SARS-CoV-2 Antibody Responses in Pediatric Patients: A Bibliometric Analysis
Abstract
1. Introduction
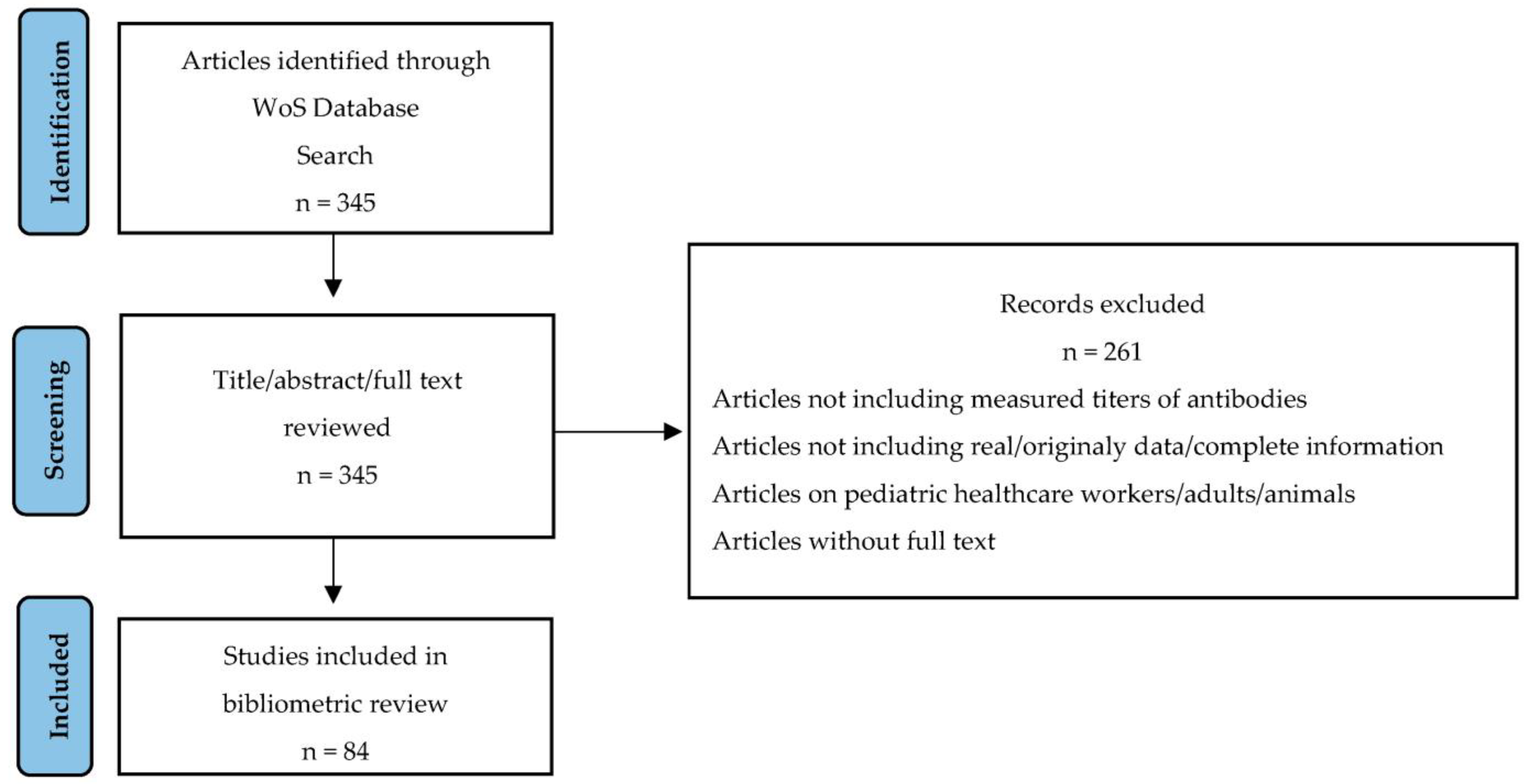
2. Materials and Methods
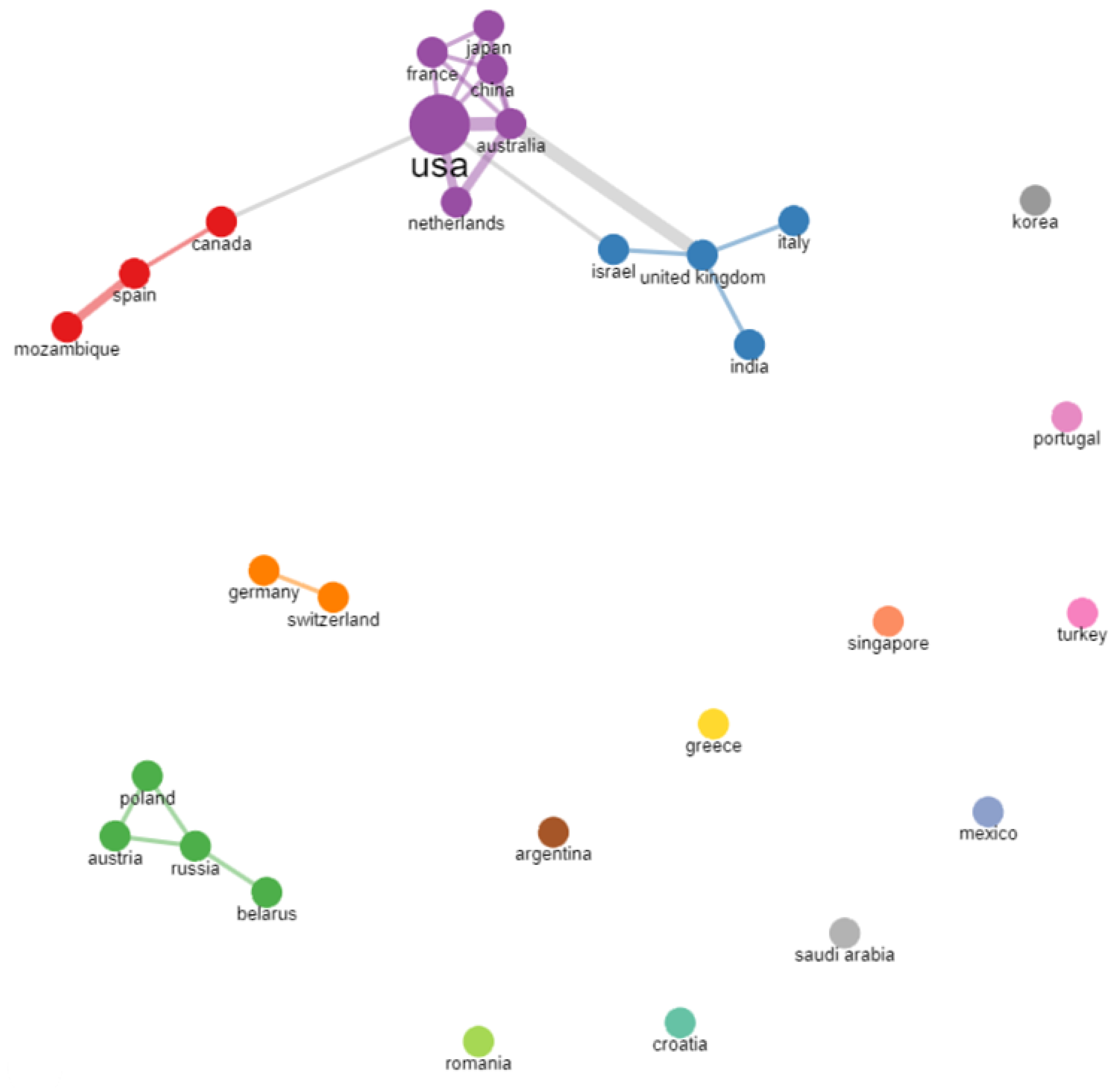
3. Results
4. Discussion
4.1. Antibodies Levels and Disease Severity
4.2. Relationship between SARS-CoV-2 Antibodies and Inflammatory Markers
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, J.; Deng, Y.; Zhao, Z.; Mao, B.; Lu, M.; Lin, Y.; Huang, A. Characterization of SARS-CoV-2-specific humoral immunity and its potential applications and therapeutic prospects. Cell. Mol. Immunol. 2021, 19, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Kudlay, D.; Kofiadi, I.; Khaitov, M. Peculiarities of the T Cell Immune Response in COVID-19. Vaccines 2022, 10, 242. [Google Scholar] [CrossRef] [PubMed]
- Deeks, J.J.; Dinnes, J.; Takwoingi, Y.; Davenport, C.; Spijker, R.; Taylor-Phillips, S.; Adriano, A.; Beese, S.; Dretzke, J.; Di Ruffano, L.F.; et al. Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst. Rev. 2020, 6, CD013652. [Google Scholar] [PubMed]
- VanEck, N.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef]
- VanEck, N.J.; Waltman, L. VOSviewer Manual; Univeristeit Leiden: Leiden, The Netherlands, 2013. [Google Scholar]
- Hosseini, M.R.; Martek, I.; Zavadskas, E.K.; Aibinu, A.A.; Arashpour, M.; Chileshe, N. Critical evaluation of off-site construction research: A Scientometric analysis. Autom. Constr. 2018, 87, 235–247. [Google Scholar] [CrossRef]
- Grosseck, G.; Tîru, L.G.; Bran, R.A. Education for sustainable development: Evolution and perspectives: A bibliometric review of research, 1992–2018. Sustainability 2019, 11, 6136. [Google Scholar] [CrossRef]
- Maniu, I.; Costea, R.; Maniu, G.; Neamtu, B.M. Inflammatory Biomarkers in Febrile Seizure: A Comprehensive Bibliometric, Review and Visualization Analysis. Brain Sci. 2021, 11, 1077. [Google Scholar] [CrossRef]
- Fiore, U.; Florea, A.; Kifor, C.V.; Zanetti, P. Digitization, Epistemic Proximity, and the Education System: Insights from a Bibliometric Analysis. J. Risk Financ. Manag. 2021, 14, 267. [Google Scholar] [CrossRef]
- Gajdosikova, D.; Valaskova, K. A Systematic Review of Literature and Comprehensive Bibliometric Analysis of Capital Structure Issue. Manag. Dyn. Knowl. Econ. 2022, 10, 210–224. [Google Scholar]
- Cretu, D.M.; Morandau, F. Initial Teacher Education for Inclusive Education: A Bibliometric Analysis of Educational Research. Sustainability 2020, 12, 4923. [Google Scholar] [CrossRef]
- Tomaszewska, E.J.; Florea, A. Urban smart mobility in the scientific literature—Bibliometric analysis. Eng. Manag. Prod. Serv. 2018, 10, 41–56. [Google Scholar] [CrossRef]
