Using the Microbiome as a Regenerative Medicine Strategy for Autoimmune Diseases
Abstract
1. Introduction
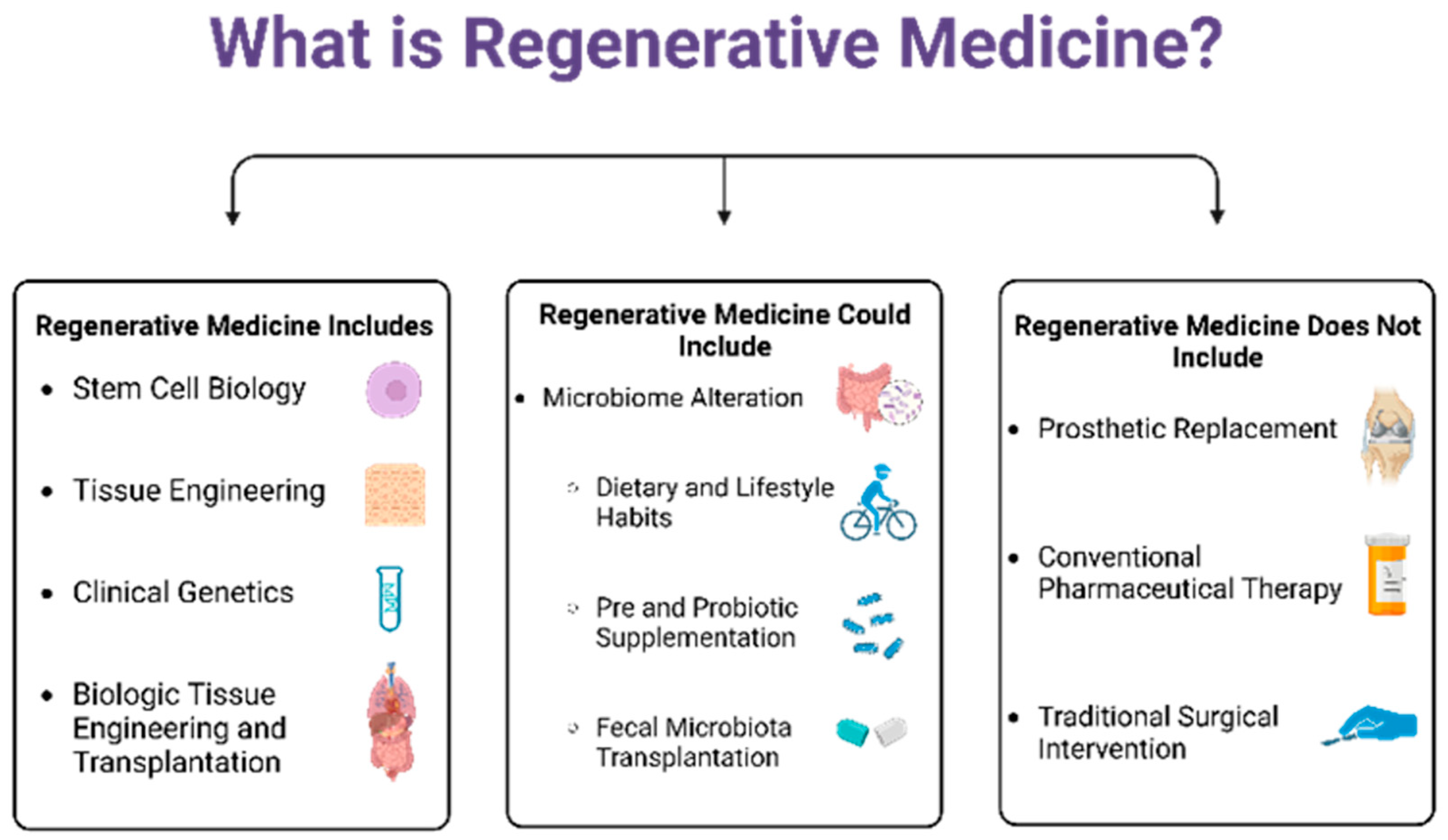
2. Concept of Regenerative Medicine
3. Disease Characteristics
3.1. Psoriatic Arthritis
3.2. Rheumatoid Arthritis
3.3. Inflammatory Bowel Disease
3.4. Systemic Lupus Erythematosus
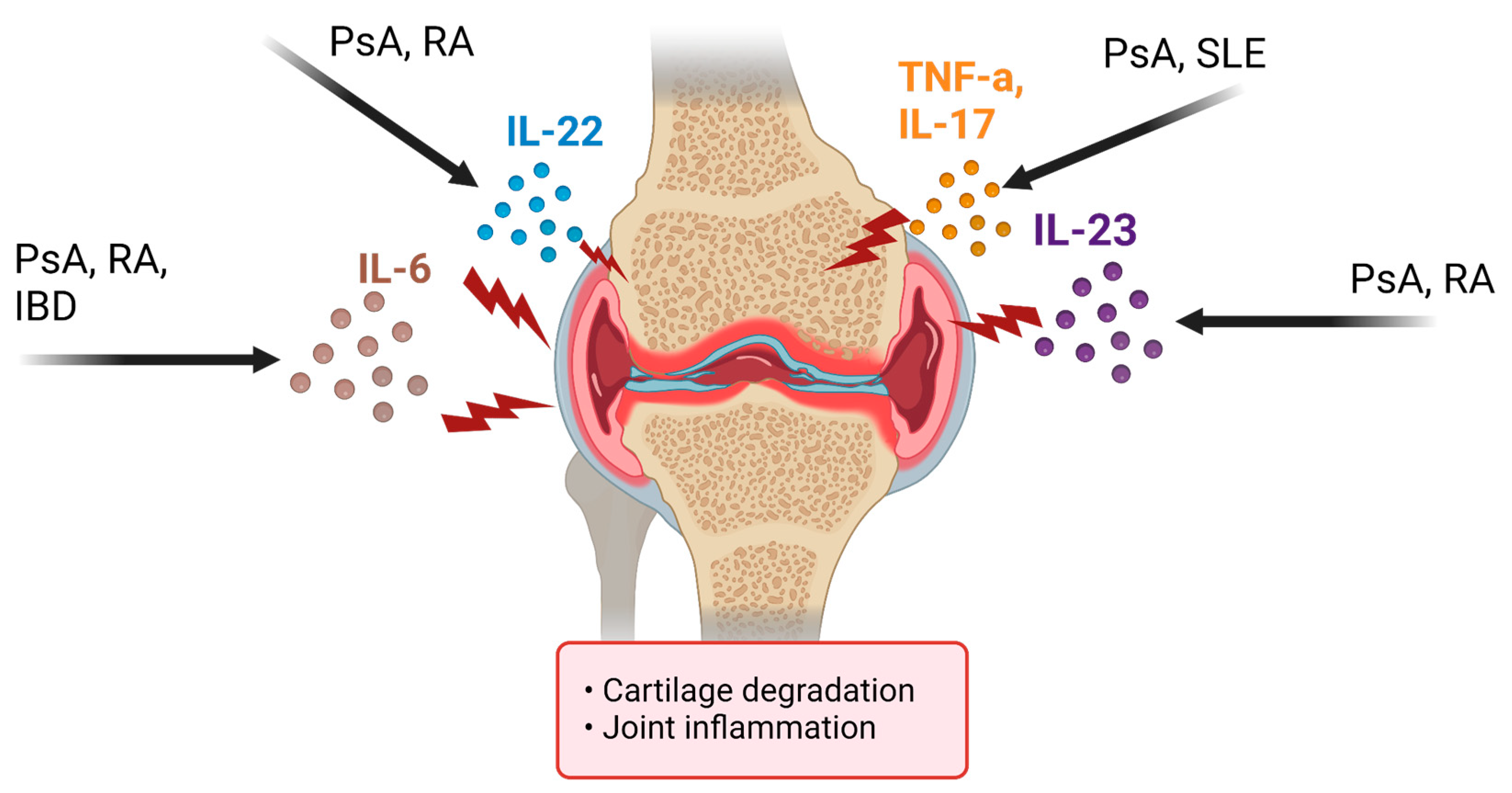
4. Disease Summary
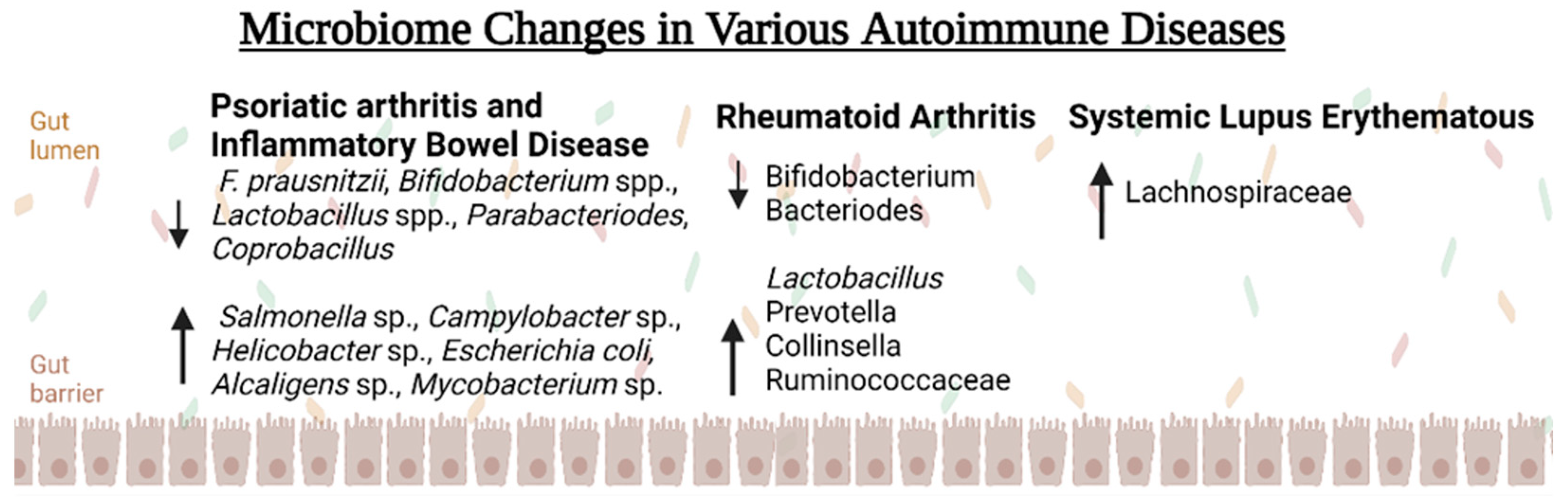
5. Microbiome Alterations Seen with Autoimmune Pathology
5.1. Psoriatic Arthritis
5.2. Inflammatory Bowel Disease
5.3. Rheumatoid Arthritis
5.4. Systemic Lupus Erythematosus
6. Discussion: Using the Microbiome as a Treatment Target
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rose, N.R. Prediction and Prevention of Autoimmune Disease in the 21st Century: A Review and Preview. Am. J. Epidemiol. 2016, 183, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Ostrov, B.E.; Amsterdam, D. Immunomodulatory interplay of the microbiome and therapy of rheumatic diseases. Immunol. Investig. 2017, 46, 769–792. [Google Scholar] [CrossRef] [PubMed]
- Behera, J.; Ison, J.; Tyagi, S.C.; Tyagi, N. The role of gut microbiota in bone homeostasis. Bone 2020, 135, 115317. [Google Scholar] [CrossRef]
