [Google Scholar] [PubMed]
- Conget, I.; Sarri, Y.; Somoza, N.; Vives, M.; Novials, A.; Ariza, A.; Fernández-Alvarez, J.; Pujol-Borrell, R.; Gomis, R. β-Cell Function Abnormalities in Islets from an Adult Subject with Nesidioblastosis and Autoantibodies against the Islet Cells. Pancreas 1997, 14, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.; Kristopaitis, T.; Yong, S.; Chejfec, G.; Pickleman, J. Cystic Glucagonoma: A Rare Variant of an Uncommon Neuroendocrine Pancreas Tumor. J. Gastrointest. Surg. 1998, 2, 533–536. [Google Scholar] [CrossRef]
- Ueda, Y.; Kurihara, K.; Kondoh, T.; Okanoue, T.; Chiba, T. Islet-Cell Hyperplasia Causing Hyperinsulinemic Hypoglycemia in an Adult. J. Gastroenterol. 1998, 33, 125–128. [Google Scholar] [CrossRef]
- Soga, J.; Yakuwa, Y.; Osaka, M. Insulinoma/Hypoglycemic Syndrome: A Statistical Evaluation of 1085 Reported Cases of a Japanese Series. J. Exp. Clin. Cancer Res. 1998, 17, 379–388. [Google Scholar]
- Pereira, P.L.; Roche, A.J.; Maier, G.W.; Huppert, P.E.; Dammann, F.; Farnsworth, C.T.; Duda, S.H.; Claussen, C.D. Insulinoma and Islet Cell Hyperplasia: Value of the Calcium Intraarterial Stimulation Test When Findings of Other Preoperative Studies Are Negative. Radiology 1998, 206, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Franksson, C.; Hellstrom, J.; Hultquist, G.; Perman, G. Primary Chief-Cell Hyperplasia of the Parathyroids, and Islet Cell Hyperplasia and Adenomata of the Pancreas Associated with Gastro-Duodeno-Jejunal Ulcer. Acta Chir. Scand. 1960, 118, 270–277. [Google Scholar] [PubMed]
- Rinker, R.D.; Friday, K.; Aydin, F.; Jaffe, B.M.; Lambiase, L. Adult Nesidioblastosis: A Case Report and Review of the Literature. Dig. Dis. Sci. 1998, 43, 1784–1790. [Google Scholar] [CrossRef]
- Pasieka, J.L.; Hershfield, N. Pancreatic Polypeptide Hyperplasia Causing Watery Diarrhea Syndrome: A Case Report. Can. J. Surg. 1999, 42, 55–58. [Google Scholar] [PubMed]
- Service, F.J.; Natt, N.; Thompson, G.B.; Grant, C.S.; Van Heerden, J.A.; Andrews, J.C.; Lorenz, E.; Terzic, A.; Lloyd, R.V. Noninsulinoma Pancreatogenous Hypoglycemia: A Novel Syndrome of Hyperinsulinemic Hypoglycemia in Adults Independent of Mutations in Kir6.2 and SUR1 Genes. J. Clin. Endocrinol. Metab. 1999, 84, 1582–1589. [Google Scholar] [CrossRef]
- Eriguchi, N.; Aoyagi, S.; Hara, M.; Tanaka, E.; Hashimoto, M.; Jimi, A. Nesidioblastosis with Hyperinsulinemic Hypoglycemia in Adults: Report of Two Cases. Surg. Today 1999, 29, 361–363. [Google Scholar] [CrossRef]
- Wängberg, B.; Nilsson, O.; Grimelius, L.; Lundell, L.; Ahlman, H. Nesidiodysplasia. A Rare Cause of Hyperinsulinemia in an Adult Patient. Lakartidningen 1999, 96, 4655–4657. [Google Scholar]
- Tomaszewska, R.; Nowak, W.; Rudnicka-Sosin, L.; Stachura, J. Nesidioblastosis in an Adult Man—Case Report. Pol. J. Pathol. 1999, 50, 43–46. [Google Scholar]
- Yeh, S.P.; Wang, Y.C.; Wu, H.; Yu, M.S.; Hsueh, E.J.; Wang, Y.C. Nesidioblastosis, Myelodysplastic Syndrome and Nodular Diabetic Glomerulosclerosis in an Elderly Nondiabetic Woman: An Autopsy Report. Diabet. Med. 1999, 16, 437–441. [Google Scholar] [CrossRef]
- Hashimoto, L.A.; Walsh, R.M. Preoperative Localization of Insulinomas Is Not Necessary. J. Am. Coll. Surg. 1999, 189, 368–373. [Google Scholar] [CrossRef]
- Habanec, B.; Růzicka, M.; Dvorák, K.; Stracár, K.; Tretinová, L. Pathology of the Pancreas. Analysis of 172 Resection Specimens. Cesk. Patol. 1999, 35, 39–44. [Google Scholar] [PubMed]
- Thompson, G.B.; Service, F.J.; Andrews, J.C.; Lloyd, R.V.; Natt, N.; Van Heerden, J.A.; Grant, C.S. Noninsulinoma Pancreatogenous Hypoglycemia Syndrome: An Update in 10 Surgically Treated Patients. Surgery 2000, 128, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Bloodworth, J.M.B. Morphologic Changes Associated with Sulfonylurea Therapy. Metabolism 1963, 12, 287–301. [Google Scholar]
- Kim, Y.W.; Park, Y.-K.; Park, J.H.; Lee, S.M.; Lee, J.; Ko, S.W.; Yang, M.H. Islet Cell Hyperplasia of the Pnacreas Presenting as Hyperinsulinemic Hypoglycemia in an Adult. Yonsei Med. J. 2000, 41, 426–429. [Google Scholar] [CrossRef] [PubMed]
- Van der Wal, B.C.H.; De Krijger, R.R.; De Herder, W.W.; Kwekkeboom, D.J.; Van der Ham, F.; Bonjer, H.J.; Van Eijck, C.H.J. Adult Hyperinsulinemic Hypoglycemia Not Caused by an Insulinoma: A Report of Two Cases. Virchows Arch. 2000, 436, 481–486. [Google Scholar] [CrossRef]
- White, S.A.; Sutton, C.D.; Robertson, G.S.; Furness, P.N.; Dennison, A.R. Incidental Adult Nesidioblastosis after Distal Pancreatectomy for Endocrine Microadenoma. Eur. J. Gastroenterol. Hepatol. 2000, 12, 1147–1149. [Google Scholar] [CrossRef] [PubMed]
- Kon, Y.C.; Loh, K.C.; Chew, S.P.; Wong, D.; Yap, W.M.; Lee, Y.S.; Low, C.H. Hypoglycaemia from Islet Cell Hyperplasia and Nesidioblastosis in a Patient with Type 2 Diabetes Mellitus—A Case Report. Ann. Acad. Med. Singapore 2000, 29, 682–687. [Google Scholar]
- Hellman, P.; Goretzki, P.; Simon, D.; Dotzenrath, C.; Roher, H.D. Therapeutic Experience of 65 Cases with Organic Hyperinsulinism. Langenbeck’s Arch. Surg. 2000, 385, 329–336. [Google Scholar] [CrossRef]
- Lecube, A.; Obiols, G.; Ramos, I.; Gémar, E. Hyperinsulinemic Hypoglycemia and Nesidioblastosis in Adults. An Exceptional Disease. Med. Clin. 2001, 116, 238–239. [Google Scholar] [CrossRef]
- Culberson, D.; Manci, E.; Shah, A.; Haynes, J.; Ballas, S.; Pegelow, C.; Vichinsky, E. Nesidioblastosis in Sickle Cell Disease. Pediatr. Pathol. Mol. Med. 2001, 20, 155–165. [Google Scholar] [CrossRef]
- Zhao, X.; Stabile, B.E.; Mo, J.; Wang, J.; French, S.W. Nesidioblastosis Coexisting with Islet Cell Tumor and Intraductal Papillary Mucinous Hyperplasia. Arch. Pathol. Lab. Med. 2001, 125, 1344–1347. [Google Scholar] [CrossRef] [PubMed]
- Sumarac-Dumanovic, M.; Micic, D.; Popovic, V. Noninsulinoma Pancreatogenous Hypoglycemia in Adults: Presentations of Two Cases. J. Clin. Endocrinol. Metab. 2001, 86, 2328–2329. [Google Scholar] [CrossRef] [PubMed]
- Witteles, R.M.; Straus, F.H.; Sugg, S.L.; Koka, M.R.; Costa, E.A.; Kaplan, E.L. Adult-Onset Nesidioblastosis Causing Hypoglycemia. Arch. Surg. 2001, 136, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Zollinger, R.M. Zollinger-Ellison Syndrome: Re-Appraisal and Evaluation of 260 Registered Cases. Ann. Surg. 1964, 160, 512–528. [Google Scholar]
- Govindarajan, M.; Mohan, V.; Deepa, R.; Ashok, S.; Pitchumoni, C.S. Histopathology and Immunohistochemistry of Pancreatic Islets in Fibrocalculous Pancreatic Diabetes. Diabetes Res. Clin. Pract. 2001, 51, 29–38. [Google Scholar] [CrossRef]
- Espinosa-de-los-Monteros, A.; Robledo, F.; Cabrera, L.; Mercado, M. Nesidioblastosis as Extracolonic Manifestation Associated with Adenomatous Familial Polyposis. Rev. Gastroenterol. Mex. 2001, 66, 46–49. [Google Scholar]
- Semakula, C.; Pambuccian, S.; Gruessner, R.; Kendall, D.; Pittenger, G.; Vinik, A.; Seaquist, E.R. Hypoglycemia after Pancreas Transplantation: Association with Allograft Nesidiodysplasia and Expression of Islet Neogenesis-Associated Peptide. J. Clin. Endocrinol. Metab. 2002, 87, 3548–3554. [Google Scholar] [CrossRef]
- Matthews, B.; Smith, T.; Kercher, K.; Holder, W.; Heniford, B. Surgical Experience with Functioning Pancreatic Neuroendocrine Tumors. Am. Surg. 2002, 68, 660–665. [Google Scholar] [CrossRef]
- Carneiro, D.M.; Levi, J.U.; Irvin, G.L. Rapid Insulin Assay for Intraoperative Confirmation of Complete Resection of Insulinomas. Surgery 2002, 132, 937–943. [Google Scholar] [CrossRef]
- Lu, J.Y.; Huang, T.S.; Wu, M.Z. Hypopituitarism and Nesidioblastosis in an Elderly Patient with Orbital Lymphoma. J. Formos. Med. Assoc. 2002, 101, 68–72. [Google Scholar]
- Kaczirek, K.; Soleiman, A.; Schindl, M.; Passler, C.; Scheuba, C.; Prager, G.; Kaserer, K.; Niederle, B. Nesidioblastosis in Adults: A Challenging Cause of Organic Hyperinsulinism. Eur. J. Clin. Investig. 2003, 33, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Vella, A.; Service, F.J.; O’Brien, P.C. Glucose Counterregulatory Hormones in the 72-Hour Fast. Endocr. Pract. 2003, 9, 115–118. [Google Scholar] [CrossRef]
- Branco, V.A.; Santos, R.M.; Cipriano, M.A.; Tralhão, G.; Otero, M.; Moura, J.A.; Sousa, F.C.; Porto, A. Adult Nesidioblastosis. Acta Med. Port. 2003, 16, 465–470. [Google Scholar] [PubMed]
- Martignoni, M.E.; Kated, H.; Stiegler, M.; Büchler, M.W.; Friess, H.; Zimmermann, A.; Schirp, U.; Nitzsche, E.U. Nesidioblastosis with Glucagon-Reactive Islet Cell Hyperplasia: A Case Report. Pancreas 2003, 26, 402–405. [Google Scholar] [CrossRef]
- Knight, P.O. Insulinoma and Generalized Islet Cell Hyperplasia in a Patient with Diabetes Mellitus. South. Med. J. 1967, 60, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Giorgadze, T.A.; Roy, S.; Fraker, D.L.; Brooks, J.S.J.; LiVolsi, V.A. Pathologic Quiz Case: A 49-Year-Old Woman with an Adrenal Mass. Arch. Pathol. Lab. Med. 2004, 128, 1294–1296. [Google Scholar] [CrossRef]
- Ito, K.; Takada, T.; Amano, H.; Toyota, N.; Yasuda, H.; Yoshida, M.; Takada, Y.; Takeshita, K.; Koutake, H.; Takada, K.; et al. Localization of Islet-Cell Hyperplasia: Value of Pre- and Intraoperative Arterial Stimulation and Venous Sampling. J. Hepatobiliary. Pancreat. Surg. 2004, 11, 203–206. [Google Scholar] [CrossRef]
- Wiesli, P.; Brändle, M.; Schmid, C.; Krähenbühl, L.; Furrer, J.; Keller, U.; Spinas, G.A.; Pfammatter, T. Selective Arterial Calcium Stimulation and Hepatic Venous Sampling in the Evaluation of Hyperinsulinemic Hypoglycemia: Potential and Limitations. J. Vasc. Interv. Radiol. 2004, 15, 1251–1256. [Google Scholar] [CrossRef]
- Wiesli, P.; Perren, A.; Saremaslani, P.; Pfammatter, T.; Spinas, G.A.; Schmid, C. Abnormalities of Proinsulin Processing in Functioning Insulinomas: Clinical Implications. Clin. Endocrinol. 2004, 61, 424–430. [Google Scholar] [CrossRef]
- Proye, C.; Stalnikiewicz, G.; Wemeau, J.L.; Porchet, N.; D’Herbomez, M.; Maunoury, V.; Bauters, C. Genetically-Driven or Supposed Genetic-Related Insulinomas in Adults: Validation of the Surgical Strategy Proposed by the A.F.C.E./G.E.N.E.M. Ann. Endocrinol. 2004, 65, 149–161. [Google Scholar] [CrossRef]
- Kaczirek, K.; Ba-Ssalamah, A.; Schima, W.; Niederle, B. The Importance of Preoperative Localisation Procedures in Organic Hyperinsulinism--Experience in 67 Patients. Wien. Klin. Wochenschr. 2004, 116, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Boulanger, C.; Vezzosi, D.; Bennet, A.; Lorenzini, F.; Fauvel, J.; Caron, P. Normal Pregnancy in a Woman with Nesidioblastosis Treated with Somatostatin Analog Octreotide. J. Endocrinol. Investig. 2004, 27, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Chetty, R.; Kennedy, M.; Ezzat, S.; Asa, S.L. Pancreatic Endocrine Pathology in von Hippel-Lindau Disease: An Expanding Spectrum of Lesions. Endocr. Pathol. 2004, 15, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Patti, M.E.; McMahon, G.; Mun, E.C.; Bitton, A.; Holst, J.J.; Goldsmith, J.; Hanto, D.W.; Callery, M.; Arky, R.; Nose, V.; et al. Severe Hypoglycaemia Post-Gastric Bypass Requiring Partial Pancreatectomy: Evidence for Inappropriate Insulin Secretion and Pancreatic Islet Hyperplasia. Diabetologia 2005, 48, 2236–2240. [Google Scholar] [CrossRef]
- Pedrazzoli, S.; Pasquali, C.; D’Andrea, A.A. Surgical Treatment of Insulinoma. Br. J. Surg. 2005, 81, 672–676. [Google Scholar] [CrossRef]
- Cavallero, C.; Solcia, E.; Sampietro, R. Cytology of Islet Tumours and Hyperplasias Associated with the Zollinger-Ellison Syndrome. Gut 1967, 8, 172–177. [Google Scholar] [CrossRef]
- Babińska, A.; Jaśkiewicz, K.; Karaszewski, B.; Łukiański, M.; Sworczak, K. Nesidioblastosis—A Rare Cause of Hypoglycaemia in Adults. Exp. Clin. Endocrinol. Diabetes 2005, 113, 350–353. [Google Scholar] [CrossRef]
- Rare Presentation of Endocrine Pancreatic Tumor: A Case of Diffuse Glucagonoma without Metastasis and Necrolytic Migratory Erythema—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/15959605/ (accessed on 7 October 2021).
