Abnormal Resting-State Network Presence in Females with Overactive Bladder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Questionnaires
2.3. Pre- and Post-Measurement Procedures
2.4. MRI Recording
2.5. Resting-State fMRI Connectivity Analysis
2.6. Statistical Analyses of Population Characteristics (Including Clinical and Behavioral Data)
3. Results
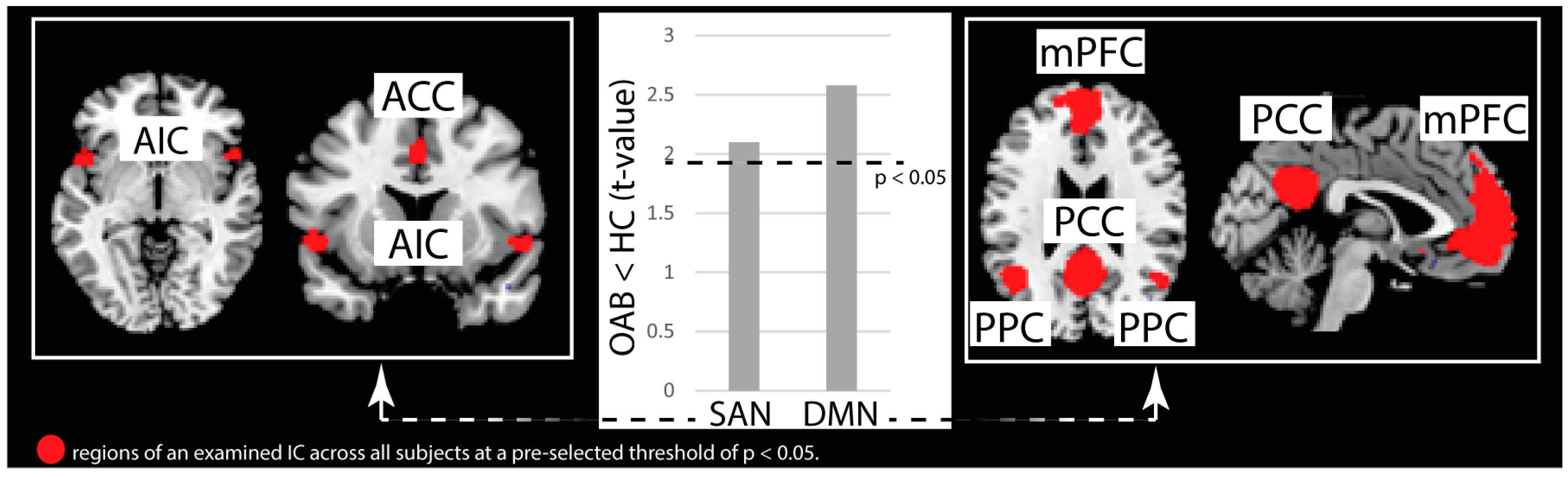
3.1. Independent Component Analysis
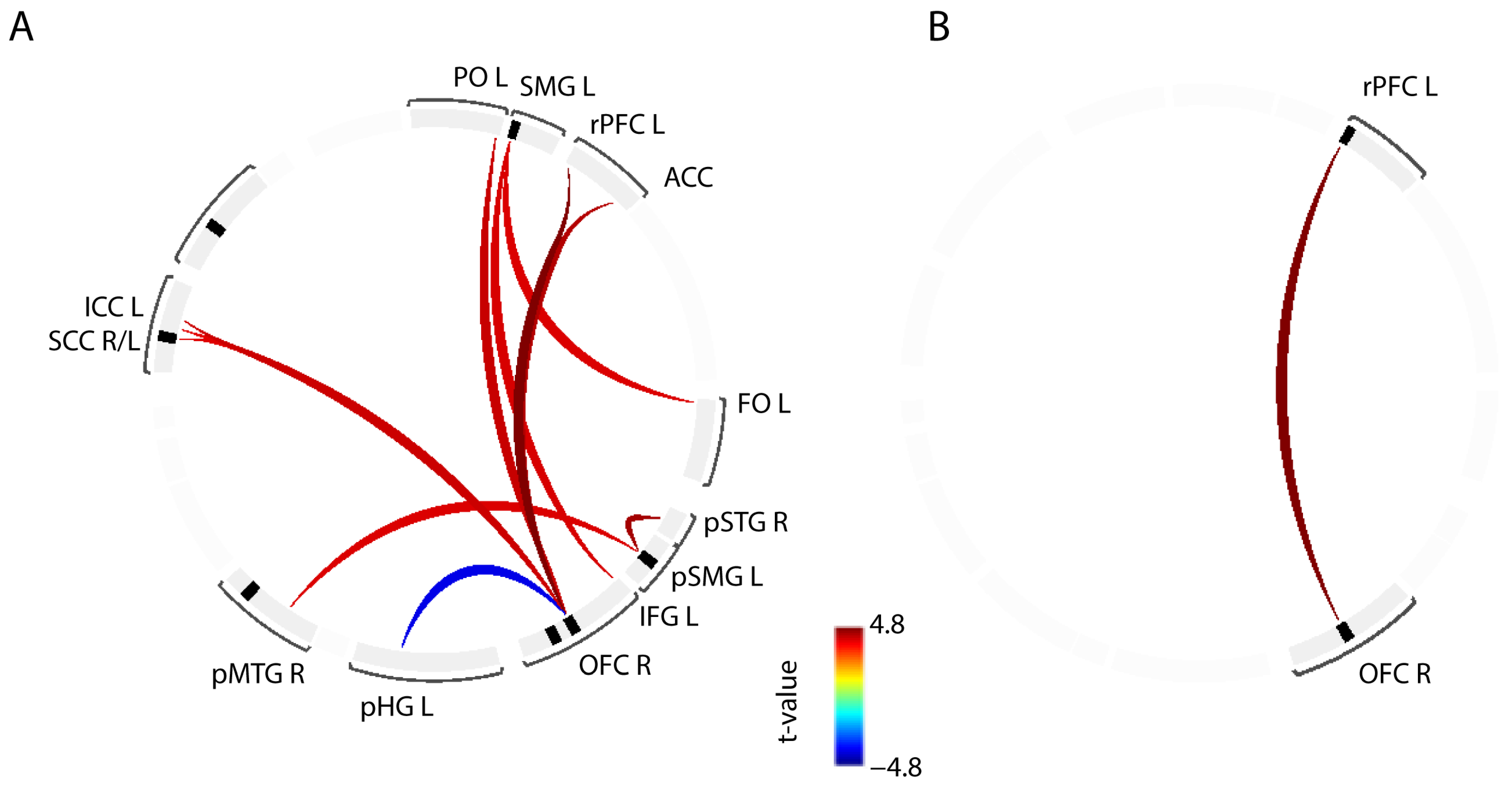
3.2. ROI-to-ROI Connectivity
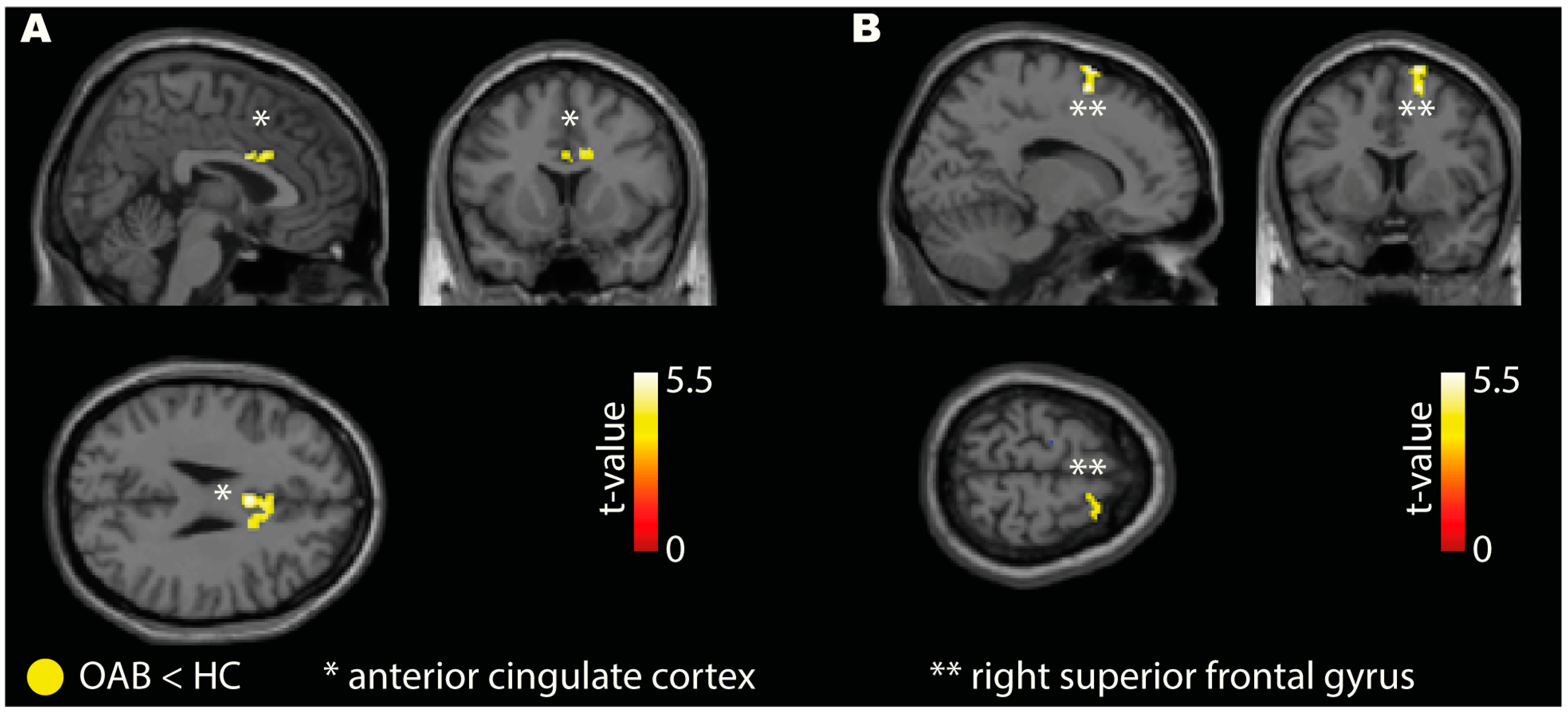
3.3. Seed-to-Voxel Connectivity
3.4. Directed Connectivity
3.5. Relation of OAB Symptoms to Functional Connectivity
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abrams, P.; Cardozo, L.; Fall, M.; Griffiths, D.; Rosier, P.; Ulmsten, U.; van Kerrebroeck, P.; Victor, A.; Wein, A.; Standardisation Sub-committee of the International Continence Society. The standardisation of terminology of lower urinary tract function: Report from the Standardisation Sub-committee of the International Continence Society. Neurourol. Urodyn. 2002, 21, 167–178. [Google Scholar] [CrossRef]
- Coyne, K.S.; Sexton, C.C.; Kopp, Z.S.; Ebel-Bitoun, C.; Milsom, I.; Chapple, C. The impact of overactive bladder on mental health, work productivity and health-related quality of life in the UK and Sweden: Results from EpiLUTS. BJU Int. 2011, 108, 1459–1471. [Google Scholar] [CrossRef] [PubMed]
- Irwin, D.E.; Milsom, I.; Hunskaar, S.; Reilly, K.; Kopp, Z.; Herschorn, S.; Coyne, K.; Kelleher, C.; Hampel, C.; Artibani, W.; et al. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: Results of the EPIC study. Eur. Urol. 2006, 50, 1306–1314; discussion 1305–1314. [Google Scholar] [CrossRef] [PubMed]
- Irwin, D.E.; Mungapen, L.; Milsom, I.; Kopp, Z.; Reeves, P.; Kelleher, C. The economic impact of overactive bladder syndrome in six Western countries. BJU Int. 2009, 103, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Ganz, M.L.; Smalarz, A.M.; Krupski, T.L.; Anger, J.T.; Hu, J.C.; Wittrup-Jensen, K.U.; Pashos, C.L. Economic costs of overactive bladder in the United States. Urology 2010, 75, 526–532.e18. [Google Scholar] [CrossRef]
- Durden, E.; Walker, D.; Gray, S.; Fowler, R.; Juneau, P.; Gooch, K. The economic burden of overactive bladder (OAB) and its effects on the costs associated with other chronic, age-related comorbidities in the United States. Neurourol. Urodyn. 2018, 37, 1641–1649. [Google Scholar] [CrossRef]
- Prasopsanti, K.; Santi-Ngamkun, A.; Pornprasit, K. Estimated cost of overactive bladder in Thailand. J. Med. Assoc. Thai. 2007, 90, 2316–2320. [Google Scholar]
- Abrams, P. Describing bladder storage function: Overactive bladder syndrome and detrusor overactivity. Urology 2003, 62, 28–37; discussion 40–22. [Google Scholar] [CrossRef]
- Tikkinen, K.A.; Auvinen, A. Does the imprecise definition of overactive bladder serve commercial rather than patient interests? Eur. Urol. 2012, 61, 746–748; discussion 749–750. [Google Scholar] [CrossRef]
