The Role of the Microbiome in the Pathogenesis and Treatment of Asthma
Abstract
1. Introduction
2. Human Microbiome
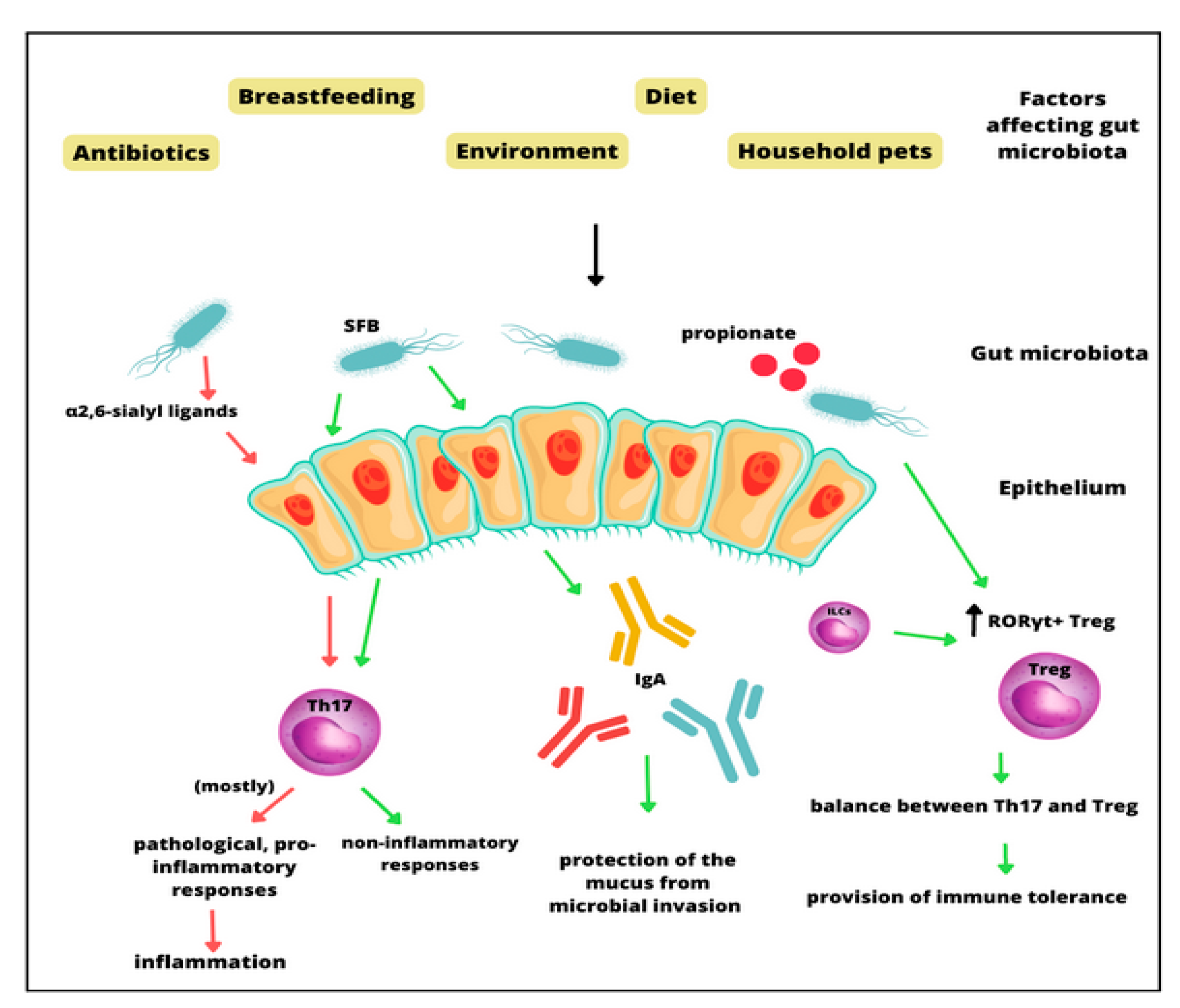
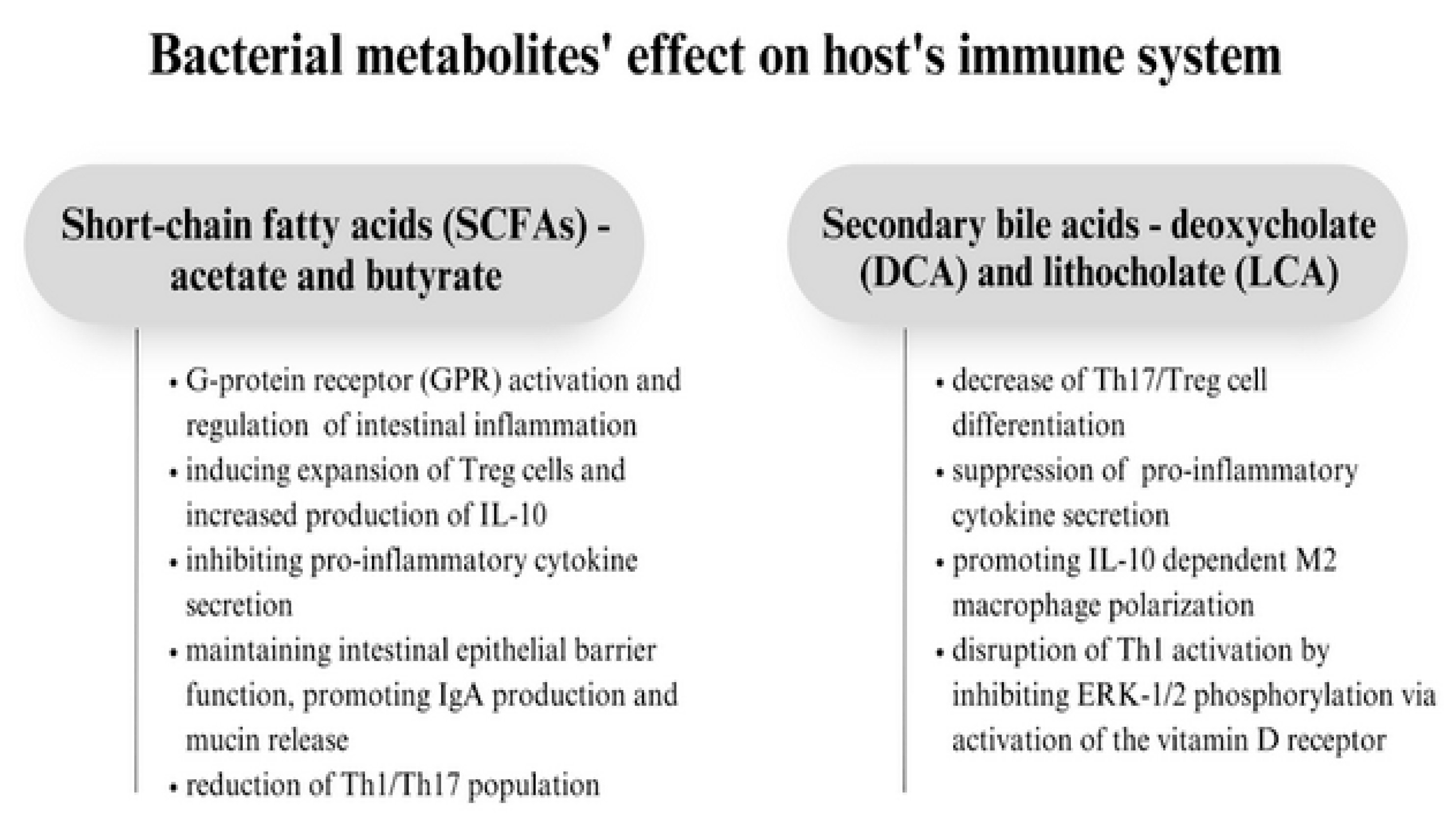
3. The Molecular Role of the Gut Microbiota in Asthma Pathogenesis
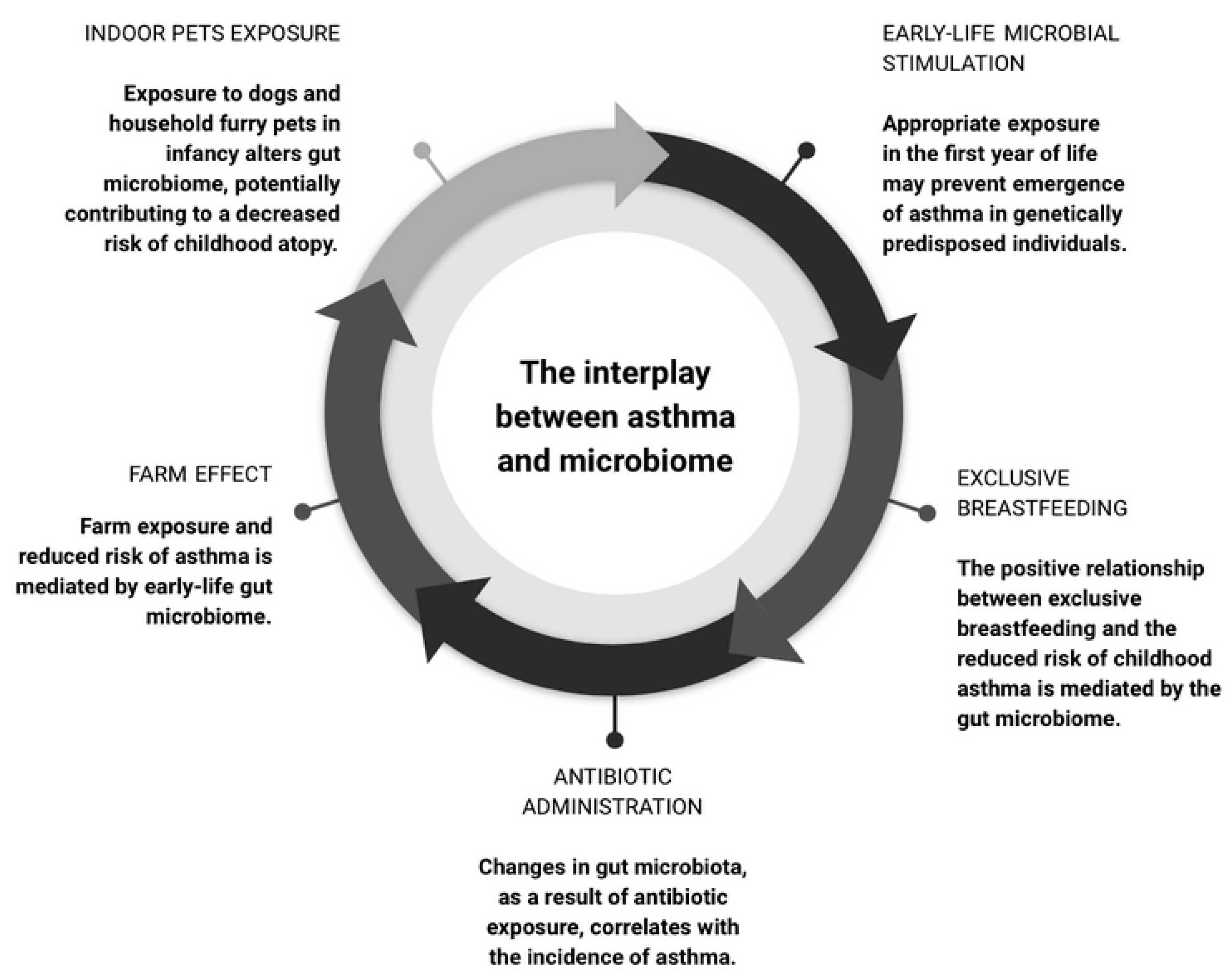
4. The Microbiome as a Moderator in Asthma Development
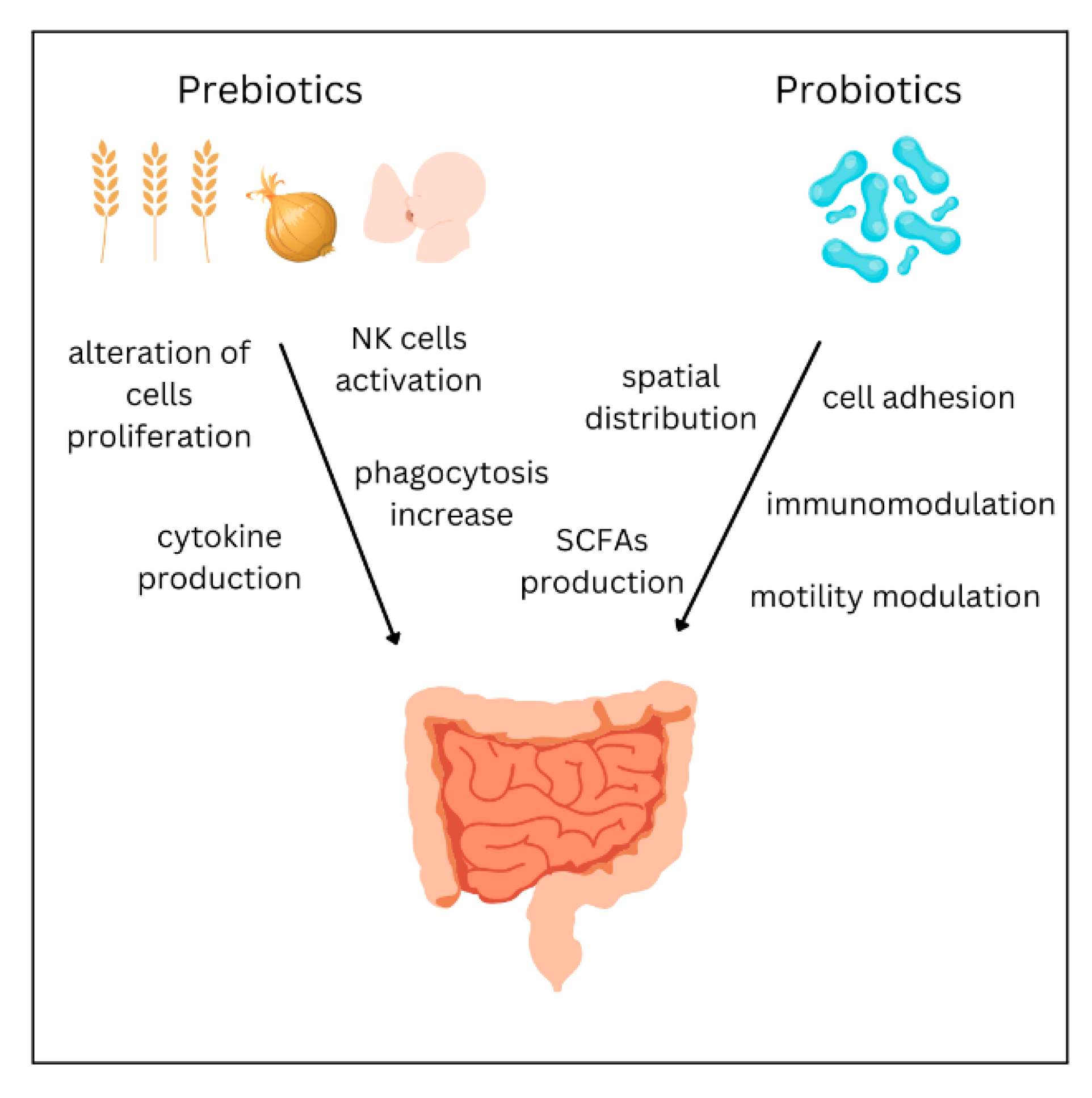
5. Treatment
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Porsbjerg, C.; Melén, E.; Lehtimäki, L.; Shaw, D. Asthma. Lancet 2023, 401, 858–873. [Google Scholar] [CrossRef] [PubMed]
- Hammad, H.; Lambrecht, B.N. The Basic Immunology of Asthma. Cell 2021, 184, 1469–1485. [Google Scholar] [CrossRef] [PubMed]
- Stern, J.; Pier, J.; Litonjua, A.A. Asthma Epidemiology and Risk Factors. Semin. Immunopathol. 2020, 42, 5–15. [Google Scholar] [CrossRef]
- Gans, M.D.; Gavrilova, T. Understanding the Immunology of Asthma: Pathophysiology, Biomarkers, and Treatments for Asthma Endotypes. Paediatr. Respir. Rev. 2020, 36, 118–127. [Google Scholar] [CrossRef]
- Jeong, J.; Lee, H.K. The Role of CD4+ T Cells and Microbiota in the Pathogenesis of Asthma. Int. J. Mol. Sci. 2021, 22, 11822. [Google Scholar] [CrossRef]
- Rogliani, P.; Ora, J.; Calzetta, L.; Matera, M.G.; Cazzola, M. Asthma and Comorbidities: Recent Advances. Polish Arch. Intern. Med. 2022, 132, 16250. [Google Scholar] [CrossRef]
- Lommatzsch, M.; Buhl, R.; Korn, S. The Treatment of Mild and Moderate Asthma in Adults. Dtsch. Arztebl. Int. 2020, 117, 434–444. [Google Scholar] [CrossRef]
- De Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut Microbiome and Health: Mechanistic Insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Human Gut Microbiota/Microbiome in Health and Diseases: A Review. Antonie Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef]
- Manos, J. The Human Microbiome in Disease and Pathology. Apmis 2022, 130, 690. [Google Scholar] [CrossRef]
- Welch, C.B.; Ryman, V.E.; Pringle, T.D.; Lourenco, J.M. Utilizing the Gastrointestinal Microbiota to Modulate Cattle Health through the Microbiome-Gut-Organ Axes. Microorganisms 2022, 10, 1391. [Google Scholar] [CrossRef]
- Brown, E.G.; Tanner, C.M.; Goldman, S.M. The Microbiome in Neurodegenerative Disease. Curr. Geriatr. Rep. 2018, 7, 81–91. [Google Scholar] [CrossRef]
- Ticinesi, A.; Tana, C.; Nouvenne, A.; Prati, B.; Lauretani, F.; Meschi, T. Gut Microbiota, Cognitive Frailty and Dementia in Older Individuals: A Systematic Review. Clin. Interv. Aging 2018, 13, 1497. [Google Scholar] [CrossRef] [PubMed]
- van den Munckhof, I.C.L.; Kurilshikov, A.; ter Horst, R.; Riksen, N.P.; Joosten, L.A.B.; Zhernakova, A.; Fu, J.; Keating, S.T.; Netea, M.G.; de Graaf, J.; et al. Role of Gut Microbiota in Chronic Low-Grade Inflammation as Potential Driver for Atherosclerotic Cardiovascular Disease: A Systematic Review of Human Studies. Obes. Rev. 2018, 19, 1719–1734. [Google Scholar] [CrossRef]
- Barcik, W.; Boutin, R.C.T.; Sokolowska, M.; Finlay, B.B. The Role of Lung and Gut Microbiota in the Pathology of Asthma. Immunity 2020, 52, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, J.R.; Ravel, J. The Vocabulary of Microbiome Research: A Proposal. Microbiome 2015, 3, 31. [Google Scholar] [CrossRef]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef]
- Barko, P.C.; McMichael, M.A.; Swanson, K.S.; Williams, D.A. The Gastrointestinal Microbiome: A Review. J. Vet. Intern. Med. 2018, 32, 9–25. [Google Scholar] [CrossRef]
- Bezirtzoglou, E. The Intestinal Microflora During the First Weeks of Life. Anaerobe 1997, 3, 173–177. [Google Scholar] [CrossRef]
