Spatial and Ecological Factors Modulate the Incidence of Anti-NMDAR Encephalitis—A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Correlating the Incidence of Anti-NMDAR Encephalitis with Different Climatic, Environmental, and Demographic Factors
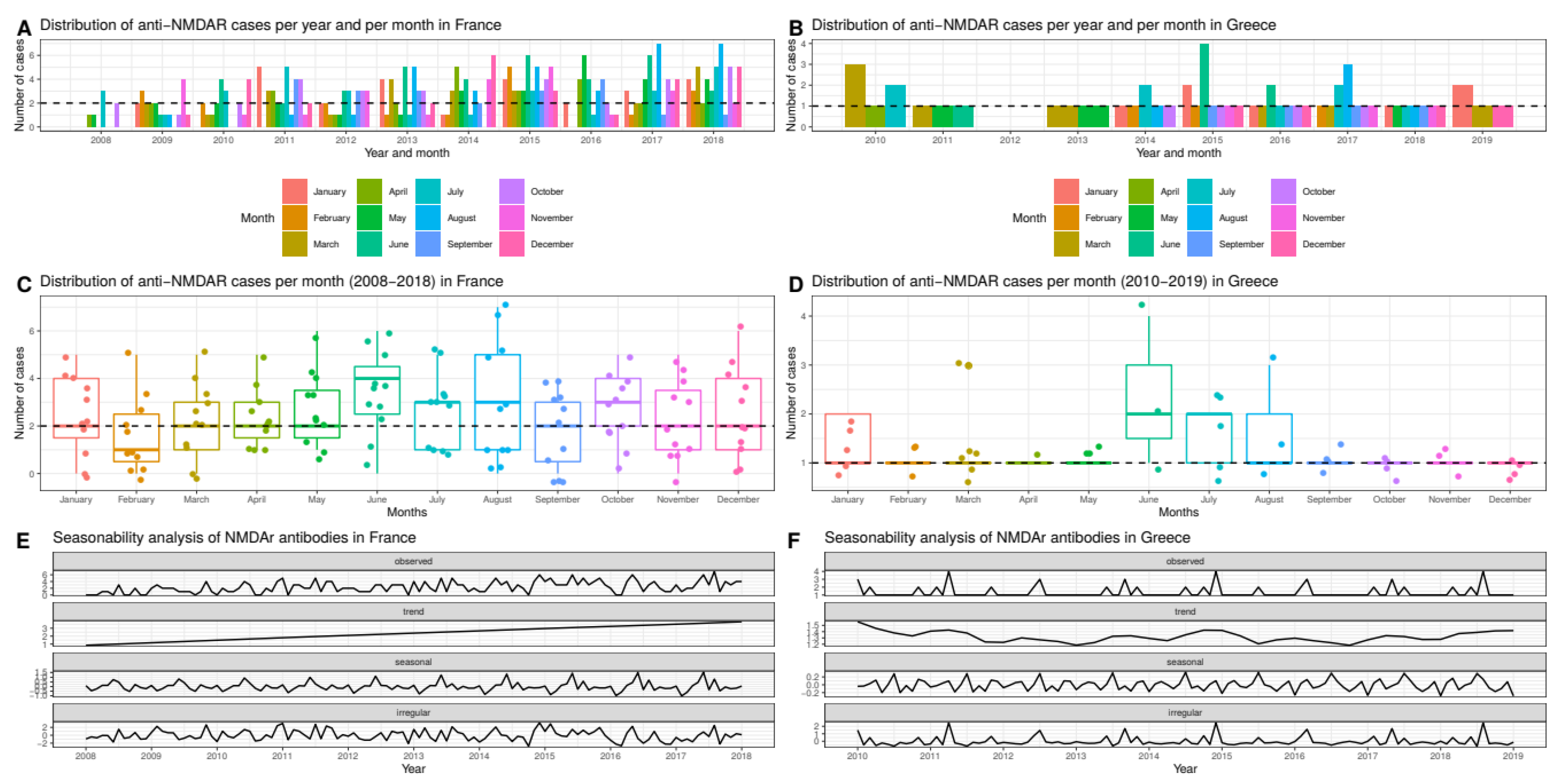
2.2. Seasonal and Monthly Trends
3. Results
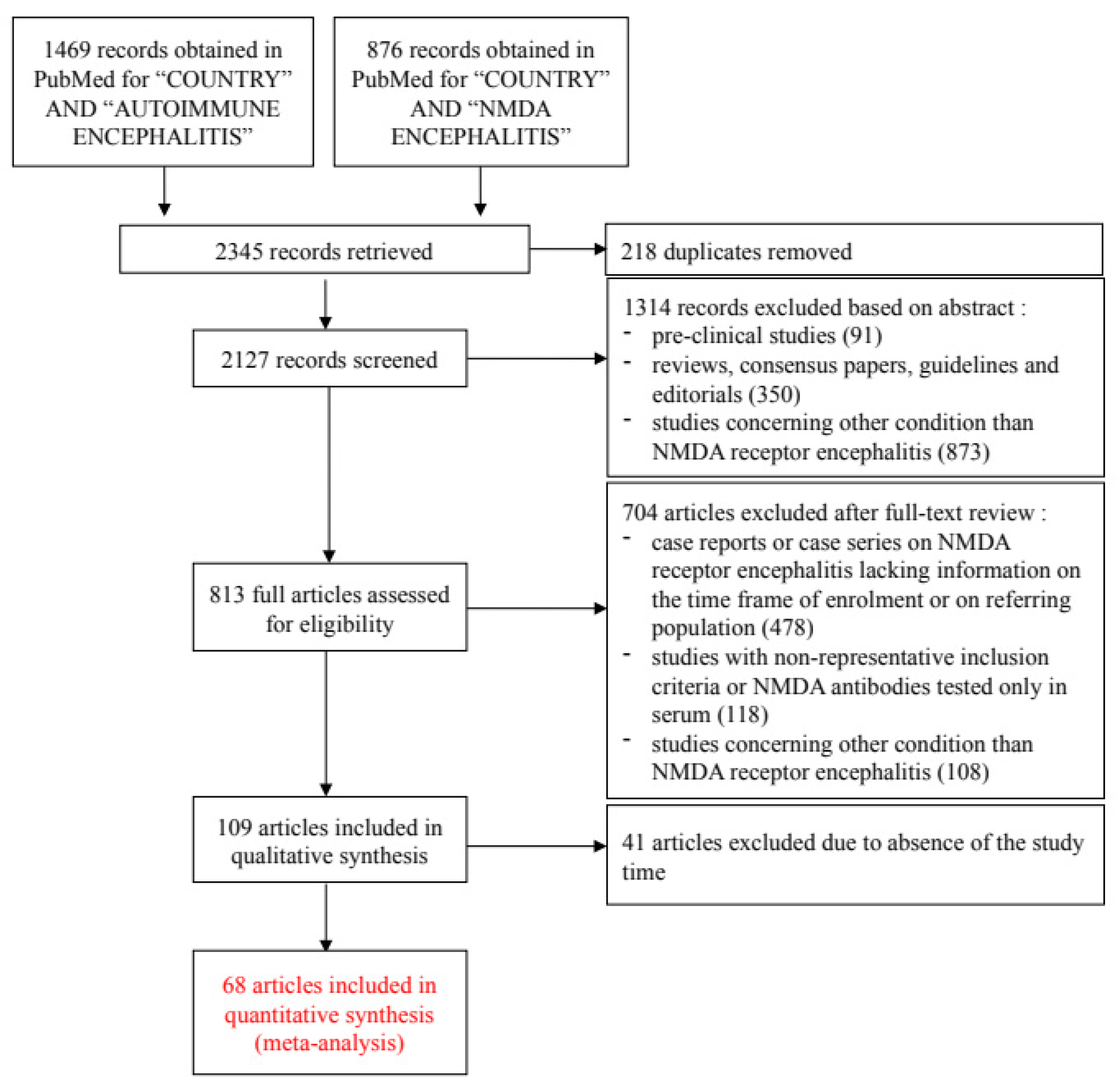
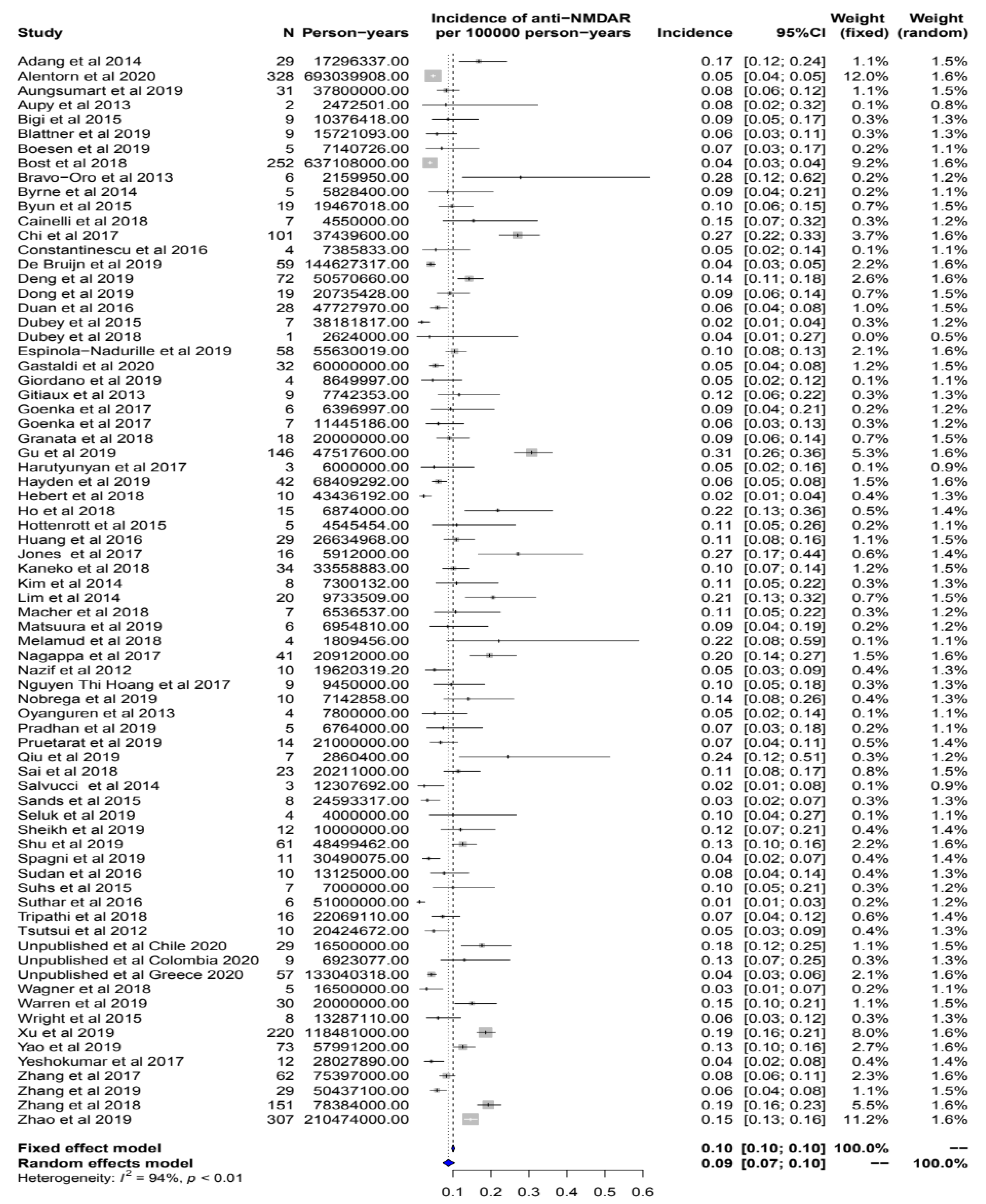
3.1. Literature Meta-Analysis
3.2. Unpublished Data
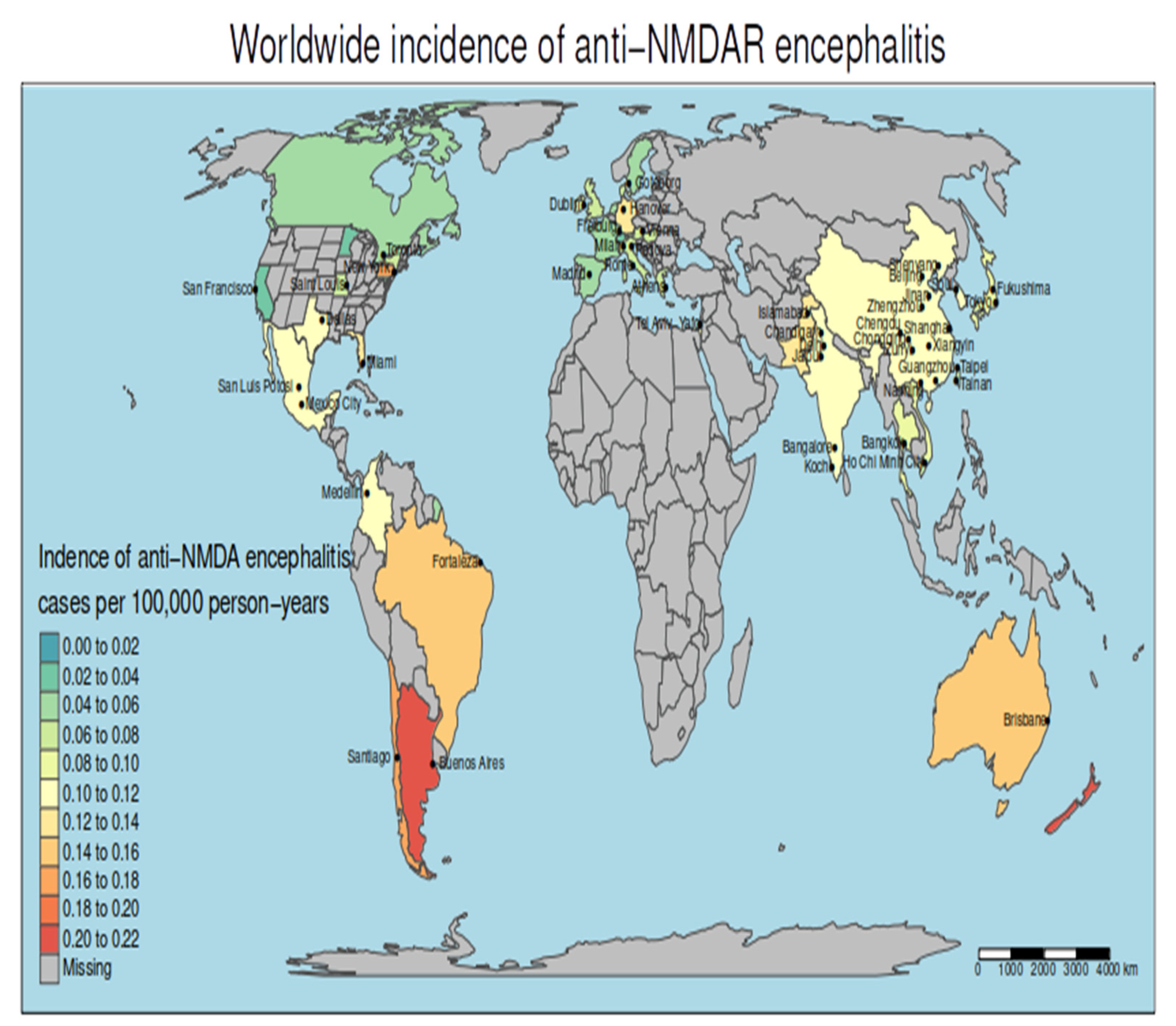
3.3. The Incidence of Anti-NMDAR Encephalitis Differs between Continents
3.4. Geographical Clusters of Higher and Lower Incidence
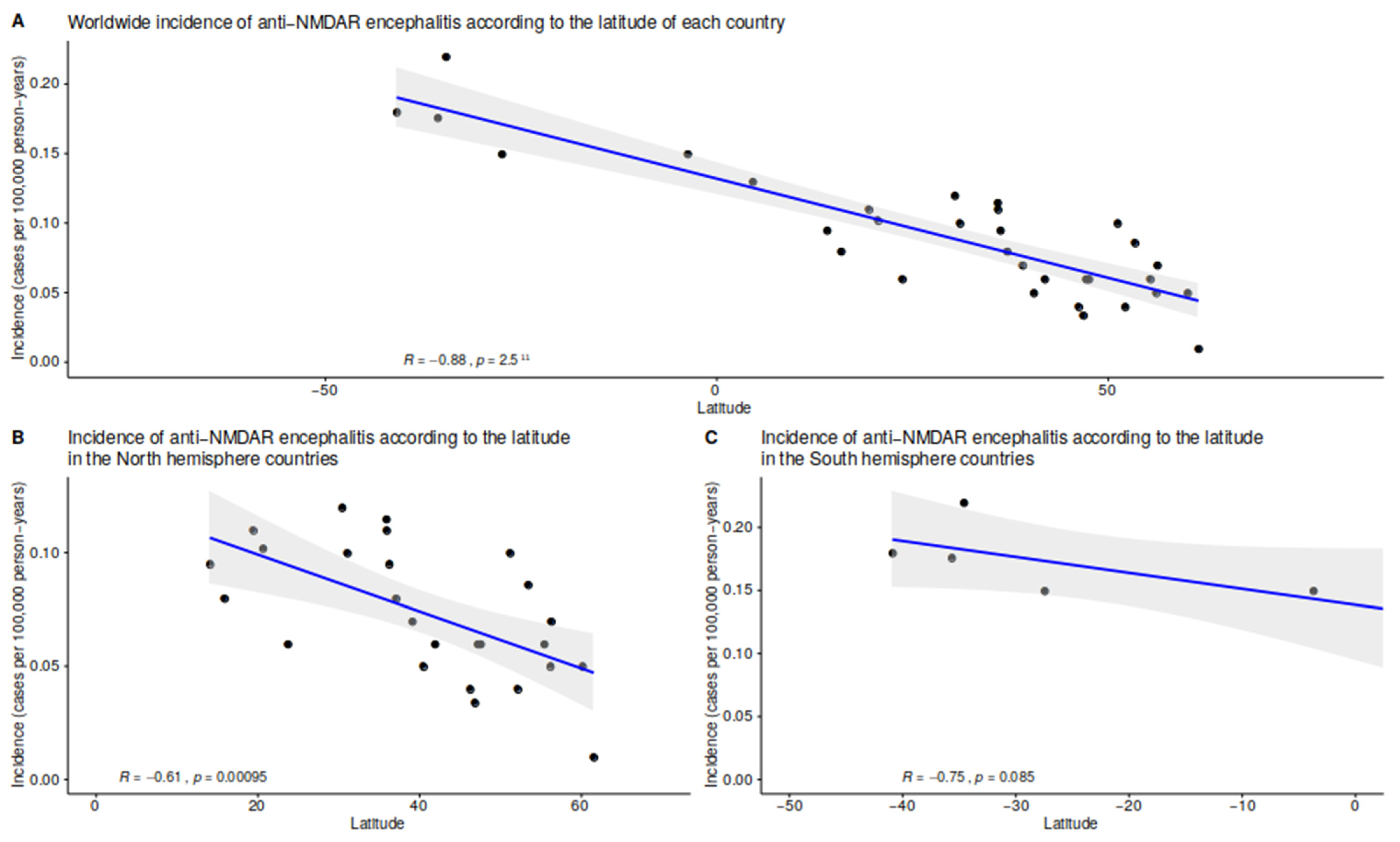
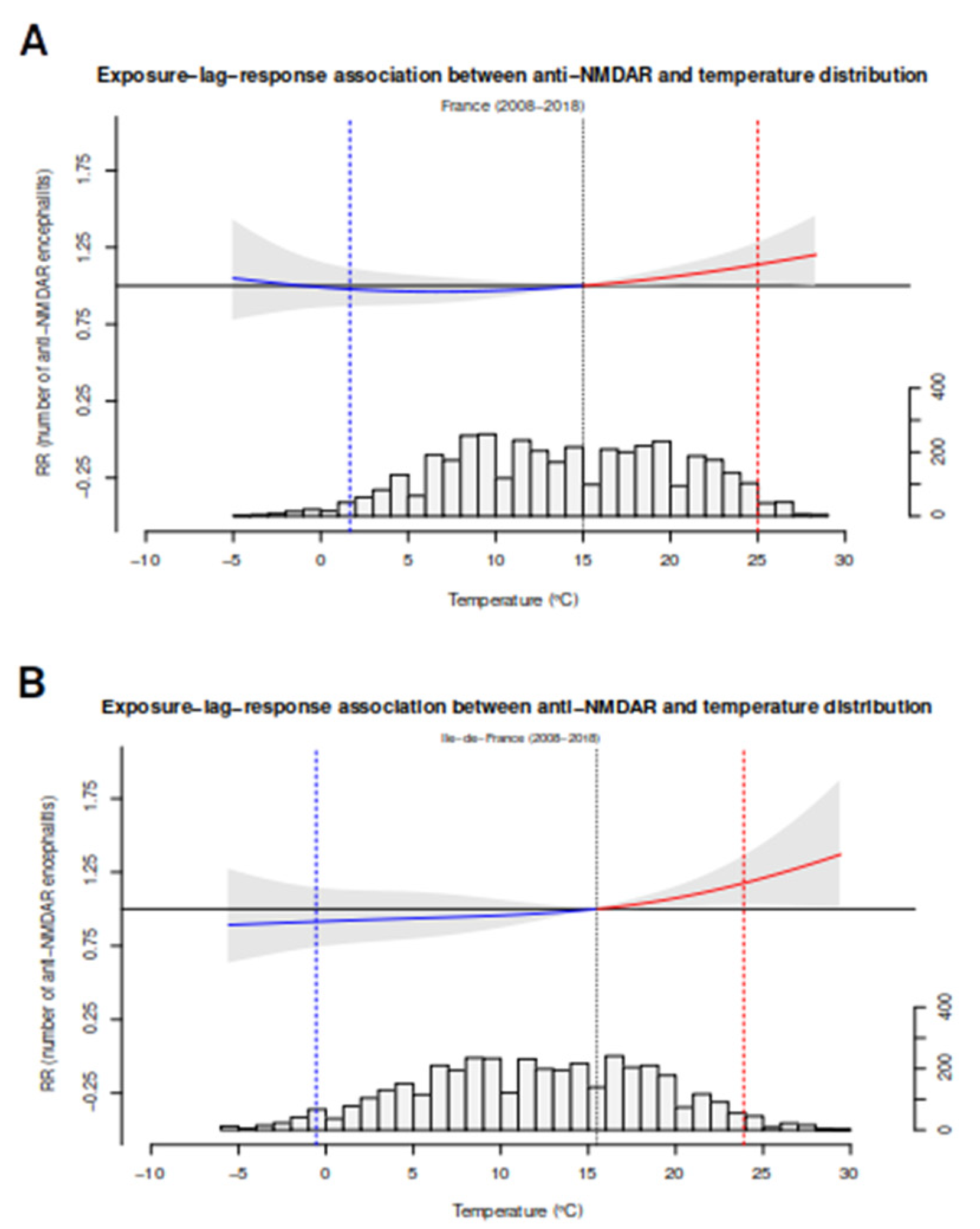
3.5. Multivariate Spatial Analyses: The Impact of Temperature and Latitude
3.6. Spatial and Temporal Analyses on the French and Greek Dataset
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dalmau, J.; Tüzün, E.; Wu, H.-Y.; Masjuan, J.; Ba, J.E.R.; Voloschin, A.; Baehring, J.M.; Shimazaki, H.; Koide, R.; King, D.; et al. Paraneoplastic anti-N-methyl-d-aspartate receptor encephalitis associated with ovarian teratoma. Ann. Neurol. 2007, 61, 25–36. [Google Scholar] [CrossRef]
- Hughes, E.G.; Peng, X.; Gleichman, A.J.; Lai, M.; Zhou, L.; Tsou, R.; Parsons, T.D.; Lynch, D.R.; Dalmau, J.; Balice-Gordon, R.J. Cellular and Synaptic Mechanisms of Anti-NMDA Receptor Encephalitis. J. Neurosci. 2010, 30, 5866–5875. [Google Scholar] [CrossRef]
- Planagumà, J.; Leypoldt, F.; Mannara, F.; Gutiérrez-Cuesta, J.; Martín-García, E.; Aguilar, E.; Titulaer, M.; Petit-Pedrol, M.; Jain, A.; Balice-Gordon, R.; et al. Human N-methyl d-aspartate receptor antibodies alter memory and behaviour in mice. Brain 2015, 138, 94–109. [Google Scholar] [CrossRef]
- Dalmau, J.; Gleichman, A.J.; Hughes, E.G.; Rossi, J.E.; Peng, X.; Lai, M.; Dessain, S.K.; Rosenfeld, M.R.; Balice-Gordon, R.; Lynch, D.R. Anti-NMDA-receptor encephalitis: Case series and analysis of the effects of antibodies. Lancet Neurol. 2008, 7, 1091–1098. [Google Scholar] [CrossRef]
- Dalmau, J.; Lancaster, E.; Martinez-Hernandez, E.; Rosenfeld, M.R.; Balice-Gordon, R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011, 10, 63–74. [Google Scholar] [CrossRef]
- Armangue, T.; Spatola, M.; Vlagea, A.; Mattozzi, S.; Cárceles-Cordon, M.; Martinez-Heras, E.; Llufriu, S.; Muchart, J.; Erro, M.E.; Abraira, L.; et al. Frequency, symptoms, risk factors, and outcomes of autoimmune encephalitis after herpes simplex encephalitis: A prospective observational study and retrospective analysis. Lancet Neurol. 2018, 17, 760–772. [Google Scholar] [CrossRef]
- Ma, J.; Han, W.; Jiang, L. Japanese encephalitis-induced anti-N-methyl-d-aspartate receptor encephalitis: A hospital-based prospective study. Brain Dev. 2020, 42, 179–184. [Google Scholar] [CrossRef]
- Mueller, S.H.; Färber, A.; Prüss, H.; Melzer, N.; Golombeck, K.S.; Kümpfel, T.; Thaler, F.; Elisak, M.; Lewerenz, J.; Kaufmann, M.; et al. Genetic predisposition in anti-LGI1 and anti-NMDA receptor encephalitis. Ann. Neurol. 2018, 83, 863–869. [Google Scholar] [CrossRef]
- Shu, Y.; Qiu, W.; Zheng, J.; Sun, X.; Yin, J.; Yang, X.; Yue, X.; Chen, C.; Deng, Z.; Li, S.; et al. HLA class II allele DRB1*16:02 is associated with anti-NMDAR encephalitis. J. Neurol. Neurosurg. Psychiatry 2019, 90, 652–658. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Wu, J.-D.; Chen, C.-C. The Association of Ovarian Teratoma and Anti-N-Methyl-D-Aspartate Receptor Encephalitis: An Updated Integrative Review. Int. J. Mol. Sci. 2021, 22, 10911. [Google Scholar] [CrossRef]