- Peyronnet, B.; Mironska, E.; Chapple, C.; Cardozo, L.; Oelke, M.; Dmochowski, R.; Amarenco, G.; Game, X.; Kirby, R.; Van Der Aa, F.; et al. A Comprehensive Review of Overactive Bladder Pathophysiology: On the Way to Tailored Treatment. Eur. Urol. 2019, 75, 988–1000. [Google Scholar] [CrossRef] [Green Version]
- Marcelissen, T.; Cornu, J.N.; Antunes-Lopes, T.; Geavlete, B.; Delongchamps, N.B.; Rashid, T.; Rieken, M.; Rahnama’i, M.S. Management of Idiopathic Overactive Bladder Syndrome: What Is the Optimal Strategy After Failure of Conservative Treatment? Eur. Urol. Focus 2018, 4, 760–767. [Google Scholar] [CrossRef]
- Yeowell, G.; Smith, P.; Nazir, J.; Hakimi, Z.; Siddiqui, E.; Fatoye, F. Real-world persistence and adherence to oral antimuscarinics and mirabegron in patients with overactive bladder (OAB): A systematic literature review. BMJ Open 2018, 8, e021889. [Google Scholar] [CrossRef] [Green Version]
- Bosch, J.L.; Kelleher, C.; van Kerrebroeck, P.E.; Schurch, B. What treatment should we use if drugs fail for OAB; and, what really works after drugs? Neurourol. Urodyn. 2010, 29, 658–661. [Google Scholar] [CrossRef]
- Blok, B.F.; Holstege, G. The central control of micturition and continence: Implications for urology. BJU Int. 1999, 83 (Suppl. 2), 1–6. [Google Scholar] [CrossRef] [Green Version]
- de Groat, W.C.; Griffiths, D.; Yoshimura, N. Neural control of the lower urinary tract. Compr. Physiol. 2015, 5, 327–396. [Google Scholar] [CrossRef] [Green Version]
- Griffiths, D.; Tadic, S.D. Bladder control, urgency, and urge incontinence: Evidence from functional brain imaging. Neurourol. Urodyn. 2008, 27, 466–474. [Google Scholar] [CrossRef]
- Griffiths, D.J.; Tadic, S.D.; Schaefer, W.; Resnick, N.M. Cerebral control of the lower urinary tract: How age-related changes might predispose to urge incontinence. Neuroimage 2009, 47, 981–986. [Google Scholar] [CrossRef] [Green Version]
- Raichle, M.E.; MacLeod, A.M.; Snyder, A.Z.; Powers, W.J.; Gusnard, D.A.; Shulman, G.L. A default mode of brain function. Proc. Natl. Acad. Sci. USA 2001, 98, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Menon, V. Large-scale brain networks and psychopathology: A unifying triple network model. Trends Cogn. Sci. 2011, 15, 483–506. [Google Scholar] [CrossRef]
- Menon, V.; Uddin, L.Q. Saliency, switching, attention and control: A network model of insula function. Brain Struct. Funct. 2010, 214, 655–667. [Google Scholar] [CrossRef] [Green Version]
- Craig, A.D. Interoception: The sense of the physiological condition of the body. Curr. Opin. Neurobiol. 2003, 13, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.D. Human feelings: Why are some more aware than others? Trends Cogn. Sci. 2004, 8, 239–241. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.D. How do you feel—Now? The anterior insula and human awareness. Nat. Rev. Neurosci. 2009, 10, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Seeley, W.W. The Salience Network: A Neural System for Perceiving and Responding to Homeostatic Demands. J. Neurosci. 2019, 39, 9878–9882. [Google Scholar] [CrossRef] [PubMed]
- Seeley, W.W.; Menon, V.; Schatzberg, A.F.; Keller, J.; Glover, G.H.; Kenna, H.; Reiss, A.L.; Greicius, M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007, 27, 2349–2356. [Google Scholar] [CrossRef] [Green Version]