- Tsujino, M.; Sugiyama, T.; Nishida, K.; Takada, Y.; Takanishi, K.; Ishizawa, M.; Hirata, Y. Noninsulinoma Pancreatogenous Hypoglycemia Syndrome: A Rare Case of Adult-Onset Nesidioblastosis. Intern. Med. 2005, 44, 843–847. [Google Scholar] [CrossRef]
- Kondo, T.; Tomita, S.; Adachi, H.; Motoshima, H.; Taketa, K.; Matsuyoshi, A.; Tokunaga, H.; Miyamura, N.; Araki, E. A Case of Hyperinsulinemia of Undetermined Origin, Successfully Treated with Long-Acting Octreotide. Endocr. J. 2005, 52, 511–517. [Google Scholar] [CrossRef]
- Won, J.G.S.; Tseng, H.S.; Yang, A.H.; Tang, K.T.; Jap, T.S.; Lee, C.H.; Da Lin, H.; Burcus, N.; Pittenger, G.; Vinik, A. Clinical Features and Morphological Characterization of 10 Patients with Noninsulinoma Pancreatogenous Hypoglycaemia Syndrome (NIPHS). Clin. Endocrinol. 2006, 65, 566–578. [Google Scholar] [CrossRef]
- Diaz, A.; Lucas, S.; Ferraina, P.; Ferraro, A.; Puchulu, F.; Paes De Lima, A.; Maselli Mdel, C.; Gomez, R.; Bruno, O. Clinical Experience in 37 Cases of Insulinoma. Medicina 2006, 66, 499–504. [Google Scholar]
- Raffel, A.; Krausch, M.M.; Anlauf, M.; Wieben, D.; Braunstein, S.; Klöppel, G.; Röher, H.D.; Knoefel, W.T. Diffuse Nesidioblastosis as a Cause of Hyperinsulinemic Hypoglycemia in Adults: A Diagnostic and Therapeutic Challenge. Surgery 2007, 141, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Starke, A.; Saddig, C.; Kirch, B.; Tschahargane, C.; Goretzki, P. Islet Hyperplasia in Adults: Challenge to Preoperatively Diagnose Non-Insulinoma Pancreatogenic Hypoglycemia Syndrome. World J. Surg. 2006, 30, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Meier, J.J.J.; Butler, A.A.E.; Galasso, R.; Butler, P.P.C. Hyperinsulinemic Hypoglycemia after Gastric Bypass Surgery Is Not Accompanied by Islet Hyperplasia or Increased β-Cell Turnover. Diabetes Care 2006, 29, 1554–1559. [Google Scholar] [CrossRef]
- Ouyang, D.; Dhall, D.; Yu, R. Pathologic Pancreatic Endocrine Cell Hyperplasia. World J. Gastroenterol. 2011, 17, 137–143. [Google Scholar] [CrossRef]
- Wiesli, P.; Pavlicek, V.; Perren, A.; Baechli, E.; Pfammatter, T.; Krahenbuhl, L.; Schulthess, G.; Schmid, C. Recurrent Hypoglycaemia in HIV-Positive Narcotic Addicts. Swiss Med. Wkly. 2006, 136, 805–810. [Google Scholar]
- Arao, T.; Okada, Y.; Hirose, A.; Tanaka, Y. A Rare Case of Adult-Onset Nesidioblastosis Treated Successfully with Diazoxide. Endocr. J. 2006, 53, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Clancy, T.E.; Moore, F.D.; Zinner, M.J.; TE, C.; FD, M.; MJ, Z. Post−Gastric Bypass Hyperinsulinism With Nesidioblastosis: Subtotal or Total Pancreatectomy May Be Needed to Prevent Recurrent Hypoglycemia. J. Gastrointest. Surg. 2006, 10, 1116–1119. [Google Scholar] [CrossRef]
- Albazaz, R.; Da Costa, P.E.; Verbeke, C.S. Pancreatic Polypeptide Cell Hyperplasia of the Pancreas. J. Clin. Pathol. 2006, 59, 1087–1090. [Google Scholar] [CrossRef]
- Raffel, A.; Anlauf, M.; Hosch, S.B.; Krausch, M.; Henopp, T.; Bauersfeld, J.; Klofat, R.; Bach, D.; Eisenberger, C.F.; Klöppel, G.; et al. Hyperinsulinemic Hypoglycemia Due to Adult Nesidioblastosis in Insulin-Dependent Diabetes. World J. Gastroenterol. 2006, 12, 7221–7224. [Google Scholar] [CrossRef]
- Nakagawa, A.; Ueno, K.; Ito, M.; Okamoto, S.; Uehara, K.; Ito, H.; Mishina, S.; Kinoshita, E.; Nojima, T.; Takahashi, H.; et al. Insulin Responses to Selective Arterial Calcium Infusion under Hyperinsulinemic Euglycemic Glucose Clamps: Case Studies in Adult Nesidioblastosis and Childhood Insulinoma. Endocr. J. 2007, 54, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, G.C.; Faria, E.N.; Beck, M.; Girardon, D.T.; Machado, A.C. Laparoscopic Spleen-Preserving Distal Pancreatectomy as Treatment for Nesidioblastosis after Gastric Bypass Surgery. Obes. Surg. 2007, 17, 550–552. [Google Scholar] [CrossRef] [PubMed]
- Tseng, L.M.; Chen, J.Y.; Won, J.G.S.; Tseng, H.S.; Yang, A.H.; Wang, S.E.; Lee, C.H. The Role of Intra-Arterial Calcium Stimulation Test with Hepatic Venous Sampling (IACS) in the Management of Occult Insulinomas. Ann. Surg. Oncol. 2007, 14, 2121–2127. [Google Scholar] [CrossRef] [PubMed]
- Bantle, J.P.; Ikramuddin, S.; Kellogg, T.A.; Buchwald, H. Hyperinsulinemic Hypoglycemia Developing Late after Gastric Bypass. Obes. Surg. 2007, 17, 592–594. [Google Scholar] [CrossRef] [PubMed]
- Sahloul, R.; Yaqub, N.; Driscoll, H.K.; Leidy, J.W.; Parkash, J.; Matthews, K.A.; Chertow, B.S. Noninsulinoma Pancreatogenous Hypoglycemia Syndrome: Quantitative and Immunohistochemical Analyses of Islet Cells for Insulin, Glucagon, Somatostatin, and Pancreatic and Duodenal Homeobox Protein. Endocr. Pract. 2007, 13, 187–193. [Google Scholar] [CrossRef]
- Brown, R.; Still, W. Nesidioblastosis and the Zollinger-Ellison Syndrome. Am. J. Dig. Dis. 1968, 13, 656–663. [Google Scholar] [CrossRef]
- Costa, R.; Maia, F.; Araújo, L. Endogenous Persistent Hypoglicemia of Adult: Case Report. Arq. Bras. Endocrinol. Metabol. 2007, 51, 125–130. [Google Scholar] [CrossRef]
- Rosman, J.; Bravo-Vera, R.; Sheikh, A.; Gouller, A. Metastatic Insulinoma in an Adult Patient with Underlying Nesidioblastosis. J. Endocrinol. Investig. 2007, 30, 521–524. [Google Scholar] [CrossRef]
- Kok, V.K.; Wang, T.K.; Lin, N.H.; Bei, J.J.; Huang, P.H.; Chen, Y.C. Adult Intussusception Caused by Heterotopic Pancreas. J. Formos. Med. Assoc. 2007, 106, 418–421. [Google Scholar] [CrossRef]
- Kauhanen, S.; Seppänen, M.; Minn, H.; Gullichsen, R.; Salonen, A.; Alanen, K.; Parkkola, R.; Solin, O.; Bergman, J.; Sane, T.; et al. Fluorine-18-L-Dihydroxyphenylalanine (18F-DOPA) Positron Emission Tomography as a Tool to Localize an Insulinoma or β-Cell Hyperplasia in Adult Patients. J. Clin. Endocrinol. Metab. 2007, 92, 1237–1244. [Google Scholar] [CrossRef]
- Vezzosi, D.; Bennet, A.; Fauvel, J.; Caron, P. Insulin, C-Peptide and Proinsulin for the Biochemical Diagnosis of Hypoglycaemia Related to Endogenous Hyperinsulinism. Eur. J. Endocrinol. 2007, 157, 75–83. [Google Scholar] [CrossRef]
- Bunning, J.; Merchant, S.H.; Crooks, L.A.; Hartshorne, M.F. Indium-111 Pentetreotide Uptake by Pancreatic Polypeptide Cell Hyperplasia: Potential Pitfall in Somatostatin Receptor Scintigraphy. Pancreas 2007, 35, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Hong, R.; Choi, D.; Lim, S. Hyperinsulinemic Hypoglycemia Due to Diffuse Nesidioblastosis in Adults: A Case Report. World J. Gastroenterol. 2008, 14, 140–142. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Nissen, N.N.; Dhall, Þ.D.; Heaney, A.P. Nesidioblastosis and Hyperplasia of Alpha Cells, Microglucagonoma, and Nonfunctioning Islet Cell Tumor of the Pancreas. Pancreas 2008, 36, 428–431. [Google Scholar] [CrossRef] [PubMed]
- Moreira, R.O.; Moreira, R.B.M.; Machado, N.A.M.; Gonçalves, T.B.; Coutinho, W.F. Post-Prandial Hypoglycemia after Bariatric Surgery: Pharmacological Treatment with Verapamil and Acarbose. Obes. Surg. 2008, 18, 1618–1621. [Google Scholar] [CrossRef] [PubMed]
- Kovács, E.; Németh, H.; Pásztor, E.; Pfliegler, G. Hyperinsulinemic Hypoglycemia in Adults. Case Reports and a Short Review. Orv. Hetil. 2008, 149, 1659–1664. [Google Scholar] [CrossRef] [PubMed]
- Sircus, W.; Brunt, P.W.; Walker, R.J.; Small, W.P.; Falconer, C.W.; Thomson, C.G. Two Cases of “Pancreatic Cholera” with Features of Peptide-Secreting Adenomatosis of the Pancreas. Gut 1970, 11, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Abellán, P.; Cámara, R.; Merino-Torres, J.F.; Pérez-Lazaro, A.; del Olmo, M.I.; Ponce, J.L.; Rayón, J.M.; Piñón, F. Severe Hypoglycemia after Gastric Bypass Surgery for Morbid Obesity. Diabetes Res. Clin. Pract. 2008, 79, 2007–2009. [Google Scholar] [CrossRef]
- Kellogg, T.A.; Bantle, J.P.; Leslie, D.B.; Redmond, J.B.; Slusarek, B.; Swan, T.; Buchwald, H.; Ikramuddin, S. Postgastric Bypass Hyperinsulinemic Hypoglycemia Syndrome: Characterization and Response to a Modified Diet. Surg. Obes. Relat. Dis. 2008, 4, 492–499. [Google Scholar] [CrossRef]
- Dissanayake, A.S.; Jones, V.; Fernando, D.J.S. Adult Hyperinsulinaemic Hypoglycaemia Caused by Coexisting Nesidioblastosis and Insulinoma. Eur. J. Intern. Med. 2008, 19, 303–306. [Google Scholar] [CrossRef]
- Z’graggen, K.; Guweidhi, A.; Steffen, R.; Potoczna, N.; Biral, R.; Walther, F.; Komminoth, P.; Horber, F. Severe Recurrent Hypoglycemia after Gastric Bypass Surgery. Obes. Surg. 2008, 18, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Karawagh, A.; Abdullah, L.; Gasim, A.; Abdelaziz, M. Noninsulinoma Pancreatogenous Hypoglycemia Syndrome in a Saudi Male. Saudi Med. J. 2008, 29, 1654–1657. [Google Scholar] [PubMed]
- Barbour, J.; Thomas, B.; Morgan, K.; Byrne, T.; Adams, D. The Practice of Pancreatic Resection after Roux-En-Y Gastric Bypass. Am. Surg. 2008, 74, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Kenney, B.; Tormey, C.A.; Qin, L.; Sosa, J.A.; Jain, D.; Neto, A. Adult Nesidioblastosis. Clinicopathologic Correlation between Pre-Operative Selective Arterial Calcium Stimulation Studies and Post-Operative Pathologic Findings. J. Pancreas 2008, 9, 504–511. [Google Scholar]
- Geraghty, M.; Draman, M.; Moran, D.; Muldoon, C.; Reynolds, J.; Cullen, M. Hypoglycaemia in an Adult Male: A Surprising Finding in Pursuit of Insulinoma. Surgeon 2008, 6, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Catton, J.A.; Zaitoun, A.M.; Aithal, G.P.; Sturrock, N.D.C.; Lobo, D.N. Diffuse Nesidioblastosis Causing Hyperinsulinemic Hypoglycemia: The Importance of Pancreatic Sampling on EUS. Gastrointest. Endosc. 2008, 68, 571–572. [Google Scholar] [CrossRef] [PubMed]
- Jabri, A.L.; Bayard, C. Nesidioblastosis Associated with Hyperinsulinemic Hypoglycemia in Adults: Review of the Literature. Eur. J. Intern. Med. 2004, 15, 407–410. [Google Scholar] [CrossRef]
- Wig, J.; Nayak, N.; Kumar, S.; Buxi, T.; Nundy, S.; Mittal, K.; Gupta, P. A 20-Year-Old Man with Recurrent Abdominal Pain and Vomiting since the Age of 5 Years. Familial Chronic Pancreatitis with Nesidioblastosis and Hyperinsulinaemic Hypoglycaemia. Natl. Med. J. India 2008, 21, 307–312. [Google Scholar]
- Bright, E.; Garcea, G.; Ong, S.L.; Madira, W.; Berry, D.P.; Dennison, A.R. An Unsual Case of Concurrent Insulinoma and Nesidioblastosis. J. Pancreas 2008, 9, 649–653. [Google Scholar]
- Zhao, Y.; Xu, J.; Wu, Y.; Han, W. The Application of Intraoperative Ultrasonography in the Diagnosis and Therapy of Insulinoma. Zhonghua Wai Ke Za Zhi 2009, 47, 337–338. [Google Scholar]
- Dong, A.; Yuan, Z.; Zhang, H.; Gao, Y.; Guo, X. Nesidioblastosis in an Adult with Type 2 Diabetes Mellitus: A Case Report. Beijing Da Xue Xue Bao 2009, 41, 590–592. [Google Scholar] [PubMed]
- Carpenter, T.; Trautmann, M.E.; Baron, A.D. Hyperinsulinemic Hypoglycemia with Nesidioblastosis after Gastric-Bypass Surgery. N. Engl. J. Med. 2009, 353, 2192–2194. [Google Scholar] [CrossRef]
- Andronesi, D.; Andronesi, A.; Tonea, A.; Andrei, S.; Herlea, V.; Lupescu, I.; Ionescu-Târgovişte, C.; Coculescu, M.; Fica, S.; Ionescu, M.; et al. Insulinoma of the Pancreas: Analysis of a Clinical Series of 30 Cases. Chirurgia 2009, 104, 675–685. [Google Scholar] [PubMed]
- Ahn, J.; Lee, S.E.; Lee, E.S.; Chung, Y.J.; Oh, Y.S.; Shinn, S.H.; Kim, J. A Case of Nesidioblastosis Causing Hypoglycaemia after Delivery. Diabetes Res. Clin. Pract. 2009, 83, e5–e7. [Google Scholar] [CrossRef]
