Importance of Electrode Selection and Number in Reconstructing Standard Twelve Lead Electrocardiograms
Abstract
1. Introduction
2. Materials and Methods
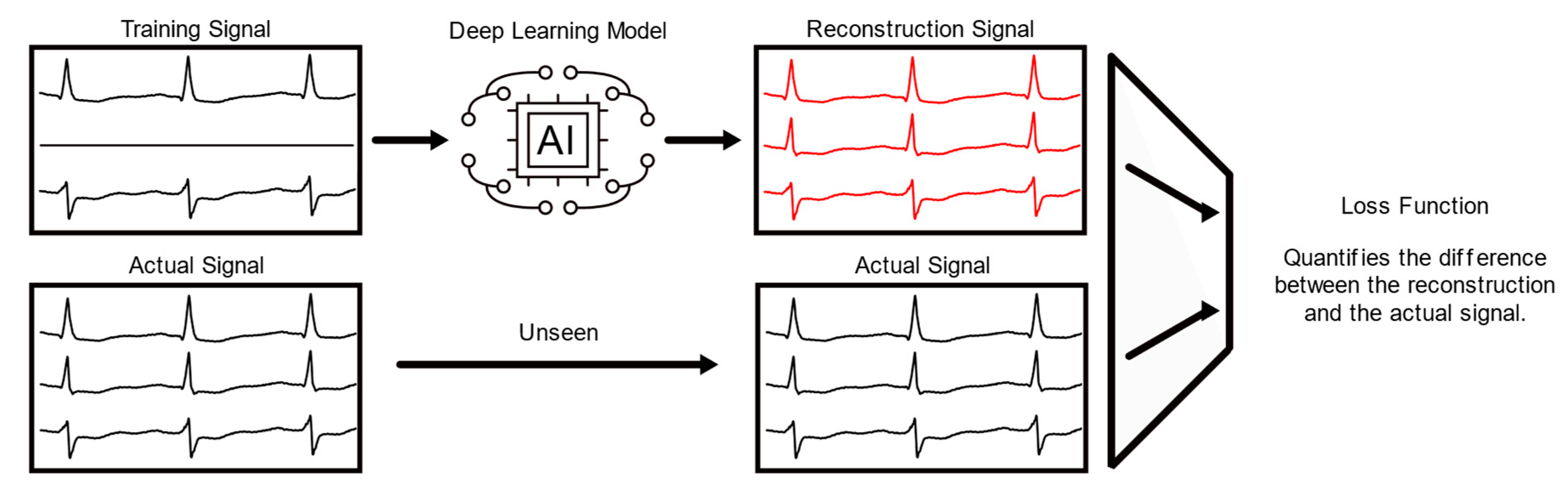
2.1. The Deep Learning Model
2.2. The PTB Database and Data Cleaning
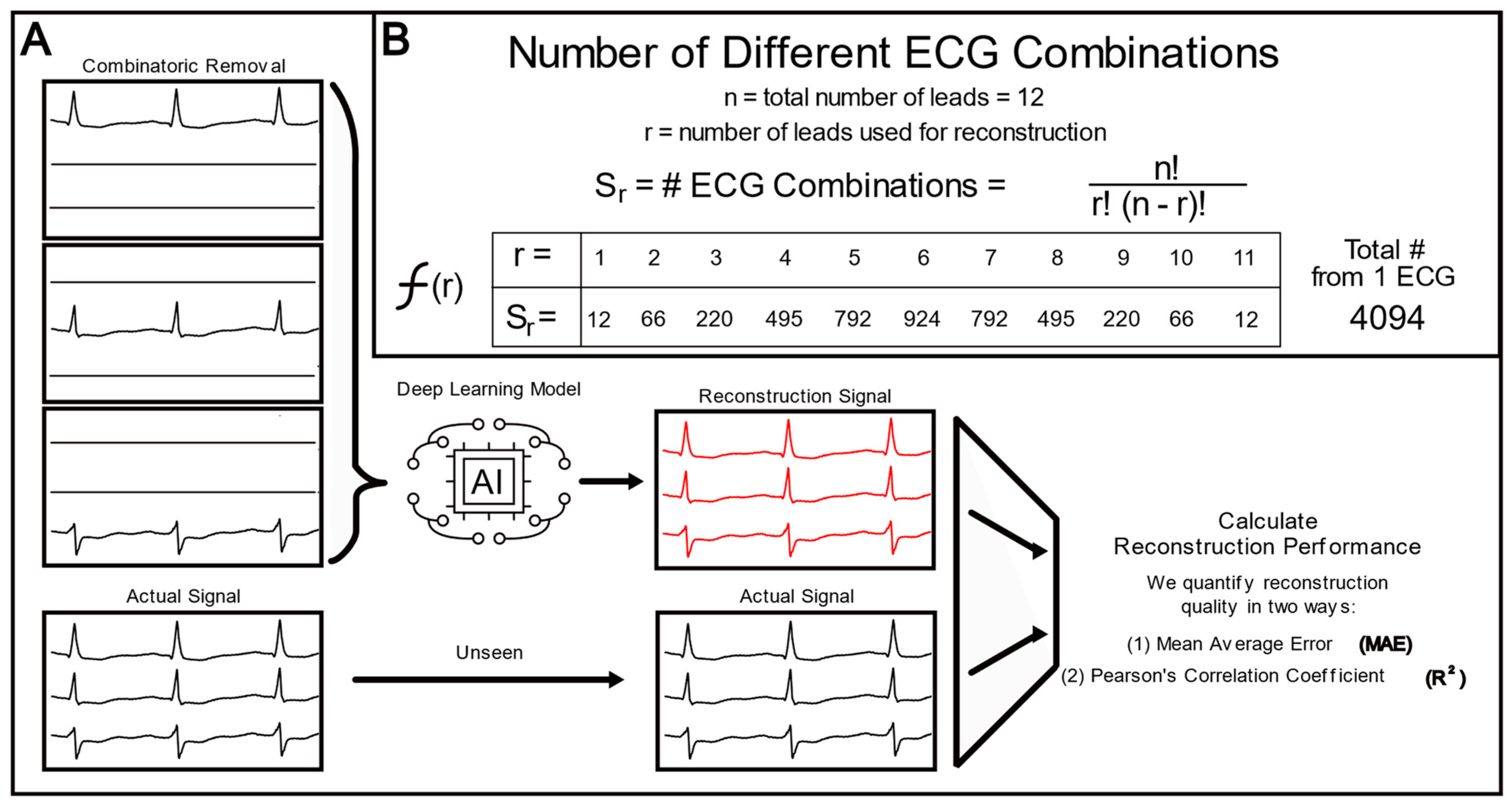
2.3. Lead Combinatorial Analysis
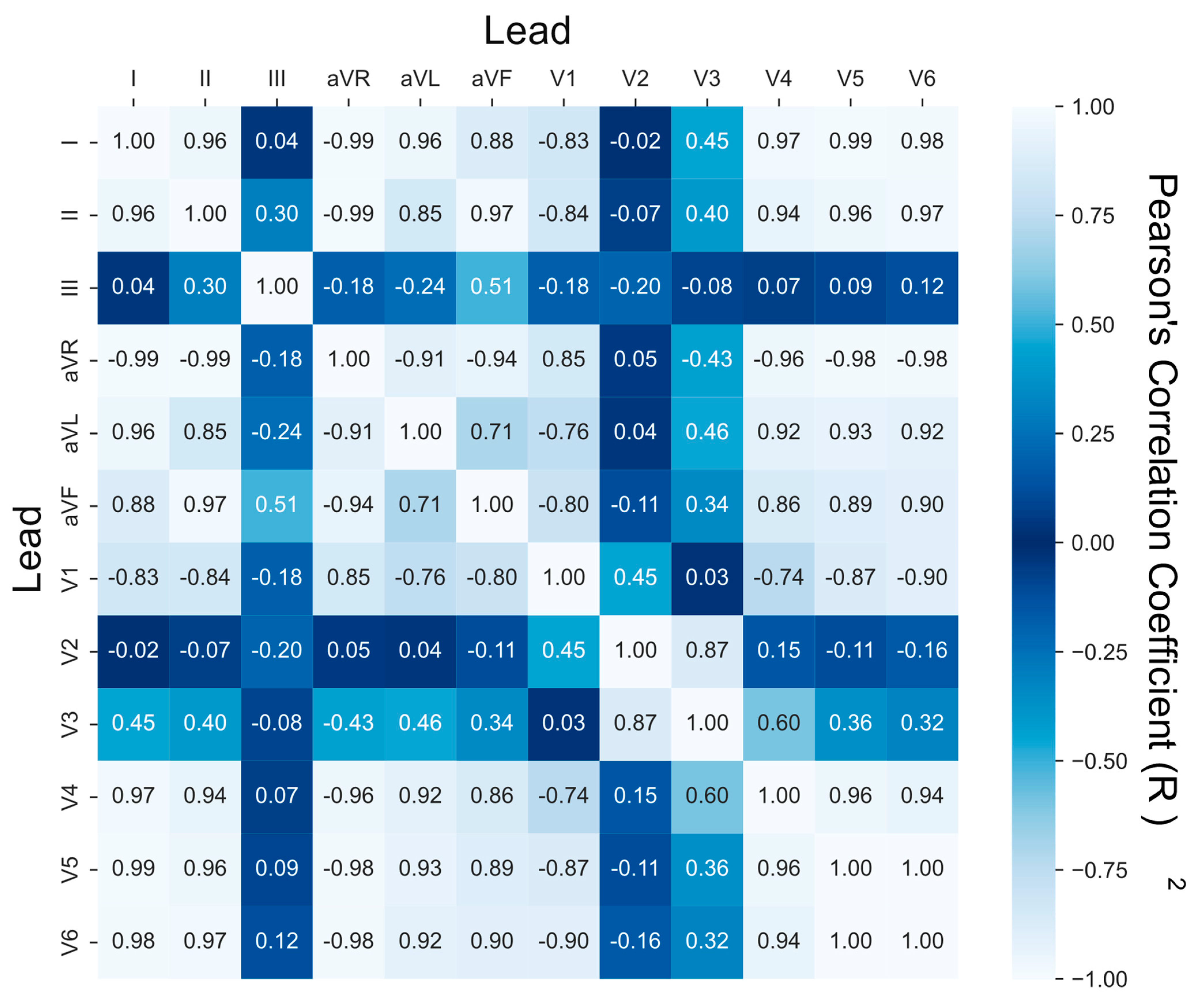
2.4. Lead Correlation Analysis
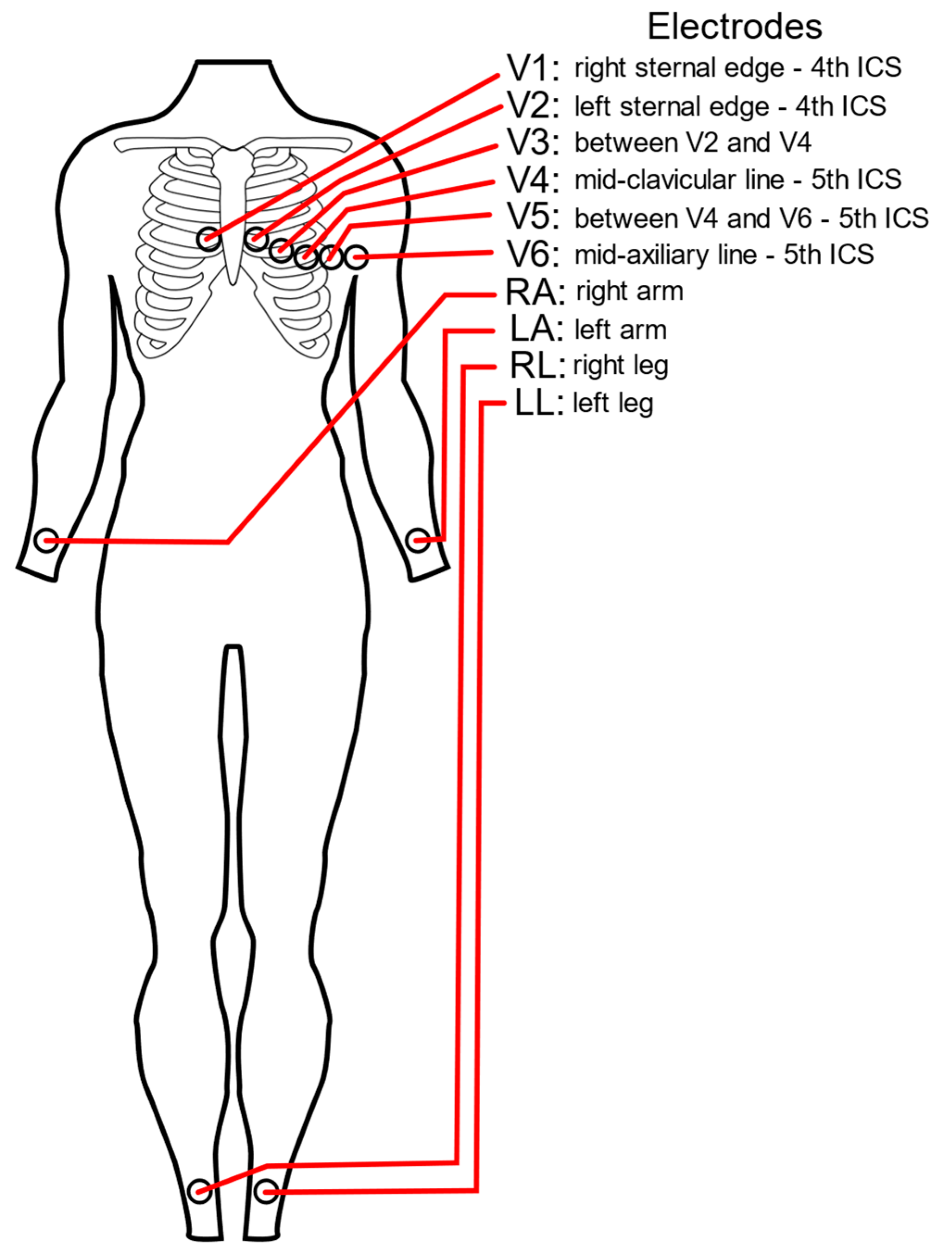
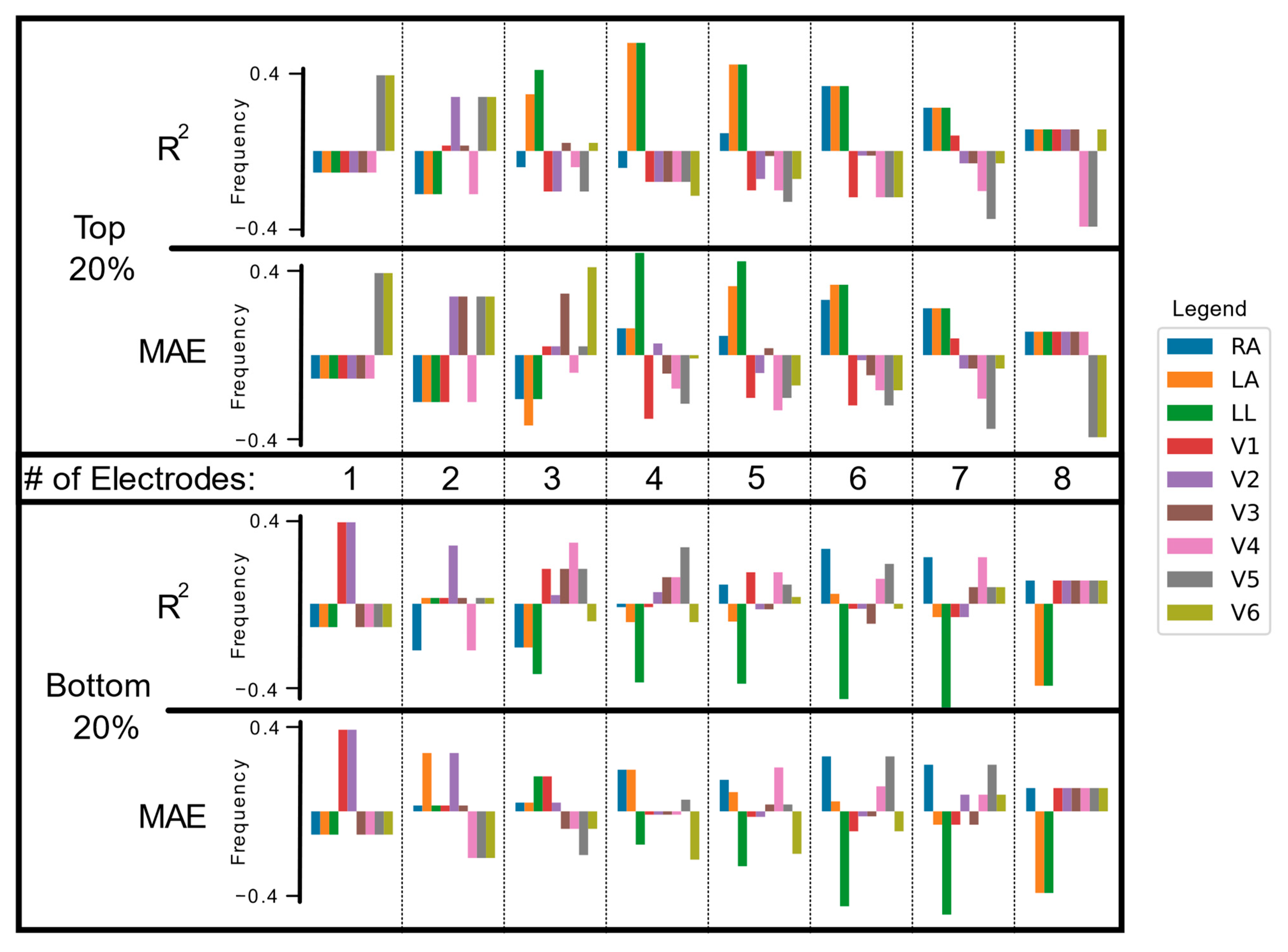
2.5. Electrode Combinatorial Analysis
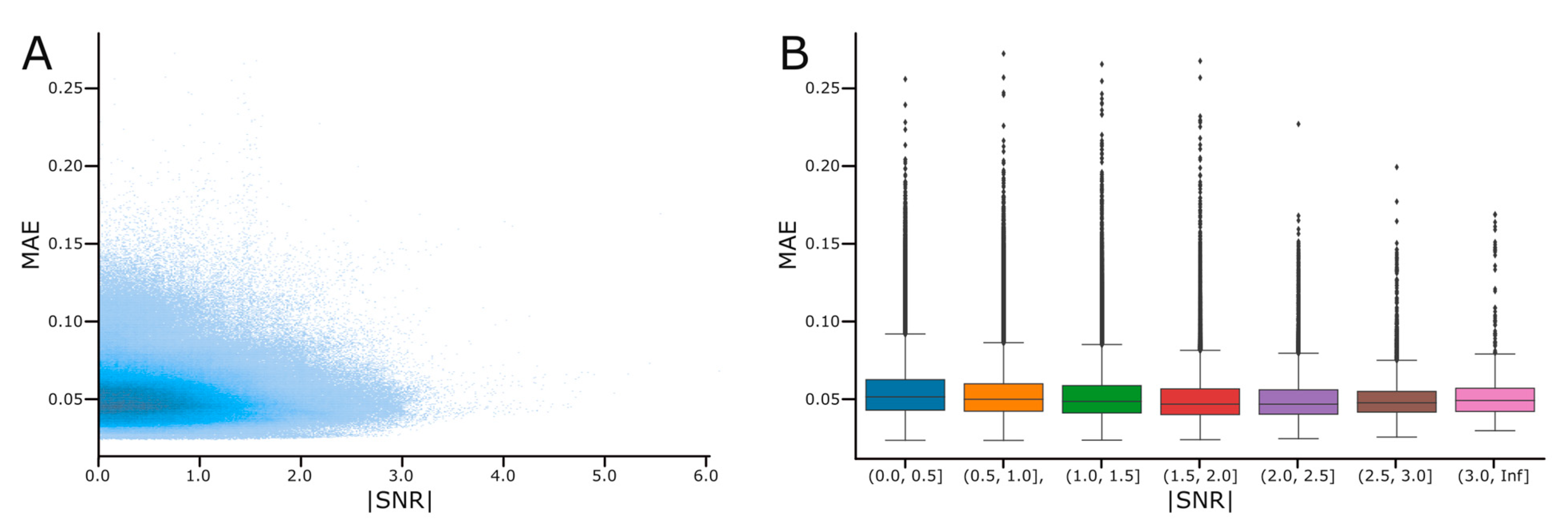
2.6. The Signal-to-Noise Ratio Calculation
2.7. Statistics and Error Calculations
3. Results
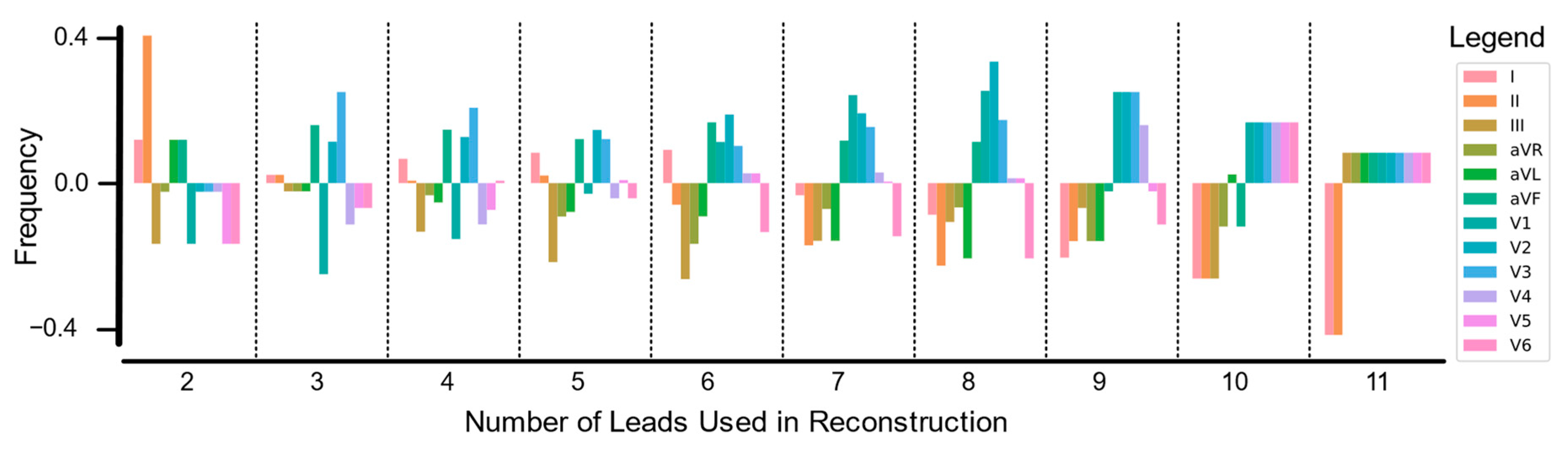
3.1. Lead Importance in Fewer Lead Reconstructions