- Daar, A.S.; Greenwood, H.L. A proposed definition of regenerative medicine. J. Tissue Eng. Regen. Med. 2007, 1, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Orlando, G.; Murphy, S.V.; Bussolati, B.; Clancy, M.; Cravedi, P.; Migliaccio, G.; Murray, P. Rethinking Regenerative Medicine from a Transplant Perspective (and Vice Versa). Transplantation 2019, 103, 237–249. [Google Scholar] [CrossRef]
- Mao, A.S.; Mooney, D.J. Regenerative medicine: Current therapies and future directions. Proc. Natl. Acad. Sci. USA 2015, 112, 14452–14459. [Google Scholar] [CrossRef]
- Preethy, S.; Ranganathan, N.; Raghavan, K.; Dedeepiya, V.D.; Ikewaki, N.; Abraham, S.J. Integrating the Synergy of the Gut Microbiome into Regenerative Medicine: Relevance to Neurological Disorders. J. Alzheimer’s Dis. 2022, 87, 1451–1460. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Lau, A.W.Y.; Tan, L.T.-H.; Ab Mutalib, N.-S.; Wong, S.H.; Letchumanan, V.; Lee, L.-H. The chemistry of gut microbiome in health and diseases. Prog. Microbes Mol. Biol. 2021, 4, 1780–1791. [Google Scholar] [CrossRef]
- Foo, J.L.; Ling, H.; Lee, Y.S.; Chang, M.W. Microbiome engineering: Current applications and its future. Biotechnol. J. 2017, 12, 1600099. [Google Scholar] [CrossRef]
- Vyas, U.; Ranganathan, N. Probiotics, Prebiotics, and Synbiotics: Gut and Beyond. Gastroenterol. Res. Pr. 2012, 2012, 872716. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Kurtzman, D.J.; Perez-Chada, L.M.; Merola, J.F. Psoriatic arthritis and the dermatologist: An approach to screening and clinical evaluation. Clin. Dermatol. 2018, 36, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Paine, A.; Ritchlin, C. Bone remodeling in psoriasis and psoriatic arthritis. Curr. Opin. Rheumatol. 2016, 28, 66–75. [Google Scholar] [CrossRef]
