Thickness and Volume of Epicardial Adipose Tissue in Relation to Stiffness and Elasticity of Aorta Assessed by Computed Tomography Angiography
Abstract
1. Introduction
2. Material and Methods
2.1. Calculation of Group Size, and Inclusion and Exclusion Criteria of the Study
2.2. Study Group Description
2.3. Criteria for Separating the Subgroups—Medians of Studied Variables of Epicardial Fat Thickness and Aortic Stiffness and Elasticity
2.4. Methodology
2.5. Statistical Analysis
3. Results
3.1. Coronary Computed Tomography Angiography Results and Measured Parameters of EAT and Aorta
3.2. Relation between Volume and Thickness of Epicardial Adipose and Aortic Stiffness and Elasticity—Higher Thickness and Volume of EAT in Group of Higher Ao Stiffness and Lower Ao Elasticity
3.3. Relation between Stiffness and Elasticity of Aorta and EAT—Higher Ao Stiffness and Lower Ao Elasticity in Subgroups of Higher EAT Thickness and Volume
3.4. Analysis of Correlations between EAT and Aortic Parameters
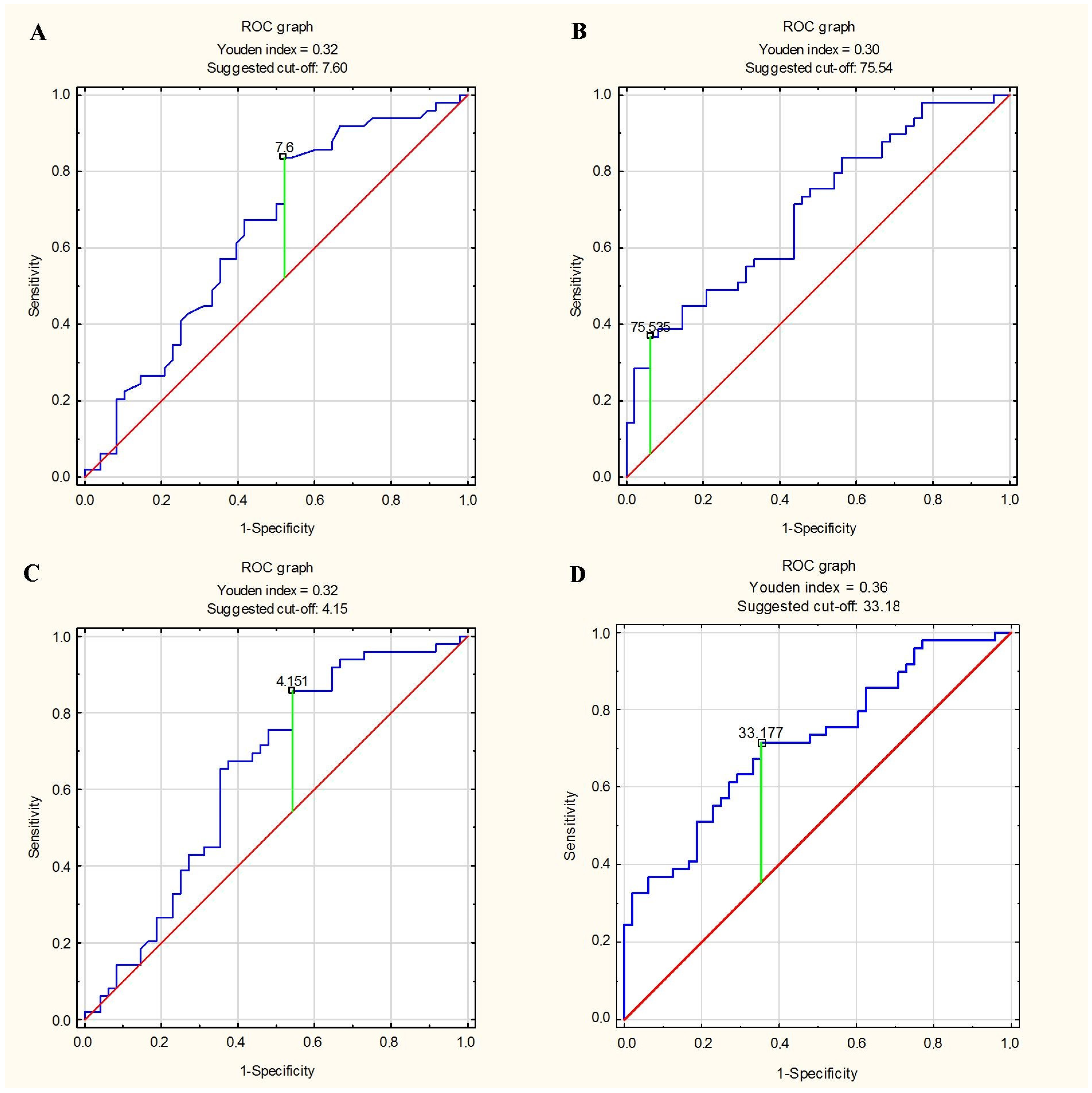
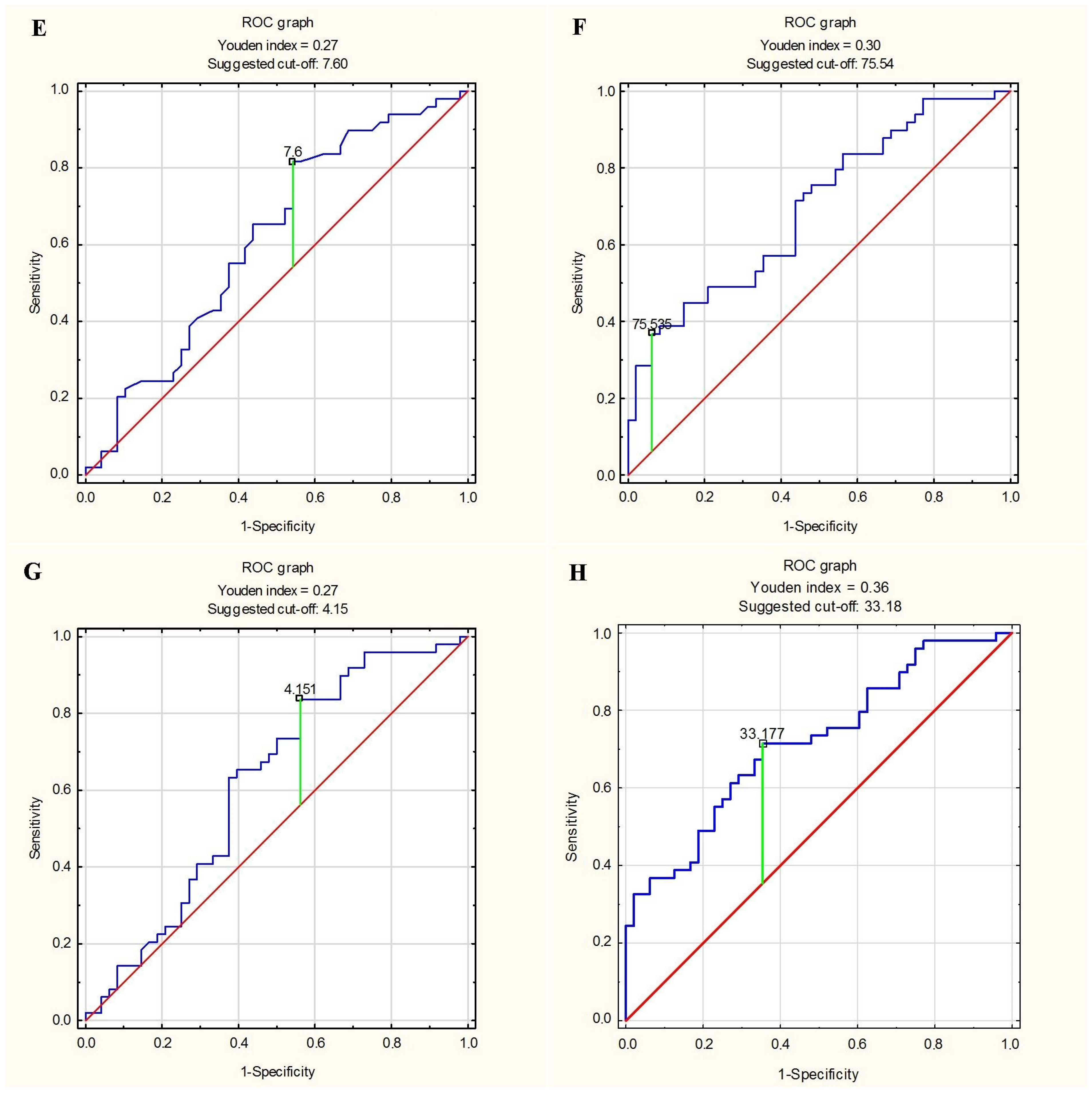
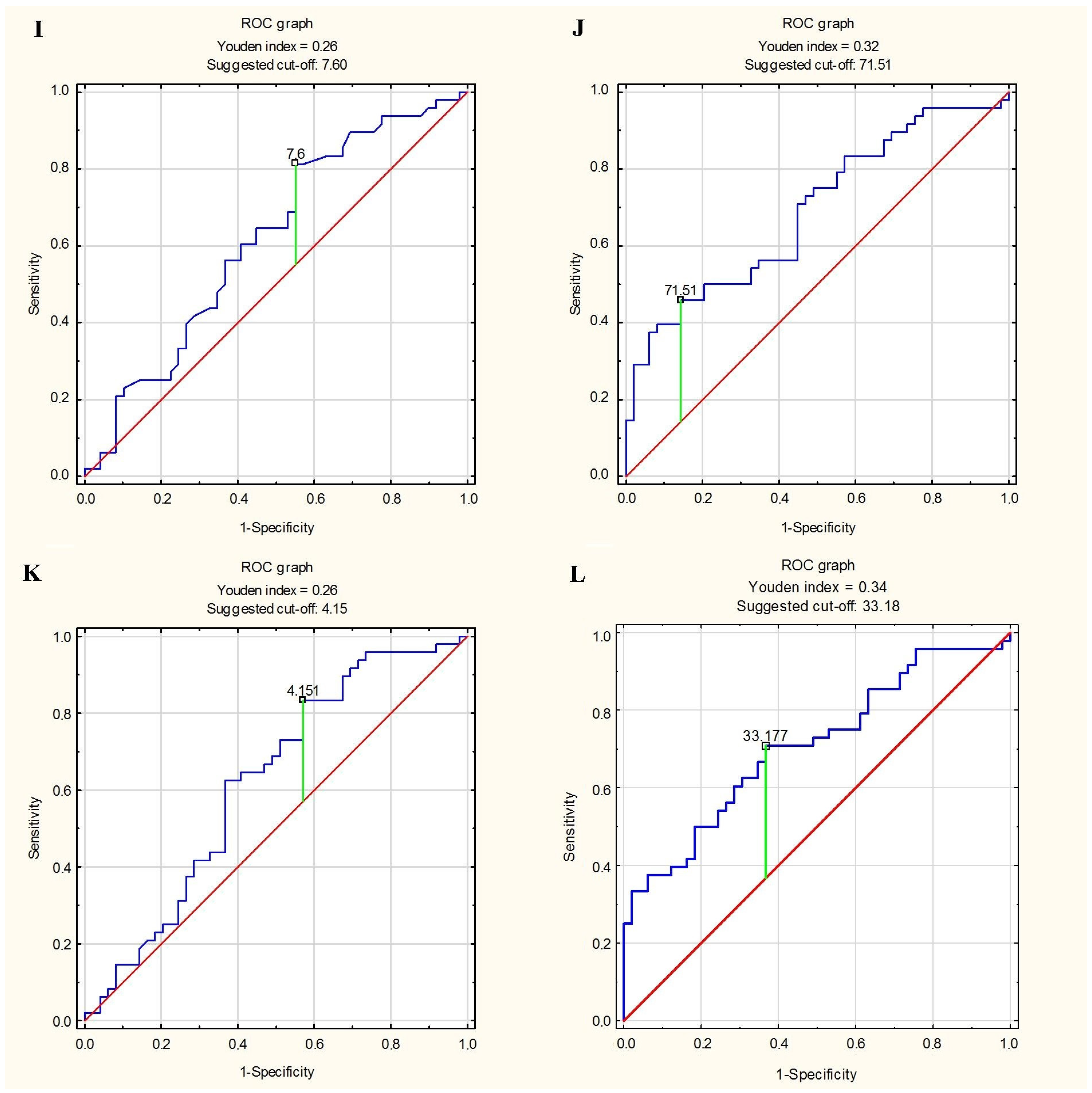
3.5. Predictor of Greater Stiffness and Lesser Elasticity of Aorta
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gueret, P.; Deux, J.-F.; Bonello, L.; Sarran, A.; Tron, C.; Christiaens, L.; Dacher, J.-N.; Bertrand, D.; Leborgne, L.; Renard, C.; et al. Diagnostic Performance of Computed Tomography Coronary Angiography (from the Prospective National Multicenter Multivendor EVASCAN Study). Am. J. Cardiol. 2013, 111, 471–478. [Google Scholar] [CrossRef]
- Hoffmann, U.; Ferencik, M.; Udelson, J.E.; Picard, M.H.; Truong, Q.A.; Patel, M.R.; Huang, M.; Pencina, M.; Mark, D.B.; Heitner, J.F.; et al. Prognostic Value of Noninvasive Cardiovascular Testing in Patients With Stable Chest Pain. Circulation 2017, 135, 2320–2332. [Google Scholar] [CrossRef] [PubMed]
- Knuuti, J.; Ballo, H.; Juarez-Orozco, L.E.; Saraste, A.; Kolh, P.; Rutjes, A.W.S.; Jüni, P.; Windecker, S.; Bax, J.J.; Wijns, W. The performance of non-invasive tests to rule-in and rule-out significant coronary artery stenosis in patients with stable angina: A meta-analysis focused on post-test disease probability. Eur. Heart J. 2018, 39, 3322–3330. [Google Scholar] [CrossRef]