- Nardos, R.; Karstens, L.; Carpenter, S.; Aykes, K.; Krisky, C.; Stevens, C.; Gregory, W.T.; Fair, D.A. Abnormal functional connectivity in women with urgency urinary incontinence: Can we predict disease presence and severity in individual women using Rs-fcMRI. Neurourol. Urodyn. 2016, 35, 564–573. [Google Scholar] [CrossRef]
- Tadic, S.D.; Griffiths, D.; Schaefer, W.; Resnick, N.M. Abnormal connections in the supraspinal bladder control network in women with urge urinary incontinence. Neuroimage 2008, 39, 1647–1653. [Google Scholar] [CrossRef] [Green Version]
- Walter, M.; Leitner, L.; Betschart, C.; Engeler, D.S.; Freund, P.; Kessler, T.M.; Kollias, S.; Liechti, M.D.; Scheiner, D.A.; Michels, L.; et al. Considering non-bladder aetiologies of overactive bladder: A functional neuroimaging study. BJU Int. 2021, 128, 586–597. [Google Scholar] [CrossRef]
- Jackson, S.; Donovan, J.; Brookes, S.; Eckford, S.; Swithinbank, L.; Abrams, P. The Bristol Female Lower Urinary Tract Symptoms questionnaire: Development and psychometric testing. Br. J. Urol. 1996, 77, 805–812. [Google Scholar] [CrossRef]
- Coyne, K.S.; Thompson, C.L.; Lai, J.S.; Sexton, C.C. An overactive bladder symptom and health-related quality of life short-form: Validation of the OAB-q SF. Neurourol. Urodyn. 2015, 34, 255–263. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Whitfield-Gabrieli, S.; Nieto-Castanon, A. Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012, 2, 125–141. [Google Scholar] [CrossRef] [Green Version]
- Behzadi, Y.; Restom, K.; Liau, J.; Liu, T.T. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 2007, 37, 90–101. [Google Scholar] [CrossRef] [Green Version]
- Chai, X.J.; Castanon, A.N.; Ongur, D.; Whitfield-Gabrieli, S. Anticorrelations in resting state networks without global signal regression. Neuroimage 2012, 59, 1420–1428. [Google Scholar] [CrossRef] [Green Version]
- Ciric, R.; Wolf, D.H.; Power, J.D.; Roalf, D.R.; Baum, G.L.; Ruparel, K.; Shinohara, R.T.; Elliott, M.A.; Eickhoff, S.B.; Davatzikos, C.; et al. Benchmarking of participant-level confound regression strategies for the control of motion artifact in studies of functional connectivity. Neuroimage 2017, 154, 174–187. [Google Scholar] [CrossRef]
- Tzourio-Mazoyer, N.; Landeau, B.; Papathanassiou, D.; Crivello, F.; Etard, O.; Delcroix, N.; Mazoyer, B.; Joliot, M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002, 15, 273–289. [Google Scholar] [CrossRef]
- Calhoun, V.D.; Adali, T.; Pearlson, G.D.; Pekar, J.J. A method for making group inferences from functional MRI data using independent component analysis. Hum. Brain Mapp. 2001, 14, 140–151. [Google Scholar] [CrossRef]
- Sakoglu, U.; Mete, M.; Esquivel, J.; Rubia, K.; Briggs, R.; Adinoff, B. Classification of cocaine-dependent participants with dynamic functional connectivity from functional magnetic resonance imaging data. J. Neurosci. Res. 2019, 97, 790–803. [Google Scholar] [CrossRef]
- Sakoglu, U.; Pearlson, G.D.; Kiehl, K.A.; Wang, Y.M.; Michael, A.M.; Calhoun, V.D. A method for evaluating dynamic functional network connectivity and task-modulation: Application to schizophrenia. MAGMA 2010, 23, 351–366. [Google Scholar] [CrossRef] [Green Version]
