Abstract
Germanium is an essential microelement, and its deficiency can result in numerous diseases, particularly oncogenic conditions. Consequently, water-soluble germanium compounds, including inorganic and coordination compounds, have attracted significant attention due to their biological activity. The review analyzes the primary research from the last decade related to the anticancer activity of germanium compounds. Furthermore, the review clarifies their actual toxicity, identifies errors and misconceptions that have contributed to the discrediting of their biological activity, and briefly suggests a putative mechanism of germanium-mediated protection from oxidative stress. Finally, the review provides clarifications on the discovery history of water-soluble organic germanium compounds, which was distorted and suppressed for a long time.
1. Introduction
At present, germanium is widely recognized as a vital trace element, which is particularly essential for the normal functioning of the immune system and plays a significant role in cancer prevention [1,2,3,4,5,6,7]. Germanium is ubiquitously present in mammalian organs and tissues, with the highest concentration in the thymus. Germanium normalizes many physiological functions, particularly blood characteristics including pH, glucose, minerals, cholesterol, uric acid, hemoglobin and leukocytes [8,9]. Conversely, germanium deficiency can result in numerous diseases, primarily oncogenic conditions [10]. Research has revealed that cancer patients exhibit anomalously low concentrations of germanium in their blood serum [7,11,12]. Additionally, germanium levels in cancerous tissues are significantly lower than those in adjacent healthy tissues [13].
Germanium is primarily introduced into the body through the consumption of vegetable-based foods with an average daily human dose of only 0.4–1.5 mg [14,15]. Research on the determination of this element in plant raw materials unexpectedly revealed an elevated content in plants and mushrooms that are traditionally used in ethnoscience, particularly in China [7,16,17,18]. Germanium compounds in natural sources have long been considered a therapeutic agent with anticancer, antitumor, antiviral and anti-inflammatory effects [19]. Thus, the highest germanium concentrations are contained in ginseng, saprophyte mushrooms, particularly lacquered polypore (Ganoderma lucidum) and chaga, as well as in garlic, aloe and echinacea [20,21,22,23,24,25]. Among these, ginseng and Ganoderma lucidum are widely used in complex therapies of oncological diseases [26,27,28,29,30]. Germanium compounds have been shown to normalize the oxygen respiration (i.e., oxidative phosphorylation) in cells, which can retard the growth of tumors [26,31,32,33]. Restoring cell oxygen respiration is key to treating Warburg-like cancers [33]. The stimulating effect of germanium on oxidizing enzymes such as aldehyde reductase [34] has also been established. Hence, germanium-containing drugs have long attracted the attention of researchers and medical practitioners.
The antitumor activity of inorganic germanium compounds was first detected in 1928 [35]. However, the field only began to intensively develop in the 1970s, when the first water-soluble organic germanium compounds were synthesized, gaining attention due to their wide range of biological activities. This topic has been addressed in several reviews [1,5,6,8,24,36,37,38,39,40,41,42,43,44], as well as a monograph [7].
This review specifically focuses on research conducted within the past decade, during which inorganic and coordination compounds of germanium have been incorporated into medical practices alongside water-soluble organic germanium compounds [3,45,46]. Moreover, the toxicity of germanium compounds has been the subject of much controversy and confusion, and the discovery history of stable water-soluble germanium compounds has been significantly distorted. Therefore, the initial focus of this review is to elucidate the tangle of errors, inaccuracies, and myths associated with germanium. At the end of this review, the authors propose a putative mechanism for germanium-mediated cancer treatment and prevention based on the unique chemical properties of germanium.
2. Historical Digression and Toxicity of Germanium Compounds
The chemical element number 32 was predicted by D.I. Mendeleev in 1871, and later, in 1886, was discovered by C. Winkler, who named it after his homeland Germany (Figure 1).

Figure 1.
D.I. Mendeleev (left) and C. Winkler (right).
Germanium has had a tumultuous history since its discovery over half a century ago. Initially, it remained an inaccessible chemical element that did not garner much scientific attention. It was not until 1948, when the first semiconductor transistors and diodes were created using germanium, that it gained significance in the field of microelectronics. However, the use of this element as a semiconductor was soon replaced by silicon and it was again forgotten. In the 1970s, the biological activity of the discovered stable organic germanium water-soluble compounds [36] attracted the attention of scientists, among which bis(carboxyethylgermanium) sesquioxide (Ge-132) was most famous. However, in the late 1980s, interest in such compounds declined sharply as a result of an ongoing discussion about the allegedly anomalously high toxicity of organic germanium compounds (similar to organic mercury compounds). Unfortunately, the interest in such compounds declined sharply in the late 1980s due to a typo in an article published in 1987 in an inaccessible journal, which listed erroneous toxicity values for Ge-132 [6,32,33,47]. This mistake was not immediately noticed and led to erroneous criticism in subsequent publications issued in highly influential scientific journals. The correction was only published in 1988; however, until recently, many authors quoted only secondary sources that cited the erroneous data about the high toxicity of organic germanium compounds. The situation was further aggravated by a barbaric experiment conducted in Japan to determine the lethal dose of Ge-132 for humans. The experiment involved the consumption of an astronomical dose of 328 g of germanium, which is not used in medical practice [32,48,49,50]. The result of this experiment showed that the toxicity of Ge-132 was due to the formation and precipitation of solid germanium dioxide (GeO2) in the renal pelvis [48,49,50]. The therapeutic doses of organic germanium derivatives are thousands of times less than this lethal dose. The situation was further exacerbated by cases of germanium poisoning in individuals suffering from severe diseases, who took Ge-132 for a long time in huge excess of the recommended daily dose values without the recommendation of a doctor. These individuals consumed Ge-132 in total quantities from 15 to 300 g over a period of up to three years or more (see review [50]).
It is evident that in the instances mentioned, high doses of Ge-132 resulted in toxic effects due to its hydrolysis in the body to form solid GeO2 [15]. However, it is now known that such poisoning, even with extremely high doses of germanium, can be successfully treated with combined blood-purification therapy [51]. The occurrence of these tragic events led to various controversial political decisions concerning organic germanium. Specifically, Ge-132 was banned in several countries, despite being universally allowed as a dietary supplement as early as the 1980s. This resulted in the long-term neglect of research on the biological activity of Ge-132, particularly its anticancer properties. Ultimately, this denial of the role of germanium in wildlife was based on erroneous toxicity data, published in influential journals. The combination of typographical errors and reliance on secondary sources of information led to the neglect of the potential clinical use of compounds of this unique microelement. These events have also delayed the study of biological activity of germanium compounds, as noted in reviews [6,47]. To date, many influential journals continue to reject work related to the physiological activity of germanium compounds. It is now time to rectify this situation and restore justice by rehabilitating germanium and its biochemical role.
As of now, low toxicity Ge-132 has been established [40,52,53,54]. In fact, the toxicity of organic germanium compounds [55,56,57,58,59,60] is lower than that of table salt and inorganic germanium dioxide, for which the oral toxicity for mice (LD50) is 5400 mg/kg [55]. For example, for the best-known organic germanium sesquioxide Ge-132 oral toxicity for mice is LD50 > 6300 mg/kg, oral for rats is >10,000 mg/kg and intravenous toxicity for rats is >1000 mg/kg [58]. Germatranol, another common germanium derivative, is also of low toxicity: oral toxicity (LD50) is 8400 mg/kg for mice; intravenous toxicity is 300 mg/kg [57]. Thus, both inorganic and organic compounds of germanium are perfectly safe in those doses in which they are usually used. It should be noted that all known chemical databases, such as PubChem, currently have correct toxicity values for these compounds.
Inorganic derivatives of germanium have also been involved in a number of incidents. Dietary supplements and elixirs containing cheap both inorganic GeO2 and Ge (IV) coordination complexes (particularly germanium citrate and citrate-lactate) have been widely sold in Japan since the early 1970s. They were advertised primarily for cancer treatment [51], wherein the recommended daily dose of 50–100 mg was completely safe. However, a number of precedents of poisoning by such germanium compounds in persons who took such elixirs for a long time have been described. In all cases, the daily dose of germanium was arbitrarily exceeded by tens and even hundreds of times (up to 5 g GeO2 per day) for a long time (up to 18–24 months or more) [48,49,61,62]. As a result, the total dose of germanium in these people was between 100 and 500 g! Some of the more common symptoms of inorganic germanium poisoning include weight loss, fatigue, gastrointestinal disorders, anemia, muscle weakness and, in all cases, kidney failure [48,49,50,61,62]. Moreover, several serious fatal cases were described (see also review [50]). Because of such cases, these elixirs were banned in many countries [60]. However, in each of the above-mentioned cases of poisoning with germanium, it is necessary to understand fully and assess not only the harm from poisoning, but also the possible benefits. Patients in the last stages of cancer took these drugs (both in the form of Ge-132 and in the form of GeO2 and other derivative compounds) in such huge doses independently at their own risk. When taking germanium medication, even in such toxic doses, oncological sufferers, who usually live no more than 3–6 months after diagnosis, have lived 1.5–3 years or more [50,63]. Moreover, during this time, they lived a full life, contrary to the application of classical chemotherapy.
Most of these poisoning cases occurred more than 25 years ago. However, they worsened the already bad reputation of the germanium compounds. In natural compounds, germanium forms very weak chemical bonds with organic molecules, primarily with oxygen atoms. At present, there are no methods to isolate, separate and purify such substances, so the natural germanium compounds and/or its complexes have not yet been isolated and characterized. At present, scientists have drawn attention to the water-soluble synthetic germanium derivatives that make them bioavailable and enable them to be used in safe doses.
The development of water-soluble organic derivatives of germanium (i.e., containing at least one Ge-C bond) is inextricably connected with the N.D. Zelinsky Institute of Organic Chemistry of the Russian Academy of Sciences (ZIOC RAS) and its scientists. Althouge germanium sesquioxides were known long ago, they were insoluble in water. The first water-soluble derivatives were discovered in 1965 by Prof. S.P. Kolesnikov [64,65,66], at that time a graduate student in the laboratory of Prof. O.M. Nefedov [67]. These water-soluble compounds were produced by the hydrolysis of HGeCl3 adducts with cyclohexanone or methyl methacrylate. Later, in 1967 Prof. V.F. Mironov, a former employee of the same laboratory, similarly synthesized another stable water-soluble germanium sesquioxide-bis(carboxyethylgermanium) sesquioxide (Ge-132, CEGS), which is now the best known [68,69].
(O1.5GeCH2CH2COOH)n
In the 1960s, the synthesis of such compounds seemed simple only on paper and, in reality, required highly qualified chemists and specialized equipment, which was available only in a few laboratories in the USSR and the USA. However, there is often a misconception in the literature that K. Asai, a well-known popularizer and author of several books about germanium, was the first to synthesize Ge-132. In 1967, at the international scientific conference, K. Asai learned about the discovery of water-soluble germanium compounds from Soviet scientists, who later gave him samples for testing. K. Asai was the first to foresee the pharmaceutical potential of the Ge-132 [24]. The history of Ge-132 is now well-known (see e.g., [6,7,24,70,71]). It was Ge-132 that led to the active study of biological activity of germanium compounds and their application in medical practice, especially in complex cancer therapy [7,19,31,36,72]. There are clinically proven cases of the successful use of these compounds in cancer treatment; for example, the complete remission of lung cancer was achieved when taking Ge-132 [73]. The spectrum of the biological activity of Ge-132 turned out to be very extensive, with the most pronounced being antitumor activity [40,52,53,54].
