The Influence of Cleaning Solutions on the Retention of Overdenture Attachment Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
Materials Used in the Study
2.2. Methods
2.2.1. Preparation of the Samples
2.2.2. Preparation of the Acrylic Testing Block
2.2.3. Protocol for Immersion in Cleaning Solutions
2.2.4. Dynamic Fatigue Test
2.3. Statistical Analysis
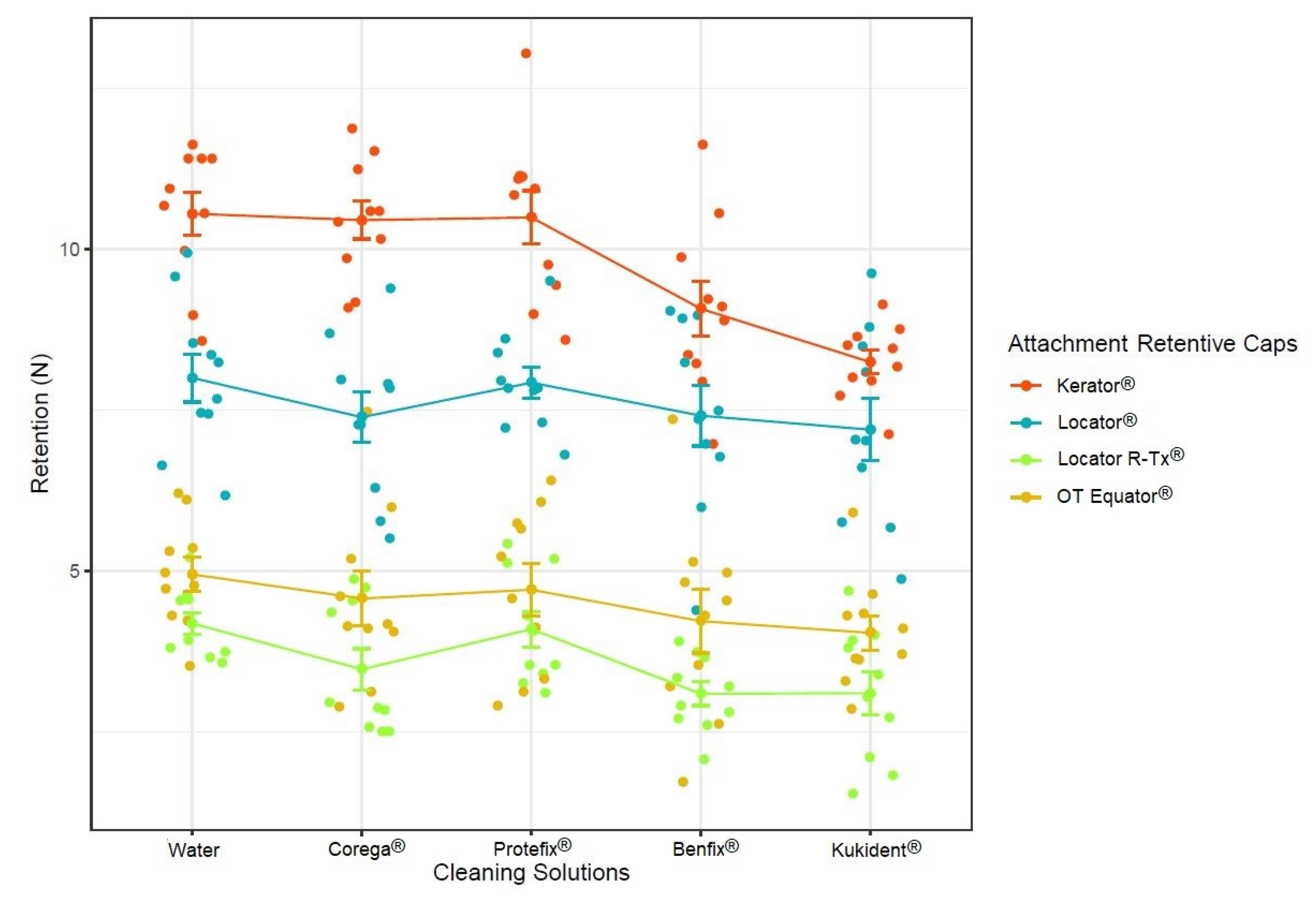
3. Results
4. Discussion
5. Conclusions
- There were no significant statistical differences between Corega® (Stafford Miller, Waterford, Ireland), Protefix® (Neuhofer Weiche, Parchim, Germany), and tap water, despite the retention decreasing in all three solutions.