- Palmer, C.; Bik, E.M.; DiGiulio, D.B.; Relman, D.A.; Brown, P.O. Development of the Human Infant Intestinal Microbiota. PLoS Biol. 2007, 5, 1556–1573. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G.; De Jesus-Laboy, K.M.; Shen, N.; Cox, L.M.; Amir, A.; Gonzalez, A.; Bokulich, N.A.; Song, S.J.; Hoashi, M.; Rivera-Vinas, J.I.; et al. Partial Restoration of the Microbiota of Cesarean-Born Infants via Vaginal Microbial Transfer. Nat. Med. 2016, 22, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Biasucci, G.; Rubini, M.; Riboni, S.; Morelli, L.; Bessi, E.; Retetangos, C. Mode of Delivery Affects the Bacterial Community in the Newborn Gut. Early Hum. Dev. 2010, 86 (Suppl. S1), 13–15. [Google Scholar] [CrossRef] [PubMed]
- Martín-Peláez, S.; Cano-Ibáñez, N.; Pinto-Gallardo, M.; Amezcua-Prieto, C. The Impact of Probiotics, Prebiotics, and Synbiotics during Pregnancy or Lactation on the Intestinal Microbiota of Children Born by Cesarean Section: A Systematic Review. Nutrients 2022, 14, 341. [Google Scholar] [CrossRef] [PubMed]
- Mohajeri, M.H.; Brummer, R.J.M.; Rastall, R.A.; Weersma, R.K.; Harmsen, H.J.M.; Faas, M.; Eggersdorfer, M. The Role of the Microbiome for Human Health: From Basic Science to Clinical Applications. Eur. J. Nutr. 2018, 57, 1–14. [Google Scholar] [CrossRef]
- Gritz, E.C.; Bhandari, V. The Human Neonatal Gut Microbiome: A Brief Review. Front. Pediatr. 2015, 3, 17. [Google Scholar]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the Human Intestinal Microbial Flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef]
- Guarner, F.; Malagelada, J.R. Gut Flora in Health and Disease. Lancet 2003, 361, 512–519. [Google Scholar] [CrossRef]
- Cui, L.; Morris, A.; Ghedin, E. The Human Mycobiome in Health and Disease. Genome Med. 2013, 5, 63. [Google Scholar] [CrossRef]
- Scarpellini, E.; Ianiro, G.; Attili, F.; Bassanelli, C.; De Santis, A.; Gasbarrini, A. The Human Gut Microbiota and Virome: Potential Therapeutic Implications. Dig. Liver Dis. 2015, 47, 1007–1012. [Google Scholar] [CrossRef]
- Karczewski, J.; Troost, F.J.; Konings, I.; Dekker, J.; Kleerebezem, M.; Brummer, R.J.M.; Wells, J.M. Regulation of Human Epithelial Tight Junction Proteins by Lactobacillus Plantarum in Vivo and Protective Effects on the Epithelial Barrier. Am. J. Physiol.-Gastrointest. Liver Physiol. 2010, 298, 851–859. [Google Scholar] [CrossRef]
- Ohno, H. Intestinal M Cells. J. Biochem. 2016, 159, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Štěpánková, R.; Kovářů, F.; Kruml, J. Lymphatic Tissue of the Intestinal Tract of Germfree and Conventional Rabbits. Folia Microbiol. 1980, 25, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, S.A.; McGinniss, J.E.; Collman, R.G. The Lung Microbiome: Progress and Promise. J. Clin. Investig. 2021, 131, e150473. [Google Scholar] [CrossRef]
- Charlson, E.S.; Bittinger, K.; Haas, A.R.; Fitzgerald, A.S.; Frank, I.; Yadav, A.; Bushman, F.D.; Collman, R.G. Topographical Continuity of Bacterial Populations in the Healthy Human Respiratory Tract. Am. J. Respir. Crit. Care Med. 2011, 184, 957–963. [Google Scholar] [CrossRef]
- Gleeson, K.; Eggli, D.F.; Maxwell, S.L. Quantitative Aspiration during Sleep in Normal Subjects. Chest 1997, 111, 1266–1272. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Yu, Y.; Du, W.; Liu, Y.; Dai, R.; Tang, W.; Wang, P.; Zhang, C.; Shi, G. Fungal and Bacterial Microbiome Dysbiosis and Imbalance of Trans-Kingdom Network in Asthma. Clin. Transl. Allergy 2020, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Jeong, K.I.; Itoh, K.; Doi, K. Regional Variations in the Distributions of Small Intestinal Intraepithelial Lymphocytes in Germ-Free and Specific Pathogen-Free Mice. Exp. Mol. Pathol. 2002, 72, 230–235. [Google Scholar] [CrossRef]
- Campbell, C.; Kandalgaonkar, M.R.; Golonka, R.M.; Yeoh, B.S.; Vijay-Kumar, M.; Saha, P. Crosstalk between Gut Microbiota and Host Immunity: Impact on Inflammation and Immunotherapy. Biomedicines 2023, 11, 294. [Google Scholar] [CrossRef]