- Ding, M.; Chen, Y.; Bressler, S.L. Granger Causality: Basic Theory and Application to Neuroscience. In Handbook of Time Series Analysis: Recent Theoretical Developments and Applications; Schelter, B., Winterhalder, M., Timmer, J., Eds.; Wiley: Hoboken, NJ, USA, 2006; pp. 437–460. [Google Scholar] [CrossRef] [Green Version]
- Slotnick, S.D.; Moo, L.R.; Segal, J.B.; Hart, J., Jr. Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Brain Res. Cogn. Brain Res. 2003, 17, 75–82. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate—A Practical and Powerful Approach to Multiple Testing. J. Roy. Stat. Soc. B. Met. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Pustejovsky, J.E. Converting from d to r to z when the design uses extreme groups, dichotomization, or experimental control. Psychol. Methods 2014, 19, 92–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howel, D.C. Statistical Methods for Psychology, 8th ed.; Wadsworth Publishing Co., Inc.: Belmont, CA, USA, 2013; p. 768. [Google Scholar]
- Rosenthal, R. Parametric measures of effect size. In The Handbook of Research Synthesis; Cooper, H., Hedges, L.V., Eds.; Russell Sage Foundation: New York, NY, USA, 1994. [Google Scholar]
- Amiri, M.; Murgas, S.; Stang, A.; Michel, M.C. Do overactive bladder symptoms and their treatment-associated changes exhibit a normal distribution? Implications for analysis and reporting. Neurourol. Urodyn. 2020, 39, 754–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erbing, F.; Schneider, T.; Igawa, Y.; Michel, M.C. Correlations of mean voided volume with other parameters of overactive bladder syndrome. Continence 2023, 5, 100577. [Google Scholar] [CrossRef]
- Dosenbach, N.U.; Fair, D.A.; Miezin, F.M.; Cohen, A.L.; Wenger, K.K.; Dosenbach, R.A.; Fox, M.D.; Snyder, A.Z.; Vincent, J.L.; Raichle, M.E.; et al. Distinct brain networks for adaptive and stable task control in humans. Proc. Natl. Acad. Sci. USA 2007, 104, 11073–11078. [Google Scholar] [CrossRef] [Green Version]
- Dosenbach, N.U.; Visscher, K.M.; Palmer, E.D.; Miezin, F.M.; Wenger, K.K.; Kang, H.C.; Burgund, E.D.; Grimes, A.L.; Schlaggar, B.L.; Petersen, S.E. A core system for the implementation of task sets. Neuron 2006, 50, 799–812. [Google Scholar] [CrossRef] [Green Version]
- Etkin, A.; Egner, T.; Peraza, D.M.; Kandel, E.R.; Hirsch, J. Resolving emotional conflict: A role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron 2006, 51, 871–882. [Google Scholar] [CrossRef] [Green Version]
- Crottaz-Herbette, S.; Menon, V. Where and when the anterior cingulate cortex modulates attentional response: Combined fMRI and ERP evidence. J. Cogn. Neurosci. 2006, 18, 766–780. [Google Scholar] [CrossRef]
- Zuo, L.; Zhou, Y.; Wang, S.; Wang, B.; Gu, H.; Chen, J. Abnormal Brain Functional Connectivity Strength in the Overactive Bladder Syndrome: A Resting-State fMRI Study. Urology 2019, 131, 64–70. [Google Scholar] [CrossRef]
- Zuo, L.; Chen, J.; Wang, S.; Zhou, Y.; Wang, B.; Gu, H. Intra- and inter-resting-state networks abnormalities in overactive bladder syndrome patients: An independent component analysis of resting-state fMRI. World J. Urol. 2020, 38, 1027–1034. [Google Scholar] [CrossRef]