3.2. Lead Similarity and Correlation
3.3. Electrode Importance in Fewer Lead Reconstructions
3.4. Effect of the Signal-to-Noise Ratio on Reconstruction Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Su, L.; Borov, S.; Zrenner, B. 12-lead Holter electrocardiography: Review of the literature and clinical application update. Herzschrittmachertherapie + Elektrophysiologie 2013, 24, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Papouchado, M.; Walker, P.; James, M.; Clarke, L. Fundamental differences between the standard 12–lead electrocardiograph and the modified (Mason—Likar) exercise lead system. Eur. Heart J. 1987, 8, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, D.; Sattar, A.; Drigalla, D.; Higgins, S. Ambulatory cardiac monitoring for discharged emergency department patients with possible cardiac arrhythmias. West. J. Emerg. Med. 2014, 15, 194. [Google Scholar] [CrossRef] [PubMed]
- Guzik, P.; Malik, M. ECG by mobile technologies. J. Electrocardiol. 2016, 49, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Lobodzinski, S.S. ECG patch monitors for assessment of cardiac rhythm abnormalities. Prog. Cardiovasc. Dis. 2013, 56, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.-H.; Huang, C.-L.; Chang, W.-W.; Chien, J.-T. Automatic classification of myocardial infarction using spline representation of single-lead derived vectorcardiography. Sensors 2020, 20, 7246. [Google Scholar] [CrossRef] [PubMed]