- Irons, E.E.; Cortes Gomez, E.; Andersen, V.L.; Lau, J.T.Y. Bacterial Colonization and TH17 Immunity Are Shaped by Intestinal Sialylation in Neonatal Mice. Glycobiology 2022, 32, 414–428. [Google Scholar] [CrossRef]
- Lyu, M.; Suzuki, H.; Kang, L.; Gaspal, F.; Zhou, W.; Goc, J.; Zhou, L.; Zhou, J.; Zhang, W.; Artis, D.; et al. ILC3s Select Microbiota-Specific Regulatory T Cells to Establish Tolerance in the Gut. Nature 2022, 610, 744–751. [Google Scholar] [CrossRef]
- Su, X.; Yin, X.; Liu, Y.; Yan, X.; Zhang, S.; Wang, X.; Lin, Z.; Zhou, X.; Gao, J.; Wang, Z.; et al. Gut Dysbiosis Contributes to the Imbalance of Treg and Th17 Cells in Graves’ Disease Patients by Propionic Acid. J. Clin. Endocrinol. Metab. 2020, 105, 3526–3547. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Ohno, H. Reciprocal Regulation of IgA and the Gut Microbiota: A Key Mutualism in the Intestine. Int. Immunol. 2021, 33, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Wolf, N.; Lavelle, E.C. Innate Immune Receptors. Methods Mol. Biol. 2016, 1417, 1–43. [Google Scholar] [CrossRef] [PubMed]
- Clarke, T.B. Early Innate Immunity to Bacterial Infection in the Lung Is Regulated Systemically by the Commensal Microbiota via Nod-Like Receptor Ligands. Infect. Immun. 2014, 82, 4596. [Google Scholar] [CrossRef] [PubMed]
- Mu, C.; Yang, Y.; Zhu, W. Crosstalk Between The Immune Receptors and Gut Microbiota. Curr. Protein Pept. Sci. 2015, 16, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Guryanova, S.V.; Gigani, O.B.; Gudima, G.O.; Kataeva, A.M.; Kolesnikova, N.V. Dual Effect of Low-Molecular-Weight Bioregulators of Bacterial Origin in Experimental Model of Asthma. Life 2022, 12, 192. [Google Scholar] [CrossRef]
- Gu, B.H.; Rim, C.Y.; Lee, S.; Kim, T.Y.; Joo, S.S.; Lee, S.J.; Park, H.K.; Kim, M. Alteration of Gut Immunity and Microbiome in Mixed Granulocytic Asthma. Biomedicines 2022, 10, 2946. [Google Scholar] [CrossRef]
- Abdel-Aziz, M.I.; Vijverberg, S.J.H.; Neerincx, A.H.; Kraneveld, A.D.; Maitland-van der Zee, A.H. The Crosstalk between Microbiome and Asthma: Exploring Associations and Challenges. Clin. Exp. Allergy 2019, 49, 1067–1086. [Google Scholar] [CrossRef]
- Mandaliya, D.K.; Patel, S.; Seshadri, S. The Combinatorial Effect of Acetate and Propionate on High-Fat Diet Induced Diabetic Inflammation or Metaflammation and T Cell Polarization. Inflammation 2021, 44, 68–79. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal Microbe-Derived Butyrate Induces the Differentiation of Colonic Regulatory T Cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Adivitiya; Kaushik, M.S.; Chakraborty, S.; Veleri, S.; Kateriya, S. Mucociliary Respiratory Epithelium Integrity in Molecular Defense and Susceptibility to Pulmonary Viral Infections. Biology 2021, 10, 95. [Google Scholar] [CrossRef] [PubMed]
- Strachan, D.P. Hay Fever, Hygiene, and Household Size. Br. Med. J. 1989, 299, 1259–1260. [Google Scholar] [CrossRef] [PubMed]
- Haspeslagh, E.; Heyndrickx, I.; Hammad, H.; Lambrecht, B.N. The Hygiene Hypothesis: Immunological Mechanisms of Airway Tolerance. Curr. Opin. Immunol. 2018, 54, 102. [Google Scholar] [CrossRef] [PubMed]
- Rook, G.A.W. 99th Dahlem Conference on Infection, Inflammation and Chronic Inflammatory Disorders: Darwinian Medicine and the ‘Hygiene’ or ‘Old Friends’ Hypothesis. Clin. Exp. Immunol. 2010, 160, 70–79. [Google Scholar] [CrossRef]
- Stokholm, J.; Blaser, M.J.; Thorsen, J.; Rasmussen, M.A.; Waage, J.; Vinding, R.K.; Schoos, A.M.M.; Kunøe, A.; Fink, N.R.; Chawes, B.L.; et al. Maturation of the Gut Microbiome and Risk of Asthma in Childhood. Nat. Commun. 2018, 9, 141. [Google Scholar] [CrossRef]
- Christensen, E.D.; Hjelmsø, M.H.; Thorsen, J.; Shah, S.; Redgwell, T.; Poulsen, C.E.; Trivedi, U.; Russel, J.; Gupta, S.; Chawes, B.L.; et al. The Developing Airway and Gut Microbiota in Early Life Is Influenced by Age of Older Siblings. Microbiome 2022, 10, 106. [Google Scholar] [CrossRef]
