The Role of Sphingolipids in Regulating Vascular Permeability in Idiopathic Pulmonary Fibrosis
Abstract
1. Introduction
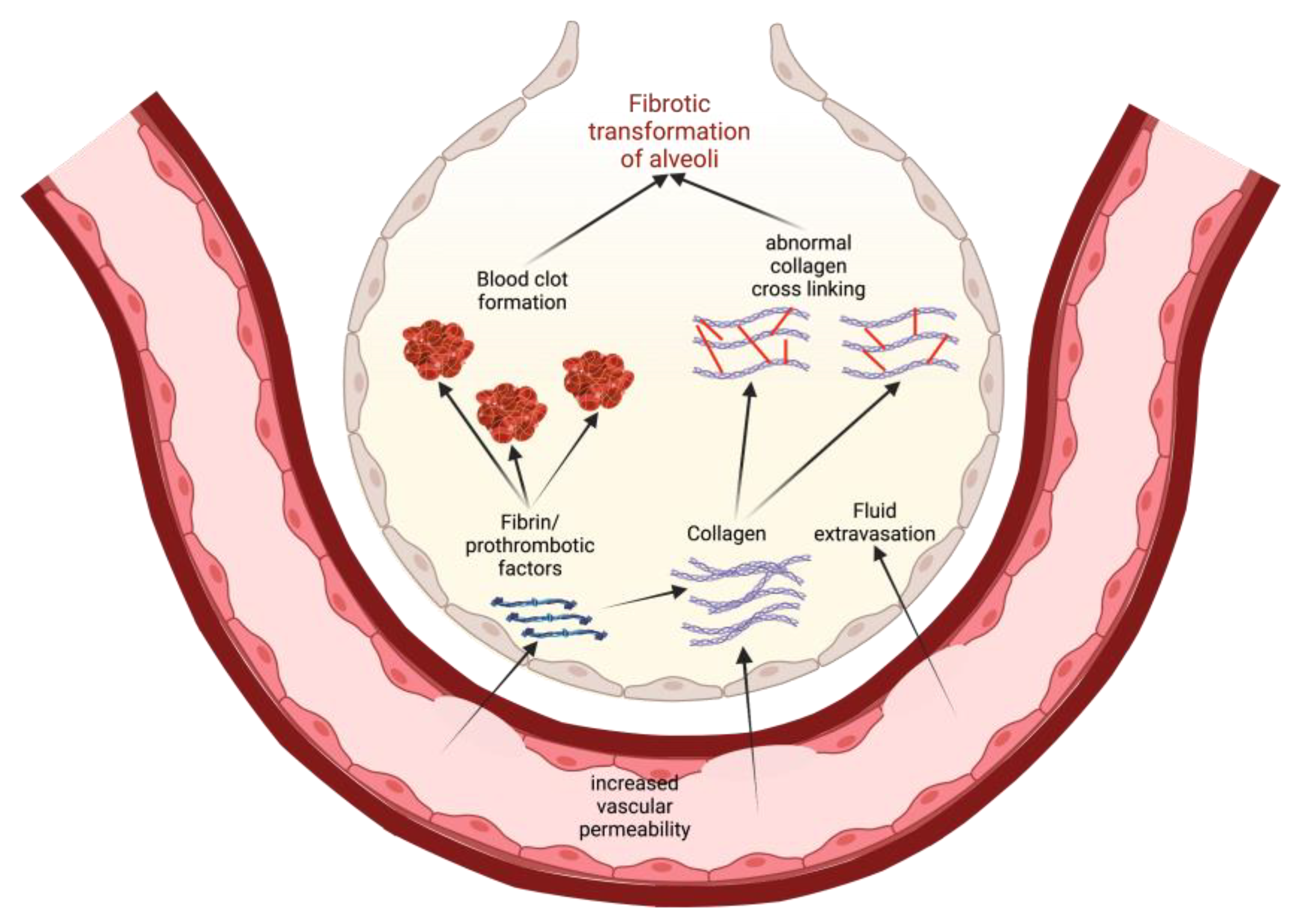
2. Models of Pathogenesis of Idiopathic Pulmonary Fibrosis
3. The Endothelial Barrier
4. The Lung Endothelium in Inflammatory States
5. Rho Kinases
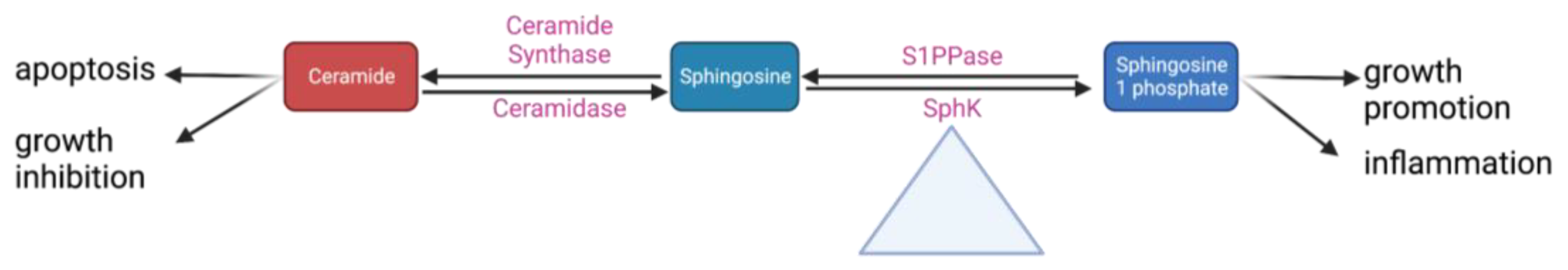
6. Sphingolipids
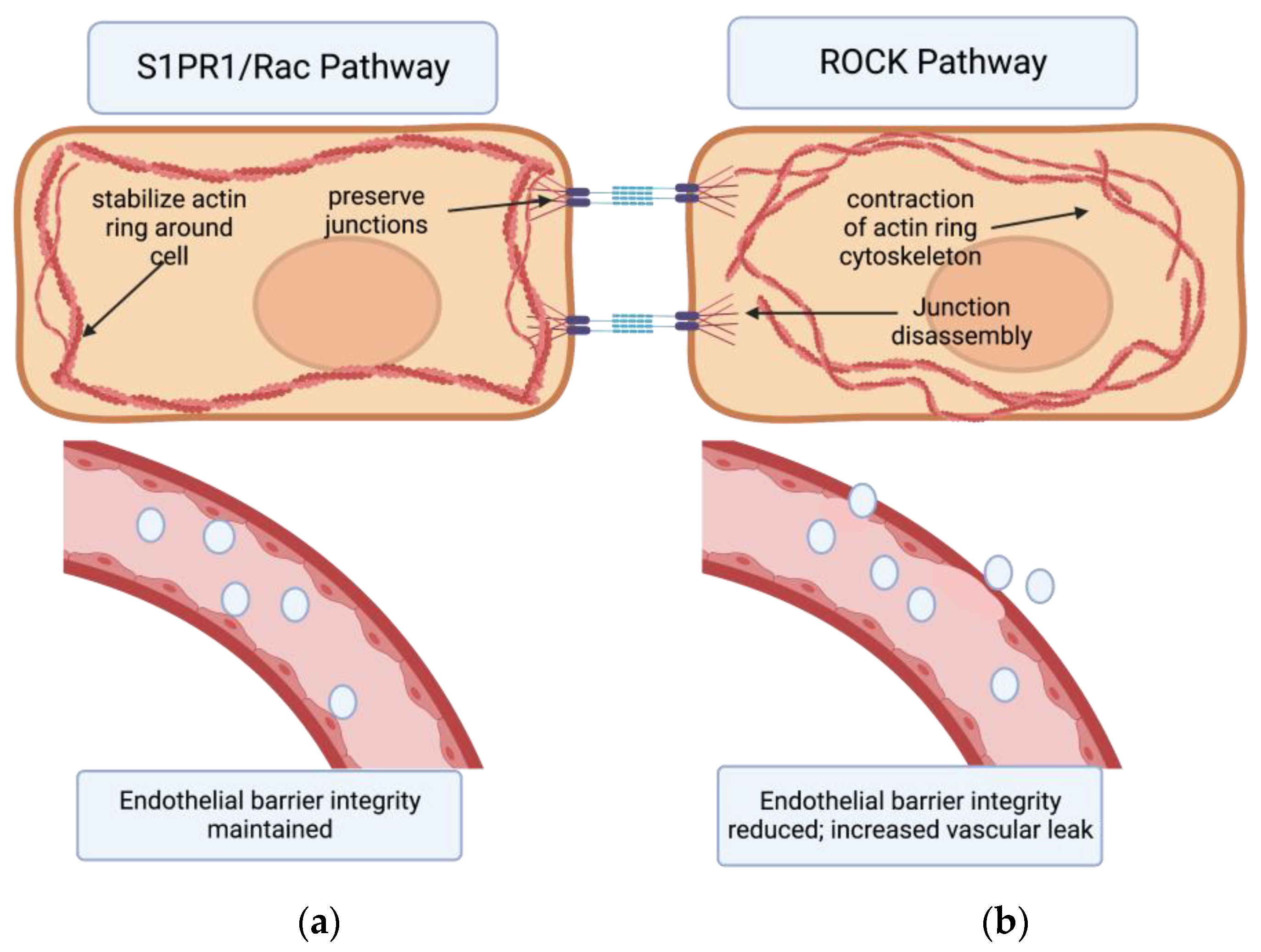
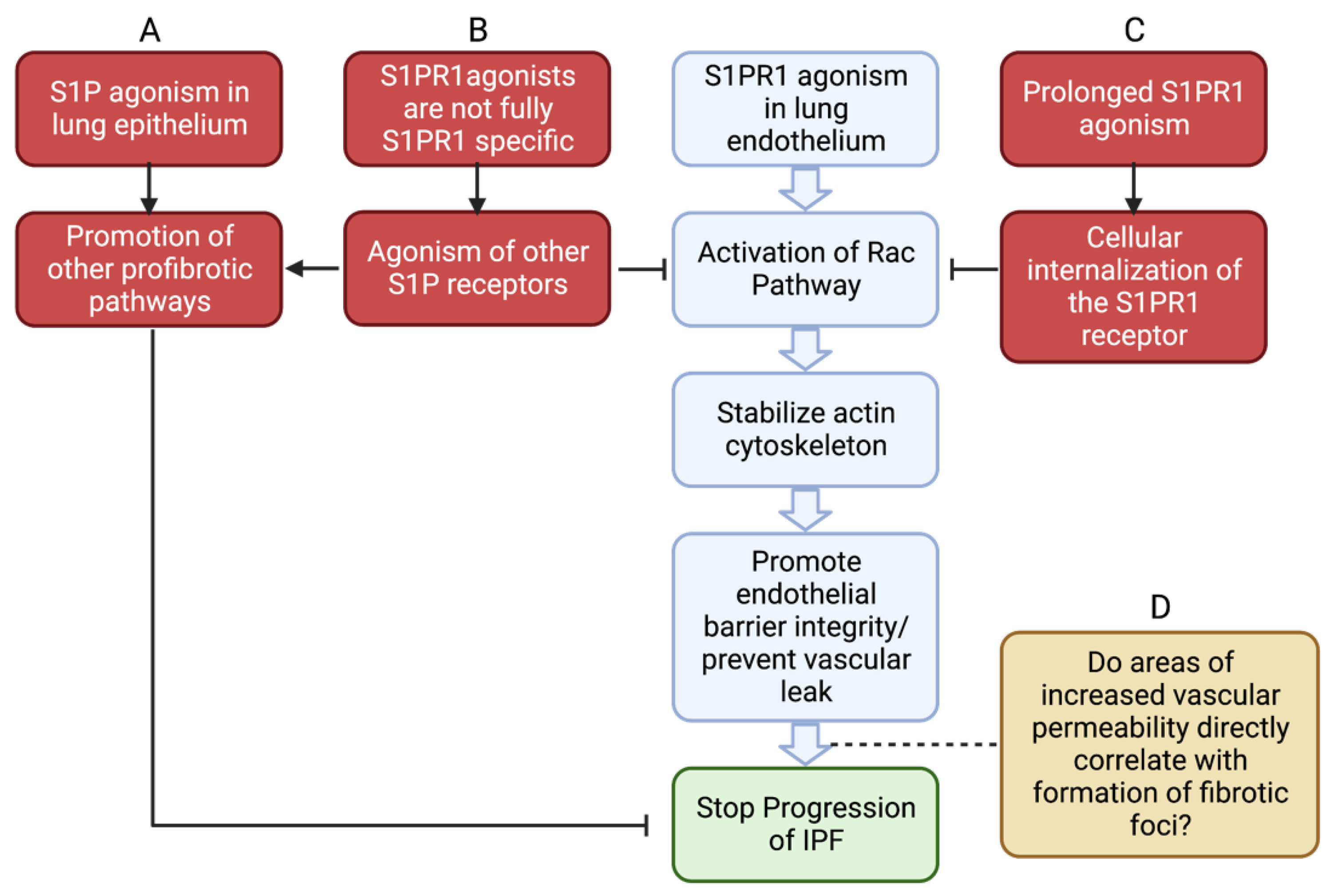
7. The Effects of S1PR1 on Vascular Permeability
8. The Effects of Other S1P Receptors and Sphingolipids on Vascular Permeability
9. S1P Modulators and Sphingolipid-Based Treatment Options
10. Angiogenesis in IPF
11. Future Study
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maher, T.M.; Bendstrup, E.; Dron, L.; Langley, J.; Smith, G.; Khalid, J.M.; Patel, H.; Kreuter, M. Global incidence and prevalence of idiopathic pulmonary fibrosis. Respir. Res. 2021, 22, 197. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Cox, I.A.; Campbell, J.A.; Xia, Q.; Otahal, P.; de Graaff, B.; Corte, T.J.; Teoh, A.K.Y.; Walters, E.H.; Palmer, A.J. Mortality and survival in idiopathic pulmonary fibrosis: A systematic review and meta-analysis. ERJ Open Res. 2022, 8, 00591–2021. [Google Scholar] [CrossRef] [PubMed]
- Nathan, S.D.; Albera, C.; Bradford, W.Z.; Costabel, U.; Glaspole, I.; Glassberg, M.K.; Kardatzke, D.R.; Daigl, M.; Kirchgaessler, K.-U.; Lancaster, L.H.; et al. Effect of pirfenidone on mortality: Pooled analyses and meta-analyses of clinical trials in idiopathic pulmonary fibrosis. Lancet Respir. Med. 2017, 5, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Spagnolo, P.; Kropski, J.A.; Jones, M.G.; Lee, J.S.; Rossi, G.; Karampitsakos, T.; Maher, T.M.; Tzouvelekis, A.; Ryerson, C.J. Idiopathic pulmonary fibrosis: Disease mechanisms and drug development. Pharmacol. Ther. 2021, 222, 107798. [Google Scholar] [CrossRef]
- Noble, P.W.; Albera, C.; Bradford, W.Z.; Costabel, U.; Glassberg, M.K.; Kardatzke, D.; King, T.E.; Lancaster, L.; Sahn, S.A.; Szwarcberg, J.; et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): Two randomised trials. Lancet 2011, 377, 1760–1769. [Google Scholar] [CrossRef]
- Taniguchi, H.; Ebina, M.; Kondoh, Y.; Ogura, T.; Azuma, A.; Suga, M.; Taguchi, Y.; Takahashi, H.; Nakata, K.; Sato, A.; et al. Pirfenidone in idiopathic pulmonary fibrosis. Eur. Respir. J. 2010, 35, 821–829. [Google Scholar] [CrossRef]
- Canestaro, W.J.; Forrester, S.H.; Raghu, G.; Ho, L.; Devine, B.E. Drug Treatment of Idiopathic Pulmonary Fibrosis: Systematic Review and Network Meta-Analysis. Chest 2016, 149, 756–766. [Google Scholar] [CrossRef]
- Ahluwalia, N.; Shea, B.S.; Tager, A.M. New therapeutic targets in idiopathic pulmonary fibrosis. Aiming to rein in runaway wound-healing responses. Am. J. Respir. Crit. Care Med. 2014, 190, 867–878. [Google Scholar] [CrossRef]
- Yang, J.; Pan, X.; Wang, L.; Yu, G. Alveolar cells under mechanical stressed niche: Critical contributors to pulmonary fibrosis. Mol. Med. 2020, 26, 95. [Google Scholar] [CrossRef]
- Hewlett, J.C.; Kropski, J.A.; Blackwell, T.S. Idiopathic pulmonary fibrosis: Epithelial-mesenchymal interactions and emerging therapeutic targets. Matrix Biol. 2018, 71–72, 112–127. [Google Scholar] [CrossRef]
