Evolving Diagnostic and Treatment Strategies for Pediatric CNS Tumors: The Impact of Lipid Metabolism
Abstract
1. Introduction
2. Cytogenetic Alterations and Lipidomic Landscape of Pediatric Neurological Tumors
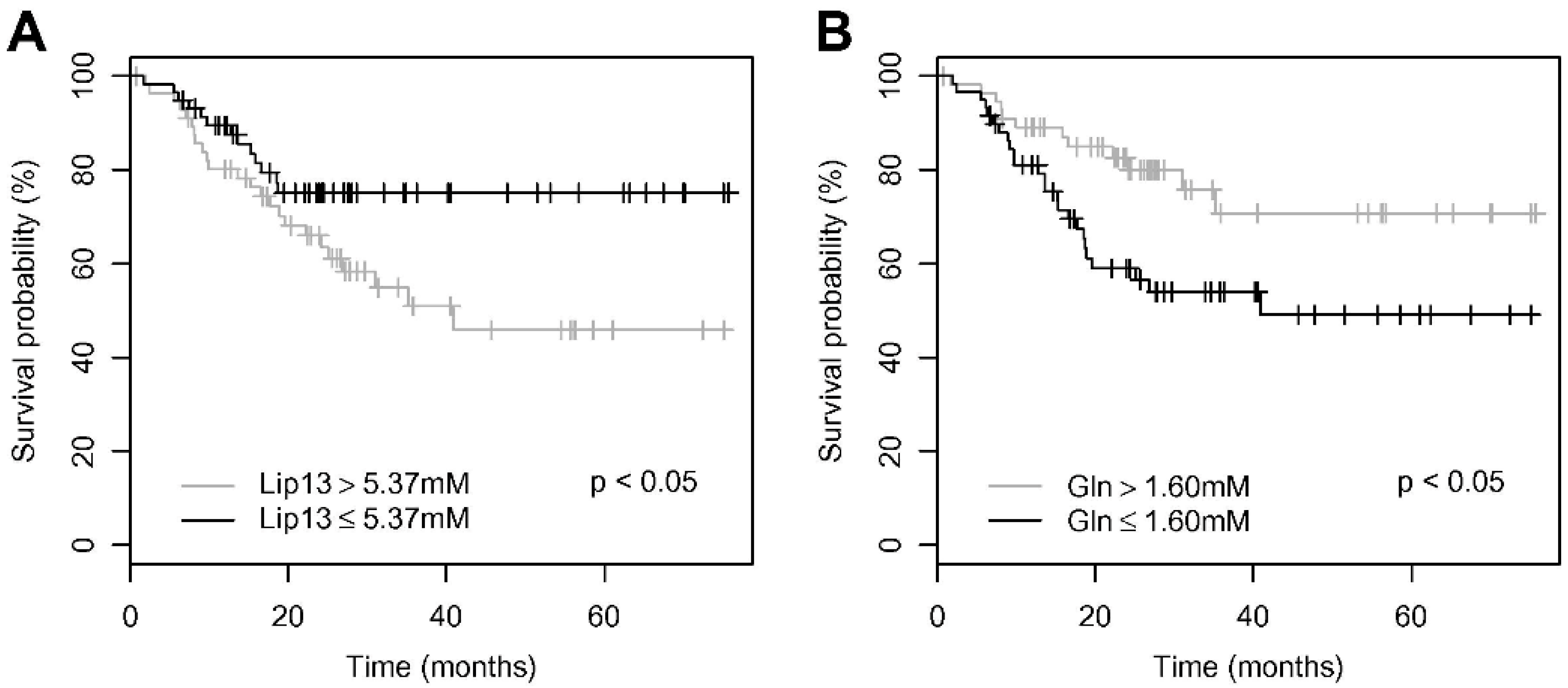
3. Lipid Metabolism and Pediatric Brain Tumor Prognosis
4. Neuroimaging of Lipids in Pediatric Brain Tumors
5. Current Trends in the Treatment of Pediatric Neurological Tumors Targeting the Lipid Metabolism
6. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2OHOA | 2-hydroxyoleic acid |
| ATRT | atypical teratoid/rhabdoid tumor |
| ATRX | α thalassemia/intellectual disability syndrome X–linked gene |
| BBB | blood–brain barrier |
| BRAF | B-Raf proto-oncogene serine/threonineprotein kinase |
| CAR | chimeric antigen receptor |
| CNS | central nervous system |
| CSF | cerebrospinal fluid |
| DIPG | diffuse intrinsic pontine glioma |
| DNETs | dysembryoplastic neuroepithelial tumors |
| ETMR | embryonal tumor with multilayer rosettes |
| FABP | fatty-acid-binding protein |
| GBMs | glioblastomas |
| HGG | high-grade glioma |
| LGG | low-grade glioma |
| MAPK | mitogen-activated protein kinase |
| MRSI | proton magnetic resonance spectroscopic imaging |
| MS | mass spectroscopy |
| NMR | nuclear magnetic resonance |
| OS | overall survival |
| PA | pilocytic astrocytoma |
| PBTs | pediatric brain tumors |
| PFS | progression-free survival |
| pLGG/HGG | pediatric low-/high-grade glioma |
| PXA | pleomorphic xanthoastrocytoma |
| SCD | stearoyl CoA desaturase |
| SEGA | subependymal giant cell astrocytomas |
| SHH | Sonic Hedgehog |
| SMO | smoothened: frizzled class receptor |
| SM | sphingomyelin |
| SUFU | suppressor of fused homolog (Drosophila) |
| TP53 | tumor protein p53 |
| WHO | World Health Organization |
| WT | wild-type |
References
- Udaka, Y.T.; Packer, R.J. Pediatric Brain Tumors. Neurol Clin. 2018, 36, 533–556. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Gittleman, H.; Xu, J.; Kromer, C.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2009–2013. Neuro. Oncol. 2016, 18, v1–v75. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA. Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Dang, M.; Phillips, P.C. Pediatric Brain Tumors. Continuum (Minneap. Minn.) 2017, 23, 1727–1757. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro. Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Pfister, S.M.; Reyes-Múgica, M.; Chan, J.K.C.; Hasle, H.; Lazar, A.J.; Rossi, S.; Ferrari, A.; Jarzembowski, J.A.; Pritchard-Jones, K.; Hill, D.A.; et al. A Summary of the Inaugural WHO Classification of Pediatric Tumors: Transitioning from the Optical into the Molecular Era. Cancer Discov. 2022, 12, 331–355. [Google Scholar] [CrossRef]
- Komori, T. The 2021 WHO classification of tumors, 5th edition, central nervous system tumors: The 10 basic principles. Brain Tumor Pathol. 2022, 39, 47–50. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Wesseling, P.; Brandner, S.; Brat, D.J.; Ellison, D.W.; Giangaspero, F.; Hattab, E.M.; Hawkins, C.; Judge, M.J.; Kleinschmidt-DeMasters, B.; et al. Data sets for the reporting of tumors of the central nervous system recommendations from the international collaboration on cancer reporting. Arch. Pathol. Lab. Med. 2020, 144, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Fangusaro, J.; Bandopadhayay, P. Advances in the classification and treatment of pediatric brain tumors. Curr. Opin. Pediatr. 2021, 33, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Parrales, A.; Iwakuma, T. p53 as a regulator of lipid metabolism in cancer. Int. J. Mol. Sci. 2016, 17, 2074. [Google Scholar] [CrossRef]
- Kang, J.G.; Lago, C.U.; Lee, J.E.; Park, J.H.; Donnelly, M.P.; Starost, M.F.; Liu, C.; Kwon, J.; Noguchi, A.C.; Ge, K.; et al. A Mouse Homolog of a Human TP53 Germline Mutation Reveals a Lipolytic Activity of p53. Cell Rep. 2020, 30, 783–792.e5. [Google Scholar] [CrossRef]
- Runkle, K.B.; Kharbanda, A.; Stypulkowski, E.; Cao, X.J.; Wang, W.; Garcia, B.A.; Witze, E.S. Inhibition of DHHC20-Mediated EGFR Palmitoylation Creates a Dependence on EGFR Signaling. Mol. Cell 2016, 62, 385–396. [Google Scholar] [CrossRef]
- Calvert, A.E.; Chalastanis, A.; Wu, Y.; Hurley, L.A.; Kouri, F.M.; Bi, Y.; Kachman, M.; May, J.L.; Bartom, E.; Hua, Y.; et al. Cancer-Associated IDH1 Promotes Growth and Resistance to Targeted Therapies in the Absence of Mutation. Cell Rep. 2017, 19, 1858–1873. [Google Scholar] [CrossRef]
- Qin, X.Y.; Su, T.; Yu, W.; Kojima, S. Lipid desaturation-associated endoplasmic reticulum stress regulates MYCN gene expression in hepatocellular carcinoma cells. Cell Death Dis. 2020, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.A.; Paton, E.L.; Gulick, R.V.; Stefanoni, D.; Cendali, F.; Reisz, J.; Tobin, R.P.; McCarter, M.; D’Alessandro, A.; Torres, R.M.; et al. BRAF Modulates Lipid Use and Accumulation. Cancers 2022, 14, 2110. [Google Scholar] [CrossRef] [PubMed]
- Talebi, A.; Dehairs, J.; Rambow, F.; Rogiers, A.; Nittner, D.; Derua, R.; Vanderhoydonc, F.; Duarte, J.A.G.; Bosisio, F.; Van Den Eynde, K.; et al. Sustained SREBP-1-dependent lipogenesis as a key mediator of resistance to BRAF-targeted therapy. Nat. Commun. 2018, 9, 2500. [Google Scholar] [CrossRef]
- Anderson, D.H. Role of lipids in the MAPK signaling pathway. Prog. Lipid Res. 2006, 45, 102–119. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, I.; Welti, S.; Bonneau, F.; Scheffzek, K. A novel bipartite phospholipid-binding module in the neurofibromatosis type 1 protein. EMBO Rep. 2006, 7, 174–179. [Google Scholar] [CrossRef]
- Wang, C.; Haas, M.A.; Yang, F.; Yeo, S.; Okamoto, T.; Chen, S.; Wen, J.; Sarma, P.; Plas, D.R.; Guan, J.L. Autophagic lipid metabolism sustains mTORC1 activity in TSC-deficient neural stem cells. Nat. Metab. 2019, 1, 1127–1140. [Google Scholar] [CrossRef]
- Priolo, C.; Ricoult, S.J.H.; Khabibullin, D.; Filippakis, H.; Yu, J.; Manning, B.D.; Clish, C.; Henske, E.P. Tuberous sclerosis complex 2 loss increases lysophosphatidylcholine synthesis in lymphangioleiomyomatosis. Am. J. Respir. Cell Mol. Biol. 2015, 53, 33–41. [Google Scholar] [CrossRef]
- Lee, H.C.; Ou, C.H.; Huang, Y.C.; Hou, P.C.; Creighton, C.J.; Lin, Y.S.; Hu, C.Y.; Lin, S.C. YAP1 overexpression contributes to the development of enzalutamide resistance by induction of cancer stemness and lipid metabolism in prostate cancer. Oncogene 2021, 40, 2407–2421. [Google Scholar] [CrossRef] [PubMed]