Microbiological methods are another direction for the synthesis of organic germanium compounds. Thus, the yeast fermentation method produces Bio-Germanium, a medicine that acts as an effective immunostimulant, increasing the cytotoxicity of NK cells and activating immunoglobulin, B-cells and the tumor necrosis factor [19]. However, such drugs will remain outside the scope of this review.
The surge in the number of publications (Figure 2) addressing the biological activity of germanium compounds until the beginning of this century was accompanied by a number of indeterminate publications containing erroneous toxicity values and reported cases of ultra-high-dose poisoning, among other issues. In the last two decades, similar peaks in publication activity were observed following the identification of novel categories of germanium compounds or the disclosure of alternative types of activity. The average number of publications has steadily risen by nearly fourfold over a span of 50 years. Thus, the discovery of novel classes of stable water-soluble germanium compounds is of significant importance.
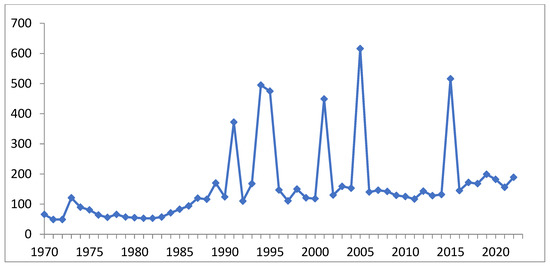
Figure 2.
Number (per year) of publications on physiological activity of organogermanium compounds (including Ge-132) (compiled by authors of the review based on Google Academy data).
3. Organic Germanium Compounds
3.1. Germanium Sesquioxides
The most-studied organic germanium compound is bis(carboxy ethylgermanium) sesquioxide (Ge-132). Its synthesis is carried out by the addition of trichlorogermane (HgeCl3) to acrylic acid to produce 3-(trichlorogermyl)propanoic acid, followed by the hydrolysis thereof. In this reaction, the trichlorogermyl group Cl3Ge regiospecifically adds to the terminal carbon atom of the vinyl group of acrylic acids (Figure 3) [64,68,69].
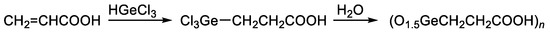
Figure 3.
Synthesis of Ge-132.
Since the first synthesis of this compound was reported 55 years ago [68] the process of producing the original trichlorogermane has gone from a technically complex synthesis from elemental germanium [74] to the development of a simple and convenient method using germanium dioxide (GeO2), HCl and H3PO2 [75]. As a result, Ge-132 and other germanium sesquioxides are now readily available.
Regarding concerns over the alleged high toxicity of Ge-132 (see Section 2), its toxicity [56,57,58] and possible carcinogenicity [76,77] have been studied many times over the past 10 years. The results of the research once again confirmed the complete safety of Ge-132. The comprehensive study of the various biological activities of Ge-132 [8], including those that were previously known [36], particularly its antitumor [78,79,80,81,82,83,84] and immunomodulating [43,52,54,85] activities, also continued. In addition, Ge-132 is proposed as a treatment for a number of diseases: haemorrhagic and ischemic stroke [86,87], viral infections [43,54,88,89,90] (including COVID-19 [43]), various inflammatory diseases, particularly mastitis [4]. Next, this is suggested in the treatment of diabetes mellitus to reduce insulin resistance [91], and as an antioxidant for various disorders caused by oxidative stress [53,54,92,93,94] as well as in dermatological practice to heal skin wounds and protect the skin from reactive oxygen species [95,96]. Finally, Ge-132, together with hydroxyapatite, is proposed for the recovery and regeneration of mineralized tissues, particularly bone marrow [97]. The biological activity of Ge-132 is described in detail in the recently published monograph [71].
Structural studies of bis(carboxy ethylgermanium) sesquioxide have shown that, in solid form, it can exist in several polymeric forms (repagermanium RGe, propagermanium PGe and linear polymer GeSP) (Figure 4) [88]. The structure of the polymer affects the rapidity and completeness of its solubility in water and, as a consequence, its biological activity and dosage. When dissolved in water, it turns into a hydrated form-3-(trihydroxygermil)propanoic acid (THGPA). PGe possesses the best water solubility.
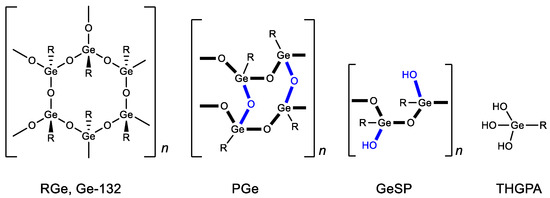
Figure 4.
Forms of Ge-132. R = CH2CH2COOH.
As a result, there has recently been increased interest in Ge-132 in its most soluble form, PGe. For example, it is currently used in Japan to treat virus-hepatitis B [88]. Another direction in the study of Ge-132 biological activity is associated with the direct use of its hydrated form, THGPA. Thus, THGPA is shown to inhibit melanoma cell proliferation through phagocytosis [98]. Furthermore, it was revealed to have analgesic [99] and anti-inflammatory effects [100].
THGPA contains three hydroxy groups in its molecule, which can react with OH- groups of vital molecules. Such interactions may explain a number of physiological effects of Ge-132. Thus, to assess the possible mechanisms of this physiological activity, the interaction of THGPA with biologically active compounds such as adrenaline and ATP, which have vicinal diol functional groups, has been studied in detail. The interaction with these diols explains the numerous physiological functions of Ge-132 at low toxicities [52,100]. It was later found that, in solution, THGPA can form complexes with nucleotides or nucleosides containing cis-diol fragments [101]. At the same time, the ability of THGPA to form complexes with nucleotides depended on the number of phosphate groups present at the ribose residue. Interestingly, THGPA inhibits the enzymatic activity of adenosine deaminase (ADA) when using adenosine as a substrate [101].
Given the presence of several reaction centers in the Ge-132 molecule, chemical modification has been explored to increase biological activity and broaden its scope of application. Several Ge-132 derivatives have been synthesized, including those substituted on the carboxylic group, 3-alkylsubstituted, and those with substitutes on the germanium atom.
It was previously shown that the introduction of aromatic and heteroaromatic substituents (quinolin, anthraquinone and naphthalene) as an ester group in Ge-132 increased their antitumor activity compared to Ge-132 itself [24,36]. At the same time, the introduction of an alkyl replacement in propionic acid position 2 (R1 = Alk) significantly reduced antitumor activity (Figure 5) [24,36].
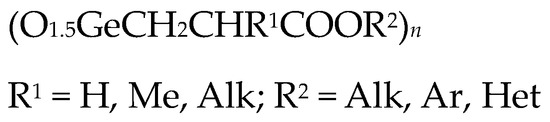
Figure 5.
Derivatives of Ge-132.
Later esters with naphthalene and phenanthrene fragments, as well as N-arylamides with anthraquinone and dibenzofuran fragments, were synthesized (Figure 6) [102,103]. The resulting compounds had a stronger cytotoxic activity than Ge-132. The derivatives of methacrylic acid (R1 = Me) were therefore less active than similar derivatives of acrylic acid (R1 = H) [102,103]. These studies demonstrate possible means of Ge-132 modification to enhance its biological activity.
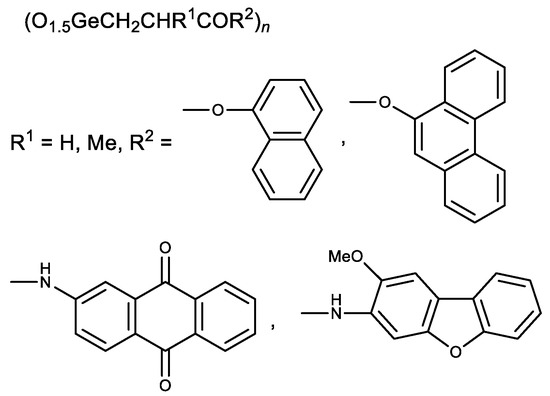
Figure 6.
Aromatic derivatives of Ge-132.
In parallel with the derivatives of Ge-132, a germanium sesquioxide with resveratrol was synthesized (Figure 7) [104]. The antioxidant activity of the resulting compound was higher than that of Ge-132 and resveratrol separately, i.e., a synergistic effect was observed.
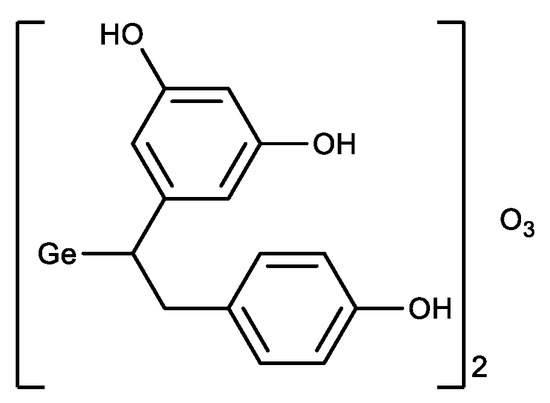
Figure 7.
Germanium complex with resveratrol.
3.2. Germatranes, Germocanes
Germatranes (1) are another interesting class of biologically active germanium compounds, which are cyclic molecules stabilized by the hypervalent germanium atom (Figure 8) [105,106,107,108,109,110].
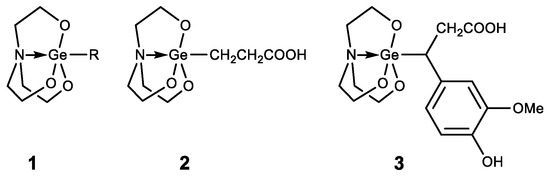
Figure 8.
Germatranes.
Several compounds were identified as having high biological activity, including a peculiar hybrid of Ge-132 and germatrane-3-germatranyl substituted propionic acid (2) and its derivatives, which showed strong activity against various tumors [111,112,113]. Based on caffeic acid 3-germatranyl-3-(4-hydroxy-3-methoxyphenyl) propionic acid (3) was synthesized, which showed strong activity against cervical tumor U14 (in vitro and in vivo). This had inhibitory activity against cervical cancer cell line U14 with an IC50 as high as 48.57 mg/L (117.32 µM), whereas the degree of inhibition of the tumor growth is 64% in the animal experiment [114]. 2-aminoethoxy-substituted germatrane (1, R = OCH2CH2NH2) inhibits the activity of mononuclear alkaline phospholipase A2, and may serve for the development of new antisclerotic drugs to prevent lipid metabolism disorders [115]. In addition, this compound has a beneficial effect on the bioenergetic characteristics of mitochondria, increasing the efficiency of oxidative phosphorylation and increasing the oxidation rate of NAD-dependent substrates by mitochondria [116,117,118]. Germatranol (1, R = OH) reveals a similar activity; it also acts as an antioxidant and reduces the content of reactive oxygen species (ROS) in plant cells [119]. Germatranol contains a hydroxy group, which (like the hydrated form of Ge-132) can interact with functional groups in vital molecules. Thus, germatranol-hydrate interacts with simple amino acids (glycine, L-alanine, β-alanine, and L-valine), resulting in corresponding aminocarboxygermanates [120].
In addition to germatranes, their bicyclic analogues—germocanes (quasigermatranes, 4) and monocyclic analogues-hypogermatranes (5) have been synthesized, and their biological activity was found to be similar to that of germatranes (Figure 9) [108,121,122,123,124,125,126].