- McKeown, S.; Richter, A.G.; O’Kane, C.; McAuley, D.F.; Thickett, D.R. MMP expression and abnormal lung permeability are important determinants of outcome in IPF. Eur. Respir. J. 2009, 33, 77–84. [Google Scholar] [CrossRef]
- Declercq, M.; Treps, L.; Carmeliet, P.; Witters, P. The role of endothelial cells in cystic fibrosis. J. Cyst. Fibros. 2019, 18, 752–761. [Google Scholar] [CrossRef]
- Murakami, A.; Takasugi, H.; Ohnuma, S.; Koide, Y.; Sakurai, A.; Takeda, S.; Hasegawa, T.; Sasamori, J.; Konno, T.; Hayashi, K.; et al. Sphingosine 1-phosphate (S1P) regulates vascular contraction via S1P3 receptor: Investigation based on a new S1P3 receptor antagonist. Mol. Pharmacol. 2010, 77, 704–713. [Google Scholar] [CrossRef]
- Huang, L.S.; Sudhadevi, T.; Fu, P.; Punathil-Kannan, P.-K.; Ebenezer, D.L.; Ramchandran, R.; Putherickal, V.; Cheresh, P.; Zhou, G.; Ha, A.W.; et al. Sphingosine Kinase 1/S1P Signaling Contributes to Pulmonary Fibrosis by Activating Hippo/YAP Pathway and Mitochondrial Reactive Oxygen Species in Lung Fibroblasts. Int. J. Mol. Sci. 2020, 21, 2064. [Google Scholar] [CrossRef]
- Khan, S.A.; Goliwas, K.F.; Deshane, J.S. Sphingolipids in Lung Pathology in the Coronavirus Disease Era: A Review of Sphingolipid Involvement in the Pathogenesis of Lung Damage. Front. Physiol. 2021, 12, 760638. [Google Scholar] [CrossRef]
- Jernigan, P.L.; Makley, A.T.; Hoehn, R.S.; Edwards, M.J.; Pritts, T.A. The role of sphingolipids in endothelial barrier function. Biol. Chem. 2015, 396, 681–691. [Google Scholar] [CrossRef]
- Kuperberg, S.J.; Wadgaonkar, R. Sepsis-Associated Encephalopathy: The Blood-Brain Barrier and the Sphingolipid Rheostat. Front. Immunol. 2017, 8, 597. [Google Scholar] [CrossRef]
- Knipe, R.S.; Spinney, J.J.; Abe, E.A.; Probst, C.K.; Franklin, A.; Logue, A.; Giacona, F.; Drummond, M.; Griffith, J.; Brazee, P.L.; et al. Endothelial-Specific Loss of Sphingosine-1-Phosphate Receptor 1 Increases Vascular Permeability and Exacerbates Bleomycin-induced Pulmonary Fibrosis. Am. J. Respir. Cell Mol. Biol. 2022, 66, 38–52. [Google Scholar] [CrossRef]
- Knipe, R.S.; Spinney, J.J.; Abe, E.; Probst, C.K.; Logue, A.; Griffith, J.; Black, K.E.; Montesi, S.B.; Shea, B.; Medoff, B.D. Loss of endothelial S1PR1 exacerbates bleomycin-induced pulmonary fibrosis through intra-alveolar coagulation and immune cell infiltration. Am. J. Respir. Crit. Care Med. 2020, 201, A7878. [Google Scholar] [CrossRef]
- Wadgaonkar, R.; Patel, V.; Grinkina, N.; Romano, C.; Liu, J.; Zhao, Y.; Sammani, S.; Garcia, J.G.N.; Natarajan, V. Differential regulation of sphingosine kinases 1 and 2 in lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2009, 296, L603–L613. [Google Scholar] [CrossRef]
- Hay, J.; Shahzeidi, S.; Laurent, G. Mechanisms of bleomycin-induced lung damage. Arch. Toxicol. 1991, 65, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Probst, C.K.; Montesi, S.B.; Medoff, B.D.; Shea, B.S.; Knipe, R.S. Vascular permeability in the fibrotic lung. Eur. Respir. J. 2020, 56, 1900100. [Google Scholar] [CrossRef] [PubMed]
- Villar, J.; Zhang, H.; Slutsky, A.S. Lung Repair and Regeneration in ARDS: Role of PECAM1 and Wnt Signaling. Chest 2019, 155, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Engelbrecht, E.; Kooistra, T.; Knipe, R.S. The Vasculature in Pulmonary Fibrosis. Curr. Tissue Microenviron. Rep. 2022, 3, 83–97. [Google Scholar] [CrossRef]