- The only statistically different results found were between the Kukident® (P&G Tech, Oxford Parkway, UK) and Benfix® (Laboratorios URGO S.L., Guipúzcoa, Spain) cleaning solution groups, suggesting that the amount of time required for the cleaning solution to work could influence the attachment retentive cap’s degradation.
- There were significant statistical differences between the different manufacturers in terms of the retention forces of the attachment retentive caps, despite the fact that the caps are made of the same material. There were different components that caused each one to have a different elasticity, resulting in retention differences, and explaining the variation between the initial retentive forces from all of the groups.
- Further studies are necessary to analyze whether the percentage of different material elements used to make the attachment influence or accelerate the attachment retentive cap’s degradation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. World Report on Ageing and Health; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Nitschke, I.; Wendland, A.; Weber, S.; Jockusch, J.; Lethaus, B.; Hahnel, S. Considerations for the Prosthetic Dental Treatment of Geriatric Patients in Germany. J. Clin. Med. 2021, 10, 304. [Google Scholar] [CrossRef]
- Gray, D.; Patel, J. Implant-supported overdentures: Part 1. Br. Dent. J. 2021, 231, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Vahidi, F.; Pinto-Sinai, G. Complications Associated with Implant-Retained Removable Prostheses. Dent. Clin. N. Am. 2015, 59, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Prasad, D.K.; Prasad, D.A.; Buch, M. Selection of attachment systems in fabricating an implant supported overdenture. J. Dent. Implant. 2014, 4, 176. [Google Scholar] [CrossRef]
- Thomason, J.M.; Kelly, S.A.M.; Bendkowski, A.; Ellis, J.S. Two implant retained overdentures–A review of the literature supporting the McGill and York consensus statements. J. Dent. 2012, 40, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Stilwell, C. Mandibular Implant Overdentures: Treatment and Medico-Legal Considerations. Prim. Dent. J. 2017, 6, 28–35. [Google Scholar] [CrossRef]
- Melescanu, I.M.; Marin, M.; Preoteasa, E.; Tancu, A.M.; Preoteasa, C.T. Two implant overdenture–the first alternative treatment for patients with complete edentulous mandible. J. Med. Life 2011, 4, 207. [Google Scholar]
- Silva, A.S.; Martins, D.; Sá, J.; Mendes, J.M. Clinical evaluation of the implant survival rate in patients subjected to immediate implant loading protocols. Dent. Med. Probl. 2021, 58, 61–68. [Google Scholar] [CrossRef]
- Zhang, L.; Lyu, C.; Shang, Z.; Niu, A.; Liang, X. Quality of Life of Implant-Supported Overdenture and Conventional Complete Denture in Restoring the Edentulous Mandible: A Systematic Review. Implant Dent. 2017, 26, 945–950. [Google Scholar] [CrossRef]
- Kutkut, A.; Bertoli, E.; Frazer, R.; Pinto-Sinai, G.; Hidalgo, R.F.; Studts, J. A systematic review of studies comparing conventional complete denture and implant retained overdenture. J. Prosthodont. Res. 2018, 62, 1–9. [Google Scholar] [CrossRef]