- Toyomasu, Y.; Fukuchi, M.; Yoshida, T.; Tajima, K.; Osawa, H.; Motegi, M.; Iijima, T.; Nagashima, K.; Ishizaki, M.; Mochiki, E.; et al. Treatment of Hyperinsulinemic Hypoglycemia Due to Diffuse Nesidioblastosis in Adults: A Case Report. Am. Surg. 2009, 75, 331–334. [Google Scholar] [CrossRef]
- Rumilla, K.M.; Erickson, L.A.; Service, F.J.; Vella, A.; Thompson, G.B.; Grant, C.S.; Lloyd, R.V. Hyperinsulinemic Hypoglycemia with Nesidioblastosis: Histologic Features and Growth Factor Expression. Mod. Pathol. 2009, 22, 239–245. [Google Scholar] [CrossRef]
- Francesconi, A.B.; Matos, M.; Lee, J.C.; Wyld, D.K.; Clouston, A.D.; Macfarlane, D. Nesidioblastosis as a Cause of Focal Pancreatic 111In-Pentetreotide Uptake in a Patient with Putative VIPoma: Another Differential Diagnosis. Ann. Nucl. Med. 2009, 23, 497–499. [Google Scholar] [CrossRef]
- Hight, D.; James, L.P.; Jahadi, M.R. Pancreatic Islet Hyperplasia as a Cause of a Severe Ulcer Diathesis. Arch. Surg. 1971, 103, 98–100. [Google Scholar] [CrossRef]
- Thaler, J.P.; Cummings, D.E. Minireview: Hormonal and Metabolic Mechanisms of Diabetes Remission after Gastrointestinal Surgery. Endocrinology 2009, 150, 2518–2525. [Google Scholar] [CrossRef]
- Henopp, T.; Anlauf, M.; Schmitt, A.; Schlenger, R.; Zalatnai, A.; Couvelard, A.; Ruszniewski, P.; Schaps, K.P.; Jonkers, Y.M.H.; Speel, E.J.M.; et al. Glucagon Cell Adenomatosis: A Newly Recognized Disease of the Endocrine Pancreas. J. Clin. Endocrinol. Metab. 2009, 94, 213–217. [Google Scholar] [CrossRef]
- Spanakis, E.; Gragnoli, C. Successful Medical Management of Status Post-Roux-En-y-Gastric-Bypass Hyperinsulinemic Hypoglycemia. Obes. Surg. 2009, 19, 1333–1334. [Google Scholar] [CrossRef]
- Aasheim, E.; Frigstad, S.; Søvik, T.; Birkeland, K.; Haukeland, J. Hyperinsulinemic Hypoglycemia and Liver Cirrhosis Presenting after Duodenal Switch: A Case Report. Surg. Obes. Relat. Dis. 2010, 6, 441–443. [Google Scholar] [CrossRef] [PubMed]
- Halperin, F.; Patti, M.E.; Goldfine, A.B. Glucagon Treatment for Post-Gastric Bypass Hypoglycemia. Obesity 2010, 18, 1858–1860. [Google Scholar] [CrossRef] [PubMed]
- Guseva, N.; Phillips, D.; Mordes, J.P. Successful Treatment of Persistent Hyperinsulinemic Hypoglycemia with Nifedipine in an Adult Patient. Endocr. Pract. 2010, 16, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Moreno Moreno, P.; Gutiérrez Alcántara, C.; Muñoz-Villanueva Mdel, C.; Ortega, R.; Corpas Jiménez Mdel, S.; Zurera Tendero, L.; Benito López, P. Usefulness of Arterial Calcium Stimulation with Hepatic Venous Sampling in the Localization Diagnosis of Endogenous Hyperinsulinism. Endocrinol. Nutr. 2010, 57, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Goretzki, P.; Starke, A.; Lammers, B.; Schwarz, K.; Röher, H.D. Pankreatischer Hyperinsulinismus Wandel Des Krankheitsbildes Mit Spezifischen Unterschieden Auch Bei Sporadischen Erkrankungsformen (Eigene Erfahrung an 144 operierten Patienten von 19862009). Zent. Chir.-Z. Allg. Visz. Gefasschir. 2010, 135, 218–225. [Google Scholar] [CrossRef]
- Ballester, M.; Pastor, B.; Pascual, H.; Pallares, J.; Albasini, J. Nesidioblastosis as a Cause of Hyperinsulinism in the Adult. Cir. Esp. 2010, 88, 423–425. [Google Scholar] [CrossRef]
- Bränström, R.; Berglund, E.; Curman, P.; Forsberg, L.; Höög, A.; Grimelius, L.; Berggren, P.-O.; Mattsson, P.; Hellman, P.; Juntti-Berggren, L. Electrical Short-Circuit in β-Cells from a Patient with Non-Insulinoma Pancreatogenous Hypoglycemic Syndrome (NIPHS): A Case Report. J. Med. Case Rep. 2010, 4, 2–6. [Google Scholar] [CrossRef]
- Greene, C.A.; Rao, V.S.; Maragos, G.D.; Mitchell, J.R. Pancreatic Islet Cell Hyperplasia. A Case Report. Nebr. Med. J. 1972, 57, 125–126. [Google Scholar]
- McElroy, M.K.; Lowy, A.M.; Weidner, N. Case Report: Focal Nesidioblastosis (“nesidioblastoma”) in an Adult. Hum. Pathol. 2010, 41, 447–451. [Google Scholar] [CrossRef]
- Kibebew, M.; Bacha, S.; Feleke, Y.; Johnson, O. Case Reports of Insulinoma and Nesidioblastosis in Ethiopia. Ethiop. Med. J. 2010, 48, 73–79. [Google Scholar] [PubMed]
- Mathavan, V.K.; Arregui, M.; Davis, C.; Singh, K.; Patel, A.; Meacham, J. Management of Postgastric Bypass Noninsulinoma Pancreatogenous Hypoglycemia. Surg. Endosc. 2010, 24, 2547–2555. [Google Scholar] [CrossRef] [PubMed]
- Reubi, J.C.; Perren, A.; Rehmann, R.; Waser, B.; Christ, E.; Callery, M.; Goldfine, A.B.; Patti, M.E. Glucagon-like Peptide-1 (GLP-1) Receptors Are Not Overexpressed in Pancreatic Islets from Patients with Severe Hyperinsulinaemic Hypoglycaemia Following Gastric Bypass. Diabetologia 2010, 53, 2641–2645. [Google Scholar] [CrossRef] [PubMed]
- Vanderveen, K.A.; Grant, C.S.; Thompson, G.B.; Farley, D.R.; Richards, M.L.; Vella, A.; Vollrath, B.; Service, F.J. Outcomes and Quality of Life after Partial Pancreatectomy for Noninsulinoma Pancreatogenous Hypoglycemia from Diffuse Islet Cell Disease. Surgery 2010, 148, 1237–1246. [Google Scholar] [CrossRef]
- Bernard, B.; Kline, G.A.; Service, F.J. Hypoglycaemia Following Upper Gastrointestinal Surgery: Case Report and Review of the Literature. BMC Gastroenterol. 2010, 10, 77. [Google Scholar] [CrossRef]
- Rabiee, A.; Magruder, J.T.; Salas-Carrillo, R.; Carlson, O.; Egan, J.M.; Askin, F.B.; Elahi, D.; Andersen, D.K. Hyperinsulinemic Hypoglycemia after Roux-En-y Gastric Bypass: Unraveling the Role of Gut Hormonal and Pancreatic Endocrine Dysfunction. J. Surg. Res. 2011, 167, 199–205. [Google Scholar] [CrossRef]
- Arafah, M.; Kfoury, H.; Naseem, S. Hypoglycemia, a Rare Presentation of a Solid Pseudopapillary Neoplasm of the Pancreas: A Case Report. Turk. J. Gastroenterol. 2011, 22, 544–547. [Google Scholar] [CrossRef]
- Nayak, H.K.; Sothwal, A.; Raizaida, N.; Daga, M.K.; Agarwal, A.K.; Durga, G. A Rare Case of Non-Insulinoma Pancreatic Hypoglycaemia Syndrome (Niphs) in an Adult Due to Localised Islet Cell Hyperplasia-Successfully Managed by Enucleation. BMJ Case Rep. 2011, 2011, bcr0720114554. [Google Scholar] [CrossRef]
- Batra, C.; Saluja, S.; Bajaj, R.; Rajshekar, U.; Thakur, V.; Gupta, V. Non-Insulinoma Pancreatogenous Hypoglycemia Syndrome. J. Assoc. Physicians India 2011, 59, 256–258. [Google Scholar]
- Jacobs, W.H.; Halperin, P.; Mantz, F.A. Watery Diarrhea and Hypokalemia Due to Nonbeta-Islet Cell Hyperplasia of the Pancreas. Am. J. Gastroenterol. 1972, 57, 333–339. [Google Scholar]
- Otto, A.I.; Marschalko, M.; Zalatnai, A.; Toth, M.; Kovacs, J.; Harsing, J.; Tulassay, Z.; Karpati, S. Glucagon Cell Adenomatosis: A New Entity Associated with Necrolytic Migratory Erythema and Glucagonoma Syndrome. J. Am. Acad. Dermatol. 2011, 65, 458–459. [Google Scholar] [CrossRef] [PubMed]
- Azemoto, N.; Kumagi, T.; Yokota, T.; Kuroda, T.; Koizumi, M.; Yamanishi, H.; Soga, Y.; Furukawa, S.; Abe, M.; Ikeda, Y.; et al. An Unusual Case of Subclinical Diffuse Glucagonoma Coexisting with Two Nodules in the Pancreas: Characteristic Features on Computed Tomography. Clin. Res. Hepatol. Gastroenterol. 2012, 36, e43–e47. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.E.; Zhao, M.; Whitelaw, B.C.; Ramage, J.; Diaz-Cano, S.; Le Roux, C.W.; Quaglia, A.; Huang, G.C.; Aylwin, S.J.B. GLP-1 and Glucagon Secretion from a Pancreatic Neuroendocrine Tumor Causing Diabetes and Hyperinsulinemic Hypoglycemia. J. Clin. Endocrinol. Metab. 2012, 97, 3039–3045. [Google Scholar] [CrossRef] [PubMed]
- Qintar, M.; Sibai, F.; Taha, M. Hypoglycemia Due to an Adult-Onset Nesidioblastosis, a Diagnostic and Management Dilemma. Avicenna J. Med. 2012, 2, 45–47. [Google Scholar] [CrossRef]
- Limongelli, P.; Belli, A.; Cioffi, L.; Russo, G.; D’Agostino, A.; Fantini, C.; Maiello, F.; Belli, G. Hepatobiliary and Pancreatic: Nesidioblastosis. J. Gastroenterol. Hepatol. 2012, 27, 1538. [Google Scholar] [CrossRef]
- De Heide, L.J.M.; Glaudemans, A.W.J.M.; Oomen, P.H.N.; Apers, J.A.; Totté, E.R.E.; Van Beek, A.P. Functional Imaging in Hyperinsulinemic Hypoglycemia after Gastric Bypass Surgery for Morbid Obesity. J. Clin. Endocrinol. Metab. 2012, 97, 963–967. [Google Scholar] [CrossRef]
- Myint, K.S.; Greenfield, J.R.; Farooqi, I.S.; Henning, E.; Holst, J.J.; Finer, N. Prolonged Successful Therapy for Hyperinsulinaemic Hypoglycaemia after Gastric Bypass: The Pathophysiological Role of GLP1 and Its Response to a Somatostatin Analogue. Eur. J. Endocrinol. 2012, 166, 951–955. [Google Scholar] [CrossRef]
- Nadelson, J.; Epstein, A. A Rare Case of Noninsulinoma Pancreatogenous Hypoglycemia Syndrome. Case Rep. Gastrointest. Med. 2012, 2012, 1–3. [Google Scholar] [CrossRef]
- Przybylik-Mazurek, E.; Pach, D.; Hubalewska-Dydejczyk, A.; Sowa-Staszczak, A.; Gilis-Januszewska, A.; Kulig, J.; Matyja, A.; Chrapczyński, P. Symptoms and Early Diagnostic Possibilities of Pancreatic Endocrine Cells Hyperplasia (Nesidioblastosis). Przegl. Lek. 2012, 69, 9–14. [Google Scholar]
- Ceppa, E.P.; Ceppa, D.P.; Omotosho, P.A.; Dickerson, J.A.; Park, C.W.; Portenier, D.D. Algorithm to Diagnose Etiology of Hypoglycemia after Roux-En-Y Gastric Bypass for Morbid Obesity: Case Series and Review of the Literature. Surg. Obes. Relat. Dis. 2012, 8, 641–647. [Google Scholar] [CrossRef]
- Vance, J.E.; Stoll, R.W.; Kitabchi, A.E.; Buchanan, K.D.; Hollander, D.; Williams, R.H. Familial Nesidioblastosis as the Predominant Manifestation of Multiple Endocrine Adenomatosis. Am. J. Med. 1972, 52, 211–227. [Google Scholar] [CrossRef]
- Ferrario, C.; Stoll, D.; Boubaker, A.; Matter, M.; Yan, P.; Puder, J.J. Diffuse Nesidioblastosis with Hypoglycemia Mimicking an Insulinoma: A Case Report. J. Med. Case Rep. 2012, 6, 332. [Google Scholar] [CrossRef]
- Lee, C.J.; Brown, T.; Magnuson, T.H.; Egan, J.M.; Carlson, O.; Elahi, D. Hormonal Response to a Mixed-Meal Challenge after Reversal of Gastric Bypass for Hypoglycemia. J. Clin. Endocrinol. Metab. 2013, 98, 1208–1212. [Google Scholar] [CrossRef]
- Christ, E.; Wild, D.; Ederer, S.; Béhé, M.; Nicolas, G.; Caplin, M.E.; Brändle, M.; Clerici, T.; Fischli, S.; Stettler, C.; et al. Glucagon-like Peptide-1 Receptor Imaging for the Localisation of Insulinomas: A Prospective Multicentre Imaging Study. Lancet Diabetes Endocrinol. 2013, 1, 115–122. [Google Scholar] [CrossRef]
- Soares, F.; Providência, R.; Pontes, M. Nesidioblastosis: An Undescribed Cause of Transient Loss of Conscience in Young Adults. Europace 2013, 15, 1506. [Google Scholar] [CrossRef] [PubMed]
- García-Santos, E.P.; Manzanares-Campillo, M.D.C.; Padilla-Valverde, D.; Villarejo-Campos, P.; Gil-Rendo, A.; Muñoz-Atienza, V.; Sánchez-García, S.; Puig-Rullán, A.M.; Rodríguez-Peralto, J.L.; Martín-Fernández, J. Nesidioblastosis. A Case of Hyperplasia of the Islets of Langerhans in the Adult. Pancreatology 2013, 13, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Then, C.; Nam-Apostolopoulos, Y.; Seissler, J.; Lechner, A. Refractory Idiopathic Non-Insulinoma Pancreatogenous Hypoglycemia in an Adult: Case Report and Review of the Literature. JOP 2013, 14, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Al-Sarireh, B.; Haidermota, M.; Verbeke, C.; Rees, D.A.; Yu, R.; Griffiths, A.P. Glucagon Cell Adenomatosis without Glucagon Receptor Mutation. Pancreas 2013, 42, 360–362. [Google Scholar] [CrossRef]
- Choi, J.E.; Noh, S.J.; Sung, J.J.; Moon, W.S. Nesidioblastosis and Pancreatic Non-Functioning Islet Cell Tumor in an Adult with Type 2 Diabetes Mellitus. Korean J. Pathol. 2013, 47, 489–491. [Google Scholar] [CrossRef]