- Simpson, J.S.; Wang, W.; Otahal, P.; Blizzard, L.; Mei, I.A.F.V.D.; Taylor, B.V. Latitude continues to be significantly associated with the prevalence of multiple sclerosis: An updated meta-analysis. J. Neurol. Neurosurg. Psychiatry 2019, 90, 1193–1200. [Google Scholar] [CrossRef]
- Bergamaschi, R.; Cortese, A.; Pichiecchio, A.; Berzolari, F.G.; Borrelli, P.; Mallucci, G.; Bollati, V.; Romani, A.; Nosari, G.; Villa, S.; et al. Air pollution is associated to the multiple sclerosis inflammatory activity as measured by brain MRI. Mult. Scler. J. 2017, 24, 1578–1584. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; the PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef]
- Adang, L.A.; Lynch, D.R.; Panzer, J.A. Pediatric anti-NMDA receptor encephalitis is seasonal. Ann. Clin. Transl. Neurol. 2014, 1, 921–925. [Google Scholar] [CrossRef]
- Aungsumart, S.; Ha, A.; Apiwattanakul, M. Abnormal level of consciousness predicts outcomes of patients with anti-NMDA encephalitis. J. Clin. Neurosci. 2018, 62, 184–187. [Google Scholar] [CrossRef]
- Aupy, J.; Collongues, N.; Blanc, F.; Tranchant, C.; Hirsch, E.; de Seze, J. Encéphalites dysimmunitaires, données cliniques, radiologiques et immunologiques. Rev. Neurol. 2013, 169, 142–153. [Google Scholar] [CrossRef]
- Bigi, S.; Hladio, M.; Twilt, M.; Dalmau, J.; Benseler, S.M. The growing spectrum of antibody-associated inflammatory brain diseases in children. Neurol. Neuroimmunol. Neuroinflamm. 2015, 2, e92. [Google Scholar] [CrossRef]
- Blattner, M.S.; de Bruin, G.S.; Bucelli, R.C.; Day, G.S. Sleep disturbances are common in patients with autoimmune encephalitis. J. Neurol. 2019, 266, 1007–1015. [Google Scholar] [CrossRef]
- Boesen, M.S.; Born, A.P.; Lydolph, M.C.; Blaabjerg, M.; Børresen, M.L. Pediatric autoimmune encephalitis in Denmark during 2011–17: A nationwide multicenter population-based cohort study. Eur. J. Paediatr. Neurol. 2019, 23, 639–652. [Google Scholar] [CrossRef]
- Bost, C.; Chanson, E.; Picard, G.; Meyronet, D.; Mayeur, M.-E.; Ducray, F.; Rogemond, V.; Psimaras, D.; Antoine, J.-C.; Delattre, J.-Y.; et al. Malignant tumors in autoimmune encephalitis with anti-NMDA receptor antibodies. J. Neurol. 2018, 265, 2190–2200. [Google Scholar] [CrossRef]
- Bravo-Oro, A.; Abud-Mendoza, C.; Quezada-Corona, A.; Dalmau, J.; Campos-Guevara, V. Anti-N-methyl-d-aspartate (NMDA) receptor encephalitis: Experience with six pediatric patients. Potential efficacy of methotrexate. Rev. Neurol. 2013, 57, 405–410. [Google Scholar]
- Byrne, S.; McCoy, B.; Lynch, B.; Webb, D.; King, M.D. Does early treatment improve outcomes in N-methyl-d-aspartate receptor encephalitis? Dev. Med. Child Neurol. 2014, 56, 794–796. [Google Scholar] [CrossRef]
- Byun, J.-I.; Lee, S.-T.; Moon, J.; Jung, K.-H.; Shin, J.-W.; Sunwoo, J.-S.; Lim, J.-A.; Shin, Y.-W.; Kim, T.-J.; Lee, K.-J.; et al. Cardiac sympathetic dysfunction in anti-NMDA receptor encephalitis. Auton. Neurosci. 2015, 193, 142–146. [Google Scholar] [CrossRef]
- Cainelli, E.; Nosadini, M.; Sartori, S.; Suppiej, A. Neuropsychological and Psychopathological Profile Of Anti-Nmdar Encephalitis: A Possible Pathophysiological Model For Pediatric Neuropsychiatric Disorders. Arch. Clin. Neuropsychol. 2018, 34, 1309–1319. [Google Scholar] [CrossRef]