- Komarova, Y.; Malik, A.B. Regulation of endothelial permeability via paracellular and transcellular transport pathways. Annu. Rev. Physiol. 2010, 72, 463–493. [Google Scholar] [CrossRef]
- Pober, J.S.; Sessa, W.C. Evolving functions of endothelial cells in inflammation. Nat. Rev. Immunol. 2007, 7, 803–815. [Google Scholar] [CrossRef]
- Mehta, D.; Malik, A.B. Signaling mechanisms regulating endothelial permeability. Physiol. Rev. 2006, 86, 279–367. [Google Scholar] [CrossRef]
- Campbell, H.K.; Maiers, J.L.; DeMali, K.A. Interplay between tight junctions & adherens junctions. Exp. Cell. Res. 2017, 358, 39–44. [Google Scholar] [CrossRef]
- De Caterina, R.; Libby, P.; Peng, H.B.; Thannickal, V.J.; Rajavashisth, T.B.; Gimbrone, M.A.; Shin, W.S.; Liao, J.K. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J. Clin. Investig. 1995, 96, 60–68. [Google Scholar] [CrossRef]
- Claesson-Welsh, L. Vascular permeability—The essentials. Ups. J. Med. Sci. 2015, 120, 135–143. [Google Scholar] [CrossRef]
- Nagy, J.A.; Benjamin, L.; Zeng, H.; Dvorak, A.M.; Dvorak, H.F. Vascular permeability, vascular hyperpermeability and angiogenesis. Angiogenesis 2008, 11, 109–119. [Google Scholar] [CrossRef]
- Wettschureck, N.; Strilic, B.; Offermanns, S. Passing the Vascular Barrier: Endothelial Signaling Processes Controlling Extravasation. Physiol. Rev. 2019, 99, 1467–1525. [Google Scholar] [CrossRef]
- Jambusaria, A.; Hong, Z.; Zhang, L.; Srivastava, S.; Jana, A.; Toth, P.T.; Dai, Y.; Malik, A.B.; Rehman, J. Endothelial heterogeneity across distinct vascular beds during homeostasis and inflammation. eLife 2020, 9, e51413. [Google Scholar] [CrossRef]
- Shimoda, L.A.; Semenza, G.L. HIF and the lung: Role of hypoxia-inducible factors in pulmonary development and disease. Am. J. Respir. Crit. Care Med. 2011, 183, 152–156. [Google Scholar] [CrossRef]
- Laddha, A.P.; Kulkarni, Y.A. VEGF and FGF-2: Promising targets for the treatment of respiratory disorders. Respir. Med. 2019, 156, 33–46. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Anand, V.; Roy, S. Vascular endothelial growth factor signaling in hypoxia and inflammation. J. Neuroimmune Pharmacol. 2014, 9, 142–160. [Google Scholar] [CrossRef]
- Broermann, A.; Winderlich, M.; Block, H.; Frye, M.; Rossaint, J.; Zarbock, A.; Cagna, G.; Linnepe, R.; Schulte, D.; Nottebaum, A.F.; et al. Dissociation of VE-PTP from VE-cadherin is required for leukocyte extravasation and for VEGF-induced vascular permeability in vivo. J. Exp. Med. 2011, 208, 2393–2401. [Google Scholar] [CrossRef]
- Nottebaum, A.F.; Cagna, G.; Winderlich, M.; Gamp, A.C.; Linnepe, R.; Polaschegg, C.; Filippova, K.; Lyck, R.; Engelhardt, B.; Kamenyeva, O.; et al. VE-PTP maintains the endothelial barrier via plakoglobin and becomes dissociated from VE-cadherin by leukocytes and by VEGF. J. Exp. Med. 2008, 205, 2929–2945. [Google Scholar] [CrossRef]
- Oldenburg, J.; de Rooij, J. Mechanical control of the endothelial barrier. Cell Tissue Res. 2014, 355, 545–555. [Google Scholar] [CrossRef]
- Nagatoya, K.; Moriyama, T.; Kawada, N.; Takeji, M.; Oseto, S.; Murozono, T.; Ando, A.; Imai, E.; Hori, M. Y-27632 prevents tubulointerstitial fibrosis in mouse kidneys with unilateral ureteral obstruction. Kidney Int. 2002, 61, 1684–1695. [Google Scholar] [CrossRef]