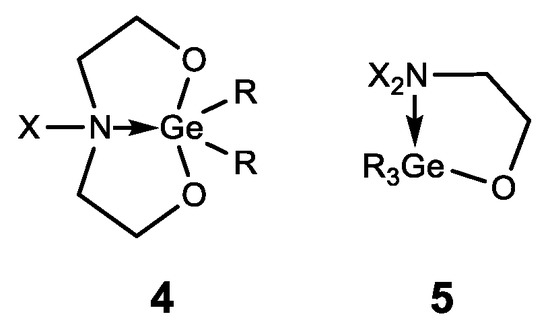
Figure 9.
Germocanes and hypogermatranes.
The hypogermatranes 6 [127] and 7 [128] obtained in this way are molecules in which the ligands are coordinated to the germanium atom (Figure 10). These compounds exhibit antimicrobial activity against various strains of fungi and bacteria. Their pesticide activity against Corcyra Cephalonica is also established.
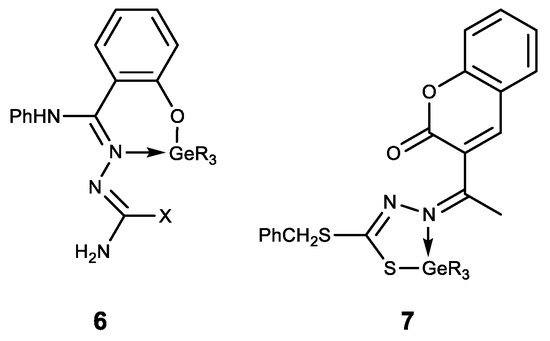
Figure 10.
Hypogermatranes. R = Me, Ph; X = OH, SH.
Hypogermatranes 8, in which the ligands are coordinated with Ge (IV) via azomethine nitrogen atom and sulfur thiol/enol oxygen atom, are also known (Figure 11) [129,130]. These compounds have strong fungicidal and bactericidal properties. Furthermore, they are antioxidants and DNA splitters, whereas the compounds 8b showed strong antifertile activity [130].
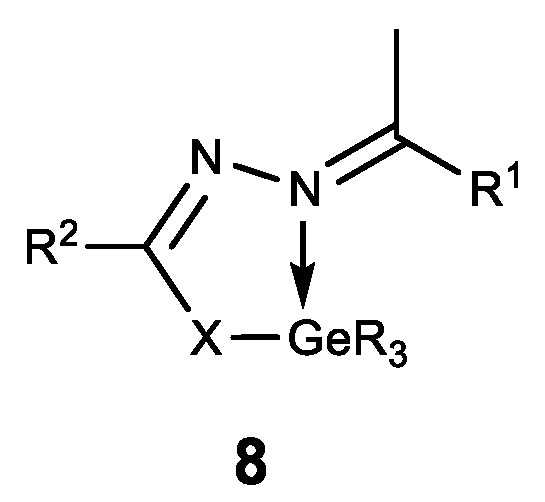
Figure 11.
Hypogermatranes. R = Me, Ph; 8a: R1 = ferrocenyl, R2 = NH2; X = O, S; 8b: R1 = furan-2-yl, pyridine-2-yl, R2 = Py; X = O.
Finally, the first stable water-soluble germylene (a compound of divalent germanium) 9 with dipyrromethane ligand was described and its biological activity was studied (Figure 12) [131]. Compound 9 has been shown to have a comparable antiproliferative effect to cisplatin. These results form the basis for further biological research using germylenes, which are highly active compounds of low-valence germanium.
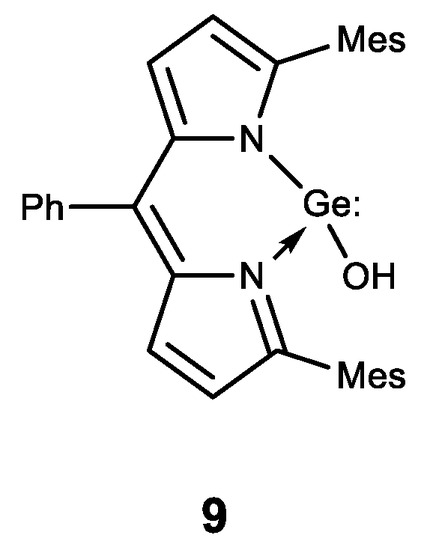
Figure 12.
Stable water-soluble germylene.
3.3. Other Germanium Compounds
Among the compounds of other classes, germanium was introduced to compounds with known physiological activity. The obtained compounds had a synergistic effect. One of these compounds is ascorbic acid, where germanium was introduced as a substituent. Thus, an amide of trimethylgermylpropionic acid 10 was synthesized (Figure 13). It possesses high antioxidant properties and is proposed for the treatment of atopic dermatitis [132,133]. Similarly, a stable lipophilic ascorbic acid 11 derivative with high antioxidant activity was obtained (Figure 13) [92].
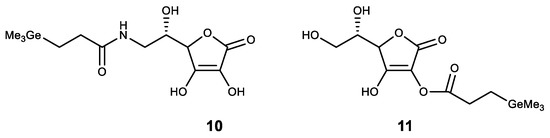
Figure 13.
Germanium complexes with ascorbic acid.
A natural flavone crysine with a wide range of biological activity was also modified in this way. The resulting germanium complex with crysine (12) exhibits a synergistic effect as an antioxidant (Figure 14) [134].
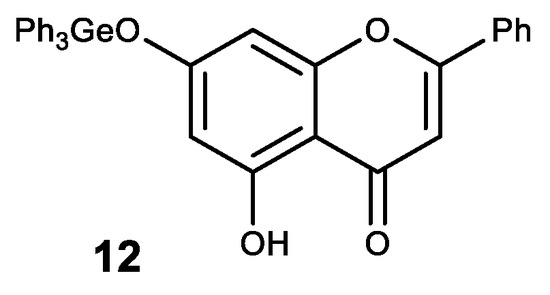
Figure 14.
Germanium complex with crysine.
Complex 12 also showed high antitcancer activity. Thus, it has a significant inhibitory effect on the proliferation and growth of human cancer cell lines MCF-7, HepG2 and Colo205 with high selectivity between cancerous and normal cells [135,136]. An inhibitory effect on the proliferation of these cell lines is thought to occur through the induction of apoptosis via the ROS-dependent mitochondrial pathway [135,136].
Germanium was also introduced into dihydroartemisinin (DHA) as an analogue of Ge-132 (product of GeHCl3 addition to crotonic acid) (Figure 15) [137]. The resulting DHA-Ge complex 13 displays a synergistic effect of DHA and Ge-132, i.e., effectively inhibits the proliferation of HepG2 cells and can induce their apoptosis. Complex 13 is regarded as a promising antitumor agent [137].
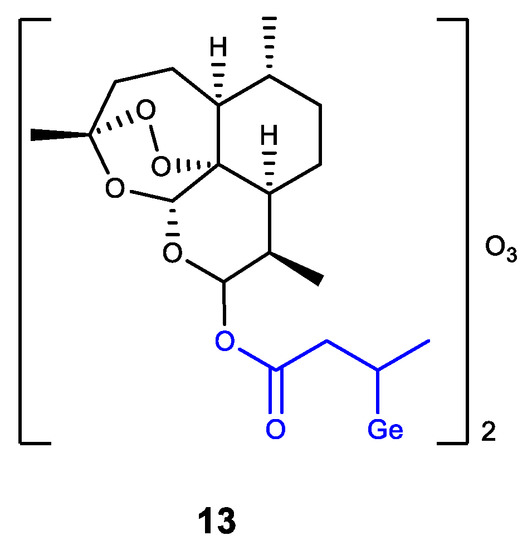
Figure 15.
Germanium complex with dihydroartemisinin.
Steroids are another class of physiologically active compounds in which germanium was introduced as a substituent to position 16 [138,139,140]. The predicted biological activity of these and a number of other similar compounds was calculated by QSAR [141]. Antitumor, antiseborrheic and dermatological activities are the most characteristic predicted biological properties for these steroids.
Apart from the modification of natural compounds, GeR3 moeity is introduced to various heterocyclic derivatives. Thus, a number of germylsubstituted hetarylbenzimidazoles (14) was synthesized, and showed high cytotoxicity on the cell lines MG-22A, HT-1080 and NIH 3T3 (Figure 16) [142]. A similar series of germylsubstituted pyrane-3-carbonitriles (15) also showed high cytotoxicity and the inhibition of matrix metalloproteinase (Figure 16) [143]. The introduction of a germyl substituent in the heterocyclic position 5 (in furan or thiophene) was demonstrated to contribute to the emergence of cytotoxicity.
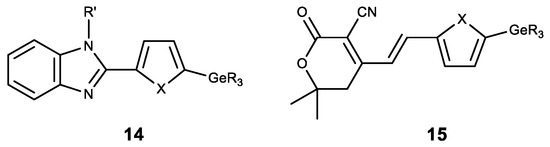
Figure 16.
Germanium complexes with heterocycles. R = Me, Et; R’ = H, Me, Alk, CH2C≡CH; X = O, S.
4. Inorganic and Coordination Germanium Compounds
The inorganic and coordination germanium compounds are now well-established in medical practice (see reviews [3,144,145] and monograph [46]). The structure of such compounds is discussed in detail in the review [146]. Problems with the use of GeO2 in medical practice in the 1980s were related to its low solubility, which required a substantial increase in the dose. It was recently shown to be possible to synthesize highly soluble forms of GeO2 [147]. This opens up new avenues for its use, including in medicine. Among the coordination germanium compounds, the most studied are germanium (IV) citrate and germanium (IV) citrate-lactate, which, like GeO2, are of low toxicity but exhibit nephrotoxicity in high doses [6,47,58]. These compounds activate the immune system and are recommended for the treatment of a wide range of diseases, primarily oncological [3,43,46,144,145,148].
There are also known complexes of germanium (IV) with acetylacetone ligand [Ge(acac)3)]+ with different anions (16) (Figure 17) [149]. The obtained complexes exhibit high activity against different cancer cell lines, with high selectivity in cancer cells compard to normal epithelial cells. Furthermore, the compounds induce significant apoptosis [149].
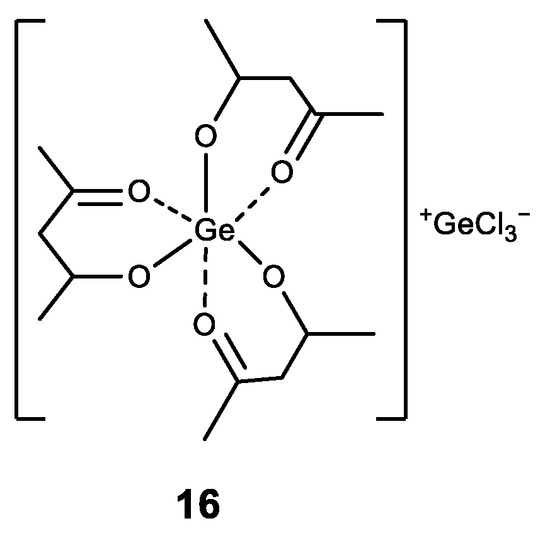
Figure 17.
Germanium complex with acetylacetone.