- Francis, J. ECG monitoring leads and special leads. Indian Pacing Electrophysiol. J. 2016, 16, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Hadzievski, L.; Bojovic, B.s.; Vukcevic, V.; Belicev, P.; Pavlovic, S.s.; Vasiljevic-Pokrajcic, Z.; Ostojic, M. A novel mobile transtelephonic system with synthesized 12-lead ECG. IEEE Trans. Inf. Technol. Biomed. 2004, 8, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Mann, S.A.; Orglmeister, R. A flexible PCA-based ECG-reconstruction algorithm with confidence estimation for ECG during exercise. In Proceedings of the Computing in Cardiology, Cambridge, MA, USA, 7–10 September 2014; pp. 33–36. [Google Scholar]
- Tomasic, I.; Trobec, R. Electrocardiographic systems with reduced numbers of leads-synthesis of the 12-lead ECG. IEEE Rev. Biomed. Eng. 2014, 7, 126–142. [Google Scholar] [CrossRef] [PubMed]
- Jain, U.; Butchy, A.A.; Leasure, M.T.; Covalesky, V.A.; McCormick, D.; Mintz, G.S. 12-Lead ECG Reconstruction via Combinatoric Inclusion of Fewer Standard ECG Leads with Implications for Lead Information and Significance. In Proceedings of the Biosignals, Virual Conference, 9–11 February 2022; pp. 133–141. [Google Scholar]
- Wagner, P.; Strodthoff, N.; Bousseljot, R.D.; Kreiseler, D.; Lunze, F.I.; Samek, W.; Schaeffter, T. PTB-XL, a large publicly available electrocardiography dataset. Sci. Data 2020, 7, 154. [Google Scholar] [CrossRef] [PubMed]
- Leasure, M.; Jain, U.; Butchy, A.; Otten, J.; Covalesky, V.A.; McCormick, D.; Mintz, G.S. Deep Learning Algorithm Predicts Angiographic Coronary Artery Disease in Stable Patients Using Only a Standard 12-Lead Electrocardiogram. Can. J. Cardiol. 