- Chinthalapudi, K.; Mandati, V.; Zheng, J.; Sharff, A.J.; Bricogne, G.; Griffin, P.R.; Kissil, J.; Izard, T. Lipid binding promotes the open conformation and tumor-suppressive activity of neurofibromin 2. Nat. Commun. 2018, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gorbenko, O.; Panayotou, G.; Zhyvoloup, A.; Volkova, D.; Gout, I.; Filonenko, V. Identification of novel PTEN-binding partners: PTEN interaction with fatty acid binding protein FABP4. Mol. Cell. Biochem. 2010, 337, 299–305. [Google Scholar] [CrossRef]
- Senni, N.; Savall, M.; Cabrerizo Granados, D.; Alves-Guerra, M.C.; Sartor, C.; Lagoutte, I.; Gougelet, A.; Terris, B.; Gilgenkrantz, H.; Perret, C.; et al. β-catenin-activated hepatocellular carcinomas are addicted to fatty acids. Gut 2019, 68, 322–334. [Google Scholar] [CrossRef] [PubMed]
- Blassberg, R.; Jacob, J. Lipid metabolism fattens up hedgehog signaling. BMC Biol. 2017, 15, 95. [Google Scholar] [CrossRef]
- Xiao, X.; Tang, J.J.; Peng, C.; Wang, Y.; Fu, L.; Qiu, Z.P.; Xiong, Y.; Yang, L.F.; Cui, H.W.; He, X.L.; et al. Cholesterol Modification of Smoothened Is Required for Hedgehog Signaling. Mol. Cell 2017, 66, 154–162.e10. [Google Scholar] [CrossRef]
- Casciano, J.C.; Perry, C.; Cohen-Nowak, A.J.; Miller, K.D.; Vande Voorde, J.; Zhang, Q.; Chalmers, S.; Sandison, M.E.; Liu, Q.; Hedley, A.; et al. MYC regulates fatty acid metabolism through a multigenic program in claudin-low triple negative breast cancer. Br. J. Cancer 2020, 122, 868–884. [Google Scholar] [CrossRef]
- Ricci, F.; Brunelli, L.; Talapatra, J.; Reddy, M.M. Lipid Metabolic Reprogramming in Embryonal Neoplasms with MYCN Amplification. Cancers 2023, 15, 2144. [Google Scholar] [CrossRef]
- Zhang, P.; Li, L.; Bao, Z.; Huang, F. Role of BAF60a/BAF60c in chromatin remodeling and hepatic lipid metabolism. Nutr. Metab. 2016, 13, 30. [Google Scholar] [CrossRef]
- Liu, M.X.; Gao, M.; Li, C.Z.; Yu, C.Z.; Yan, H.; Peng, C.; Li, Y.; Li, C.G.; Ma, Z.L.; Zhao, Y.; et al. Dicer1/miR-29/HMGCR axis contributes to hepatic free cholesterol accumulation in mouse non-alcoholic steatohepatitis. Acta Pharmacol. Sin. 2017, 38, 660–671. [Google Scholar] [CrossRef]
- Huang, T.C.; Sahasrabuddhe, N.A.; Kim, M.S.; Getnet, D.; Yang, Y.; Peterson, J.M.; Ghosh, B.; Chaerkady, R.; Leach, S.D.; Marchionni, L.; et al. Regulation of lipid metabolism by dicer revealed through SILAC mice. J. Proteome Res. 2012, 11, 2193–2205. [Google Scholar] [CrossRef]
- Li, Z.; Martin, M.; Zhang, J.; Huang, H.Y.; Bai, L.; Zhang, J.; Kang, J.; He, M.; Li, J.; Maurya, M.R.; et al. Krüppel-Like Factor 4 Regulation of Cholesterol-25-Hydroxylase and Liver X Receptor Mitigates Atherosclerosis Susceptibility. Circulation 2017, 136, 1315–1320. [Google Scholar] [CrossRef] [PubMed]
- Lutz, K.; Jünger, S.T.; Messing-Jünger, M. Essential Management of Pediatric Brain Tumors. Children 2022, 9, 498. [Google Scholar] [CrossRef]
- Laetsch, T.W.; Dubois, S.G.; Bender, J.G.; Macy, M.E.; Moreno, L. Opportunities and challenges in drug development for pediatric cancers. Cancer Discov. 2021, 11, 545–559. [Google Scholar] [CrossRef] [PubMed]
- Aldape, K.; Brindle, K.M.; Chesler, L.; Chopra, R.; Gajjar, A.; Gilbert, M.R.; Gottardo, N.; Gutmann, D.H.; Hargrave, D.; Holland, E.C.; et al. Challenges to curing primary brain tumours. Nat. Rev. Clin. Oncol. 2019, 16, 509–520. [Google Scholar] [CrossRef]
- Girardi, F.; Allemani, C.; Coleman, M.P. Worldwide Trends in Survival From Common Childhood Brain Tumors: A Systematic Review. J. Glob. Oncol. 2019. [Google Scholar] [CrossRef]
- Cheng, C.; Geng, F.; Cheng, X.; Guo, D. Lipid metabolism reprogramming and its potential targets in cancer. Cancer Commun. (Lond. Engl.) 2018, 38, 27. [Google Scholar] [CrossRef]
- Koundouros, N.; Poulogiannis, G. Reprogramming of fatty acid metabolism in cancer. Br. J. Cancer 2019, 122, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Bai, L.; Li, W.; Cui, J. The Lipid Metabolic Landscape of Cancers and New Therapeutic Perspectives. Front. Oncol. 2020, 10, 2686. [Google Scholar] [CrossRef]
- Snaebjornsson, M.T.; Janaki-Raman, S.; Schulze, A. Greasing the Wheels of the Cancer Machine: The Role of Lipid Metabolism in Cancer. Cell Metab. 2020, 31, 62–76. [Google Scholar] [CrossRef]
- Santos, C.R.; Schulze, A. Lipid metabolism in cancer. FEBS J. 2012, 279, 2610–2623. [Google Scholar] [CrossRef]
- Wang, B.; Tontonoz, P. Phospholipid Remodeling in Physiology and Disease. Annu. Rev. Physiol. 2019, 81, 165–188. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Liu, R.; Meng, Y.; Xing, D.; Xu, D.; Lu, Z. Lipid metabolism and cancer. J. Exp. Med. 2021, 218, e20201606. [Google Scholar] [CrossRef] [PubMed]
- Casares, D.; Escribá, P.V.; Rosselló, C.A. Membrane lipid composition: Effect on membrane and organelle structure, function and compartmentalization and therapeutic avenues. Int. J. Mol. Sci. 2019, 20, 2167. [Google Scholar] [CrossRef]
- Escribá, P.V.; Busquets, X.; Inokuchi, J.I.; Balogh, G.; Török, Z.; Horváth, I.; Harwood, J.L.; Vígh, L. Membrane lipid therapy: Modulation of the cell membrane composition and structure as a molecular base for drug discovery and new disease treatment. Prog. Lipid Res. 2015, 59, 38–53. [Google Scholar] [CrossRef]
- Agarwala, P.K.; Aneja, R.; Kapoor, S. Lipidomic landscape in cancer: Actionable insights for membrane-based therapy and diagnoses. Med. Res. Rev. 2022, 42, 983–1018. [Google Scholar] [CrossRef]
- Preta, G. New Insights Into Targeting Membrane Lipids for Cancer Therapy. Front. Cell Dev. Biol. 2020, 8, 876. [Google Scholar] [CrossRef] [PubMed]
- Gröbner, S.N.; Worst, B.C.; Weischenfeldt, J.; Buchhalter, I.; Kleinheinz, K.; Rudneva, V.A.; Johann, P.D.; Balasubramanian, G.P.; Segura-Wang, M.; Brabetz, S.; et al. The landscape of genomic alterations across childhood cancers. Nature 2018, 555, 321–327. [Google Scholar] [CrossRef]
- Ma, X.; Liu, Y.; Liu, Y.; Alexandrov, L.B.; Edmonson, M.N.; Gawad, C.; Zhou, X.; Li, Y.; Rusch, M.C.; John, E.; et al. Pan-cancer genome and transcriptome analyses of 1,699 paediatric leukaemias and solid tumours. Nature 2018, 555, 371–376. [Google Scholar] [CrossRef]
- Śledzińska, P.; Bebyn, M.G.; Furtak, J.; Kowalewski, J.; Lewandowska, M.A. Prognostic and Predictive Biomarkers in Gliomas. Int. J. Mol. Sci. 2021, 22, 10373. [Google Scholar] [CrossRef] [PubMed]