- Thorsen, J.; Rasmussen, M.A.; Waage, J.; Mortensen, M.; Brejnrod, A.; Bønnelykke, K.; Chawes, B.L.; Brix, S.; Sørensen, S.J.; Stokholm, J.; et al. Infant Airway Microbiota and Topical Immune Perturbations in the Origins of Childhood Asthma. Nat. Commun. 2019, 10, 5001. [Google Scholar] [CrossRef]
- Rosas-Salazar, C.; Shilts, M.H.; Tang, Z.Z.; Hong, Q.; Turi, K.N.; Snyder, B.M.; Wiggins, D.A.; Lynch, C.E.; Gebretsadik, T.; Peebles, R.S.; et al. Exclusive Breast-Feeding, the Early-Life Microbiome and Immune Response, and Common Childhood Respiratory Illnesses. J. Allergy Clin. Immunol. 2022, 150, 612–621. [Google Scholar] [CrossRef]
- Biagi, E.; Quercia, S.; Aceti, A.; Beghetti, I.; Rampelli, S.; Turroni, S.; Faldella, G.; Candela, M.; Brigidi, P.; Corvaglia, L. The Bacterial Ecosystem of Mother’s Milk and Infant’s Mouth and Gut. Front. Microbiol. 2017, 8, 1214. [Google Scholar] [CrossRef]
- Laursen, M.F.; Sakanaka, M.; von Burg, N.; Mörbe, U.; Andersen, D.; Moll, J.M.; Pekmez, C.T.; Rivollier, A.; Michaelsen, K.F.; Mølgaard, C.; et al. Bifidobacterium Species Associated with Breastfeeding Produce Aromatic Lactic Acids in the Infant Gut. Nat. Microbiol. 2021, 6, 1367–1382. [Google Scholar] [CrossRef]
- Arrieta, M.C.; Stiemsma, L.T.; Dimitriu, P.A.; Thorson, L.; Russell, S.; Yurist-Doutsch, S.; Kuzeljevic, B.; Gold, M.J.; Britton, H.M.; Lefebvre, D.L.; et al. Early Infancy Microbial and Metabolic Alterations Affect Risk of Childhood Asthma. Sci. Transl. Med. 2015, 7, 307ra152. [Google Scholar] [CrossRef] [PubMed]
- Stokholm, J.; Thorsen, J.; Blaser, M.J.; Rasmussen, M.A.; Hjelmsø, M.; Shah, S.; Christensen, E.D.; Chawes, B.L.; Bønnelykke, K.; Brix, S.; et al. Delivery Mode and Gut Microbial Changes Correlate with an Increased Risk of Childhood Asthma. Sci. Transl. Med. 2020, 12, eaax9929. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Friedman, H.; Boyd, B.C.; McGurn, A.; Babinski, P.; Markossian, T.; Dugas, L.R. Early Antibiotic Exposure and Development of Asthma and Allergic Rhinitis in Childhood. BMC Pediatr. 2019, 19, 225. [Google Scholar] [CrossRef] [PubMed]
- Patrick, D.M.; Sbihi, H.; Dai, D.L.Y.; Al Mamun, A.; Rasali, D.; Rose, C.; Marra, F.; Boutin, R.C.T.; Petersen, C.; Stiemsma, L.T.; et al. Decreasing Antibiotic Use, the Gut Microbiota, and Asthma Incidence in Children: Evidence from Population-Based and Prospective Cohort Studies. Lancet Respir. Med. 2020, 8, 1094–1105. [Google Scholar] [CrossRef]
- Von Ehrenstein, O.S.; Von Mutius, E.; Illi, S.; Baumann, L.; Böhm, O.; Von Kries, R. Reduced Risk of Hay Fever and Asthma among Children of Farmers. Clin. Exp. Allergy 2000, 30, 187–193. [Google Scholar] [CrossRef]
- Von Mutius, E.; Vercelli, D. Farm Living: Effects on Childhood Asthma and Allergy. Nat. Rev. Immunol. 2010, 10, 861–868. [Google Scholar] [CrossRef]
- Ege, M.J.; Mayer, M.; Normand, A.-C.; Genuneit, J.; Cookson, W.O.C.M.; Braun-Fahrländer, C.; Heederik, D.; Piarroux, R.; von Mutius, E. Exposure to Environmental Microorganisms and Childhood Asthma. N. Engl. J. Med. 2011, 364, 701–709. [Google Scholar] [CrossRef]
- Pivniouk, V.; Gimenes Junior, J.A.; Honeker, L.K.; Vercelli, D. The Role of Innate Immunity in Asthma Development and Protection: Lessons from the Environment. Clin. Exp. Allergy 2020, 50, 282–290. [Google Scholar] [CrossRef]
- Valkonen, M.; Wouters, I.M.; Täubel, M.; Rintala, H.; Lenters, V.; Vasara, R.; Genuneit, J.; Braun-Fahrländer, C.; Piarroux, R.; Von Mutius, E.; et al. Bacterial Exposures and Associations with Atopy and Asthma in Children. PLoS ONE 2015, 10, e0131594. [Google Scholar] [CrossRef]