- SCOT-HEART Investigators; Newby, D.E.; Adamson, P.D.; Berry, C.; Boon, N.A.; Dweck, M.R.; Flather, M.; Forbes, J.; Hunter, A.; Lewis, S.; et al. Coronary CT Angiography and 5-Year Risk of Myocardial Infarction. N. Engl. J. Med. 2018, 379, 924–933. [Google Scholar]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Straube, F.; Pongratz, J.; Hartl, S.; Brueck, B.; Tesche, C.; Ebersberger, U.; Helmberger, T.; Crispin, A.; Wankerl, M.; Dorwarth, U.; et al. Cardiac computed tomography angiography-derived analysis of left atrial appendage morphology and left atrial dimensions for the prediction of atrial fibrillation recurrence after pulmonary vein isolation. Clin. Cardiol. 2021, 44, 1636–1645. [Google Scholar] [CrossRef] [PubMed]
- Foldyna, B.; Sandri, M.; Luecke, C.; Garbade, J.; Gohmann, R.; Hahn, J.; Fischer, J.; Gutberlet, M.; Lehmkuhl, L. Quantitative coronary computed tomography angiography for the detection of cardiac allograft vasculopathy. Eur. Radiol. 2020, 30, 4317–4326. [Google Scholar] [CrossRef][Green Version]
- Tweet, M.S.; Akhtar, N.J.; Hayes, S.N.; Best, P.J.; Gulati, R.; A Araoz, P. Spontaneous coronary artery dissection: Acute findings on coronary computed tomography angiography. Eur. Heart J. Acute Cardiovasc. Care 2019, 8, 467–475. [Google Scholar] [CrossRef]
- Alqahtani, A.M.; Boczar, K.E.; Kansal, V.; Chan, K.; Dwivedi, G.; Chow, B.J. Quantifying Aortic Valve Calcification using Coronary Computed Tomography Angiography. J. Cardiovasc. Comput. Tomogr. 2017, 11, 99–104. [Google Scholar] [CrossRef]
- Öztürk, E.; Kafadar, C.; Tutar, S.; Bozlar, U.; Hagspiel, K.D. Non-coronary abnormalities of the left heart: CT angiography findings. Anatol. J. Cardiol. 2016, 16, 720–727. [Google Scholar]
- Szymczyk, K.; Polguj, M.; Szymczyk, E.; Bakoń, L.; Pacho, R.; Stefańczyk, L. Assessment of aortic valve in regard to its anatomical variants morphology in 2053 patients using 64-slice CT retrospective coronary angiography. BMC Cardiovasc. Disord. 2016, 16, 89. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Iacobellis, G. Epicardial adipose tissue in contemporary cardiology. Nat. Rev. Cardiol. 2022, 19, 593–606. [Google Scholar] [CrossRef] [PubMed]
- le Jemtel, T.H.; Samson, R.; Ayinapudi, K.; Singh, T.; Oparil, S. Epicardial Adipose Tissue and Cardiovascular Disease. Curr. Hypertens. Rep. 2019, 21, 36. [Google Scholar] [CrossRef]
- Iacobellis, G.; Barbaro, G. Epicardial adipose tissue feeding and overfeeding the heart. Nutrition 2019, 59, 1–6. [Google Scholar] [CrossRef]