A number of Ge (IV) complexes with natural polyphenols were synthesized and shown to be promising pharmacologically active substances for cancer treatment. The quercetin–germanium complex (17) (Figure 18) showed high cytotoxicity against four tumor cell lines (PC-3, Hela, EC9706 and SPC-A-1) [150,151].
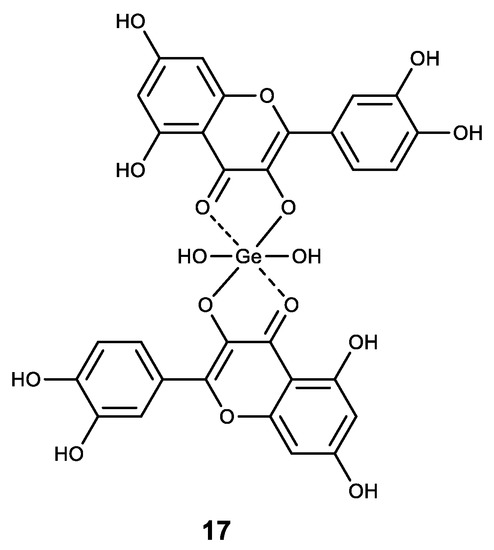
Figure 18.
Germanium complex with quercetin.
Among the other polyphenolic compounds that were used in the synthesis of complexes with Ge (IV), we noted a natural coumarin daphnetin (18) and glucosylxanthone mangiferin (19) (Figure 19) [152]. The resulting Ge (IV) complexes made with the above compounds exhibit high antioxidant activity and demonstrate a strong intercalating ability in calf thymus DNA molecules. In addition, these two complexes have a strong inhibitory proliferative effect on cancer cells HepG2 [152].
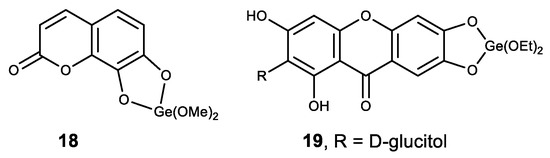
Figure 19.
Germanium polyphenolic complexes.
Last but not least, the germanium (IV) complex with hesperidin, a flavanon glycoside, was synthesized; however the structure was not established [153]. This complex showed high activity in hepatocellular carcinoma of rats.
5. A Possible Mechanism of Anticancer Action of Germanium Compounds
A century ago, the Nobel Prize winner Otto Warburg observed that tumors produce excess lactate in the presence of oxygen. He proposed that the cancer’s origin lies in the replacement of oxidation phosphorylation by glucose fermentation, which he interpreted as mitochondrial dysfunction [154,155,156,157,158]. This phenomenon was called aerobic glycolysis or the “Warburg effect”. Later, the concept of mitochondrial oxidative stress was developed [159,160,161,162,163]. The mitochondrial oxidative stress leads to the over-production of ROS, which, at teh cellular level, causes aerobic glycolysis, DNA damage, autophagy/mitophagy, and protection against apoptosis [163]. During oxidative stress, the most reactive and damaging ROS is hydroxyl radical (HO•), which is produced from hydrogen peroxide by the Fenton reaction [164]. To protect against/prevent oxidative stress, antioxidants should be applied. Antioxidants stoichiometrically react with ROS. They are required in large amounts to suppress oxidative stress and can have side effects [165,166,167,168].
Germanium compounds were found to be effective against oxidative stress [43,71,96]. Old publications describe the unique properties of germanium derivatives, which led us to suggest a putative mechanism of oxidative stress suppression/prevention. In 1930, R. Schwarz and H. Giese studied the reaction of alkali germanates with hydrogen peroxide and obtained peroxyhygrates [169]. Later, in 1935, R. Schwarz and F. Heinrich proved that these peroxyhygrates are coordination germanium compounds (not peroxides), with H2O and H2O2 as ligands [170]: K2Ge2O5·2H2O2·2H2O, Na2Ge2O5·2H2O2·2H2O, Na2GeO3··2H2O2·2H2O. Such complexes do not oxidize iodides and evolve oxygen. By this means, germanium derivatives catalytically decompose hydrogen peroxide, and germanium trace quantities can keep hydrogen peroxide at low levels, thus dramatically reducing the formation of the HO•, the most damaging ROS, by the Fenton reaction (Figure 20).
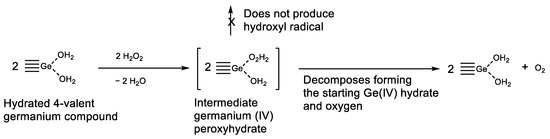
Figure 20.
Putative mechanism of suppression/prevention of oxidative stress. Hydrogen peroxide decomposition catalyzed by Ge (IV).
Therefore, germanium derivatives can dramatically reduce hydrogen peroxide levels in cells, suppressing/preventing oxidative stress. This explains the important role of germanium in the restoration of oxygen respiration in Warburg-like cancers.
6. Conclusions
Germanium is a vital ultra-microelement that participates in the fundamental biochemical reactions of a living cell, determining the broadest range of biological activity in its compounds. Germanium normalizes the immune system, which is essential for cancer prevention. Germanium’s ability to restore cell oxygen respiration is particularly attractive, and can serve as the basis for the treatment of Warburg-like cancers. In addition to organic compounds, germanium’s other classes, particularly the well-known coordination compounds, have become the subject of studies of physiological activity in the last decade.
Based on the knowledge at present, it is anticipated that the exploration of biologically active germanium compounds will progress in two main directions: Firstly, through comprehensive investigations of established compounds, primarily Ge-132, aiming to obtain a more thorough understanding of their properties. Secondly, through the synthesis of novel derivatives of known compounds to enhance their biological activity and broaden their range of effects. Furthermore, research in germanium chemistry holds the potential to unveil new categories of water-soluble germanium compounds and their associated properties. Of particular relevance is the study of the mechanism of action of germanium compounds in living cells. It has been observed that germanium is integral to the active centers of certain enzymes and is involved in oxidative reactions, primarily with hydrogen peroxide, without generating detrimental reactive oxygen species, including free radicals. Consequently, germanium compounds facilitate the restoration of oxygen respiration (i.e., oxidative phosphorylation) in cancer cells, thereby impeding or even halting the growth of Warburg-like tumors. Understanding this mechanism in depth will enable the purposeful synthesis of novel germanium compounds with a targeted biological activity, yielding more significant and directed therapeutic outcomes.
Despite being neglected in a number of influential journals (see Section 2), research on the biological activity of germanium compounds continues. The reliance on secondary sources of information with erroneous data on the toxicity of organic germanium compounds is the real reason for the neglect of its biological activity to date. The publication of the review [171] has sparked further discussion on germanium, its role in wildlife, and its associated errors and misperceptions in the scientific literature [172,173].
Author Contributions
Conceptualization, writing–original draft, writing–review and editing L.G.M. and A.V.P. Funding acquisition A.V.P. All authors have read and agreed to the published version of the manuscript.
Funding
Supported in part (AVP) by Grant #IRG-78-002-31 from the American Cancer Society and by the National Center for Advancing Translational Sciences under Award Number UL1TR001878 (A Pilot Grant from by the Institute for Translational Medicine and Therapeutics (ITMAT) Transdisciplinary Program in Translational Medicine and Therapeutics at the University of Pennsylvania). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors thank R.K. Isuri (University of Pennsylvania) for a fruitful discussion. Color photo of D.I. Mendeleev and C. Winkler created by L.G. Menchikov, A.V. Popov © 2023.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lukevics, E.; Ignatovich, L. Biological activity of organogermanium compounds. In The Chemistry of Organic Germanium, Tin and Lead Compounds; Rappoport, Z., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2002; Volume 2, pp. 1653–1683. [Google Scholar] [CrossRef]
- World Health Organization. WHO Drug Information 2014. WHO Drug Inf. 2014, 28, 125–210. [Google Scholar]
- Kadomtseva, A.V.; Mochalov, G.M.; Kuzina, O.V. Biologically Active Coordination Compounds of Germanium. Synthesis and Physicochemical Properties. Russ. J. Org. Chem. 2021, 57, 879–888. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Teng, G.-Q.; Zhou, H.; Dong, C.-L. Germanium Reduces Inflammatory Damage in Mammary Glands During Lipopolysaccharide-Induced Mastitis in Mice. Biol. Trace Elem. Res. 2020, 198, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ruan, T.; Lyu, Y.; Wu, B. Advances in effect of germanium or germanium compounds on animals—A review. J. Biosci. Med. 2017, 5, 56–73. [Google Scholar] [CrossRef]
- Kaplan, B.J.; Parish, W.W.; Andrus, G.M.; Simpson, J.S.A.; Field, C.J. Germane Facts About Germanium Sesquioxide: I. Chemistry and Anticancer Properties. J. Altern. Complement. Med. 2004, 10, 337–344. [Google Scholar] [CrossRef]
- Lukevics, E.; Gar, T.K.; Ignatovich, L.M.; Mironov, V.F. Biological Activity of Germanium Compounds; Zinatne: Riga, Latvia, 1990; p. 191. (In Russian) [Google Scholar]
- Rosenberg, E. Germanium-Containing Compounds, Current Knowledge and Applications. In Encyclopedia of Metalloproteins; Kretsinger, R., Uversky, V., Permyakov, E., Eds.; Springer: New York, NY, USA, 2013; pp. 847–855. [Google Scholar] [CrossRef]
- Ali, M.M. Germanium l-Cysteine Alpha-Tocopherol Complex as Stimulator to Antioxidant Defense System. In Encyclopedia of Metalloproteins; Kretsinger, R., Uversky, V., Permyakov, E., Eds.; Springer: New York, NY, USA, 2013; pp. 836–841. [Google Scholar] [CrossRef]
- Marczynski, B. Carcinogenesis as the result of the deficiency of some essential trace elements. Med. Hypotheses 1988, 26, 239–249. [Google Scholar] [CrossRef]