- Bosch, F.V.D.; Coates, L. Clinical management of psoriatic arthritis. Lancet 2018, 391, 2285–2294. [Google Scholar] [CrossRef] [PubMed]
- Ogdie, A.; Coates, L.C.; Gladman, D.D. Treatment guidelines in psoriatic arthritis. Rheumatology 2020, 59, i37–i46. [Google Scholar] [CrossRef]
- Veale, D.J.; Fearon, U. The pathogenesis of psoriatic arthritis. Lancet 2018, 391, 2273–2284. [Google Scholar] [CrossRef]
- Aletaha, D.; Smolen, J.S. Diagnosis and Management of Rheumatoid Arthritis: A Review. JAMA 2018, 320, 1360–1372. [Google Scholar] [CrossRef]
- Mateen, S.; Zafar, A.; Moin, S.; Khan, A.Q.; Zubair, S. Understanding the role of cytokines in the pathogenesis of rheumatoid arthritis. Clin. Chim. Acta 2016, 455, 161–171. [Google Scholar] [CrossRef]
- Lee, N.; Kim, W.-U. Microbiota in T-cell homeostasis and inflammatory diseases. Exp. Mol. Med. 2017, 49, e340. [Google Scholar] [CrossRef]
- Gupta, V.K.; Cunningham, K.Y.; Hur, B.; Bakshi, U.; Huang, H.; Warrington, K.J.; Taneja, V.; Myasoedova, E.; Davis, J.M.; Sung, J. Gut microbial determinants of clinically important improvement in patients with rheumatoid arthritis. Genome Med. 2021, 13, 149. [Google Scholar] [CrossRef]