- Gupta, R.; Patel, R.; Nagral, S. Adult Onset Nesidioblastosis Treated by Subtotal Pancreatectomy. JOP 2013, 14, 286–288. [Google Scholar] [CrossRef]
- Sowa-Staszczak, A.; Pach, D.; Mikołajczak, R.; Mäcke, H.; Jabrocka-Hybel, A.; Stefańska, A.; Tomaszuk, M.; Janota, B.; Gilis-Januszewska, A.; Małecki, M.; et al. Glucagon-like Peptide-1 Receptor Imaging with [Lys40(Ahx-HYNIC- 99mTc/EDDA)NH2]-Exendin-4 for the Detection of Insulinoma. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Larsson, L.I.; Ljungberg, O.; Sundler, F.; Håkanson, R.; Svensson, S.O.; Rehfeld, J.; Stadil, R.; Holst, J. Antro Pyloric Gastrinoma Associated with Pancreatic Nesidioblastosis and Proliferation of Islets. Virchows Arch. A Pathol. Pathol. Anat. 1973, 360, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Pongprasobchai, S.; Lertwattanarak, R.; Pausawasdi, N.; Prachayakul, V. Diagnosis and Localization of Insulinoma in Thai Patients: Performance of Endoscopic Ultrasonography Compared to Computed Tomography and Magnetic Resonance Imaging. J. Med. Assoc. Thai. 2013, 96 (Suppl. 2), S187–S193. [Google Scholar] [PubMed]
- Maeda, Y.; Yokoyama, K.; Takeda, K.; Takada, J.; Hamada, H.; Hujioka, Y.; Kudo, S.E. Adult-Onset Diffuse Nesidioblastosis Causing Hypoglycemia. Clin. J. Gastroenterol. 2013, 6, 50–54. [Google Scholar] [CrossRef]
- Grönberg, M.; Tsolakis, A.V.; Holmbäck, U.; Stridsberg, M.; Grimelius, L.; Janson, E.T. Ghrelin and Obestatin in Human Neuroendocrine Tumors: Expression and Effect on Obestatin Levels after Food Intake. Neuroendocrinology 2013, 97, 291–299. [Google Scholar] [CrossRef]
- Challis, B.G.; Harris, J.; Sleigh, A.; Isaac, I.; Orme, S.M.; Seevaratnam, N.; Dhatariya, K.; Simpson, H.L.; Semple, R.K. Familial Adult Onset Hyperinsulinism Due to an Activating Glucokinase Mutation: Implications for Pharmacological Glucokinase Activation. Clin. Endocrinol. 2014, 81, 855–861. [Google Scholar] [CrossRef]
- Campos, G.M.; Ziemelis, M.; Paparodis, R.; Ahmed, M.; Davis, D.B. Laparoscopic Reversal of Roux-En-Y Gastric Bypass: Technique and Utility for Treatment of Endocrine Complications. Surg. Obes. Relat. Dis. 2014, 10, 36–43. [Google Scholar] [CrossRef]
- Kang, H.; Kim, S.; Lim, T.S.; Lee, H.W.; Choi, H.; Kang, C.M.; Kim, H.G.; Bang, S. A Case of Alpha-Cell Nesidioblastosis and Hyperplasia with Multiple Glucagon-Producing Endocrine Cell Tumor of the Pancreas. Korean J. Gastroenterol. 2014, 63, 253–257. [Google Scholar] [CrossRef]
- Pathak, R.; Karmacharya, P.; Salman, A.; Alweis, R. An Unusual Cause of Hypoglycemia in a Middle-Aged Female after Bariatric Surgery. J. Community Hosp. Intern. Med. Perspect. 2014, 4, 23118. [Google Scholar] [CrossRef]
- Rao, B.B.; Click, B.; Codario, R. Successful Management of Refractory Noninsulinoma Pancreatogenous Hypoglycemia Syndrome with Gastric Bypass Reversal: A Case Report. Am. J. Gastroenterol. 2014, 109, S309. [Google Scholar] [CrossRef]
- de Santibañes, M.; Cristiano, A.; Mazza, O.; Grossenbacher, L.; de Santibañes, E.; Sánchez Clariá, R.; Sivori, E.; García Mónaco, R.; Pekolj, J. Endogenous Hyperinsulinemic Hypoglycemia Syndrome: Surgical Treatment. Cir. Esp. 2014, 92, 547–552. [Google Scholar] [CrossRef] [PubMed]
- García, B.F.; Peromingo, R.; Galindo, J.; Arrieta, F.; Sánchez, J.; Vázquez, C.; Botella-Carretero, J.I. Tratamiento de Las Hipoglucemias Graves Tras Bypass Gástrico Con Pancreatectomía Subtotal: A Propósito de 2 Casos. Endocrinol. Nutr. 2014, 61, 391–393. [Google Scholar] [CrossRef] [PubMed]
- Creutzfeldt, W.; Arnold, R.; Creutzfeldt, C.; Track, N.S. Pathomorphologic, Biochemical, and Diagnostic Aspects of Gastrinomas (Zollinger-Ellison Syndrome). Hum. Pathol. 1975, 6, 47–76. [Google Scholar] [CrossRef] [PubMed]
- Woo, C.Y.; Jeong, J.Y.; Jang, J.E.; Leem, J.; Jung, C.H.; Koh, E.H.; Lee, W.J.; Kim, M.S.; Park, J.Y.; Lee, J.B.; et al. Clinical Features and Causes of Endogenous Hyperinsulinemic Hypoglycemia in Korea. Diabetes Metab. J. 2015, 39, 126–131. [Google Scholar] [CrossRef]
- Martin-Grace, J.; O’Tuathail, M.; Hannon, M.J.; Swan, N.; O’Shea, D.; Tamagno, G. Amlodipine for the Medical Treatment of Adult-Onset Diffuse Nesidioblastosis. Pancreas 2015, 44, 1162–1164. [Google Scholar] [CrossRef]
- Valli, V.; Blandamura, S.; Pastorelli, D.; Merigliano, S.; Sperti, C. Nesidioblastosis Coexisting with Non-Functioning Islet Cell Tumour in an Adult. Endokrynol. Pol. 2015, 66, 356–360. [Google Scholar] [CrossRef]
- Ramírez-González, L.R.; Sotelo-Álvarez, J.A.; Rojas-Rubio, P.; MacÍas-Amezcua, M.D.; Orozco-Rubio, R.; Fuentes-Orozco, C. Nesidioblastosis En El Adulto: Reporte de Un Caso. Cir. Cir. 2015, 83, 324–328. [Google Scholar] [CrossRef]
- Thompson, S.M.; Vella, A.; Thompson, G.B.; Rumilla, K.M.; Service, F.J.; Grant, C.S.; Andrews, J.C. Selective Arterial Calcium Stimulation with Hepatic Venous Sampling Differentiates Insulinoma from Nesidioblastosis. J. Clin. Endocrinol. Metab. 2015, 100, 4189–4197. [Google Scholar] [CrossRef]
- Maguire, D. Lesson of the Month 2: An Unusual Presentation of Hyperinsulinaemic Hypoglycaemia with Possible Underlying Diagnosis of Glucose-Sensitive Insulinoma or Islet Cell Hyperplasia. Clin. Med. J. R. Coll. Physicians Lond. 2015, 15, 495–496. [Google Scholar] [CrossRef]
- Ünal, B.; Uzun, Ö.C.; Başsorgun, C.I.; Erdoğan, O.; Elpek, G.Ö. A Rare Complication of Gastric Bypass (Weight Loss) Surgery: Nesidioblastosis. Int. J. Surg. Pathol. 2015, 23, 68–70. [Google Scholar] [CrossRef]
- Fountoulakis, S.; Malliopoulos, D.; Papanastasiou, L.; Pappa, T.; Karydas, G.; Piaditis, G. Reversal of Impaired Counterregulatory Cortisol Response Following Diazoxide Treatment in a Patient with Non Insulinoma Pancreatogenous Hypoglycemia Syndrome: Case Report and Overview of Pathogenetic Mechanisms. Hormones 2015, 14, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Li, Z.; Qu, L.; Liu, Y.; Gao, Y.; Li, F.; Wang, G. A Rare Case of Focal Nesidioblastosis Causing Adult-Onset Hypoglycemia. Exp. Ther. Med. 2015, 10, 723–726. [Google Scholar] [CrossRef] [PubMed]
- Sampaio-Neto, J.; Branco-Filho, A.J.; Nassif, L.S.; Nassif, A.T.; Masi, F.D.J.; Ximenez, D.R. Proposal of a Revisional Surgery to Treat Non-Insulinoma Hyperinsulinemic Hypoglicemia Postgastric Bypass. ABCD Arq. Bras. Cir. Dig. 2015, 28, 278–281. [Google Scholar] [CrossRef]
- Verner, J.V.; Morrison, A.B. Endocrine Pancreatic Islet Disease With Diarrhea: Report of a Case Due to Diffuse Hyperplasia of Nonbeta Islet Tissue with a Review of 54 Additional Cases. Arch. Intern. Med. 1974, 133, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Christ, E.; Wild, D.; Antwi, K.; Waser, B.; Fani, M.; Schwanda, S.; Heye, T.; Schmid, C.; Baer, H.U.; Perren, A.; et al. Preoperative Localization of Adult Nesidioblastosis Using 68Ga-DOTA-Exendin-4-PET/CT. Endocrine 2015, 50, 821–823. [Google Scholar] [CrossRef] [PubMed]
- Mordes, J.; Alonso, L. Evaluation, Medical Therapy, and Course of Adult Persistent Hyperinsulinemic Hypoglycemia after Roux-En-Y Gastric Bypass Surgery: A Case Series. Endocr. Pract. 2015, 21, 237–246. [Google Scholar] [CrossRef]
- Mihai, B.M.; Lăcătuşu, C.M.; Arhire, L.I.; Graur, M.; Scripcariu, V.; Aniţei, M.G.; Radu, I.; Ferariu, D.; Danciu, M. Pathological Aspects Underlying Pancreatogenous Hyperinsulinemic Hypoglycemia—Report of Three Cases. Rom. J. Morphol. Embryol. 2015, 56, 251–256. [Google Scholar]
- de Vasconcellos Macedo, A.L.; Hidal, J.T.; Marcondes, W.; Mauro, F.C. Robotic Near-Total Pancreatectomy for Nesidioblastosis after Bariatric Surgery. Obes. Surg. 2016, 26, 3082–3083. [Google Scholar] [CrossRef]
- Kim, J.R.; Jang, J.Y.; Shin, Y.C.; Cho, Y.M.; Kim, H.; Kwon, W.; Han, Y.M.; Kim, S.W. Difficult Diagnosis and Localization of Focal Nesidioblastosis: Clinical Implications of 68Gallium-DOTAD-Phe1-Tyr3-Octreotide PET Scanning. Ann. Surg. Treat. Res. 2016, 91, 51–55. [Google Scholar] [CrossRef]
- Yang, Z.; Tan, H.; Sun, Y.; Si, S.; Xu, L.; Liu, X.; Liu, L.; Zhou, W.; Huang, J. Intraoperative Portal Vein Insulin Assay Combined with Occlusion of the Pancreas for Complex Pancreatogenous Hypoglycemia. Medicine 2016, 95, e3928. [Google Scholar] [CrossRef]
- Luo, Y.; Pan, Q.; Yao, S.; Yu, M.; Wu, W.; Xue, H.; Kiesewetter, D.O.; Zhu, Z.; Li, F.; Zhao, Y.; et al. Glucagon-like Peptide-1 Receptor PET/CT with 68Ga-NOTA-Exendin-4 for Detecting Localized Insulinoma: A Prospective Cohort Study. J. Nucl. Med. 2016, 57, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Schwetz, V.; Horvath, K.; Kump, P.; Lackner, C.; Perren, A.; Forrer, F.; Pieber, T.R.; Treiber, G.; Sourij, H.; Mader, J.K. Successful Medical Treatment of Adult Nesidioblastosis with Pasireotide over 3 Years. Medicine 2016, 95, e3272. [Google Scholar] [CrossRef] [PubMed]
- Prasad, V.; Sainz-Esteban, A.; Arsenic, R.; Plöckinger, U.; Denecke, T.; Pape, U.F.; Pascher, A.; Kühnen, P.; Pavel, M.; Blankenstein, O. Role of 68Ga Somatostatin Receptor PET/CT in the Detection of Endogenous Hyperinsulinaemic Focus: An Explorative Study. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1593–1600. [Google Scholar] [CrossRef]
- Krieger, A.; Smirnov, A.; Berelavichus, S.; Gorin, D.; Kaldarov, A.; Karel’skaya, N.; Vetsheva, N.; Kalinin, D.; Lebedeva, A.; Dugarova, R. Organic Hyperinsulinism: Radiological Diagnostics and Surgical Treatment. Khirurgiia 2016, 6, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Stefanini, P.; Carboni, M.; Patrassi, N.; Basoli, A. Hypoglycemia and Insular Hyperplasia. Review of 148 Cases. Ann. Surg. 1974, 180, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Vilarrasa, N.; Goday, A.; Rubio, M.A.; Caixàs, A.; Pellitero, S.; Ciudin, A.; Calañas, A.; Botella, J.I.; Bretón, I.; Morales, M.J.; et al. Hyperinsulinemic Hypoglycemia after Bariatric Surgery: Diagnosis and Management Experience from a Spanish Multicenter Registry. Obes. Facts 2016, 9, 41–51. [Google Scholar] [CrossRef]
- Sowa-Staszczak, A.; Trofimiuk-Müldner, M.; Stefańska, A.; Tomaszuk, M.; Buziak-Bereza, M.; Gilis-Januszewska, A.; Jabrocka-Hybel, A.; Głowa, B.; Małecki, M.; Bednarczuk, T.; et al. 99mTc Labeled Glucagon-like Peptide-1-Analogue (99mTc-GLP1) Scintigraphy in the Management of Patients with Occult Insulinoma. PLoS ONE 2016, 11, e0160714. [Google Scholar] [CrossRef]
- Gunnarsdóttir, G.; Guðmundsdóttir, A.; Hellmanm, P.; Stålberg, P. Repeated Non-Epileptic Seizures in a Previously Healthy Young Woman—A Case Report. Laeknabladid 2016, 102, 339–342. [Google Scholar] [CrossRef]
- De Sousa, S.M.C.; Haghighi, K.S.; Qiu, M.R.; Greenfield, J.R.; Chen, D.L.T. Synchronous Nesidioblastosis, Endocrine Microadenoma, and Intraductal Papillary Mucinous Neoplasia in a Man Presenting with Hyperinsulinemic Hypoglycemia. Pancreas 2016, 45, 154–159. [Google Scholar] [CrossRef]
- Anderson, B.; Nostedt, J.; Girgis, S.; Dixon, T.; Agrawal, V.; Wiebe, E.; Senior, P.A.; Shapiro, A.M.J. Insulinoma or Non-Insulinoma Pancreatogenous Hypoglycemia? A Diagnostic Dilemma. J. Surg. Case Rep. 2016, 2016, rjw188. [Google Scholar] [CrossRef]
- Preechasuk, L.; Pongpaibul, A.; Kunavisarut, T. Non-Insulinoma Pancreatogeneous Hypoglycemia Syndrome with False-Positive Somatostatin Receptor Scintigraphy: A Case Report and Review of Literature. J. Med. Assoc. Thai. 2016, 99, 354–359. [Google Scholar] [PubMed]