- Fairbridge, N.A.; Southall, T.M.; Ayre, D.C.; Komatsu, Y.; Raquet, P.I.; Brown, R.J.; Randell, E.; Kovacs, C.S.; Christian, S.L. Loss of CD24 in mice leads to metabolic dysfunctions and a reduction in white adipocyte tissue. PLoS ONE 2015, 10, e0141966. [Google Scholar] [CrossRef] [PubMed]
- Sweet-Cordero, E.A.; Biegel, J.A. The genomic landscape of pediatric cancers: Implications for diagnosis and treatment. Science 2019, 363, 1170–1175. [Google Scholar] [CrossRef]
- Mackay, A.; Burford, A.; Carvalho, D.; Izquierdo, E.; Fazal-Salom, J.; Taylor, K.R.; Bjerke, L.; Clarke, M.; Vinci, M.; Nandhabalan, M.; et al. Integrated Molecular Meta-Analysis of 1,000 Pediatric High-Grade and Diffuse Intrinsic Pontine Glioma. Cancer Cell 2017, 32, 520–537.e5. [Google Scholar] [CrossRef]
- Jones, C.; Baker, S.J. Unique genetic and epigenetic mechanisms driving paediatric diffuse high-grade glioma. Nat. Rev. Cancer 2014, 14, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Liu, A.P.Y.; Orr, B.A.; Northcott, P.A.; Robinson, G.W. Advances in the classification of pediatric brain tumors through DNA methylation profiling: From research tool to frontline diagnostic. Cancer 2018, 124, 4168–4180. [Google Scholar] [CrossRef]
- Capper, D.; Jones, D.T.W.; Sill, M.; Hovestadt, V.; Schrimpf, D.; Sturm, D.; Koelsche, C.; Sahm, F.; Chavez, L.; Reuss, D.E.; et al. DNA methylation-based classification of central nervous system tumours. Nature 2018, 555, 469–474. [Google Scholar] [CrossRef]
- Pasqualetti, F.; Bertero, L.; Buccoliero, A.M.; Giunti, L.; Moscardi, S.; Castiglione, F.; Provenzano, A.; Sardi, I.; Scagnet, M.; Genitori, L.; et al. Pediatric High Grade Glioma Classification Criteria and Molecular Features of a Case Series. Genes 2022, 13, 624. [Google Scholar] [CrossRef]
- Bočkaj, I.; Martini, T.E.I.; De Camargo Magalhães, E.S.; Bakker, P.L.; Meeuwsen-De Boer, T.G.J.; Armandari, I.; Meuleman, S.L.; Mondria, M.T.; Stok, C.; Kok, Y.P.; et al. The H3.3K27M oncohistone affects replication stress outcome and provokes genomic instability in pediatric glioma. PLOS Genet. 2021, 17, e1009868. [Google Scholar] [CrossRef]
- Huang, T.Y.T.; Piunti, A.; Qi, J.; Morgan, M.; Bartom, E.; Shilatifard, A.; Saratsis, A.M. Effects of H3.3G34V mutation on genomic H3K36 and H3K27 methylation patterns in isogenic pediatric glioma cells. Acta Neuropathol. Commun. 2020, 8, 1–13. [Google Scholar] [CrossRef]
- Liu, X.; McEachron, T.A.; Schwartzentruber, J.; Wu, G. Histone H3 mutations in pediatric brain tumors. Cold Spring Harb. Perspect. Biol. 2014, 6. [Google Scholar] [CrossRef] [PubMed]
- Bale, T.A.; Rosenblum, M.K. The 2021 WHO Classification of Tumors of the Central Nervous System: An update on pediatric low-grade gliomas and glioneuronal tumors. Brain Pathol. 2022, 32, e13060. [Google Scholar] [CrossRef]
- Ryall, S.; Tabori, U.; Hawkins, C. Pediatric low-grade glioma in the era of molecular diagnostics. Acta Neuropathol. Commun. 2020, 8, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.T.W.; Kocialkowski, S.; Liu, L.; Pearson, D.M.; Bäcklund, L.M.; Ichimura, K.; Collins, V.P. Tandem duplication producing a novel oncogenic BRAF fusion gene defines the majority of pilocytic astrocytomas. Cancer Res. 2008, 68, 8673–8677. [Google Scholar] [CrossRef] [PubMed]
- Lassaletta, A.; Zapotocky, M.; Mistry, M.; Ramaswamy, V.; Honnorat, M.; Krishnatry, R.; Stucklin, A.G.; Zhukova, N.; Arnoldo, A.; Ryall, S.; et al. JOURNAL OF CLINICAL ONCOLOGY Therapeutic and Prognostic Implications of BRAF V600E in Pediatric Low-Grade Gliomas. J. Clin. Oncol. 2017, 35, 2934–2941. [Google Scholar] [CrossRef] [PubMed]
- Egbivwie, N.; Cockle, J.V.; Humphries, M.; Ismail, A.; Esteves, F.; Taylor, C.; Karakoula, K.; Morton, R.; Warr, T.; Short, S.C.; et al. FGFR1 expression and role in migration in low and high grade pediatric gliomas. Front. Oncol. 2019, 9, 103. [Google Scholar] [CrossRef]
- Kouhara, H.; Hadari, Y.R.; Spivak-Kroizman, T.; Schilling, J.; Bar-Sagi, D.; Lax, I.; Schlessinger, J. A Lipid-Anchored Grb2-Binding Protein That Links FGF-Receptor Activation to the Ras/MAPK Signaling Pathway. Cell 1997, 89, 693–702. [Google Scholar] [CrossRef]
- Zaytseva, M.; Papusha, L.; Novichkova, G.; Druy, A. Molecular Stratification of Childhood Ependymomas as a Basis for Personalized Diagnostics and Treatment. Cancers 2021, 13, 4954. [Google Scholar] [CrossRef]
- Hommelberg, P.P.H.; Plat, J.; Langen, R.C.J.; Schols, A.M.W.J.; Mensink, R.P. Fatty acid-induced NF-κB activation and insulin resistance in skeletal muscle are chain length dependent. Am. J. Physiol.-Endocrinol. Metab. 2009, 296, 114–120. [Google Scholar] [CrossRef]
- Yadav, U.C.S.; Ramana, K.V. Regulation of NF-κB-induced inflammatory signaling by lipid peroxidation-derived aldehydes. Oxid. Med. Cell. Longev. 2013, 2013. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Taouk, G.M. A Potential Role of YAP/TAZ in the Interplay Between Metastasis and Metabolic Alterations. Front. Oncol. 2020, 10, 928. [Google Scholar] [CrossRef]
- Stucklin, A.S.G.; Ramaswamy, V.; Daniels, C.; Taylor, M.D. Review of molecular classification and treatment implications of pediatric brain tumors. Curr. Opin. Pediatr. 2018, 30, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Bacci, M.; Lorito, N.; Smiriglia, A.; Morandi, A. Fat and Furious: Lipid Metabolism in Antitumoral Therapy Response and Resistance. Trends Cancer 2021, 7, 198–213. [Google Scholar] [CrossRef] [PubMed]
- Lladó, V.; López, D.J.; Ibarguren, M.; Alonso, M.; Soriano, J.B.; Escribá, P.V.; Busquets, X. Regulation of the cancer cell membrane lipid composition by NaCHOleate: Effects on cell signaling and therapeutical relevance in glioma. Biochim. Biophys. Acta-Biomembr. 2014, 1838, 1619–1627. [Google Scholar] [CrossRef]
- Hanauer, D.; Rhodes, D.; Sinha-Kumar, C.; Chinnaiyan, A. Bioinformatics Approaches in the Study of Cancer. Curr. Mol. Med. 2007, 7, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Owada, Y. Fatty acid binding protein: Localization and functional significance in the brain. Tohoku J. Exp. Med. 2008, 214, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.; Rosselló, C.A.; Fernández-García, P.; Lladó, V.; Kakhlon, O.; Escribá, P.V. The Implications for Cells of the Lipid Switches Driven by Protein–Membrane Interactions and the Development of Membrane Lipid Therapy. Int. J. Mol. Sci. 2020, 21, 2322. [Google Scholar] [CrossRef]