- Depner, M.; Taft, D.H.; Kirjavainen, P.V.; Kalanetra, K.M.; Karvonen, A.M.; Peschel, S.; Schmausser-Hechfellner, E.; Roduit, C.; Frei, R.; Lauener, R.; et al. Maturation of the Gut Microbiome during the First Year of Life Contributes to the Protective Farm Effect on Childhood Asthma. Nat. Med. 2020, 26, 1766–1775. [Google Scholar] [CrossRef]
- Haahtela, T. Biodiversity for Resilience-What Is Needed for Allergic Children. Pediatr. Allergy Immunol. 2022, 33, e13779. [Google Scholar] [CrossRef]
- Hanski, I.; Von Hertzen, L.; Fyhrquist, N.; Koskinen, K.; Torppa, K.; Laatikainen, T.; Karisola, P.; Auvinen, P.; Paulin, L.; Mäkelä, M.J.; et al. Environmental Biodiversity, Human Microbiota, and Allergy Are Interrelated. Proc. Natl. Acad. Sci. USA 2012, 109, 8334–8339. [Google Scholar] [CrossRef] [PubMed]
- Lehtimäki, J.; Thorsen, J.; Rasmussen, M.A.; Hjelmsø, M.; Shah, S.; Mortensen, M.S.; Trivedi, U.; Vestergaard, G.; Bønnelykke, K.; Chawes, B.L.; et al. Urbanized Microbiota in Infants, Immune Constitution, and Later Risk of Atopic Diseases. J. Allergy Clin. Immunol. 2021, 148, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Zhang, B.; Zhang, K.; Lv, X.; Wang, Q.; Bai, X. The Impact of Air Pollution on Intestinal Microbiome of Asthmatic Children: A Panel Study. BioMed Res. Int. 2020, 2020, 5753427. [Google Scholar] [CrossRef] [PubMed]
- Çolak, Y.; Afzal, S.; Nordestgaard, B.G.; Lange, P. Characteristics and Prognosis of Never-Smokers and Smokers with Asthma in the Copenhagen General Population Study. A Prospective Cohort Study. Am. J. Respir. Crit. Care Med. 2015, 192, 172–181. [Google Scholar] [CrossRef]
- Panzer, A.R.; Sitarik, A.R.; Fadrosh, D.; Havstad, S.L.; Jones, K.; Davidson, B.; Finazzo, S.; Wegienka, G.R.; Woodcroft, K.; Lukacs, N.W.; et al. The Impact of Prenatal Dog Keeping on Infant Gut Microbiota Development. Clin. Exp. Allergy 2023, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tun, H.M.; Konya, T.; Takaro, T.K.; Brook, J.R.; Chari, R.; Field, C.J.; Guttman, D.S.; Becker, A.B.; Mandhane, P.J.; Turvey, S.E.; et al. Exposure to Household Furry Pets Influences the Gut Microbiota of Infant at 3–4 Months Following Various Birth Scenarios. Microbiome 2017, 5, 40. [Google Scholar] [CrossRef]
- Shailesh, H.; Janahi, I.A. Role of Obesity in Inflammation and Remodeling of Asthmatic Airway. Life 2022, 12, 948. [Google Scholar] [CrossRef]
- Michalovich, D.; Rodriguez-Perez, N.; Smolinska, S.; Pirozynski, M.; Mayhew, D.; Uddin, S.; Van Horn, S.; Sokolowska, M.; Altunbulakli, C.; Eljaszewicz, A.; et al. Obesity and Disease Severity Magnify Disturbed Microbiome-Immune Interactions in Asthma Patients. Nat. Commun. 2019, 10, 5711. [Google Scholar] [CrossRef]
- Cloutier, M.M.; Baptist, A.P.; Blake, K.V.; Brooks, E.G.; Bryant-Stephens, T.; DiMango, E.; Dixon, A.E.; Elward, K.S.; Hartert, T.; Krishnan, J.A.; et al. 2020 Focused Updates to the Asthma Management Guidelines: A Report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group. J. Allergy Clin. Immunol. 2020, 146, 1217–1270. [Google Scholar] [CrossRef]
- Khan, L. Overview of the Updates for the Management of Asthma Guidelines. Pediatr. Ann. 2022, 51, e132–e135. [Google Scholar] [CrossRef]
- Durack, J.; Lynch, S.V.; Nariya, S.; Bhakta, N.R.; Beigelman, A.; Castro, M.; Dyer, A.M.; Israel, E.; Kraft, M.; Martin, R.J.; et al. Features of the Bronchial Bacterial Microbiome Associated with Atopy, Asthma and Responsiveness to Inhaled Corticosteroid Treatment. J. Allergy Clin. Immunol. 2017, 140, 63. [Google Scholar] [CrossRef]