- Sun, X.; Zhai, J.-J.; Liao, J.; Teng, M.-H.; Tian, A.; Liang, X. Masticatory efficiency and oral health-related quality of life with implant-retained mandibular overdentures. Saudi Med. J. 2014, 35, 1195–1202. [Google Scholar]
- Renvert, S.; Aghazadeh, A.; Hallström, H.; Persson, G.R. Factors related to peri-implantitis–A retrospective study. Clin. Oral Implants Res. 2014, 25, 522–529. [Google Scholar] [CrossRef]
- Serino, G.; Ström, C. Peri-implantitis in partially edentulous patients: Association with inadequate plaque control. Clin. Oral Implants Res. 2009, 20, 169–174. [Google Scholar] [CrossRef]
- De Araújo Nobre, M.; Mano Azul, A.; Rocha, E.; Maló, P. Risk factors of peri-implant pathology. Eur. J. Oral Sci. 2015, 123, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Matthys, C.; Vervaeke, S.; Besseler, J.; Doornewaard, R.; Dierens, M.; De Bruyn, H. Five years follow-up of mandibular 2-implant overdentures on locator or ball abutments: Implant results, patient-related outcome, and prosthetic aftercare. Clin. Implant Dent. Relat. Res. 2019, 21, 835–844. [Google Scholar] [CrossRef]
- Felton, D.; Cooper, L.; Duqum, I.; Minsley, G.; Guckes, A.; Haug, S.; Meredith, P.; Solie, C.; Avery, D.; Chandler, N.D. Evidence-Based Guidelines for the Care and Maintenance of Complete Dentures: A Publication of the American College of Prosthodontists. J. Prosthodont. 2011, 20, S1–S12. [Google Scholar] [CrossRef] [PubMed]
- Nishi, Y.; Seto, K.; Kamashita, Y.; Kaji, A.; Kurono, A.; Nagaoka, E. Survival of microorganisms on complete dentures following ultrasonic cleaning combined with immersion in peroxide-based cleanser solution. Gerodontology 2012, 31, 202–209. [Google Scholar] [CrossRef]
- Budtz-Jørgensen, E. Materials and methods for cleaning dentures. J. Prosthet. Dent. 1979, 42, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.S.; Singh, S.; Hari, P.A.; Amarnath, G.S.; Kundapur, V.; Pasha, N.; Anand, M. Evaluate the Effect of Commercially Available Denture Cleansers on Surface Hardness and Roughness of Denture Liners at Various Time Intervals. Int. J. Biomed. Sci. 2016, 12, 130–142. [Google Scholar] [PubMed]
- Valentini-Mioso, F.; Maske, T.T.; Cenci, M.S.; Boscato, N.; Pereira-Cenci, T. Chemical hygiene protocols for complete dentures: A crossover randomized clinical trial. J. Prosthet. Dent. 2019, 121, 83–89. [Google Scholar] [CrossRef]
- Axe, A.S.; Varghese, R.; Bosma, M.; Kitson, N.; Bradshaw, D.J. Dental health professional recommendation and consumer habits in denture cleansing. J. Prosthet. Dent. 2015, 115, 183–188. [Google Scholar] [CrossRef]
- Hayran, Y.; Sarikaya, I.; Aydin, A.; Tekin, Y.H. Determination of the effective anticandidal concentration of denture cleanser tablets on some denture base resins. J. Appl. Oral Sci. 2018, 26, e20170077. [Google Scholar] [CrossRef] [PubMed]
- Papadiochou, S.; Polyzois, G. Hygiene practices in removable prosthodontics: A systematic review. Int. J. Dent. Hyg. 2018, 16, 179–201. [Google Scholar] [CrossRef] [PubMed]
