Abstract
Infections are important factors contributing to the morbidity and mortality among elderly patients. High rates of consumption of antimicrobial agents by the elderly may result in increased risk of toxic reactions, deteriorating functions of various organs and systems and leading to the prolongation of hospital stay, admission to the intensive care unit, disability, and lethal outcome. Both safety and efficacy of antibiotics are determined by the values of their plasma concentrations, widely affected by physiologic and pathologic age-related changes specific for the elderly population. Drug absorption, distribution, metabolism, and excretion are altered in different extents depending on functional and morphological changes in the cardiovascular system, gastrointestinal tract, liver, and kidneys. Water and fat content, skeletal muscle mass, nutritional status, use of concomitant drugs are other determinants of pharmacokinetics changes observed in the elderly. The choice of a proper dosing regimen is essential to provide effective and safe antibiotic therapy in terms of attainment of certain pharmacodynamic targets. The objective of this review is to perform a structure of evidence on the age-related changes contributing to the alteration of pharmacokinetic parameters in the elderly.
1. Introduction
The global population aging in the 21st century is unprecedented. In the Western world persons over 65 years are the fastest growing cohort [1], which outnumbers the population of children below five years old and attracts the attention of researchers all over the world [2]. It is predicted that by 2100 in Europe people over 65 will make up 31% of the total population, and people over 80 will reach about 15% [3].
Age-related physiological and pathological changes, poor functional status, poor nutrition, and comorbidities predispose older adults to infections and their complications [4,5]. The incidence and severity of infections increase with advancing age [6,7]. Compared to younger age groups, elderly patients are more prone to pneumonia, skin and soft tissue infections, urinary tract infections and septicemia [1,4]. An additional problem is a substantial risk of antibiotic (AB) resistance; its typical risk factors in the elderly include frequent contact with the healthcare system, frequent AB exposure, depressed immune system, frailty, and comorbidity [4]. Elderly patients are considered the high-risk group for the development of healthcare-associated infections caused by multidrug-resistant (MDR) bacteria [8,9,10]. The elderly population has longer hospital stays compared to younger adults and a significantly higher mortality rate (25%) compared to the general population (10%) [6,11,12]. Infections aggravate the course of concomitant chronic diseases, including cardiovascular and cognitive disorders, and contribute to the emergence of a new comorbidity [13].
High infectious morbidity leads to high consumption of antimicrobial agents by the elderly. ABs are among the most frequently prescribed medicines to seniors [14,15,16] and their use is accompanied by a significant rate of side effects and clinically relevant drug-drug interactions compared to younger counterparts [14]. Adverse drug reactions (ADRs) are an important cause of morbidity and mortality in the elderly [17,18,19] and their risk is significantly increased in the presence of comorbidity and polypharmacy [19,20,21,22].
The choice of optimal antimicrobial agent for the elderly is challenging [23]. Finding the right balance between efficacy, safety and tolerability of antibiotics is difficult for several reasons including significant changes in body tissue composition, a progressive physiological decline of organ functions, frailty, comorbidity, and polypharmacy [24]. All these factors can cause significant alterations in antimicrobials pharmacokinetics (PK) and pharmacodynamics (PD) leading to altered efficacy, safety, and tolerance. The problem is compounded by the fact that elderly patients represent a heterogeneous group that should be treated individually [25,26].
This review includes an analysis of the available data on the PK of antibacterial agents in the elderly and a consideration of the critical issues of AB use in this vulnerable heterogenous population. We used the PubMed database to retrieve relevant articles dedicated to the pharmacokinetic studies of antibacterial agents in the elderly published during the period 980–2023 years.
2. Factors Influencing AB Prescribing in the Elderly
Infections in the elderly may be caused by a more diverse group of pathogens compared to the younger population [6,27,28]. For example, there is a higher prevalence of Gram-negative bacilli in pneumonia and a lower prevalence of E. coli in urinary tract infections [6].
Common infections do not manifest with classic symptoms in the elderly. Aged patients may have neither fever nor leukocytosis [29]. An absence of fever and a lack of respiratory symptoms has been described in 40–60% of elderly patients with community-acquired pneumonia [30]. The only clinical presentation of pneumonia in up to 20–50% of the elderly may be an altered mental status including delirium and confusion, a sudden decline in functional capacity, and worsening of underlying diseases [28]. The high prevalence of unusual and/or multidrug-resistant pathogens in the elderly makes AB susceptibility testing highly desirable, though in the real clinical practice, antibiotics may be prescribed empirically as even subtle clinical manifestations may herald the onset of life-threatening infectious disease and delayed therapy can worsen treatment outcomes [28].
Appropriate AB dosing requires knowledge of the pharmacokinetic and pharmacodynamic properties of AB which are often altered due to aging processes, including age- or disease-related decline of kidney and liver functions. To select an optimal AB for the concrete patient, it is necessary to identify all comorbidities, concomitant drugs, and dietary supplements, and collect the patient’s allergic history. Consideration should be given to the factors associated with poor treatment compliance such as poor vision and/or hearing, physical dexterity, cognitive impairment, or mental illness [21,31,32,33]. These patients require treatment supervision by relatives or caregivers.
Elderly patients are at high risk of potential harm associated both with missed treatment and excessive AB therapy [31,34]. In long-term care facilities 50–75% of residents receive at least one course of AB each year with 30–50% of AB prescriptions being unnecessary or inappropriate in terms of drug choice, dosing regimen and/or duration of treatment [35]. Inappropriate drug selection and use may lead to medication-related problems, including ADRs, therapy failure and withdrawal events alongside the spread of AB resistance [34,36].
Compared to younger counterparts, older patients are more vulnerable to AB side effects and clinically relevant consequences of drug interactions [14]. The risk of ADR development is especially high in patients with comorbidity and polypharmacy [19,20,21,22]. ADRs are an important cause of morbidity and mortality in elderly patients [17,18,19].
Increased risk of AB-induced toxicity, ADRs, and negative outcomes in elderly patients with infectious diseases may be mediated by the changed PK of AB resulting in the changed PD.
3. General Considerations on AB Pharmacokinetics in the Elderly
The development of knowledge of the antimicrobial PK/PD relationship is essential to provide maximization of the efficacy, minimization of the toxicity, and preservation of the lifespan of currently available antibiotics [37].
PK characterizes the concentration time course of antibiotics based on absorption, distribution, metabolism, and elimination [38]. The value of plasma concentration being the result of PK processes is the main determinant of PD. PD characterizes parameters of antibacterial activity and describes the effect of AB on the target pathogens, relying on the minimum inhibitory concentration (MIC) [38,39,40].
The quantitative relationship between PK parameters and PD parameters is described by pharmacokinetic/pharmacodynamic (PK/PD) indices [41]. These indices should be used in patients with critical illness, central nervous system infections, severe burns, severe impairment of renal function, severe hypoalbuminemia, morbid obesity, and other medically complicated conditions. AB efficacy is described by the next PK/PD indices: the ratio of the area under the concentration-time curve (AUC) from zero to 24 h (AUC0–24) to the MIC, the ratio of the maximum plasma concentration (Cmax) to the MIC and % of the time during which the free plasma concentration exceeds the MIC (% fT > MIC) [42].
Based on PK/PD indices antibacterials can be divided into 3 groups [38]:
- time-dependent (β-lactams, natural macrolides, lincosamides, oxazolidinones),
- concentration-dependent (aminoglycosides, fluoroquinolones, nitroimidazoles, daptomycin, quinupristin/dalfopristin),
- concentration-dependent with time-dependence (tetracyclines, glycylcyclines, glycopeptides, semisynthetic macrolides).
The efficacy of time-dependent antibiotics is mainly related to %fT > MIC. A significant increase in concentration does not enhance the antibacterial effect of these antibiotics, hence dosing regimens maintaining stable drug concentrations above the MIC are preferred [43].
The efficacy of concentration-dependent antibiotics is defined by Cmax/MIC ratio. Antibacterial activity of this group increases with increasing concentration of AB; therefore, treatment success is determined by a larger dose of AB with less frequency of administration [43].
The efficacy of concentration-dependent drugs with time-dependence is determined mainly by the ratio AUC0-24/MIC [44]. The aim of treatment with this type of antibiotic is to maximize the patient’s overall exposure to the drug [44].
The PK of antibiotics in the elderly may significantly differ from that in younger adults. Age-associated PK changes strongly depend on the patient’s individual characteristics, including individual rate of senescence of organs and tissues, comorbidity profile, presence, and rate of progression of geriatric syndromes (frailty is one of the most important), and severity of the main disease. The main contributors to altered pharmacokinetics in the elderly are age-related changes in organ mass and blood circulation alongside changes in body composition, and disease-associated changes in the organs and systems functioning.
3.1. Absorption
Drug absorption is an essential process of drug transport from the site of administration to the systemic blood flow. Depending on the route of administration, the rate of absorption may vary, resulting in a certain value of bioavailability—the fraction of unmetabolized drug that reaches systemic circulation. The main PK parameters describing absorption are AUC and Cmax. Decreased absorption leads to the decreased concentration of AB, and thus, to the decrease of treatment efficacy. On the contrary, increased absorption may result in increased concentrations and, thus, increased risks of toxic effects.
The most convenient route of drug administration is oral. In the elderly, there are both structural and functional changes of the gastrointestinal tract (GIT) which may affect absorption, and, thus, bioavailability of AB. Senescence results in the decrease of salivary glands secretion and changed the quality of saliva, atrophic changes in the GIT mucosa, damage of enteric neurons, gastric secretion, and GIT motility [45]. Elderly patients are characterized by delayed gastric emptying, reduced GIT blood flow, and alterations in pH, typically hypochlorhydria [8]. Since elderly patients are characterized by the presence of different chronic diseases and a high burden of polypharmacy, these factors should also be considered together with the physiological changes. Age-associated GIT alterations may be worsened in the presence of some comorbidities, drug interactions and ADRs. Senescence-related changes and drug-induced changes of GIT contributing to the PK changes are demonstrated in Table 1.

Table 1.
Senescence-related changes of GIT, drug-induced changes of GIT and their effects on PK.
Age-associated and PPI-induced hypochlorhydria may result in the decreased absorption, and, thus, bioavailability of such antibacterials, as azithromycin, erythromycin, cefaclor, ceftibuten, sulfonamides [8], decreased intestinal motility and blood flow may lead to the decreased bioavailability of cefpodoxime proxetil [8]. The reduced first-pass metabolism in the elderly may lead to some decrease in active moiety formation from the prodrug form. Among antibacterials, there are several prodrugs. Ampicillin prodrugs include pivampicillin, talampicillin bacampicillin, and hetacillin (all are esters), another is sultamicillin (ampicillin linked to sulbactam by a methylene group) [77]. Examples of prodrugs among cephalosporines include ceftaroline fosamil and a combined form, novel cephalosporin-fluoroquinolone prodrug, including ciprofloxacin linked to the cephem core via the carboxylic acid [78]. An old antibacterial agent in the prodrug form is metronidazole, which is activated through reduction with redox active form production. The effect of the changed intestinal permeability on the absorption of antibacterials may be well illustrated with an example of inflammatory bowel disease, which incidence is rising in the elderly [79]. For patients with inflammatory bowel disease, a decrease of metronidazole exposure after oral intake was demonstrated as well as alterations of bioavailability of many other drugs administered orally [80].
Absorption is also affected by the number and function of transport proteins in the GIT. One of the most important transport proteins, P-glycoprotein (P-gp) revealed nearly no change in the elderly compared with younger adults, though intestinal P-gp activity was significantly reduced in the elderly with renal failure [81]. The same change was demonstrated for the organic anion transporter polypeptides 1B1 (OATP1B1), those activity was reduced in the elderly with chronic kidney disease (CKD) compared to healthy young participants and healthy elderly patients [81]. The activity of another intestinal efflux transporter, breast cancer resistant protein (BCRP), revealed a marked decrease both in the healthy elderly and those with CKD [81]. Estimation of BCRP expression in the liver demonstrated the same decline with age, being the lowest in the elderly compared with adults and children [82]. Aging also may also affect the expression of peptide transporters 1 and 2 (PepT1 and 2), which are involved in the uptake of di- or tripeptide substrates in such locations, as the intestine, kidneys, bile duct epithelium (PepT1), brain, lung, and mammary gland (PepT2) [83,84]. PepT1 and Pept2 expression may be altered in the presence of age-related diseases including diabetes mellitus (downregulation of PepT1) and obesity (leptin-dependent activation of PepT1 activity and expression) [83].
Some age-associated changes of transport proteins may contribute to the altered PK of AB, though genetic polymorphism is considered to be a more important factor resulting in these changes [85]. The only transport protein whose expression was found to be age-dependent was revealed with a quantitative review of age- vs. genotype-related differences, including P-gp [85]. Different results were demonstrated with liquid chromatography tandem mass spectroscopy transport proteins quantification: no age correlation was estimated for the hepatic protein expression of OATP1B1, OATP1B3, OATP2B1, or P-gp (p < 0.05) [86]. In general, there are limited studies aimed at the estimation of age-associated transport protein changes in humans. Animal studies declare a decline of gene expression and mRNA expression of most OATPs with aging (OATP1A1 OATP1A2, OATP1A4, OATP1A5 and OATP1B2), as well as of P-gp and multidrug-resistance like proteins 1 and 2 (MRP1, MRP2) [87,88,89].
The summary of age-associated changes of the main transport proteins involved in AB transport along with data on antibiotic-substrates is given in Table 2.

Table 2.
Age-associated changes of transport proteins and data on their antibiotic-substrates.
Absorption of orally administered antibiotics may be affected by food intake. For highly lipophilic drugs absorption is increased in the fed state. Food also changes acidity values in different departments of GIT, affecting the absorption of weak acids and bases. Passive diffusion and thus a high rate of absorption is specific for the unionized forms, and their formation depends upon the dissociation constant of the drug in the physiological pH range [108]. Fed state results in the increase of acidity in the stomach and, in less extent, in the colon. On the opposite, in the duodenum, jejunum and ileum fed state lead to the decrease of acidity. With pH greater than 5 ionization rate of weak bases is dramatically decreased, while for weak acids it is significantly increased [109]. Depending on the drug, taking on an empty stomach (preprandial) or with a meal (postprandial) may be recommended. In the elderly, for whom cognitive disorders are specific, as well as multiple comorbidities and polypharmacy, the risk of not meeting these recommendations is high, resulting in the decreased efficacy of treatment or increased risks of toxic reactions [109]. Age-associated changes also may aggravate fast-fed variabilities. Choosing antibiotics with high absorption regardless of food is strongly recommended for the elderly. Table 3 contains information about food effects on antibiotics absorption and related PK parameters along with recommendations on the proper administration.

Table 3.
Food effects on PK parameters of antibiotics.
Drug absorption in non-oral routes of administration (injection, inhalation) may also be affected by age-associated changes. Considering intramuscular drug administration skeletal muscle mass decrease and hypoperfusion are the main factor altering absorption. For intranasal administration and for inhalation the decreased blood flow is also a key determinant resulting in the reduced absorption. Table 4 includes data on the age-associated changes of absorption for non-oral routes of drug administration in the elderly.