- Al-Zamely, O.M.Y. New approach for evaluation of Co, Ni, Mo, V, and Ge levels in serum of no Hodgkin lymphoma patients. Int. J. Biotechnol. Biochem. 2011, 7, 39–60. [Google Scholar]
- Kamil, Z.H.; Ewadh, M.J. Determination of serum trace elements and haematological parameters in lymphoma patients receiving chemotherapy. J. Babylon Univ. Pure Appl. Sci. 2016, 24, 2489–2500. [Google Scholar]
- Cheng, X.; Zhou, Y.-C.; Zhou, B.; Huang, Y.-C.; Wang, G.-Z.; Zhou, G.-B. Systematic analysis of concentrations of 52 elements in tumor and counterpart normal tissues of patients with non-small cell lung cancer. Cancer Med. 2019, 8, 7720–7727. [Google Scholar] [CrossRef]
- Schroeder, H.A.; Balassa, J.J. Abnormal trace metals in man: Germanium. J. Chronic Dis. 1967, 20, 211–224. [Google Scholar] [CrossRef]
- Chen, T.J.; Lin, C.H. Germanium: Environmental Pollution and Health Effects. In Encyclopedia of Environmental Health; Nriagu, J.O., Ed.; Elsevier: Burlington, NJ, USA, 2011; pp. 927–933. [Google Scholar] [CrossRef]
- Siwulski, M.; Rzymski, P.; Niedzielski, P.; Budka, A.; Gąsecka, M.; Kalač, P.; Jasińska, A.; Budzyńska, S.; Kozak, L.; Mleczek, M. Comparison of multielemental composition of Polish and Chinese mushrooms (Ganoderma spp.). Eur. Food Res. Technol. 2017, 243, 1555–1566. [Google Scholar] [CrossRef]
- Wiche, O.; Székely, B.; Moschner, C.; Heilmeier, H. Germanium in the soil-plant system—A review. Environ. Sci. Pollut. Res. 2018, 25, 31938–31956. [Google Scholar] [CrossRef] [PubMed]
- Murthy, H.N.; Kim, Y.-S.; Jeong, C.-S.; Kim, S.-J.; Zhong, J.-J.; Paek, K.-Y. Production of Ginsenosides from Adventitious Root Cultures of Panax ginseng. In Production of Biomass and Bioactive Compounds Using Bioreactor Technology; Paek, K.-Y., Murthy, H.N., Zhong, J.-J., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 625–651. [Google Scholar] [CrossRef]
- Cho, J.M.; Chae, J.; Jeong, S.R.; Moon, M.J.; Shin, D.Y.; Lee, J.H. Immune activation of Bio-Germanium in a randomized, double-blind, placebo-controlled clinical trial with 130 human subjects: Therapeutic opportunities from new insights. PLoS ONE 2020, 15, e0240358. [Google Scholar] [CrossRef]
- McMahon, M.; Regan, F.; Hughes, H. The determination of total germanium in real food samples including Chinese herbal remedies using graphite furnace atomic absorption spectroscopy. Food Chem. 2006, 97, 411–417. [Google Scholar] [CrossRef]
- Ohri, L.K.; Vicari, S.M.; Malone, P.M. Germanium Use and Associated Adverse Effects: A Review. J. Pharm. Technol. 1993, 9, 237–241. [Google Scholar] [CrossRef]
- Yajing, Z.; Xuan, G.; Sueichin, H.; Lihua, W. Determination of Germanium in Lucid Ganoderma and Ginseng by GFASS. Chin. J. Mod. Appl. Pharm. 1993, 10, 11–12. [Google Scholar]
- Avula, B.; Wang, Y.-H.; Smillie, T.J.; Duzgoren-Aydin, N.S.; Khan, I.A. Quantitative Determination of Multiple Elements in Botanicals and Dietary Supplements Using ICP-MS. J. Agric. Food Chem. 2010, 58, 8887–8894. [Google Scholar] [CrossRef]
- Rosenberg, E. Germanium: Environmental occurrence, importance and speciation. Rev. Environ. Sci. Biotechnol. 2009, 8, 29–57. [Google Scholar] [CrossRef]
- Yang, L.-L.; Zhang, D.-Q. Direct determination of germanium in botanical samples by graphite furnace atomic absorption spectrometry with palladium–zirconium as chemical modifier. Talanta 2002, 56, 1123–1129. [Google Scholar] [CrossRef]
- Chen, X.-C.; Zhu, Y.-G.; Zhu, L.-A.; Huang, C.; Chen, Y.; Chen, L.-M.; Fang, F.; Zhou, Y.-C.; Zhao, C.-H. Ginsenoside Rg1 attenuates dopamine-induced apoptosis in PC12 cells by suppressing oxidative stress. Eur. J. Pharmacol. 2003, 473, 1–7. [Google Scholar] [CrossRef]
- Ahuja, A.; Kim, J.H.; Kim, J.-H.; Yi, Y.-S.; Cho, J.Y. Functional role of ginseng-derived compounds in cancer. J. Ginseng Res. 2018, 42, 248–254. [Google Scholar] [CrossRef]
- Najafi, T.F.; Bahri, N.; Tohidinik, H.R.; Feyz, S.; Bloki, F.; Savarkar, S.; Jahanfar, S. Treatment of cancer-related fatigue with ginseng: A systematic review and meta-analysis. J. Herb. Med. 2021, 28, 100440. [Google Scholar] [CrossRef]
- Sadeghian, M.; Rahmani, S.; Zendehdel, M.; Hosseini, S.A.; Zare Javid, A. Ginseng and Cancer-Related Fatigue: A Systematic Review of Clinical Trials. Nutr. Cancer 2021, 73, 1270–1281. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Baatar, D.; Hwang, S.G. Anticancer Activities of Ginsenosides, the Main Active Components of Ginseng. Evid.-Based Complement. Altern. Med. 2021, 2021, 8858006. [Google Scholar] [CrossRef] [PubMed]
- Awais, M.; Aizaz, A.; Nazneen, A.; Bhatti, Q.u.A.; Akhtar, M.; Wadood, A.; Rehman, M.A.U. A Review on the Recent Advancements on Therapeutic Effects of Ions in the Physiological Environments. Prosthesis 2022, 4, 263–316. [Google Scholar] [CrossRef]
- Popov, A.V.; Menchikov, L.G. The Warburg Effect Is a Guide to Multipurpose Cancer Therapy Including Trace Element Delivery. In Drug Delivery Systems: Advanced Technologies Potentially Applicable in Personalised Treatment; Coelho, J., Ed.; Springer: Dordrecht, The Netherlands, 2013; Volume 4, pp. 255–270. [Google Scholar] [CrossRef]
- Menchikov, L.G.; Shestov, A.A.; Popov, A.V. Warburg effect revisited: Embodiment of classical biochemistry and organic chemistry. Current state and prospects. Biochemistry 2023, 88 (Suppl. 1), S1–S20. [Google Scholar] [CrossRef]
- Unakar, N.J.; Tsui, J.; Johnson, M. Effect of pretreatment of germanium-132 on Na(+)-K(+)-ATPase and galactose cataracts. Curr. Eye Res. 1997, 16, 832–837. [Google Scholar] [CrossRef]
- Ishiwara, F. The influence of different types of metals on mouse carcinoma (Uber die Beeinflussung von Mausecarcinom durch verschiedene Metallarten). Ber. Gesamte Physiol. Exp. Pharmakol. 1928, 49, 615. [Google Scholar]
- Menchikov, L.G.; Ignatenko, M.A. Biological Activity of Organogermanium Compounds (A Review). Pharm. Chem. J. 2012, 46, 635–638. [Google Scholar] [CrossRef]
- Thayer, J.S. Germapharmaca: Some recent studies on biologically active organogermanium compounds. Appl. Organomet. Chem. 1987, 1, 227–234. [Google Scholar] [CrossRef]
- Patai, S. (Ed.) The Chemistry of Organic Germanium, Tin and Lead Compounds; John Wiley & Sons, Ltd.: Chichester, UK, 1995; Volume 1. [Google Scholar]
- Rappoport, Z. (Ed.) The Chemistry of Organic Germanium, Tin and Lead Compounds; John Wiley & Sons, Ltd.: Chichester, UK, 2002; Volume 2. [Google Scholar]
- Chase, T.A.; Cupp, M.J.; Tracy, T.S. Germanium. In Dietary Supplements: Toxicology and Clinical Pharmacology; Cupp, M.J., Tracy, T.S., Eds.; Humana Press: Totowa, NJ, USA, 2003; pp. 197–207. [Google Scholar] [CrossRef]
- Satge, J. Some Applications of Germanium and its Derivatives. Main Group Met. Chem. 2004, 27, 301–308. [Google Scholar] [CrossRef]
- Lukevics, E.; Ignatovich, L. Biological Activity of Organogermanium Compounds. In Metallotherapeutic Drugs and Metal-Based Diagnostic Agents. The Use of Metals in Medicine; Gielen, M., Tiekink, E.R.T., Eds.; J. Wiley: Chichester, UK, 2005; Volume 15, pp. 279–295. [Google Scholar] [CrossRef]
- Narokha, V.; Nizhenkovska, I.; Kuznetsova, O. Potential of germanium-based compounds in coronavirus infection. Acta Pharm. 2022, 72, 245–258. [Google Scholar] [CrossRef]
- Lee, V.Y. (Ed.) Organogermanium Compounds: Theory, Experiment, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2023. [Google Scholar] [CrossRef]
- Singh, R.V.; Gupta, P.; Chaudhary, P.; Deshmukh, C.N. Coordination compounds of germanium(IV) formed with soft and hard donor atoms: A look into the past and present work. Main Group Met. Chem. 2005, 28, 93–118. [Google Scholar] [CrossRef]
- Seifullina, I.I.; Martsinko, E.E. Coordination Compounds of Germanium (IV) with Anions of Citric, Tartaric and Xylaric Acids; Odessa Mechnikov National University: Odessa, Ukraine, 2015. (In Russian) [Google Scholar]
- Kaplan, B.J.; Andrus, G.M.; Parish, W.W. Germane facts about germanium sesquioxide: II. Scientific error and misrepresentation. J. Altern. Complement. Med. 2004, 10, 345–348. [Google Scholar] [CrossRef]
- Schauss, A.G. Nephrotoxicity and neurotoxicity in humans from organogermanium compounds and germanium dioxide. Biol. Trace Elem. Res. 1991, 29, 267–280. [Google Scholar] [CrossRef]
- Schauss, A.G. Nephrotoxicity in Humans by the Ultratrace Element Germanium. Ren. Fail. 1991, 13, 1–4. [Google Scholar] [CrossRef]
- Tao, S.-H.; Bolger, P.M. Hazard Assessment of Germanium Supplements. Regul. Toxicol. Pharmacol. 1997, 25, 211–219. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, C.; Zhao, D. Successful management of germanium poisoning-induced multiple organ dysfunctions by combined blood purification therapy. Curr. Med. Res. Opin. 2020, 36, 687–691. [Google Scholar] [CrossRef]
- Nakamura, T.; Shimada, Y.; Takeda, T.; Sato, K.; Akiba, M.; Fukaya, H. Organogermanium compound, Ge-132, forms complexes with adrenaline, ATP and other physiological cis-diol compounds. Future Med. Chem. 2015, 7, 1233–1246. [Google Scholar] [CrossRef]