- Chen, X.; Kamel, D.; Barnett, B.; Yung, E.; Quinn, A.; Nguyen, C. An Unusual Presentation of Post Gastric Bypass Hypoglycemia with Both Postprandial and Fasting Hypoglycemia. Endocrinol. Diabetes Metab. Case Rep. 2018, 2018, 18–89. [Google Scholar] [CrossRef] [PubMed]
- Dadheech, N.; Garrel, D.; Buteau, J. Evidence of Unrestrained Beta-Cell Proliferation and Neogenesis in a Patient with Hyperinsulinemic Hypoglycemia after Gastric Bypass Surgery. Islets 2018, 10, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Antwi, K.; Fani, M.; Heye, T.; Nicolas, G.; Rottenburger, C.; Kaul, F.; Merkle, E.; Zech, C.J.; Boll, D.; Vogt, D.R.; et al. Comparison of Glucagon-like Peptide-1 Receptor (GLP-1R) PET/CT, SPECT/CT and 3T MRI for the Localisation of Occult Insulinomas: Evaluation of Diagnostic Accuracy in a Prospective Crossover Imaging Study. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2318–2327. [Google Scholar] [CrossRef]
- Luca, V.G.; Kwadwo, A.; Guillaume, N.P.; Damian, W.; Emanuel, C. Clinical Presentation of 54 Patients with Endogenous Hyperinsulinaemic Hypoglycaemia: A Neurological Chameleon (Observational Study). Swiss Med. Wkly. 2018, 148, 1–5. [Google Scholar] [CrossRef]
- Jahnke, K.; Sawitzki, E. Deafness Due to Hyperinsulinism (Author’s Transl). HNO 1975, 23, 152–155. [Google Scholar]
- Kérékou, A.; El Aziz, S.; Dédjan, A.; Chadli, A.; Farouqi, A. Hypoglycemia Controlled by Prednisone in an Occult Insulinoma or a Nesidioblastosis. Open J. Endocr. Metab. Dis. 2019, 9, 69–73. [Google Scholar] [CrossRef]
- Wiesli, P.; Pavlicek, V.; Brändle, M.; Pfammatter, T.; Perren, A.; Schmid, C. Distinct Mechanisms of Hypoglycaemia in Patients with Somatostatin-secreting Neuroendocrine Tumours. Endocrinol. Diabetes Metab. 2019, 2, 2–5. [Google Scholar] [CrossRef]
- Lozano-Melendez, E.; Aguilar-Soto, M.; Graniel-Palafox, L.E.; Ceceña-Martínez, L.E.; Valdez-Ortiz, R.; Solis-Jimenez, F. Adult Nesidioblastosis in Chronic Kidney Disease. Case Rep. Endocrinol. 2019, 2019, 1–6. [Google Scholar] [CrossRef]
- Dauriz, M.; Maneschi, C.; Castelli, C.; Tomezzoli, A.; Fuini, A.; Landoni, L.; Malleo, G.; Ferdeghini, M.; Bonora, E.; Moghetti, P. A Case Report of Insulinoma Relapse on Background Nesidioblastosis: A Rare Cause of Adult Hypoglycemia. J. Clin. Endocrinol. Metab. 2019, 104, 773–778. [Google Scholar] [CrossRef]
- Allué, M.; Ros, S.; Lamata, F. Pancreatic Nesidioblastosis: Case Report. Med. Clin. 2019, 152, 245–246. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.; Conway, L.; Cooper, C.; Sinha, A.; Nandi, N. Nesidioblastosis in an Adult with Short Gut Syndrome and Type 2 Diabetes. AACE Clin. Case Rep. 2019, 5, e375–e379. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Snaith, J.R.; Mahajan, H.; Holmes-Walker, D.J. Nesidioblastosis Following Laparoscopic Sleeve Gastrectomy. Clin. Endocrinol. 2019, 91, 906–908. [Google Scholar] [CrossRef]
- Orujov, M.; Lai, K.K.; Forse, C.L. Concurrent Adult-Onset Diffuse β-Cell Nesidioblastosis and Pancreatic Neuroendocrine Tumor: A Case Report and Review of the Literature. Int. J. Surg. Pathol. 2019, 27, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Delbeke, D.; Newman, G.; Deppen, S.; Shah, C.; Ndolo, J.; Shi, C.; Bailey, C.E.; Jessop, A.C.; Sandler, M.P. 68Ga-DOTATATE: Significance of Uptake in the Tail of the Pancreas in Patients without Lesions. Clin. Nucl. Med. 2019, 44, 851–854. [Google Scholar] [CrossRef] [PubMed]
- Kalff, V.; Iravani, A.; Ackhurst, T.; Pattison, D.A.; Eu, P.; Hofman, M.S.; Hicks, R.J.; Akhurst, T.; Pattison, D.A.; Eu, P.; et al. Utility of 68 Ga-DOTA-Exendin-4 PET/CT Imaging in Distinguishing between Insulinoma and Nesidioblastosis in Patients with Confirmed Endogenous Hyperinsulinaemic Hypoglycaemia. Intern. Med. J. 2020, 51, 1657–1664. [Google Scholar] [CrossRef]
- Rastogi, G.K.; Sialy, R.; Sinha, M.K.; Dash, R.; Chopra, J.S.; Kataria, R.N. Serum and Pancreatic Immunoreactive Insulin (IRI) and Proinsulin-like Component (PLC), Serum IRI and PLC Response to Different Stimuli in Normal Subjects and Organic Hyperinsulinism. Acta Diabetol. Lat. 1975, 12, 309–323. [Google Scholar] [CrossRef]
- Lopes, A.A.; Miranda, A.C.; Maior, M.S.; de Mello, R.V.; Bandeira, F. Nesidioblastosis Associated with Pancreatic Heterotopia as a Differential Diagnosis of Hypoglycemia: A Literature Review and Case Report. Am. J. Case Rep. 2020, 21, e922778-1. [Google Scholar] [CrossRef]
- Antwi, K.; Hepprich, M.; Müller, N.A.; Reubi, J.C.; Fani, M.; Rottenburger, C.; Nicolas, G.; Kaul, F.; Christ, E.R.; Wild, D. Pitfalls in the Detection of Insulinomas with Glucagon-like Peptide-1 Receptor Imaging. Clin. Nucl. Med. 2020, 45, e386–e392. [Google Scholar] [CrossRef]
- Boss, M.; Buitinga, M.; Jansen, T.J.P.; Brom, M.; Visser, E.P.; Gotthardt, M. PET-Based Human Dosimetry of 68Ga-NODAGA-Exendin-4, a Tracer for β-Cell Imaging. J. Nucl. Med. 2020, 61, 112–116. [Google Scholar] [CrossRef]
- Awramiszyn-Fernández, N.; Paz-Ibarra, J.; Suárez-Rojas, J.; Rodríguez-Alegría, C.; Somocurcio-Peralta, J.; Ramírez-Delpino, E.; Teruya-Gibu, A. Hyperinsulinemic Hypoglycemia Caused by Nesidioblastosis Associated with Non-Surgical Weight Loss. Rev. Med. Inst. Mex. Seguro Soc. 2020, 58, 528–535. [Google Scholar] [CrossRef] [PubMed]
- McManus, N.M.; Margart, K.M.; Offman, R.P. Hypoglycemia Worsened by Glucose Administration: A Case of Hypoglycemia Years After Gastric Surgery. J. Emerg. Med. 2021, 60, e77–e79. [Google Scholar] [CrossRef] [PubMed]
- Kato, R.; Nishimura, A.; Matsumura, K.; Kikuno, S.; Nagasawa, K.; Mori, Y. Successful Treatment of Adult-Onset Nesidioblastosis by Continuous Subcutaneous Octreotide Infusion in a Patient on Hemodialysis. Clin. Case Rep. 2021, 9, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Snaith, J.R.; McLeod, D.; Richardson, A.; Chipps, D. Multifocal Insulinoma Secondary to Insulinomatosis: Persistent Hypoglycaemia despite Total Pancreatectomy. Endocrinol. Diabetes Metab. Case Rep. 2021, 2020. [Google Scholar] [CrossRef] [PubMed]
- Torres-Arano, S.E.; Fernández-Baez, N.; Torres-González, M.G.; González-Cabrera, I.G.; López-Rosales, F. Hyperinsulinemic Hypoglycemia in Adults. Nesidioblastosis Case Report and Review of the Literature. Cir. Cir. Engl. Ed. 2021, 89, 70–75. [Google Scholar] [CrossRef]
- Castillo-López, M.G.; Fernandez, M.F.; Sforza, N.; Barbás, N.C.; Pattin, F.; Mendez, G.; Ogresta, F.; Gondolesi, I.; Barros Schelotto, P.; Musso, C.; et al. Hyperinsulinemic Hypoglycemia in Adolescents: Case Report and Systematic Review. Clin. Diabetes Endocrinol. 2022, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Koneshamoorthy, A.; Seniveratne-Epa, D.; Calder, G.; Sawyer, M.; Kay, T.W.H.; Farrell, S.; Loudovaris, T.; Mariana, L.; McCarthy, D.; Lyu, R.; et al. Case Report: Hypoglycemia Due to a Novel Activating Glucokinase Variant in an Adult—A Molecular Approach. Front. Endocrinol. 2022, 13, 842937. [Google Scholar] [CrossRef]
- Dobosz, P.; Dzieciątkowski, T. The Intriguing History of Cancer Immunotherapy. Front. Immunol. 2019, 10, 2965. [Google Scholar] [CrossRef]
- Doi, S.; Yamada, T.; Kito, Y.; Obara, S.; Fujii, Y.; Nishimura, T.; Kato, T.; Nakayama, H.; Tsutsumi, M.; Okamura, R. Adult-Onset Focal Nesidioblastosis with Nodular Formation Mimicking Insulinoma. J. Endocr. Soc. 2022, 6, bvab185. [Google Scholar] [CrossRef]
- Efendić, S.; Lins, P.E.; Sigurdsson, G.; Ivemark, B.; Granberg, P.O.; Luft, R. Effect of Somatostatin on Basal and Glucose Induced Insulin Release in Five Patients with Hyperinsulinaemia. Acta Endocrinol. 1976, 81, 525–529. [Google Scholar] [CrossRef]
- Mendelsohn, G. Multiple Tumors in a Renal Transplant Recipient. Johns Hopkins Med. J. 1976, 139, 253–256. [Google Scholar] [PubMed]
- Larsson, L.I. Two Distinct Types of Islet Abnormalities Associated with Endocrine Pancreatic Tumours. Virchows Arch. A Pathol. Anat. Histol. 1977, 376, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Greider, M.H.; Lacy, P.E.; Kissane, J.M.; Rieders, E.; Thomas, G. Pancreatic Perinuclear Inclusions in Diabetes Mellitus and Other Diseases. Diabetes 1977, 26, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Lesna, M.; Hamlyn, A.N.; Venables, C.W.; Record, C.O. Chronic Laxative Abuse Associated with Pancreatic Islet Cell Hyperplasia. Gut 1977, 18, 1032–1035. [Google Scholar] [CrossRef] [PubMed]
- Ingemansson, S.; Kühl, C.; Larsson, L.I.; Lunderquist, A.; Nobin, A. Islet Cell Hyperplasia Localized by Pancreatic Vein Catheterization and Insulin Radioimmunoassay. Am. J. Surg. 1977, 133, 643–645. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Floyd, J.C.; Pek, S.; Fajans, S.S. Insulin, Proinsulin, Glucagon and Gastrin in Pancreatic Tumors and in Plasma of Patients with Organic Hyperinsulinism. J. Clin. Endocrinol. Metab. 1977, 44, 681–694. [Google Scholar] [CrossRef]
- Schwartz, S.E.; Fitzgerald, M.A.; Levine, R.A.; Schwartzel, E.H. Normal Jejunal Cyclic Nucleotide Content in a Patient With Secretory Diarrhea. Arch. Intern. Med. 1978, 138, 1403–1405. [Google Scholar] [CrossRef]
- Schikman, C.H.; Chertow, B.S.; Fabris, B.L. Paradoxical Enhancement of Tolbutamide-Induced Insulin Release by Diazoxide in a Patient with Islet Cell Hyperplasia. Acta Paediatr. Scand. 1978, 67, 671–676. [Google Scholar] [CrossRef]
- Ingemansson, S.; Kühl, C.; Larsson, L.I.; Lunderquist, A.; Lundquist, I. Localization of Insulinomas and Islet Cell Hyperplasias by Pancreatic Vein Catheterization and Insulin Assay. Surg. Gynecol. Obstet. 1978, 146, 725–734. [Google Scholar]
- Kidd, G.S.; Donowitz, M.; O’Dorisio, T.; Cataland, S.; Newman, F. Mild Chronic Watery Diarrhea-Hypokalemia Syndrome Associated with Pancreatic Islet Cell Hyperplasia. Elevated Plasma and Tissue Levels of Gastric Inhibitory Polypeptide and Successful Management with Nicotinic Acid. Am. J. Med. 1979, 66, 883–888. [Google Scholar] [CrossRef]
- Varas Lorenzo, M.J.; Curto Cardús, J.A.; Sans Segarra, M.; Carrera Plans, M. Zollinger-Ellison Syndrome Type II Due to Diffuse Hyperplasia of the Pancreatic Islet Cells (Author’s Transl). Med. Clin. 1979, 73, 68–72. [Google Scholar]
- Dahms, B.B.; Landing, B.H.; Blashovics, M.; Roe, T.F. Nesidioblastosis and Other Islet Cell Abnormalities in Hyperinsulinemic Hypoglycemia of Childhood. Hum. Pathol. 1980, 11, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Tomita, T.; Kimmel, J.; Friesen, S.; Mantz, F. Pancreatic Polypeptide Cell Hyperplasia with and without Watery Diarrhea Syndrome. J. Surg. Oncol. 1980, 14, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Friesen, S.R.; Kimmel, J.R.; Tomita, T. Pancreatic Polypeptide as Screening Marker for Pancreatic Polypeptide Apudomas in Multiple Endocrinopathies. Am. J. Surg. 1980, 139, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Leong, A.S.; Slavotinek, A.H.; Higgins, B.A. Nesidioblastosis, Islet Cell Hyperplasia, and Adenomatosis in a Case of Metastasizing Insulinoma: Contribution to the Genesis of the Islets of Langerhans. Diabetes Care 1980, 3, 537–542. [Google Scholar] [CrossRef]
- Brennan, M.D.; Service, F.J.; Carpenter, A.M.; Rubenstein, A.H.; Edis, A.J. Diagnosis of Pancreatic Islet Hyperplasia Causing Hypoglycemia in a Patient with Portacaval Anastomosis. Am. J. Med. 1980, 68, 941–948. [Google Scholar] [CrossRef]
- Reichardt, W.; Ingemansson, S. Selective Vein Catheterization for Hormone Assay in Endocrine Tumours of the Pancreas. Technique and Results. Acta Radiol. Diagn. 1980, 21, 177–187. [Google Scholar] [CrossRef]