- Ansaldo, A.M.; Montecucco, F.; Sahebkar, A.; Dallegri, F.; Carbone, F. Epicardial adipose tissue and cardiovascular diseases. Int. J. Cardiol. 2019, 278, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, Y.; Xu, L.; Greenwald, S.E.; Zhang, L.; Zhang, R.; You, H.; Yang, B. Automatic quantification of epicardial adipose tissue volume. Med. Phys. 2021, 48, 4279–4290. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Guo, B.J.; Lei, Y.; Wang, T.; Fu, Y.; Curran, W.J.; Zhang, L.J.; Liu, T.; Yang, X. Automatic segmentation and quantification of epicardial adipose tissue from coronary computed tomography angiography. Phys. Med. Biol. 2020, 65, 095012. [Google Scholar] [CrossRef]
- Yuvaraj, J.; Isa, M.; Che, Z.C.; Lim, E.; Nerlekar, N.; Nicholls, S.J.; Seneviratne, S.; Lin, A.; Dey, D.; Wong, D.T.L. Atherogenic index of plasma is associated with epicardial adipose tissue volume assessed on coronary computed tomography angiography. Sci. Rep. 2022, 12, 9626. [Google Scholar] [CrossRef]
- Konwerski, M.; Postuła, M.; Barczuk-Falęcka, M.; Czajkowska, A.; Mróz, A.; Witek, K.; Bakalarski, W.; Gąsecka, A.; Małek, A.; Mazurek, T. Epicardial Adipose Tissue and Cardiovascular Risk Assessment in Ultra-Marathon Runners: A Pilot Study. Int. J. Environ. Res. Public Health 2021, 18, 3136. [Google Scholar] [CrossRef]
- Villasante Fricke, A.C.; Iacobellis, G. Epicardial Adipose Tissue: Clinical Biomarker of Cardio-Metabolic Risk. Int. J. Mol. Sci. 2019, 20, 5989. [Google Scholar] [CrossRef][Green Version]
- Pugliese, N.R.; Paneni, F.; Mazzola, M.; De Biase, N.; Del Punta, L.; Gargani, L.; Mengozzi, A.; Virdis, A.; Nesti, L.; Taddei, S.; et al. Impact of epicardial adipose tissue on cardiovascular haemodynamics, metabolic profile, and prognosis in heart failure. Eur. J. Heart Fail. 2021, 23, 1858–1871. [Google Scholar] [CrossRef] [PubMed]
- Senoo, A.; Kitagawa, T.; Torimaki, S.; Yamamoto, H.; Sentani, K.; Takahashi, S.; Kubo, Y.; Yasui, W.; Sueda, T.; Kihara, Y. Association between histological features of epicardial adipose tissue and coronary plaque characteristics on computed tomography angiography. Heart Vessels 2018, 33, 827–836. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dong, D.-D.; Wang, K.; Wang, D.; Zhang, T.; Tu, Y.-F.; Shen, B.-Z. Relationship between epicardial adipose tissue volume measured using coronary computed tomography angiography and atherosclerotic plaque characteristics in patients with severe coronary artery stenosis. J. Int. Med. Res. 2013, 41, 1520–1531. [Google Scholar] [CrossRef] [PubMed]