- Kim, E.; Hwang, S.-U.; Yoon, J.D.; Jeung, E.-B.; Lee, E.; Kim, D.Y.; Hyun, S.-H. Carboxyethylgermanium sesquioxide (Ge-132) treatment during in vitro culture protects fertilized porcine embryos against oxidative stress induced apoptosis. J. Reprod. Dev. 2017, 63, 581–590. [Google Scholar] [CrossRef]
- Wada, T.; Hanyu, T.; Nozaki, K.; Kataoka, K.; Kawatani, T.; Asahi, T.; Sawamura, N. Antioxidant Activity of Ge-132, a Synthetic Organic Germanium, on Cultured Mammalian Cells. Biol. Pharm. Bull. 2018, 41, 749–753. [Google Scholar] [CrossRef]
- Gerber, G.B.; Leonard, A. Mutagenicity, carcinogenicity and teratogenicity of germanium compounds. Mutat. Res. Rev. Mutat. Res. 1997, 387, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Keith, L.S.; Faroon, O.M.; Maples-Reynolds, N.; Fowler, B.A. Germanium. In Handbook on the Toxicology of Metals, 4th ed.; Nordberg, G.F., Fowler, B.A., Nordberg, M., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 799–816. [Google Scholar] [CrossRef]
- Keith, L.S.; Maples-Reynolds, N. Germanium. In Handbook on the Toxicology of Metals. Volume II: Specific Metals, 5th ed.; Nordberg, G.F., Costa, M., Eds.; Academic Press: London, UK; San Diego, CA, USA, 2022; pp. 289–316. [Google Scholar] [CrossRef]
- Reddeman, R.A.; Glávits, R.; Endres, J.R.; Murbach, T.S.; Hirka, G.; Vértesi, A.; Béres, E.; Szakonyiné, I.P. A Toxicological Evaluation of Germanium Sesquioxide (Organic Germanium). J. Toxicol. 2020, 2020, 6275625. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.H.; Macintosh, D.; Allen, J.; McCarthy, J. Germanium, Tin, and Copper. In Patty’s Toxicology; Bingham, E., Cohrssen, B., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 355–380. [Google Scholar] [CrossRef]
- Banasik, M. Germanium. In Hamilton & Hardy’s Industrial Toxicology; Harbison, R.D., Bourgeois, M.M., Johnson, G.T., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 119–122. [Google Scholar] [CrossRef]
- Matsusaka, T.; Fujii, M.; Nakano, T.; Terai, T.; Kurata, A.; Imaizumi, M.; Abe, H. Germanium-induced nephropathy: Report of two cases and review of the literature. Clin. Nephrol. 1988, 30, 341–345. [Google Scholar]
- Sanai, T.; Okuda, S.; Onoyama, K.; Oochi, N.; Oh, Y.; Kobayashi, K.; Shimamatsu, K.; Fujimi, S.; Fujishima, M. Germanium Dioxide-Induced Nephropathy: A New Type of Renal Disease. Nephron 1990, 54, 53–60. [Google Scholar] [CrossRef]
- Takeuchi, A.; Yoshizawa, N.; Oshima, S.; Kubota, T.; Oshikawa, Y.; Akashi, Y.; Oda, T.; Niwa, H.; Imazeki, N.; Seno, A.; et al. Nephrotoxicity of germanium compounds: Report of a case and review of the literature. Nephron 1992, 60, 436–442. [Google Scholar] [CrossRef]
- Kolesnikov, S.P. Research in Chemistry of Trihalogermans and Germanium Analogues of Dihalocarbenes; N.D. Zelinsky Institute of Organic Chemistry, Academy of Sciencies of USSR: Moscow, Russia, 1966. (In Russian) [Google Scholar]
- Kolesnikov, S.P.; Nefedov, O.M. The reaction of trichlorogermane with ketones (in Connection with the Paper by T.K.Gar and V.F.Mironov). J. Gen. Chem. USSR Engl. Transl. 1967, 37, 701. [Google Scholar]
- Nefedov, O.M.; Kolesnikov, S.P.; Perlmutter, B.L. Reactions of Trichlorogermane with Ketones and Alcohols. Angew. Chem. Int. Ed. 1967, 6, 628–629. [Google Scholar] [CrossRef]
- Tomilov, Y.V.; Menchikov, L.G.; Shapiro, E.A.; Gvozdev, V.D.; Shavrin, K.N.; Volchkov, N.V.; Lipkind, M.B.; Egorov, M.P.; Boganov, S.E.; Khabashesku, V.N.; et al. Carbenes, related intermediates, and small-sized cycles: Contribution from Professor Nefedov’s laboratory. Mendeleev Commun. 2021, 31, 750–768. [Google Scholar] [CrossRef]
- Mironov, V.F.; Berliner, E.M.; Gar, T.K. Reactions of trichlorogermane with acrylic acid and its derivatives. J. Gen. Chem. USSR Engl. Transl. 1967, 37, 911–912. [Google Scholar]
- Mironov, V.F.; Berliner, E.M.; Gar, T.K.; Rybakov, E.A. Reactions of Trichlorogermane with Unsaturated Carboxylic Acids. J. Gen. Chem. USSR Engl. Transl. 1968, 38, 2218. [Google Scholar]
- Zhang, C.L.; Li, T.H.; Niu, S.H.; FuWang, R.; Fu, Z.L.; Guo, F.Q.; Yang, M. Synthesis and Evaluation of Novel Organogermanium Sesquioxides As Antitumor Agents. Bioinorg. Chem. Appl. 2009, 2009, 908625. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Shimada, Y.; Sato, K. Bioorganic and Medicinal Organogermanium Chemistry. In Organogermanium Compounds: Theory, Experiment, and Applications; Lee, V.Y., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2023; pp. 839–865. [Google Scholar] [CrossRef]
- Lahans, T. Integrating Conventional and Chinese Medicine in Cancer Care: A Clinical Guide; Elsevier Health Sciences: Philadelphia, PA, USA, 2007; p. 405. [Google Scholar]
- Mainwaring, M.G.; Poor, C.; Zander, D.S.; Harman, E. Complete remission of pulmonary spindle cell carcinoma after treatment with oral germanium sesquioxide. Chest 2000, 117, 591–593. [Google Scholar] [CrossRef] [PubMed]
- Nefedov, O.M.; Kolesnikov, S.P. Trichlorogermane etherates. Bull. Acad. Sci. USSR Div. Chem. Sci. 1963, 12, 1910. [Google Scholar] [CrossRef]
- Shangguan, G.Q.; Zhang, S.G.; Ni, J.Z. Synthesis, structures and antitumor activities of beta-phenol ester propyl germanium sesquioxides. Chin. Chem. Lett. 1995, 6, 945–946. [Google Scholar]
- Doi, Y.; Imai, N.; Suguro, M.; Numano, T.; Furukawa, F. No carcinogenicity of poly-trans-[(2-carboxyethyl) germasesquioxane](Ge-132): 26-week feeding study using rasH2 mice. Fundam. Toxicol. Sci. 2017, 4, 137–150. [Google Scholar] [CrossRef]
- Iwadate, K.; Yamaguchi, Y.; Sasaki, M.; Nakatani, M.; Doi, Y.; Imai, N.; Tamano, S.; Nishihori, Y. Carcinogenicity study of poly-trans-[(2-carboxyethyl)germasesquioxane] (Ge-132) in F344 rats. Fundam. Toxicol. Sci. 2018, 5, 127–140. [Google Scholar] [CrossRef]
- Ogwapit, S.M. Analysis of Ge-132 and development of a simple oral anticancer formulation. Biosci. Horiz. 2011, 4, 128–139. [Google Scholar] [CrossRef]
- Vinodhini, J.; Kumar, D.S.R.S.; Sudha, S. Cytotoxic Effect of Carboxyethylgermanium Sesquioxide (Ge-132) on MCF-7 Human Breast Cancer Cell Line. Int. J. Curr. Sci. Res. IJCSR 2011, 2, 178–181. [Google Scholar]
- Masuda, T.; Noda, M.; Kogawa, T.; Kitagawa, D.; Hayashi, N.; Jomori, T.; Nakanishi, Y.; Nakayama, K.I.; Ohno, S.; Mimori, K. Phase I dose-escalation trial to repurpose propagermanium, an oral CCL2 inhibitor, in patients with breast cancer. Cancer Sci. 2020, 111, 924–931. [Google Scholar] [CrossRef]
- Masuda, T.; Tsuruda, Y.; Matsumoto, Y.; Uchida, H.; Nakayama, K.I.; Mimori, K. Drug repositioning in cancer: The current situation in Japan. Cancer Sci. 2020, 111, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Vinodhini, J.; Sudha, S. Effect of bis-carboxy ethyl germanium sesquoxide on N-nitroso-N-methylurea-induced rat mammary carcinoma. Asian J. Pharm. Clin. Res. 2013, 6, 242–244. [Google Scholar]
- Ayodele, O.T. Anticancer activities of organogermanium compounds. Int. J. Progress. Sci. Technol. 2016, 3, 23–25. [Google Scholar] [CrossRef]
- Shimada, Y.; Sato, K.; Tokuji, Y.; Nakamura, T. Nuclear magnetic resonance studies of the interactions between the organic germanium compound Ge-132 and saccharides. Carbohydr. Res. 2015, 407, 10–15. [Google Scholar] [CrossRef]
- Nakamura, T.; Takeda, T.; Tokuji, Y. The Oral Intake of Organic Germanium, Ge-132, Elevates α-Tocopherol Levels in the Plasma and Modulates Hepatic Gene Expression Profiles to Promote Immune Activation in Mice. Int. J. Vitam. Nutr. Res. 2014, 84, 0183–0195. [Google Scholar] [CrossRef]
- Guo, F.; Xu, D.; Lin, Y.; Wang, G.; Wang, F.; Gao, Q.; Wei, Q.; Lei, S. Chemokine CCL2 contributes to BBB disruption via the p38 MAPK signaling pathway following acute intracerebral hemorrhage. FASEB J. 2020, 34, 1872–1884. [Google Scholar] [CrossRef]
- He, S.; Liu, R.; Li, B.; Huang, L.; Fan, W.; Tembachako, C.R.; Zheng, X.; Xiong, X.; Miyata, M.; Xu, B.; et al. Propagermanium, a CCR2 inhibitor, attenuates cerebral ischemia/reperfusion injury through inhibiting inflammatory response induced by microglia. Neurochem. Int. 2019, 125, 99–110. [Google Scholar] [CrossRef]
- Mizuno, N.; Nishibori, E.; Oka, M.; Jomori, T.; Takata, M.; Kumasaka, T. Structural Basis for Polymer Packing and Solvation Properties of the Organogermanium Crystalline Polymer Propagermanium and Its Derivatives. J. Pharm. Sci. 2015, 104, 2482–2488. [Google Scholar] [CrossRef]
- Baidya, S.; Nishimoto, Y.; Sato, S.; Shimada, Y.; Sakurai, N.; Nonaka, H.; Noguchi, K.; Kido, M.; Tadano, S.; Ishikawa, K.; et al. Dual Effect of Organogermanium Compound THGP on RIG-I-Mediated Viral Sensing and Viral Replication during Influenza a Virus Infection. Viruses 2021, 13, 1674. [Google Scholar] [CrossRef]
- Alimbarova, L.M.; Ambrosov, I.V.; Matelo, S.K.; Barinsky, I.F. Antiviral activity of the organic germanium complex with aciclovir against herpes simplex virus (Herpesviridae: Alphaherpesvirinae: Simplexvirus: Human alphaherpesvirus 1/2) in the in vitro and in vivo systems. Probl. Virol. 2021, 66, 368–382. [Google Scholar] [CrossRef]
- Mulder, P.; van den Hoek, A.M.; Kleemann, R. The CCR2 Inhibitor Propagermanium Attenuates Diet-Induced Insulin Resistance, Adipose Tissue Inflammation and Non-Alcoholic Steatohepatitis. PLoS ONE 2017, 12, e0169740. [Google Scholar] [CrossRef] [PubMed]