- Knipe, R.S.; Probst, C.K.; Lagares, D.; Franklin, A.; Spinney, J.J.; Brazee, P.L.; Grasberger, P.; Zhang, L.; Black, K.E.; Sakai, N.; et al. The Rho Kinase Isoforms ROCK1 and ROCK2 Each Contribute to the Development of Experimental Pulmonary Fibrosis. Am. J. Respir. Cell Mol. Biol. 2018, 58, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, K.; Tada, S.; Nakamoto, N.; Toda, K.; Horikawa, H.; Kurita, S.; Tsunematsu, S.; Kumagai, N.; Ishii, H.; Saito, H.; et al. Rho/Rho kinase is a key enzyme system involved in the angiotensin II signaling pathway of liver fibrosis and steatosis. J. Gastroenterol. Hepatol. 2007, 22, 2022–2033. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Kano, Y.; Noda, Y. Rho-associated kinase-dependent contraction of stress fibres and the organization of focal adhesions. J. R. Soc. Interface 2011, 8, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Wojciak-Stothard, B.; Ridley, A.J. Rho GTPases and the regulation of endothelial permeability. Vascul. Pharmacol. 2002, 39, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Mong, P.Y.; Wang, Q. Activation of Rho kinase isoforms in lung endothelial cells during inflammation. J. Immunol. 2009, 182, 2385–2394. [Google Scholar] [CrossRef]
- Abbès, M.; Sabatier, P. [Statistical study of 154 plastic operations following excision of head and neck tumors]. Ann. Chir. Plast. 1970, 15, 205–213. [Google Scholar]
- Del Gaudio, I.; Camerer, E. Distinct GEFs Couple S1PR1 to Rac for Endothelial Barrier Enhancement and Lymphocyte Trafficking. Arterioscler Thromb. Vasc. Biol. 2022, 42, 903–905. [Google Scholar] [CrossRef]
- Wadgaonkar, R.; Geraghty, P.; Kabir, I.; Foronjy, R. Role of sphingomyelin synthase regulated micro domain signaling in cigarette smoke induced inflammation. Am. J. Respir. Crit. Care Med. 2017, 195, A6339. [Google Scholar]
- Gowda, S.; Yeang, C.; Wadgaonkar, S.; Anjum, F.; Grinkina, N.; Cutaia, M.; Jiang, X.-C.; Wadgaonkar, R. Sphingomyelin synthase 2 (SMS2) deficiency attenuates LPS-induced lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2011, 300, L430–L440. [Google Scholar] [CrossRef]
- Wattenberg, B.W.; Pitson, S.M.; Raben, D.M. The sphingosine and diacylglycerol kinase superfamily of signaling kinases: Localization as a key to signaling function. J. Lipid Res. 2006, 47, 1128–1139. [Google Scholar] [CrossRef]
- Siow, D.L.; Anderson, C.D.; Berdyshev, E.V.; Skobeleva, A.; Natarajan, V.; Pitson, S.M.; Wattenberg, B.W. Sphingosine kinase localization in the control of sphingolipid metabolism. Adv. Enzyme Regul. 2011, 51, 229–244. [Google Scholar] [CrossRef]
- Bravo, G.A.; Cedeno, R.R.; Casadevall, M.P.; Ramio-Torrenta, L. Sphingosine-1-Phosphate (S1P) and S1P Signaling Pathway Modulators, from Current Insights to Future Perspectives. Cells 2022, 11, 2058. [Google Scholar] [CrossRef]
- Sanchez, T.; Skoura, A.; Wu, M.T.; Casserly, B.; Harrington, E.O.; Hla, T. Induction of vascular permeability by the sphingosine-1-phosphate receptor-2 (S1P2R) and its downstream effectors ROCK and PTEN. Arterioscler Thromb. Vasc. Biol. 2007, 27, 1312–1318. [Google Scholar] [CrossRef]
- Sanchez, T.; Hla, T. Structural and functional characteristics of S1P receptors. J. Cell. Biochem. 2004, 92, 913–922. [Google Scholar] [CrossRef]