Table 4.
Age-associated changes of absorption for non-oral routes of drugs administration in the elderly.
3.2. Distribution
Drug distribution through different body fluids, organs, and systems is markedly affected by the aging process resulting in the change of such PK parameters, as the volume of distribution (Vd). The main contributors to Vd alterations are changes in the cardiovascular system specifically for the elderly population. Cardiovascular senescence includes the cascade of physiological changes which may precipitate the formation of cardiovascular diseases. Advanced age results in the thickening of the walls of arteries with a decrease in vessel compliance and an increase in pulse wave velocity resulting in systolic blood pressure increase. These changes lead to the left ventricular afterload contributing to myocardial remodeling and congestive heart failure (HF) as well as to ischemic heart disease [132]. HF mediates hypoperfusion first in the peripheral tissues, but with HF progression blood flow will be reduced in the GIT, liver and kidneys resulting in significant PK changes [128]. Oedema secondary to HF, ascites secondary to cirrhosis, and chronic liver disease—all can worsen a fluid accumulation in the site of infection and adjacent tissues. This leads to the dilution of AB concentrations at the infection site and predisposes to a treatment failure [133]. Systemic inflammation in the elderly can promote endothelial dysfunction, leading to increased capillary permeability and plasma leakage into the interstitial space, which in critically ill patients can be further aggravated by fluid administration. Consequently, Vd especially of hydrophilic antibiotics (e.g., beta-lactams) can increase and a loading dose may be required [134].
Another concern is a change in the body’s water and fat composition. In the elderly, there is an increase in the body’s total fat mass, fat redistribution (increase in the abdominal fat and reduction in subcutaneous fat), and fat infiltration of various organs (liver, pancreas) [135]. Total body water content is decreased in the elderly and obese patients, and intracellular water is also reduced reflecting skeletal muscle loss [136]. An increase in fat mass may affect drug distribution, since the presence of obesity mediates changes in the tissue blood flow and perfusion. Considering the distribution of ABs, tissue penetration may be altered, as shown in the studies with cefuroxime and ciprofloxacin in obese patients [137].
Physicochemical drug characteristics are other determinants affecting Vd. Lipophilic agents can easily cross cellular membranes and blood-tissue barriers distributing to the various organs and tissues and resulting in the high values of Vd, while hydrophilic drugs are mainly concentrated in the body fluids and are typically characterized by the low values of Vd. Lipophilic antibacterial agents include fluoroquinolones, macrolides, tetracyclines and glycylcyclines, oxazolidinones, rifampicin, and chloramphenicol. Hydrophilic antibiotics are β-lactams, aminoglycosides, and glycopeptides. With increased fat content Vd for highly lipophilic drugs increases. Advanced age is associated with the decrease of total body water resulting in Vd contraction of hydrophilic drugs (e.g., aminoglycosides, β-lactams, and glycopeptides), though in critically ill patients Vd of hydrophilic agents may be increased with hemodynamic insufficiency development, excessive permeability of capillaries, and ongoing infusion therapy [138]. The ability of both lipophilic and hydrophilic antibiotics to cross blood-tissue barriers may be increased in the presence of some diseases. The most important barrier is the blood-brain barrier, and its breakdown may result in an increased risk of drug-induced neurotoxicity. The elderly population is characterized by a high prevalence of pathological states resulting in increased permeability of the blood-brain barrier, such as Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, amyotrophic lateral sclerosis, multiple sclerosis, and ischemic stroke [139]. AB therapy in patients with listed diseases may be accompanied by the rise of various neurotoxic reactions. The highest risk of encephalopathy was reported for penicillins, cephalosporines, carbapenems, oxazolidinones, fluoroquinolones, polymyxins, sulfonamides, metronidazole. Tetracyclines can induce cranial nerve toxicity and intracranial hypertension [140].
Another factor affecting drug distribution is plasma protein binding rate (PPB). Serum albumin, lipoproteins and alpha-1 acid glycoprotein are the main plasma proteins sequestering drugs in plasma [141]. A bound fraction of a drug is pharmacologically inactive and stays in the intravascular space, while an unbound fraction penetrates the extravascular space, reaches corresponding molecular targets, and causes a pharmacological response. Changes in the quantity and quality of plasma proteins may result in a disbalance between the bound and unbound fractions leading to altered pharmacological effects. Age is one of many factors that can influence drug protein binding, but the clinical significance of age-related hypoalbuminemia seems to be minimal. In healthy noninstitutionalized individuals, a gradual small decrease in serum albumin level (approximately 4% per decade) was found [142]. It is not noticeable until people reach 70 years of age [142], when the level of serum albumins decreases by 20% [143].
Hypoalbuminemia is more specific for elderly hospitalized patients. Serum albumins decrease below 3.5 mg/dL was observed with aging, and among cases of marked hypoalbuminemia at hospital discharge (<2.5 mg/dL) 74.2% were reported in persons over 65 years of age [144]. Serum globulins are also affected by aging, in 47.6% of patients aged between 60 and 85 years an increased gamma gap was observed (>3.1 g/dL) [145]. More pronounced alterations of serum proteins may be caused by acute or chronic disease, proteinuria, and malnutrition [142].
Hypoalbuminemia may play an important role in critically ill patients treated with intravenous highly protein-bound antimicrobials such as cefazolin, ceftriaxone, ertapenem, sulfonamides, clindamycin and daptomycin [146]. Decreased PPB may result in a significant increase in free serum concentrations of these antibiotics [146,147] which may require direct measurement of free drug levels [147]. In general, hypoalbuminemia may lead to the increase of Vd of highly albumin-bound antibiotics and hydrophilic drugs (e.g., streptomycin) and to the decrease of Vd of the α1-acid glycoprotein-bound drugs (e.g., rifabutin) [43]. In a retrospective observational study, older patients with methicillin-resistant Staphylococcus aureus hospital-acquired pneumonia and severe hypoalbuminemia had significantly longer vancomycin half-life (T1/2), high values of AUC, more frequent nephrotoxicity episodes, and greater risk of 28-day mortality compared with patients with mild hypoalbuminemia [148]. The authors recommended individual vancomycin dose adjustment to senior patients with low body weight and severe hypoalbuminemia. In the presence of hypoalbuminemia highly protein-bound ertapenem (normal PPB is 85–95%) showed a higher incidence of 30-day mortality in patients with carbapenems-susceptible Enterobacteriaceae compared to less protein-bound imipenem or meropenem [149].
Other studies demonstrated a decreased probability of target attainment with ceftriaxone in critically ill patients with severe hypoalbuminemia [150], and an increased risk of clinical failure with this AB [151].
Older individuals have increased plasma levels of α1-acid glycoprotein that are associated with a reduced unbound fraction of basic antibiotics, e.g., macrolides [8]. The synthesis of α1-acid glycoprotein may be augmented by infections and malnutrition [43] and an increase of its levels from 2 to 6-fold is seen in severe inflammation and cancer [141], which can affect Vd of antibiotics binding mainly to alpha-1-acid glycoprotein, such as clindamycin [152].
Nutritional status has a significant impact on antibiotics distribution and to a lesser extent on other PK parameters. Both obesity and malnutrition (undernutrition) are highly prevalent among older adults [153]. Rates of obesity vary in different age groups and are the highest among the young-old individuals (65–74 years) [154]. Obesity (BMI > 30 kg/m2) and particularly morbid obesity (BMI > 40 kg/m2) influence various physiological processes including gut permeability, gastric emptying, cardiac output, liver and renal function, and is associated with the different physiological compositions of muscle and fat compared to non-obese patients [137,155]. An increase of both body fat tissue and lean body mass in patients with obesity leads to an increase of Vd particularly of lipophilic drugs. The elimination of highly lipophilic agents, such as fluoroquinolones, macrolides, oxazolidinones, tetracyclines and rifampin can decrease. Morbid obesity (BMI > 40 kg/m2) can profoundly affect both antibiotics distribution and clearance [156]. PK changes in obese patients can potentially reduce the efficacy of standard AB doses used for the treatment of non-obese individuals [157]. In general, the dosing of lipophilic antibiotics in obese patients is recommended to be based on actual body weight, and that of hydrophilic antibiotics—on ideal body weight [158], but optimal dosing in obese elderly patients needs further study.
Malnutrition is another important concern in the elderly [159]. In malnourished individuals, there is a decrease in adipose tissue content and lean body mass with an increase in total body water. Malnutrition is associated with other pathophysiological changes which can impact PK such as hypochlorhydria, delayed gastrointestinal emptying time, increased, or decreased intestinal transit time, gastric and mucosal atrophy and dysfunction, gastrointestinal inflammation, and pancreatic insufficiency [43]. P-gp activity in the enterocytes of malnourished patients is decreased and tight junctions are enlarged, influencing the uptake of food and drugs [43]. A common feature of malnutrition especially in elderly patients with infections is pronounced hypoalbuminemia [7,43], those effects on Vd were discussed above.
The list of factors affecting Vd and clearance in the elderly with severe infections and their interrelationships are shown in Figure 1.
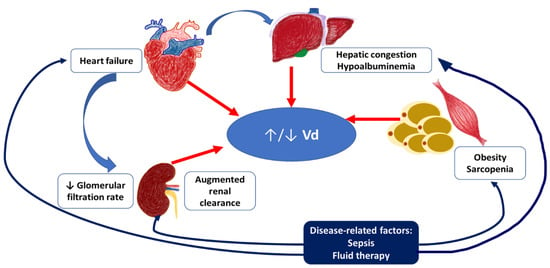
Figure 1.
The list of the main factors affecting Vd and clearance in the elderly with severe infections and their interrelationships. Severe infections may worsen heart failure and hepatic congestion, contribute to sarcopenia progression and fat redistribution. Exacerbation of heart failure leads to the decreased renal function, while septic changes may result in the augmented renal clearance. In critically ill patients Vd of hydrophilic agents may be increased with hemodynamic insufficiency development, excessive permeability of capillaries, and infusion therapy, while Vd of lipophilic agents may be decreased due to the decrease of fat tissue mass. These changes are different from those observed in a healthy elderly patient (typically decreased Vd of hydrophilic agents and increased Vd of lipophilic agents).
3.3. Metabolism
Age is associated with significant changes in drug metabolism. In healthy aging the mass of the liver reduces by 20–40% resulting in a reduction of drug clearance [31]. A decrease in liver mass and liver function is mainly related to the significant hepatic blood flow decline (40 to 60%) in the elderly [24]. Both decreased hepatic function and reduced hepatic blood flow contribute to increased T1/2 of hepatically metabolized antibiotics in the elderly [8]. The age-related loss of surfaced endoplasmic reticulum causes a strong negative correlation between age and hepatic microsomal phase I drug metabolizing activity [160]. In people aged ≥70 years activity of the cytochrome P450 (CYP450) oxidases may decrease by 30% [161] resulting in the decline of clearance of CYP substrates. CYP3A activity decrease was reported in the elderly compared with healthy adults, resulting in the midazolam and atorvastatin Cmax nearly twice increase [81]. In the elderly 30 to 50% clearance reduction was reported for the CYP3A4 metabolized drugs [24] and 20% reduction for CYP2D6 substrates [23].
Changes in the body composition specific to the elderly may affect the functions of drug metabolizing enzymes. Kaburaki S et al. (2022) observed associations between the skeletal muscle mass index (SMI), handgrip strength (HGS), hepatic steatosis index, and activity of CYP2C19 and CYP3A4. In male patients ≥65 years of age a reduction in SMI and HGS below the sarcopenia diagnostic criteria correlated with a decline in CYP2C19 and CYP3A4 activity. In elderly female patients, a decline in CYP2C19 metabolic activity was associated with fatty liver disease presence [162].
Liver pathology being highly prevalent in the elderly population is the most obvious factor altering CYP450 expression and activity. In this respect, it is interesting to consider the results of the estimation of the protein abundance and gene expression of various CYPs in the liver samples of patients with hepatitis C, alcoholic liver disease, autoimmune hepatitis, primary biliary cholangitis and primary sclerosing cholangitis. CYP2E1 was defined as the most vulnerable enzyme in which protein levels were significantly reduced in Child–Pugh class A cirrhosis. The most prominent downregulation of metabolizing enzymes was associated with alcoholic liver disease (CYP1A2, CYP2C8, CYP2D6, CYP2E1, CYP3A4, UGT2B7) and primary biliary cholangitis (CYP1A1, CYP2B6, CYP2C8, CYP2E1, CYP3A4). The protein abundance most of UDP-glucuronosyltransferases (UGT) was unaffected by liver pathology (UGT1A1, UGT1A3, UGT2B15) [163].
Decreased function of CYP450 enzymes may lead to the reduction of the first-pass metabolism of orally taken macrolides, fluoroquinolones (except levofloxacin), clindamycin, tetracyclines, sulfamethoxazole/trimethoprim, and rifampin, resulting in the increase of bioavailability and serum concentrations of these agents [8]. Though these changes in enzymes metabolizing activity vary significantly from drug to drug and from person to person they might be an important cause of ADRs [160].
The activity of the phase II enzymes, such as sulfotransferases, UDP-glucuronosyltransferases (UGTs), and glutathione s-transferases (GSTs) is usually less affected by age and therefore, the clearance of moderately lipophilic antibiotics, such as fluoroquinolones or linezolid is similar to that among young adults [24]. There is some evidence that phase II metabolism might be affected by frailty [24].
Malnutrition can cause a decrease in the content of hepatic cytochromes, which was proved by the observed reduction of drug metabolism in patients with cachexia [164].
Substrates, inhibitors, and inducers of CYP450 isoenzymes among antibacterial agents and CYP450 aging changes are indicated in Table 5.

Table 5.
Substrates, inhibitors, and inducers of CYP450 isoenzymes among antibacterial agents and CYP450 aging changes.
3.4. Excretion
Elderly patients have a high risk of decreased antibiotics clearance from the body due to declining functions of the lung, bladder, liver, GIT and circulatory system, but deterioration of the kidney function is the most important [207]. The kidneys are the major route of elimination for many classes of antibiotics including beta-lactams, aminoglycosides, glycopeptides, fluoroquinolones (except moxifloxacin), lipoglycopeptides, lipopeptides (daptomycin), trimethoprim/sulfamethoxazole [8].
A gradual decrease in the kidney size and weight, renal blood flow, estimated glomerular filtration rate (eGFR), altered renal tubular secretion and age-related anatomic abnormalities (e.g., glomerulosclerosis, arteriosclerosis, arteriolar hyalinosis, medial hypertrophy, tubular atrophy) leads to a progressive decline of the renal function in the elderly [208]. Renal mass reaches about 400 g in the fourth decade of life and declines gradually to about 300 g [209].
Both age-related physiological changes and pathological changes (due to hypertension, diabetes mellitus, and heart failure) lead to the amplification of the cellular signaling pathways involved in renal cell senescence resulting in the imbalance between the proliferation and apoptosis with the intensification of the last one [210]. Renal aging results in increased susceptibility to acute kidney injury (AKI) and increased risk of formation of chronic kidney disease (CKD). In the age group 40 to 49 years CKD stage 3–5 was reported only in 1.4%, in the group 50 to 59 years—in 5.4%, while for the group 70 to 79 years—in 35.4%, and in the group 80 to 89 years—in 30.9% [211]. By the eighth decade of life approximately 30–40% of all glomeruli become sclerotic and by the ninth decade kidney size and the total number of glomeruli may be about 70% of that of the third decade [212]. Patients ≥80 years have a 40–50% decline in renal function compared to adults of middle age [1,213]. The decline in renal function is accelerated in patients with frailty [214].
Another factor contributing to the altered renal function and AKI is the use of nephrotoxic drugs. Antibiotics may result in nephrotoxic reactions with various mechanisms, including acute tubular necrosis and acute interstitial nephritis (Table 6). In the elderly decreased renal clearance mediates the amplification of nephrotoxicity, since for the majority of associated antibiotics it has a concentration-dependent character.