- Oh, C.; Li, M.; Kim, E.H.; Park, J.S.; Lee, J.C.; Ham, S.W. Antioxidant and Radical Scavenging Activities of Ascorbic Acid Derivatives Conjugated with Organogermanium. Bull. Korean Chem. Soc. 2010, 31, 3513–3514. [Google Scholar] [CrossRef]
- Tezuka, T.; Higashino, A.; Akiba, M.; Nakamura, T. Organogermanium (Ge-132) Suppresses Activities of Stress Enzymes Responsible for Active Oxygen Species in Monkey Liver Preparation. Adv. Enzym. Res. 2017, 5, 13. [Google Scholar] [CrossRef]
- Kim, E.; Jeon, Y.; Kim, D.Y.; Lee, E.; Hyun, S.-H. Antioxidative effect of carboxyethylgermanium sesquioxide (Ge-132) on IVM of porcine oocytes and subsequent embryonic development after parthenogenetic activation and IVF. Theriogenology 2015, 84, 226–236. [Google Scholar] [CrossRef]
- Matsumoto, H.; Iwafuji, H.; Yamane, J.; Takeuchi, R.; Utsunomiya, T.; Fujii, A. Restorative effect of organic germanium compound (Ge-132) on dermal injury. Wound Med. 2016, 15, 6–10. [Google Scholar] [CrossRef]
- Takeda, T.; Doiyama, S.; Azumi, J.; Shimada, Y.; Tokuji, Y.; Yamaguchi, H.; Nagata, K.; Sakamoto, N.; Aso, H.; Nakamura, T. Organogermanium suppresses cell death due to oxidative stress in normal human dermal fibroblasts. Sci. Rep. 2019, 9, 13637. [Google Scholar] [CrossRef] [PubMed]
- Elango, J.; Bushin, R.; Lijnev, A.; De Aza, P.N.; Martínez, C.P.-A.; Marín, J.M.G.; Hernandez, A.B.; Olmo, L.R.M.; Val, J.E.M.S.D. The Effect of Germanium-Loaded Hydroxyapatite Biomaterials on Bone Marrow Mesenchymal Stem Cells Growth. Cells 2022, 11, 2993. [Google Scholar] [CrossRef] [PubMed]
- Azumi, J.; Takeda, T.; Shimada, Y.; Zhuang, T.; Tokuji, Y.; Sakamoto, N.; Aso, H.; Nakamura, T. Organogermanium THGP Induces Differentiation into M1 Macrophages and Suppresses the Proliferation of Melanoma Cells Via Phagocytosis. Int. J. Mol. Sci. 2023, 24, 1885. [Google Scholar] [CrossRef]
- Sekiguchi, F.; Koike, N.; Shimada, Y.; Sugimoto, K.; Masuda, H.; Nakamura, T.; Yamaguchi, H.; Tanabe, G.; Marumoto, S.; Kasanami, Y.; et al. A hydrolysate of poly-trans-[(2-carboxyethyl)germasesquioxane] (Ge-132) suppresses Cav3.2-dependent pain by sequestering exogenous and endogenous sulfide. Redox Biol. 2023, 59, 102579. [Google Scholar] [CrossRef]
- Azumi, J.; Shimada, Y.; Takeda, T.; Aso, H.; Nakamura, T. The Organogermanium Compound 3-(Trihydroxygermyl) Propanoic Acid (THGP) Suppresses Inflammasome Activation Via Complexation with ATP. Int. J. Mol. Sci. 2022, 23, 13364. [Google Scholar] [CrossRef]
- Shimada, Y.; Sato, K.; Takeda, T.; Tokuji, Y. The Organogermanium Compound Ge-132 Interacts with Nucleic Acid Components and Inhibits the Catalysis of Adenosine Substrate by Adenosine Deaminase. Biol. Trace Elem. Res. 2018, 181, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Zhao, Y.; Sui, B.; Shangguan, G. Studies on the interaction of novel organogermanium sesquioxides with DNA. Chem. Res. Chin. Univ. 2015, 31, 31–37. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, L.; Sui, B.; Shangguan, G. Synthesis and cytotoxicity of novel organogermanium sesquioxides. J. Jining Med. Univ. 2017, 40, 14. [Google Scholar] [CrossRef]
- Yao, S.; Jiang, J.; Yang, P.; Cai, J.-Y. Synthesis, characterization and antioxidant activity of a novel organogermanium sesquioxide with resveratrol. Bull. Korean Chem. Soc. 2012, 33, 1121–1122. [Google Scholar] [CrossRef]
- Chernyshev, E.A.; Knyazev, S.P.; Kirin, V.N.; Vasilev, I.M.; Alekseev, N.V. Structural features of silatranes and germatranes. Russ. J. Gen. Chem. 2004, 74, 58–65. [Google Scholar] [CrossRef]
- Ignatyev, I.S.; Sundius, T.R.; Vrazhnov, D.V.; Kochina, T.A.; Voronkov, M.G. Bonding in germatranyl cation and germatranes. J. Organomet. Chem. 2007, 692, 5697–5700. [Google Scholar] [CrossRef]
- Zabalov, M.; Karlov, S.; Zaitseva, G.; Lemenovskii, D. The molecular and electronic structure features of silatranes, germatranes, and their carbon analogs. Russ. Chem. Bull. 2006, 55, 464–476. [Google Scholar] [CrossRef]
- Voronkov, M.G.; Korlyukov, A.A.; Samokhin, G.S.; Vrazhnov, D.V.; Kochina, T.A. Germatranes and their quasi and hypo analogs with highly electronegative substituent at the Ge atom. Russ. Chem. Bull. 2012, 61, 992–998. [Google Scholar] [CrossRef]
- Karlov, S.S.; Zaitseva, G.S. Germatranes and their Analogs. Synthesis, Structure, and Reactivity. (Review). Chem. Heterocycl. Compd. 2001, 37, 1325–1357. [Google Scholar] [CrossRef]
- Xu, M.-Y.; Xiao, B. Germatranes and carbagermatranes: (hetero)aryl and alkyl coupling partners in Pd-catalyzed cross-coupling reactions. Chem. Commun. 2021, 57, 11764–11775. [Google Scholar] [CrossRef]
- Lukevics, E.; Germane, S.; Zidermane, A.; Dauvarte, A.; Kravchenko, I.M.; Trusule, M.; Mironov, V.F.; Gar, T.K.; Khromova, N.Y. Synthesis, neurotropic and antitumor activity of some germatranes, germasesquioxanes and their organotin analogs. Pharm. Chem. J. 1984, 18, 89–94. [Google Scholar] [CrossRef]
- Kakimoto, N.; Sato, K.; Takada, T.; Akiba, M. Organogermanium compounds: Synthesis, structure, and properties of masked ethoxycarbonylgermanium sesquioxide (GE-132) and related compounds with one triethanolamine component. Heterocycles 1987, 26, 347–353. [Google Scholar] [CrossRef]
- Song, X.; Yang, Z.; Su, G.; Xieqinglan. Synthesis, characterization and anticancer activity of some bis(germylpropionato-di-n-butyltin) oxides. Phosphorus Sulfur Silicon Relat. Elem. 1999, 150–151, 367–374. [Google Scholar] [CrossRef]
- Ye, L.; Ou, X.; Peng, X.; Luo, Y. Synthesis of 3-(2, 8, 9-trioxa-5-aza-1-germatricyclo [3.3.3.0] Undecane-1- yl)-3-(4-hydroxyl-3-methoxyphenyl)-propionic Acid and its Inhibitory Effect on the Cervical Tumor U14 in vitro and in vivo. Med. Chem. 2012, 8, 595–598. [Google Scholar] [CrossRef]
- Zhigacheva, I.V.; Baryshok, V.P.; Rasulov, M.M.; Storozhenko, P.A. 2-(Germatran-1-yloxy)ethylamine as an inhibitor of the total activity of mononuclear alkaline phospholipase A2. Russ. Chem. Bull. 2021, 70, 444–448. [Google Scholar] [CrossRef]
- Binyukov, V.I.; Mil, E.M.; Zhigacheva, I.V.; Generozova, I.P.; Rasulov, M.M. Morphological and bioenergetic characteristics of mitochondria under stress and action of organogermanium compounds. J. Nat. Sci. Sustain. Technol. 2015, 9, 439. [Google Scholar]
- Binyukov, V.I.; Mil, E.M.; Zhigacheva, I.V.; Generozova, I.P.; Rasulov, M.M. Morphological and bioenergetical characteristics of mitochondria. In Chemical and Biochemical Physics: A Systematic Approach to Experiments, Evaluation, and Modeling; Schiraldi, D., Zaikov, G.E., Eds.; Apple Academic Press: Oakville, ON, Canada, 2016; pp. 259–276. [Google Scholar]
- Zhigacheva, I.V.; Burlakova, E.B. Plant Growth and Development Regulators and their Effect on the Functional State of Mitochondria. In Chemistry and Technology of Plant Substances; Kutchin, A.V., Shishkina, L.N., Weisfeld, L.I., Eds.; Apple Academic Press: New York, NY, USA, 2017; pp. 243–278. [Google Scholar] [CrossRef]
- Shigarova, A.M.; Grabelnych, O.I.; Baryshok, V.P.; Borovskii, G.B. Impossible mechanisms of germatranol influence on the thermal stability of wheat germs. Appl. Biochem. Microbiol. 2016, 52, 429–434. [Google Scholar] [CrossRef]
- Ignatyev, I.S.; Lezov, D.V.; Kondratenko, Y.A.; Kochina, T.A. Interaction of simple amino acids (glycine, α-alanine, β-alanine and L-valine) with germatranol hydrate. J. Mol. Struct. 2022, 1253, 132245. [Google Scholar] [CrossRef]
- Baukov, Y.I.; Tandura, S.N. Hypervalent Compounds of Organic Germanium, Tin and Lead Derivatives. In The Chemistry of Organic Germanium, Tin and Lead Compounds; Rappoport, Z., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2002; Volume 2, pp. 963–1239. [Google Scholar] [CrossRef]
- Selina, A.; Karlov, S.; Zaitseva, G. Metallocanes of group 14 elements. 1. Derivatives of silicon and germanium. (Review). Chem. Heterocycl. Compd. 2006, 42, 1518–1556. [Google Scholar] [CrossRef]
- Lermontova, E.K.; Churakov, A.V.; Quan, M.; Oprunenko, Y.F.; Karlov, S.S.; Zaitseva, G.S. Synthesis, structure, and reactivity of germanium-containing derivatives of substituted diethanolamines. Russ. J. Inorg. Chem. 2009, 54, 211–218. [Google Scholar] [CrossRef]
- de Velasco, D.O.-G.; Sánchez-Jiménez, R.; Hernández-Ortega, S.; Toscano, R.A.; García-Montalvo, V. Study on the transannular bond formation and hypercoordination in tin and germanium spirometallocanes. Polyhedron 2010, 29, 2435–2439. [Google Scholar] [CrossRef]
- Shainyan, B.A. Structural and Conformational Aspects in the Chemistry of Heterocycles. Molecules 2020, 25, 3461. [Google Scholar] [CrossRef] [PubMed]
- Lukevics, E.; Ignatovich, L. Comparative study of the biological activity of organosilicon and organogermanium compounds. Appl. Organomet. Chem. 1992, 6, 113–126. [Google Scholar] [CrossRef]
- Dawara, L.; Singh, D.; Singh, R.V. Antimicrobial and pesticidal activity of some organogermanium (IV) complexes synthesized under microwave irradiation. Main Group Met. Chem. 2011, 34, 69–75. [Google Scholar] [CrossRef]