- Ketai, L.H.; Komesu, Y.M.; Dodd, A.B.; Rogers, R.G.; Ling, J.M.; Mayer, A.R. Urgency urinary incontinence and the interoceptive network: A functional magnetic resonance imaging study. Am. J. Obs. Gynecol. 2016, 215, 449.e1–449.e17. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Zuo, L.; Zhou, Y.; Gu, H.; Wang, S. Abnormal resting-state brain activity and connectivity of brain-bladder control network in overactive bladder syndrome. Acta Radiol. 2022, 63, 1695–1702. [Google Scholar] [CrossRef]
- Aminoff, E.M.; Kveraga, K.; Bar, M. The role of the parahippocampal cortex in cognition. Trends Cogn. Sci. 2013, 17, 379–390. [Google Scholar] [CrossRef] [Green Version]
- Qin, C.; Greenwood-Van Meerveld, B.; Foreman, R.D. Spinal neuronal responses to urinary bladder stimulation in rats with corticosterone or aldosterone onto the amygdala. J. Neurophysiol. 2003, 90, 2180–2189. [Google Scholar] [CrossRef] [Green Version]
- Randich, A.; DeWitte, C.; DeBerry, J.J.; Robbins, M.T.; Ness, T.J. Lesions of the central amygdala and ventromedial medulla reduce bladder hypersensitivity produced by acute but not chronic foot shock. Brain Res. 2017, 1675, 1–7. [Google Scholar] [CrossRef]
- Blok, B.F.; Groen, J.; Bosch, J.L.; Veltman, D.J.; Lammertsma, A.A. Different brain effects during chronic and acute sacral neuromodulation in urge incontinent patients with implanted neurostimulators. BJU Int. 2006, 98, 1238–1243. [Google Scholar] [CrossRef]
- Uddin, L.Q.; Yeo, B.T.T.; Spreng, R.N. Towards a Universal Taxonomy of Macro-scale Functional Human Brain Networks. Brain Topogr. 2019, 32, 926–942. [Google Scholar] [CrossRef]
- Griffiths, D.; Derbyshire, S.; Stenger, A.; Resnick, N. Brain control of normal and overactive bladder. J. Urol. 2005, 174, 1862–1867. [Google Scholar] [CrossRef]
- Zhang, H.; Reitz, A.; Kollias, S.; Summers, P.; Curt, A.; Schurch, B. An fMRI study of the role of suprapontine brain structures in the voluntary voiding control induced by pelvic floor contraction. Neuroimage 2005, 24, 174–180. [Google Scholar] [CrossRef]
- Kuhtz-Buschbeck, J.P.; van der Horst, C.; Wolff, S.; Filippow, N.; Nabavi, A.; Jansen, O.; Braun, P.M. Activation of the supplementary motor area (SMA) during voluntary pelvic floor muscle contractions—An fMRI study. Neuroimage 2007, 35, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Tadic, S.D.; Griffiths, D.; Schaefer, W.; Murrin, A.; Clarkson, B.; Resnick, N.M. Brain activity underlying impaired continence control in older women with overactive bladder. Neurourol. Urodyn. 2012, 31, 652–658. [Google Scholar] [CrossRef] [Green Version]
- Gross, J.; Vetter, J.M.; Lai, H.H. Clustering of patients with overactive bladder syndrome. BMC Urol. 2021, 21, 41. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, D.; Clarkson, B.; Tadic, S.D.; Resnick, N.M. Brain Mechanisms Underlying Urge Incontinence and its Response to Pelvic Floor Muscle Training. J. Urol. 2015, 194, 708–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martins, R.; Joanette, Y.; Monchi, O. The implications of age-related neurofunctional compensatory mechanisms in executive function and language processing including the new Temporal Hypothesis for Compensation. Front. Hum. Neurosci. 2015, 9, 221. [Google Scholar] [CrossRef] [Green Version]