- Wu, X.; He, B.; Liu, J.; Feng, H.; Ma, Y.; Li, D.; Guo, B.; Liang, C.; Dang, L.; Wang, L.; et al. Molecular Insight into Gut Microbiota and Rheumatoid Arthritis. Int. J. Mol. Sci. 2016, 17, 431. [Google Scholar] [CrossRef] [PubMed]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O., 3rd; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010, 62, 2569–2581. [Google Scholar] [CrossRef] [PubMed]
- Fraenkel, L.; Bathon, J.M.; England, B.R.; St Clair, E.W.; Arayssi, T.; Carandang, K.; Deane, K.D.; Genovese, M.; Huston, K.K.; Kerr, G.; et al. 2021 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Rheumatol. 2021, 73, 1108–1123. [Google Scholar] [CrossRef] [PubMed]
- Schönenberger, K.A.; Schüpfer, A.-C.; Gloy, V.L.; Hasler, P.; Stanga, Z.; Kaegi-Braun, N.; Reber, E. Effect of Anti-Inflammatory Diets on Pain in Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 4221. [Google Scholar] [CrossRef] [PubMed]
- Flynn, S.; Eisenstein, S. Inflammatory Bowel Disease Presentation and Diagnosis. Surg. Clin. North Am. 2019, 99, 1051–1062. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.; Blumberg, R.S. Inflammatory Bowel Disease. In Harrison’s Principles of Internal Medicine, 21st ed.; Loscalzo, J., Fauci, A., Kasper, D., Hauser, S., Longo, D., Jameson, J., Eds.; McGraw Hill: New York, NY, USA, 2022; Available online: https://accessmedicine-mhmedical-com.proxy.rvu.edu/content.aspx?bookid=3095§ionid=265428110 (accessed on 5 January 2023).