Table 6.
Antibacterial agents associated with AKI development and mechanisms of their nephrotoxic effects.
AKI risk in patients using antibiotics is increased with increasing age: odds ratio (OR) was 4.38 (p = 0.002) for those older than 75 years [219]. High rates of AKI development are associated with the use of penicillins (piperacillin tazobactam, cloxacillin, flucloxacillin) and vancomycin. A combination of piperacillin tazobactam with vancomycin was associated with a significantly higher incidence of AKI compared with piperacillin tazobactam plus meropenem combination (16.5% vs 3.6%; p = 0.009). Finally, piperacillin tazobactam with vancomycin was associated with a 6.8-fold increased risk of developing AKI (OR: 6.8, 95% confidence interval [CI] 1.5–30.9), and the higher plasma concentration of vancomycin was also a determinant of AKI risk [217]. A systematic review and meta-analysis of observational studies (12 studies included, 14,511 patients) revealed significantly higher odds of AKI development in patients treated with a combination of vancomycin plus piperacillin tazobactam compared with vancomycin plus meropenem combination (OR = 2.31; 95%CI 1.69–3.15) [237,238]. Similar results were demonstrated in another study (retrospective cohort study, period 20134—2019 years), where AKI incidence was reported to be 33.3% in patients receiving vancomycin with piperacillin tazobactam compared with 9.1% for those who received vancomycin with meropenem or doripenem [238]. Estimating AKI risks for meropenem, it is worth noting that the comparison of vancomycin plus meropenem versus vancomycin plus cefepime revealed a nearly 2-fold increase in AKI incidence for the first combination (38% versus 19.1%; p = 0.049) [239].
The highest AKI odds associated with piperacillin tazobactam were demonstrated in another study, with OR = 1.89 (95% CI: 1.73–2.06) [240]. Considering vancomycin monotherapy, overall incidence of AKI was 9.3 (95% CI 0.78–1.22) per 100 person-years, and the adjusted hazard ratio versus all other comparator antibiotics was 0.74 (95% CI: 0.45–1.21) [241]. Comparison of nephrotoxicity induced by glycopeptides revealed less AKI risks for teicoplanin compared with vancomycin (relative risk, RR = 0.66; 95% CI: 0.48–0.90; I2 = 10%) as it was established in the Cochrane systematic review [242], suggesting possible benefits of its inclusion in the combined AB schemes instead of vancomycin. This benefit was proved by the recent study, which revealed that piperacillin tazobactam with vancomycin combination compared with piperacillin tazobactam plus teicoplanin or vancomycin plus meropenem was associated with 3.96 times (95% CI, 1.48–10.63, p = 0.006) and 3.11 times (95% CI, 1.12–8.62; p = 0.028) increased risk of AKI, respectively [243].
The incidence rate of linezolid-induced AKI is considered to be lower than that of vancomycin, though there are results of a small study (63 patients with vancomycin and 38 with linezolid) indicating no difference in risk of AKI between these groups (p = 0.773). AKI occurred in 19 (30.2%) patients from the vancomycin group and in 14 (36.8%) patients from linezolid groups (p = 0.448) [223].
Considering imipenem, it is important to note the protective effect of cilastatin, and favorable outcomes for a combination of imipenem/cilastatin with relebactam compared with colistin adding. For the first antibiotics combination, AKI incidence was zero, for the second—31.3% [244].
Aminoglycosides are a common reason for nephrotoxicity. Amikacin-induced AKI in mechanically ventilated critically ill patients with sepsis was 26.7%, and among factors independently associated with an increased risk of amikacin-induced AKI were concurrent use of colistin (OR = 25.51, 95%CI: 6.99–93.05, p < 0.001), presence of septic shock (OR = 4.22, 95%CI: 1.76–10.11, p = 0.001), and Charlson Comorbidity Index (OR = 1.14, 95%CI: 1.02–1.28, p = 0.025) [226].
Analysis of the Food and Drug Administration Adverse Event Reporting System (FAERS) database from 2000 to 2021 year revealed antibacterial agents associated with AKI in older adults. The highest reporting odds ratios (ROR) were determined for the next ones: vancomycin (5.73 (95% CI: 5.30–6.21)), sulfamethoxazole (5.30 (95% CI: 4.80–5.85)), trimethoprim (5.25 (95% CI: 4.27–6.45), colistin (5.11 (95% CI: 3.17–8.22)), amoxicillin (2.75 (95% CI: 2.50–3.04)), ciprofloxacin (2.66 (95% CI: 2.45–2.89)), clarithromycin (2.75 (95% CI: 2.46–3.07)) [215]. AKI occurred in 68.5% of 412 enrolled patients with an incidence rate of 10.6 per 100 patients-days and a median time was 6 (3–13) days. Stages I–III of AKI were 38.3, 24.5, and 37.2%.
Estimation of patients with COVID-19 treated with antibiotics revealed a significantly higher incidence of AKI in those who received linezolid (p < 0.0001), vancomycin (p < 0.0001), carbapenem (p < 0.0001), cephalosporin (p < 0.0001), and piperacillin/tazobactam (p = 0.028). AKI was associated with prolonged hospitalization (OR = 3.37; 95% CI: 1.76–6.45) [245].
Antibiotic-induced AKI is also associated with increased mortality, especially in the elderly, as was demonstrated for patients who used intravenous colistin (hazard ratio, HR = 1.74, 95% CI: 1.06–2.86, p = 0.028). Colistin-induced AKI incidence rate was estimated as 10.6 per 100 patients-days, stage 1 was seen in 38.3%, stage 2 in 24.5%, and stage 3 in 37.2% [235].
Patients with severe malnutrition accompanied by dehydration are at increased risk of diminished glomerular filtration rate (GFR), renal blood flow decline, and impaired tubular excretion and reabsorption [43]. The existing evidence suggests that elimination of streptomycin may decrease and that of rifampicin increases in malnourished adults [43].
Hepatic impairment may directly or indirectly decrease protein binding, metabolism, and renal elimination of antibiotics [133]. Dose adjustment is needed for drugs that undergo hepatobiliary clearance, especially those that undergo phase I metabolism, have high protein binding, or are associated with high hepatotoxicity [133]. Liver cirrhosis has a significant impact on antibiotics disposition due to numerous pathological changes including liver cell necrosis, portosystemic shunt, reduction in the concentration of drug-binding proteins, atypical Vd, altered metabolism and elimination, altered PD, drug-drug interactions, and frequent association with renal failure) [246]. The percentage of antibiotics bound by albumin may be altered in cirrhotic patients [133]. Elimination of tigecycline which is lipophilic and highly protein bound (71–89%) demonstrated a reduction in patients with hepatic failure, accompanied by a 43% increase in elimination half-life in severe hepatic impairment [247]. In patients with severe liver disease, it is recommended to half the standard maintenance dose of tigecycline but no changes in its usual loading dose are needed [247].
Decompensated hepatic failure causes renal vasoconstriction and subsequent renal failure, leading to the reduction of renally eliminated antibiotics excretion and an increase of their serum concentrations [248]. Diminished renal elimination in liver cirrhosis was shown for ofloxacin, ampicillin, aminoglycosides, and vancomycin [246].
Aging and age-related diseases may affect the levels of circulating proteins modifying their renal excretion. The work by Lind L et al. (2019) revealed inverse relation of the change in eGFR to the change in most of the evaluated plasma proteins (74%), among which the most significant inverse relationships were reported for cystatin-B (CSTB), tumor necrosis factor receptor 1 (TNF-R1), CD40L receptor (CD40), tumor necrosis factor receptor 2 (TNF-R2), TNF-related apoptosis-inducing ligand receptor 2 (TRAIL-R2); study population included persons aged 70 at baseline, the study period was 10 years. A positive relationship was revealed between the change in eGFR and the change in hemoglobin (beta 0.10, SE 0.03, Pearson’s correlation coefficient 0.11, p-value = 7.9 × 10−4) [249].
Reduction of renal clearance mediated by different reasons leads to the increase in the half-life period of various drugs and indicates the need to decrease the daily dose of some antibiotics [213]. This is of paramount importance for antibiotics with narrow therapeutic index (NTI) including glycopeptides, aminoglycosides, and chloramphenicol succinate [213,250]. The risk of toxicity of NTI antibiotics is extremely increased in the elderly, especially in those with frailty [251], suggesting the actual need for therapeutic drug monitoring (TDM) [252].
Age-related changes in AB PK may be illustrated with data derived from linezolid TDM. Comparison of TDM samples from adult patients (<50 years) and from the elderly (>90 years) revealed a highly significant, progressive increment in the linezolid trough concentrations (5.8 ± 5.6 mg/L versus 16.6 ± 10.0 mg/L), an overall increment was 30% per decade of age. Increased trough concentrations contribute to the increased overdose and toxicity risks; they were found to exceed therapeutic levels in 30%, 50%, and 65% of patients aged <65 years, 65–80 years, and >80 years, respectively [253].
Investigation of the efficacy and safety of vancomycin in patients ≥ 80 years revealed a failure of treatment in 34.4%. The increased trough concentrations of vancomycin (VTC) were associated with increased 30-day mortality rates: for VTC at <10 μg/mL mortality rate was 2.8%, at 10 to 15 μg/mL—15.0%, at 15 to 20 μg/mL—15.3%, at ≥20 μg/mL—37.8%. The multivariate analysis determined blood urea nitrogen ≥ 11 g/dL and heart failure as independent factors associated with treatment failure (p = 0.004, 0.016, respectively). Nephrotoxicity was observed in 12.0% of patients treated with vancomycin. Independent factors associated with increased nephrotoxicity were VTC ≥ 15 μg/mL; treatment duration ≥ 15 d; and concomitant aminoglycosides administration (p = 0.024, 0.035, 0.029, respectively) [254]. Comparison of the VTC and AUC/MIC in the patients with the mean age (+standard deviation) 50.9 ± 12.4 versus 76.9 ± 8 years revealed their significant increase in the elderly. Rapid achievement of VTC ≥ 15 mg/L (within 4 days) was significantly more specific for the elderly compared with younger patients (54.1% vs. 36.5%, p = 0.004) as did 30-day mortality (40.9% vs. 12.5%, p < 0.001) [255]. In the work by Hatti M et al. (2018) a considerable variation of trough AB concentrations in older adults was demonstrated for cefotaxime, meropenem, and piperacillin-tazobactam, which was mainly related to the low eGFR. Increased trough concentrations of cefotaxime were significantly associated with older age, diabetes with end organ damage, moderate/severe kidney disease, and higher sepsis severity [256].
For beta-lactams renal function is an important factor affecting PK parameters and creatinine clearance was reported to be the most significant covariate altering beta-lactams PK in late elderly patients. Population PK methods revealed decreased levels of clearance for both piperacillin and tazobactam compared with younger population [257]. Doripenem in the elderly with nosocomial pneumonia was characterized by increased AUC and prolonged T1/2, reflecting a decrease in the renal clearance related to aging [258]. The same tendency was demonstrated for meropenem in the elderly: clearance was significantly lower than in younger patients due to the decline of renal function [259].
Aminoglycosides are also among antibiotics whose PK is dramatically affected by renal function decrease. Elimination of amikacin was delayed with increasing age, reflecting glomerular filtration rate decline [260].
Population pharmacokinetic modeling for levofloxacin in patients with a mean age of 81.2 years and impaired renal function demonstrated decreased mean clearance compared to healthy volunteers [261].
Another concern regarding renal function effects on PK is augmented renal clearance (ARC), defined as a creatinine clearance of more than 130 mL/min/1.73 m2 [262]. ARC is mediated by the changes in kidney function arising in a critically ill state. The meta-analysis of 70 studies revealed a pooled prevalence of ARC of 39% (95% CI: 34.9–43.3) and its risk factors in populations with apparently normal renal function including young age, male sex, and trauma [263]. In critically ill patients with cancer ARC development on the first day of intensive care unit (ICU) admission demonstrated a significant association with younger age (OR 1.028, 95% CI: 1.005–1.051) [264]. Despite the association with young age stated above a retrospective study of 2592 critically ill patients admitted to the ICU revealed that the median age of patients with ARC was 70 (55–79) years, with a prevalence of 33.4% [265], pointing out the importance of the ARC for the elderly population. This state can lead to decreased AB exposure and thus to treatment failure with beta-lactams, aminoglycosides, glycopeptides, and other, mainly hydrophilic agents [262]. Vancomycin use in ARC patients demonstrated achievement of the trough concentration in only 19.23% [266], and the same trend was revealed in the vancomycin population pharmacokinetic model, affirming that ARC was significantly associated with subtherapeutic serum concentrations [267]. Renal function is a significant predictor of proper meropenem exposure. In critically ill septic patients with ARC (median age 63 years, interquartile range, 55 to 68 years) poor PK/PD target attainment was demonstrated [268]. Linezolid clearance demonstrated a significant increase in ARC patients, resulting in sub-therapeutic concentrations after standard doses [269].
Age-related changes in the main PK parameters of antibacterial agents compared to the younger population are given in Table 7, Table 8, Table 9, Table 10, Table 11, Table 12 and Table 13.

Table 7.
Comparative data on PK parameters of β-lactam antibiotics in the elderly and adults.

Table 8.
Comparative data on PK parameters of glycopeptides, lipopeptides, and lipoglycopeptides in the elderly and adults.

Table 9.
Comparative data on PK parameters of oxazolidinones in the elderly and adults.

Table 10.
Comparative data on PK parameters of tigecycline in the elderly and adults.

Table 11.
Comparative data on PK parameters of fluoroquinolones in the elderly and adults.

Table 12.
Comparative data on PK parameters of aminoglycosides in the elderly and adults.

Table 13.
Comparative data on PK parameters of polymyxin B in the elderly and adults.
4. AB Dosing Regimens in the Elderly
The main aim of AB therapy in elderly patients is to provide a proper balance between efficacy (PK/PD target attainment) and safety. Changed PK parameters may lead both to decreased or increased AB exposure contributing to negative treatment outcomes. Dose adjustment is a typical approach in the management of the elderly with infections. Decreased metabolizing capacity and declined renal clearance result in the need to decrease the standard adult dose of AB, while ARC specific for critically ill patients may dictate the necessity to use a higher dose.
Table 14, Table 15, Table 16, Table 17 and Table 18 include information about the proposed regimens of AB dosing depending on age, renal function, and hepatic function along with data on concentrations reported to cause toxic reactions.

Table 14.
Beta-lactam AB dosing depending on age, renal function, and hepatic impairment.

Table 15.
Aminoglycosides dosing depending on age, renal function, and hepatic impairment.

Table 16.
Glycopeptides and Lipopeptides dosing depending on age, renal function, and hepatic impairment.

Table 17.
Fluoroquinolones dosing depending on age, renal function, and hepatic impairment.