- Bruschi, M.; Petretto, A.; Cama, A.; Pavanello, M.; Bartolucci, M.; Morana, G.; Ramenghi, L.A.; Garré, M.L.; Ghiggeri, G.M.; Panfoli, I.; et al. Potential biomarkers of childhood brain tumor identified by proteomics of cerebrospinal fluid from extraventricular drainage (EVD). Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef]
- Lee, B.; Mohamad, I.; Pokhrel, R.; Murad, R.; Yuan, M.; Stapleton, S.; Bettegowda, C.; Jallo, G.; Eberhart, C.G.; Garrett, T.; et al. Medulloblastoma cerebrospinal fluid reveals metabolites and lipids indicative of hypoxia and cancer-specific RNAs. Acta Neuropathol. Commun. 2022, 10, 1–14. [Google Scholar] [CrossRef]
- Puget, S.; Philippe, C.; Bax, D.A.; Job, B.; Varlet, P.; Junier, M.P.; Andreiuolo, F.; Carvalho, D.; Reis, R.; Guerrini-Rousseau, L.; et al. Mesenchymal transition and pdgfra amplification/mutation are key distinct oncogenic events in pediatric diffuse intrinsic pontine gliomas. PLoS ONE 2012, 7, e3031. [Google Scholar] [CrossRef]
- Ullman, M.D.; Radin, N.S. The enzymatic formation of sphingomyelin from ceramide and lecithin in mouse liver. J. Biol. Chem. 1974, 249, 1506–1512. [Google Scholar] [CrossRef] [PubMed]
- Fernández-García, P.; Rosselló, C.A.; Rodríguez-Lorca, R.; Beteta-Göbel, R.; Fernández-Díaz, J.; Lladó, V.; Busquets, X.; Escribá, P.V. The opposing contribution of SMS1 and SMS2 to glioma progression and their value in the therapeutic response to 2OHOA. Cancers 2019, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Kuang, S.; Cao, R.; Wang, J.; Peng, Q.; Sun, C. Sorafenib kills liver cancer cells by disrupting SCD1-mediated synthesis of monounsaturated fatty acids via the ATP-AMPK-mTOR-SREBP1 signaling pathway. FASEB J. 2019, 33, 10089–10103. [Google Scholar] [CrossRef]
- Martin, M.L.; Barceló-Coblijn, G.; De Almeida, R.F.M.; Noguera-Salvà, M.A.; Terés, S.; Higuera, M.; Liebisch, G.; Schmitz, G.; Busquets, X.; Escribá, P.V. The role of membrane fatty acid remodeling in the antitumor mechanism of action of 2-hydroxyoleic acid. Biochim. Biophys. Acta-Biomembr. 2013, 1828, 1405–1413. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chu, H.J.; Liang, Y.C.; Huang, J.M.; Shang, C.L.; Tan, H.; Liu, D.; Zhao, Y.H.; Liu, T.Y.; Yao, S.Z. FABP5 correlates with poor prognosis and promotes tumor cell growth and metastasis in cervical cancer. Tumour Biol. 2016, 37, 14873–14883. [Google Scholar] [CrossRef]
- Wang, Y.; Wahafu, A.; Wu, W.; Xiang, J.; Huo, L.; Ma, X.; Wang, N.; Liu, H.; Bai, X.; Xu, D.; et al. FABP5 enhances malignancies of lower-grade gliomas via canonical activation of NF-κB signaling. J. Cell. Mol. Med. 2021, 25, 4487–4500. [Google Scholar] [CrossRef]
- Dyer, C.A.; Benjamins, J.A. Organization of oligodendroglial membrane sheets: II. Galactocerebroside: Antibody interactions signal changes in cytoskeleton and myelin basic protein. J. Neurosci. Res. 1989, 24, 212–221. [Google Scholar] [CrossRef]
- Liu, D.G.; Xue, L.; Li, J.; Yang, Q.; Peng, J.Z. Epithelial-mesenchymal transition and GALC expression of circulating tumor cells indicate metastasis and poor prognosis in non-small cell lung cancer. Cancer Biomark. 2018, 22, 417–426. [Google Scholar] [CrossRef]
- Chen, W.C.; Wang, C.Y.; Hung, Y.H.; Weng, T.Y.; Yen, M.C.; Lai, M.D. Systematic Analysis of Gene Expression Alterations and Clinical Outcomes for Long-Chain Acyl-Coenzyme A Synthetase Family in Cancer. PLoS ONE 2016, 11, e0155660. [Google Scholar] [CrossRef]
- Tea, M.N.; Poonnoose, S.I.; Pitson, S.M. Targeting the Sphingolipid System as a Therapeutic Direction for Glioblastoma. Cancers 2020, 12, 111. [Google Scholar] [CrossRef]
- Sheridan, M.; Ogretmen, B. The Role of Ceramide Metabolism and Signaling in the Regulation of Mitophagy and Cancer Therapy. Cancers 2021, 13, 2475. [Google Scholar] [CrossRef] [PubMed]
- Kuwata, H.; Hara, S. Role of acyl-CoA synthetase ACSL4 in arachidonic acid metabolism. Prostaglandins Other Lipid Mediat. 2019, 144, 106363. [Google Scholar] [CrossRef]
- Röhrig, F.; Schulze, A. The multifaceted roles of fatty acid synthesis in cancer. Nat. Rev. Cancer 2016, 16, 732–749. [Google Scholar] [CrossRef] [PubMed]
- Gabriely, G.; Wheeler, M.A.; Takenaka, M.C.; Quintana, F.J. Role of AHR and HIF-1$α$ in Glioblastoma Metabolism. Trends Endocrinol. Metab. 2017, 28, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Parveen, F.; Bender, D.; Law, S.H.; Mishra, V.K.; Chen, C.C.; Ke, L.Y. Role of Ceramidases in Sphingolipid Metabolism and Human Diseases. Cells 2019, 8, 1573. [Google Scholar] [CrossRef]
- Bonica, J.; Mao, C.; Obeid, L.M.; Hannun, Y.A. Transcriptional Regulation of Sphingosine Kinase 1. Cells 2020, 9, 2437. [Google Scholar] [CrossRef]
- Dyer, M.A.; Qadeer, Z.A.; Valle-Garcia, D.; Bernstein, E.; Armstrong, S.A.; Henikoff, S.; Vakoc, C.R. ATRX and DAXX: Mechanisms and Mutations. Cold Spring Harb. Perspect. Med. 2017, 7, a026567. [Google Scholar] [CrossRef] [PubMed]
- Pyne, N.J.; Pyne, S. Recent advances in the role of sphingosine 1-phosphate in cancer. FEBS Lett. 2020, 594, 3583–3601. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, Z.; Liu, C.; Wang, B.; Liu, P.; Fang, S.; Yang, F.; You, Y.; Li, X. ATF4-dependent fructolysis fuels growth of glioblastoma forme n.d. Nat. Commun. 2022, 13, 6108. [Google Scholar] [CrossRef] [PubMed]
- Arlotta, P.; Molyneaux, B.J.; Jabaudon, D.; Yoshida, Y.; Macklis, J.D. Ctip2 Controls the Differentiation of Medium Spiny Neurons and the Establishment of the Cellular Architecture of the Striatum. J. Neurosci. 2008, 28, 622–632. [Google Scholar] [CrossRef]
- Yoon, J.; Grinchuk, O.V.; Tirado-Magallanes, R.; Ngian, Z.K.; Tay, E.X.Y.; Chuah, Y.H.; Lee, B.W.L.; Feng, J.; Crasta, K.C.; Ong, C.T.; et al. E2F and STAT3 provide transcriptional synergy for histone variant H2AZ activation to sustain glioblastoma chromatin accessibility and tumorigenicity. Cell Death Differ. 2022, 29, 1379–1394. [Google Scholar] [CrossRef]
- Stoffel, W.; Jenke, B.; Schmidt-Soltau, I.; Binczek, E.; Brodesser, S.; Hammels, I. SMPD3 deficiency perturbs neuronal proteostasis and causes progressive cognitive impairment. Cell Death Dis. 2018, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Sheng, S.; Ye, L.; Xu, X.; Ma, Y.; Feng, X.; Qiu, L.; Fan, Z.; Wang, Y.; Xia, X.; et al. Extracellular vesicles derived from glioblastoma promote proliferation and migration of neural progenitor cells via PI3K-Akt pathway. Cell Commun. Signal. 2022, 20, 1–16. [Google Scholar] [CrossRef]