- Maniu, I.; Maniu, G.; Totan, M. Clinical and Laboratory Characteristics of Pediatric COVID-19 Population—A Bibliometric Analysis. J. Clin. Med. 2022, 11, 5987. [Google Scholar] [CrossRef] [PubMed]
- Morante-Carballo, F.; Montalván-Burbano, N.; Arias-Hidalgo, M.; Domínguez-Granda, L.; Apolo-Masache, B.; Carrión-Mero, P. Flood Models: An Exploratory Analysis and Research Trends. Water 2022, 14, 2488. [Google Scholar] [CrossRef]
- Fiore, U.; Florea, A.; Pérez Lechuga, G. An Interdisciplinary Review of Smart Vehicular Traffic and Its Applications and Challenges. J. Sens. Actuator Netw. 2019, 8, 13. [Google Scholar] [CrossRef]
- Florea, A. Digital design skills for factories of the future. In Proceedings of the 9th International Conference on Manufacturing Science and Education—MSE 2019 “Trends in New Industrial Revolution”, Sibiu, Romania, 5–7 June 2019; Volume 290, p. 14002. [Google Scholar]
- Ratiu, A.; Maniu, I.; Pop, E.-L. EntreComp Framework: A Bibliometric Review and Research Trends. Sustainability 2023, 15, 1285. [Google Scholar] [CrossRef]
- Aria, M.; Cuccurullo, C. Bibliometrix: An R-tool for comprehensive science mapping analysis. J. Informetr. 2017, 11, 959–975. [Google Scholar] [CrossRef]
- Yonker, L.M.; Neilan, A.M.; Bartsch, Y.; Patel, A.B.; Regan, J.; Arya, P.; Gootkind, E.; Park, G.; Hardcastle, M.; St. John, A.; et al. Pediatric severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): Clinical presentation, infectivity, and immune responses. J. Pediatr. 2020, 227, 45–52. [Google Scholar] [CrossRef]
- Bartsch, Y.C.; Wang, C.; Zohar, T.; Fischinger, S.; Atyeo, C.; Burke, J.S.; Kang, J.; Edlow, A.G.; Fasano, A.; Baden, L.R.; et al. Humoral signatures of protective and pathological SARS-CoV-2 infection in children. Nat. Med. 2021, 27, 454–462. [Google Scholar] [CrossRef]
- Pierce, C.A.; Preston-Hurlburt, P.; Dai, Y.; Aschner, C.B.; Cheshenko, N.; Galen, B.; Garforth, S.J.; Herrera, N.G.; Jangra, R.K.; Morano, N.C.; et al. Immune responses to SARS-CoV-2 infection in hospitalized pediatric and adult patients. Sci. Transl. Med. 2020, 12, eabd5487. [Google Scholar] [CrossRef]
- Pierce, C.A.; Sy, S.; Galen, B.; Goldstein, D.Y.; Orner, E.; Keller, M.J.; Herold, K.C.; Herold, B.C. Natural mucosal barriers and COVID-19 in children. JCI Insight 2021, 6, e148694. [Google Scholar] [CrossRef]
- Fox, A.; Marino, J.; Amanat, F.; Krammer, F.; Hahn-Holbrook, J.; Zolla-Pazner, S.; Powell, R.L. Robust and specific secretory IgAagainst SARS-CoV-2 detected in human milk. iScience 2020, 23, 101735. [Google Scholar] [CrossRef] [PubMed]
- Rostad, C.A.; Chahroudi, A.; Mantus, G.; Lapp, S.A.; Teherani, M.; Macoy, L.; Tarquinio, K.M.; Basu, K.R.; Kao, C.; Linam, W.M.; et al. Quantitative SARS-CoV-2 serology in children with multisystem inflammatory syndrome (MIS-C). Pediatrics 2020, 146, e2020018242. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.S.; Costa, V.; Racine-Brzostek, S.E.; Acker, K.P.; Yee, J.; Chen, Z.; Karbaschi, M.; Zuk, R.; Rand, S.; Sukhu, A.; et al. Association of age with SARS-CoV-2 antibody response. JAMA Netw. Open 2021, 4, e214302. [Google Scholar] [CrossRef]