Table 18.
Linezolid and polymyxin B dosing depending on age, renal function, and hepatic impairment.
5. Conclusions
Optimization of the management of elderly patients with infectious diseases is a complex process, and successful performance demands knowledge of the main age-related and pathology-related changes in the patient’s organism. Altered PK parameters may contribute to the decreased efficacy of the treatments with suboptimal antibiotic exposure or to the increased risks of toxic reactions ameliorating further response to drugs with overexposure. Individualization of the pharmacotherapy based on the unique characteristics of the elderly patients may ensure the attainment of an optimal PK/PD target and treatment success. The existing level of evidence on PK changes in the elderly clearly indicates a significant difference in most PK parameters compared to younger adults. The last decade is characterized by a tendency to increase the participation of the elderly in clinical trials. However, the number of such trials is still insufficient to cover all the classes of ABs and to provide full evidence-based background to choose proper dosing regimens in all the pathologic states specific to the elderly and senile patients. Global aging indicates an urgent need to extend inclusion of the elderly and senile patients with various comorbidity profiles and geriatric syndromes in clinical trials and PK studies.
Author Contributions
Conceptualization, O.I.B., S.K.Z.; resources, O.I.B. and E.A.B.; data curation, S.K.Z. and E.A.U.; search of data sources—O.I.B. and M.S.C.; writing—original draft preparation, O.I.B.; writing—review and editing, O.I.B. and E.A.U.; supervision, S.K.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This publication has been supported by the RUDN project No. 0321040000.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Sources of information used in this review are listed in the References.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chinzowu, T.; Roy, S.; Nishtala, P.S. Antimicrobial-associated organ injury among the elderly: A systematic review and meta-analysis protocol. BMJ Open 2022, 11, e055210. [Google Scholar] [CrossRef] [PubMed]
- Sabri, S.M.; Annuar, N.; Rahman, N.L.A.; Musairah, S.K.; Mutalib, H.A.; Subagja, I.K. Major Trends in Ageing Population Research: A Bibliometric Analysis from 2001 to 2021. Proceedings 2022, 82, 19. [Google Scholar] [CrossRef]
- Veimer Jensen, M.L.; Aabenhus, R.M.; Holzknecht, B.J.; Bjerrum, L.; Jensen, J.N.; Siersma, V.; Córdoba, G. Antibiotic prescribing in Danish general practice in the elderly population from 2010 to 2017. Scand. J. Prim. Health Care 2021, 39, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Petrosillo, N.; Cataldo, M.A.; Pea, F. Treatment options for community-acquired pneumonia in the elderly people. Expert Rev. Anti-Infect. Ther. 2015, 13, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Bradley, S.F. Principles of Antimicrobial Therapy in Older Adults. Clin. Geriatr. Med. 2016, 32, 443–457. [Google Scholar] [CrossRef]
- Bouza, E.; Brenes, F.J.; Díez Domingo, J.; Eiros Bouza, J.M.; González, J.; Gracia, D.; Juárez González, R.; Muñoz, P.; Petidier Torregrossa, R.; Casado, J.M.R.; et al. The situation of infection in the elderly in Spain: A multidisciplinary opinion document. Rev. Española Quimioter. 2020, 33, 327–349. [Google Scholar] [CrossRef]
- Barber, K.E.; Bell, A.M.; Stover, K.R.; Wagner, J.L. Intravenous Vancomycin Dosing in the Elderly: A Focus on Clinical Issues and Practical Application. Drugs Aging 2016, 33, 845–854. [Google Scholar] [CrossRef]
- Giarratano, A.; Green, S.E.; Nicolau, D.P. Review of antimicrobial use and considerations in the elderly population. Clin. Interv. Aging 2018, 13, 657–667. [Google Scholar] [CrossRef]
- Pagani, L. Appropriate antimicrobial therapy in the elderly: When half-size does not fit all frail patients. Clin. Microbiol. Infect. 2015, 21, 1–2. [Google Scholar] [CrossRef]
- Cristina, M.L.; Spagnolo, A.M.; Giribone, L.; Demartini, A.; Sartini, M. Epidemiology and Prevention of Healthcare-Associated Infections in Geriatric Patients: A Narrative Review. Int. J. Environ. Res. Public Health 2021, 18, 5333. [Google Scholar] [CrossRef]
- Sanz, F.; Morales-Suarez-Varela, M.; Fernandez, E.; Force, L.; Perez-Lozano, M.J.; Martin, V.; Egurrola, M.; Castilla, J.; Astray, J.; Toledo, D.; et al. A Composite of Functional Status and Pneumonia Severity Index Improves the Prediction of Pneumonia Mortality in Older Patients. J. Gen. Intern. Med. 2018, 33, 437–444. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Yong, Y.; Tan, W.C.; Shen, L.; Ng, H.S.; Fong, K.Y. Prognostic factors for mortality due to pneumonia among adults from different age groups in Singapore and mortality predictions based on PSI and CURB-65. Singap. Med. J. 2018, 59, 190–198. [Google Scholar] [CrossRef]
- Torres, A.; Cilloniz, C.; Niederman, M.S.; Menéndez, R.; Chalmers, J.D.; Wunderink, R.G.; van der Poll, T. Pneumonia. Nat. Rev. Dis. Prim. 2021, 7, 25. [Google Scholar] [CrossRef]
- Galimberti, F.; Casula, M.; Olmastroni, E.; Catapano, A.L.; Tragni, E.; On Behalf Of Edu Re Drug Group. Antibiotic Prescription in the Community-Dwelling Elderly Population in Lombardy, Italy: A Sub-Analysis of the EDU.RE.DRUG Study. Antibiotics 2022, 11, 1369. [Google Scholar] [CrossRef]
- Kusuma, I.Y.; Matuz, M.; Bordás, R.; Juhasz Haverinen, M.; Bahar, M.A.; Hajdu, E.; Visnyovszki, Á.; Ruzsa, R.; Doró, P.; Engi, Z.; et al. Antibiotic use in elderly patients in ambulatory care: A comparison between Hungary and Sweden. Front. Pharmacol. 2022, 13, 1042418. [Google Scholar] [CrossRef]
- Cruz, S.P.; Cebrino, J. Prevalence and Determinants of Antibiotic Consumption in the Elderly during 2016–2017. Int. J. Environ. Res. Public Health 2020, 17, 3243. [Google Scholar] [CrossRef]
- Insani, W.N.; Whittlesea, C.; Alwafi, H.; Man, K.K.C.; Chapman, S.; Wei, L. Prevalence of adverse drug reactions in the primary care setting: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0252161. [Google Scholar] [CrossRef]
- Vrdoljak, D.; Borovac, J.A. Medication in the elderly—Considerations and therapy prescription guidelines. Acta Med. Acad. 2015, 44, 159–168. [Google Scholar] [CrossRef]
- Lexow, M.; Wernecke, K.; Schmid, G.L.; Sultzer, R.; Bertsche, T.; Schiek, S. Considering additive effects of polypharmacy: Analysis of adverse events in geriatric patients in long-term care facilities. Wien. Klin. Wochenschr. 2021, 133, 816–824. [Google Scholar] [CrossRef]
- Dovjak, P. Polypharmacy in elderly people. Wien. Med. Wochenschr. 2022, 172, 109–113. [Google Scholar] [CrossRef]
- Kim, J.; Parish, A.L. Polypharmacy and Medication Management in Older Adults. Nurs. Clin. N. Am. 2017, 52, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Yang-Huang, J.; Franse, C.B.; Rukavina, T.; Vasiljev, V.; Mattace-Raso, F.; Verma, A.; Borrás, T.A.; Rentoumis, T.; Raat, H. Factors associated with polypharmacy and the high risk of medication-related problems among older community-dwelling adults in European countries: A longitudinal study. BMC Geriatr. 2022, 22, 841. [Google Scholar] [CrossRef] [PubMed]
- Corsonello, A.; Abbatecola, A.M.; Fusco, S.; Luciani, F.; Marino, A.; Catalano, S.; Maggio, M.G.; Lattanzio, F. The impact of drug interactions and polypharmacy on antimicrobial therapy in the elderly. Clin. Microbiol. Infect. 2015, 21, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Pea, F. Pharmacokinetics and drug metabolism of antibiotics in the elderly. Expert Opin. Drug Metab. Toxicol. 2018, 14, 1087–1100. [Google Scholar] [CrossRef] [PubMed]
- Hoff, B.M.; Maker, J.H.; Dager, W.E.; Heintz, B.H. Antibiotic Dosing for Critically Ill Adult Patients Receiving Intermittent Hemodialysis, Prolonged Intermittent Renal Replacement Therapy, and Continuous Renal Replacement Therapy: An Update. Ann. Pharmacother. 2020, 54, 43–55. [Google Scholar] [CrossRef]
- Tannenbaum, C.; Day, D.; on behalf of the Matera Alliance. Age and sex in drug development and testing for adults. Pharmacol. Res. 2017, 121, 83–93. [Google Scholar] [CrossRef]
- Appaneal, H.J.; Shireman, T.I.; Lopes, V.V.; Mor, V.; Dosa, D.M.; LaPlante, K.L.; Caffrey, A.R. Poor clinical outcomes associated with suboptimal antibiotic treatment among older long-term care facility residents with urinary tract infection: A retrospective cohort study. BMC Geriatr. 2021, 21, 436. [Google Scholar] [CrossRef]
- Simonetti, A.F.; Viasus, D.; Garcia-Vidal, C.; Carratalà, J. Management of community-acquired pneumonia in older adults. Ther. Adv. Infect. Dis. 2014, 2, 3–16. [Google Scholar] [CrossRef]
- Compté, N.; Dumont, L.; Bron, D.; De Breucker, S.; Praet, J.P.; Bautmans, I.; Pepersack, T. White blood cell counts in a geriatric hospitalized population: A poor diagnostic marker of infection. Exp. Gerontol. 2018, 114, 87–92. [Google Scholar] [CrossRef]
- Chong, C.P.; Street, P.R. Pneumonia in the elderly: A review of the epidemiology, pathogenesis, microbiology, and clinical features. South Med. J. 2008, 101, 1141–1145; quiz 1132, 1179. [Google Scholar] [CrossRef]
- Davies, E.A.; O’Mahony, M.S. Adverse drug reactions in special populations—The elderly. Br. J. Clin. Pharmacol. 2015, 80, 796–807. [Google Scholar] [CrossRef]
- Völter, C.; Götze, L.; Dazert, S.; Wirth, R.; Thomas, J.P. Impact of Hearing Loss on Geriatric Assessment. Clin. Interv. Aging 2020, 15, 2453–2467. [Google Scholar] [CrossRef]
- Kim, L.D.; Koncilja, K.; Nielsen, C. Medication management in older adults. Clevel. Clin. J. Med. 2018, 85, 129–135. [Google Scholar] [CrossRef]
- Zullo, A.R.; Gray, S.L.; Holmes, H.M.; Marcum, Z.A. Screening for Medication Appropriateness in Older Adults. Clin. Geriatr. Med. 2018, 34, 39–54. [Google Scholar] [CrossRef]
- Morrill, H.J.; Caffrey, A.R.; Jump, R.L.; Dosa, D.; LaPlante, K.L. Antimicrobial Stewardship in Long-Term Care Facilities: A Call to Action. J. Am. Med. Dir. Assoc. 2016, 17, 183.e1–183.e16. [Google Scholar] [CrossRef]
- Janssen, M.W.H.; de Bont, E.G.P.M.; Hoebe, C.J.P.A.; Cals, J.W.L.; den Heijer, C.D.J. Trends in antibiotic prescribing in Dutch general practice and determinants of nonprudent antibiotic prescriptions. Fam. Pract. 2023, 40, 61–67. [Google Scholar] [CrossRef]
- Palmer, M.E.; Andrews, L.J.; Abbey, T.C.; Dahlquist, A.E.; Wenzler, E. The importance of pharmacokinetics and pharmacodynamics in antimicrobial drug development and their influence on the success of agents developed to combat resistant gram negative pathogens: A review. Front. Pharmacol. 2022, 13, 888079. [Google Scholar] [CrossRef]
- Póvoa, P.; Moniz, P.; Pereira, J.G.; Coelho, L. Optimizing Antimicrobial Drug Dosing in Critically Ill Patients. Microorganisms 2021, 9, 1401. [Google Scholar] [CrossRef]
- Kowalska-Krochmal, B.; Dudek-Wicher, R. The Minimum Inhibitory Concentration of Antibiotics: Methods, Interpretation, Clinical Relevance. Pathogens 2021, 10, 165. [Google Scholar] [CrossRef]
- Pruskowski, K.A. Pharmacokinetics and Pharmacodynamics of Antimicrobial Agents in Burn Patients. Surg. Infect. 2021, 22, 77–82. [Google Scholar] [CrossRef]
- Asín-Prieto, E.; Rodríguez-Gascón, A.; Isla, A. Applications of the pharmacokinetic/pharmacodynamic (PK/PD) analysis of antimicrobial agents. J. Infect. Chemother. 2015, 21, 319–329. [Google Scholar] [CrossRef]
- Butranova, O.I.; Ushkalova, E.A.; Zyryanov, S.K.; Chenkurov, M.S. Developmental Pharmacokinetics of Antibiotics Used in Neonatal ICU: Focus on Preterm Infants. Biomedicines 2023, 11, 940. [Google Scholar] [CrossRef] [PubMed]
- Verrest, L.; Wilthagen, E.A.; Beijnen, J.H.; Huitema, A.D.R.; Dorlo, T.P.C. Influence of Malnutrition on the Pharmacokinetics of Drugs Used in the Treatment of Poverty-Related Diseases: A Systematic Review. Clin. Pharmacokinet. 2021, 60, 1149–1169. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Bunachita, S.; Agarwal, A.A.; Bhamidipati, A.; Patel, U.K. A Comprehensive Overview of Antibiotic Selection and the Factors Affecting It. Cureus 2021, 13, e13925. [Google Scholar] [CrossRef] [PubMed]
- Dumic, I.; Nordin, T.; Jecmenica, M.; Stojkovic Lalosevic, M.; Milosavljevic, T.; Milovanovic, T. Gastrointestinal Tract Disorders in Older Age. Can. J. Gastroenterol. Hepatol. 2019, 2019, 6757524. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Izumi, N.; Funayama, S.; Nohno, K.; Katsura, K.; Kaneko, N.; Inoue, M. Characteristics of medication-induced xerostomia and effect of treatment. PLoS ONE 2023, 18, e0280224. [Google Scholar] [CrossRef]
- Tan, E.C.K.; Lexomboon, D.; Sandborgh-Englund, G.; Haasum, Y.; Johnell, K. Medications That Cause Dry Mouth as an Adverse Effect in Older People: A Systematic Review and Metaanalysis. J. Am. Geriatr. Soc. 2018, 66, 76–84. [Google Scholar] [CrossRef]
- Abdi, S.; Masbough, F.; Nazari, M.; Abbasinazari, M. Drug-induced esophagitis and helpful management for healthcare providers. Gastroenterol. Hepatol. Bed Bench 2022, 15, 219–224. [Google Scholar] [CrossRef]
- Hughes, J.; Lockhart, J.; Joyce, A. Do calcium antagonists contribute to gastro-oesophageal reflux disease and concomitant noncardiac chest pain? Br. J. Clin. Pharmacol. 2007, 64, 83–89. [Google Scholar] [CrossRef]
- Zhou, Y.; Dai, Y.; Lu, L.; Fu, Z. Dabigatran-induced esophagitis: A case report. Medicine 2020, 99, e19890. [Google Scholar] [CrossRef]
- Kono, Y.; Miyahara, K.; Nakagawa, M. A case of esophagitis induced by apixaban. J. Gastrointest. Liver Dis. 2020, 29, 471. [Google Scholar] [CrossRef]
- Nagata, K.; Akazawa, Y. Exfoliative Esophagitis Induced By Sunitinib. Mayo Clin. Proc. 2019, 94, 557–558. [Google Scholar] [CrossRef]
- Kooshkaki, O.; Derakhshani, A.; Hosseinkhani, N.; Torabi, M.; Safaei, S.; Brunetti, O.; Racanelli, V.; Silvestris, N.; Baradaran, B. Combination of Ipilimumab and Nivolumab in Cancers: From Clinical Practice to Ongoing Clinical Trials. Int. J. Mol. Sci. 2020, 21, 4427. [Google Scholar] [CrossRef]
- Endo, R.; Nakamura, Y.; Ishizuki, S.; Ishitsuka, Y.; Watanabe, R.; Okiyama, N.; Ito, Y.; Nagafuchi, M.; Mizokami, Y.; Fujisawa, Y. Ulcerative esophagitis associated with combined nivolumab and ipilimumab therapy. J. Dermatol. 2020, 47, e299–e300. [Google Scholar] [CrossRef]
- Nasir, U.M.; Rodgers, B.; Panchal, D.; Choi, C.; Ahmed, S.; Ahlawat, S. Ferrous Sulfate-Induced Esophageal Injury Leading to Esophagitis Dissecans Superficialis. Case Rep. Gastroenterol. 2020, 14, 172–177. [Google Scholar] [CrossRef]
- Lanas, A.; Boers, M.; Nuevo, J. Gastrointestinal events in at-risk patients starting non-steroidal anti-inflammatory drugs (NSAIDs) for rheumatic diseases: The EVIDENCE study of European routine practice. Ann. Rheum. Dis. 2015, 74, 675–681. [Google Scholar] [CrossRef]
- Helgadottir, H.; Bjornsson, E.S. Problems Associated with Deprescribing of Proton Pump Inhibitors. Int. J. Mol. Sci. 2019, 20, 5469. [Google Scholar] [CrossRef]
- Xun, X.; Yin, Q.; Fu, Y.; He, X.; Dong, Z. Proton Pump Inhibitors and the Risk of Community-Acquired Pneumonia: An Updated Meta-analysis. Ann. Pharmacother. 2022, 56, 524–532. [Google Scholar] [CrossRef]
- Lin, S.H.; Chang, Y.S.; Lin, T.M.; Hu, L.-F.; Hou, T.-Y.; Hsu, H.-C.; Shen, Y.-C.; Kuo, P.-I.; Chen, W.-S.; Lin, Y.-C.; et al. Proton Pump Inhibitors Increase the Risk of Autoimmune Diseases: A Nationwide Cohort Study. Front. Immunol. 2021, 12, 736036. [Google Scholar] [CrossRef]
- Ariel, H.; Cooke, J.P. Cardiovascular Risk of Proton Pump Inhibitors. Methodist Debakey Cardiovasc. J. 2019, 15, 214–219. [Google Scholar] [CrossRef]
- Novotny, M.; Klimova, B.; Valis, M. PPI Long Term Use: Risk of Neurological Adverse Events? Front. Neurol. 2019, 9, 1142. [Google Scholar] [CrossRef] [PubMed]