- Forrest, M.; Sun, S.Y.; Hajdu, R.; Bergstrom, J.; Card, D.; Doherty, G.; Hale, J.; Keohane, C.; Meyers, C.; Milligan, J.; et al. Immune cell regulation and cardiovascular effects of sphingosine 1-phosphate receptor agonists in rodents are mediated via distinct receptor subtypes. J. Pharmacol. Exp. Ther. 2004, 309, 758–768. [Google Scholar] [CrossRef]
- Jin, F.; Hagemann, N.; Sun, L.; Wu, J.; Doeppner, T.R.; Dai, Y.; Hermann, D.M. High-density lipoprotein (HDL) promotes angiogenesis via S1P3-dependent VEGFR2 activation. Angiogenesis 2018, 21, 381–394. [Google Scholar] [CrossRef]
- Gräler, M.H.; Grosse, R.; Kusch, A.; Kremmer, E.; Gudermann, T.; Lipp, M. The sphingosine 1-phosphate receptor S1P4 regulates cell shape and motility via coupling to Gi and G12/13. J. Cell. Biochem. 2003, 89, 507–519. [Google Scholar] [CrossRef]
- Niedernberg, A.; Scherer, C.R.; Busch, A.E.; Kostenis, E. Comparative analysis of human and rat S1P(5) (edg8): Differential expression profiles and sensitivities to antagonists. Biochem. Pharmacol. 2002, 64, 1243–1250. [Google Scholar] [CrossRef]
- Garcia, J.G.; Liu, F.; Verin, A.D.; Birukova, A.; Dechert, M.A.; Gerthoffer, W.T.; Bamberg, J.R.; English, D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J. Clin. Investig. 2001, 108, 689–701. [Google Scholar] [CrossRef]
- Berdyshev, E.V.; Gorshkova, I.; Usatyuk, P.; Kalari, S.; Zhao, Y.; Pyne, N.J.; Pyne, S.; Sabbadini, R.A.; Garcia, J.G.N.; Natarajan, V. Intracellular S1P generation is essential for S1P-induced motility of human lung endothelial cells: Role of sphingosine kinase 1 and S1P lyase. PLoS ONE 2011, 6, e16571. [Google Scholar] [CrossRef]
- Adyshev, D.M.; Moldobaeva, N.K.; Elangovan, V.R.; Garcia, J.G.N.; Dudek, S.M. Differential involvement of ezrin/radixin/moesin proteins in sphingosine 1-phosphate-induced human pulmonary endothelial cell barrier enhancement. Cell Signal. 2011, 23, 2086–2096. [Google Scholar] [CrossRef] [PubMed]
- Donati, C.; Bruni, P. Sphingosine 1-phosphate regulates cytoskeleton dynamics: Implications in its biological response. Biochim. Biophys. Acta 2006, 1758, 2037–2048. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Shikata, Y.; Wang, L.; Ohmori, K.; Watanabe, N.; Wada, J.; Shikata, K.; Birukov, K.G.; Makino, H.; Jacobson, J.R.; et al. Enhanced interaction between focal adhesion and adherens junction proteins: Involvement in sphingosine 1-phosphate-induced endothelial barrier enhancement. Microvasc. Res. 2009, 77, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Hla, T.; Brinkmann, V. Sphingosine 1-phosphate (S1P): Physiology and the effects of S1P receptor modulation. Neurology 2011, 76, S3–S8. [Google Scholar] [CrossRef]
- Bazzoni, G.; Dejana, E. Endothelial cell-to-cell junctions: Molecular organization and role in vascular homeostasis. Physiol. Rev. 2004, 84, 869–901. [Google Scholar] [CrossRef]
- Natarajan, V.; Dudek, S.M.; Jacobson, J.R.; Moreno-Vinasco, L.; Huang, L.S.; Abassi, T.; Mathew, B.; Zhao, Y.; Wang, L.; Bittman, R.; et al. Sphingosine-1-phosphate, FTY720, and sphingosine-1-phosphate receptors in the pathobiology of acute lung injury. Am. J. Respir. Cell Mol. Biol. 2013, 49, 6–17. [Google Scholar] [CrossRef]