- Tosif, S.; Neeland, M.R.; Sutton, P.; Licciardi, P.V.; Sarkar, S.; Selva, K.J.; Do, L.A.H.; Donato, C.; Quan Toh, Z.; Higgins, R.; et al. Immune Responses to SARS-CoV-2 in Three Children of Parents with Symptomatic COVID-19. Nat. Commun. 2020, 11, 5703. [Google Scholar] [CrossRef] [PubMed]
- Selva, K.J.; van de Sandt, C.E.; Lemke, M.M.; Lee, C.Y.; Shoffner, S.K.; Chua, B.Y.; Davis, S.K.; Nguyen, T.H.O.; Rowntree, L.C.; Hensen, L.; et al. Systems Serology Detects Functionally Distinct Coronavirus Antibody Features in Children and Elderly. Nat. Commun. 2021, 12, 2037. [Google Scholar] [CrossRef]
- Cohen, C.A.; Li, A.P.Y.; Hachim, A.; Hui, D.S.C.; Kwan, M.Y.W.; Tsang, O.T.Y.; Chiu, S.S.; Chan, W.H.; Yau, Y.S.; Kavian, N.; et al. SARS-CoV-2 Specific T Cell Responses Are Lower in Children and Increase with Age and Time after Infection. Nat. Commun. 2021, 12, 4678. [Google Scholar] [CrossRef]
- Anderson, E.M.; Diorio, C.; Goodwin, E.C.; McNerney, K.O.; Weirick, M.E.; Gouma, S.; Bolton, M.J.; Arevalo, C.P.; Chase, J.; Hicks, P. Severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) antibody responses in children with multisystem inflammatory syndrome in children (MIS-C) and mild and severe coronavirus disease 2019 (COVID-19). J. Pediatr. Infect. Dis. Soc. 2021, 10, 669–673. [Google Scholar] [CrossRef]
- Isoldi, S.; Mallardo, S.; Marcellino, A.; Bloise, S.; Dilillo, A.; Iorfida, D.; Testa, A.; Del Giudice, E.; Martucci, V.; Sanseviero, M.; et al. The comprehensive clinic, laboratory, and instrumental evaluation of children with COVID-19: A 6-months prospective study. J. Med. Virol. 2021, 93, 3122–3132. [Google Scholar] [CrossRef]
- Lau, E.H.; Hui, D.S.; Tsang, O.T.; Chan, W.H.; Kwan, M.Y.; Chiu, S.S.; Cheng, S.M.; Ko, R.W.; Li, J.K.; Chaothai, S.; et al. Long-term persistence of SARS-CoV-2 neutralizing antibody responses after infection and estimates of the duration of protection. EClinicalMedicine 2021, 41, 101174. [Google Scholar] [CrossRef]
- Woudenberg, T.; Pelleau, S.; Anna, F.; Attia, M.; Donnadieu, F.; Gravet, A.; Lohmann, C.; Seraphin, H.; Guiheneuf, R.; Delamare, C.; et al. Humoral immunity to SARS-CoV-2 and seasonal coronaviruses in children and adults in north-eastern France. EBioMedicine 2021, 70, 103495. [Google Scholar] [CrossRef]
- Shrwani, K.; Sharma, R.; Krishnan, M.; Jones, T.; Mayora-Neto, M.; Cantoni, D.; Temperton, N.J.; Dobson, S.L.; Subramaniam, K.; McNamara, P.S.; et al. Detection of Serum Cross-Reactive Antibodies and Memory Response to SARS-CoV-2 in Prepandemic and Post–COVID-19 Convalescent Samples. J. Infect. Dis. 2021, 224, 1305–1315. [Google Scholar] [CrossRef] [PubMed]