- Briganti, S.I.; Naciu, A.M.; Tabacco, G.; Cesareo, R.; Napoli, N.; Trimboli, P.; Castellana, M.; Manfrini, S.; Palermo, A. Proton Pump Inhibitors and Fractures in Adults: A Critical Appraisal and Review of the Literature. Int. J. Endocrinol. 2021, 2021, 8902367. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, M.; Fukahori, M.; Ikemura, A.; Kubota, A.; Higashino, H.; Sakuma, S.; Yamashita, S. Effects of gastric pH on oral drug absorption: In vitro assessment using a dissolution/permeation system reflecting the gastric dissolution process. Eur. J. Pharm. Biopharm. 2016, 101, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Claesson, M.J.; Cusack, S.; O’Sullivan, O.; Greene-Diniz, R.; De Weerd, H.; Flannery, E.; Marchesi, J.R.; Falush, D.; Dinan, T.G.; Fitzgerald, G.F.; et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4586–4591. [Google Scholar] [CrossRef]
- Walrath, T.; Dyamenahalli, K.U.; Hulsebus, H.J.; McCullough, R.L.; Idrovo, J.-P.; Boe, D.M.; McMahan, R.H.; Kovacs, E.J. Age-related changes in intestinal immunity and the microbiome. J. Leukoc. Biol. 2021, 109, 1045–1061. [Google Scholar] [CrossRef]
- Wang, Q.; Qi, Y.; Shen, W.; Xu, J.; Wang, L.; Chen, S.; Hou, T.; Si, J. The Aged Intestine: Performance and Rejuvenation. Aging Dis. 2021, 12, 1693–1712. [Google Scholar] [CrossRef]
- Brechmann, T.; Günther, K.; Neid, M.; Schmiegel, W.; Tannapfel, A. Triggers of histologically suspected drug-induced colitis. World J. Gastroenterol. 2019, 25, 967–979. [Google Scholar] [CrossRef]
- Herlihy, N.; Feakins, R. Gut inflammation induced by drugs: Can pathology help to differentiate from inflammatory bowel disease? United Eur. Gastroenterol. J. 2022, 10, 451–464. [Google Scholar] [CrossRef]
- Tawam, D.; Baladi, M.; Jungsuwadee, P.; Earl, G.; Han, J. The Positive Association between Proton Pump Inhibitors and Clostridium Difficile Infection. Innov. Pharm. 2021, 12, 21. [Google Scholar] [CrossRef]
- Rémond, D.; Shahar, D.R.; Gille, D.; Pinto, P.; Kachal, J.; Peyron, M.-A.; dos Santos, C.N.; Walther, B.; Bordoni, A.; Dupont, D.; et al. Understanding the gastrointestinal tract of the elderly to develop dietary solutions that prevent malnutrition. Oncotarget 2015, 6, 13858–13898. [Google Scholar] [CrossRef]
- Löhr, J.M.; Panic, N.; Vujasinovic, M.; Verbeke, C.S. The ageing pancreas: A systematic review of the evidence and analysis of the consequences. J. Intern. Med. 2018, 283, 446–460. [Google Scholar] [CrossRef]
- Wolfe, D.; Kanji, S.; Yazdi, F.; Barbeau, P.; Rice, D.; Beck, A.; Butler, C.; Esmaeilisaraji, L.; Skidmore, B.; Moher, D.; et al. Drug induced pancreatitis: A systematic review of case reports to determine potential drug associations. PLoS ONE 2020, 15, e0231883. [Google Scholar] [CrossRef]
- Olesen, A.E.; Brokjaer, A.; Fisher, I.W.; Larsen, I.M. Pharmacological challenges in chronic pancreatitis. World J. Gastroenterol. 2013, 19, 7302–7307. [Google Scholar] [CrossRef]
- Kim, I.H.; Kisseleva, T.; Brenner, D.A. Aging and liver disease. Curr. Opin. Gastroenterol. 2015, 31, 184–191. [Google Scholar] [CrossRef]
- Björnsson, E.S.; Stephens, C.; Atallah, E.; Robles-Diaz, M.; Alvarez-Alvarez, I.; Gerbes, A.; Weber, S.; Stirnimann, G.; Kullak-Ublick, G.; Cortez-Pinto, H.; et al. A new framework for advancing in drug-induced liver injury research. The Prospective European DILI Registry. Liver Int. 2023, 43, 115–126. [Google Scholar] [CrossRef]
- Drenth-van Maanen, A.C.; Wilting, I.; Jansen, P.A.F. Prescribing medicines to older people-How to consider the impact of ageing on human organ and body functions. Br. J. Clin. Pharmacol. 2020, 86, 1921–1930. [Google Scholar] [CrossRef]
- Jubeh, B.; Breijyeh, Z.; Karaman, R. Antibacterial Prodrugs to Overcome Bacterial Resistance. Molecules 2020, 25, 1543. [Google Scholar] [CrossRef]
- Evans, L.E.; Krishna, A.; Ma, Y.; Webb, T.E.; Marshall, D.C.; Tooke, C.L.; Spencer, J.; Clarke, T.B.; Armstrong, A.; Edwards, A.M. Exploitation of antibiotic resistance as a novel drug target: Development of a β-lactamase-activated antibacterial prodrug. J. Med. Chem. 2019, 62, 4411–4425. [Google Scholar] [CrossRef]
- Sousa, P.; Bertani, L.; Rodrigues, C. Management of inflammatory bowel disease in the elderly: A review. Dig. Liver Dis. 2023. advance online publication. [Google Scholar] [CrossRef]
- Alrubia, S.; Mao, J.; Chen, Y.; Barber, J.; Rostami-Hodjegan, A. Altered Bioavailability and Pharmacokinetics in Crohn’s Disease: Capturing Systems Parameters for PBPK to Assist with Predicting the Fate of Orally Administered Drugs. Clin. Pharmacokinet. 2022, 61, 1365–1392. [Google Scholar] [CrossRef]
- Rattanacheeworn, P.; Kerr, S.J.; Kittanamongkolchai, W.; Townamchai, N.; Udomkarnjananun, S.; Praditpornsilpa, K.; Thanusuwannasak, T.; Udomnilobol, U.; Jianmongkol, S.; Ongpipattanakul, B.; et al. Quantification of CYP3A and Drug Transporters Activity in Healthy Young, Healthy Elderly and Chronic Kidney Disease Elderly Patients by a Microdose Cocktail Approach. Front. Pharmacol. 2021, 12, 726669. [Google Scholar] [CrossRef] [PubMed]
- Riches, Z.; Abanda, N.; Collier, A.C. BCRP protein levels do not differ regionally in adult human livers, but decline in the elderly. Chem. Biol. Interact. 2015, 242, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Viennois, E.; Pujada, A.; Zen, J.; Merlin, D. Function, Regulation, and Pathophysiological Relevance of the POT Superfamily, Specifically PepT1 in Inflammatory Bowel Disease. Compr. Physiol. 2018, 8, 731–760. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chu, C.; Ji, X.; Luo, G.; Xu, C.; He, H.; Yao, J.; Wu, J.; Hu, J.; Jin, Y. Biology of Peptide Transporter 2 in Mammals: New Insights into Its Function, Structure and Regulation. Cells 2022, 11, 2874. [Google Scholar] [CrossRef] [PubMed]
- Dücker, C.M.; Brockmöller, J. Genomic Variation and Pharmacokinetics in Old Age: A Quantitative Review of Age- vs. Genotype-Related Differences. Clin. Pharmacol. Ther. 2019, 105, 625–640. [Google Scholar] [CrossRef]
- Prasad, B.; Evers, R.; Gupta, A.; Hop, C.E.C.A.; Salphati, L.; Shukla, S.; Ambudkar, S.V.; Unadkat, J.D. Interindividual variability in hepatic organic anion-transporting polypeptides and P-glycoprotein (ABCB1) protein expression: Quantification by liquid chromatography tandem mass spectroscopy and influence of genotype, age, and sex. Drug Metab. Dispos. 2014, 42, 78–88. [Google Scholar] [CrossRef]
- Hou, W.Y.; Xu, S.F.; Zhu, Q.N.; Lu, Y.F.; Cheng, X.G.; Liu, J. Age- and sex-related differences of organic anion-transporting polypeptide gene expression in livers of rats. Toxicol. Appl. Pharmacol. 2014, 280, 370–377. [Google Scholar] [CrossRef]
- Zhang, Y.K.; Saupe, K.W.; Klaassen, C.D. Energy restriction does not compensate for the reduced expression of hepatic drug-processing genes in mice with aging. Drug Metab. Dispos. 2010, 38, 1122–1131. [Google Scholar] [CrossRef]
- Rosati, A.; Maniori, S.; Decorti, G.; Candussio, L.; Giraldi, T.; Bartoli, F. Physiological regulation of P-glycoprotein, MRP1, MRP2 and cytochrome P450 3A2 during rat ontogeny. Dev. Growth Differ. 2003, 45, 377–387. [Google Scholar] [CrossRef]
- Qian, X.; Cheng, Y.H.; Mruk, D.D.; Cheng, C.Y. Breast cancer resistance protein (Bcrp) and the testis—An unexpected turn of events. Asian J. Androl. 2013, 15, 455–460. [Google Scholar] [CrossRef]
- DrugBank Online. BCRP/ABCG2 Substrates. Available online: https://go.drugbank.com/categories/DBCAT002663 (accessed on 10 April 2023).
- Brenner, S.S.; Klotz, U. P-glycoprotein function in the elderly. Eur. J. Clin. Pharmacol. 2004, 60, 97–102. [Google Scholar] [CrossRef]
- Klaassen, C.D.; Aleksunes, L.M. Xenobiotic, bile acid, and cholesterol transporters: Function and regulation. Pharmacol. Rev. 2010, 62, 1–96. [Google Scholar] [CrossRef]
- Sugie, M.; Asakura, E.; Zhao, Y.L.; Torita, S.; Nadai, M.; Baba, K.; Kitaichi, K.; Takagi, K.; Takagi, K.; Hasegawa, T. Possible involvement of the drug transporters P glycoprotein and multidrug resistance-associated protein Mrp2 in disposition of azithromycin. Antimicrob. Agents Chemother. 2004, 48, 809–814. [Google Scholar] [CrossRef]
- Sakaeda, T.; Nakamura, T.; Okumura, K. MDR1 genotype-related pharmacokinetics and pharmacodynamics. Biol. Pharm. Bull. 2002, 25, 1391–1400. [Google Scholar] [CrossRef]
- Putnam, W.S.; Woo, J.M.; Huang, Y.; Benet, L.Z. Effect of the MDR1 C3435T variant and P-glycoprotein induction on dicloxacillin pharmacokinetics. J. Clin. Pharmacol. 2005, 45, 411–421. [Google Scholar] [CrossRef]
- Stage, T.B.; Graff, M.; Wong, S.; Rasmussen, L.L.; Nielsen, F.; Pottegård, A.; Brøsen, K.; Kroetz, D.L.; Khojasteh, S.C.; Damkier, P. Dicloxacillin induces CYP2C19, CYP2C9 and CYP3A4 in vivo and in vitro. Br. J. Clin. Pharmacol. 2018, 84, 510–519. [Google Scholar] [CrossRef]
- Human Transporters MRP2. Available online: https://www.solvobiotech.com/transporters/mrp2 (accessed on 10 April 2023).
- Maeda, T.; Takahashi, K.; Ohtsu, N.; Oguma, T.; Ohnishi, T.; Atsumi, R.; Tamai, I. Identification of influx transporter for the quinolone antibacterial agent levofloxacin. Mol. Pharm. 2007, 4, 85–94. [Google Scholar] [CrossRef]
- Franke, R.M.; Baker, S.D.; Mathijssen, R.H.; Schuetz, E.G.; Sparreboom, A. Influence of solute carriers on the pharmacokinetics of CYP3A4 probes. Clin. Pharmacol. Ther. 2008, 84, 704–709. [Google Scholar] [CrossRef]
- Mahalingam, A.; Shenoy, B. Tebipenem: A Novel Oral Carbapenem. Pediatr. Infect. Dis. 2020, 2, 25–28. [Google Scholar] [CrossRef]
- Badée, J.; Achour, B.; Rostami-Hodjegan, A.; Galetin, A. Meta-analysis of expression of hepatic organic anion-transporting polypeptide (OATP) transporters in cellular systems relative to human liver tissue. Drug Metab. Dispos. Biol. Fate Chem. 2015, 43, 424–432. [Google Scholar] [CrossRef]
- Nakakariya, M.; Shimada, T.; Irokawa, M.; Maeda, T.; Tamai, I. Identification and species similarity of OATP transporters responsible for hepatic uptake of beta-lactam antibiotics. Drug Metab. Pharmacokinet. 2008, 23, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Tamai, I.; Nezu, J.; Uchino, H.; Sai, Y.; Oku, A.; Shimane, M.; Tsuji, A. Molecular identification and characterization of novel members of the human organic anion transporter (OATP) family. Biochem. Biophys. Res. Commun. 2000, 273, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Shirasaka, Y.; Kuraoka, E.; Kikuchi, A.; Iguchi, M.; Suzuki, H.; Shibasaki, S.; Kurosawa, T.; Tamai, I. Intestinal absorption mechanism of tebipenem pivoxil, a novel oral carbapenem: Involvement of human OATP family in apical membrane transport. Mol. Pharm. 2010, 7, 1747–1756. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, O.A.; King, N.; Andronicos, N.M.; Jones, G.L.; Chami, B.; Witting, P.K.; Moens, P.D.J. Molecular changes to the rat renal cotransporters PEPT1 and PEPT2 due to ageing. Mol. Cell. Biochem. 2019, 452, 71–82. [Google Scholar] [CrossRef]
- Lu, X.; Chan, T.; Xu, C.; Zhu, L.; Zhou, Q.T.; Roberts, K.D.; Chan, H.K.; Li, J.; Zhou, F. Human oligopeptide transporter 2 (PEPT2) mediates cellular uptake of polymyxins. J. Antimicrob. Chemother. 2016, 71, 403–412. [Google Scholar] [CrossRef]
- Shekhawat, P.B.; Pokharkar, V.B. Understanding peroral absorption: Regulatory aspects and contemporary approaches to tackling solubility and permeability hurdles. Acta Pharm. Sin. B 2017, 7, 260–280. [Google Scholar] [CrossRef]
- Rangaraj, N.; Sampathi, S.; Junnuthula, V.; Kolimi, P.; Mandati, P.; Narala, S.; Nyavanandi, D.; Dyawanapelly, S. Fast-Fed Variability: Insights into Drug Delivery, Molecular Manifestations, and Regulatory Aspects. Pharmaceutics 2022, 14, 1807. [Google Scholar] [CrossRef]
- Genser, D. Food and drug interaction: Consequences for the nutrition/health status. Ann. Nutr. Metab. 2008, 52 (Suppl. S1), 29–32. [Google Scholar] [CrossRef]
- Thambavita, D.D.; Galappatthy, P.; Jayakody, R.L. Pharmacokinetics and Bioequivalence of Two Amoxicillin 500 mg Products: Effect of Food on Absorption and Supporting Scientific Justification for Biowaiver. J. Pharm. Sci. 2021, 110, 3735–3741. [Google Scholar] [CrossRef]
- Weitschies, W.; Friedrich, C.; Wedemeyer, R.S.; Schmidtmann, M.; Kosch, O.; Kinzig, M.; Trahms, L.; Sörgel, F.; Siegmund, W.; Horkovics-Kovats, S.; et al. Bioavailability of amoxicillin and clavulanic acid from extended release tablets depends on intragastric tablet deposition and gastric emptying. Eur. J. Pharm. Biopharm. 2008, 70, 641–648. [Google Scholar] [CrossRef]
- Gardiner, S.J.; Drennan, P.G.; Begg, R.; Zhang, M.; Green, J.K.; Isenman, H.L.; Everts, R.J.; Chambers, S.T.; Begg, E.J. In healthy volunteers, taking flucloxacillin with food does not compromise effective plasma concentrations in most circumstances. PLoS ONE 2018, 13, e0199370. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Deng, Q.; Li, Y.; Li, P.; Liu, G.; Wang, Y.; Liu, Z.; Yu, S.; Cheng, Y.; Zhou, Y.; et al. Pharmacokinetics and safety of the two oral cefaclor formulations in healthy chinese subjects in the fasting and postprandial states. Front. Pharmacol. 2022, 13, 1012294. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Omata, H.; Nishimura, H.; Tanaka, S.; Matsumoto, H.; Fujii, A.; Akimoto, Y.; Komiya, M. Cefpodoxime Concentrations in Human Serum and Oral Tissues Following a Single Oral Administration of Cefpodoxime Proxetil. Int. J. Oral-Med. Sci. 2015, 14, 48–53. [Google Scholar] [CrossRef]
- Borin, M.T.; Forbes, K.K. Effect of food on absorption of cefpodoxime proxetil oral suspension in adults. Antimicrob. Agents Chemother. 1995, 39, 273–275. [Google Scholar] [CrossRef] [PubMed]
- Borin, M.T.; Ferry, J.J.; Forbes, K.K.; Hughes, G.S. Pharmacokinetics of cefpodoxime proxetil in healthy young and elderly volunteers. J. Clin. Pharmacol. 1994, 34, 774–781. [Google Scholar] [CrossRef]
- Curatolo, W.; Foulds, G.; Labadie, R. Mechanistic study of the azithromycin dosage-form-dependent food effect. Pharm. Res. 2010, 27, 1361–1366. [Google Scholar] [CrossRef]
- Kshirsagar, A.S.; Patil, V.M.; Patil, S. A Study on Effect of Food on Pharmacokinetics of Clindamycin: A Research. Int. J. Sci. Res. 2022, 11, 304–307. [Google Scholar] [CrossRef]
- Stalker, D.J.; Jungbluth, G.L. Clinical pharmacokinetics of linezolid, a novel oxazolidinone antibacterial. Clin. Pharmacokinet. 2003, 42, 1129–1140. [Google Scholar] [CrossRef]
- Imaoka, A.; Abiru, K.; Akiyoshi, T.; Ohtani, H. Food intake attenuates the drug interaction between new quinolones and aluminum. J. Pharm. Health Care Sci. 2018, 4, 11. [Google Scholar] [CrossRef]
- Williams, N.T. Medication administration through enteral feeding tubes. Am. J. Health Syst. Pharm. 2008, 65, 2347–2357. [Google Scholar] [CrossRef]
- Lee, L.J.; Hafkin, B.; Lee, I.D.; Hoh, J.; Dix, R. Effects of food and sucralfate on a single oral dose of 500 milligrams of levofloxacin in healthy subjects. Antimicrob. Agents Chemother. 1997, 41, 2196–2200. [Google Scholar] [CrossRef]
- Amsden, G.W.; Whitaker, A.M.; Johnson, P.W. Lack of bioequivalence of levofloxacin when coadministered with a mineral-fortified breakfast of juice and cereal. J. Clin. Pharmacol. 2003, 43, 990–995. [Google Scholar] [CrossRef]
- Taubel, J.; Ferber, G.; Lorch, U.; Batchvarov, V.; Savelieva, I.; Camm, A.J. Pharmacokinetics of 400 mg oral moxifloxacin in the fed and fasted state in TQT studies. Br. J. Clin. Pharmacol. 2014, 77, 170–179. [Google Scholar] [CrossRef]
- Pal, A.; Matzneller, P.; Gautam, A.; Österreicher, Z.; Wulkersdorfer, B.; Reiter, B.; Stimpfl, T.; Zeitlinger, M. Target site pharmacokinetics of doxycycline for rosacea in healthy volunteers is independent of the food effect. Br. J. Clin. Pharmacol. 2018, 84, 2625–2633. [Google Scholar] [CrossRef]
- McCormick, R.; Vasilaki, A. Age-related changes in skeletal muscle: Changes to life-style as a therapy. Biogerontology 2018, 19, 519–536. [Google Scholar] [CrossRef]
- Mangoni, A.A.; Jarmuzewska, E.A. The influence of heart failure on the pharmacokinetics of cardiovascular and non-cardiovascular drugs: A critical appraisal of the evidence. Br. J. Clin. Pharmacol. 2019, 85, 20–36. [Google Scholar] [CrossRef]
- Kaestli, L.Z.; Wasilewski-Rasca, A.F.; Bonnabry, P.; Vogt-Ferrier, N. Use of transdermal drug formulations in the elderly. Drugs Aging 2008, 25, 269–280. [Google Scholar] [CrossRef]
- Cho, S.J.; Stout-Delgado, H.W. Aging and Lung Disease. Annu. Rev. Physiol. 2020, 82, 433–459. [Google Scholar] [CrossRef]