- Marques, C.; Unterkircher, T.; Kroon, P.; Oldrini, B.; Izzo, A.; Dramaretska, Y.; Ferrarese, R.; Kling, E.; Schnell, O.; Nelander, S.; et al. Nf1 regulates mesenchymal glioblastoma plasticity and aggressiveness through the ap-1 transcription factor fosl1. Elife 2021, 10, e64846. [Google Scholar] [CrossRef]
- Prucca, C.G.; Racca, A.C.; Velazquez, F.N.; Gizzi, A.M.C.; Berdini, L.R.; Caputto, B.L. Impairing activation of phospholipid synthesis by c-Fos interferes with glioblastoma cell proliferation. Biochem. J. 2020, 477, 4675–4688. [Google Scholar] [CrossRef] [PubMed]
- Giacopelli, F.; Cappato, S.; Tonachini, L.; Mura, M.; Di Lascio, S.; Fornasari, D.; Ravazzolo, R.; Bocciardi, R. Identification and characterization of regulatory elements in the promoter of ACVR1, the gene mutated in Fibrodysplasia Ossificans Progressiva. Orphanet J. Rare Dis. 2013, 8, 1–16. [Google Scholar] [CrossRef]
- Peng, G.; Wang, Y.; Ge, P.; Bailey, C.; Zhang, P.; Zhang, D.; Meng, Z.; Qi, C.; Chen, Q.; Chen, J.; et al. The HIF1$α$-PDGFD-PDGFR$α$ axis controls glioblastoma growth at normoxia/mild-hypoxia and confers sensitivity to targeted therapy by echinomycin. J. Exp. Clin. Cancer Res. 2021, 40, 1–16. [Google Scholar] [CrossRef]
- Renfrow, J.J.; Soike, M.H.; West, J.L.; Ramkissoon, S.H.; Metheny-Barlow, L.; Mott, R.T.; Kittel, C.A.; D’Agostino, R.B.; Tatter, S.B.; Laxton, A.W.; et al. Attenuating hypoxia driven malignant behavior in glioblastoma with a novel hypoxia-inducible factor 2 alpha inhibitor. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Gan, B. ACSL4, PUFA, and Ferroptosis: New Arsenal in Anti-Tumor Immunity. Signal. Transduct. Target. Ther. 2022, 7, 1–3. [Google Scholar] [CrossRef]
- Liang, J.; Piao, Y.; Henry, V.; Tiao, N.; de Groot, J.F.; Liang, J.; Piao, Y.; Henry, V.; Tiao, N.; de Groot, J.F. Interferon-Regulatory Factor-1 (IRF1) Regulates Bevacizumab Induced Autophagy. Oncotarget 2015, 6, 31479–31492. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, E.J.; Hitomi, M.; Oh, S.Y.; Jin, X.; Jeon, H.M.; Beck, S.; Jin, X.; Kim, J.K.; Park, C.G.; et al. The LIM-only transcription factor LMO2 determines tumorigenic and angiogenic traits in glioma stem cells. Cell Death Differ. 2015, 22, 1517–1525. [Google Scholar] [CrossRef]
- Nishi-Tatsumi, M.; Yahagi, N.; Takeuchi, Y.; Toya, N.; Takarada, A.; Murayama, Y.; Aita, Y.; Sawada, Y.; Piao, X.; Oya, Y.; et al. A key role of nuclear factor Y in the refeeding response of fatty acid synthase in adipocytes. FEBS Lett. 2017, 591, 965–978. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Zhang, M.; Wang, Y.; Wang, Y. NF-YC in glioma cell proliferation and tumor growth and its role as an independent predictor of patient survival. Neurosci. Lett. 2016, 631, 40–49. [Google Scholar] [CrossRef]
- Wegner, M.S.; Gruber, L.; Mattjus, P.; Geisslinger, G.; Grösch, S. The UDP-glucose ceramide glycosyltransferase (UGCG) and the link to multidrug resistance protein 1 (MDR1). BMC Cancer 2018, 18, 1–10. [Google Scholar] [CrossRef]
- Gopal, K.; Grossi, E.; Paoletti, P.; Usardi, M. Lipid composition of human intracranial tumors: A biochemical study. Acta Neurochir. 1963, 11, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Campenella, R. Membrane lipids modifications in human gliomas of different degree of malignancy. J. Neurosurg. Sci. 1992, 36, 11–25. [Google Scholar]
- Martin, D.D.; Robbins, M.E.C.; Spector, A.A.; Wen, B.C.; Hussey, D.H. The fatty acid composition of human gliomas differs from that found in nonmalignant brain tissue. Lipids 1996, 31, 1283–1288. [Google Scholar] [CrossRef]
- Aramesh, M.; Stoycheva, D.; Sandu, I.; Ihle, S.J.; Zünd, T.; Shiu, J.Y.; Forró, C.; Asghari, M.; Bernero, M.; Lickert, S.; et al. Nanoconfinement of microvilli alters gene expression and boosts T cell activation. Proc. Natl. Acad. Sci. USA 2021, 118, e2107535118. [Google Scholar] [CrossRef]
- Polis, B.; Imiela, A.; Polis, L.; Abramczyk, H. Raman spectroscopy for medulloblastoma. Child’s Nerv. Syst. 2018, 34, 2425–2430. [Google Scholar] [CrossRef]
- Leslie, D.G.; Kast, R.E.; Poulik, J.M.; Rabah, R.; Sood, S.; Auner, G.W.; Klein, M.D. Identification of pediatric brain neoplasms using Raman spectroscopy. Pediatr. Neurosurg. 2012, 48, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Glunde, K.; Bhujwalla, Z.M. Metabolic tumor imaging using magnetic resonance spectroscopy. Semin. Oncol. 2011, 38, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Gillies, R.J.; Morse, D.L. In Vivo Magnetic Resonance Spectroscopy in Cancer. Annu. Rev. Biomed. Eng. 2005, 7, 287–326. [Google Scholar] [CrossRef]
- Cuellar-Baena, S.; Morales, J.M.; Martinetto, H.; Calvar, J.; Sevlever, G.; Castellano, G.; Cerdá-Nicolás, M.; Celda, B.; Monle-on, D. Comparative metabolic profiling of paediatric ependymoma, medulloblastoma and pilocytic astrocytoma. Int. J. Mol. Med. 2010, 26, 941–948. [Google Scholar] [CrossRef]
- Liserre, R.; Pinelli, L.; Gasparotti, R. MR spectroscopy in pediatric neuroradiology. Transl. Pediatr. 2021, 10, 1169. [Google Scholar] [CrossRef] [PubMed]
- Orphanidou-Vlachou, E.; Kohe, S.E.; Brundler, M.A.; MacPherson, L.; Sun, Y.; Davies, N.; Wilson, M.; Pan, X.; Arvanitis, T.N.; Grundy, R.G.; et al. Metabolite Levels in Paediatric Brain Tumours Correlate with Histological Features. Pathobiology 2018, 85, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Galanaud, D.; Nicoli, F.; Confort-Gouny, S.; Le Fur, Y.; Dormont, D.; Girard, N.; Ranjeva, J.P.; Cozzone, P.J. Spectroscopie par résonance magnétique cérébrale. J. Radiol. 2007, 88, 483–496. [Google Scholar] [CrossRef]
- Clark, A.R.; Calligaris, D.; Regan, M.S.; Pomeranz Krummel, D.; Agar, J.N.; Kallay, L.; MacDonald, T.; Schniederjan, M.; Santagata, S.; Pomeroy, S.L.; et al. Rapid discrimination of pediatric brain tumors by mass spectrometry imaging. J. Neurooncol. 2018, 140, 269–279. [Google Scholar] [CrossRef]
- Srivastava, N.K.; Pradhan, S.; Gowda, G.A.N.; Kumar, R. In vitro, high-resolution 1H and 31P NMR based analysis of the lipid components in the tissue, serum, and CSF of the patients with primary brain tumors: One possible diagnostic view. NMR Biomed. 2010, 23, 113–122. [Google Scholar] [CrossRef]
- Wilson, M.; Cummins, C.L.; MacPherson, L.; Sun, Y.; Natarajan, K.; Grundy, R.G.; Arvanitis, T.N.; Kauppinen, R.A.; Peet, A.C. Magnetic resonance spectroscopy metabolite profiles predict survival in paediatric brain tumours. Eur. J. Cancer 2013, 49, 457–464. [Google Scholar] [CrossRef]