- Sermet-Gaudelus, I.; Temmam, S.; Huon, C.; Behillil, S.; Gajdos, V.; Bigot, T.; Lurier, T.; Chrétien, D.; Backovic, M.; Delaunay-Moisan, A.; et al. Prior Infection by Seasonal Coronaviruses, as Assessed by Serology, Does Not Prevent SARS-CoV-2 Infection and Disease in Children, France, April to June 2020. Eurosurveillance 2021, 26, 2001782. [Google Scholar] [CrossRef] [PubMed]
- Toh, Z.Q.; Anderson, J.; Mazarakis, N.; Neeland, M.; Higgins, R.A.; Rautenbacher, K.; Dohle, K.; Nguyen, J.; Overmars, I.; Donato, C.; et al. Comparison of Seroconversion in Children and Adults with Mild COVID-19. JAMA Netw. Open. 2022, 5, e221313. [Google Scholar] [CrossRef]
- Bloise, S.; Marcellino, A.; Testa, A.; Dilillo, A.; Mallardo, S.; Isoldi, S.; Martucci, V.; Sanseviero, M.T.; Giudice, E.D.; Iorfida, D.; et al. Serum IgG Levels in Children 6 Months after SARS-CoV-2 Infection and Comparison with Adults. Eur. J. Pediatr. 2021, 1850, 3335–3342. [Google Scholar] [CrossRef]
- Seery, V.; Raiden, S.C.; Algieri, S.C.; Grisolía, N.A.; Filippo, D.; De Carli, N.; Di Lalla, S.; Cairoli, H.; Chiolo, M.J.; Meregalli, C.N.; et al. Blood neutrophils from children with COVID-19 exhibit both inflammatory and anti-inflammatory markers. EBioMedicine 2021, 67, 103357. [Google Scholar] [CrossRef] [PubMed]
- Keuning, M.W.; Grobben, M.; de Groen, A.-E.C.; Berman-de Jong, E.P.; Bijlsma, M.W.; Cohen, S.; Felderhof, M.; Pajkrt, D. Saliva SARS-CoV-2 Antibody Prevalence in Children. Microbiol. Spectr. 2021, 9, e0073121. [Google Scholar] [CrossRef]
- Vilibic-Cavlek, T.; Stevanovic, V.; Ilic, M.; Barbic, L.; Capak, K.; Tabain, I.; Krleza, J.L.; Ferenc, T.; Hruskar, Z.; Topic, R.Z.; et al. SARS-CoV-2 Seroprevalence and Neutralizing Antibody Response after the First and Second COVID-19 Pandemic Wave in Croatia. Pathogens 2021, 10, 774. [Google Scholar] [CrossRef]
- Han, M.S.; Um, J.; Lee, E.J.; Kim, K.M.; Chang, S.H.; Lee, H.; Kim, Y.K.; Choi, Y.Y.; Cho, E.Y.; Kim, D.H.; et al. Antibody Responses to SARS-CoV-2 in Children With COVID-19. J. Pediatr. Infect. Dis Soc. 2022, 11, 267–273. [Google Scholar] [CrossRef]
- Gong, F.; Dai, Y.; Zheng, T.; Cheng, L.; Zhao, D.; Wang, H.; Liu, M.; Pei, H.; Jin, T.; Yu, D.; et al. Peripheral CD4+ T cell subsets and antibody response in COVID-19 convalescent individuals. J. Clin. Investig. 2020, 130, 6588–6599. [Google Scholar] [CrossRef]
- Lucas, C.; Klein, J.; Sundaram, M.; Liu, F.; Wong, P.; Silva, J.; Mao, T.; Oh, J.E.; Tokuyama, M.; Lu, P.; et al. Kinetics of antibody responses dictate COVID-19 outcome. medRxiv 2020. [Google Scholar]