- Chi, X.; Wang, W.; Huang, C.; Wu, M.; Zhang, L.; Li, J.; Zhou, D. Risk factors for mortality in patients with anti-NMDA receptor encephalitis. Acta Neurol. Scand. 2016, 136, 298–304. [Google Scholar] [CrossRef]
- Constantinescu, R.; Krýsl, D.; Bergquist, F.; Andrén, K.; Malmeström, C.; Asztély, F.; Axelsson, M.; Menachem, E.B.; Blennow, K.; Rosengren, L.; et al. Cerebrospinal fluid markers of neuronal and glial cell damage to monitor disease activity and predict long-term outcome in patients with autoimmune encephalitis. Eur. J. Neurol. 2016, 23, 796–806. [Google Scholar] [CrossRef]
- De Bruijn, M.A.; van Sonderen, A.; van Coevorden-Hameete, M.H.; Bastiaansen, A.E.; Schreurs, M.W.; Rouhl, R.P.; van Donselaar, C.A.; Majoie, M.H.; Neuteboom, R.F.; Smitt, P.A.S.; et al. Evaluation of seizure treatment in anti-LGI1, anti-NMDAR, and anti-GABABR encephalitis. Neurology 2019, 92, e2185–e2196. [Google Scholar] [CrossRef]
- Deng, S.; Qiu, K.; Liu, H.; Wu, X.; Lei, Q.; Lu, W. Clinical Characteristics and Short-Term Prognosis of Autoimmune Encephalitis: A Single-Center Cohort Study in Changsha, China. Front. Neurol. 2019, 10, 539. [Google Scholar] [CrossRef]
- Dong, X.; Zheng, D.; Nao, J. Clinical characteristics and factors associated with short-term prognosis in adult patients with autoimmune encephalitis of non-neoplastic etiology. Neurol. Sci. 2019, 40, 1567–1575. [Google Scholar] [CrossRef]
- Duan, B.-C.; Weng, W.-C.; Lin, K.-L.; Wong, L.C.; Li, S.-T.; Hsu, M.-H.; Lin, J.-J.; Fan, P.-C.; Lin, M.-I.; Chiu, N.-C.; et al. Variations of movement disorders in anti-N-methyl-d-aspartate receptor encephalitis. Medicine 2016, 95, e4365. [Google Scholar] [CrossRef]
- Dubey, D.; Pittock, S.J.; Kelly, C.R.; McKeon, A.; Lopez-Chiriboga, A.S.; Lennon, V.A.; Gadoth, A.; Smith, C.Y.; Bryant, S.C.; Klein, C.J.; et al. Autoimmune encephalitis epidemiology and a comparison to infectious encephalitis. Ann. Neurol. 2018, 83, 166–177. [Google Scholar] [CrossRef]
- Dubey, D.; Samudra, N.; Gupta, P.; Agostini, M.; Ding, K.; Van Ness, P.C.; Vernino, S.; Hays, R. Retrospective case series of the clinical features, management and outcomes of patients with autoimmune epilepsy. Seizure 2015, 29, 143–147. [Google Scholar] [CrossRef]
- Nadurille, M.E.; Flores-Rivera, J.; Rivas-Alonso, V.; Vargas-Cañas, S.; Fricchione, G.L.; Bayliss, L.; Martínez-Juárez, I.E.; Hernandez-Vanegas, L.E.; Martinez-Hernandez, R.; Bautista-Gomez, P.; et al. Catatonia in patients with anti-NMDA receptor encephalitis. Psychiatry Clin. Neurosci. 2019, 73, 574–580. [Google Scholar] [CrossRef]
- Fominykh, V.; Brylev, L.; Gaskin, V.; Luzin, R.; Yakovlev, A.; Komoltsev, I.; Belousova, I.; Rosliakova, A.; Guekht, A.; Gulyaeva, N. Neuronal damage and neuroinflammation markers in patients with autoimmune encephalitis and multiple sclerosis. Metab. Brain Dis. 2019, 34, 1473–1485. [Google Scholar] [CrossRef]
- Gastaldi, M.; Mariotto, S.; Giannoccaro, M.P.; Iorio, R.; Zoccarato, M.; Nosadini, M.; Benedetti, L.; Casagrande, S.; Di Filippo, M.; Valeriani, M.; et al. Subgroup comparison according to clinical phenotype and serostatus in autoimmune encephalitis: A multicenter retrospective study. Eur. J. Neurol. 2019, 27, 633–643. [Google Scholar] [CrossRef]