- Glaser, B.; Valtysson, G.; Fajans, S.S.; Vinik, A.I.; Cho, K.; Thompson, N. Gastrointestinal/Pancreatic Hormone Concentrations in the Portal Venous System of Nine Patients with Organic Hyperinsulinism. Metabolism 1981, 30, 1001–1010. [Google Scholar] [CrossRef]
- Duncan, W.E.; Duncan, T.G.; DeLaurentis, D.A.; Kryston, L.; Kaminski, K.; Paskin, D.L. Artificial Pancreas as an Aid during Insulinoma Resection. Am. J. Surg. 1981, 142, 528–531. [Google Scholar] [CrossRef]
- Bonfils, S.; Landor, J.H.; Mignon, M.; Hervoir, P. Results of Surgical Management in 92 Consecutive Patients with Zollinger Ellison Syndrome. Ann. Surg. 1981, 194, 692–697. [Google Scholar] [CrossRef]
- Nathan, D.M.; Axelrod, L.; Proppe, K.H.; Wald, R.; Hirsch, H.J.; Martin, D.B. Nesidioblastosis Associated with Insulin-Mediated Hypoglycemia in an Adult. Diabetes Care 1981, 4, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Harness, J.K.; Geelhoed, G.W.; Thompson, N.W.; Nishiyama, R.H.; Fajans, S.S.; Kraft, R.O.; Howard, D.R.; Clark, K.A. Nesidioblastosis in Adults: A Surgical Dilemma. Arch. Surg. 1981, 116, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Jennette, J.C.; Wilkman, A.S.; Bagnell, C.R. Insulin Receptor Autoantibody-Induced Pancreatic Islet Beta (B) Cell Hyperplasia. Arch. Pathol. Lab. Med. 1982, 106, 218–220. [Google Scholar] [PubMed]
- Cho, K.J.; Vinik, A.I.; Thompson, N.W.; Sgields, J.J.; Porter, D.J.; Brady, T.M.; Cadavid, G.; Fajans, S.S.; Shields, J.J.; Porter, D.J.; et al. Localization of the Source of Hyperinsulinism: Percutaneous Transhepatic Portal and Pancreatic Vein Catheterization with Hormone Assay. Am. J. Radiol. 1982, 139, 237–245. [Google Scholar] [CrossRef]
- Zhu, Y.; Zeng, X.J.; Zhang, J.X.; Cai, L. Reoperation for Insulinoma and Islet Hyperplasia. Chin. Med. J. 1983, 96, 525–532. [Google Scholar]
- Oliver, M.H.; Drury, P.L.; Van’t Hoff, W. A Case of Multiple Endocrine Adenomatosis (Type 1) with Nesidioblastosis, Terminating with an Exocrine Pancreatic Carcinoma. Clin. Endocrinol. 1983, 18, 495–503. [Google Scholar] [CrossRef]
- Keller, A.; Stone, A.M.; Valderrama, E.; Kolodny, H. Pancreatic Nesidioblastosis in Adults. Report of a Patient with Hyperinsulinemic Hypoglycemia. Am. J. Surg. 1983, 145, 412–416. [Google Scholar] [CrossRef]
- Rayman, G.; Santo, M.; Salomon, F.; Almog, S.; Paradinas, F.J.; Pinkhas, J.; Reynolds, K.W.; Wise, P.H. Hyperinsulinaemic Hypoglycaemia Due to Chlorpropamide-Induced Nesidioblastosis. J. Clin. Pathol. 1984, 37, 651–654. [Google Scholar] [CrossRef]
- Bauman, W.; Merkle, L.; Rachman, R.; Mitsuto, S. Hypoglycemia in a Diabetes Nurse Care Coordinator. Diabetes Care 1984, 7, 88–91. [Google Scholar] [CrossRef]
- Harrison, T.S.; Fajans, S.S.; Floyd, J.C.; Thompson, N.W.; Rasbach, D.A.; Santen, R.J.; Cohen, C. Prevalence of Diffuse Pancreatic Beta Islet Cell Disease with Hyperinsulinism: Problems in Recognition and Management. World J. Surg. 1984, 8, 583–587. [Google Scholar] [CrossRef]
- Tomita, T.; Kimmel, J.R.; Friesen, S.R.; Doull, V.; Pollock, H.G. Pancreatic Polypeptide in Islet Cell Tumors. Morphologic and Functional Correlations. Cancer 1985, 56, 1649–1657. [Google Scholar] [CrossRef] [PubMed]
- Bani, D.; Bani Sacchi, T.; Biliotti, G. Nesidioblastosis and Intermediate Cells in the Pancreas of Patients with Hyperinsulinemic Hypoglycemia. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1985, 48, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Bani Sacchi, T.; Bani, D.; Biliotti, G. Nesidioblastosis and Islet Cell Changes Related to Endogenous Hypergastrinemia. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1985, 48, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Campbell, I.L.; Harrison, L.C.; Ley, C.J.; Colman, P.G.; Ellis, D.W. Nesidioblastosis and Multifocal Pancreatic Islet Cell Hyperplasia in an Adult. Clinicopathologic Features and in Vitro Pancreatic Studies. Am. J. Clin. Pathol. 1985, 84, 534–541. [Google Scholar] [CrossRef]
- Madeira, M.D.; Reis, L.; Medina, J.L.; Sambade, C.; Carneiro, F.; de Oliveira, C. Nesidioblastose e Insulinoma. Uma Associação Pouco Frequente. Acta Med. Port. 1986, 7, 165–170. [Google Scholar]
- Illyés, G.; Bálint, A.; Kiss, S. Nesidioblastosis in an Adult. Orv. Hetil. 1986, 127, 2633–2636. [Google Scholar]
- Klöppel, G.; Willemer, S.; Stamm, B.; Häcki, W.H.; Heitz, P.U. Pancreatic Lesions and Hormonal Profile of Pancreatic Tumors in Multiple Endocrine Neoplasia Type I. An Immunocytochemical Study of Nine Patients. Cancer 1986, 57, 1824–1832. [Google Scholar] [CrossRef]
- Ray, M.B.; Zumwalt, R. Islet-Cell Hyperplasia in Genetic Deficiency of Alpha-1-Proteinase Inhibitor. Am. J. Clin. Pathol. 1986, 85, 681–687. [Google Scholar] [CrossRef]
- Kovacs, K.; Horvath, E.; Asa, S.L.; Murray, D.; Singer, W.; Reddy, S.S. Microscopic Peliosis of Pancreatic Islets in a Woman with MEN-1 Syndrome. Arch. Pathol. Lab. Med. 1986, 110, 607–610. [Google Scholar]
- Odaira, C.; Choux, R.; Payan, M.J.; Bockman, D.E.; Sarles, H. Chronic Obstructive Pancreatitis, Nesidioblastosis, and Small Endocrine Pancreatic Tumor. Dig. Dis. Sci. 1987, 32, 770–774. [Google Scholar] [CrossRef]
- Jerkins, T.W.; Sacks, H.S.; O’dorisio, T.M.; Tuttle, S.; Solomon, S.S. Medullary Carcinoma of the Thyroid, Pancreatic Nesidioblastosis and Microadenosis, and Pancreatic Polypeptide Hypersecretion: A New Association and Clinical and Hormonal Responses to Long-Acting Somatostatin Analog SMS 201-995*. J. Clin. Endocrinol. Metab. 1987, 64, 1313–1319. [Google Scholar] [CrossRef]
- Carlson, T.; Eckhauser, M.; DeBaz, B.; Khiyami, A.; Park, C. Nesidioblastosis in an Adult: An Illustrative Case and Collective Review. Am. J. Gastroenterol. 1987, 82, 566–571. [Google Scholar]
- Asa, S.L.; Singer, W.; Kovacs, K.; Horvath, E.; Murray, D.; Colapinto, N.; Thorner, M.O. Pancreatic Endocrine Tumour Producing Growth Hormone-Releasing Hormone Associated with Multiple Endocrine Neoplasia Type I Syndrome. Acta Endocrinol. 1987, 115, 331–337. [Google Scholar] [CrossRef]
- Glaser, B.; Shapiro, B.; Glowniak, J.; Fajans, S.S.; Vinik, A.I. Effects of Secretin on the Normal and Pathological β-Cell. J. Clin. Endocrinol. Metab. 1988, 66, 1138–1143. [Google Scholar] [CrossRef] [PubMed]
- Roncari, D.A.; Yoon, J.W.; Pak, C.Y.; Keane, P.M.; Preshaw, R.M. Excessive Growth of Cultured Beta Cells from an Adult Patient with Beta Cell Hyperplasia. Clin. Investig. Med. 1988, 11, 129–133. [Google Scholar]
- Kohnert, K.D.; Fält, K.; Odselius, R.; Ziegler, M.; Madsen, O.D.; Grimelius, L.; Falkmer, S. Production of Pro-Insulin, C-Peptide, and Insulin in Nesidioblastosis, Focal Islet-Cell Adenomatosis, and Genuine Insulomas. A Correlated Radioimmunochemical, Immunohistochemical, and Ultrastructural Investigation with Particular Regard to the Occurrence. Diabetes Res. 1988, 8, 151–163. [Google Scholar] [PubMed]
- Balas, D.; Senegas-Balas, F.; Delvaux, M.; Bertrand, C.; Fagot-Revurat, P.; Rumeau, J.L.; Ribet, A. Silent Human Pancreatic Glucagonoma and “a” Nesidioblastosis. Pancreas 1988, 3, 734–739. [Google Scholar] [CrossRef]
- Lamberts, S.W.J. Somatostatin Analogs in the Management of Gastrointestinal Tumors. Horm. Res. Paediatr. 1988, 29, 118–120. [Google Scholar] [CrossRef]
- Woltering, E.A.; Mozell, E.J.; O’Dorisio, T.M.; Fletcher, W.S.; Howe, B. Suppression of Primary and Secondary Peptides with Somatostatin Analog in the Therapy of Functional Endocrine Tumors. Surg. Gynecol. Obstet. 1988, 167, 453–462. [Google Scholar]
- Derizhanova, I.S. Clinico-Anatomical Comparisons in Duodenal Carcinoid Tumors. Arkh. Patol. 1989, 51, 53–58. [Google Scholar]
- McHenry, C.; Newell, K.; Chejfec, G.; Barbato, A.; Lawrence, A.M.; Brooks, M.; Emanuele, M.; Paloyan, E. Adult Nesidioblastosis. An Unusual Cause of Fasting Hypoglycemia. Am. Surg. 1989, 55, 366–369. [Google Scholar] [PubMed]
- Sawady, J.; Mendelsohn, G. Extrapancreatic Gastrinoma with Pancreatic Islet Cell Hyperplasia. Arch. Pathol. Lab. Med. 1989, 113, 536–538. [Google Scholar] [PubMed]
- Risaliti, A.; Pizzolitto, S. Nesidioblastosis Arising from Heterotopic Pancreas and Presenting with Hypertension. A Clinical, Immunohistochemical and Ultrastructural Study. Ann. Chir. 1989, 43, 459–464. [Google Scholar] [PubMed]
- Chines, A.; Fogelfeld, L.; Zaidel, L.; Feigl, D. Nesidioblastosis in Adults. Isr. J. Med. Sci. 1989, 25, 450–453. [Google Scholar] [PubMed]
- Albers, N.; Lohr, M.; Bogner, U.; Loy, V.; Kloppel, G.; Klöppel, G.; Kloppel, G. Nesidioblastosis of the Pancreas in an Adult with Persistent Hyperinsulinemic Hypoglycemia. Am. J. Clin. Pathol. 1989, 91, 336–340. [Google Scholar] [CrossRef]
- Fong, T.L.; Warner, N.E.; Kumar, D. Pancreatic Nesidioblastosis in Adults. Diabetes Care 1989, 12, 108–114. [Google Scholar] [CrossRef]
- Martínez Valls, J.F.; Ascaso, J.F.; Ferrández, A.; Hernández, A.; González Bayo, E.; Carmena, R. Hyperplasia of the Pancreatic Islets or Nesidioblastosis in Adults? Apropos 2 Cases. Med. Clin. 1990, 95, 341–343. [Google Scholar]
- Mozell, E.J.; Woltering, E.A.; O’Dorisio, T.M.; Phillipson, B.E.; Fletcher, J.; Fletcher, W.S.; Howe, B.; Hill, D.; Rhea, D. Adult Onset Nesidioblastosis: Response of Glucose, Insulin, and Secondary Peptides to Therapy with Sandostatin. Am. J. Gastroenterol. 1990, 85, 181–188. [Google Scholar]
- Alhindawi, R.; Deitel, M.; Kempston, J.; Bendago, M. Severe Secretory Diarrhea with Elevated Gastrin-Releasing Peptide Controlled by Somatostatin Analogue: A Case Report. Can. J. Surg. 1990, 33, 139–142. [Google Scholar]
- Kohnert, K.; Fält, K.; Rosolski, T.; Ziegler, M.; Warzok, R.; Weirich, J.; Falkmer, S. Argyrophil and Beta-Endorphin Immunoreactive Cells in Focal Islet-Cell Adenomatosis and Insulin-Producing Islet-Cell Adenomata. Acta Histochem. 1990, 89, 57–60. [Google Scholar] [CrossRef]
- Bani, D.; Biliotti, G.; Sacchi, T.B. Morphological Changes in the Human Endocrine Pancreas Induced by Chronic Excess of Endogenous Glucagon. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1991, 60, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Kimura, W.; Kuroda, A.; Morioka, Y. Clinical Pathology of Endocrine Tumors of the Pancreas. Analysis of Autopsy Cases. Dig. Dis. Sci. 1991, 36, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Böttger, T.; Gabbert, H.; Beyer, J.; Schweden, F.; Junginger, T. Nesidioblastosis and Adenomatosis of the Pancreas as an Unusual Cause of Organic Hyperinsulinism. Chirurg 1992, 63, 139–142. [Google Scholar] [PubMed]
- Burman, W.J.; McDermott, M.T.; Bornemann, M. Familial Hyperinsulinism Presenting in Adults. Arch. Intern. Med. 1992, 152, 2125–2127. [Google Scholar] [CrossRef]
- Andrews, R.; Balsitis, M.; Shurrock, K.; Jeffcoate, W.J. Nesidioblastosis in Adults. Postgrad. Med. J. 1992, 68, 390. [Google Scholar] [CrossRef]
- Tibaldi, J.M.; Lorber, D.; Lomasky, S.; Steinberg, J.J.; Reisman, R.; Shamoon, H. Postprandial Hypoglycemia in Islet Beta Cell Hyperplasia with Adenomatosis of the Pancreas. J. Surg. Oncol. 1992, 50, 53–57. [Google Scholar] [CrossRef]
- Gaulier, A.; Cahen, J.; Buisson, J.L.; Périé, G.; Vacher, G.; Poulet, B. Pancreatic Insulinoma, Adenomatosis of the Wirsung’s Duct and Chronic Pancreatitis. Apropos of a Case. Arch. Anat. Cytol. Pathol. 1993, 41, 245–250. [Google Scholar]
- Weinand, J.; Kemp, W. Pancreatic Islet Hyperplasia: A Potential Marker for Anabolic-Androgenic Steroid Use. Acad. Forensic Pathol. 2018, 8, 777–785. [Google Scholar] [CrossRef]