- Peart Akindele, N.; Kouo, T.; Karaba, A.H.; Gordon, O.; Fenstermacher, K.Z.J.; Beaudry, J.; Rubens, J.H.; Atik, C.C.; Zhou, W.; Ji, H.; et al. Distinct cytokine and chemokine dysregulation in hospitalized children with acute Coronavirus Disease 2019 and Multisystem Inflammatory Syndrome with similar levels of nasopharyngeal severe acute respiratory syndrome Coronavirus 2 shedding. J. Infect. Dis. 2021, 224, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, A.; Balasubramanian, S.; Putilibai, S.; Lakshan Raj, S.; Amperayani, S.; Senthilnathan, S.; Manoharan, A.; Sophi, A.; Amutha, R.; Sadasivam, K.; et al. Correlation of SARS-CoV-2 Serology and Clinical Phenotype Amongst Hospitalised Children in a Tertiary Children’s Hospital in India. J. Trop. Pediatr. 2021, 67, fmab015. [Google Scholar] [CrossRef] [PubMed]
- Goenka, A.; Halliday, A.; Gregorova, M.; Milodowski, E.; Thomas, A.; Williamson, M.K.; Baum, H.; Oliver, E.; Long, A.E.; Knezevic, L.; et al. Young Infants Exhibit Robust Functional Antibody Responses and Restrained IFN-γ Production to SARS-CoV-2. Cell Rep. Med. 2021, 2, 100327. [Google Scholar] [CrossRef] [PubMed]
- Jakuszko, K.; Kościelska-Kasprzak, K.; Żabińska, M.; Bartoszek, D.; Poznański, P.; Rukasz, D.; Kłak, R.; Królak-Olejnik, B.; Krajewska, M. Immune Response to Vaccination against COVID-19 in Breastfeeding Health Workers. Vaccines 2021, 9, 663. [Google Scholar] [CrossRef] [PubMed]
- De la Torre, E.P.; Obando, I.; Vidal, M.; de Felipe, B.; Aguilar, R.; Izquierdo, L.; Carolis, C.; Olbrich, P.; Capilla-Miranda, A.; Serra, P.; et al. SARS-CoV-2 Seroprevalence Study in Pediatric Patients and Health Care Workers Using Multiplex Antibody Immunoassays. Viruses 2022, 14, 2039. [Google Scholar] [CrossRef]
- Dobaño, C.; Alonso, S.; Vidal, M.; Jiménez, A.; Rubio, R.; Santano, R.; Barrios, D.; Tomas, G.P.; Casas, M.M.; García, M.H.; et al. Multiplex antibody analysis of IgM, IgA and IgG to SARS-CoV-2 in saliva and serum from infected children and their close contacts. Front. Immunol. 2022, 13, 85. [Google Scholar] [CrossRef]
- Li, D.; Calderone, R.; Nsouli, T.M.; Reznikov, E.; Bellanti, J.A. Salivary and serum IgA and IgG responses to SARS-CoV-2-spike protein following SARS-CoV-2 infection and after immunization with COVID-19 vaccines. Allergy Asthma Proc. 2022, 43, 419–430. [Google Scholar] [CrossRef]
- Chao, Y.X.; Rötzschke, O.; Tan, E.K. The role of IgA in COVID-19. Brain Behav. Immun. 2020, 87, 182. [Google Scholar] [CrossRef]
- Rangel-Ramírez, V.V.; Macías-Piña, K.A.; Garrido, R.R.S.; de Alba-Aguayo, D.R.; Moreno-Fierros, L.; Rubio-Infante, N. A systematic review and meta-analysis of the IgA seroprevalence in COVID-19 patients; Is there a role for IgA in COVID-19 diagnosis or severity? Microbiol. Res. 2022, 263, 127105. [Google Scholar] [CrossRef]
- Schultze, J.L.; Aschenbrenner, A.C. COVID-19 and the human innate immune system. Cell 2021, 184, 1671–1692. [Google Scholar] [CrossRef]