- Giordano, A.; Fazio, R.; Gelibter, S.; Minicucci, F.; Vabanesi, M.; Anzalone, N.; Magnani, G.; Filippi, M.; Martinelli, V. Diagnosing autoimmune encephalitis in a real-world single-centre setting. J. Neurol. 2019, 267, 449–460. [Google Scholar] [CrossRef]
- Gitiaux, C.; Simonnet, H.; Eisermann, M.; Leunen, D.; Dulac, O.; Nabbout, R.; Chevignard, M.; Honnorat, J.; Gataullina, S.; Musset, L.; et al. Early electro-clinical features may contribute to diagnosis of the anti-NMDA receptor encephalitis in children. Clin. Neurophysiol. 2013, 124, 2354–2361. [Google Scholar] [CrossRef]
- Goenka, A.; Jain, V.; Nariai, H.; Spiro, A.; Steinschneider, M. Extended Clinical Spectrum of Anti-N-Methyl-d-Aspartate Receptor Encephalitis in Children: A Case Series. Pediatr. Neurol. 2017, 72, 51–55. [Google Scholar] [CrossRef]
- Granata, T.; Matricardi, S.; Ragona, F.; Freri, E.; Zibordi, F.; Andreetta, F.; Binelli, S.; Nardocci, N. Pediatric NMDAR encephalitis: A single center observation study with a closer look at movement disorders. Eur. J. Paediatr. Neurol. 2018, 22, 301–307. [Google Scholar] [CrossRef]
- Gu, Y.; Zhong, M.; He, L.; Li, W.; Huang, Y.; Liu, J.; Chen, Y.; Xiao, Z. Epidemiology of Antibody-Positive Autoimmune Encephalitis in Southwest China: A Multicenter Study. Front. Immunol. 2019, 10, 2611. [Google Scholar] [CrossRef]
- Harutyunyan, G.; Hauer, L.; Dünser, M.W.; Moser, T.; Pikija, S.; Leitinger, M.; Novak, H.F.; Aichhorn, W.; Trinka, E.; Sellner, J. Risk Factors for Intensive Care Unit Admission in Patients with Autoimmune Encephalitis. Front. Immunol. 2017, 8, 835. [Google Scholar] [CrossRef]
- Hayden, Z.; Böröcz, K.; Csizmadia, Z.; Balogh, P.; Kellermayer, Z.; Bodó, K.; Najbauer, J.; Berki, T. Single-center study of autoimmune encephalitis-related autoantibody testing in Hungary. Brain Behav. 2019, 9, e01454. [Google Scholar] [CrossRef]
- Hébert, J.; Day, G.S.; Steriade, C.; Wennberg, R.A.; Tang-Wai, D.F. Long-Term Cognitive Outcomes in Patients with Autoimmune Encephalitis. Can. J. Neurol. Sci. 2018, 45, 540–544. [Google Scholar] [CrossRef]
- Ho, A.C.-C.; Chan, S.H.-S.; Chan, E.; Wong, S.S.-N.; Fung, S.T.-H.; Cherk, S.W.-W.; Fung, E.L.-W.; Ma, K.-H.; Tsui, K.-W.; Yau, E.K.-C.; et al. Anti-N-methyl-d-aspartate receptor encephalitis in children: Incidence and experience in Hong Kong. Brain Dev. 2018, 40, 473–479. [Google Scholar] [CrossRef]
- Hottenrott, T.; Dersch, R.; Berger, B.; Rauer, S.; Eckenweiler, M.; Huzly, D.; Stich, O. The intrathecal, polyspecific antiviral immune response in neurosarcoidosis, acute disseminated encephalomyelitis and autoimmune encephalitis compared to multiple sclerosis in a tertiary hospital cohort. Fluids Barriers CNS 2015, 12, 27. [Google Scholar] [CrossRef]
- Huang, Q.; Wu, Y.; Qin, R.; Wei, X.; Ma, M. Clinical characteristics and outcomes between children and adults with anti-N-Methyl-d-Aspartate receptor encephalitis. J. Neurol. 2016, 263, 2446–2455. [Google Scholar] [CrossRef]
- Jones, H.F.; Mohammad, S.S.; Reed, P.W.; Dunn, P.P.J.; Steele, R.H.; Dale, R.C.; Sharpe, C. Anti-N-methyl-d-aspartate receptor encephalitis in Māori and Pacific Island children in New Zealand. Dev. Med. Child Neurol. 2017, 59, 719–724. [Google Scholar] [CrossRef]
- Kaneko, A.; Kaneko, J.; Tominaga, N.; Kanazawa, N.; Hattori, K.; Ugawa, Y.; Moriya, A.; Kuzume, D.; Ishima, D.; Kitamura, E.; et al. Pitfalls in clinical diagnosis of anti-NMDA receptor encephalitis. J. Neurol. 2018, 265, 586–596. [Google Scholar] [CrossRef]