- Peng, X.; Hassoun, P.M.; Sammani, S.; McVerry, B.J.; Burne, M.J.; Rabb, H.; Pearse, D.; Tuder, R.M.; Garcia, J.G.N. Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. Am. J. Respir. Crit. Care Med. 2004, 169, 1245–1251. [Google Scholar] [CrossRef]
- McVerry, B.J.; Peng, X.; Hassoun, P.M.; Sammani, S.; Simon, B.A.; Garcia, J.G.N. Sphingosine 1-phosphate reduces vascular leak in murine and canine models of acute lung injury. Am. J. Respir. Crit. Care Med. 2004, 170, 987–993. [Google Scholar] [CrossRef]
- Knipe, R.S.; Spinney, J.J.; Abe, E.; Probst, C.K.; Franklin, A.; Griffith, J.W.; Liao, J.K.; McCarthy, J.R.; Shea, B.S.; Medoff, B.D. The pulmonary endothelium plays a critical role in the fibrotic response to lung injury through S1PR1 and rock mediated cytoskeletal rearrangements. Am. J. Respir. Crit. Care Med. 2019, 199, A4020. [Google Scholar] [CrossRef]
- Shea, B.S.; Probst, C.K.; Brazee, P.L.; Rotile, N.J.; Blasi, F.; Weinreb, P.H.; Black, K.E.; Sosnovik, D.E.; Van Cott, E.M.; Violette, S.M.; et al. Uncoupling of the profibrotic and hemostatic effects of thrombin in lung fibrosis. JCI Insight 2017, 2, e86608. [Google Scholar] [CrossRef]
- Milara, J.; Navarro, R.; Juan, G.; Peiro, T.; Serrano, A.; Ramon, M.; Morcillo, E.; Cortijo, J. Sphingosine-1-phosphate is increased in patients with idiopathic pulmonary fibrosis and mediates epithelial to mesenchymal transition. Thorax 2012, 67, 147–156. [Google Scholar] [CrossRef]
- Brinkmann, V. FTY720 (fingolimod) in Multiple Sclerosis: Therapeutic effects in the immune and the central nervous system. Br. J. Pharmacol. 2009, 158, 1173–1182. [Google Scholar] [CrossRef]
- Garnier, O.; Vilgrain, I. Dialogue between VE-Cadherin and Sphingosine 1 Phosphate Receptor1 (S1PR1) for Protecting Endothelial Functions. Int. J. Mol. Sci. 2023, 24, 4018. [Google Scholar] [CrossRef]
- Leonard, A.; Grose, V.; Paton, A.W.; Paton, J.C.; Yule, D.I.; Rahman, A.; Fazal, F. Selective Inactivation of Intracellular BiP/GRP78 Attenuates Endothelial Inflammation and Permeability in Acute Lung Injury. Sci. Rep. 2019, 9, 2096. [Google Scholar] [CrossRef]
- Shea, B.S.; Brooks, S.F.; Fontaine, B.A.; Chun, J.; Luster, A.D.; Tager, A.M. Prolonged exposure to sphingosine 1-phosphate receptor-1 agonists exacerbates vascular leak, fibrosis, and mortality after lung injury. Am. J. Respir. Cell Mol. Biol. 2010, 43, 662–673. [Google Scholar] [CrossRef]
- Zhao, J.; Okamoto, Y.; Asano, Y.; Ishimaru, K.; Aki, S.; Yoshioka, K.; Takuwa, N.; Wada, T.; Inagaki, Y.; Takahashi, C.; et al. Sphingosine-1-phosphate receptor-2 facilitates pulmonary fibrosis through potentiating IL-13 pathway in macrophages. PLoS ONE 2018, 13, e0197604. [Google Scholar] [CrossRef]
- Sammani, S.; Moreno-Vinasco, L.; Mirzapoiazova, T.; Singleton, P.A.; Chiang, E.T.; Evenoski, C.L.; Wang, T.; Mathew, B.; Husain, A.; Moitra, J.; et al. Differential effects of sphingosine 1-phosphate receptors on airway and vascular barrier function in the murine lung. Am. J. Respir. Cell Mol. Biol. 2010, 43, 394–402. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, T.-T.; Xu, D.-F.; Zhu, X.-Y.; Dong, W.-W.; Lv, Z.; Liu, Y.-J.; Jiang, L. Upregulation of sphingosine kinase 1 contributes to ventilator-associated lung injury in a two-hit model. Int. J. Mol. Med. 2019, 44, 2077–2090. [Google Scholar] [CrossRef]