2021, 37, 1715–1724. [Google Scholar] [CrossRef] [PubMed]
- Jain, U.; Butchy, A.A.; Leasure, M.T. Medical Diagnostic Tool with Neural Model Trained through Machine Learning for Predicting Coronary Disease from ECG Signals. U.S. Patent 11,568,991, 31 January 2023. [Google Scholar]
- Tantithamthavorn, C.; McIntosh, S.; Hassan, A.E.; Matsumoto, K. An empirical comparison of model validation techniques for defect prediction models. IEEE Trans. Softw. Eng. 2016, 43, 1–18. [Google Scholar] [CrossRef]
- Crawford, J.; Doherty, L. Practical Aspects of ECG Recording; M&K Update Ltd.: Keswick, UK, 2012. [Google Scholar]
- Ricciardi, D.; Cavallari, I.; Creta, A.; Di Giovanni, G.; Calabrese, V.; Di Belardino, N.; Mega, S.; Colaiori, I.; Ragni, L.; Proscia, C. Impact of the high-frequency cutoff of bandpass filtering on ECG quality and clinical interpretation: A comparison between 40 Hz and 150 Hz cutoff in a surgical preoperative adult outpatient population. J. Electrocardiol. 2016, 49, 691–695. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Deng, G.; Wei, W.; Wang, H.; Ming, Z. Design of a real-time ECG filter for portable mobile medical systems. IEEE Access 2016, 5, 696–704. [Google Scholar] [CrossRef]
- N. I. o. S. a. Technology, “Signal to Noise Ratio”. 2017. Available online: https://www.itl.nist.gov/div898/software/dataplot/refman2/auxillar/snr.htm (accessed on 15 January 2023).