- Kim, S.Y.; Choi, S.A.; Ryu, H.W.; Kim, H.; Lim, B.C.; Hwang, H.; Chae, J.-H.; Choi, J.; Kim, K.J.; Hwang, Y.S.; et al. Screening Autoimmune Anti-neuronal Antibodies in Pediatric Patients with Suspected Autoimmune Encephalitis. J. Epilepsy Res. 2014, 4, 55–61. [Google Scholar] [CrossRef]
- Lim, J.-A.; Lee, S.-T.; Jung, K.-H.; Kim, S.; Shin, J.-W.; Moon, J.; Byun, J.-I.; Kim, T.-J.; Shin, Y.-W.; Lee, K.-J.; et al. Anti-N-Methyl-D-Aspartate Receptor Encephalitis in Korea: Clinical Features, Treatment, and Outcome. J. Clin. Neurol. 2014, 10, 157–161. [Google Scholar] [CrossRef]
- Macher, S.; Zimprich, F.; De Simoni, D.; Höftberger, R.; Rommer, P.S. Management of Autoimmune Encephalitis: An Observational Monocentric Study of 38 Patients. Front. Immunol. 2018, 9, 2708. [Google Scholar] [CrossRef]
- Matsuura, R.; Hamano, S.-I.; Daida, A.; Nonoyama, H.; Kubota, J.; Ikemoto, S.; Hirata, Y.; Koichihara, R.; Kikuchi, K.; Yamaguchi, A.; et al. Serum matrix metallopeptidase-9 and tissue inhibitor of metalloproteinase-1 levels in autoimmune encephalitis. Brain Dev. 2020, 42, 264–269. [Google Scholar] [CrossRef]
- Melamud, L.I.; Fernández, V.C.; Manin, A.; Villa, A.M. Autoimmune encephalitis and immune therapy: Lessons from Argentina. Acta Neurol. Belg. 2018, 120, 565–572. [Google Scholar] [CrossRef]
- Nagappa, M.; Parayil, S.B.; Mahadevan, A.; Sinha, S.; Mathuranath, P.S.; Taly, A.B. Management of Anti- N-Methyl-d-Aspartate (NMDA) Receptor Encephalitis in Children. J. Child Neurol. 2017, 32, 513–514. [Google Scholar] [CrossRef]
- Nazif, T.M.; Vázquez, J.; Honig, L.S.; Dizon, J.M. Anti-N-methyl-D-aspartate receptor encephalitis: An emerging cause of centrally mediated sinus node dysfunction. Europace 2012, 14, 1188–1194. [Google Scholar] [CrossRef]
- Hoang, M.N.T.; Hoan, P.N.; Le Van, T.; McBride, A.; Trung, N.H.D.; Tan, T.T.; Thu, H.N.T.; Heemskerk, D.; Day, J.; Vincent, A.; et al. First reported cases of anti-NMDA receptor encephalitis in Vietnamese adolescents and adults. J. Neurol. Sci. 2017, 373, 250–253. [Google Scholar] [CrossRef]
- Nóbrega, P.R.; Pitombeira, M.S.; Mendes, L.S.; Krueger, M.B.; Santos, C.F.; Morais, N.M.D.M.; Simabukuro, M.M.; Maia, F.M.; Braga-Neto, P. Clinical Features and Inflammatory Markers in Autoimmune Encephalitis Associated with Antibodies Against Neuronal Surface in Brazilian Patients. Front. Neurol. 2019, 10, 472. [Google Scholar] [CrossRef]
- Oyanguren, B.; Sánchez, V.; González, F.J.; De Felipe, A.; Esteban, L.; López-Sendón, J.L.; Garcia-Barragán, N.; Millán, J.M.-S.; Masjuán, J.; Corral, I. Limbic encephalitis: A clinical-radiological comparison between herpetic and autoimmune etiologies. Eur. J. Neurol. 2013, 20, 1566–1570. [Google Scholar] [CrossRef]
- Pradhan, S.; Das, A.; Das, A.; Mulmuley, M. Antibody negative autoimmune encephalitis—Does it differ from definite one? Ann. Indian Acad. Neurol. 2019, 22, 401–408. [Google Scholar] [CrossRef]
- Pruetarat, N.; Netbaramee, W.; Pattharathitikul, S.; Veeravigrom, M. Clinical manifestations, treatment outcomes, and prognostic factors of pediatric anti-NMDAR encephalitis in tertiary care hospitals: A multicenter retrospective/prospective cohort study. Brain Dev. 2019, 41, 436–442. [Google Scholar] [CrossRef]