- Arya, N.G.; Weissbart, S.J.; Xu, S.; Rao, H. Brain activation in response to bladder filling in healthy adults: An activation likelihood estimation meta-analysis of neuroimaging studies. Neurourol. Urodyn. 2017, 36, 960–965. [Google Scholar] [CrossRef]
- Griffiths, D. Neural control of micturition in humans: A working model. Nat. Rev. Urol. 2015, 12, 695–705. [Google Scholar] [CrossRef]
- Aron, A.R.; Robbins, T.W.; Poldrack, R.A. Inhibition and the right inferior frontal cortex. Trends Cogn. Sci. 2004, 8, 170–177. [Google Scholar] [CrossRef]
- Michels, L.; Blok, B.F.; Gregorini, F.; Kurz, M.; Schurch, B.; Kessler, T.M.; Kollias, S.; Mehnert, U. Supraspinal Control of Urine Storage and Micturition in Men—An fMRI Study. Cereb. Cortex 2015, 25, 3369–3380. [Google Scholar] [CrossRef]
- Griffiths, D.; Tadic, S.D.; Schaefer, W.; Resnick, N.M. Cerebral control of the bladder in normal and urge-incontinent women. Neuroimage 2007, 37, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Cavanna, A.E.; Trimble, M.R. The precuneus: A review of its functional anatomy and behavioural correlates. Brain 2006, 129, 564–583. [Google Scholar] [CrossRef] [Green Version]
- Nabi, G.; Cody, J.D.; Ellis, G.; Herbison, P.; Hay-Smith, J. Anticholinergic drugs versus placebo for overactive bladder syndrome in adults. Cochrane Database Syst. Rev. 2006, 2006, CD003781. [Google Scholar] [CrossRef]
- Pang, D.; Liao, L.; Chen, G.; Wang, Y. Sacral Neuromodulation Improves Abnormal Prefrontal Brain Activity in Patients with Overactive Bladder: A Possible Central Mechanism. J. Urol. 2022, 207, 1256–1267. [Google Scholar] [CrossRef]
- Gill, B.C.; Pizarro-Berdichevsky, J.; Bhattacharyya, P.K.; Brink, T.S.; Marks, B.K.; Quirouet, A.; Vasavada, S.P.; Jones, S.E.; Goldman, H.B. Real-Time Changes in Brain Activity during Sacral Neuromodulation for Overactive Bladder. J. Urol. 2017, 198, 1379–1385. [Google Scholar] [CrossRef]
- Peng, L.; Yan, L.; Benkang, S.; Qiujie, Z.; Hu, G. Comparison of different types of therapy for overactive bladder: A systematic review and network meta-analysis. Front. Med. 2022, 9, 1014291. [Google Scholar] [CrossRef]
- Fowler, C.J.; Griffiths, D.J. A decade of functional brain imaging applied to bladder control. Neurourol. Urodyn. 2010, 29, 49–55. [Google Scholar] [CrossRef]
- Zare, A.; Jahanshahi, A.; Rahnama′i, M.S.; Schipper, S.; van Koeveringe, G.A. The Role of the Periaqueductal Gray Matter in Lower Urinary Tract Function. Mol. Neurobiol. 2019, 56, 920–934. [Google Scholar] [CrossRef]
- Coulombe, M.A.; Erpelding, N.; Kucyi, A.; Davis, K.D. Intrinsic functional connectivity of periaqueductal gray subregions in humans. Hum. Brain Mapp. 2016, 37, 1514–1530. [Google Scholar] [CrossRef]
- World Medical Association. Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Subjects. Available online: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ (accessed on 22 February 2023).