- Huang, Y.J.; Nariya, S.; Harris, J.M.; Lynch, S.V.; Choy, D.F.; Arron, J.R.; Boushey, H. The Airway Microbiome in Severe Asthma: Associations with Disease Features and Severity. J. Allergy Clin. Immunol. 2015, 136, 874. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, A.B.; Turturice, B.A.; Perkins, D.L.; Finn, P.W. The Potential for Emerging Microbiome-Mediated Therapeutics in Asthma. Curr. Allergy Asthma Rep. 2017, 17, 62. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Roberfroid, M.B. Dietary Modulation of the Human Colonic Microbiota: Introducing the Concept of Prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Enam, F.; Mansell, T.J. Prebiotics: Tools to Manipulate the Gut Microbiome and Metabolome. J. Ind. Microbiol. Biotechnol. 2019, 46, 1445–1459. [Google Scholar] [CrossRef] [PubMed]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef]
- Carlson, J.L.; Erickson, J.M.; Lloyd, B.B.; Slavin, J.L. Health Effects and Sources of Prebiotic Dietary Fiber. Curr. Dev. Nutr. 2018, 2, nzy005. [Google Scholar] [CrossRef]
- Panesar, P.S.; Kumari, S.; Panesar, R. Biotechnological Approaches for the Production of Prebiotics and Their Potential Applications. Crit. Rev. Biotechnol. 2013, 33, 345–364. [Google Scholar] [CrossRef]
- Comstock, S.S.; Wang, M.; Hester, S.N.; Li, M.; Donovan, S.M. Select Human Milk Oligosaccharides Directly Modulate Peripheral Blood Mononuclear Cells Isolated from 10-d-Old Pigs. Br. J. Nutr. 2014, 111, 819–828. [Google Scholar] [CrossRef]
- Coppa, G.V.; Zampini, L.; Galeazzi, T.; Gabrielli, O. Prebiotics in Human Milk: A Review. Dig. Liver Dis. 2006, 38 (Suppl. S2), S291–S294. [Google Scholar] [CrossRef] [PubMed]
- Vohra, Y.; Vasan, M.; Venot, A.; Boons, G.J. One-Pot Synthesis of Oligosaccharides by Combining Reductive Openings of Benzylidene Acetals and Glycosylations. Org. Lett. 2008, 10, 3247–3250. [Google Scholar] [CrossRef] [PubMed]
- Osborn, D.A.; Sinn, J.K. Prebiotics in Infants for Prevention of Allergy. Cochrane Database Syst. Rev. 2013, 2013, CD006474. [Google Scholar] [CrossRef]
- Wawryk-Gawda, E.; Markut-Miotła, E.; Emeryk, A. Postnatal Probiotics Administration Does Not Prevent Asthma in Children, but Using Prebiotics or Synbiotics May Be the Effective Potential Strategies to Decrease the Frequency of Asthma in High-Risk Children—A Meta-Analysis of Clinical Trials. Allergol. Immunopathol. 2021, 49, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Cuello-Garcia, C.; Fiocchi, A.; Pawankar, R.; Yepes-Nuñez, J.J.; Morgano, G.P.; Zhang, Y.; Agarwal, A.; Gandhi, S.; Terracciano, L.; Schünemann, H.J.; et al. Prebiotics for the Prevention of Allergies: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Clin. Exp. Allergy 2017, 47, 1468–1477. [Google Scholar] [CrossRef]
- Suez, J.; Zmora, N.; Segal, E.; Elinav, E. The Pros, Cons, and Many Unknowns of Probiotics. Nat. Med. 2019, 25, 716–729. [Google Scholar] [CrossRef]
- Williams, N.T. Probiotics. Am. J. Health Syst. Pharm. 2010, 67, 449–458. [Google Scholar] [CrossRef]
- Chapman, C.M.C.; Gibson, G.R.; Rowland, I. Health Benefits of Probiotics: Are Mixtures More Effective than Single Strains? Eur. J. Nutr. 2011, 50, 1–17. [Google Scholar] [CrossRef]
- Chapman, C.M.C.; Gibson, G.R.; Rowland, I. In Vitro Evaluation of Single- and Multi-Strain Probiotics: Inter-Species Inhibition between Probiotic Strains, and Inhibition of Pathogens. Anaerobe 2012, 18, 405–413. [Google Scholar] [CrossRef]
- McFarland, L.V. Efficacy of Single-Strain Probiotics Versus Multi-Strain Mixtures: Systematic Review of Strain and Disease Specificity. Dig. Dis. Sci. 2021, 66, 694–704. [Google Scholar] [CrossRef]
- Stavropoulou, E.; Bezirtzoglou, E. Probiotics in Medicine: A Long Debate. Front. Immunol. 2020, 11, 2192. [Google Scholar] [CrossRef] [PubMed]
- Taibi, A.; Comelli, E.M. Practical Approaches to Probiotics Use. Appl. Physiol. Nutr. Metab. 2014, 39, 980–986. [Google Scholar] [CrossRef] [PubMed]
- Krishna Rao, R.; Samak, G. Protection and Restitution of Gut Barrier by Probiotics: Nutritional and Clinical Implications. Curr. Nutr. Food Sci. 2013, 9, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Hertzberger, R.Y.; Knaus, U.G. Hydrogen Peroxide Production by Lactobacilli Promotes Epithelial Restitution during Colitis. Redox Biol. 2018, 16, 11–20. [Google Scholar] [CrossRef]