- Verhaeghe, T.V.; Wyatt, C.C.; Mostafa, N.Z. The effect of overnight storage conditions on complete denture colonization by Candida albicans and dimensional stability: A systematic review. J. Prosthet. Dent. 2020, 124, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.H.; Ochiai, K.T.; Hojo, S.; Nishimura, R.; Caputo, A.A. Retention of maxillary implant overdenture bars of different designs. J. Prosthet. Dent. 2001, 86, 603–607. [Google Scholar] [CrossRef]
- Warreth, A.; Alkadhimi, A.; Sultan, A.; Byrne, C.; Woods, E. Mandibular implant-supported overdentures: Attachment systems, and number and locations of implants–Part I. J. Ir. Dent. Assoc. 2015, 61, 93–97. [Google Scholar]
- Driscoll, C.F.; Freilich, M.A.; Guckes, A.D.; Knoernschild, K.L.; McGarry, T.J. The Glossary of Prosthodontic Terms: Ninth Edition. J. Prosthet. Dent. 2017, 117, e1–e105. [Google Scholar]
- Daou, E. Biomaterial aspects: A key factor in the longevity of implant overdenture attachment systems. J. Int. Soc. Prev. Community Dent. 2015, 5, 255–262. [Google Scholar] [CrossRef]
- Al-Zubeidi, M.I.; Alsabeeha, N.H.; Thomson, W.M.; Payne, A.G. Patient Satisfaction and Dissatisfaction with Mandibular Two-Implant Overdentures Using Different Attachment Systems: 5-Year Outcomes. Clin. Implant. Dent. Relat. Res. 2010, 14, 696–707. [Google Scholar] [CrossRef]
- Passia, N.; Ghazal, M.; Kern, M. Long-term retention behaviour of resin matrix attachment systems for overdentures. J. Mech. Behav. Biomed. Mater. 2016, 57, 88–94. [Google Scholar] [CrossRef] [PubMed]
- De Campos, M.R.; Agnelli, J.A.M.; dos Reis, A.C. Factors influencing retention and durability of attachments for overdentures–Adverse effects of cleansings, pH, and temperature: A systematic review. Heliyon 2022, 8, e12411. [Google Scholar] [CrossRef]
- Silva, A.S.; Aroso, C.; Ustrell, R.; Braga, A.C.; Mendes, J.M.; Escuin, T. The influence of saliva pH value on the retention and durability of bar-clip attachments. J. Adv. Prosthodont. 2015, 7, 32–38. [Google Scholar] [CrossRef]
- Ozyilmaz, O.Y.; Kara, O.; Akin, C. Evaluation of various denture cleansers on color stability and surface topography of polyetherketoneketone, polyamide, and polymethylmethacrylate. Microsc. Res. Tech. 2020, 84, 3–11. [Google Scholar] [CrossRef]
- You, W.; Masri, R.; Romberg, E.; Driscoll, C.F.; You, T. The Effect of Denture Cleansing Solutions on the Retention of Pink Locator Attachments after Multiple Pulls: An In Vitro Study. J. Prosthodont. 2011, 20, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Ayyıldız, S.; Şahin, C.; Emir, F.; Ersu, B. Effect of Denture Cleansing Solutions on the Retention of Locator Attachments Over Time. J. Prosthodont. 2020, 29, 237–242. [Google Scholar] [CrossRef]
- Derafshi, R.; Mohaghegh, M.; Saki, M.; Safari, A.; Haghighi, M.R. The Effects of Denture Cleansing Solutions on the Retention of Attachments of Implant Supported Overdentures. J. Dent. 2015, 16 (Suppl. 1), 68–72. [Google Scholar]