- Swiss Federal Office of Public Health. Swiss Federal Human Research Act (SR 810.30). Available online: https://www.bag.admin.ch/bag/en/home/gesetze-und-bewilligungen/gesetzgebung/gesetzgebung-mensch-gesundheit/gesetzgebung-forschung-am-menschen.html (accessed on 22 February 2023).

| Group | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| All |
|
|
| OAB |
| |
| HC |
|
|
| Baseline Characteristics | OAB Group (n = 12) | HC Group (n = 12) | Group Difference (95% CI) [OAB − HC Group] |
|---|---|---|---|
| Demographics | |||
| Age (years) | 40 (31–43) | 34 (28–45) | 6 (−6, 18) |
| Weight (kg) | 70 (57–77) | 58 (56–65) | 12 (1, 23) |
| 3-day bladder diary | |||
| Fluid intake per 72 h (mL) | 6075 (4550–6470) | 5550 (4405–7900) | 525 (−1833, 2883) |
| Urinary frequency (no. of micturitions) per 72 h | 27 (25–32) | 18 (16–19) | 9 (5, 13) |
| Average voided volume per micturition (mL) | 242 (161–261) | 302 (236–419) | −60 (−192, 72) |
| Voided volume per 72 h (mL) | 6285 (4805–7580) | 5020 (4145–7163) | 1265 (−924, 3454) |
| No. of urinary urgency episodes per 72 h | 13 (9–18) | 0 (0–0) | 13 (7, 18) |
| No. of urgency urinary incontinence episodes per 72 h | 2 (0–5) | 0 (0–0) | 2 (−1, 5) |
| Questionnaires | |||
| ICIQ-FLUTS (score range 0–48) | 13 (12–16) | 2 (1–3) | 11 (8, 13) |
| Filling (score range 0–16) | 7 (5–8) | 1 (1–2) | 6 (4, 8) |
| Voiding (score range 0–12) | 1 (0–3) | 0 (0–1) | 1 (−1, 3) |
| Incontinence (score range 0–20) | 5 (4–7) | 0 (0–1) | 5 (3, 6) |
| OAB-q SF | |||
| Symptoms (transformed score range 0–100) | 52 (35–70) | 3 (0–8) | 48 (32, 65) |
| Quality of life (transformed score range 0–100) | 65 (58–69) | 98 (97–100) | −34 (−42, −25) |
| HADS | |||
| Anxiety (score range 0–21, cut-off ≤ 7) | 6 (4.5–8.5) | 2.5 (1.5–4) | 3.5 (1.2, 5.8) |
| Depression (score range 0–21, cut-off ≤ 7) | 3.5 (2.5–4) | 0 (0–0.5) | 3.5 (2.2, 4.8) |
| MMSE (score range 0–30, cut-off ≥ 24) | 29.5 (29–30) | 29 (29–30) | 0.5 (−0.7, 1.7) |
| Urodynamic investigation | |||
| Filling cystometry | |||
| First sensation of filling (mL) | 80 (65–110) | 23 (8–150) | 58 (−34, 149) |
| First desire to void (mL) | 163 (120–293) | 268 (128–373) | −105 (−267, 57) |
| Strong desire to void (mL) | 305 (190–420) | 520 (388–660) | −215 (−413, −17) |
| Maximum cystometric capacity (mL) | 378 (258–498) | 643 (550–720) | −265 (−418, −112) |
| Maximum detrusor pressure (cmH2O) | 10 (3–22) | 3.5 (2.5–8) | 7 (−7, 20) |
| Detrusor overactivity, n (proportion) | 7 (0.58) | 0 (0) | 0.58 (0.3, 0.86) |
| Pressure-Flow-Study | |||
| Voided volume (mL) | 360 (155–390) | 643 (550–723) | −283 (−413, −152) |
| Maximum flow rate (mL/s) | 21 (11–33) | 23 (21–33) | −2 (−15, 12) |
| Maximum detrusor pressure (cmH2O) | 33 (17–74) | 37 (26–42) | −4 (−30, 22) |
| Detrusor pressure at maximum flow rate (cmH2O) | 25 (11–40) | 26 (19–32) | −1 (−21, 19) |
| Post void residual volume (mL) | 0 (0–45) | 0 (0–0) | 0 (−28, 28) |
| ROI-to-ROI | t-Value | Cohen’s d |
|---|---|---|
| rPFC L—OFC R | 4.80 | 2.05 |
| pSTG R—pSMG L | 4.46 | 1.90 |
| ACC—OFC R | 4.31 | 1.84 |
| PO L—OFC R | 4.13 | 1.76 |
| ICC L—OFC R | 4.10 | 1.75 |
| IFG L—SMG L | 3.98 | 1.70 |
| pSMG L—pMTG R | 3.94 | 1.68 |
| FO L—SMG L | 3.92 | 1.67 |
| SCC R—OFC R | 3.91 | 1.67 |
| SCC L—OFC R | 3.85 | 1.64 |
| pHG L—OFC R | −3.82 | −1.63 |