- Wallin, M.; Tagami, T.; Chen, L.; Yang, M.; Chan, H.K. Pulmonary drug delivery to older people. Adv. Drug Deliv. Rev. 2018, 135, 50–61. [Google Scholar] [CrossRef]
- Singam, N.S.V.; Fine, C.; Fleg, J.L. Cardiac changes associated with vascular aging. Clin. Cardiol. 2020, 43, 92–98. [Google Scholar] [CrossRef]
- Falcone, M.; Paul, M.; Tiseo, G.; Yahav, D.; Prendki, V.; Friberg, L.E.; Guerri, R.; Gavazzi, G.; Mussini, C.; Tinelli, M.; et al. Considerations for the optimal management of antibiotic therapy in elderly patients. J. Glob. Antimicrob. Resist. 2020, 22, 325–333. [Google Scholar] [CrossRef]
- Wicha, S.G.; Märtson, A.G.; Nielsen, E.I.; Koch, B.C.; Friberg, L.E.; Alffenaar, J.; Minichmayr, I.K. From Therapeutic Drug Monitoring to Model-Informed Precision Dosing for Antibiotics. Clin. Pharmacol. Ther. 2021, 109, 928–941. [Google Scholar] [CrossRef] [PubMed]
- Ponti, F.; Santoro, A.; Mercatelli, D.; Gasperini, C.; Conte, M.; Martucci, M.; Sangiorgi, L.; Franceschi, C.; Bazzocchi, A. Aging and Imaging Assessment of Body Composition: From Fat to Facts. Front. Endocrinol. 2020, 10, 861. [Google Scholar] [CrossRef] [PubMed]
- Serra-Prat, M.; Lorenzo, I.; Palomera, E.; Ramírez, S.; Yébenes, J.C. Total Body Water and Intracellular Water Relationships with Muscle Strength, Frailty and Functional Performance in an Elderly Population. A Cross-Sectional Study. J. Nutr. Health Aging 2019, 23, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Smit, C.; De Hoogd, S.; Brüggemann, R.J.M.; Knibbe, C.A.J. Obesity and drug pharmacology: A review of the influence of obesity on pharmacokinetic and pharmacodynamic parameters. Expert Opin. Drug Metab. Toxicol. 2018, 14, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Udy, A.A.; Roberts, J.A.; Lipman, J. Clinical implications of antibiotic pharmacokinetic principles in the critically ill. Intensive Care Med. 2013, 39, 2070–2082. [Google Scholar] [CrossRef] [PubMed]
- Knox, E.G.; Aburto, M.R.; Clarke, G.; Cryan, J.F.; O’Driscoll, C.M. The blood-brain barrier in aging and neurodegeneration. Mol. Psychiatry 2022, 27, 2659–2673. [Google Scholar] [CrossRef]
- Hurkacz, M.; Dobrek, L.; Wiela-Hojeńska, A. Antibiotics and the Nervous System-Which Face of Antibiotic Therapy Is Real, Dr. Jekyll (Neurotoxicity) or Mr. Hyde (Neuroprotection)? Molecules 2021, 26, 7456. [Google Scholar] [CrossRef]
- Smith, S.A.; Waters, N.J. Pharmacokinetic and Pharmacodynamic Considerations for Drugs Binding to Alpha-1-Acid Glycoprotein. Pharm. Res. 2018, 36, 30. [Google Scholar] [CrossRef]
- Celestin, M.N.; Musteata, F.M. Impact of Changes in Free Concentrations and Drug-Protein Binding on Drug Dosing Regimens in Special Populations and Disease States. J. Pharm. Sci. 2021, 110, 3331–3344. [Google Scholar] [CrossRef]
- Brock, F.; Bettinelli, L.A.; Dobner, T.; Stobbe, J.C.; Pomatti, G.; Telles, C.T. Prevalence of hypoalbuminemia and nutritional issues in hospitalized elders. Rev. Lat. Am. Enfermagem. 2016, 24, e2736. [Google Scholar] [CrossRef]
- Moramarco, S.; Morciano, L.; Morucci, L.; Messinese, M.; Gualtieri, P.; Carestia, M.; Ciccacci, F.; Orlando, S.; Buonomo, E.; Legramante, J.M.; et al. Epidemiology of Hypoalbuminemia in Hospitalized Patients: A Clinical Matter or an Emerging Public Health Problem? Nutrients 2020, 12, 3656. [Google Scholar] [CrossRef]
- Frith, E.; Loprinzi, P.D. Physical Activity and Cognitive Function among Older Adults with an Elevated Gamma Gap. Med. Princ. Pract. 2018, 27, 531–536. [Google Scholar] [CrossRef]
- Ulldemolins, M.; Roberts, J.A.; Rello, J.; Paterson, D.L.; Lipman, J. The effects of hypoalbuminaemia on optimizing antibacterial dosing in critically ill patients. Clin. Pharmacokinet. 2011, 50, 99–110. [Google Scholar] [CrossRef]
- Pereira, J.G.; Fernandes, J.; Duarte, A.R.; Fernandes, S.M. β-Lactam Dosing in Critical Patients: A Narrative Review of Optimal Efficacy and the Prevention of Resistance and Toxicity. Antibiotics 2022, 11, 1839. [Google Scholar] [CrossRef]
- Mizuno, T.; Mizokami, F.; Fukami, K.; Ito, K.; Shibasaki, M.; Nagamatsu, T.; Furuta, K. The influence of severe hypoalbuminemia on the half-life of vancomycin in elderly patients with methicillin-resistant Staphylococcus aureus hospital-acquired pneumonia. Clin. Interv. Aging 2013, 8, 1323–1328. [Google Scholar] [CrossRef]
- Zusman, O.; Farbman, L.; Tredler, Z.; Daitch, V.; Lador, A.; Leibovici, L.; Paul, M. Association between hypoalbuminemia and mortality among subjects treated with ertapenem versus other carbapenems: Prospective cohort study. Clin. Microbiol. Infect. 2014, 21, 54–58. [Google Scholar] [CrossRef]
- Heffernan, A.J.; Sime, F.B.; Kumta, N.; Wallis, S.C.; McWhinney, B.; Ungerer, J.; Wong, G.; Joynt, G.M.; Lipman, J.; Roberts, J.A. Multicenter Population Pharmacokinetic Study of Unbound Ceftriaxone in Critically Ill Patients. Antimicrob. Agents Chemother. 2022, 66, e0218921. [Google Scholar] [CrossRef]
- Baalbaki, N.; Blum, S.; Akerman, M.; Johnson, D. Ceftriaxone 1 g Versus 2 g Daily for the Treatment of Enterobacterales Bacteremia: A Retrospective Cohort Study. J. Pharm. Technol. 2022, 38, 326–334. [Google Scholar] [CrossRef]
- Allegaert, K.; Muller, A.E.; Russo, F.; Schoenmakers, S.; Deprest, J.; Koch, B.C.P. Pregnancy-related pharmacokinetics and antimicrobial prophylaxis during fetal surgery, cefazolin and clindamycin as examples. Prenat. Diagn. 2020, 40, 1178–1184. [Google Scholar] [CrossRef]
- Tucker, L.A.; Parker, K. 10-Year Weight Gain in 13,802 US Adults: The Role of Age, Sex, and Race. J. Obes. 2022, 2022, 7652408. [Google Scholar] [CrossRef] [PubMed]
- Fakhouri, T.; Ogden, C.L.; Carroll, M.D.; Kit, B.K.; Flegal, K.M. Prevalence of obesity among older adults in the United States, 2007–2010. Natl. Center Health Stat. Data Brief. 2012, 106, 1–8. [Google Scholar]
- Meng, L.; Mui, E.; Holubar, M.K.; Deresinski, S.C. Comprehensive Guidance for Antibiotic Dosing in Obese Adults. Pharmacotherapy 2017, 37, 1415–1431. [Google Scholar] [CrossRef] [PubMed]
- Tucker, C.E.; Lockwood, A.M.; Nguyen, N.H. Antibiotic dosing in obesity: The search for optimum dosing strategies. Clin. Obes. 2014, 4, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Coates, M.; Shield, A.; Peterson, G.M.; Hussain, Z. Prophylactic Cefazolin Dosing in Obesity-a Systematic Review. Obes. Surg. 2022, 32, 3138–3149. [Google Scholar] [CrossRef] [PubMed]
- Janson, B.; Thursky, K. Dosing of antibiotics in obesity. Curr. Opin. Infect. Dis. 2012, 25, 634–649. [Google Scholar] [CrossRef]
- Donini, L.M.; Stephan, B.C.M.; Rosano, A.; Molfino, A.; Poggiogalle, E.; Lenzi, A.; Siervo, M.; Muscaritoli, M. What Are the Risk Factors for Malnutrition in Older-Aged Institutionalized Adults? Nutrients 2020, 12, 2857. [Google Scholar] [CrossRef]
- Alvis, B.D.; Hughes, C.G. Physiology Considerations in Geriatric Patients. Anesthesiol. Clin. 2015, 33, 447–456. [Google Scholar] [CrossRef]
- Tan, J.L.; Eastment, J.G.; Poudel, A.; Hubbard, R.E. Age-Related Changes in Hepatic Function: An Update on Implications for Drug Therapy. Drugs Aging 2015, 32, 999–1008. [Google Scholar] [CrossRef]
- Kaburaki, S.; Yoshimura, E.; Miyamoto, Y.; Imai, S.; Kashiwagi, H.; Ueno, H.; Sugawara, M.; Takekuma, Y. Hepatic drug metabolism in older people with body composition changes. Geriatr. Gerontol. Int. 2022, 22, 449–454. [Google Scholar] [CrossRef]
- Drozdzik, M.; Lapczuk-Romanska, J.; Wenzel, C.; Szelag-Pieniek, S.; Post, M.; Skalski, Ł.; Kurzawski, M.; Oswald, S. Gene Expression and Protein Abundance of Hepatic Drug Metabolizing Enzymes in Liver Pathology. Pharmaceutics 2021, 13, 1334. [Google Scholar] [CrossRef]
- Trobec, K.; Kerec Kos, M.; von Haehling, S.; Springer, J.; Anker, S.D.; Lainscak, M. Pharmacokinetics of drugs in cachectic patients: A systematic review. PLoS ONE 2013, 8, e79603. [Google Scholar] [CrossRef]
- Obach, R.S. Linezolid Metabolism Is Catalyzed by Cytochrome P450 2J2, 4F2, and 1B1. Drug Metab. Dispos. 2022, 50, 413–421. [Google Scholar] [CrossRef]
- DrugBank Online. Cytochrome P450 1A1. Available online: https://go.drugbank.com/polypeptides/P04798 (accessed on 13 April 2023).
- Lu, J.; Shang, X.; Zhong, W.; Xu, Y.; Shi, R.; Wang, X. New insights of CYP1A in endogenous metabolism: A focus on single nucleotide polymorphisms and diseases. Acta Pharm. Sin. B 2020, 10, 91–104. [Google Scholar] [CrossRef]
- DrugBank Online. Cytochrome P-450 CYP1A2 Substrates. Available online: https://go.drugbank.com/categories/DBCAT002609 (accessed on 13 April 2023).
- DrugBank Online. Cytochrome P-450 CYP1A2. Available online: Inhibitorshttps://go.drugbank.com/categories/DBCAT000402 (accessed on 13 April 2023).
- DrugBank Online. Cytochrome P-450 CYP1A2 Inducers. Available online: https://go.drugbank.com/categories/DBCAT000614 (accessed on 13 April 2023).
- DrugBank Online. Cytochrome P-450 CYP2A6 Substrates. Available online: https://go.drugbank.com/categories/DBCAT002613 (accessed on 13 April 2023).
- DrugBank Online. Cytochrome P-450 CYP2A6 Inhibitors. Available online: https://go.drugbank.com/categories/DBCAT002614 (accessed on 13 April 2023).
- DrugBank Online. Cytochrome P-450 CYP2A6 Inducers. Available online: https://go.drugbank.com/categories/DBCAT002617 (accessed on 13 April 2023).
- Tanner, J.A.; Prasad, B.; Claw, K.G.; Stapleton, P.; Chaudhry, A.; Schuetz, E.G.; Thummel, K.E.; Tyndale, R.F. Predictors of Variation in CYP2A6 mRNA, Protein, and Enzyme Activity in a Human Liver Bank: Influence of Genetic and Nongenetic Factors. J. Pharmacol. Exp. Ther. 2017, 360, 129–139. [Google Scholar] [CrossRef]
- Li, F.; Zhu, W.; Gonzalez, F.J. Potential role of CYP1B1 in the development and treatment of metabolic diseases. Pharmacol. Ther. 2017, 178, 18–30. [Google Scholar] [CrossRef]
- DrugBank Online. Cytochrome P-450 CYP2B6 Inhibitors. Available online: https://go.drugbank.com/categories/DBCAT001285 (accessed on 13 April 2023).
- DrugBank Online. Cytochrome P-450 CYP2B6 Inducers. Available online: https://go.drugbank.com/categories/DBCAT001015 (accessed on 13 April 2023).
- Torgersen, J.; Bellamy, S.L.; Ratshaa, B.; Han, X.; Mosepele, M.; Zuppa, A.F.; Vujkovic, M.; Steenhoff, A.P.; Bisson, G.P.; Gross, R. Impact of Efavirenz Metabolism on Loss to Care in Older HIV+ Africans. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 179–187. [Google Scholar] [CrossRef]
- DrugBank Online. Cytochrome P-450 CYP2C8 Substrates. Available online: https://go.drugbank.com/categories/DBCAT002642 (accessed on 13 April 2023).
- DrugBank Online. Cytochrome P-450 CYP2C8 Inhibitors. Available online: https://go.drugbank.com/categories/DBCAT000868 (accessed on 13 April 2023).
- DrugBank Online. Cytochrome P-450 CYP2C8 Inducers. Available online: https://go.drugbank.com/categories/DBCAT001247 (accessed on 13 April 2023).
- Liu, W.; Wang, B.; Ding, H.; Wang, D.W.; Zeng, H. A potential therapeutic effect of CYP2C8 overexpression on anti-TNF-α activity. Int. J. Mol. Med. 2014, 34, 725–732. [Google Scholar] [CrossRef]
- DrugBank Online. Cytochrome P-450 CYP2C9 Substrates. Available online: https://go.drugbank.com/categories/DBCAT002634 (accessed on 13 April 2023).
- DrugBank Online. Cytochrome P450 2C9. Available online: https://go.drugbank.com/polypeptides/P11712 (accessed on 13 April 2023).
- DrugBank Online. Cytochrome P-450 CYP2C19 Inhibitors. Available online: https://go.drugbank.com/categories/DBCAT000403 (accessed on 13 April 2023).
- DrugBank Online. Cytochrome P-450 CYP2C19 Inducers. Available online: https://go.drugbank.com/categories/DBCAT001246 (accessed on 13 April 2023).
- DrugBank Online. Cytochrome P-450 CYP2D6 Substrates. Available online: https://go.drugbank.com/categories/DBCAT002623 (accessed on 13 April 2023).
- DrugBank Online. Cytochrome P-450 CYP2D6 Inhibitors. Available online: https://go.drugbank.com/categories/DBCAT000911 (accessed on 13 April 2023).
- Waade, R.B.; Hermann, M.; Moe, H.L.; Molden, E. Impact of age on serum concentrations of venlafaxine and escitalopram in different CYP2D6 and CYP2C19 genotype subgroups. Eur. J. Clin. Pharmacol. 2014, 70, 933–940. [Google Scholar] [CrossRef]
- DrugBank Online. Cytochrome P-450 CYP2E1 Substrates. Available online: https://go.drugbank.com/categories/DBCAT002628 (accessed on 13 April 2023).
- DrugBank Online. Cytochrome P-450 CYP2E1 Inhibitors. Available online: https://go.drugbank.com/categories/DBCAT002629 (accessed on 13 April 2023).
- DrugBank Online. Cytochrome P-450 CYP2E1 Inducers. Available online: https://go.drugbank.com/categories/DBCAT002632 (accessed on 13 April 2023).
- DrugBank Online. Erythromycin. Available online: https://go.drugbank.com/drugs/DB00199 (accessed on 13 April 2023).
- Wynalda, M.A.; Hutzler, J.M.; Koets, M.D.; Podoll, T.; Wienkers, L.C. In Vitro Metabolism Of Clindamycin in Human Liver and Intestinal Microsomes. Drug Metab. Dispos. 2003, 31, 878–887. [Google Scholar] [CrossRef]
- DrugBank Online. Cytochrome P-450 CYP3A4 Substrates. Available online: https://go.drugbank.com/categories/DBCAT002646 (accessed on 13 April 2023).
- DrugBank Online. Cytochrome P-450 CYP3A4 Inhibitors. Available online: https://go.drugbank.com/categories/DBCAT003232 (accessed on 13 April 2023).
- DrugBank Online. Cytochrome P-450 CYP3A4 Inducers. Available online: https://go.drugbank.com/categories/DBCAT003896 (accessed on 13 April 2023).
- Álvarez, L.A.; Van de Sijpe, G.; Desmet, S.; Metsemakers, W.-J.; Spriet, I.; Allegaert, K.; Rozenski, J. Ways to Improve Insights into Clindamycin Pharmacology and Pharmacokinetics Tailored to Practice. Antibiotics 2022, 11, 701. [Google Scholar] [CrossRef] [PubMed]
- DrugBank Online. Cytochrome P-450 CYP3A5 Substrates. Available online: https://go.drugbank.com/categories/DBCAT003807 (accessed on 13 April 2023).
- DrugBank Online. Cytochrome P-450 CYP3A5 Inhibitors. Available online: https://go.drugbank.com/categories/DBCAT003893 (accessed on 13 April 2023).
- DrugBank Online. Cytochrome P-450 CYP3A5 Inducers. Available online: https://go.drugbank.com/categories/DBCAT004489 (accessed on 13 April 2023).
- Williams, J.A.; Ring, B.J.; Cantrell, V.E.; Jones, D.R.; Eckstein, J.; Ruterbories, K.; Hamman, M.A.; Hall, S.D.; Wrighton, S.A. Comparative metabolic capabilities of CYP3A4, CYP3A5, and CYP3A7. Drug Metab. Dispos. 2002, 30, 883–891. [Google Scholar] [CrossRef]
- DrugBank Online. Cytochrome P-450 CYP3A7 Substrates. Available online: https://go.drugbank.com/categories/DBCAT003800 (accessed on 13 April 2023).
- DrugBank Online. Cytochrome P-450 CYP3A7 Inhibitors. Available online: https://go.drugbank.com/categories/DBCAT003892 (accessed on 13 April 2023).
- DrugBank Online. Cytochrome P-450 CYP3A7 Inducers. Available online: https://go.drugbank.com/categories/DBCAT004499 (accessed on 13 April 2023).
- Sim, S.C.; Edwards, R.J.; Boobis, A.R.; Ingelman-Sundberg, M. CYP3A7 protein expression is high in a fraction of adult human livers and partially associated with the CYP3A7*1C allele. Pharm. Genom. 2005, 15, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Jarrell, A.S.; Kruer, R.M.; Johnson, D.; Lipsett, P.A. Antimicrobial Pharmacokinetics and Pharmacodynamics. Surg. Infect. 2015, 16, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Karam, Z.; Tuazon, J. Anatomic and physiologic changes of the aging kidney. Clin. Geriatr. Med. 2013, 29, 555–564. [Google Scholar] [CrossRef]
- Lerma, E.V. Anatomic and physiologic changes of the aging kidney. Clin. Geriatr. Med. 2009, 25, 325–329. [Google Scholar] [CrossRef]
- Fang, Y.; Gong, A.Y.; Haller, S.T.; Dworkin, L.D.; Liu, Z.; Gong, R. The ageing kidney: Molecular mechanisms and clinical implications. Ageing Res. Rev. 2020, 63, 101151. [Google Scholar] [CrossRef]
- Kampmann, J.D.; Heaf, J.G.; Mogensen, C.B.; Mickley, H.; Wolff, D.L.; Brandt, F. Prevalence and incidence of chronic kidney disease stage 3–5—Results from KidDiCo. BMC Nephrol. 2023, 24, 17. [Google Scholar] [CrossRef]
- Bridges, C.C.; Zalups, R.K. The aging kidney and the nephrotoxic effects of mercury. J. Toxicol. Environ. Health B Crit. Rev. 2017, 20, 55–80. [Google Scholar] [CrossRef]