- Calabresi, P.A.; Radue, E.-W.; Goodin, D.; Jeffery, D.; Rammohan, K.W.; Reder, A.T.; Vollmer, T.; Agius, M.A.; Kappos, L.; Stites, T.; et al. Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2014, 13, 545–556. [Google Scholar] [CrossRef]
- Cohen, J.A.; Khatri, B.; Barkhof, F.; Comi, G.; Hartung, H.-P.; Montalban, X.; Pelletier, J.; Stites, T.; Ritter, S.; von Rosenstiel, P.; et al. Long-term (up to 4.5 years) treatment with fingolimod in multiple sclerosis: Results from the extension of the randomised TRANSFORMS study. J. Neurol. Neurosurg. Psychiatry 2016, 87, 468–475. [Google Scholar] [CrossRef]
- Khatri, B.; Barkhof, F.; Comi, G.; Hartung, H.-P.; Kappos, L.; Montalban, X.; Pelletier, J.; Stites, T.; Wu, S.; Holdbrook, F.; et al. Comparison of fingolimod with interferon beta-1a in relapsing-remitting multiple sclerosis: A randomised extension of the TRANSFORMS study. Lancet Neurol. 2011, 10, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Lublin, F.; Miller, D.H.; Freedman, M.S.; Cree, B.A.C.; Wolinsky, J.S.; Weiner, H.; Lubetzki, C.; Hartung, H.-P.; Montalban, X.; Uitdehaag, B.M.J.; et al. Oral fingolimod in primary progressive multiple sclerosis (INFORMS): A phase 3, randomised, double-blind, placebo-controlled trial. Lancet 2016, 387, 1075–1084. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Wang, H.; Wang, S.; Tang, N. FTY720, a sphingosine-1 phosphate receptor modulator, improves liver fibrosis in a mouse model by impairing the motility of bone marrow-derived mesenchymal stem cells. Inflammation 2014, 37, 1326–1336. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Chen, J.; Pan, M.; Zhang, M.; Zhang, J.; Chen, P.; Liu, B. FTY720 prevents progression of renal fibrosis by inhibiting renal microvasculature endothelial dysfunction in a rat model of chronic kidney disease. J. Mol. Histol. 2013, 44, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Ye, Y.; Lv, L.; Zhu, C.; Ye, S. FTY720 attenuates paraquat-induced lung injury in mice. Int. Immunopharmacol. 2014, 21, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.D.; Gao, G.; Liu, H.Y.; Yan, G.H.; Li, L.C.; Zhang, J.Y.; Cui, H. Effects of fty-720 on pulmonary fibrosis in mice via tgf-pl/p38 mapk/nf-kb signaling pathway. Chin. Pharmacol. Bull. 2020, 36, 250–256. [Google Scholar] [CrossRef]
- Muller, H.C.; Hocke, A.C.; Hellwig, K.; Gutbier, B.; Peters, H.; Schonrock, S.M.; Tschernig, T.; Schmiedl, A.; Hippenstiel, S.; N’Guessan, P.D.; et al. The Sphingosine-1 Phosphate receptor agonist FTY720 dose dependently affected endothelial integrity in vitro and aggravated ventilator-induced lung injury in mice. Pulm. Pharmacol. Ther. 2011, 24, 377–385. [Google Scholar] [CrossRef]
- Gendron, D.R.; Lemay, A.-M.; Lecours, P.B.; Perreault-Vallières, V.; Huppé, C.-A.; Bossé, Y.; Blanchet, M.-R.; Dion, G.; Marsolais, D. FTY720 promotes pulmonary fibrosis when administered during the remodelling phase following a bleomycin-induced lung injury. Pulm. Pharmacol. Ther. 2017, 44, 50–56. [Google Scholar] [CrossRef]
- Sobel, K.; Menyhart, K.; Killer, N.; Renault, B.; Bauer, Y.; Studer, R.; Steiner, B.; Bolli, M.H.; Nayler, O.; Gatfield, J. Sphingosine 1-phosphate (S1P) receptor agonists mediate pro-fibrotic responses in normal human lung fibroblasts via S1P2 and S1P3 receptors and Smad-independent signaling. J. Biol. Chem. 2013, 288, 14839–14851. [Google Scholar] [CrossRef]