- Němcová, A.; Smíšek, R.; Maršánová, L.; Smital, L.; Vítek, M. A comparative analysis of methods for evaluation of ECG signal quality after compression. BioMed Res. Int. 2018, 2018, 1868519. [Google Scholar] [CrossRef] [PubMed]
- Chai, T.; Draxler, R.R. Root mean square error (RMSE) or mean absolute error (MAE)?–Arguments against avoiding RMSE in the literature. Geosci. Model Dev. 2014, 7, 1247–1250. [Google Scholar] [CrossRef]
- Janse, R.J.; Hoekstra, T.; Jager, K.J.; Zoccali, C.; Tripepi, G.; Dekker, F.W.; van Diepen, M. Conducting correlation analysis: Important limitations and pitfalls. Clin. Kidney J. 2021, 14, 2332–2337. [Google Scholar] [CrossRef] [PubMed]
- Marinucci, D.; Sbrollini, A.; Marcantoni, I.; Morettini, M.; Swenne, C.A.; Burattini, L. Artificial neural network for atrial fibrillation identification in portable devices. Sensors 2020, 20, 3570. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-M.; Tsai, Y.-H.; Tseng, H.-H.; Liu, K.-C.; Chen, J.-Y.; Huang, C.-H.; Li, G.-Y.; Shen, C.-Y.; Tsao, Y. SRECG: ECG Signal Super-resolution Framework for Portable/Wearable Devices in Cardiac Arrhythmias Classification. IEEE Trans. Consum. Electron. 2023; Early Access. [Google Scholar]
| N (%) | Age | |
|---|---|---|
| Male | 209 (72.1%) | 55.5 |
| Female | 81 (27.9%) | 61.6 |
| Total | 290 (100%) | 57.2 |
| Electrode | Reconstruction | p-Value | |
|---|---|---|---|
| w/Lead | w/o Lead | ||
| RA | 0.836 | 0.754 | 0.000 |
| LA | 0.836 | 0.743 | 0.000 |
| LL | 0.836 | 0.737 | 0.000 |
| V1 | 0.841 | 0.826 | 0.157 |
| V2 | 0.843 | 0.824 | 0.059 |
| V3 | 0.844 | 0.823 | 0.049 |
| V4 | 0.841 | 0.825 | 0.112 |
| V5 | 0.839 | 0.827 | 0.188 |
| V6 | 0.839 | 0.828 | 0.261 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butchy, A.A.; Jain, U.; Leasure, M.T.; Covalesky, V.A.; Mintz, G.S. Importance of Electrode Selection and Number in Reconstructing Standard Twelve Lead Electrocardiograms. Biomedicines 2023, 11, 1526. https://doi.org/10.3390/biomedicines11061526
Butchy AA, Jain U, Leasure MT, Covalesky VA, Mintz GS. Importance of Electrode Selection and Number in Reconstructing Standard Twelve Lead Electrocardiograms. Biomedicines. 2023; 11(6):1526. https://doi.org/10.3390/biomedicines11061526
Chicago/Turabian StyleButchy, Adam A., Utkars Jain, Michael T. Leasure, Veronica A. Covalesky, and Gary S. Mintz. 2023. "Importance of Electrode Selection and Number in Reconstructing Standard Twelve Lead Electrocardiograms" Biomedicines 11, no. 6: 1526. https://doi.org/10.3390/biomedicines11061526
APA StyleButchy, A. A., Jain, U., Leasure, M. T., Covalesky, V. A., & Mintz, G. S. (2023). Importance of Electrode Selection and Number in Reconstructing Standard Twelve Lead Electrocardiograms. Biomedicines, 11(6), 1526. https://doi.org/10.3390/biomedicines11061526