- Nguyen, C.T.; Masri, R.; Driscoll, C.F.; Romberg, E. The Effect of Denture Cleansing Solutions on the Retention of Pink Locator Attachments: An In Vitro Study. J. Prosthodont. 2010, 19, 226–230. [Google Scholar] [CrossRef]
- Mariotto, L.; Valente, M.; de Castro, D.; dos Reis, A. Effects of Denture Cleansing Solutions on Different Materials Used for Fabrication of Polymer Attachment Components. Int. J. Prosthodont. 2020, 33, 74–80. [Google Scholar] [CrossRef]
- Varghese, R.M.; Masri, R.; Driscoll, C.F.; Romberg, E. The Effect of Denture Cleansing Solutions on the Retention of Yellow Hader Clips: An In Vitro Study. J. Prosthodont. 2007, 16, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Berger, D.; Rakhamimova, A.; Pollack, A.; Loewy, Z. Oral Biofilms: Development, Control, and Analysis. High Throughput 2018, 7, 24. [Google Scholar]
- WHO. Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
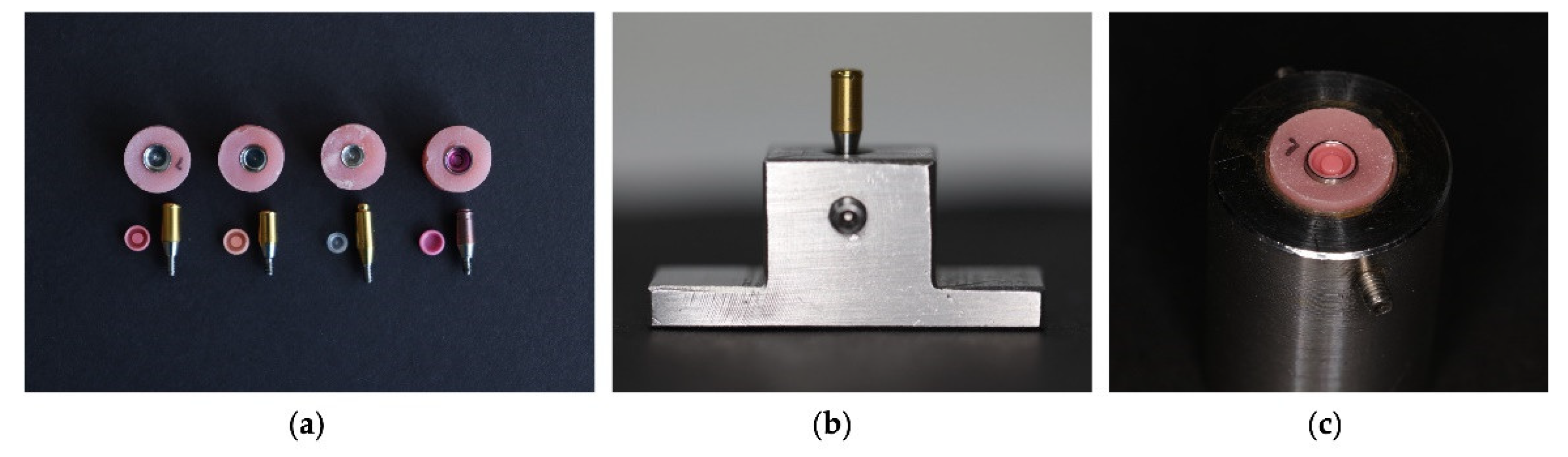

| Brand | Color | Force |
|---|---|---|
| Locator® | Pink  | 1360 g |
| OT Equator® | Clear  | 1300 g |
| Kerator® | Pink  | 1088 g |
| Locator R-Tx® | Pink  | 907 g |
| Locator® | OT Equator® | Kerator® | Locator R-Tx® | |
|---|---|---|---|---|
| Corega® (1460 tablets) | 10 | 10 | 10 | 10 |
| Benfix® (1460 tablets) | 10 | 10 | 10 | 10 |
| Protefix® (1460 tablets) | 10 | 10 | 10 | 10 |
| Kukident® (1460 tablets) | 10 | 10 | 10 | 10 |
| Control | 10 | 10 | 10 | 10 |
| Total | 50 | 50 | 50 | 50 |
| Daily Hygiene (1 Day) | One Year (365 Days) | |
|---|---|---|
| Corega® | 5 min | 1825 min |
| Protefix® | 10 min | 3650 min |
| Benfix® | 15 min | 5475 min |
| Kukident® | 30 min | 10,950 min |
| Corega®. | Dissolve one Corega Cleanser® tablet in warm (not hot) water to cover the denture. | For an antifungal action, leave it submerged for 5 min. You can also leave it overnight. | Rinse the denture with plenty of running water before putting it in your mouth. |
| Protefix® | Dissolve one Protefix Active Cleanser® tablet in a glass of lukewarm water (100–200 mL, about 35 °C). | Clean and fresh in 3 min, disinfected in 10 min. Cleaning is also possible overnight. | Rinse the dental prosthesis well with running water before putting it in the mouth. |
| Benfix® | Introduce a single cleaning tablet in a glass of warm water. | Let the product act for a minimum of 15 min. For deep cleaning, you can leave your denture in the cup overnight. | Rinse with plenty of water to eliminate possible product residue. |
| Kukident® | Put the tablet in enough warm water to cover the denture. | Place the denture in the solution and let it sit for 30 mor overnight. | Remove the dentures and rinse in plenty of running water. |
| Locator® | Kerator® | OT Equator® | Locator R-Tx® | |||||
|---|---|---|---|---|---|---|---|---|
| Time | Solution | Time | Solution | Time | Solution | Time | Solution | |
| Control (water) | - | - | - | - | - | - | - | - |
| Experiment 1 | 5 min | Corega | 5 min | Corega | 5 min | Corega | 5 min | Corega |
| Experiment 2 | 10 min | Protefix | 10 min | Protefix | 10 min | Protefix | 10 min | Protefix |