- Cereda, A.; Toselli, M.; Palmisano, A.; Vignale, D.; Khokhar, A.; Campo, G.; Bertini, M.; Loffi, M.; Andreini, D.; Pontone, G.; et al. Coronary calcium score as a predictor of outcomes in the hypertensive COVID-19 population: Results from the Italian (S) Core-COVID-19 Registry. Hypertens. Res. 2022, 45, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Nalliah, C.J.; Bell, J.R.; Raaijmakers, A.J.; Waddell, H.M.; Wells, S.P.; Bernasochi, G.B.; Montgomery, M.K.; Binny, S.; Watts, T.; Joshi, S.B.; et al. Epicardial Adipose Tissue Accumulation Confers Atrial Conduction Abnormality. J. Am. Coll. Cardiol. 2020, 76, 1197–1211. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, H.; Chen, J.; Zhao, L. Epicardial adipose tissue and atrial fibrillation: Possible mechanisms, potential therapies, and future directions. Pacing Clin. Electrophysiol. 2020, 43, 133–145. [Google Scholar] [CrossRef]
- Hajdusianek, W.; Żórawik, A.; Poręba, R.; Gać, P. Assessment of aortic stiffness in computed tomography–methodology of radiological examination from 2000 to 2020. Pol. J. Radiol. 2022, 87, 635–640. [Google Scholar] [CrossRef]
- Birudaraju, D.; Cherukuri, L.; Kinninger, A.; Chaganti, B.T.; Haroun, P.; Pidikiti, S.; Lakshmanan, S.; Hamal, S.; Flores, F.; Dailing, C.; et al. Relationship between cardio-ankle vascular index and obstructive coronary artery disease. Coron. Artery Dis. 2020, 31, 550–555. [Google Scholar] [CrossRef]
- Park, H.E.; Choi, S.-Y.; Kim, H.S.; Kim, M.K.; Cho, S.-H.; Oh, B.-H. Epicardial Fat Reflects Arterial Stiffness: Assessment Using 256-Slice Multidetector Coronary Computed Tomography and Cardio-Ankle Vascular Index. J. Atheroscler. Thromb. 2012, 19, 570–576. [Google Scholar] [CrossRef][Green Version]
- Pereira, T.; Maldonado, J.; Pereira, L.; Conde, J. Aortic Stiffness is an Independent Predictor of Stroke in Hypertensive Patients. Arq. Bras. Cardiol. 2013, 100, 437–443. [Google Scholar] [CrossRef]
- Jia, C.-F.; Jiang, Y.-N.; Yang, Z.-Q.; Sun, X.-X.; Yu, Y.; Wang, H.; Lu, Y.; Chen, A.-J.; Wang, Z.-Q. Ascending Aortic Elasticity and Related Risk Factors Study on Prehypertension Patients. Am. J. Hypertens. 2017, 30, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Gürel, E.; Tigen, K.; Karaahmet, T.; Gecmen, C.; Mutlu, B.; Bulut, M.; Basaran, Y. Aortic elastic properties predict occult coronary artery disease: A multidetector row computed tomography study. Kardiol. Pol. 2015, 73, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Foldyna, B.; Szilveszter, B.; Scholtz, J.-E.; Banerji, D.; Maurovich-Horvat, P.; Hoffmann, U. CAD-RADS—A new clinical decision support tool for coronary computed tomography angiography. Eur. Radiol. 2018, 28, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Altun, I.; Unal, Y.; Başaran, Ö.; Akin, F.; Emir, G.K.; Kutlu, G.; Biteker, M. Increased Epicardial Fat Thickness Correlates with Aortic Stiffness and N-Terminal Pro-Brain Natriuretic Peptide Levels in Acute Ischemic Stroke Patients. Tex. Heart Inst. J. 2016, 43, 220–226. [Google Scholar] [CrossRef][Green Version]