- Nakase, H.; Uchino, M.; Shinzaki, S.; Matsuura, M.; Matsuoka, K.; Kobayashi, T.; Saruta, M.; Hirai, F.; Hata, K.; Hiraoka, S.; et al. Evidence-based clinical practice guidelines for inflammatory bowel disease. J. Gastroenterol. 2021, 56, 489–526. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Qing, P.; Yang, H.; Wu, Y.; Liu, Y.; Luo, Y. Gut Microbiome and Metabolites in Systemic Lupus Erythematosus: Link, Mechanisms and Intervention. Front. Immunol. 2021, 12, 686501. [Google Scholar] [CrossRef] [PubMed]
- Vaillant, J.A.A.; Goyal, A.; Varacallo, M. Systemic Lupus Erythematosus; StatPearls Publishing: Tampa, FL, USA, 2022. Available online: http://www.ncbi.nlm.nih.gov/books/NBK535405/ (accessed on 2 May 2022).
- Thong, B.; Olsen, N.J. Systemic lupus erythematosus diagnosis and management. Rheumatology 2016, 56, i3–i13. [Google Scholar] [CrossRef]
- Scher, J.U.; Ubeda, C.; Artacho, A.; Isaac, S.; Reddy, S.M.; Marmon, S.; Neimann, A.; Brusca, S.; Patel, T.; Manasson, J.; et al. Decreased Bacterial Diversity Characterizes the Altered Gut Microbiota in Patients with Psoriatic Arthritis, Resembling Dysbiosis in Inflammatory Bowel Disease. Arthritis Rheumatol. 2015, 67, 128–139. [Google Scholar] [CrossRef]
- Paine, A.; Ritchlin, C. Altered Bone Remodeling in Psoriatic Disease: New Insights and Future Directions. Calcif. Tissue Int. 2018, 102, 559–574. [Google Scholar] [CrossRef]
- Blauvelt, A.; Chiricozzi, A. The Immunologic Role of IL-17 in Psoriasis and Psoriatic Arthritis Pathogenesis. Clin. Rev. Allergy Immunol. 2018, 55, 379–390. [Google Scholar] [CrossRef]
- Olejniczak-Staruch, I.; Ciążyńska, M.; Sobolewska-Sztychny, D.; Narbutt, J.; Skibińska, M.; Lesiak, A. Alterations of the Skin and Gut Microbiome in Psoriasis and Psoriatic Arthritis. Int. J. Mol. Sci. 2021, 22, 3998. [Google Scholar] [CrossRef] [PubMed]
- Ciccia, F.; Guggino, G.; Ferrante, A.; Raimondo, S.; Bignone, R.; Rodolico, V.; Peralta, S.; Van Tok, M.; Cannizzaro, A.; Schinocca, C.; et al. Interleukin-9 Overexpression and Th9 Polarization Characterize the Inflamed Gut, the Synovial Tissue, and the Peripheral Blood of Patients with Psoriatic Arthritis. Arthritis Rheumatol. 2016, 68, 1922–1931. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Wu, X.; Rocha, C.S.; Rolston, M.; Garcia-Melchor, E.; Huynh, M.; Nguyen, M.; Law, T.; Haas, K.N.; Yamada, D.; et al. Short-Term Western Diet Intake Promotes IL-23-Mediated Skin and Joint Inflammation Accompanied by Changes to the Gut Microbiota in Mice. J. Investig. Dermatol. 2021, 141, 1780–1791. [Google Scholar] [CrossRef] [PubMed]
- Santana, P.T.; Rosas, S.L.B.; Ribeiro, B.E.; Marinho, Y.; de Souza, H.S.P. Dysbiosis in Inflammatory Bowel Disease: Pathogenic Role and Potential Therapeutic Targets. Int. J. Mol. Sci. 2022, 23, 3464. [Google Scholar] [CrossRef]
- Rogler, G.; Singh, A.; Kavanaugh, A.; Rubin, D.T. Extraintestinal Manifestations of Inflammatory Bowel Disease: Current Concepts, Treatment, and Implications for Disease Management. Gastroenterology 2021, 161, 1118–1132. [Google Scholar] [CrossRef]