- Qiu, X.; Zhang, H.; Li, D.; Wang, J.; Jiang, Z.; Zhou, Y.; Xu, P.; Zhang, J.; Feng, Z.; Yu, C.; et al. Analysis of Clinical Characteristics and Poor Prognostic Predictors in Patients with an Initial Diagnosis of Autoimmune Encephalitis. Front. Immunol. 2019, 10, 1286. [Google Scholar] [CrossRef]
- Sai, Y.; Zhang, X.; Feng, M.; Tang, J.; Liao, H.; Tan, L. Clinical diagnosis and treatment of pediatric anti-N-methyl-d-aspartate receptor encephalitis: A single center retrospective study. Exp. Ther. Med. 2018, 16, 1442–1448. [Google Scholar] [CrossRef]
- Salvucci, A.; Devine, I.M.; Hammond, D.; Sheth, R.D. Pediatric Anti-NMDA (N-methyl d-Aspartate) Receptor Encephalitis. Pediatr. Neurol. 2014, 50, 507–510. [Google Scholar] [CrossRef]
- Sands, T.T.; Nash, K.; Tong, S.; Sullivan, J. Focal seizures in children with anti-NMDA receptor antibody encephalitis. Epilepsy Res. 2015, 112, 31–36. [Google Scholar] [CrossRef]
- Seluk, L.; Taliansky, A.; Yonath, H.; Gilburd, B.; Amital, H.; Shoenfeld, Y.; Kivity, S. A large screen for paraneoplastic neurological autoantibodies; diagnosis and predictive values. Clin. Immunol. 2018, 199, 29–36. [Google Scholar] [CrossRef]
- Sheikh, S.; Ahmad, A.; Ahmed, T.A. Anti NMDA receptor antibody encephalitis in Pakistan: Clinicopathological features and treatment outcomes. J. Pak. Med. Assoc. 2019, 69, 1910–1914. [Google Scholar] [CrossRef]
- Spagni, G.; Iorio, R. Grading the severity of autoimmune encephalitis: Advances and pitfalls. Ann. Neurol. 2019, 86, 150. [Google Scholar] [CrossRef]
- Sudan, Y.S.; Vinayan, K.P.; Roy, A.G.; Wagh, A.; Kannoth, S.; Patil, S. Clinical Characteristics and Follow-Up of South Indian Children with Autoimmune Encephalopathy. Indian J. Pediatr. 2016, 83, 1367–1373. [Google Scholar] [CrossRef]
- Sühs, K.-W.; Wegner, F.; Skripuletz, T.; Trebst, C.; Ben Tayeb, S.; Raab, P.; Stangel, M. Heterogeneity of clinical features and corresponding antibodies in seven patients with anti-NMDA receptor encephalitis. Exp. Ther. Med. 2015, 10, 1283–1292. [Google Scholar] [CrossRef]
- Suthar, R.; Saini, A.G.; Sankhyan, N.; Sahu, J.K.; Singhi, P. Childhood Anti-NMDA Receptor Encephalitis. Indian J. Pediatr. 2016, 83, 628–633. [Google Scholar] [CrossRef]
- Tripathi, M.; Tripathi, M.; Roy, S.G.; Parida, G.K.; Ihtisham, K.; Dash, D.; Damle, N.; Shamim, S.A.; Bal, C. Metabolic topography of autoimmune non-paraneoplastic encephalitis. Neuroradiology 2017, 60, 189–198. [Google Scholar] [CrossRef]
- Tsutsui, K.; Kanbayashi, T.; Tanaka, K.; Boku, S.; Ito, W.; Tokunaga, J.; Mori, A.; Hishikawa, Y.; Shimizu, T.; Nishino, S. Anti-NMDA-receptor antibody detected in encephalitis, schizophrenia, and narcolepsy with psychotic features. BMC Psychiatry 2012, 12, 37. [Google Scholar] [CrossRef]
- Wagner, J.N.; Kalev, O.; Sonnberger, M.; Krehan, I.; Von Oertzen, T.J. Evaluation of Clinical and Paraclinical Findings for the Differential Diagnosis of Autoimmune and Infectious Encephalitis. Front. Neurol. 2018, 9, 434. [Google Scholar] [CrossRef]
- Warren, N.; O’Gorman, C.; McKeon, G.; Swayne, A.; Blum, S.; Siskind, D. Psychiatric management of anti-NMDAR encephalitis: A cohort analysis. Psychol. Med. 2019, 51, 435–440. [Google Scholar] [CrossRef]
- Wright, S.; Hacohen, Y.; Jacobson, L.; Agrawal, S.; Gupta, R.; Philip, S.; Smith, M.; Lim, M.; Wassmer, E.; Vincent, A. N-methyl-d-aspartate receptor antibody-mediated neurological disease: Results of a UK-based surveillance study in children. Arch. Dis. Child. 2015, 100, 521–526. [Google Scholar] [CrossRef]