- Homsi, R.; Sprinkart, A.M.; Gieseke, J.; Meier-Schroers, M.; Yuecel, S.; Fischer, S.; Nadal, J.; Dabir, D.; A Luetkens, J.; Kuetting, D.L.; et al. Cardiac magnetic resonance based evaluation of aortic stiffness and epicardial fat volume in patients with hypertension, diabetes mellitus, and myocardial infarction. Acta Radiol. 2018, 59, 65–71. [Google Scholar] [CrossRef]
- Homsi, R.; Thomas, D.; Gieseke, J.; Meier-Schroers, M.; Dabir, D.; Kuetting, D.; Luetkens, J.A.; Marx, C.; Schild, H.H.; Sprinkart, A. Epicardial Fat Volume and Aortic Stiffness in Healthy Individuals: A Quantitative Cardiac Magnetic Resonance Study. Rofo 2016, 188, 853–858. [Google Scholar] [CrossRef][Green Version]
- Liu, Z.; Hu, W.; Zhang, H.; Tao, H.; Lei, P.; Liu, J.; Yu, Y.; Dong, Q.; Gao, L.; Zhang, D. EAT Thickness as a Predominant Feature for Evaluating Arterial Stiffness in Patients with Heart Failure with Preserved Ejection Fraction. Diabetes Metab. Syndr. Obes. 2022, 15, 1217–1226. [Google Scholar] [CrossRef]
- Doğan, M.; Turak, O.; Akyel, A.; Grbovic, E.; Mendi, M.A.; Öksüz, F.; Dogan, A.; Cimen, T.; Bilgin, M.; Sunman, H.; et al. Increased epicardial adipose tissue thickness is linked to aortic stiffness in patients with primary hypertension. Blood Press. 2014, 23, 222–227. [Google Scholar] [CrossRef]
- Kim, B.J.; Kim, B.S.; Kang, J.H. Echocardiographic epicardial fat thickness is associated with arterial stiffness. Int. J. Cardiol. 2013, 167, 2234–2238. [Google Scholar] [CrossRef]
- Petra, C.-V.; Albu, A.; Pamfil, C.; Tamas, M.M.; Vesa, S.C.; Rednic, S. The relationship between epicardial adipose tissue and arterial stiffness in patients with rheumatoid arthritis. Med. Ultrason. 2019, 21, 427–434. [Google Scholar] [CrossRef]
- Argan, O.; Avci, E.; Yildirim, T.; Safak, O. Epicardial adipose tissue is a predictor of ascending aortic dilatation in hypertensive patients, but not paracardial adipose tissue. BMC Cardiovasc. Disord. 2020, 20, 142. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Çanga, A.; Kocaman, S.A.; Çetin, M.; Erdoğan, T.; Durakoğlugil, M.E.; Çiçek, Y.; Uğurlu, Y.; Satiroğlu, Ö. Increased epicardial adipose tissue thickness is correlated with ascending aortic diameter. Tohoku J. Exp. Med. 2012, 226, 183–190. [Google Scholar] [CrossRef][Green Version]