- Tay, M.Z.; Poh, C.M.; Rénia, L.; MacAry, P.A.; Ng, L.F. The trinity of COVID-19: Immunity, inflammation and intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Lucas, C.; Wong, P.; Klein, J.; Castro, T.B.; Silva, J.; Sundaram, M.; Ellingson, F.K.; Mao, T.; Oh, J.E.; Israelow, B.; et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature 2020, 584, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Xu, M.; Song, D.; Yue, T.; Wang, Y.; Zhang, P.; Zhang, Y.; Zhang, M.; Lam, T.T.-K.; Faria, N.R.; et al. Association between inflammatory cytokines and anti-SARS-CoV-2 antibodies in hospitalized patients with COVID-19. Immun. Ageing 2022, 19, 12. [Google Scholar] [CrossRef] [PubMed]
- Ngo, B.; Lapp, S.A.; Siegel, B.; Patel, V.; Hussaini, L.; Bora, S.; Philbrook, B.; Weinschenk, K.; Wright, L.; Anderson, E.J.; et al. Cerebrospinal fluid cytokine, chemokine, and SARS-CoV-2 antibody profiles in children with neuropsychiatric symptoms associated with COVID-19. Mult. Scler. Relat. Disord. 2021, 55, 103169. [Google Scholar] [CrossRef]
- Monk, P.D.; Marsden, R.J.; Tear, V.J.; Brookes, J.; Batten, T.N.; Mankowski, M.; Gabbay, F.J.; Davies, D.E.; Holgate, S.T.; Ho, L.-P.; et al. Safety and efficacy of inhaled nebulised interferon beta-1a (SNG001) for treatment of SARS-CoV-2 infection: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Respir. Med. 2021, 9, 196–206. [Google Scholar] [CrossRef]
- Guo, Y.; Li, T.; Xia, X.; Su, B.; Li, H.; Feng, Y.; Han, J.; Wang, X.; Jia, L.; Bao, Z.; et al. Different profiles of antibodies and cytokines were found between severe and moderate COVID-19 patients. Front. Immunol. 2021, 12, 3344. [Google Scholar] [CrossRef]
- Coomes, E.A.; Haghbayan, H. Interleukin-6 in COVID-19: A systematic review and meta-analysis. Rev. Med. Virol. 2020, 30, 1–9. [Google Scholar] [CrossRef]
- Schultheiß, C.; Willscher, E.; Paschold, L.; Gottschick, C.; Klee, B.; Henkes, S.S.; Bosurgi, L.; Dutzmann, J.; Sedding, D.; Frese, T.; et al. The IL-1β, IL-6, and TNF cytokine triad is associated with post-acute sequelae of COVID-19. Cell Rep. Med. 2022, 3, 100663. [Google Scholar] [CrossRef]
- Okba, N.M.; Müller, M.A.; Li, W.; Wang, C.; GeurtsvanKessel, C.H.; Corman, V.M.; Lamers, M.M.; Sikkema, R.S.; De Bruin, E.; Chandler, F.D.; et al. Severe acute respiratory syndrome coronavirus 2− specific antibody responses in coronavirus disease patients. Emerg. Infect. Dis. 2020, 26, 1478. [Google Scholar] [CrossRef]
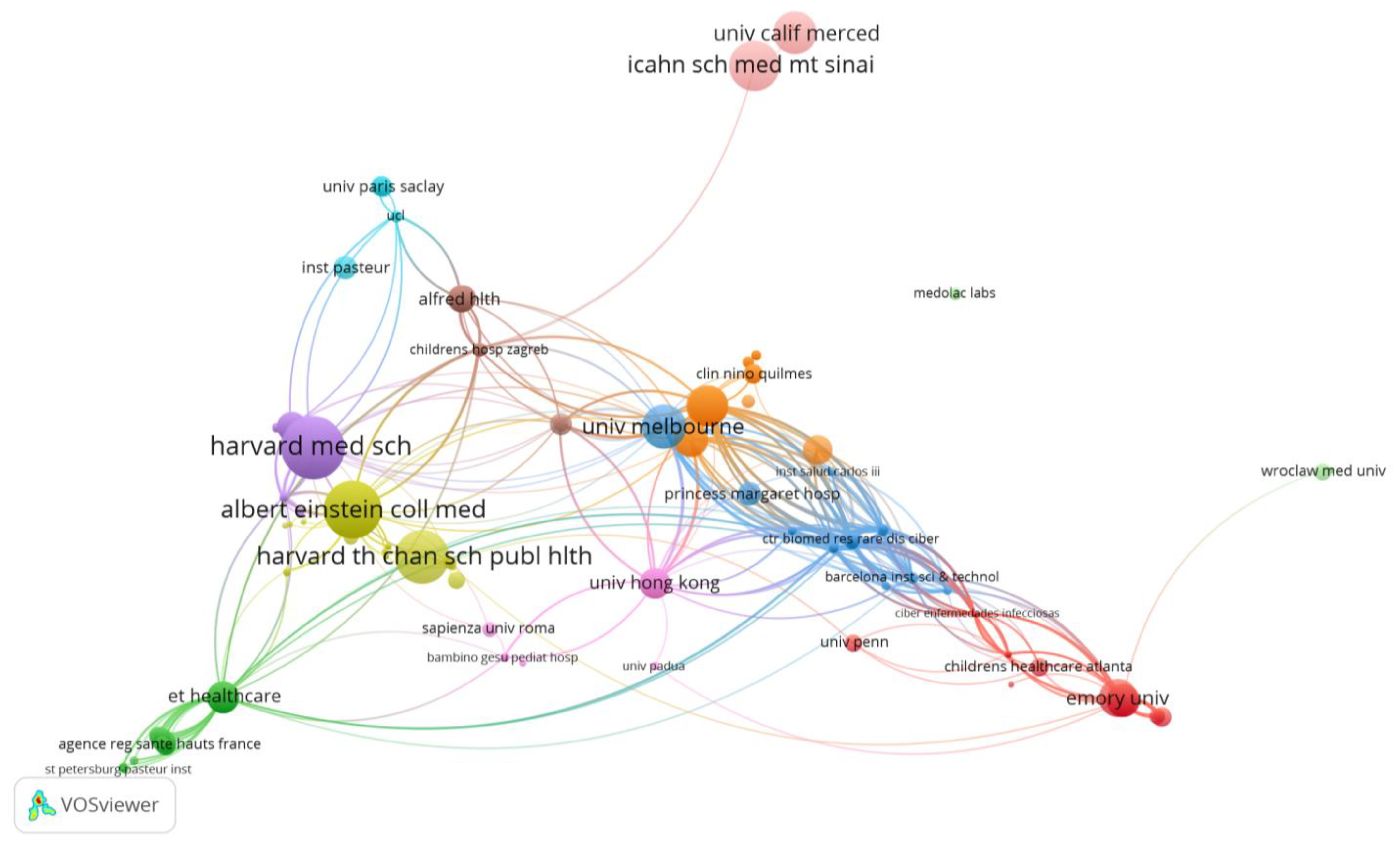

| Rank | Organizations Publications | Rank | Organizations Citations | Rank | Organizations Affiliations |
|---|---|---|---|---|---|
| 1 | Univ Melbourne (5) | 1 | Harvard Med Sch (257) | 1 | Emory Univ (18) |