- Weir, G.C.; Bonner-Weir, S. Islet β Cell Mass in Diabetes and How It Relates to Function, Birth, and Death. Ann. N. Y. Acad. Sci. 2013, 1281, 92–105. [Google Scholar] [CrossRef]
- Meckler, K.; Brentnall, T.; Haggitt, R.; Crispin, D.; Byrd, D.; Kimmey, M.; Bronner, M. Familial Fibrocystic Pancreatic Atrophy with Endocrine Cell Hyperplasia and Pancreatic Carcinoma. Am. J. Surg. Pathol. 2001, 25, 1047–1053. [Google Scholar] [CrossRef]
- Iannucci, A.; Mukai, K.; Johnson, D.; Burke, B. Endocrine Pancreas in Cystic Fibrosis: An Immunohistochemical Study. Hum. Pathol. 1984, 15, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, E.; Schmid, C.; Mortara, G. Anatomopathologic Pictures of Chronic Pancreatitis. Pathologica 1990, 82, 687–693. [Google Scholar] [PubMed]
- Christ, E.; Antwi, K.; Fani, M.; Wild, D. Innovative Imaging of Insulinoma: The End of Sampling? A Review. Endocr. Relat. Cancer 2020, 27, R79–R92. [Google Scholar] [CrossRef]
- Pattison, D.A.; Hicks, R.J. Molecular Imaging in the Investigation of Hypoglycaemic Syndromes and Their Management. Endocr. Relat. Cancer 2017, 24, R203–R221. [Google Scholar] [CrossRef] [PubMed]
- Harris, S. Hyperinsulinism and Dysinsulinism. JAMA 1924, 83, 729–733. [Google Scholar] [CrossRef]
- Wilder, R.; Allan, F.; Power, M.; Robertson, H. Carcinoma of the Islands of the Pancreas: Hyperinsulinism and Hypoglycemia. J. Am. Med. Assoc. 1927, 89, 348–355. [Google Scholar] [CrossRef]
- Bartow, S.A.; Mukai, K.; Rosai, J. Pseudoneoplastic Proliferation of Endocrine Cells in Pancreatic Fibrosis. Cancer 1981, 47, 2627–2633. [Google Scholar] [CrossRef]
- Goudswaard, W.B.; Houthoff, H.J.; Koudstaal, J.; Zwierstra, R.P. Nesidioblastosis and Endocrine Hyperplasia of the Pancreas: A Secondary Phenomenon. Hum. Pathol. 1986, 17, 46–54. [Google Scholar] [CrossRef]
- Hirshberg, B.; Libutti, S.K.; Alexander, H.R.; Bartlett, D.L.; Cochran, C.; Livi, A.; Chang, R.; Shawker, T.; Skarulis, M.C.; Gorden, P. Blind Distal Pancreatectomy for Occult Insulinoma, an Inadvisable Procedure. J. Am. Coll. Surg. 2002, 194, 761–764. [Google Scholar] [CrossRef]
- Yakovac, W.C.; Baker, L.; Hummeler, K. Beta Cell Nesidioblastosis in Idiopathic Hypoglycemia of Infancy. J. Pediatr. 1971, 79, 226–231. [Google Scholar] [CrossRef]
- Bouwens, L.; Pipeleers, D.G. Extra-Insular Beta Cells Associated with Ductules Are Frequent in Adult Human Pancreas. Diabetologia 1998, 41, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Ariel, I.; Kerem, E.; Bartfeld, E. Nesidiodysplasia A Histologic Entity? Hum. Pathol. 1988, 19, 1215–1218. [Google Scholar] [CrossRef] [PubMed]
- Gilbert-Barness, E.; Potter, E.L. Potter’s Pathology of the Fetus and Infant; Yearbook Publisher: Chicago, IL, USA, 1997; p. 54. [Google Scholar]
- Jiang, C.X.; Tan, Y.B. Morphogenetic Study on Hyperplastic and Neoplastic Diseases of Endocrine Pancreas. Zhonghua Bing Li Xue Za Zhi = Chin. J. Pathol. 1990, 19, 175–178. [Google Scholar]
- Yu, R. Pancreatic α-Cell Hyperplasia: Facts and Myths. J. Clin. Endocrinol. Metab. 2014, 99, 748–756. [Google Scholar] [CrossRef]
- Zhou, C.; Dhall, D.; Nissen, N.N.; Chen, C.R.; Yu, R. Homozygous P86s Mutation of the Human Glucagon Receptor Is Associated with Hyperglucagonemia, α Cell Hyperplasia, and Islet Cell Tumor. Pancreas 2009, 38, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Fathia, G.; Michael, S.; Andrea, P.; Jensen, R.T. Multiple Endocrine Neoplasia Type 1 and Zollinger-Ellison Syndrome: A Prospective Study of 107 Cases and Comparison with 1009 Cases from the Literature. Medicine 2004, 83, 43–83. [Google Scholar] [CrossRef]
- Meier, J.J.; Butler, A.E.; Galasso, R.; Rizza, R.A.; Butler, P.C. Increased Islet Beta Cell Replication Adjacent to Intrapancreatic Gastrinomas in Humans. Diabetologia 2006, 49, 2689–2696. [Google Scholar] [CrossRef]
- Rosenberg, L.; Brown, R.A.; Duguid, W.P. A New Approach to the Induction of Duct Epithelial Hyperplasia and Nesidioblastosis by Cellophane Wrapping of the Hamster Pancreas. J. Surg. Res. 1983, 35, 63–72. [Google Scholar] [CrossRef]
- Rosenberg, L.; Duguid, W.; Brown, R.; Vinik, A. Induction of Nesidioblastosis Will Reverse Diabetes in Syrian Golden Hamster. Diabetes 1988, 37, 334–341. [Google Scholar] [CrossRef]
- Donev, S.; Petkov, P.; Zhablenska, R.; Katala, Z.; Bonafus, R.; Fisho, F.; Desbal, B. Morphological Changes in the Pancreas after Ligation of the Excretory Duct. Eksp. Med. Morfol. 1994, 32, 9–18. [Google Scholar]
- Meissner, H. The Islands of Langerhans in Cystic Pancreatic Fibrosis and Morphologically Related Pancreatic Conditions. Beitr. Pathol. Anat. 1954, 114, 192–211. [Google Scholar]
- Soejima, K.; Landing, B. Pancreatic Islets in Older Patients with Cystic Fibrosis with and without Diabetes Mellitus: Morphometric and Immunocytologic Studies. Pediatr. Pathol. 1986, 6, 25–46. [Google Scholar] [CrossRef] [PubMed]
- Larghi, A.; Stobinski, M.; Galasso, D.; Lecca, P.G.; Costamagna, G. Concomitant Intraductal Papillary Mucinous Neoplasm and Pancreatic Endocrine Tumour: Report of Two Cases and Review of the Literature. Dig. Liver Dis. 2009, 41, 759–761. [Google Scholar] [CrossRef] [PubMed]
- Palladino, A.A.; Stanley, C.A. Nesidioblastosis No Longer! It’s All about Genetics. J. Clin. Endocrinol. Metab. 2011, 96, 617–619. [Google Scholar] [CrossRef]
- Rosenfeld, E.; Ganguly, A.; De Leon, D.D. Congenital Hyperinsulinism Disorders: Genetic and Clinical Characteristics. Am. J. Med. Genet. Part C Semin. Med. Genet. 2019, 181, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Gilis-Januszewska, A.; Piatkowski, J.; Skalniak, A.; Piwonska-Solska, B.; Nazim, J.; Pach, D.; Przybylik-Mazurek, E.; Sowa-Staszczak, A.; Starzyk, J.; Hubalewska-Dydejczyk, A. Noninsulinoma Pancreatogenous Hypoglycaemia in Adults—A Spotlight on Its Genetics. Endokrynol. Pol. 2015, 66, 344–354. [Google Scholar] [CrossRef]
- Dunger, D.B.; Burns, C.; Ghale, G.K.; Muller, D.P.R.; Spitz, L.; Grant, D.B. Pancreatic Exocrine and Endocrine Function after Subtotal Pancreatectomy for Nesidioblastosis. J. Pediatr. Surg. 1988, 23, 112–115. [Google Scholar] [CrossRef]
- Rother, K.I.; Carney, J.A.; Couce, M.; Charlesworth, J.; Butler, P.C. Islet Amyloid Polypeptide in Pancreatic Tissue of Children with Persistent Hyperinsulinemic Hypoglycemia Caused by Primary Islet Hyperplasia and Nesidioblastosis. J. Clin. Endocrinol. Metab. 1995, 80, 1956–1959. [Google Scholar] [CrossRef]
- Leibowitz, G.; Glaser, B.; Higazi, A.A.; Salameh, M.; Cerasi, E.; Landau, H. Hyperinsulinemic Hypoglycemia of Infancy (Nesidioblastosis) in Clinical Remission: High Incidence of Diabetes Mellitus and Persistent Beta-Cell Dysfunction at Long-Term Follow-Up. J. Clin. Endocrinol. Metab. 1995, 80, 386–392. [Google Scholar] [CrossRef]
- Dacou-Voutetakis, C.; Psychou, F.; Maniati-Christidis, M. Persistent Hyperinsulinemic Hypoglycemia of Infancy: Long-Term Results. J. Pediatr. Endocrinol. Metab. 1998, 11 (Suppl. 1), 131–141. [Google Scholar] [CrossRef]
- Martinez-Ibanez, V.; Gussinyer, M.; Toran, N.; Lloret, J.; Abad, P.; Carrascosa, A.; Boix-Ochoa, J. Pancreatectomy Extension in Persistent Hyperinsulinaemic Hypoglycaemia: A New Strategy. Eur. J. Pediatr. Surg. 2002, 12, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Bin-Abbas, B.S.; Al-Ashwal, A.A. Diabetes in a Nonpancreatectomized Child with Nesidioblastosis. Diabetes Care 2004, 27, 626–627. [Google Scholar] [CrossRef] [PubMed]
- Restrepo, K.; Garavito, G.; Rojas, L.; Romero, A.; Neira, F.; Oliveros, R.; Abisambra, N. Nesidioblastosis Del Adulto Coexistente Con Insulinoma. Rev. Colomb. Cancerol. 2009, 13, 49–60. [Google Scholar] [CrossRef]
- De Jesús, J.; Fung, L.; Garcia, F.; Núñez, M. Nesidioblastosis En Adolescentes: A Propósito de Un Caso. Rev. Venez. Endocrinol. Metab. 2015, 13, 48–53. [Google Scholar]
- Glaser, B.; Kesavan, P.; Heyman, M.; Davis, E.; Cuesta, A.; Buchs, A.; Stanley, C.A.; Thornton, P.S.; Permutt, M.A.; Matschinsky, F.M.; et al. Familial Hyperinsulinism Caused by an Activating Glucokinase Mutation. N. Engl. J. Med. 1998, 338, 226–230. [Google Scholar] [CrossRef]
- Christesen, H.B.T.; Jacobsen, B.B.; Odili, S.; Buettger, C.; Cuesta-Munoz, A.; Hansen, T.; Brusgaard, K.; Massa, O.; Magnuson, M.A.; Shiota, C.; et al. The Second Activating Glucokinase Mutation (A456V): Implications for Glucose Homeostasis and Diabetes Therapy. Diabetes 2002, 51, 1240–1246. [Google Scholar] [CrossRef]
- Gloyn, A.L.; Noordam, K.; Willemsen, M.A.A.P.; Ellard, S.; Lam, W.W.K.; Campbell, I.W.; Midgley, P.; Shiota, C.; Buettger, C.; Magnuson, M.A.; et al. Insights into the Biochemical and Genetic Basis of Glucokinase Activation from Naturally Occurring Hypoglycemia Mutations. Diabetes 2003, 52, 2433–2440. [Google Scholar] [CrossRef]
- Heredia, V.V.; Carlson, T.J.; Garcia, E.; Sun, S. Biochemical Basis of Glucokinase Activation and the Regulation by Glucokinase Regulatory Protein in Naturally Occurring Mutations. J. Biol. Chem. 2006, 281, 40201–40207. [Google Scholar] [CrossRef]
- Gersing, S.; Cagiada, M.; Gebbia, M.; Gjesing, A.P. A Comprehensive Map of Human Glucokinase Variant Activity. Genome Biol. 2022, 24, 97. [Google Scholar] [CrossRef]
- Christesen, H.B.T.; Tribble, N.D.; Molven, A.; Siddiqui, J.; Sandal, T.; Brusgaard, K.; Ellard, S.; Njølstad, P.R.; Alm, J.; Jacobsen, B.B.; et al. Activating Glucokinase (GCK) Mutations as a Cause of Medically Responsive Congenital Hyperinsulinism: Prevalence in Children and Characterisation of a Novel GCK Mutation. Eur. J. Endocrinol. 2008, 159, 27–34. [Google Scholar] [CrossRef]
- Labriola, L.; Peters, M.G.; Krogh, K.; Stigliano, I.; Terra, L.F.; Buchanan, C.; Machado, M.C.C.; Bal de Kier Joffé, E.; Puricelli, L.; Sogayar, M.C. Generation and Characterization of Human Insulin-Releasing Cell Lines. BMC Cell Biol. 2009, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Götz, W.; Schucht, C.; Roth, J.; Theuring, F.; Herken, R. Endocrine Pancreatic Tumors in MSV-SV40 Large T Transgenic Mice. Am. J. Pathol. 1993, 142, 1493–1503. [Google Scholar] [PubMed]
- Gacar, A.; Pekmezci, D.; Karayigit, M.O.; Kabak, Y.B.; Gulbahar, M.Y. Nesidioblastosis in a Simmental Calf. J. Comp. Pathol. 2012, 147, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Son, W.C.; Faki, K.; Mowat, V.; Gopinath, C. Spontaneously Occurring Extra-Islet Endocrine Cell Proliferation in the Pancreas of Young Beagle Dogs. Toxicol. Lett. 2010, 193, 179–182. [Google Scholar] [CrossRef]
- Polansky, B.J.; Martinez, S.A.; Chalkley, M.D. Resolution of Hyperinsulinemic Hypoglycemia Following Partial Pancreatectomy in a Dog with Nesidioblastosis. J. Am. Vet. Med. Assoc. 2018, 253, 893–896. [Google Scholar] [CrossRef]
- Hawkins, K.L.; Summers, B.A.; Kuhajda, F.P.; Smith, C.A. Immunocytochemistry of Normal Pancreatic Islets and Spontaneous Islet Cell Tumors in Dogs. Vet. Pathol. 1987, 24, 170–179. [Google Scholar] [CrossRef]
- King, C.; Streett, J.; Moody, K.; Barthold, S. Nesidioblastosis Associated with Hyperglycemia in Two Squirrel Monkeys (Saimiri sciureus). J. Med. Primatol. 1996, 25, 361–366. [Google Scholar] [CrossRef]
- Hambrook, L.E.; Ciavarella, A.A.; Nimmo, J.S.; Wayne, J. Hyperinsulinaemic, Hypoglycaemic Syndrome Due to Acquired Nesidioblastosis in a Cat. J. Feline Med. Surg. Open Rep. 2016, 2, 205511691665784. [Google Scholar] [CrossRef]