- Song, X.-T.; Rui, Y.-F.; Fan, L.; Yan, Z.-N. Echocardiographic Association of Epicardial Adipose Tissue with Ascending Aorta Elasticity in Patients with Type 2 Diabetes Mellitus. Angiology 2022, 74, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Mancio, J.; Azevedo, D.; Saraiva, F.; Azevedo, A.I.; Pires-Morais, G.; Leite-Moreira, A.; Falcão-Pires, I.; Lunet, N.; Bettencourt, N. Epicardial adipose tissue volume assessed by computed tomography and coronary artery disease: A systematic review and meta-analysis. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 490–497. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Groves, E.M.; Erande, A.S.; Le, C.; Salcedo, J.; Hoang, K.C.; Kumar, S.; Mohar, D.S.; Saremi, F.; Im, J.; Agrawal, Y.; et al. Comparison of epicardial adipose tissue volume and coronary artery disease severity in asymptomatic adults with versus without diabetes mellitus. Am. J. Cardiol. 2014, 114, 686–691. [Google Scholar] [CrossRef] [PubMed][Green Version]
| X | SD | |
|---|---|---|
| age [years] | 63.48 | 8.50 |
| height [m] | 1.67 | 0.07 |
| body mass [kg] | 74.16 | 11.03 |
| BMI [kg/m2] | 25.97 | 3.81 |
| BSA [m2] | 1.82 | 0.15 |
| % | n | |
| gender | ||
| men | 49.5 | 48 |
| women | 50.5 | 49 |
| body mass | ||
| normal | 47.4 | 46 |
| overweight/obesity | 52.6 | 51 |
| indication to CCTA | ||
| chronic CAD suspicion | 70.1 | 68 |
| chest pain | 39.2 | 38 |
| low intermediate CAD risk | 39.1 | 37 |
| numerous CAD risk factors | 26.8 | 26 |
| inconclusive exercise test | 13.4 | 13 |
| non-diagnostic exercise test | 8.2 | 8 |
| regional wall motion abnormalities of left ventricular | 2.1 | 2 |
| sudden cardiac death in the family history | 1.0 | 1 |
| X | SD | |
| SBP [mmHg] | 143.20 | 7.78 |
| DBP [mmHg] | 88.40 | 5.66 |
| X | SD | |
|---|---|---|
| CACS | 162.24 | 317.69 |
| n | % | |
| significant CAD risk | ||
| practically non-existent | 31 | 31.9 |
| minimal | 23 | 23.7 |
| mild | 15 | 15.5 |
| moderate | 16 | 16.5 |
| high | 12 | 12.4 |
| CAD-RADS | ||
| 0 | 29 | 29.9 |
| 1 | 27 | 27.8 |
| 2 | 24 | 24.7 |
| 3 | 9 | 9.3 |
| 4 | 5 | 5.1 |
| 5 | 1 | 1.0 |
| N | 2 | 2.1 |
| LVEF [%] | 69.58 | 8.18 |
| EAT thickness [mm] | 9.51 | 3.33 |
| EAT volume [mL] | 60.03 | 21.07 |
| EAT thickness index [mm/m2] | 5.23 | 1.83 |
| EAT volume index [mL/m2] | 33.08 | 11.98 |
| Ao diastolic diameter [mm] | 33.42 | 4.36 |
| Ao systolic diameter [mm] | 34.50 | 4.42 |
| Ao stiffness index | 4.18 | 0.81 |
| Ao strain [%] | 3.29 | 2.37 |
| Ao distensibility [cm2/dyn] | 0.12 | 0.09 |