- Keller, C.D.; Rivera Gil, P.; Tolle, M.; van der Giet, M.; Chun, J.; Radeke, H.H.; Schafer-Korting, M.; Kleuser, B. Immunomodulator FTY720 induces myofibroblast differentiation via the lysophospholipid receptor S1P3 and Smad3 signaling. Am. J. Pathol. 2007, 170, 281–292. [Google Scholar] [CrossRef]
- Clemons, B.; Bain, G.; Lai, A.; Santini, A.M.; Goulet, L.; Boyett, M.; Roberts, E.; Rosen, H.; Opiteck, G.J.; Scott, F.L.; et al. Favourable S1P1R/5R selectivity profile of ozanimod confers safety benefit relating to S1P3R-mediated pro-fibrotic changes in fibroblasts. Mult. Scler. J. 2018, 24, 39. [Google Scholar] [CrossRef]
- Pérez-Jeldres, T.; Alvarez-Lobos, M.; Rivera-Nieves, J. Targeting Sphingosine-1-Phosphate Signaling in Immune-Mediated Diseases: Beyond Multiple Sclerosis. Drugs 2021, 81, 985–1002. [Google Scholar] [CrossRef]
- Karnati, S.; Seimetz, M.; Kleefeldt, F.; Sonawane, A.; Madhusudhan, T.; Bachhuka, A.; Kosanovic, D.; Weissmann, N.; Krüger, K.; Ergün, S. Chronic Obstructive Pulmonary Disease and the Cardiovascular System: Vascular Repair and Regeneration as a Therapeutic Target. Front. Cardiovasc. Med. 2021, 8, 649512. [Google Scholar] [CrossRef]
- Ebina, M.; Shimizukawa, M.; Shibata, N.; Kimura, Y.; Suzuki, T.; Endo, M.; Sasano, H.; Kondo, T.; Nukiwa, T. Heterogeneous increase in CD34-positive alveolar capillaries in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2004, 169, 1203–1208. [Google Scholar] [CrossRef]
- Simler, N.R.; Brenchley, P.E.; Horrocks, A.W.; Greaves, S.M.; Hasleton, P.S.; Egan, J.J. Angiogenic cytokines in patients with idiopathic interstitial pneumonia. Thorax 2004, 59, 581–585. [Google Scholar] [CrossRef]
- Cosgrove, G.P.; Brown, K.K.; Schiemann, W.P.; Serls, A.E.; Parr, J.E.; Geraci, M.W.; Schwarz, M.I.; Cool, C.D.; Worthen, G.S. Pigment epithelium-derived factor in idiopathic pulmonary fibrosis: A role in aberrant angiogenesis. Am. J. Respir. Crit. Care Med. 2004, 170, 242–251. [Google Scholar] [CrossRef]
- Liu, X.; Qin, X.; Qin, H.; Jia, C.; Yuan, Y.; Sun, T.; Chen, B.; Chen, C.; Zhang, H. Characterization of the heterogeneity of endothelial cells in bleomycin-induced lung fibrosis using single-cell RNA sequencing. Angiogenesis 2021, 24, 809–821. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jayant, G.; Kuperberg, S.; Somnay, K.; Wadgaonkar, R. The Role of Sphingolipids in Regulating Vascular Permeability in Idiopathic Pulmonary Fibrosis. Biomedicines 2023, 11, 1728. https://doi.org/10.3390/biomedicines11061728
Jayant G, Kuperberg S, Somnay K, Wadgaonkar R. The Role of Sphingolipids in Regulating Vascular Permeability in Idiopathic Pulmonary Fibrosis. Biomedicines. 2023; 11(6):1728. https://doi.org/10.3390/biomedicines11061728
Chicago/Turabian StyleJayant, Girish, Stephen Kuperberg, Kaumudi Somnay, and Raj Wadgaonkar. 2023. "The Role of Sphingolipids in Regulating Vascular Permeability in Idiopathic Pulmonary Fibrosis" Biomedicines 11, no. 6: 1728. https://doi.org/10.3390/biomedicines11061728
APA StyleJayant, G., Kuperberg, S., Somnay, K., & Wadgaonkar, R. (2023). The Role of Sphingolipids in Regulating Vascular Permeability in Idiopathic Pulmonary Fibrosis. Biomedicines, 11(6), 1728. https://doi.org/10.3390/biomedicines11061728