| Contrast | Seed | Connected Brain Region | Cluster Size | MNI—Coordinates | t-Value | Cohen’s d | ||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | ||||||
| OAB < HC | right AIC | ACC | 77 | 0 | 8 | 28 | 5.24 | 2.23 |
| IFG | 28 | 48 | 24 | 16 | 6.27 | 2.67 | ||
| SPL | 23 | 20 | −46 | 54 | 5.77 | 2.46 | ||
| MFG | 20 | 40 | 0 | 46 | 4.59 | 1.96 | ||
| Calcarine cortex | 19 | 14 | −86 | 2 | 5.14 | 2.19 | ||
| Cerebellum | 18 | −24 | −78 | −26 | 4.82 | 2.06 | ||
| right VLPFC | SFG | 63 | 16 | 8 | 62 | 5.40 | 2.30 | |
| OAB > HC | right AIC | No connectivity differences at the specified threshold | ||||||
| right VLPFC | No connectivity differences at the specified threshold | |||||||
| (a) | ||||||||||||||
| Correlation Direction | Seed: Right AIC | Seed: Right VLPFC | ||||||||||||
| Brain Region | Cluster Size | MNI—Coordinates | Brain Region | Cluster Size | MNI—Coordinates | |||||||||
| X | Y | Z | X | Y | Z | |||||||||
| positive | MTG | 56 | 36 | 2 | −26 | THA | 27 | −14 | −24 | 14 | ||||
| MTG | 42 | −66 | −22 | −10 | ||||||||||
| TP | 35 | −42 | 8 | −40 | ||||||||||
| OP | 31 | −28 | −96 | 10 | ||||||||||
| LOC | 26 | −40 | −86 | 10 | ||||||||||
| negative | postCG | 58 | 14 | −42 | 78 | No correlations at the specified threshold | ||||||||
| postCG | 41 | −12 | −42 | 80 | ||||||||||
| preCG | 33 | −56 | 0 | 20 | ||||||||||
| IFG | 30 | 52 | 10 | 10 | ||||||||||
| (b) | ||||||||||||||
| Correlation Direction | Seed: Right AIC | Seed: Right VLPFC | ||||||||||||
| Brain Region | Cluster Size | MNI—Coordinates | Brain Region | Cluster Size | MNI—Coordinates | |||||||||
| X | Y | Z | X | Y | Z | |||||||||
| positive | LOC | 15 | −56 | −8 | 26 | No correlations at the specified threshold | ||||||||
| negative | postCG | 21 | −22 | 48 | −6 | |||||||||
| FP | 11 | 30 | −88 | 4 | ||||||||||
| (c) | ||||||||||||||
| Correlation Direction | Seed: Right AIC | Seed: Right VLPFC | ||||||||||||
| Brain Region | Cluster Size | MNI—Coordinates | Brain Region | Cluster Size | MNI—Coordinates | |||||||||
| X | Y | Z | X | Y | Z | |||||||||
| positive | CERE | 13 | 40 | −62 | −24 | Brain stem | 14 | 4 | −12 | −36 | ||||
| negative | LOC | 37 | −16 | −64 | 46 | PUT | 17 | 22 | 8 | −4 | ||||
| PREC | 13 | −6 | −46 | 56 | LG | 13 | −28 | −48 | −6 | |||||
| SPL | 12 | 46 | −42 | 58 | IFG | 12 | −46 | 14 | 16 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehnert, U.; Walter, M.; Leitner, L.; Kessler, T.M.; Freund, P.; Liechti, M.D.; Michels, L. Abnormal Resting-State Network Presence in Females with Overactive Bladder. Biomedicines 2023, 11, 1640. https://doi.org/10.3390/biomedicines11061640
Mehnert U, Walter M, Leitner L, Kessler TM, Freund P, Liechti MD, Michels L. Abnormal Resting-State Network Presence in Females with Overactive Bladder. Biomedicines. 2023; 11(6):1640. https://doi.org/10.3390/biomedicines11061640
Chicago/Turabian StyleMehnert, Ulrich, Matthias Walter, Lorenz Leitner, Thomas M. Kessler, Patrick Freund, Martina D. Liechti, and Lars Michels. 2023. "Abnormal Resting-State Network Presence in Females with Overactive Bladder" Biomedicines 11, no. 6: 1640. https://doi.org/10.3390/biomedicines11061640
APA StyleMehnert, U., Walter, M., Leitner, L., Kessler, T. M., Freund, P., Liechti, M. D., & Michels, L. (2023). Abnormal Resting-State Network Presence in Females with Overactive Bladder. Biomedicines, 11(6), 1640. https://doi.org/10.3390/biomedicines11061640