- Marcus, K.J.; Astrakas, L.G.; Zurakowski, D.; Zarifi, M.K.; Mintzopoulos, D.; Poussaint, T.Y.; Anthony, D.C.; De Girolami, U.; Black, P.M.L.; Tarbell, N.J.; et al. Predicting survival of children with CNS tumors using proton magnetic resonance spectroscopic imaging biomarkers. Int. J. Oncol. 2007, 30, 651–657. [Google Scholar] [CrossRef]
- Bennett, C.D.; Gill, S.K.; Kohe, S.E.; Wilson, M.P.; Davies, N.P.; Arvanitis, T.N.; Tennant, D.A.; Peet, A.C. Ex vivo metabolite profiling of paediatric central nervous system tumours reveals prognostic markers. Sci. Rep. 2019, 9, 10473. [Google Scholar] [CrossRef] [PubMed]
- Tzika, A.A.; Zurakowski, D.; Poussaint, T.Y.; Goumnerova, L.; Astrakas, L.G.; Barnes, P.D.; Anthony, D.C.; Billett, A.L.; Tarbell, N.J.; Scott, R.M.; et al. Proton magnetic spectroscopic imaging of the child’s brain: The response of tumors to treatment. Neuroradiology 2001, 43, 169–177. [Google Scholar] [CrossRef]
- Paine, M.R.L.; Liu, J.; Huang, D.; Ellis, S.R.; Trede, D.; Kobarg, J.H.; Heeren, R.M.A.; Fernández, F.M.; MacDonald, T.J. Three-Dimensional Mass Spectrometry Imaging Identifies Lipid Markers of Medulloblastoma Metastasis. Sci. Rep. 2019, 9, 2205. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.H.D.; Aquilina, K. Surgical approaches in pediatric neuro-oncology. Cancer Metastasis Rev. 2019, 38, 723–747. [Google Scholar] [CrossRef]
- Zebian, B.; Vergani, F.; Lavrador, J.P.; Mukherjee, S.; Kitchen, W.J.; Stagno, V.; Chamilos, C.; Pettorini, B.; Mallucci, C. Recent technological advances in pediatric brain tumor surgery. CNS Oncol. 2017, 6, 71. [Google Scholar] [CrossRef] [PubMed]
- Funakoshi, Y.; Hata, N.; Kuga, D.; Hatae, R.; Sangatsuda, Y.; Fujioka, Y.; Takigawa, K.; Mizoguchi, M. Pediatric Glioma: An Update of Diagnosis, Biology, and Treatment. Cancers 2021, 13, 758. [Google Scholar] [CrossRef]
- Laprie, A.; Hu, Y.; Alapetite, C.; Carrie, C.; Habrand, J.L.; Bolle, S.; Bondiau, P.Y.; Ducassou, A.; Huchet, A.; Bertozzi, A.I.; et al. Paediatric brain tumours: A review of radiotherapy, state of the art and challenges for the future regarding protontherapy and carbontherapy. Cancer Radiother. 2015, 19, 775–789. [Google Scholar] [CrossRef]
- Baliga, S.; Yock, T.I. Proton beam therapy in pediatric oncology. Curr. Opin. Pediatr. 2019, 31, 28–34. [Google Scholar] [CrossRef]
- Kortmann, R.D.; Seidel, C.; Müller, K.; Hirsch, F.W. Irradiation of Intracranial Gliomas in Children. Prog. Neurol. Surg. 2018, 31, 87–101. [Google Scholar] [CrossRef]
- Lobón, M.J.; Bautista, F.; Riet, F.; Dhermain, F.; Canale, S.; Dufour, C.; Blauwblomme, T.; Zerah, M.; Beccaria, K.; Saint-Rose, C.; et al. Re-irradiation of recurrent pediatric ependymoma: Modalities and outcomes: A twenty-year survey. Springerplus 2016, 5, 879. [Google Scholar] [CrossRef]
- Cohen, K.J.; Pollack, I.F.; Zhou, T.; Buxton, A.; Holmes, E.J.; Burger, P.C.; Brat, D.J.; Rosenblum, M.K.; Hamilton, R.L.; Lavey, R.S.; et al. Temozolomide in the treatment of high-grade gliomas in children: A report from the Children’s Oncology Group. Neuro. Oncol. 2011, 13, 317–323. [Google Scholar] [CrossRef]
- Chatwin, H.V.; Cruz, J.C.; Green, A.L.; Chatwin, H.V.; Cruz, J.C.; Green, A.L. Pediatric high-grade glioma: Moving toward subtype-specific multimodal therapy. FEBS J. 2021, 288, 6127–6141. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.I.; Jakacki, R.I.; Fisher, M.J.; Kilburn, L.B.; Horn, M.; Vezina, G.; Rood, B.R.; Packer, R.J. Long-term efficacy and toxicity of bevacizumab-based therapy in children with recurrent low-grade gliomas. Pediatr. Blood Cancer 2013, 60, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Rashed, W.M.; Maher, E.; Adel, M.; Saber, O.; Zaghloul, M.S. Pediatric diffuse intrinsic pontine glioma: Where do we stand? Cancer Metastasis Rev. 2019, 38, 759–770. [Google Scholar] [CrossRef]
- Stanić, D.; Grujičić, D.; Pekmezović, T.; Bokun, J.; Popović-Vuković, M.; Janić, D.; Paripović, L.; Ilić, V.; Slović, M.P.; Ilić, R.; et al. Clinical profile, treatment and outcome of pediatric brain tumors in Serbia in a 10-year period: A national referral institution experience. PLoS ONE 2021, 16, e0259095. [Google Scholar] [CrossRef] [PubMed]
- Mathew, R.K.; O’kane, R.; Parslow, R.; Stiller, C.; Kenny, T.; Picton, S.; Chumas, P.D. Comparison of survival between the UK and US after surgery for most common pediatric CNS tumors. Neuro. Oncol. 2014, 16, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The blood-brain barrier and blood-tumour barrier in brain tumours and metastases. Nat. Rev. Cancer 2020, 20, 26–41. [Google Scholar] [CrossRef]
- Patel, J.P.; Spiller, S.E.; Barker, E.D. Drug penetration in pediatric brain tumors: Challenges and opportunities. Pediatr. Blood Cancer 2021, 68, e28983. [Google Scholar] [CrossRef]
- Power, E.A.; Rechberger, J.S.; Gupta, S.; Schwartz, J.D.; Daniels, D.J.; Khatua, S. Drug delivery across the blood-brain barrier for the treatment of pediatric brain tumors—An update. Adv. Drug Deliv. Rev. 2022, 185, 114303. [Google Scholar] [CrossRef]
- De Blank, P.; Fouladi, M.; Huse, J.T. Molecular markers and targeted therapy in pediatric low-grade glioma. J. Neurooncol. 2020, 150, 5–15. [Google Scholar] [CrossRef]
- Smith, A.; Onar-Thomas, A.; Ellison, D.; Owens-Pickle, E.; Wu, S.; Leary, S.E.S.; Fouladi, M.; Merchant, T.; Gajjar, A.; Foreman, N. EPEN-54. ACNS0831, phase III randomized trial of post-radiation chemotherapy in patients with newly diagnosed ependymoma ages 1 to 21 years. Neuro. Oncol. 2020, 22, iii318. [Google Scholar] [CrossRef]
- Franz, D.N.; Belousova, E.; Sparagana, S.; Bebin, E.M.; Frost, M.; Kuperman, R.; Witt, O.; Kohrman, M.H.; Flamini, J.R.; Wu, J.Y.; et al. Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): A multicentre, randomised, placebo-controlled phase 3 trial. Lancet (Lond. Engl.) 2013, 381, 125–132. [Google Scholar] [CrossRef]
- Brabetz, S.; Leary, S.E.S.; Gröbner, S.N.; Nakamoto, M.W.; Şeker-Cin, H.; Girard, E.J.; Cole, B.; Strand, A.D.; Bloom, K.L.; Hovestadt, V.; et al. A biobank of patient-derived pediatric brain tumor models. Nat. Med. 2018, 24, 1752–1761. [Google Scholar] [CrossRef]
- Xu, J.; Margol, A.; Asgharzadeh, S.; Erdreich-Epstein, A. Pediatric Brain Tumor Cell Lines. J. Cell. Biochem. 2015, 116, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Hanz, S.Z.; Adeuyan, O.; Lieberman, G.; Hennika, T. Clinical trials using molecular stratification of pediatric brain tumors. Transl. Pediatr. 2020, 9, 144. [Google Scholar] [CrossRef]