| 1 | Emory Univ (5) | 2 | Brigham and Women’s Hospital (252) | 2 | Univ Hong Kong (17) |
| 1 | Univ Hong Kong (5) | 2 | Massachusetts Gen Hosp (252) | 3 | Johns Hopkins Univ (16) |
| 2 | Chinese Univ Hong Kong (4) | 3 | Albert Einstein Coll Med (214) | 4 | Inst Pasteur (14) |
| 2 | Childrens Healthcare Atlanta (4) | 3 | Child. Hosp Montefiore (214) | 5 | Univ Bristol (11) |
| 3 | Harvard Med Sch (3) | 3 | Montefiore Med Ctr (214) | 5 | Univ Melbourne (11) |
| 3 | Univ Amsterdam (3) | 3 | Yale Univ (214) | 5 | Univ Penn (11) |
| 3 | Murdoch Child. Res Inst (3) | 4 | Harvard Th Chan Sch Publ Hlth (192) | 6 | Univ Barcelona (10) |
| 3 | Royal Child. Hosp (3) | 5 | Hosp Sick Child. (167) | 7 | Univ Med Ctr Hamburg Eppendorf (8) |
| 3 | Hosp Author Hong Kong (3) | 6 | Icahn Sch Med Mt Sinai (163) | 8 | Hosp Author Hong Kong (7) |
| 3 | London Sch Hyg and Trop med (3) | 7 | Univ Melbourne (128) | 8 | Hosp Infantil Mexico Dr Federico Gomez (7) |
| 3 | Univ Bristol (3) | 8 | Univ Calif Merced (119) | 8 | Med Univ Lublin (7) |
| 3 | Barcelona Inst Sci and Techno (3) | 9 | Univ Amsterdam (113) | 8 | Royal Child. Hosp (7) |
| 3 | Huazhong Univ Sci and Techno (3) | 10 | Murdoch Child. Res Inst (109) | ||
| 3 | Queen Mary Hosp (3) |
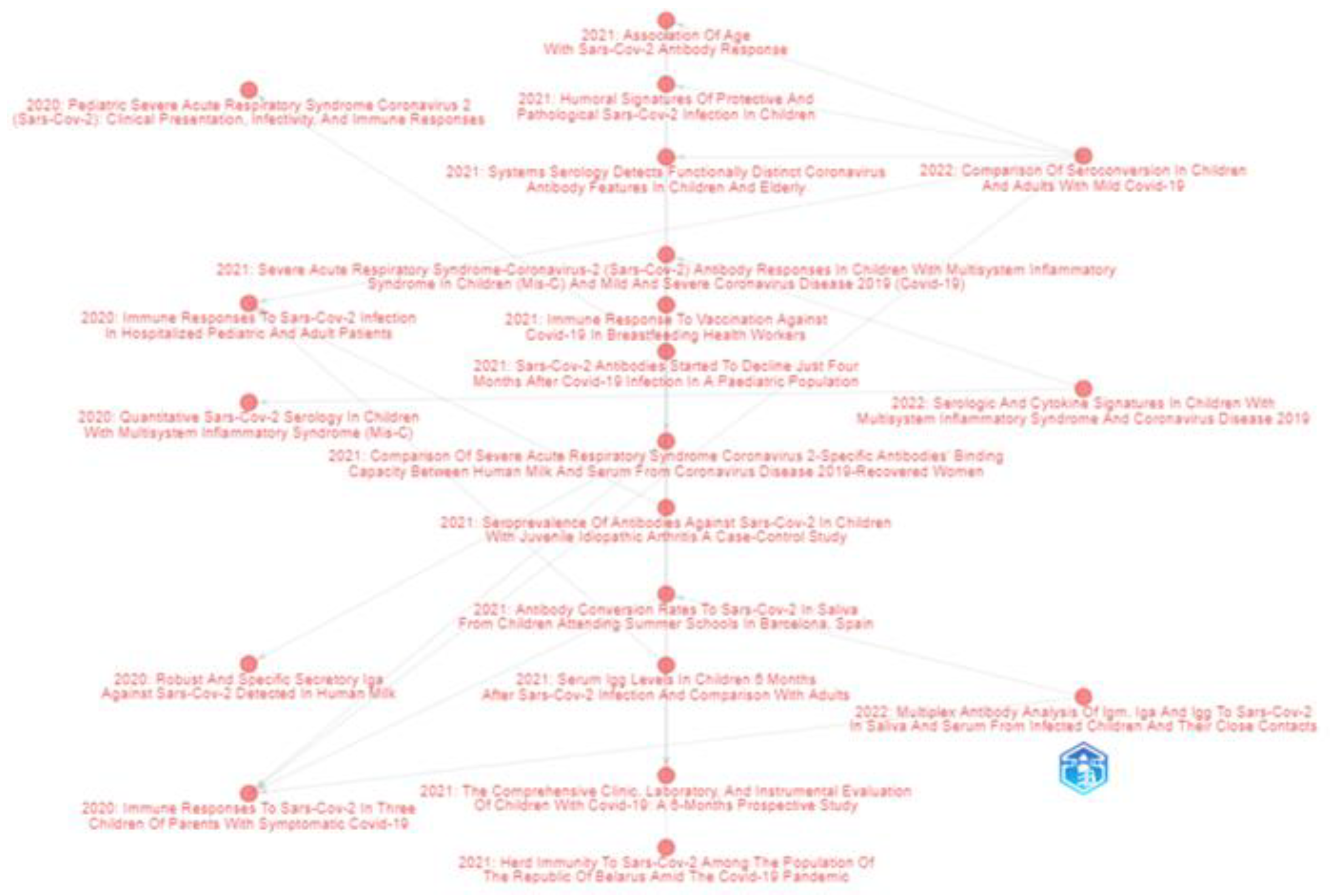
| First Author Year [Ref.] | Document Title | Journal | Citations |
|---|---|---|---|
| Yonker 2020 [19] | Pediatric Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): Clinical Presentation, Infectivity, and Immune Responses | JOURNAL OF PEDIATRICS | 192 |
| Pierce 2020 [21] | Immune responses to SARS-CoV-2 infection in hospitalized pediatric and adult patients | SCIENCE TRANSLATIONAL MEDICINE | 167 |
| Fox 2020 [23] | Robust and Specific Secretory IgA Against SARS-CoV-2 Detected in Human Milk | ISCIENCE | 119 |
| Rostad 2020 [24] | Quantitative SARS-CoV-2 Serology in Children With Multisystem Inflammatory Syndrome (MIS-C) | PEDIATRICS | 71 |
| Yang 2021 [25] | Association of Age With SARS-CoV-2 Antibody Response | JAMA NETWORK OPEN | 68 |
| Bartsch 2021 [20] | Humoral signatures of protective and pathological SARS-CoV-2 infection in children | NATURE MEDICINE | 60 |
| Tosif 2020 [26] | Immune responses to SARS-CoV-2 in three children of parents with symptomatic COVID-19 | NATURE COMMUNICATIONS | 56 |
| Pierce 2021 [22] | Natural mucosal barriers and COVID-19 in children | JCI INSIGHT | 47 |
| Selva 2021 [27] | Systems serology detects functionally distinct coronavirus antibody features in children and elderly | NATURE COMMUNICATIONS | 44 |
| Cohen 2021 [28] | SARS-CoV-2 specific T cell responses are lower in children and increase with age and time after infection | NATURE COMMUNICATIONS | 37 |