- Xu, X.; Lu, Q.; Huang, Y.; Fan, S.; Zhou, L.; Yuan, J.; Yang, X.; Ren, H.; Sun, D.; Dai, Y.; et al. Anti-NMDAR encephalitis. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e633. [Google Scholar] [CrossRef]
- Yao, L.; Yue, W.; Xunyi, W.; Jianhong, W.; Guoxing, Z.; Zhen, H. Clinical features and long-term outcomes of seizures associated with autoimmune encephalitis: A follow-up study in East China. J. Clin. Neurosci. 2019, 68, 73–79. [Google Scholar] [CrossRef]
- Yeshokumar, A.K.; Gordon-Lipkin, E.; Arenivas, A.; Cohen, J.; Venkatesan, A.; Saylor, D.; Probasco, J.C. Neurobehavioral outcomes in autoimmune encephalitis. J. Neuroimmunol. 2017, 312, 8–14. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, C.; Zhu, W.; Wang, B.; Liang, H.; Guo, S. Factors Affecting the Response to First-Line Treatments in Patients with Anti-N-Methyl-d-Aspartate Receptor Encephalitis. J. Clin. Neurol. 2019, 15, 369–375. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, G.; Di Jiang, M.; Li, L.P.; Su, Y.Y. Analysis of electroencephalogram characteristics of anti-NMDA receptor encephalitis patients in China. Clin. Neurophysiol. 2017, 128, 1227–1233. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, C.; Xu, X.; Zhang, Y.; Ren, H.; Ren, Z.; Li, G.; Zhang, J.; Guan, H. Coexistence of Autoimmune Encephalitis and Other Systemic Autoimmune Diseases. Front. Neurol. 2019, 10, 1142. [Google Scholar] [CrossRef]
- Graus, F.; Titulaer, M.J.; Balu, R.; Benseler, S.; Bien, C.G.; Cellucci, T.; Cortese, I.; Dale, R.C.; Gelfand, J.M.; Geschwind, M.; et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016, 15, 391–404. [Google Scholar] [CrossRef]
- Fay, M.P.; Feuer, E.J. Confidence intervals for directly standardized rates: A method based on the gamma distribution. Stat. Med. 1997, 16, 791–801. [Google Scholar] [CrossRef]
- Rothman, K.J. Epidemiology: An Introduction; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials revisited. Contemp. Clin. Trials 2015, 45, 139–145. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Harbord, R.M.; Egger, M.; Sterne, J. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat. Med. 2005, 25, 3443–3457. [Google Scholar] [CrossRef]
- GBD 2016 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1260–1344. [Google Scholar] [CrossRef]
- Gasparrini, A.; Guo, Y.; Hashizume, M.; Lavigne, E.; Zanobetti, A.; Schwartz, J.; Tobías, A.; Tong, S.; Rocklöv, J.; Forsberg, B.; et al. Mortality risk attributable to high and low ambient temperature: A multicountry observational study. Lancet 2015, 386, 369–375. [Google Scholar] [CrossRef]
- Findley, D.F.; Monsell, B.C.; Bell, W.R.; Otto, M.C.; Chen, B.-C. New Capabilities and Methods of the X-12-ARIMA Seasonal-Adjustment Program. J. Bus. Econ. Stat. 1998, 16, 127. [Google Scholar]
- Cleveland, R.B.; Cleveland, W.S.; McRae, J.E.; Terpenning, I. STL: A seasonal-trend decomposition. J. Off. Stat. 1990, 6, 3–73. [Google Scholar]
- Shapira, Y.; Agmon-Levin, N.; Shoenfeld, Y. Defining and analyzing geoepidemiology and human autoimmunity. J. Autoimmun. 2010, 34, J168–J177. [Google Scholar] [CrossRef]
- Shapira, Y.; Agmon-Levin, N.; Shoenfeld, Y. Geoepidemiology of autoimmune rheumatic diseases. Nat. Rev. Rheumatol. 2010, 6, 468–476. [Google Scholar] [CrossRef]