- Findlay, I.J.; De Iuliis, G.N.; Duchatel, R.J.; Jackson, E.R.; Vitanza, N.A.; Cain, J.E.; Waszak, S.M.; Dun, M.D. Pharmaco-proteogenomic profiling of pediatric diffuse midline glioma to inform future treatment strategies. Oncogene 2021, 41, 461–475. [Google Scholar] [CrossRef]
- Bahmad, H.F.; Elajami, M.K.; El Zarif, T.; Bou-Gharios, J.; Abou-Antoun, T.; Abou-Kheir, W. Drug repurposing towards targeting cancer stem cells in pediatric brain tumors. Cancer Metastasis Rev. 2020, 39, 127–148. [Google Scholar] [CrossRef]
- Hwang, E.I.; Sayour, E.J.; Flores, C.T.; Grant, G.; Wechsler-Reya, R.; Hoang-Minh, L.B.; Kieran, M.W.; Salcido, J.; Prins, R.M.; Figg, J.W.; et al. The current landscape of immunotherapy for pediatric brain tumors. Nat. Cancer 2022, 3, 11–24. [Google Scholar] [CrossRef]
- Wick, W.; Osswald, M.; Wick, A.; Winkler, F. Treatment of glioblastoma in adults. Ther. Adv. Neurol. Disord. 2018, 11. [Google Scholar] [CrossRef] [PubMed]
- Van den Bent, M.; Gan, H.K.; Lassman, A.B.; Kumthekar, P.; Merrell, R.; Butowski, N.; Lwin, Z.; Mikkelsen, T.; Nabors, L.B.; Papadopoulos, K.P.; et al. Efficacy of depatuxizumab mafodotin (ABT-414) monotherapy in patients with EGFR-amplified, recurrent glioblastoma: Results from a multi-center, international study. Cancer Chemother. Pharmacol. 2017, 80, 1209. [Google Scholar] [CrossRef] [PubMed]
- Hartman, L.L.R.; Crawford, J.R.; Makale, M.T.; Milburn, M.; Joshi, S.; Salazar, A.M.; Hasenauer, B.; Vandenberg, S.R.; Macdonald, T.J.; Durden, D.L. Pediatric Phase II Trials of Poly-ICLC in the Management of Newly Diagnosed and Recurrent Brain Tumors. J. Pediatr. Hematol. Oncol. 2014, 36, 451. [Google Scholar] [CrossRef] [PubMed]
- Patterson, J.D.; Henson, J.C.; Breese, R.O.; Bielamowicz, K.J.; Rodriguez, A. CAR T Cell Therapy for Pediatric Brain Tumors. Front. Oncol. 2020, 10, 1582. [Google Scholar] [CrossRef]
- Abdel-Khaleq, S.; Alim, L.; Johnston, A.; Adam, K.; Galvin, R.; Maeser, D.; Gruener, R.; Huang, S.; Johnson, T.S.; Pacholczyk, R.; et al. Immu-04. First-in-children phase 1b study using the ido pathway inhibitor indoximod in combination with radiation and chemotherapy for children with newly diagnosed DIPG (NCT02502708, NLG2105). Neuro. Oncol. 2021, 23, i27. [Google Scholar] [CrossRef]
- Varela-Guruceaga, M.; Tejada-Solís, S.; García-Moure, M.; Fueyo, J.; Gomez-Manzano, C.; Patiño-García, A.; Alonso, M.M. Oncolytic Viruses as Therapeutic Tools for Pediatric Brain Tumors. Cancers 2018, 10, 226. [Google Scholar] [CrossRef]
- Olsen, H.E.; Lynn, G.M.; Valdes, P.A.; Cerecedo Lopez, C.D.; Ishizuka, A.S.; Arnaout, O.; Bi, W.L.; Peruzzi, P.P.; Chiocca, E.A.; Friedman, G.K.; et al. Therapeutic cancer vaccines for pediatric malignancies: Advances, challenges, and emerging technologies. Neuro-Oncology Adv. 2021, 3, 1–14. [Google Scholar] [CrossRef]
- Banerjee, K.; Núñez, F.J.; Haase, S.; McClellan, B.L.; Faisal, S.M.; Carney, S.V.; Yu, J.; Alghamri, M.S.; Asad, A.S.; Candia, A.J.N.; et al. Current Approaches for Glioma Gene Therapy and Virotherapy. Front. Mol. Neurosci. 2021, 14, 30. [Google Scholar] [CrossRef] [PubMed]
- El Moukhtari, S.H.; Rodríguez-Nogales, C.; Blanco-Prieto, M.J. Oral lipid nanomedicines: Current status and future perspectives in cancer treatment. Adv. Drug Deliv. Rev. 2021, 173, 238–251. [Google Scholar] [CrossRef]
- Iturrioz-Rodríguez, N.; Bertorelli, R.; Ciofani, G. Lipid-Based Nanocarriers for The Treatment of Glioblastoma. Adv. NanoBiomed Res. 2021, 1, 2000054. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhang, L.; Chen, W.; Shen, L.; Jiang, J.; Sun, S.; Chen, Z. Role of Intra- and Extracellular Lipid Signals in Cancer Stemness and Potential Therapeutic Strategy. Front. Pharmacol. 2021, 12, 2504. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.; Parets, S.; Fernández-Díaz, J.; Beteta-Göbel, R.; Rodríguez-Lorca, R.; Román, R.; Lladó, V.; Rosselló, C.A.; Fernández-García, P.; Escribá, P.V. Lipids in Pathophysiology and Development of the Membrane Lipid Therapy: New Bioactive Lipids. Membranes 2021, 11, 919. [Google Scholar] [CrossRef]
- Fu, Y.; Zou, T.; Shen, X.; Nelson, P.J.; Li, J.; Wu, C.; Yang, J.; Zheng, Y.; Bruns, C.; Zhao, Y.; et al. Lipid metabolism in cancer progression and therapeutic strategies. MedComm 2020, 2, 27–59. [Google Scholar] [CrossRef] [PubMed]
- Matsui, Y. Mbrs-23. Significance of mi-r33 in generation and progression of medulloblastoma. Neuro. Oncol. 2020, 22, iii402. [Google Scholar] [CrossRef]
- Rahal, F.; Capdevielle, C.; Rousseau, B.; Izotte, J.; Dupuy, J.-W.; Cappellen, D.; Chotard, G.; Ménard, M.; Charpentier, J.; Jecko, V.; et al. An EZH2 blocker sensitizes histone mutated diffuse midline glioma to cholesterol metabolism inhibitors through an off-target effect. Neuro-Oncology Adv. 2022, 4, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Chen, J.; Lin, Z.; Wang, Z.; Mu, S.; Qin, Z. Targeting sphingosine kinase by ABC294640 against diffuse intrinsic pontine glioma (DIPG). J. Cancer 2020, 11, 4683–4691. [Google Scholar] [CrossRef] [PubMed]
- Daggubati, V.; Hochstelter, J.; Bommireddy, A.; Choudhury, A.; Krup, A.L.; Kaur, P.; Tong, P.; Li, A.; Xu, L.; Reiter, J.F.; et al. Smoothened-activating lipids drive resistance to CDK4/6 inhibition in Hedgehog-associated medulloblastoma cells and preclinical models. J. Clin. Invest. 2021, 131, e141171. [Google Scholar] [CrossRef] [PubMed]
- Sabnis, D.H.; Liu, J.-F.; Simmonds, L.; Blackburn, S.; Grundy, R.G.; Kerr, I.D.; Coyle, B. BLBP Is Both a Marker for Poor Prognosis and a Potential Therapeutic Target in Paediatric Ependymoma. Cancers 2021, 13, 2100. [Google Scholar] [CrossRef]
- Ljungblad, L.; Bergqvist, F.; Tümmler, C.; Madawala, S.; Olsen, T.K.; Andonova, T.; Jakobsson, P.J.; Johnsen, J.I.; Pickova, J.; Strandvik, B.; et al. Omega-3 fatty acids decrease CRYAB, production of oncogenic prostaglandin E2 and suppress tumor growth in medulloblastoma. Life Sci. 2022, 295, 120394. [Google Scholar] [CrossRef]
- Jendrossek, V. Erucylphosphocholine, a novel antineoplastic ether lipid, blocks growth and induces apoptosis in brain tumor cell lines in vitro. Int. J. Oncol. 1999, 14, 15–22. [Google Scholar] [CrossRef]