- Clooney, A.G.; Eckenberger, J.; Laserna-Mendieta, E.; Sexton, K.A.; Bernstein, M.T.; Vagianos, K.; Sargent, M.; Ryan, F.J.; Moran, C.; Sheehan, D.; et al. Ranking microbiome variance in inflammatory bowel disease: A large longitudinal intercontinental study. Gut 2021, 70, 499–510. [Google Scholar] [CrossRef]
- Viladomiu, M.; Kivolowitz, C.; Abdulhamid, A.; Dogan, B.; Victorio, D.; Castellanos, J.G.; Woo, V.; Teng, F.; Tran, N.L.; Sczesnak, A.; et al. IgA-coated E. coli enriched in Crohn’s disease spondyloarthritis promote T H 17-dependent inflammation. Sci. Transl. Med. 2017, 9, eaaf9655. [Google Scholar] [CrossRef]
- Horta-Baas, G.; Romero-Figueroa, M.D.S.; Montiel-Jarquín, A.J.; Pizano-Zárate, M.L.; García-Mena, J.; Ramírez-Durán, N. Intestinal Dysbiosis and Rheumatoid Arthritis: A Link between Gut Microbiota and the Pathogenesis of Rheumatoid Arthritis. J. Immunol. Res. 2017, 2017, 4835189. [Google Scholar] [CrossRef]
- Bennike, T.B.; Ellingsen, T.; Glerup, H.; Bonderup, O.K.; Carlsen, T.G.; Meyer, M.K.; Bøgsted, M.; Christiansen, G.; Birkelund, S.; Andersen, V.; et al. Proteome Analysis of Rheumatoid Arthritis Gut Mucosa. J. Proteome Res. 2016, 16, 346–354. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, S.-X.; Yin, X.-F.; Zhang, M.-X.; Qiao, J.; Xin, X.-H.; Chang, M.-J.; Gao, C.; Li, Y.-F.; Li, X.-F. The Gut Microbiota and Its Relevance to Peripheral Lymphocyte Subpopulations and Cytokines in Patients with Rheumatoid Arthritis. J. Immunol. Res. 2021, 2021, 6665563. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, F. Role of Intestinal Microbiota on Gut Homeostasis and Rheumatoid Arthritis. J. Immunol. Res. 2021, 2021, 8167283. [Google Scholar] [CrossRef] [PubMed]
- Maeda, Y.; Kurakawa, T.; Umemoto, E.; Motooka, D.; Ito, Y.; Gotoh, K.; Hirota, K.; Matsushita, M.; Furuta, Y.; Narazaki, M.; et al. Dysbiosis Contributes to Arthritis Development via Activation of Autoreactive T Cells in the Intestine. Arthritis Rheumatol. 2016, 68, 2646–2661. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Jhun, J.; Lee, S.-Y.; Na, H.S.; Choi, J.; Cho, K.-H.; Lee, S.Y.; Lee, A.R.; Park, S.-J.; You, H.J.; et al. Therapeutic Potential of a Novel Bifidobacterium Identified Through Microbiome Profiling of RA Patients with Different RF Levels. Front. Immunol. 2021, 12, 736196. [Google Scholar] [CrossRef]
- Zhang, H.; Liao, X.; Sparks, J.B.; Luo, X.M. Dynamics of Gut Microbiota in Autoimmune Lupus. Appl. Environ. Microbiol. 2014, 80, 7551–7560. [Google Scholar] [CrossRef]
- Hevia, A.; Milani, C.; López, P.; Cuervo, A.; Arboleya, S.; Duranti, S.; Turroni, F.; González, S.; Suárez, A.; Gueimonde, M.; et al. Intestinal Dysbiosis Associated with Systemic Lupus Erythematosus. mBio 2014, 5, e01548-14. [Google Scholar] [CrossRef]
- Kinashi, Y.; Hase, K. Partners in Leaky Gut Syndrome: Intestinal Dysbiosis and Autoimmunity. Front. Immunol. 2021, 12, 673708. [Google Scholar] [CrossRef]
- Matei, D.E.; Menon, M.; Alber, D.G.; Smith, A.M.; Nedjat-Shokouhi, B.; Fasano, A.; Magill, L.; Duhlin, A.; Bitoun, S.; Gleizes, A.; et al. Intestinal barrier dysfunction plays an integral role in arthritis pathology and can be targeted to ameliorate disease. Med 2021, 2, 864–883.e9. [Google Scholar] [CrossRef]