| Ao Stiffness Index ≥ Me (≥4.09, n = 49) | Ao Stiffness Index < Me (<4.09, n = 48) | p | |||
|---|---|---|---|---|---|
| X | SD | X | SD | ||
| EAT thickness [mm] | 10.26 | 3.06 | 8.74 | 3.45 | <0.05 |
| EAT volume [mL] | 67.84 | 19.68 | 52.06 | 19.55 | <0.05 |
| EAT thickness index [mm/m2] | 5.64 | 1.60 | 4.81 | 1.96 | <0.05 |
| EAT volume index [mL/m2] | 37.66 | 11.63 | 28.40 | 10.53 | <0.05 |
| Ao strain ≥ Me (≥2.67%, n = 48) | Ao strain < Me (<2.67%, n = 49) | p | |||
| X | SD | X | SD | ||
| EAT thickness [mm] | 8.89 | 3.47 | 10.12 | 3.11 | ns |
| EAT volume [mL] | 52.10 | 19.58 | 67.81 | 19.69 | <0.05 |
| EAT thickness index [mm/m2] | 4.88 | 1.97 | 5.57 | 1.63 | ns |
| EAT volume index [mL/m2] | 28.41 | 10.54 | 37.64 | 11.63 | <0.05 |
| Ao distensibility ≥ Me (≥0.10 cm2/dyn, n = 49) | Ao distensibility < Me (<0.10 cm2/dyn, n = 48) | p | |||
| X | SD | X | SD | ||
| EAT thickness [mm] | 8.89 | 3.43 | 10.14 | 3.13 | ns |
| EAT volume [mL] | 52.67 | 18.90 | 67.55 | 20.68 | <0.05 |
| EAT thickness index [mm/m2] | 4.89 | 1.95 | 5.58 | 1.64 | ns |
| EAT volume index [mL/m2] | 28.76 | 10.20 | 37.48 | 12.15 | <0.05 |
| EAT Thickness ≥ Me (≥9.40 mm, n = 49) | EAT Thickness < Me (<9.40 mm, n = 48) | p | |||
|---|---|---|---|---|---|
| X | SD | X | SD | ||
| Ao stiffness index | 4.35 | 0.71 | 4.01 | 0.87 | ns |
| Ao strain [%] | 2.64 | 1.84 | 3.96 | 2.66 | <0.05 |
| Ao distensibility [cm2/dyn] | 0.10 | 0.07 | 0.14 | 0.10 | <0.05 |
| EAT volume ≥ Me (≥61.95 mL, n = 49) | EAT volume < Me (<61.95 mL, n = 48) | p | |||
| X | SD | X | SD | ||
| Ao stiffness index | 4.39 | 0.78 | 3.96 | 0.79 | <0.05 |
| Ao strain [%] | 2.61 | 1.82 | 3.98 | 2.67 | <0.05 |
| Ao distensibility [cm2/dyn] | 0.10 | 0.07 | 0.15 | 0.11 | <0.05 |
| EAT thickness index ≥ Me (≥5.08 mm/m2, n = 49) | EAT thickness index < Me (<5.08 mm/m2, n = 48) | p | |||
| X | SD | X | SD | ||
| Ao stiffness index | 4.41 | 0.72 | 3.95 | 0.84 | <0.05 |
| Ao strain [%] | 2.50 | 1.85 | 4.09 | 2.58 | <0.05 |
| Ao distensibility [cm2/dyn] | 0.09 | 0.07 | 0.15 | 0.10 | <0.05 |
| EAT volume index ≥ Me (≥34.33 mL/m2, n = 49) | EAT volume index < Me (<34.33 mL/m2, n = 48) | p | |||
| X | SD | X | SD | ||
| Ao stiffness index | 4.56 | 0.81 | 3.79 | 0.60 | <0.05 |
| Ao strain [%] | 2.27 | 4.33 | 1.78 | 2.46 | ns |
| Ao distensibility [cm2/dyn] | 0.09 | 0.07 | 0.16 | 0.09 | <0.05 |
| Ao Stiffness Index | Ao Strain [%] | Ao Distensibility [cm2/dyn] | |
|---|---|---|---|
| EAT thickness [mm] | 0.21 | −0.32 | −0.30 |
| EAT volume [mL] | 0.51 | −0.49 | −0.48 |
| EAT thickness index [mm/m2] | 0.24 | −0.34 | −0.31 |