- Benson, J.M. Antimicrobial Pharmacokinetics and Pharmacodynamics in Older Adults. Infect. Dis. Clin. N. Am. 2017, 31, 609–617. [Google Scholar] [CrossRef]
- Otobe, Y.; Rhee, C.M.; Nguyen, M.; Kalantar-Zadeh, K.; Kopple, J.D. Current status of the assessment of sarcopenia, frailty, physical performance and functional status in chronic kidney disease patients. Curr. Opin. Nephrol. Hypertens. 2022, 31, 109–128. [Google Scholar] [CrossRef]
- Chinzowu, T.; Chyou, T.Y.; Nishtala, P.S. Antibacterial-associated acute kidney injury among older adults: A post-marketing surveillance study using the FDA adverse events reporting system. Pharmacoepidemiol. Drug Saf. 2022, 31, 1190–1198. [Google Scholar] [CrossRef]
- Liu, J.; Tong, S.Y.C.; Davis, J.S.; Rhodes, N.J.; Scheetz, M.H.; CAMERA2 Study Group. Vancomycin Exposure and Acute Kidney Injury Outcome: A Snapshot From the CAMERA2 Study. Open Forum Infect. Dis. 2020, 7, ofaa538. [Google Scholar] [CrossRef]
- Robertson, A.D.; Li, C.; Hammond, D.A.; Dickey, T.A. Incidence of Acute Kidney Injury among Patients Receiving the Combination of Vancomycin with Piperacillin-Tazobactam or Meropenem. Pharmacotherapy 2018, 38, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, B.; Davies, M.R.P.; Trubiano, J.A.; Pellicano, R. Time to Acute Kidney Injury in β-Lactam-Induced Acute Interstitial Nephritis. Kidney Int. Rep. 2020, 5, 1068–1070. [Google Scholar] [CrossRef] [PubMed]
- Crochette, R.; Ravaiau, C.; Perez, L.; Coindre, J.P.; Piccoli, G.B.; Blanchi, S. Incidence and Risk Factors for Acute Kidney Injury during the Treatment of Methicillin-Sensitive Staphylococcus aureus Infections with Cloxacillin Based Antibiotic Regimens: A French Retrospective Study. J. Clin. Med. 2021, 10, 2603. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.; Chan-Tompkins, N.H.; Como, J.; Guarascio, A.J. Retrospective Analysis of Adverse Drug Events between Nafcillin Versus Cefazolin for Treatment of Methicillin-Susceptible Staphylococcus aureus Infections. Ann. Pharmacother. 2020, 54, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Khalili, H.; Bairami, S.; Kargar, M. Antibiotics induced acute kidney injury: Incidence, risk factors, onset time and outcome. Acta Med. Iran. 2013, 51, 871–878. [Google Scholar]
- Bausch, S.; Araschmid, L.J.; Hardmeier, M.; Osthoff, M. Cefepime-Induced Neurotoxicity in the Setting of Acute Kidney Injury: A Case Series and Discussion of Preventive Measures. Cureus 2022, 14, e26392. [Google Scholar] [CrossRef]
- Billups, K.B.; Reed, E.E.; Phillips, G.S.; Stevenson, K.B.; Steinberg, S.M.; Murphy, C.V. Risk of acute kidney injury in critically ill surgical patients with presumed pneumonia is not impacted by choice of methicillin-resistant staphylococcus aureus therapy. Int. J. Crit. Illn. Inj. Sci. 2018, 8, 22–27. [Google Scholar] [CrossRef]
- Perazella, M.A. Drug-induced acute kidney injury: Diverse mechanisms of tubular injury. Curr. Opin. Crit. Care 2019, 25, 550–557. [Google Scholar] [CrossRef]
- Kan, W.C.; Chen, Y.C.; Wu, V.C.; Shiao, C.C. Vancomycin-Associated Acute Kidney Injury: A Narrative Review from Pathophysiology to Clinical Application. Int. J. Mol. Sci. 2022, 23, 2052. [Google Scholar] [CrossRef]
- Ergün, B.; Esenkaya, F.; Küçük, M.; Yakar, M.N.; Uzun, Ö.; Heybeli, C.; Hanci, V.; Ergan, B.; Cömert, B.; Gökmen, A.N. Amikacin-induced acute kidney injury in mechanically ventilated critically ill patients with sepsis. J. Chemother. 2022, 1–9. [Google Scholar] [CrossRef]
- Russell, W.; Smith, W. Clarithromycin-induced acute interstitial nephritis and minimal change disease. NDT Plus 2009, 2, 382–383. [Google Scholar] [CrossRef]
- Mishima, E.; Maruyama, K.; Nakazawa, T.; Abe, T.; Ito, S. Acute Kidney Injury from Excessive Potentiation of Calcium-channel Blocker via Synergistic CYP3A4 Inhibition by Clarithromycin Plus Voriconazole. Intern. Med. 2017, 56, 1687–1690. [Google Scholar] [CrossRef]
- Nolin, T.D.; Himmelfarb, J. Mechanisms of drug-induced nephrotoxicity. Handb. Exp. Pharmacol. 2010, 196, 111–130. [Google Scholar] [CrossRef]
- Hajji, M.; Jebali, H.; Mrad, A.; Blel, Y.; Brahmi, N.; Kheder, R.; Beji, S.; Ben Fatma, L.; Smaoui, W.; Krid, M.; et al. Nephrotoxicity of Ciprofloxacin: Five Cases and a Review of the Literature. Drug Saf. Case Rep. 2018, 5, 17. [Google Scholar] [CrossRef]
- Ansari, F.A.; Manuel, S.; Dwivedi, R.; Boraiah, S.K.; Raju, S.B.; Uppin, M.; Sharma, A. A Rare Case of Acute Kidney Injury Due to Levofloxacin-induced Crystal Nephropathy. Indian J. Nephrol. 2019, 29, 424–426. [Google Scholar] [CrossRef]
- Shimizu, Y.; Hirai, T.; Ogawa, Y.; Yamada, C.; Kobayashi, E. Characteristics of risk factors for acute kidney injury among inpatients administered sulfamethoxazole/trimethoprim: A retrospective observational study. J. Pharm. Health Care Sci. 2022, 8, 20. [Google Scholar] [CrossRef]
- Fraser, T.N.; Avellaneda, A.A.; Graviss, E.A.; Musher, D.M. Acute kidney injury associated with trimethoprim/sulfamethoxazole. J. Antimicrob. Chemother. 2012, 67, 1271–1277. [Google Scholar] [CrossRef]
- Sepúlveda, R.A.; Anghileri, F.; Huidobro, E.J.P.; Julio, R.; Ávila, E.; Figueroa, C. Acute kidney injury associated to sulfamethoxazole urine crystal: The importance of clinical suspicion. Clin. Nephrol. Case Stud. 2022, 10, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Arrayasillapatorn, N.; Promsen, P.; Kritmetapak, K.; Anunnatsiri, S.; Chotmongkol, W.; Anutrakulchai, S. Colistin-Induced Acute Kidney Injury and the Effect on Survival in Patients with Multidrug-Resistant Gram-Negative Infections: Significance of Drug Doses Adjusted to Ideal Body Weight. Int. J. Nephrol. 2021, 2021, 7795096. [Google Scholar] [CrossRef] [PubMed]
- Gai, Z.; Samodelov, S.L.; Kullak-Ublick, G.A.; Visentin, M. Molecular Mechanisms of Colistin-Induced Nephrotoxicity. Molecules 2019, 24, 653. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, A.M.; Alzahrani, M.Y.; Abujamal, M.A.; Abdalla, M.H.; Alowais, S.A.; Alfayez, O.M.; Alyami, M.S.; Almutairi, A.R.; Almohammed, O.A. Comparative Risk of Acute Kidney Injury Following Concurrent Administration of Vancomycin with Piperacillin/Tazobactam or Meropenem: A Systematic Review and Meta-Analysis of Observational Studies. Antibiotics 2022, 11, 526. [Google Scholar] [CrossRef] [PubMed]
- Ide, N.; Sato, S.; Sawaguchi, K. Risk of Acute Kidney Injury in Patients Treated with Vancomycin and Piperacillin/Tazobactam Compared to Vancomycin and Meropenem or Doripenem: A Retrospective Cohort Study. Yakugaku Zasshi 2019, 139, 1609–1614. [Google Scholar] [CrossRef]
- Sussman, M.S.; Mulder, M.B.; Ryon, E.L.; Urrechaga, E.M.; Lama, G.A.; Bahga, A.; Eidelson, S.A.; Lieberman, H.M.; Schulman, C.I.; Namias, N.; et al. Acute Kidney Injury Risk in Patients Treated with Vancomycin Combined with Meropenem or Cefepime. Surg. Infect. 2021, 22, 415–420. [Google Scholar] [CrossRef]
- Le, P.; Navaneethan, S.D.; Yu, P.C.; Pallotta, A.M.; Rastogi, R.; Patel, P.; Brateanu, A.; Imrey, P.B.; Rothberg, M.B. Association of antibiotic use and acute kidney injury in patients hospitalized with community-acquired pneumonia. Curr. Med. Res. Opin. 2022, 38, 443–450. [Google Scholar] [CrossRef]
- Gaggl, M.; Pate, V.; Stürmer, T.; Kshirsagar, A.V.; Layton, J.B. The comparative risk of acute kidney injury of vancomycin relative to other common antibiotics. Sci. Rep. 2020, 10, 17282. [Google Scholar] [CrossRef]
- Cavalcanti, A.B.; Goncalves, A.R.; Almeida, C.S.; Bugano, D.D.; Silva, E. Teicoplanin versus vancomycin for proven or suspected infection. Cochrane Database Syst. Rev. 2010, CD007022. [Google Scholar] [CrossRef]
- Aslan, A.T.; Pashayev, T.; Dağ, O.; Akova, M. Comparison of teicoplanin versus vancomycin in combination with piperacillin-tazobactam or meropenem for the risk of acute kidney injury. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 1953–1961. [Google Scholar] [CrossRef]
- Brown, M.L.; Motsch, J.; Kaye, K.S.; File, T.M.; Boucher, H.W.; Vendetti, N.; Aggrey, A.; Joeng, H.-K.; Tipping, R.W.; Du, J.; et al. Evaluation of Renal Safety between Imipenem/Relebactam and Colistin Plus Imipenem in Patients with Imipenem-Nonsusceptible Bacterial Infections in the Randomized, Phase 3 RESTORE-IMI 1 Study. Open Forum Infect. Dis. 2020, 7, ofaa054. [Google Scholar] [CrossRef]
- Mousavi Movahed, S.M.; Akhavizadegan, H.; Dolatkhani, F.; Nejadghaderi, S.A.; Aghajani, F.; Gangi, M.F.; Ghazi, Z.; Ghasemi, H. Different incidences of acute kidney injury (AKI) and outcomes in COVID-19 patients with and without non-azithromycin antibiotics: A retrospective study. J. Med. Virol. 2021, 93, 4411–4419. [Google Scholar] [CrossRef]
- Zoratti, C.; Moretti, R.; Rebuzzi, L.; Albergati, I.V.; Di Somma, A.; Decorti, G.; Di Bella, S.; Crocè, L.S.; Giuffrè, M. Antibiotics and Liver Cirrhosis: What the Physicians Need to Know. Antibiotics 2021, 11, 31. [Google Scholar] [CrossRef]
- Cotta, M.O.; Roberts, J.A.; Lipman, J. Antibiotic dose optimization in critically ill patients. Med. Intensiv. 2015, 39, 563–572. (In English)(In Spanish) [Google Scholar] [CrossRef]
- Halilovic, J.; Heintz, B.H. Antibiotic dosing in cirrhosis. Am. J. Health Syst. Pharm. 2014, 71, 1621–1634. [Google Scholar] [CrossRef]
- Lind, L.; Sundström, J.; Larsson, A.; Lampa, E.; Ärnlöv, J.; Ingelsson, E. Longitudinal effects of aging on plasma proteins levels in older adults—Associations with kidney function and hemoglobin levels. PLoS ONE 2019, 14, e0212060. [Google Scholar] [CrossRef]
- Drugbank Online. Narrow Therapeutic Index Drugs. Available online: https://go.drugbank.com/categories/DBCAT003972 (accessed on 16 April 2023).
- Hilmer, S.N. ADME-tox issues for the elderly. Expert Opin. Drug Metab. Toxicol. 2008, 4, 1321–1331. [Google Scholar] [CrossRef]
- Cattaneo, D.; Falcone, M.; Gervasoni, C.; Marriott, D.J.E. Therapeutic Drug Monitoring of Antibiotics in the Elderly: A Narrative Review. Ther. Drug Monit. 2022, 44, 75–85. [Google Scholar] [CrossRef]
- Cattaneo, D.; Fusi, M.; Cozzi, V.; Baldelli, S.; Bonini, I.; Gervasoni, C.; Clementi, E. Supra-therapeutic Linezolid Trough Concentrations in Elderly Patients: A Call for Action? Clin. Pharmacokinet. 2021, 60, 603–609. [Google Scholar] [CrossRef]
- Wang, Y.; Dai, N.; Wei, W.; Jiang, C. Outcomes and Nephrotoxicity Associated with Vancomycin Treatment in Patients 80 Years and Older. Clin. Interv. Aging 2021, 16, 1023–1035. [Google Scholar] [CrossRef]
- Yahav, D.; Abbas, M.; Nassar, L.; Ghrayeb, A.; Shepshelovich, D.; Kurnik, D.; Leibovici, L.; Paul, M. Attention to age: Similar dosing regimens lead to different vancomycin levels among older and younger patients. Age Ageing 2019, 49, 26–31. [Google Scholar] [CrossRef]
- Hatti, M.; Solomonidi, N.; Odenholt, I.; Tham, J.; Resman, F. Considerable variation of trough β-lactam concentrations in older adults hospitalized with infection–A prospective observational study. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, N.; Nishimura, N.; Ikawa, K.; Karino, F.; Miura, K.; Tamaki, H.; Yano, T.; Isobe, T.; Morikawa, N.; Naora, K. Population Pharmacokinetic Modeling and Pharmacodynamic Target Attainment Simulation of Piperacillin/Tazobactam for Dosing Optimization in Late Elderly Patients with Pneumonia. Antibiotics 2020, 9, 113. [Google Scholar] [CrossRef] [PubMed]
- Harada, M.; Inui, N.; Suda, T.; Nakamura, Y.; Wajima, T.; Matsuo, Y.; Chida, K. Pharmacokinetic analysis of doripenem in elderly patients with nosocomial pneumonia. Int. J. Antimicrob. Agents 2013, 42, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Frey, O.R.; Hempel, G. Population pharmacokinetics of meropenem in elderly patients: Dosing simulations based on renal function. Eur. J. Clin. Pharmacol. 2017, 73, 333–342. [Google Scholar] [CrossRef]
- Medellín-Garibay, S.E.; Romano-Aguilar, M.; Parada, A.; Suárez, D.; Romano-Moreno, S.; Barcia, E.; Cervero, M.; García, B. Amikacin pharmacokinetics in elderly patients with severe infections. Eur. J. Pharm. Sci. 2022, 175, 106219. [Google Scholar] [CrossRef]
- Cojutti, P.G.; Ramos-Martin, V.; Schiavon, I.; Rossi, P.; Baraldo, M.; Hope, W.; Pea, F. Population Pharmacokinetics and Pharmacodynamics of Levofloxacin in Acutely Hospitalized Older Patients with Various Degrees of Renal Function. Antimicrob. Agents Chemother. 2017, 61, e02134-16. [Google Scholar] [CrossRef]
- Chen, I.H.; Nicolau, D.P. Augmented Renal Clearance and How to Augment Antibiotic Dosing. Antibiotics 2020, 9, 393. [Google Scholar] [CrossRef]
- Hefny, F.; Stuart, A.; Kung, J.Y.; Mahmoud, S.H. Prevalence and Risk Factors of Augmented Renal Clearance: A Systematic Review and Meta-Analysis. Pharmaceutics 2022, 14, 445. [Google Scholar] [CrossRef]
- Nazer, L.H.; AbuSara, A.K.; Kamal, Y. Augmented renal clearance in critically ill patients with cancer (ARCCAN Study): A prospective observational study evaluating prevalence and risk factors. Pharmacol. Res. Perspect. 2021, 9, e00747. [Google Scholar] [CrossRef]
- Mikami, R.; Hayakawa, M.; Imai, S.; Sugawara, M.; Takekuma, Y. Onset timing and duration of augmented renal clearance in a mixed intensive care unit. J. Intensive Care 2023, 11, 13. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, L.; Zhu, M. Effect of augmented renal clearance on the therapeutic drug monitoring of vancomycin in patients after neurosurgery. J. Int. Med. Res. 2020, 48, 300060520949076. [Google Scholar] [CrossRef]
- Yu, Y.X.; Lu, J.; Lu, H.D.; Li, L.; Li, J.J.; Shi, L.; Duan, L.F.; Zhuang, Z.W.; Xue, S.D.; Shen, Y.; et al. Predictive performance of reported vancomycin population pharmacokinetic model in patients with different renal function status, especially those with augmented renal clearance. Eur. J. Hosp. Pharm. Sci. Pract. 2022, 29, e6–e14. [Google Scholar] [CrossRef]
- Gijsen, M.; Elkayal, O.; Annaert, P.; Van Daele, R.; Meersseman, P.; Debaveye, Y.; Wauters, J.; Dreesen, E.; Spriet, I. Meropenem Target Attainment and Population Pharmacokinetics in Critically Ill Septic Patients with Preserved or Increased Renal Function. Infect. Drug Resist. 2022, 15, 53–62. [Google Scholar] [CrossRef]
- Barrasa, H.; Soraluce, A.; Usón, E.; Sainz, J.; Martín, A.; Sánchez-Izquierdo, J.Á.; Maynar, J.; Rodríguez-Gascón, A.; Isla, A. Impact of augmented renal clearance on the pharmacokinetics of linezolid: Advantages of continuous infusion from a pharmacokinetic/pharmacodynamic perspective. Int. J. Infect. Dis. 2020, 93, 329–338. [Google Scholar] [CrossRef]
- Janknegt, R.; Boogaard-Van den Born, J.; Hameleers, B.A.; Hooymans, P.M.; Rang, J.; Smits, C.A.; Willems-Thissen, M.E. Pharmacokinetics of amoxycillin in elderly in-patients. Pharm. Weekbl. Sci. 1992, 14, 27–29. [Google Scholar] [CrossRef]
- Huttner, A.; Bielicki, J.; Clements, M.N.; Frimodt-Møller, N.; Muller, A.E.; Paccaud, J.P.; Mouton, J.W. Oral amoxicillin and amoxicillin-clavulanic acid: Properties, indications and usage. Clin. Microbiol. Infect. 2020, 26, 871–879. [Google Scholar] [CrossRef]
- Meyers, B.R.; Wilkinson, P.; Mendelson, M.H.; Walsh, S.; Bournazos, C.; Hirschman, S.Z. Pharmacokinetics of ampicillin-sulbactam in healthy elderly and young volunteers. Antimicrob. Agents Chemother. 1991, 35, 2098–2101. [Google Scholar] [CrossRef]
- Matsubara, K.; Matsumoto, K.; Yokoyama, Y.; Watanabe, E.; Enoki, Y.; Shigemi, A.; Ikawa, K.; Terazono, H.; Morikawa, N.; Ohshige, T.; et al. Dosing Optimization of Ampicillin-Sulbactam Based on Cystatin C in Elderly Patients with Pneumonia. Biol. Pharm. Bull. 2021, 44, 732–736. [Google Scholar] [CrossRef]
- Riccobene, T.; Jakate, A.; Rank, D. A series of pharmacokinetic studies of ceftaroline fosamil in select populations: Normal subjects, healthy elderly subjects, and subjects with renal impairment or end-stage renal disease requiring hemodialysis. J. Clin. Pharmacol. 2014, 54, 742–752. [Google Scholar] [CrossRef]
- Barbhaiya, R.H.; Knupp, C.A.; Pittman, K.A. Effects of age and gender on pharmacokinetics of cefepime. Antimicrob. Agents Chemother. 1992, 36, 1181–1185. [Google Scholar] [CrossRef] [PubMed]