- LeBlanc, J.G.; Chain, F.; Martín, R.; Bermúdez-Humarán, L.G.; Courau, S.; Langella, P. Beneficial Effects on Host Energy Metabolism of Short-Chain Fatty Acids and Vitamins Produced by Commensal and Probiotic Bacteria. Microb. Cell Fact. 2017, 16, 79. [Google Scholar] [CrossRef]
- He, S.; Ran, C.; Qin, C.; Li, S.; Zhang, H.; De Vos, W.M.; Ringø, E.; Zhou, Z. Anti-Infective Effect of Adhesive Probiotic Lactobacillus in Fish Is Correlated With Their Spatial Distribution in the Intestinal Tissue. Sci. Rep. 2017, 7, 13195. [Google Scholar] [CrossRef]
- Górska, A.; Przystupski, D.; Niemczura, M.J.; Kulbacka, J. Probiotic Bacteria: A Promising Tool in Cancer Prevention and Therapy. Curr. Microbiol. 2019, 76, 939–949. [Google Scholar] [CrossRef]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of Action of Probiotics. Adv. Nutr. 2019, 10, S49–S66. [Google Scholar] [CrossRef]
- Hassanzad, M.; Maleki Mostashari, K.; Ghaffaripour, H.; Emami, H.; Limouei, S.R.; Velayati, A.A. Synbiotics and Treatment of Asthma: A Double-Blinded, Randomized, Placebo-Controlled Clinical Trial. Galen Med. J. 2019, 8, 1350. [Google Scholar] [CrossRef]
- Del Giudice, M.M.; Indolfi, C.; Capasso, M.; Maiello, N.; Decimo, F.; Ciprandi, G. Bifidobacterium Mixture (B Longum BB536, B Infantis M-63, B Breve M-16V) Treatment in Children with Seasonal Allergic Rhinitis and Intermittent Asthma. Ital. J. Pediatr. 2017, 43, 25. [Google Scholar] [CrossRef]
- Elazab, N.; Mendy, A.; Gasana, J.; Vieira, E.R.; Quizon, A.; Forno, E. Probiotic Administration in Early Life, Atopy, and Asthma: A Meta-Analysis of Clinical Trials. Pediatrics 2013, 132, e666–e676. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Jiang, P.; Liu, J.; Sun, R.; Zhu, L. Association between Probiotic Supplementation and Asthma Incidence in Infants: A Meta-Analysis of Randomized Controlled Trials. J. Asthma 2020, 57, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhang, Y.; He, C.; Dai, J. Probiotics Supplementation in Children with Asthma: A Systematic Review and Meta-Analysis. J. Paediatr. Child Health 2018, 54, 953–961. [Google Scholar] [CrossRef] [PubMed]
- Grandinetti, R.; Fainardi, V.; Caffarelli, C.; Capoferri, G.; Lazzara, A.; Tornesello, M.; Meoli, A.; Bergamini, B.M.; Bertelli, L.; Biserna, L.; et al. Risk Factors Affecting Development and Persistence of Preschool Wheezing: Consensus Document of the Emilia-Romagna Asthma (ERA) Study Group. J. Clin. Med. 2022, 11, 6558. [Google Scholar] [CrossRef] [PubMed]
- Ciprandi, G.; Tosca, M.A. Probiotics in Children with Asthma. Children 2022, 9, 978. [Google Scholar] [CrossRef] [PubMed]
- Sangkanjanavanich, S.; Pradubpongsa, P.; Mitthamsiri, W.; Sangasapaviliya, A.; Boonpiyathad, T. Bifidobacterium Infantis 35624 Efficacy in Patients with Uncontrolled Asthma: A Randomized Placebo-Controlled Trial. Ann. Allergy. Asthma Immunol. 2022, 129, 790–792. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Liu, F.; Gao, Q.; Wang, R.; Zhang, L.; Li, Y. A Meta-Analysis of Probiotics for the Treatment of Allergic Airway Diseases in Children and Adolescents. Am. J. Rhinol. Allergy 2022, 36, 480–490. [Google Scholar] [CrossRef]
- Wood, L.G. Diet, Obesity, and Asthma. Ann. Am. Thorac. Soc. 2017, 14, S332–S338. [Google Scholar] [CrossRef]
- Alwarith, J.; Kahleova, H.; Crosby, L.; Brooks, A.; Brandon, L.; Levin, S.M.; Barnard, N.D. The Role of Nutrition in Asthma Prevention and Treatment. Nutr. Rev. 2020, 78, 928–938. [Google Scholar] [CrossRef]
- Okoniewski, W.; Lu, K.D.; Forno, E. Weight Loss for Children and Adults with Obesity and Asthma. A Systematic Review of Randomized Controlled Trials. Ann. Am. Thorac. Soc. 2019, 16, 613–625. [Google Scholar] [CrossRef]