| Anderson 2021 [29] | Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2) Antibody Responses in Children With Multisystem Inflammatory Syndrome in Children (MIS-C) and Mild and Severe Coronavirus Disease 2019 (COVID-19) | JOURNAL OF THE PEDIATRIC INFECTIOUS DISEASES SOCIETY | 21 |
| Isoldi 2021 [30] | The comprehensive clinic, laboratory, and instrumental evaluation of children with COVID-19: A 6-months prospective study | JOURNAL OF MEDICAL VIROLOGY | 19 |
| Lau 2021 [31] | Long-term persistence of SARS-CoV-2 neutralizing antibody responses after infection and estimates of the duration of protection | ECLINICALMEDICINE | 19 |
| Woudenberg 2021 [32] | Humoral immunity to SARS-CoV-2 and seasonal coronaviruses in children and adults in north-eastern France | EBIOMEDICINE | 18 |
| Shrwani 2021 [33] | Detection of Serum Cross-Reactive Antibodies and Memory Response to SARS-CoV-2 in Prepandemic and Post-COVID-19 Convalescent Samples | JOURNAL OF INFECTIOUS DISEASES | 17 |
| Sermet-Gaudelus 2021 [34] | Prior infection by seasonal coronaviruses, as assessed by serology, does not prevent SARS-CoV-2 infection and disease in children, France, April to June 2020 | EUROSURVEILLANCE | 17 |
| Toh 2022 [35] | Comparison of Seroconversion in Children and Adults With Mild COVID-19 | JAMA NETWORK OPEN | 16 |
| Bloise 2021 [36] | Serum IgG levels in children 6 months after SARS-CoV-2 infection and comparison with adults | EUROPEAN JOURNAL OF PEDIATRICS | 14 |
| Seery 2021 [37] | Blood neutrophils from children with COVID-19 exhibit both inflammatory and anti-inflammatory markers | EBIOMEDICINE | 13 |
| Keuning 2021 [38] | Saliva SARS-CoV-2 Antibody Prevalence in Children | MICROBIOLOGY SPECTRUM | 13 |
| Vilibic-Cavlek 2021 [39] | SARS-CoV-2 Seroprevalence and Neutralizing Antibody Response after the First and Second COVID-19 Pandemic Wave in Croatia | PATHOGENS | 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maniu, I.; Maniu, G.C.; Antonescu, E.; Duica, L.; Grigore, N.; Totan, M. SARS-CoV-2 Antibody Responses in Pediatric Patients: A Bibliometric Analysis. Biomedicines 2023, 11, 1455. https://doi.org/10.3390/biomedicines11051455
Maniu I, Maniu GC, Antonescu E, Duica L, Grigore N, Totan M. SARS-CoV-2 Antibody Responses in Pediatric Patients: A Bibliometric Analysis. Biomedicines. 2023; 11(5):1455. https://doi.org/10.3390/biomedicines11051455
Chicago/Turabian StyleManiu, Ionela, George Constantin Maniu, Elisabeta Antonescu, Lavinia Duica, Nicolae Grigore, and Maria Totan. 2023. "SARS-CoV-2 Antibody Responses in Pediatric Patients: A Bibliometric Analysis" Biomedicines 11, no. 5: 1455. https://doi.org/10.3390/biomedicines11051455
APA StyleManiu, I., Maniu, G. C., Antonescu, E., Duica, L., Grigore, N., & Totan, M. (2023). SARS-CoV-2 Antibody Responses in Pediatric Patients: A Bibliometric Analysis. Biomedicines, 11(5), 1455. https://doi.org/10.3390/biomedicines11051455