- Dawara, L.; Fahmi, N.; Singh, R.V. Synthesis, characterization, antimicrobial, pesticidal and DNA cleavage activity of germanium (IV) derivatives of 3-(2-methyl-2, 3-dihydro-benzthiazo-2-yl)-chromen-2-one and N′-[1-2-oxo-2H-chrome-3yl-ethylidene]-hydrazinecarbodithionic acid benzyl ester ligands. Main Group Met. Chem. 2011, 34, 139–146. [Google Scholar] [CrossRef]
- Fahmi, N.; Khedar, R.; Singh, R.V. Green synthesis of new Ge(IV) complexes with bio-potent ligands and their antimicrobial, DNA cleavage, and antioxidant activities. Russ. J. Gen. Chem. 2016, 86, 958–964. [Google Scholar] [CrossRef]
- Singh, N.; Watts, S.; Joshi, S.C.; Singh, R.V. Pesticidal and antifertility activities of triorganogermanium(IV) complexes synthesized using a green chemical approach. Appl. Organomet. Chem. 2013, 27, 269–276. [Google Scholar] [CrossRef]
- Mahawar, P.; Wasson, M.K.; Sharma, M.K.; Jha, C.K.; Mukherjee, G.; Vivekanandan, P.; Nagendran, S. A Prelude to Biogermylene Chemistry. Angew. Chem. Int. Ed. 2020, 59, 21377–21381. [Google Scholar] [CrossRef]
- Lim, D.H.; Li, M.; Seo, J.-A.; Lim, K.-M.; Ham, S.W. A novel organogermanium protected atopic dermatitis induced by oxazolone. Bioorg. Med. Chem. Lett. 2010, 20, 4032–4034. [Google Scholar] [CrossRef]
- Lim, D.H.; Li, M.; Kim, E.-h.; Ham, S.W. Synthesis of Novel Organogermanium Derivative Conjugated with Vitamin C and Study of its Antioxidant Effects. Bull. Korean Chem. Soc. 2010, 31, 1839–1840. [Google Scholar] [CrossRef]
- Jiang, J.; Yao, S.; Cai, H.-H.; Yang, P.-H.; Cai, J. Synthesis and synergetic effects of chrysin–organogermanium (IV) complex as potential anti-oxidant. Bioorg. Med. Chem. Lett. 2013, 23, 5727–5732. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Jin, H.; Pi, J.; Jiang, J.-H.; Liu, L.; Bai, H.-H.; Yang, P.-H.; Cai, J.-Y. Anti-tumor activity evaluation of novel chrysin–organogermanium(IV) complex in MCF-7 cells. Bioorg. Med. Chem. Lett. 2013, 23, 5544–5551. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Gong, L.; Jin, H.; Pi, J.; Bai, H.; Wang, H.; Cai, H.; Yang, P.; Cai, J. Chrysin–organogermanium (IV) complex induced Colo205 cell apoptosis-associated mitochondrial function and anti-angiogenesis. Scanning 2015, 37, 246–257. [Google Scholar] [CrossRef]
- Lu, P.; Yao, S.; Cai, J.; Yang, P.-h. Synthesis and synergetic anti-tumor activity evaluation of dihydroartemisinin-organogermanium(IV) compound. Bioorg. Med. Chem. Lett. 2014, 24, 5294–5297. [Google Scholar] [CrossRef] [PubMed]
- Karpenko, R.G.; Kolesnikov, S.P. Germylated steroids. 1. Hydrogermylation of conjugated steroid enones. Russ. Chem. Bull. 1998, 47, 180–182. [Google Scholar] [CrossRef]
- Karpenko, R.G.; Kolesnikov, S.P. Germylated steroids. 2. Synthesis of steroid germatranes. Russ. Chem. Bull. 1999, 48, 1185–1186. [Google Scholar] [CrossRef]
- Karpenko, R.G.; Krylova, I.V.; Kamernitskii, A.V. Germylated steroids. 3. Synthesis of trialkylgermylated steroids. Russ. Chem. Bull. 2011, 60, 2100–2102. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Gloriozova, T.A.; Poroikov, V.V. Biological Activities of Organometalloid (As, At, B, Ge, Si, Se, Te) Steroids. J. Appl. Pharm. Sci. Vol 2017, 7, 184–202. [Google Scholar] [CrossRef]
- Ignatovich, L.; Starkova, O.; Romanovs, V.; Sleiksha, I.; Shestakova, I.; Popelis, J.; Lukevics, E. Novel trialkylsilyl(germyl)-substituted thienyl- and furylbenzimidazoles and their N-substituted derivatives—Synthesis, structure and cytotoxic activity. Comptes Rendus Chim. 2013, 16, 621–627. [Google Scholar] [CrossRef]
- Ignatovich, L.; Spura, J.; Muravenko, V.; Belyakov, S.; Popelis, J.; Shestakova, I.; Domrachova, I.; Gulbe, A.; Rudevica, Z.; Leonchiks, A. Synthesis, structure and biological activity of new 6,6-dimethyl-2-oxo-4-{2-[5-organylsilyl(germyl)]furan(thiophen)-2-yl}vinyl-5,6-dihydro-2H-pyran-3-carbonitriles. Appl. Organomet. Chem. 2015, 29, 756–763. [Google Scholar] [CrossRef]
- Fedoruk, R.S.; Kovalchuk, I.I.; Mezentseva, L.M.; Tesarivska, U.I.; Pylypets, A.Z.; Kaplunenko, V.H. Germanium compounds and their role in animal body. Anim. Biol. 2022, 24, 51. [Google Scholar] [CrossRef]
- Seifullina, I.I. New page in coordination chemistry of germanium. Odesa Natl. Univ. Herald. Chem. 2003, 8, 8–25. (In Russian) [Google Scholar]
- Korlyukov, A.A. Coordination compounds of tetravalent silicon, germanium and tin: The structure, chemical bonding and intermolecular interactions in them. Russ. Chem. Rev. 2015, 84, 422. [Google Scholar] [CrossRef]
- Grishanov, D.A.; Churakov, A.V.; Medvedev, A.G.; Mikhaylov, A.A.; Lev, O.; Prikhodchenko, P.V. Crystalline Ammonium Peroxogermanate as a Waste-Free, Fully Recyclable Versatile Precursor for Germanium Compounds. Inorg. Chem. 2019, 58, 1905–1911. [Google Scholar] [CrossRef]
- Fedoruk, R.S.; Khrabko, M.I.; Dolaychuk, O.P. Effect of Germanium Citrate on Immunophysiological Activity of the Rats’ Organism. Int. J. Physiol. Pathophysiol. 2018, 9, 17–26. [Google Scholar] [CrossRef]
- Mertens, R.T.; Parkin, S.; Awuah, S.G. Exploring six-coordinate germanium(IV)-diketonate complexes as anticancer agents. Inorg. Chim. Acta 2020, 503, 119375. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-P.; Xie, W.-L.; Cai, H.-H.; Cai, J.-Y.; Yang, P.-H. Hydroxyl radical scavenging mechanism of human erythrocytes by quercetin–germanium (IV) complex. Eur. J. Pharm. Sci. 2012, 47, 28–34. [Google Scholar] [CrossRef]
- Zhai, G.; Zhu, W.; Duan, Y.; Qu, W.; Yan, Z. Synthesis, characterization and antitumor activity of the germanium-quercetin complex. Main Group Met. Chem. 2012, 35, 103–109. [Google Scholar] [CrossRef]
- Pi, J.; Zeng, J.; Luo, J.-J.; Yang, P.-H.; Cai, J.-Y. Synthesis and biological evaluation of Germanium(IV)–polyphenol complexes as potential anti-cancer agents. Bioorg. Med. Chem. Lett. 2013, 23, 2902–2908. [Google Scholar] [CrossRef]
- Mukherjee, A.; Mana, S. Pharmacological evaluation germanium (IV)-hesperidin complex for hepatocellular carcinoma on rats. World J. Pharm. Res. 2021, 10, 1795–1805. [Google Scholar] [CrossRef]
- Warburg, O. On the Origin of Cancer Cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Warburg, O. On Respiratory Impairment in Cancer Cells. Science 1956, 124, 269–270. [Google Scholar] [CrossRef]
- Warburg, O.H. The Prime Cause and Prevention of Cancer (with Two Prefaces on Prevention); National Cancer Institute: Bethesda, MD, USA, 1967.
- Warburg, O. Otto Warburg on the Prime Cause & Prevention of Cancer: Respiration of Oxygen in Normal Body Cells vs. Fermentation of Sugar in Cancer Cells, 2nd ed.; Konrad Triltsch: Würzburg, Germany, 1969. [Google Scholar]
- Wallace, D.C. Mitochondria and cancer. Nat. Rev. Cancer 2012, 12, 685–698. [Google Scholar] [CrossRef]
- Chakraborti, S.; Ray, B.K.; Roychoudhury, S. (Eds.) Handbook of Oxidative Stress in Cancer: Mechanistic Aspects; Springer: Singapore, 2022; p. LVI, 2675. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef]
- Guo, C.; Sun, L.; Chen, X.; Zhang, D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen. Res. 2013, 8, 2003–2014. [Google Scholar] [CrossRef]
- Kuo, C.-L.; Ponneri Babuharisankar, A.; Lin, Y.-C.; Lien, H.-W.; Lo, Y.K.; Chou, H.-Y.; Tangeda, V.; Cheng, L.-C.; Cheng, A.N.; Lee, A.Y.-L. Mitochondrial oxidative stress in the tumor microenvironment and cancer immunoescape: Foe or friend? J. Biomed. Sci. 2022, 29, 74. [Google Scholar] [CrossRef]
- Sotgia, F.; Martinez-Outschoorn, U.E.; Lisanti, M.P. Mitochondrial oxidative stress drives tumor progression and metastasis: Should we use antioxidants as a key component of cancer treatment and prevention? BMC Med. 2011, 9, 62. [Google Scholar] [CrossRef]
- Thomas, C.; Mackey, M.M.; Diaz, A.A.; Cox, D.P. Hydroxyl radical is produced via the Fenton reaction in submitochondrial particles under oxidative stress: Implications for diseases associated with iron accumulation. Redox Rep. 2009, 14, 102–108. [Google Scholar] [CrossRef]
- Betül, Ç.; Ali Cengiz, Ç. Antioxidant and Oxidative Stress. In Antioxidants; Viduranga, W., Ed.; IntechOpen: Rijeka, Croatia, 2021; Chapter 6. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.-P.-P.; Sulaiman Rahman, H. Antioxidant and Oxidative Stress: A Mutual Interplay in Age-Related Diseases. Front. Pharmacol. 2018, 9, 1162. [Google Scholar] [CrossRef]
- Schwarz, R.; Giese, H. Beiträge zur Chemie des Germaniums, III. Mitteil.: Sulfo-und Pergermanate. Ber. Der Dtsch. Chem. Ges. A B Ser. 1930, 63, 778–782. [Google Scholar] [CrossRef]
- Schwarz, R.; Heinrich, F. Zur Kenntnis der peroxydischen Verbindungen. Z. Für Anorg. Und Allg. Chem. 1935, 223, 387–392. [Google Scholar] [CrossRef]
- Zheng, J.; Yang, L.; Deng, Y.; Zhang, C.; Zhang, Y.; Xiong, S.; Ding, C.; Zhao, J.; Liao, C.; Gong, D. A review of public and environmental consequences of organic germanium. Crit. Rev. Environ. Sci. Technol. 2020, 50, 1384–1409. [Google Scholar] [CrossRef]
- Filella, M. Comment on “A review of public and environmental consequences of organic germanium” by Zheng and co-workers. Crit. Rev. Environ. Sci. Technol. 2022, 53, 1279–1287. [Google Scholar] [CrossRef]
- Zheng, J.; Deng, Y. Response to comment on “A review of public and environmental consequences of organic germanium”. Crit. Rev. Environ. Sci. Technol. 2022, 53, 1478–1488. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).