- Pais, G.M.; Chang, J.; Barreto, E.F.; Stitt, G.; Downes, K.J.; Alshaer, M.H.; Lesnicki, E.; Panchal, V.; Bruzzone, M.; Bumanglag, A.V.; et al. Clinical Pharmacokinetics and Pharmacodynamics of Cefepime. Clin. Pharmacokinet. 2022, 61, 929–953. [Google Scholar] [CrossRef] [PubMed]
- Geny, F.; Costa, P.; Bressolle, F.; Galtier, M. Ceftriaxone pharmacokinetics in elderly subjects and penetration into epididymis. Biopharm. Drug Dispos. 1993, 14, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.H.; Chan, Y.P.; Arnold, K.; Sun, M. Single-dose pharmacokinetics of ceftriaxone in healthy Chinese adults. Antimicrob. Agents Chemother. 1985, 27, 192–196. [Google Scholar] [CrossRef]
- Wong, G.; Briscoe, S.; Adnan, S.; McWhinney, B.; Ungerer, J.; Lipman, J.; Roberts, J.A. Protein binding of β-lactam antibiotics in critically ill patients: Can we successfully predict unbound concentrations? Antimicrob. Agents Chemother. 2013, 57, 6165–6170. [Google Scholar] [CrossRef]
- Drug Bank Online. Doripenem. Available online: https://go.drugbank.com/drugs/DB06211 (accessed on 15 April 2023).
- Paterson, D.L.; Depestel, D.D. Doripenem. Clin. Infect. Dis. 2009, 49, 291–298. [Google Scholar] [CrossRef]
- Finch, R.G.; Craddock, C.; Kelly, J.; Deaney, N.B. Pharmacokinetic studies of imipenem/cilastatin in elderly patients. J. Antimicrob. Chemother. 1986, 18 (Suppl. E), 103–107. [Google Scholar] [CrossRef]
- Drusano, G.L.; Standiford, H.C.; Bustamante, C.; Forrest, A.; Rivera, G.; Leslie, J.; Tatem, B.; Delaportas, D.; MacGregor, R.R.; Schimpff, S.C. Multiple-dose pharmacokinetics of imipenem-cilastatin. Antimicrob. Agents Chemother. 1984, 26, 715–721. [Google Scholar] [CrossRef]
- DrugBank Online. Imipenem. Available online: https://go.drugbank.com/drugs/DB01598 (accessed on 13 April 2023).
- Ljungberg, B.; Nilsson-Ehle, I. Pharmacokinetics of meropenem and its metabolite in young and elderly healthy men. Antimicrob. Agents Chemother. 1992, 36, 1437–1440. [Google Scholar] [CrossRef]
- Cunha, B.A. Meropenem in elderly and renally impaired patients. Int. J. Antimicrob. Agents. 1998, 10, 107–117, Corrected in Int. J. Antimicrob. Agents. 1999, 11, 167–177. [Google Scholar] [CrossRef]
- Namkoong, H.; Kameyama, Y.; Yasuda, H.; Nakayama, S.; Kaneko, H.; Kawashima, C.; Terajima, T.; Maezawa, K.; Hayashi, T.; Sandoh, M.; et al. The efficacy, safety, and pharmacokinetics of biapenem administered thrice daily for the treatment of pneumonia in the elderly. J. Infect. Chemother. 2014, 20, 356–360. [Google Scholar] [CrossRef]
- Kozawa, O.; Uematsu, T.; Matsuno, H.; Niwa, M.; Takiguchi, Y.; Matsumoto, S.; Minamoto, M.; Niida, Y.; Yokokawa, M.; Nagashima, S.; et al. Pharmacokinetics and safety of a new parenteral carbapenem antibiotic, biapenem (L-627), in elderly subjects. Antimicrob. Agents Chemother. 1998, 42, 1433–1436. [Google Scholar] [CrossRef]
- Griffith, D.C.; Morgan, E.E.; Dudley, M.N.; Loutit, J.S. A Phase 1 Study of the Safety, Tolerability, and Pharmacokinetics of Biapenem in Healthy Adult Subjects. Antimicrob. Agents Chemother. 2023, 65, e02612-20. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, F.; Chen, C.; Ma, L.; Yang, T.; Liu, X.; Liu, Y.; Wang, X.; Zhao, X.; Que, C.; et al. Development of a Population Pharmacokinetic Model of Vancomycin and its Application in Chinese Geriatric Patients with Pulmonary Infections. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 361–370. [Google Scholar] [CrossRef]
- Guay, D.R.; Vance-Bryan, K.; Gilliland, S.; Rodvold, K.; Rotschafer, J. Comparison of vancomycin pharmacokinetics in hospitalized elderly and young patients using a Bayesian forecaster. J. Clin. Pharmacol. 1993, 33, 918–922. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, A.J.; Germano, A.; Sime, F.B.; Roberts, J.A.; Kimura, E. Vancomycin population pharmacokinetics for adult patients with sepsis or septic shock: Are current dosing regimens sufficient? Eur. J. Clin. Pharmacol. 2019, 75, 1219–1226. [Google Scholar] [CrossRef]
- DrugBank Online. Vancomycin. Available online: https://go.drugbank.com/drugs/DB00512 (accessed on 13 April 2023).
- Patel, S.; Preuss, C.V.; Bernice, F. Vancomycin. [Updated 2023 Jan 14]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459263/ (accessed on 13 April 2023).
- Kasai, H.; Tsuji, Y.; Hiraki, Y.; Tsuruyama, M.; To, H.; Yamamoto, Y. Population pharmacokinetics of teicoplanin in hospitalized elderly patients using cystatin C as an indicator of renal function. J. Infect. Chemother. 2018, 24, 284–291. [Google Scholar] [CrossRef]
- Outman, W.R.; Nightingale, C.H.; Sweeney, K.R.; Quintiliani, R. Teicoplanin pharmacokinetics in healthy volunteers after administration of intravenous loading and maintenance doses. Antimicrob. Agents Chemother. 1990, 34, 2114–2117. [Google Scholar] [CrossRef]
- DrugBank Online. Teicoplanin. Available online: https://go.drugbank.com/drugs/DB06149 (accessed on 13 April 2023).
- Dvorchik, B.; Damphousse, D. Single-dose pharmacokinetics of daptomycin in young and geriatric volunteers. J. Clin. Pharmacol. 2004, 44, 612–620. [Google Scholar] [CrossRef]
- Goldberg, M.R.; Wong, S.L.; Shaw, J.P.; Kitt, M.M.; Barriere, S.L. Single-dose pharmacokinetics and tolerability of telavancin in elderly men and women. Pharmacotherapy 2010, 30, 806–811. [Google Scholar] [CrossRef]
- Das, B.; Sarkar, C.; Das, D.; Gupta, A.; Kalra, A.; Sahni, S. Telavancin: A novel semisynthetic lipoglycopeptide agent to counter the challenge of resistant Gram-positive pathogens. Ther. Adv. Infect. Dis. 2017, 4, 49–73. [Google Scholar] [CrossRef] [PubMed]
- Sisson, T.L.; Jungbluth, G.L.; Hopkins, N.K. Age and sex effects on the pharmacokinetics of linezolid. Eur. J. Clin. Pharmacol. 2002, 57, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Bouza, E.; Muñoz, P. Linezolid: Pharmacokinetic characteristics and clinical studies. Clin. Microbiol. Infect. 2001, 7 (Suppl. S4), 75–82. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, S.D.; Minassian, S.L.; Prokocimer, P. Pharmacokinetics, Safety, and Tolerability of Tedizolid Phosphate in Elderly Subjects. Clin. Pharmacol. Drug Dev. 2018, 7, 788–794. [Google Scholar] [CrossRef]
- Iqbal, K.; Milioudi, A.; Wicha, S.G. Pharmacokinetics and Pharmacodynamics of Tedizolid. Clin. Pharmacokinet. 2022, 61, 489–503. [Google Scholar] [CrossRef]
- Muralidharan, G.; Fruncillo, R.J.; Micalizzi, M.; Raible, D.G.; Troy, S.M. Effects of age and sex on single-dose pharmacokinetics of tigecycline in healthy subjects. Antimicrob. Agents Chemother. 2005, 49, 1656–1659. [Google Scholar] [CrossRef]
- Dorn, C.; Kratzer, A.; Liebchen, U.; Schleibinger, M.; Murschhauser, A.; Schlossmann, J.; Kees, F.; Simon, P.; Kees, M.G. Impact of Experimental Variables on the Protein Binding of Tigecycline in Human Plasma as Determined by Ultrafiltration. J. Pharm. Sci. 2018, 107, 739–744. [Google Scholar] [CrossRef]
- Chow, A.T.; Fowler, C.; Williams, R.R.; Morgan, N.; Kaminski, S.; Natarajan, J. Safety and pharmacokinetics of multiple 750-milligram doses of intravenous levofloxacin in healthy volunteers. Antimicrob. Agents Chemother. 2001, 45, 2122–2125. [Google Scholar] [CrossRef]
- Fish, D.N.; Chow, A.T. The clinical pharmacokinetics of levofloxacin. Clin. Pharmacokinet. 1997, 32, 101–119. [Google Scholar] [CrossRef]
- Sullivan, J.T.; Lettieri, J.T.; Liu, P.; Heller, A.H. The influence of age and gender on the pharmacokinetics of moxifloxacin. Clin. Pharmacokinet. 2001, 40 (Suppl. S1), 11–18. [Google Scholar] [CrossRef]
- Gai, X.; Shen, N.; He, B.; Zhou, Q.; Bo, S.; Li, X.; Zhai, S.; Yin, A.; Lu, W. Population pharmacokinetics of ciprofloxacin in Chinese elderly patients with lower respiratory tract infection. Zhonghua Yi Xue Za Zhi 2015, 95, 1581–1585. (In Chinese) [Google Scholar]
- Drug Bank Online. Ciprofloxacin. Available online: https://go.drugbank.com/drugs/DB00537 (accessed on 15 May 2023).
- Kato, H.; Parker, S.L.; Roberts, J.A.; Hagihara, M.; Asai, N.; Yamagishi, Y.; Paterson, D.L.; Mikamo, H. Population Pharmacokinetics Analysis of Amikacin Initial Dosing Regimen in Elderly Patients. Antibiotics 2021, 10, 100. [Google Scholar] [CrossRef]
- Ghaffari, S.; Hadi, A.M.; Najmeddin, F.; Shahrami, B.; Rouini, M.R.; Najafi, A.; Mojtahedzadeh, M. Evaluation of amikacin dosing schedule in critically ill elderly patients with different stages of renal dysfunction. Eur. J. Hosp. Pharm. 2022, 29, e67–e71. [Google Scholar] [CrossRef]
- Bauer, L.A.; Blouin, R.A. Influence of age on amikacin pharmacokinetics in patients without renal disease. Comparison with gentamicin and tobramycin. Eur. J. Clin. Pharmacol. 1983, 24, 639–642. [Google Scholar] [CrossRef]
- DrugBank Online. Amikacin. Available online: https://go.drugbank.com/drugs/DB00479 (accessed on 13 April 2023).
- Hilmer, S.N.; Tran, K.; Rubie, P.; Wright, J.; Gnjidic, D.; Mitchell, S.J.; Matthews, S.; Carroll, P.R. Gentamicin pharmacokinetics in old age and frailty. Br. J. Clin. Pharmacol. 2011, 71, 224–231. [Google Scholar] [CrossRef]
- Triggs, E.; Charles, B. Pharmacokinetics and therapeutic drug monitoring of gentamicin in the elderly. Clin. Pharmacokinet. 1999, 37, 331–341. [Google Scholar] [CrossRef]
- Wang, P.; Liu, D.; Sun, T.; Zhang, X.; Yang, J. Pharmacokinetics and pharmacodynamics of polymyxin B and proposed dosing regimens in elderly patients with multi-drug-resistant Gram-negative bacterial infections. Int. J. Antimicrob. Agents. 2022, 60, 106693. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, X.; Du, X.; Chen, H.; Zhao, F.; Zhou, Z.; Wang, Y.; Zheng, Y.; Bergen, P.J.; Li, X.; et al. Pharmacokinetics/pharmacodynamics of polymyxin B in patients with bloodstream infection caused by carbapenem-resistant Klebsiella pneumoniae. Front. Pharmacol. 2022, 13, 975066. [Google Scholar] [CrossRef]
- Avedissian, S.N.; Liu, J.; Rhodes, N.J.; Lee, A.; Pais, G.M.; Hauser, A.R.; Scheetz, M.H. A Review of the Clinical Pharmacokinetics of Polymyxin B. Antibiotics 2019, 8, 31. [Google Scholar] [CrossRef]
- Abodakpi, H.; Gohlke, J.; Chang, K.T.; Chow, D.S.; Tam, V.H. Analytical and functional determination of polymyxin B protein binding in serum. Antimicrob. Agents Chemother. 2015, 59, 7121–7123. [Google Scholar] [CrossRef]
- Majcher-Peszynska, J.; Loebermann, M.; Klammt, S.; Frimmel, S.; Mundkowski, R.G.; Welte, T.; Reisinger, E.C.; Drewelow, B.; CAPNETZ Study Group. Ampicillin/sulbactam in elderly patients with community-acquired pneumonia. Infection 2014, 42, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Sugiyama, E.; Nozawa, K.; Tajima, M.; Takahashi, K.; Yoshii, M.; Suzuki, H.; Sato, V.H.; Sato, H. Effects of dosing frequency on the clinical efficacy of ampicillin/sulbactam in Japanese elderly patients with pneumonia: A single-center retrospective observational study. Pharmacol. Res. Perspect. 2021, 9, e00746. [Google Scholar] [CrossRef] [PubMed]
- Cojutti, P.G.; Morandin, E.; Baraldo, M.; Pea, F. Population pharmacokinetics of continuous infusion of piperacillin/tazobactam in very elderly hospitalized patients and considerations for target attainment against Enterobacterales and Pseudomonas aeruginosa. Int. J. Antimicrob. Agents. 2021, 58, 106408. [Google Scholar] [CrossRef] [PubMed]
- Ulldemolins, M.; Roberts, J.A.; Lipman, J.; Rello, J. Antibiotic dosing in multiple organ dysfunction syndrome. Chest 2011, 139, 1210–1220. [Google Scholar] [CrossRef] [PubMed]
- Quinton, M.C.; Bodeau, S.; Kontar, L.; Zerbib, Y.; Maizel, J.; Slama, M.; Masmoudi, K.; Lemaire-Hurtel, A.S.; Bennis, Y. Neurotoxic Concentration of Piperacillin during Continuous Infusion in Critically Ill Patients. Antimicrob. Agents Chemother. 2017, 61, e00654-17. [Google Scholar] [CrossRef]
- Ruiz-Ramos, J.; Herrera-Mateo, S.; López-Vinardell, L.; Juanes-Borrego, A.; Puig-Campmany, M.; Mangues-Bafalluy, M.A. Cefepime Dosing Requirements in Elderly Patients Attended in the Emergency Rooms. Dose Response 2022, 20, 15593258221078393. [Google Scholar] [CrossRef]
- Boschung-Pasquier, L.; Atkinson, A.; Kastner, L.K.; Banholzer, S.; Haschke, M.; Buetti, N.; Furrer, D.I.; Hauser, C.; Jent, P.; Que, Y.A.; et al. Cefepime neurotoxicity: Thresholds and risk factors. A retrospective cohort study. Clin. Microbiol. Infect. 2020, 26, 333–339. [Google Scholar] [CrossRef]
- Tan, S.J.; Cockcroft, M.; Page-Sharp, M.; Arendts, G.; Davis, T.M.E.; Moore, B.R.; Batty, K.T.; Salman, S.; Manning, L. Population Pharmacokinetic Study of Ceftriaxone in Elderly Patients, Using Cystatin C-Based Estimates of Renal Function To Account for Frailty. Antimicrob. Agents Chemother. 2020, 64, e00874-20. [Google Scholar] [CrossRef]
- Jadot, L.; Judong, A.; Canivet, J.L.; Lorenzo-Villalba, N.; Damas, P. Ceftriaxone-induced Encephalopathy: A Pharmacokinetic Approach. Eur. J. Case Rep. Intern. Med. 2021, 8, 003011. [Google Scholar] [CrossRef]
- Veillette, J.J.; Truong, J.; Forland, S.C. Pharmacokinetics of Ceftazidime-Avibactam in Two Patients with KPC-Producing Klebsiella pneumoniae Bacteremia and Renal Impairment. Pharmacotherapy 2016, 36, e172–e177. [Google Scholar] [CrossRef]
- Pingue, V.; Penati, R.; Nardone, A.; Franciotta, D. Ceftazidime/avibactam neurotoxicity in an adult patient with normal renal function. Clin. Microbiol. Infect. 2020. [Google Scholar] [CrossRef]
- Aloy, B.; Launay-Vacher, V.; Bleibtreu, A.; Bortolotti, P.; Faure, E.; Filali, A.; Gauzit, R.; Gilbert, M.; Lesprit, P.; Mahieu, R.; et al. Antibiotics and chronic kidney disease: Dose adjustment update for infectious disease clinical practice. Med. Mal. Infect. 2020, 50, 323–331. [Google Scholar] [CrossRef]
- Musson, D.G.; Majumdar, A.; Holland, S.; Birk, K.; Xi, L.; Mistry, G.; Sciberras, D.; Muckow, J.; Deutsch, P.; Rogers, J.D. Pharmacokinetics of total and unbound ertapenem in healthy elderly subjects. Antimicrob. Agents Chemother. 2004, 48, 521–524. [Google Scholar] [CrossRef]
- Lee, K.H.; Ueng, Y.F.; Wu, C.W.; Chou, Y.C.; Ng, Y.Y.; Yang, W.C. The recommended dose of ertapenem poses a potential risk for central nervous system toxicity in haemodialysis patients—Case reports and literature reviews. J. Clin. Pharm. Ther. 2015, 40, 240–244. [Google Scholar] [CrossRef]
- Steffens, N.A.; Zimmermann, E.S.; Nichelle, S.M.; Brucker, N. Meropenem use and therapeutic drug monitoring in clinical practice: A literature review. J. Clin. Pharm. Ther. 2021, 46, 610–621. [Google Scholar] [CrossRef]
- Karino, F.; Deguchi, N.; Kanda, H.; Ohe, M.; Kondo, K.; Tada, M.; Kuraki, T.; Isobe, T.; Nishimura, N.; Moriyama, H.; et al. Evaluation of the efficacy and safety of biapenem against pneumonia in the elderly and a study on its pharmacokinetics. J. Infect. Chemother. 2013, 19, 98–102. [Google Scholar] [CrossRef]
- Bourguignon, L.; Goutelle, S.; De Saint-Martin, J.B.; Maire, P.; Ducher, M. Evaluation of various gentamicin dosage regimens in geriatric patients: A simulation study. Fundam. Clin. Pharmacol. 2010, 24, 109–113. [Google Scholar] [CrossRef]
- Samura, M.; Takada, K.; Yamamoto, R.; Ito, H.; Nagumo, F.; Uchida, M.; Kurata, T.; Koshioka, S.; Enoki, Y.; Taguchi, K.; et al. Population Pharmacokinetic Analysis and Dosing Optimization Based on Unbound Daptomycin Concentration and Cystatin C in Nonobese Elderly Patients with Hypoalbuminemia and Chronic Kidney Disease. Pharm. Res. 2021, 38, 1041–1055. [Google Scholar] [CrossRef]
- Balice, G.; Passino, C.; Bongiorni, M.G.; Segreti, L.; Russo, A.; Lastella, M.; Luci, G.; Falcone, M.; Di Paolo, A. Daptomycin Population Pharmacokinetics in Patients Affected by Severe Gram-Positive Infections: An Update. Antibiotics 2022, 11, 914. [Google Scholar] [CrossRef]
- Ito, F.; Ohno, Y.; Toyoshi, S.; Kaito, D.; Koumei, Y.; Endo, J.; Kamamiya, F.; Mori, H.; Mori, M.; Morishita, M.; et al. Pharmacokinetics of consecutive oral moxifloxacin (400 mg/day) in patients with respiratory tract infection. Ther. Adv. Respir. Dis. 2016, 10, 34–42. [Google Scholar] [CrossRef]
- Qu, J.; Qi, T.T.; Qu, Q.; Long, W.M.; Chen, Y.; Luo, Y.; Wang, Y. Polymyxin B-Based Regimens for Patients Infected with Carbapenem-Resistant Gram-Negative Bacteria: Clinical and Microbiological Efficacy, Mortality, and Safety. Infect. Drug Resist. 2022, 15, 1205–1218. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).