| EAT volume index [mL/m2] | 0.55 | −0.51 | −0.49 |
| Prediction Conditions | Sensitivity | Specificity | Accuracy | |
|---|---|---|---|---|
| Predictive Factor | Real State | |||
| EAT thickness ≥ 7.6 mm | Ao stiffness index ≥Me (≥4.09) | 0.479 | 0.837 | 0.660 |
| EAT volume ≥ 75.54 mL | Ao stiffness index ≥Me (≥4.09) | 0.938 | 0.347 | 0.639 |
| EAT thickness index ≥ 4.15 mm/m2 | Ao stiffness index ≥Me (≥4.09) | 0.458 | 0.857 | 0.660 |
| EAT volume index ≥ 33.18 mL/m2 | Ao stiffness index ≥Me (≥4.09) | 0.646 | 0.694 | 0.670 |
| EAT thickness ≥ 7.6 mm | Ao strain <Me (<2.67%) | 0.458 | 0.816 | 0.639 |
| EAT volume ≥ 75.54 mL | Ao strain <Me (<2.67%) | 0.938 | 0.347 | 0.639 |
| EAT thickness index ≥ 4.15 mm/m2 | Ao strain <Me (<2.67%) | 0.438 | 0.837 | 0.639 |
| EAT volume index ≥ 33.18 mL/m2 | Ao strain <Me (<2.67%) | 0.646 | 0.694 | 0.670 |
| EAT thickness ≥ 7.6 mm | Ao distensibility <Me (<0.10 cm2/dyn) | 0.449 | 0.813 | 0.629 |
| EAT volume ≥ 71.51 mL | Ao distensibility <Me (<0.10 cm2/dyn) | 0.857 | 0.438 | 0.649 |
| EAT thickness index ≥ 4.15 mm/m2 | Ao distensibility <Me (<0.10 cm2/dyn) | 0.429 | 0.833 | 0.629 |
| EAT volume index ≥ 33.18 mL/m2 | Ao distensibility <Me (<0.10 cm2/dyn) | 0.633 | 0.633 | 0.660 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gać, P.; Hajdusianek, W.; Żórawik, A.; Macek, P.; Poręba, M.; Poręba, R. Thickness and Volume of Epicardial Adipose Tissue in Relation to Stiffness and Elasticity of Aorta Assessed by Computed Tomography Angiography. Biomedicines 2023, 11, 1617. https://doi.org/10.3390/biomedicines11061617
Gać P, Hajdusianek W, Żórawik A, Macek P, Poręba M, Poręba R. Thickness and Volume of Epicardial Adipose Tissue in Relation to Stiffness and Elasticity of Aorta Assessed by Computed Tomography Angiography. Biomedicines. 2023; 11(6):1617. https://doi.org/10.3390/biomedicines11061617
Chicago/Turabian StyleGać, Paweł, Wojciech Hajdusianek, Aleksandra Żórawik, Piotr Macek, Małgorzata Poręba, and Rafał Poręba. 2023. "Thickness and Volume of Epicardial Adipose Tissue in Relation to Stiffness and Elasticity of Aorta Assessed by Computed Tomography Angiography" Biomedicines 11, no. 6: 1617. https://doi.org/10.3390/biomedicines11061617
APA StyleGać, P., Hajdusianek, W., Żórawik, A., Macek, P., Poręba, M., & Poręba, R. (2023). Thickness and Volume of Epicardial Adipose Tissue in Relation to Stiffness and Elasticity of Aorta Assessed by Computed Tomography Angiography. Biomedicines, 11(6), 1617. https://doi.org/10.3390/biomedicines11061617