- Furuoka, H.; Shirakawa, T.; Taniyama, H.; Ohishi, H.; Satoh, H.; Itakura, C. Histogenesis of Neoformation in the Endocrine Pancreas of Aging Horses. Vet. Pathol. 1989, 26, 40–46. [Google Scholar] [CrossRef]
- Novaes, G.; Chetto-de-Queiroz, A.; De-Carvalho-Cardoso, C.; Burlacchini-de-Carvalho, M.; Ribeiro-Filho, L.; Oliveira-Gonçalves, A. Nesidioblastosis Associated with Chronic Experimental Pancreatitis Produced by a Scorpion Toxin, Tityustoxin, in Rats. Braz. J. Med. Biol. Res. Rev. Bras. Pesqui. Med. Biol. 1990, 23, 1149–1151. [Google Scholar]
- Grube, D.; Jörns, A. The Endocrine Pancreas of Glucagon-and Somatostatin-Immunized Rabbits. I. Light Microscopy and Immunohistochemistry. Cell Tissue Res. 1991, 265, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, L.; AI, V. Induction of Endocrine Cell Differentiation: A New Approach to Management of Diabetes. J. Lab. Clin. Med. 1989, 114, 75–83. [Google Scholar] [PubMed]
- Simon, B.; Merchant, J.L.; Eissele, R.; Köhler, K.; Arnold, R. Transient Transcriptional Activation of Gastrin during Sodium Butyrate-Induced Differentiation of Islet Cells. Regul. Pept. 1997, 70, 143–148. [Google Scholar] [CrossRef]
- William Traverso, L.; Bockman, D.E.; Pleis, S.K.; Dail, D.H. Nesidioblastosis as a Mechanism to Prevent Fibrosis-Induced Diabetes after Pancreatic Duct Obstruction. Pancreas 1993, 8, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Gerbitz, K.; Boenke, B.; Paprotta, A.; Chemnitz, U.; Hübner, G. Human Nesidioblastosis Tissue as an Immunogen for Generation of Islet Cell Specific Monoclonal Antibodies. J. Clin. Chem. Clin. Biochem. 1988, 26, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, L.; Dafoe, D.; Turcotte, J. Enhancing Hamster Pancreatic Islet Isolation by Induction of Nesidioblastosis. Transplant. Proc. 1987, 19, 907–908. [Google Scholar]
- Kerr-Conte, J.; Pattou, F.; Lecomte-Houcke, M.; Xia, Y.; Boilly, B.; Proye, C.; Lefebvre, J. Ductal Cyst Formation in Collagen-Embedded Adult Human Islet Preparations. A Means to the Reproduction of Nesidioblastosis in Vitro. Diabetes 1996, 45, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Papaccio, G.; Esposito, V.; Mezzogiorno, V. Islet B Cell Neoproliferation in Early Low Dose Streptozocin Induced Diabetes in Mice: A Ducto-Endocrine Proliferation? Acta Morphol. Hung. 1991, 39, 43–52. [Google Scholar] [PubMed]
- Matsushita, K.; Okita, H.; Suzuki, A.; Shimoda, K.; Fukuma, M.; Yamada, T.; Urano, F.; Honda, T.; Sano, M.; Iwanaga, S.; et al. Islet Cell Hyperplasia in Transgenic Mice Overexpressing EAT/Mcl-1, a Bcl-2 Related Gene. Mol. Cell Endocrinol. 2003, 203, 105–116. [Google Scholar] [CrossRef]
- Pour, P.M.; Kazakoff, K. Stimulation of Islet Cell Proliferation Enhances Pancreatic Ductal Carcinogenesis in the Hamster Model. Am. J. Pathol. 1996, 149, 1017–1025. [Google Scholar]
- Pour, P. Islet Cells as a Component of Pancreatic Ductal Neoplasms. Am. J. Pathol. 1978, 90, 295–316. [Google Scholar] [PubMed]
- Luca, G.; Calvitti, M.; Basta, G.; Baroni, T.; Neri, L.M.; Becchetti, E.; Capitani, S.; Novaes, G.; Correa-Giannella, M.L.; Kalapothakis, E.; et al. Mitogenic Effects of Brazilian Arthropod Venom on Isolated Islet Beta Cells: In Vitro Morphologic Ultrastructural and Functional Studies. J. Investig. Med. 2003, 51, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Sandhyamani, S.; Vijayakumari, A.; Balaraman Nair, M. Bonnet Monkey Model for Pancreatic Changes in Induced Malnutrition. Pancreas 1999, 18, 84–95. [Google Scholar] [CrossRef]
- Rosenberg, L.; Clas, D.; Duguid, W. Trophic Stimulation of the Ductal/Islet Cell Axis: A New Approach to the Treatment of Diabetes. Surgery 1990, 108, 191–197. [Google Scholar]
- Novaes, G.; Cardozo Cde, C.; Costa, N.; de Falco, C.; de Carvalho, M.; de Queiroz, A. Experimental Chronic Interstitial Pancreatitis Induced by Scorpion Toxin in Rats. Arq. Gastroenterol. 1990, 27, 187–190. [Google Scholar]
- Young, M.T.; Phelan, M.J.; Nguyen, N.T. A Decade Analysis of Trends and Outcomes of Male vs Female Patients Who Underwent Bariatric Surgery. J. Am. Coll. Surg. 2016, 222, 226–231. [Google Scholar] [CrossRef]
- Michaels, A.D.; Hunter Mehaffey, J.; Brenton French, W.; Schirmer, B.D.; Kirby, J.L.; Hallowell, P.T. Hypoglycemia Following Bariatric Surgery: Our 31-Year Experience. Obes. Surg. 2017, 27, 3118–3123. [Google Scholar] [CrossRef]
- Singh, E.; Vella, A. Hypoglycemia after Gastric Bypass Surgery. Diabetes Spectr. 2012, 25, 217–221. [Google Scholar] [CrossRef]
- Berger, C.; Zdzieblo, D. Glucose Transporters in Pancreatic Islets. Pflug. Arch. Eur. J. Physiol. 2020, 472, 1249–1272. [Google Scholar] [CrossRef]
- Yan, H.; Yang, W.; Zhou, F.; Li, X.; Pan, Q.; Shen, Z.; Han, G.; Newell-Fugate, A.; Tian, Y.; Majeti, R.; et al. Estrogen Improves Insulin Sensitivity and Suppresses Gluconeogenesis via the Transcription Factor Foxo1. Diabetes 2019, 68, 291–304. [Google Scholar] [CrossRef]
- De Paoli, M.; Zakharia, A.; Werstuck, G.H. The Role of Estrogen in Insulin Resistance: A Review of Clinical and Preclinical Data. Am. J. Pathol. 2021, 191, 1490–1498. [Google Scholar] [CrossRef] [PubMed]
- Mathur, A.; Gorden, P.; Libutti, S. Insulinoma. Surg. Clin. N. Am. 2009, 89, 1105–1121. [Google Scholar] [CrossRef] [PubMed]
- Gill, J. Pancreatitis: A Forensic Perspective. Acad. Forensic Pathol. 2016, 6, 237–248. [Google Scholar] [CrossRef]
- Harris, J.P.; Ricker, A.T.; Gray, R.S.; Steed, R.D.; Gutal, J.J.P. Reversible Hypertrophic Cardiomyopathy Associated with Nesidioblastosis. J. Pediatr. 1992, 120, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Paauw, N.D.; Stegeman, R.; de Vroede, M.A.M.J.; Termote, J.U.M.; Freund, M.W.; Breur, J.M.P.J. Neonatal Cardiac Hypertrophy: The Role of Hyperinsulinism—A Review of Literature. Eur. J. Pediatr. 2020, 179, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Kaczirek, K.; Niederle, B. Nesidioblastosis: An Old Term and a New Understanding. World J. Surg. 2004, 28, 1227–1230. [Google Scholar] [CrossRef]
- Hirshberg, B.; Livi, A.; Bartlett, D.L.; Libutti, S.K.; Alexander, H.R.; Doppman, J.L.; Skarulis, M.C.; Gorden, P. Forty-Eight-Hour Fast: The Diagnostic Test for Insulinoma. J. Clin. Endocrinol. Metab. 2000, 85, 3222–3226. [Google Scholar] [CrossRef]
- Wild, D.; Mäcke, H.; Christ, E.; Gloor, B.; Reubi, J.C. Glucagon-like Peptide 1–Receptor Scans to Localize Occult Insulinomas. N. Engl. J. Med. 2009, 359, 766–768. [Google Scholar] [CrossRef]
- Hepprich, M.; Antwi, K.; Waser, B.; Reubi, J.C.; Wild, D.; Christ, E.R. Brunner’s Gland Hyperplasia in a Patient after Roux-Y Gastric Bypass: An Important Pitfall in GLP-1 Receptor Imaging. Case Rep. Endocrinol. 2020, 2020, 4510910. [Google Scholar] [CrossRef]
- Ahluwalia, N.; Attia, R.; Green, A.; Cane, P.; Routledge, T. Doege-Potter Syndrome. Ann. R. Coll. Surg. Engl. 2015, 97, e105–e107. [Google Scholar] [CrossRef]
- Schröder, G.; Blaas, V.; Boldt, R.; Schober, H.-C. Recurrent Episodes of Hypoglycaemia with Increased Production of the Tissue Hormone Adiponectin: A Case Report. Intern. Med. Care 2019, 3, 112. [Google Scholar] [CrossRef]
- Patel, A.; Daniels, G. Hypoglycemia Secondary to Factitious Hyperinsulinism in a Foster Care Adolescent—A Case Report of Munchausen Syndrome in a Community Hospital Emergency Department Setting. BMC Emerg. Med. 2018, 18, 53. [Google Scholar] [CrossRef] [PubMed]
- Douillard, C.; Mention, K.; Dobbelaere, D.; Wemeau, J.L.; Saudubray, J.M.; Vantyghem, M.C. Hypoglycaemia Related to Inherited Metabolic Diseases in Adults. Orphanet J. Rare Dis. 2012, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Douillard, C.; Jannin, A.; Vantyghem, M.C. Rare Causes of Hypoglycemia in Adults. Ann. Endocrinol. 2020, 81, 110–117. [Google Scholar] [CrossRef]
- Kandaswamy, L.; Raghavan, R.; Pappachan, J.M. Spontaneous Hypoglycemia: Diagnostic Evaluation and Management. Endocrine 2016, 53, 47–57. [Google Scholar] [CrossRef]
- Daví, M.V.; Pia, A.; Guarnotta, V.; Pizza, G.; Colao, A.; Faggiano, A. The Treatment of Hyperinsulinemic Hypoglycaemia in Adults: An Update. J. Endocrinol. Investig. 2017, 40, 9–20. [Google Scholar] [CrossRef]
- Szymanowski, M.; Estebanez, M.S.; Padidela, R.; Han, B.; Mosinska, K.; Stevens, A.; Damaj, L.; Pihan-Le Bars, F.; Lascouts, E.; Reynaud, R.; et al. MTOR Inhibitors for the Treatment of Severe Congenital Hyperinsulinism: Perspectives on Limited Therapeutic Success. J. Clin. Endocrinol. Metab. 2016, 101, 4719–4729. [Google Scholar] [CrossRef]
- McLaughlin, T.; Peck, M.; Holst, J.; Deacon, C. Reversible Hyperinsulinemic Hypoglycemia after Gastric Bypass: A Consequence of Altered Nutrient Delivery. J. Clin. Endocrinol. Metab. 2010, 95, 1851–1855. [Google Scholar] [CrossRef]
- Salehi, M.; Gastaldelli, A.; D’Alessio, D. Blockade of Glucagon-like Peptide 1 Receptor Corrects Postprandial Hypoglycemia after Gastric Bypass. Gastroenterology 2014, 146, 669–680.e2. [Google Scholar] [CrossRef]
- Craig, C.M.; Liu, L.F.; Deacon, C.F.; Holst, J.J.; McLaughlin, T.L. Critical Role for GLP-1 in Symptomatic Post-Bariatric Hypoglycaemia. Diabetologia 2017, 60, 531–540. [Google Scholar] [CrossRef]
- Hackert, T.; Strobel, O.; Michalski, C.W.; Mihaljevic, A.L.; Mehrabi, A.; Müller-Stich, B.; Berchtold, C.; Ulrich, A.; Büchler, M.W. The TRIANGLE Operation—Radical Surgery after Neoadjuvant Treatment for Advanced Pancreatic Cancer: A Single Arm Observational Study. Hpb 2017, 19, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- Weitz, J.; Rahbari, N.; Koch, M.; Büchler, M.W. The “Artery First” Approach for Resection of Pancreatic Head Cancer. J. Am. Coll. Surg. 2010, 210, e1. [Google Scholar] [CrossRef] [PubMed]
- Boss, M.; Bos, D.L.; Frielink, C.; Sandker, G.; Bronkhorst, P.; van Lith, S.A.; Brom, M.; Buitinga, M.; Gotthardt, M. Receptor-Targeted Photodynamic Therapy of Glucagon-like Peptide 1 Receptor Positive Lesions. J. Nucl. Med. 2020, 61, 1588–1593. [Google Scholar] [CrossRef]
- Soldin, O.P.; Mattison, D.R. Sex Differences in Pharmacokinetics and Pharmacodynamics. Clin. Pharmacokinet. 2009, 48, 143–157. [Google Scholar] [CrossRef]
- Rentzeperi, E.; Pegiou, S.; Koufakis, T.; Grammatiki, M.; Kotsa, K. Sex Differences in Response to Treatment with Glucagon-like Peptide 1 Receptor Agonists: Opportunities for a Tailored Approach to Diabetes and Obesity Care. J. Pers. Med. 2022, 12, 454. [Google Scholar] [CrossRef]
- Gulde, S.; Wiedemann, T.; Schillmaier, M.; Valença, I.; Lupp, A.; Steiger, K.; Yen, H.Y.; Bäuerle, S.; Notni, J.; Luque, R.; et al. Gender-Specific Efficacy Revealed by Head-to-Head Comparison of Pasireotide and Octreotide in a Representative in Vivo Model of Nonfunctioning Pituitary Tumors. Cancers 2021, 13, 3097. [Google Scholar] [CrossRef] [PubMed]
- Gatto, F.; Barbieri, F.; Arvigo, M.; Thellung, S.; Amarù, J.; Albertelli, M.; Ferone, D.; Florio, T. Biological and Biochemical Basis of the Differential Efficacy of First and Second Generation Somatostatin Receptor Ligands in Neuroendocrine Neoplasms. Int. J. Mol. Sci. 2019, 20, 3940. [Google Scholar] [CrossRef]
- Rorsman, P.; Huising, M.O. The Somatostatin-Secreting Pancreatic δ-Cell in Health and Disease. Nat. Rev. Endocrinol. 2018, 14, 404–414. [Google Scholar] [CrossRef]
- Asahara, S.I.; Inoue, H.; Watanabe, H.; Kido, Y. Roles of MTOR in the Regulation of Pancreatic β-Cell Mass and Insulin Secretion. Biomolecules 2022, 12, 614. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).