- Mills, R.H.; Dulai, P.S.; Vázquez-Baeza, Y.; Sauceda, C.; Daniel, N.; Gerner, R.R.; Batachari, L.E.; Malfavon, M.; Zhu, Q.; Weldon, K.; et al. Multi-omics analyses of the ulcerative colitis gut microbiome link Bacteroides vulgatus proteases with disease severity. Nat. Microbiol. 2022, 7, 262–276. [Google Scholar] [CrossRef]
- Mikucka, A.; Deptuła, A.; Bogiel, T.; Chmielarczyk, A.; Nurczyńska, E.; Gospodarek-Komkowska, E. Bacteraemia Caused by Probiotic Strains of Lacticaseibacillus rhamnosus—Case Studies Highlighting the Need for Careful Thought before Using Microbes for Health Benefits. Pathogens 2022, 11, 977. [Google Scholar] [CrossRef]
- Doron, S.; Snydman, D.R. Risk and Safety of Probiotics. Clin. Infect. Dis. 2015, 60, S129–S134. [Google Scholar] [CrossRef] [PubMed]
- Boyle, R.J.; Robins-Browne, R.M.; Tang, M.L. Probiotic use in clinical practice: What are the risks? Am. J. Clin. Nutr. 2006, 83, 1256–1264. [Google Scholar] [CrossRef] [PubMed]
- Besselink, M.G.; Van Santvoort, H.C.; Buskens, E.; Boermeester, M.A.; Van Goor, H.; Timmerman, H.M.; Nieuwenhuijs, V.B.; Bollen, T.L.; van Ramshorst, B.; Witterman, B.J.; et al. Probiotic prophylaxis in predicted severe acute pancreatitis: A randomised, double-blind, placebo-controlled trial. Lancet 2008, 371, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Weersma, R.K.; Zhernakova, A.; Fu, J. Interaction between drugs and the gut microbiome. Gut 2020, 69, 1510–1519. [Google Scholar] [CrossRef] [PubMed]
- Nayak, R.R.; Alexander, M.; Deshpande, I.; Stapleton-Gray, K.; Rimal, B.; Patterson, A.D.; Ubeda, C.; Scher, J.U.; Turnbaugh, P.J. Methotrexate impacts conserved pathways in diverse human gut bacteria leading to decreased host immune activation. Cell Host Microbe 2021, 29, 362–377.e11. [Google Scholar] [CrossRef] [PubMed]
- Sorbara, M.T.; Pamer, E.G. Microbiome-based therapeutics. Nat. Rev. Genet. 2022, 20, 365–380. [Google Scholar] [CrossRef]
- Nigam, M.; Panwar, A.S.; Singh, R.K. Orchestrating the fecal microbiota transplantation: Current technological advancements and potential biomedical application. Front. Med. Technol. 2022, 4, 961569. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Williams, K.L.; Enslow, R.; Suresh, S.; Beaton, C.; Hodge, M.; Brooks, A.E. Using the Microbiome as a Regenerative Medicine Strategy for Autoimmune Diseases. Biomedicines 2023, 11, 1582. https://doi.org/10.3390/biomedicines11061582
Williams KL, Enslow R, Suresh S, Beaton C, Hodge M, Brooks AE. Using the Microbiome as a Regenerative Medicine Strategy for Autoimmune Diseases. Biomedicines. 2023; 11(6):1582. https://doi.org/10.3390/biomedicines11061582
Chicago/Turabian StyleWilliams, Kaitlin L., Ryan Enslow, Shreyas Suresh, Camille Beaton, Mitchell Hodge, and Amanda E. Brooks. 2023. "Using the Microbiome as a Regenerative Medicine Strategy for Autoimmune Diseases" Biomedicines 11, no. 6: 1582. https://doi.org/10.3390/biomedicines11061582
APA StyleWilliams, K. L., Enslow, R., Suresh, S., Beaton, C., Hodge, M., & Brooks, A. E. (2023). Using the Microbiome as a Regenerative Medicine Strategy for Autoimmune Diseases. Biomedicines, 11(6), 1582. https://doi.org/10.3390/biomedicines11061582