- Nuzzi, G.; Di Cicco, M.; Trambusti, I.; Agosti, M.; Peroni, D.G.; Comberiati, P. Primary Prevention of Pediatric Asthma through Nutritional Interventions. Nutrients 2022, 14, 754. [Google Scholar] [CrossRef] [PubMed]
- Venter, C.; Agostoni, C.; Arshad, S.H.; Ben-Abdallah, M.; Du Toit, G.; Fleischer, D.M.; Greenhawt, M.; Glueck, D.H.; Groetch, M.; Lunjani, N.; et al. Dietary Factors during Pregnancy and Atopic Outcomes in Childhood: A Systematic Review from the European Academy of Allergy and Clinical Immunology. Pediatr. Allergy Immunol. 2020, 31, 889. [Google Scholar] [CrossRef] [PubMed]
- Best, K.P.; Gold, M.; Kennedy, D.; Martin, J.; Makrides, M. Omega-3 Long-Chain PUFA Intake during Pregnancy and Allergic Disease Outcomes in the Offspring: A Systematic Review and Meta-Analysis of Observational Studies and Randomized Controlled Trials. Am. J. Clin. Nutr. 2016, 103, 128–143. [Google Scholar] [CrossRef] [PubMed]
- Venter, C.; Meyer, R.W.; Nwaru, B.I.; Roduit, C.; Untersmayr, E.; Adel-Patient, K.; Agache, I.; Agostoni, C.; Akdis, C.A.; Bischoff, S.C.; et al. EAACI Position Paper: Influence of Dietary Fatty Acids on Asthma, Food Allergy, and Atopic Dermatitis. Allergy 2019, 74, 1429–1444. [Google Scholar] [CrossRef] [PubMed]
- Forte, G.C.; da Silva, D.T.R.; Hennemann, M.L.; Sarmento, R.A.; Almeida, J.C.; de Tarso Roth Dalcin, P. Diet Effects in the Asthma Treatment: A Systematic Review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1878–1887. [Google Scholar] [CrossRef]
- Wang, J.W.; Kuo, C.H.; Kuo, F.C.; Wang, Y.K.; Hsu, W.H.; Yu, F.J.; Hu, H.M.; Hsu, P.I.; Wang, J.Y.; Wu, D.C. Fecal Microbiota Transplantation: Review and Update. J. Formos. Med. Assoc. 2019, 118 (Suppl. S1), S23–S31. [Google Scholar] [CrossRef]
- Xu, M.Q.; Cao, H.L.; Wang, W.Q.; Wang, S.; Cao, X.C.; Yan, F.; Wang, B.M. Fecal Microbiota Transplantation Broadening Its Application beyond Intestinal Disorders. World J. Gastroenterol. 2015, 21, 102. [Google Scholar] [CrossRef]
- Kang, Y.; Cai, Y. Future Prospect of Faecal Microbiota Transplantation as a Potential Therapy in Asthma. Allergol. Immunopathol. 2018, 46, 307–309. [Google Scholar] [CrossRef]
| Bacterial Genera/Phyla | Fungal Genera/Fungus Taxa | Virus Genera | |
|---|---|---|---|
| Gastrointestinal microbiome | Bacteroides, Eubacterium, Peptococcus, Peptostreptococcus, Clostridium, Ruminococcus, Faecalibacterium, Escherichia, Lactobacillus | Candida, Saccharomyces, Aspergillus, Penicillium, Bullera, Pleospora, Rhodotorula, Trametes, Galactomyces | Bacteriophages |
| Respiratory microbiome | Prevotella, Streptococcus, Veillonella, Firmicutes, Bacteroidetes | Ascomycota, Basidiomycota, Candida, Saccharomyces, Penicillium, Cladosporium, Fusarium | Understudied |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Logoń, K.; Świrkosz, G.; Nowak, M.; Wrześniewska, M.; Szczygieł, A.; Gomułka, K. The Role of the Microbiome in the Pathogenesis and Treatment of Asthma. Biomedicines 2023, 11, 1618. https://doi.org/10.3390/biomedicines11061618
Logoń K, Świrkosz G, Nowak M, Wrześniewska M, Szczygieł A, Gomułka K. The Role of the Microbiome in the Pathogenesis and Treatment of Asthma. Biomedicines. 2023; 11(6):1618. https://doi.org/10.3390/biomedicines11061618
Chicago/Turabian StyleLogoń, Katarzyna, Gabriela Świrkosz, Monika Nowak, Martyna Wrześniewska, Aleksandra Szczygieł, and Krzysztof Gomułka. 2023. "The Role of the Microbiome in the Pathogenesis and Treatment of Asthma" Biomedicines 11, no. 6: 1618. https://doi.org/10.3390/biomedicines11061618
APA StyleLogoń, K., Świrkosz, G., Nowak, M., Wrześniewska, M., Szczygieł, A., & Gomułka, K. (2023). The Role of the Microbiome in the Pathogenesis and Treatment of Asthma. Biomedicines, 11(6), 1618. https://doi.org/10.3390/biomedicines11061618