| Experiment 3 | 15 min | Benfix | 15 min | Benfix | 15 min | Benfix | 15 min | Benfix |
| Experiment 4 | 30 min | Kukident | 30 min | Kukident | 30 min | Kukident | 30 min | Kukident |
| Tap Water Composition | Data |
|---|---|
| pH (Sørensen’s scale) | 6.9–8.7 |
| Hardness (mg/L CaCO3) | 38 |
| Calcium (mg/L Ca) | 13–16 |
| Magnesium (mg/L Mg) | 0.52–0.75 |
| Chlorine (mg/L Cl2) | 0.01–1.02 |
| Df | Sum Sq | Mean Sq | F Value | Pr (>F) | |
|---|---|---|---|---|---|
| Cleaning solutions | 4 | 48.1 | 12.0 | 9.616 | 4.15 × 10−7 *** |
| Attachment retentive caps | 3 | 1208.6 | 402.9 | 322.066 | <2 × 10−16 *** |
| Residuals | 192 | 240.2 | 1.3 | 10 |
| Cleaning Solution Attachment System | Water (Control) | |
| Mean | SD | |
| Locator® | 8.00 N | 1.18 N |
| Kerator® | 10.6 N | 1.07 N |
| OT Equator® | 4.95 N | 0.834 N |
| Locator R-Tx® | 4.19 N | 0.534 N |
| Cleaning Solution Attachment System | Corega® | |
| Mean | SD | |
| Locator® | 7.39 N | 1.24 N |
| Kerator® | 10.5 N | 0.926 N |
| OT Equator® | 4.58 N | 1.35 N |
| Locator R-Tx® | 3.48 N | 1.01 N |
| Cleaning Solution Attachment System | Protefix® | |
| Mean | SD | |
| Locator® | 7.93 N | 0.769 N |
| Kerator® | 10.5 N | 1.31 N |
| OT Equator® | 4.71 N | 1.29 N |
| Locator R-Tx® | 4.10 N | 0.871 N |
| Cleaning Solution Attachment System | Benfix® | |
| Mean | SD | |
| Locator® | 7.42 N | 1.49 N |
| Kerator® | 9.07 N | 1.34 N |
| OT Equator® | 4.23 N | 1.56 N |
| Locator R-Tx® | 3.10 N | 0.580 N |
| Cleaning Solution Attachment System | Kukident® | |
| Mean | SD | |
| Locator® | 7.20 N | 1.53 N |
| Kerator® | 8.25 N | 0.578 N |
| OT Equator® | 4.05 N | 0.843 N |
| Locator R-Tx® | 3.11 N | 1.04 N |
| Fmax (Mean ± SD) | Multiple Comparison Results | |
|---|---|---|
| 1. Kerator® | 9.76 ± 1.40 N | 1 vs. 2 (<0.001) 1 vs. 3 (<0.001) 1 vs. 4 (<0.001) |
| 2. Locator® | 7.59 ± 1.26 N | 2 vs. 3 (<0.001) 2 vs. 4 (<0.001) |
| 3. OT Equator® | 4.50 ± 1.21 N | 3 vs. 4 (<0.001) |
| 4. Locator R-Tx® | 3.60 ± 0.93 N |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monteiro, S.; Barreiros, P.; Mendes, J.; Aroso, C.; Silva, A.S.; Mendes, J.M. The Influence of Cleaning Solutions on the Retention of Overdenture Attachment Systems. Biomedicines 2023, 11, 1681. https://doi.org/10.3390/biomedicines11061681
Monteiro S, Barreiros P, Mendes J, Aroso C, Silva AS, Mendes JM. The Influence of Cleaning Solutions on the Retention of Overdenture Attachment Systems. Biomedicines. 2023; 11(6):1681. https://doi.org/10.3390/biomedicines11061681
Chicago/Turabian StyleMonteiro, Sofia, Pedro Barreiros, Joana Mendes, Carlos Aroso, António Sérgio Silva, and José Manuel Mendes. 2023. "The Influence of Cleaning Solutions on the Retention of Overdenture Attachment Systems" Biomedicines 11, no. 6: 1681. https://doi.org/10.3390/biomedicines11061681
APA StyleMonteiro, S., Barreiros, P., Mendes, J., Aroso, C., Silva, A. S., & Mendes, J. M. (2023). The Influence of Cleaning Solutions on the Retention of Overdenture Attachment Systems. Biomedicines, 11(6), 1681. https://doi.org/10.3390/biomedicines11061681