- Kurtzke, J.F. A reassessment of the distribution of multiple sclerosis. Acta Neurol. Scand. 1975, 51, 137–157. [Google Scholar] [CrossRef]
- Koch-Henriksen, N.; Sørensen, P.S. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol. 2010, 9, 520–532. [Google Scholar] [CrossRef]
- Jin, Y.-P.; De Pedro-Cuesta, J.; Söderström, M.; Link, H. Incidence of Optic Neuritis in Stockholm, Sweden, 1990–1995: II. Time and Space Patterns. Arch. Neurol. 1999, 56, 975–980. [Google Scholar] [CrossRef]
- Waernbaum, I.; Dahlquist, G. Low mean temperature rather than few sunshine hours are associated with an increased incidence of type 1 diabetes in children. Eur. J. Epidemiol. 2015, 31, 61–65. [Google Scholar] [CrossRef]
- George, B.P.; Schneider, E.B.; Venkatesan, A. Encephalitis Hospitalization Rates and Inpatient Mortality in the United States, 2000–2010. PLoS ONE 2014, 9, e104169. [Google Scholar] [CrossRef]
- Barbadoro, P.; Marigliano, A.; Ricciardi, A.; D’Errico, M.M.; Prospero, E. Trend of hospital utilization for encephalitis. Epidemiol. Infect. 2011, 140, 753–764. [Google Scholar] [CrossRef]
- Vora, N.M.; Holman, R.C.; Mehal, J.M.; Steiner, C.A.; Blanton, J.; Sejvar, J. Burden of encephalitis-associated hospitalizations in the United States, 1998-2010. Neurology 2014, 82, 443–451. [Google Scholar] [CrossRef]
- Dehner, C.; Fine, R.; Kriegel, M. The microbiome in systemic autoimmune disease: Mechanistic insights from recent studies. Curr. Opin. Rheumatol. 2019, 31, 201–207. [Google Scholar] [CrossRef]
- Hammond, S.R.; English, D.R.; McLeod, J.G. The age-range of risk of developing multiple sclerosis: Evidence from a migrant population in Australia. Brain 2000, 123, 968–974. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alentorn, A.; Berzero, G.; Alexopoulos, H.; Tzartos, J.; Reyes Botero, G.; Morales Martínez, A.; Muñiz-Castrillo, S.; Vogrig, A.; Joubert, B.; García Jiménez, F.A.; et al. Spatial and Ecological Factors Modulate the Incidence of Anti-NMDAR Encephalitis—A Systematic Review. Biomedicines 2023, 11, 1525. https://doi.org/10.3390/biomedicines11061525
Alentorn A, Berzero G, Alexopoulos H, Tzartos J, Reyes Botero G, Morales Martínez A, Muñiz-Castrillo S, Vogrig A, Joubert B, García Jiménez FA, et al. Spatial and Ecological Factors Modulate the Incidence of Anti-NMDAR Encephalitis—A Systematic Review. Biomedicines. 2023; 11(6):1525. https://doi.org/10.3390/biomedicines11061525
Chicago/Turabian StyleAlentorn, Agustí, Giulia Berzero, Harry Alexopoulos, John Tzartos, Germán Reyes Botero, Andrea Morales Martínez, Sergio Muñiz-Castrillo, Alberto Vogrig, Bastien Joubert, Francisco A. García Jiménez, and et al. 2023. "Spatial and Ecological Factors Modulate the Incidence of Anti-NMDAR Encephalitis—A Systematic Review" Biomedicines 11, no. 6: 1525. https://doi.org/10.3390/biomedicines11061525
APA StyleAlentorn, A., Berzero, G., Alexopoulos, H., Tzartos, J., Reyes Botero, G., Morales Martínez, A., Muñiz-Castrillo, S., Vogrig, A., Joubert, B., García Jiménez, F. A., Cabrera, D., Tobon, J. V., Delgado, C., Sandoval, P., Troncoso, M., Galleguillos, L., Giry, M., Benazra, M., Hernández Verdin, I., ... Psimaras, D. (2023). Spatial and Ecological Factors Modulate the Incidence of Anti-NMDAR Encephalitis—A Systematic Review. Biomedicines, 11(6), 1525. https://doi.org/10.3390/biomedicines11061525