- Bi, J.; Khan, A.; Tang, J.; Armando, A.M.; Wu, S.; Zhang, W.; Gimple, R.C.; Reed, A.; Jing, H.; Koga, T.; et al. Targeting glioblastoma signaling and metabolism with a re-purposed brain-penetrant drug. Cell Rep. 2021, 37, 109957. [Google Scholar] [CrossRef]
- Xu, C.; Zhao, J.; Song, J.; Xiao, M.; Cui, X.; Xin, L.; Xu, J.; Zhang, Y.; Yi, K.; Hong, B.; et al. lncRNA PRADX is a Mesenchymal Glioblastoma Biomarker for Cellular Metabolism Targeted Therapy. Front. Oncol. 2022, 12, 1865. [Google Scholar] [CrossRef]
- Cruz Flores, V.A.; Menghani, H.; Mukherjee, P.K.; Marrero, L.; Obenaus, A.; Dang, Q.; Khoutorova, L.; Reid, M.M.; Belayev, L.; Bazan, N.G. Combined Therapy With Avastin, a PAF Receptor Antagonist and a Lipid Mediator Inhibited Glioblastoma Tumor Growth. Front. Pharmacol. 2021, 12, 2554. [Google Scholar] [CrossRef] [PubMed]
- Yi, K.; Zhan, Q.; Wang, Q.; Tan, Y.; Fang, C.; Wang, Y.; Zhou, J.; Yang, C.; Li, Y.; Kang, C. PTRF/cavin-1 remodels phospholipid metabolism to promote tumor proliferation and suppress immune responses in glioblastoma by stabilizing cPLA2. Neuro. Oncol. 2021, 23, 387–399. [Google Scholar] [CrossRef]
- Oatman, N.; Dasgupta, N.; Arora, P.; Choi, K.; Gawali, M.V.; Gupta, N.; Parameswaran, S.; Salomone, J.; Reisz, J.A.; Lawler, S.; et al. Mechanisms of stearoyl CoA desaturase inhibitor sensitivity and acquired resistance in cancer. Sci. Adv. 2021, 7, 7459. [Google Scholar] [CrossRef] [PubMed]
- Eyme, K.M.; Sammarco, A.; Jha, R.; Mnatsakanyan, H.; Pechdimaljian, C.; Carvalho, L.; Neustadt, R.; Moses, C.; Alnasser, A.; Tardiff, D.F.; et al. Targeting de novo lipid synthesis induces lipotoxicity and impairs DNA damage repair in glioblastoma mouse models. Sci. Transl. Med. 2023, 15, eabq6288. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Patel, S.; Affeck, V.S.; Wilson, I.; Turnbull, D.M.; Joshi, A.R.; Maxwell, R.; Stoll, E.A. Fatty acid oxidation is required for the respiration and proliferation of malignant glioma cells. Neuro. Oncol. 2017, 19, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.J.; Kim, Y.E.; Moon, B.S.; Kim, H.Y.; Jung, D.; Choi, S.; Jang, J.W.; Nam, D.H.; Cho, H. Azathioprine antagonizes aberrantly elevated lipid metabolism and induces apoptosis in glioblastoma. iScience 2021, 24, 102238. [Google Scholar] [CrossRef] [PubMed]
- Dasari, R.; Masi, M.; Lisy, R.; Ferdérin, M.; English, L.R.; Cimmino, A.; Mathieu, V.; Brenner, A.J.; Kuhn, J.G.; Whitten, S.T.; et al. Fungal metabolite ophiobolin A as a promising anti-glioma agent: In vivo evaluation, structure-activity relationship and unique pyrrolylation of primary amines. Bioorg. Med. Chem. Lett. 2015, 25, 4544–4548. [Google Scholar] [CrossRef] [PubMed]
- Villa, G.R.; Hulce, J.J.; Zanca, C.; Bi, J.; Ikegami, S.; Cahill, G.L.; Gu, Y.; Lum, K.M.; Masui, K.; Yang, H.; et al. An LXR-Cholesterol Axis Creates a Metabolic Co-Dependency for Brain Cancers. Cancer Cell 2016, 30, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Seyfried, T.N.; Mukherjee, P. Ganglioside GM3 is antiangiogenic in malignant brain cancer. J. Oncol. 2010, 2010, 961243. [Google Scholar] [CrossRef]
- Barceló-Coblijn, G.; Martin, M.L.; De Almeida, R.F.M.; Noguera-Salvà, M.A.; Marcilla-Etxenike, A.; Guardiola-Serrano, F.; Lüth, A.; Kleuser, B.; Halver, J.E.; Escribá, P.V. Sphingomyelin and sphingomyelin synthase (SMS) in the malignant transformation of glioma cells and in 2-hydroxyoleic acid therapy. Proc. Natl. Acad. Sci. USA 2011, 108, 19569–19574. [Google Scholar] [CrossRef] [PubMed]
- Lladó, V.; Terés, S.; Higuera, M.; Álvarez, R.; Noguera-Salva, M.A.; Halver, J.E.; Escribá, P.V.; Busquetsa, X. Pivotal role of dihydrofolate reductase knockdown in the anticancer activity of 2-hydroxyoleic acid. Proc. Natl. Acad. Sci. USA 2009, 106, 13754. [Google Scholar] [CrossRef]
- Terés, S.; Lladó, V.; Higuera, M.; Barceló-Coblijn, G.; Martin, M.L.; Noguera-Salvà, M.A.; Marcilla-Etxenike, A.; García-Verdugo, J.M.; Soriano-Navarro, M.; Saus, C.; et al. 2-Hydroxyoleate, anontoxic membrane binding anticancer drug, induces glioma cell differentiation and autophagy. Proc. Natl. Acad. Sci. USA 2012, 109, 8489–8494. [Google Scholar] [CrossRef]
- Kaynak, A.; Davis, H.W.; Vallabhapurapu, S.D.; Pak, K.Y.; Gray, B.D.; Qi, X. SapC–DOPS as a Novel Therapeutic and Diagnostic Agent for Glioblastoma Therapy and Detection: Alternative to Old Drugs and Agents. Pharmaceuticals 2021, 14, 1193. [Google Scholar] [CrossRef]
- Davis, H.W.; Kaynak, A.; Vallabhapurapu, S.D.; Qi, X. Targeting of elevated cell surface phosphatidylserine with saposin C-dioleoylphosphatidylserine nanodrug as individual or combination therapy for pancreatic cancer. World J. Gastrointest. Oncol. 2021, 13, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.W.; Vallabhapurapu, S.D.; Chu, Z.; Vallabhapurapu, S.L.; Franco, R.S.; Mierzwa, M.; Kassing, W.; Barrett, W.L.; Qi, X. Enhanced phosphatidylserine-selective cancer therapy with irradiation and SapC-DOPS nanovesicles. Oncotarget 2019, 10, 856–868. [Google Scholar] [CrossRef] [PubMed]
- Felix, F.H.C.; De Araujo, O.L.; Da Trindade, K.M.; Trompieri, N.M.; Fontenele, J.B. Retrospective evaluation of the outcomes of children with diffuse intrinsic pontine glioma treated with radiochemotherapy and valproic acid in a single center. J. Neurooncol. 2014, 116, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wong, J.Y.C.; Wong, P.; Radany, E.H. Low Dose Valproic Acid Enhances Radiosensitivity of Prostate Cancer through Acetylated p53-Dependent Modulation of Mitochondrial Membrane Potential and Apoptosis. Mol. Cancer Res. 2011, 9, 448. [Google Scholar] [CrossRef]
- Pajovic, S.; Siddaway, R.; Bridge, T.; Sheth, J.; Rakopoulos, P.; Kim, B.; Ryall, S.; Agnihotri, S.; Phillips, L.; Yu, M.; et al. Epigenetic activation of a RAS/MYC axis in H3.3K27M-driven cancer. Nat. Commun. 2020, 11, 1–16. [Google Scholar] [CrossRef]
- Chavan, T.S.; Muratcioglu, S.; Marszalek, R.; Jang, H.; Keskin, O.; Gursoy, A.; Nussinov, R.; Gaponenko, V. Plasma membrane regulates Ras signaling networks. Cell. Logist. 2016, 5, e1136374. [Google Scholar] [CrossRef]
- Bagchi, S.; Rathee, P.; Jayaprakash, V.; Banerjee, S. Farnesyl Transferase Inhibitors as Potential Anticancer Agents. Mini Rev. Med. Chem. 2018, 18, 1611–1623. [Google Scholar] [CrossRef]
- Chidley, C.; Trauger, S.A.; Birsoy, K.; O’Shea, E.K. The anticancer natural product ophiobolin A induces cytotoxicity by covalent modification of phosphatidylethanolamine. Elife 2016, 5, e14601. [Google Scholar] [CrossRef] [PubMed]
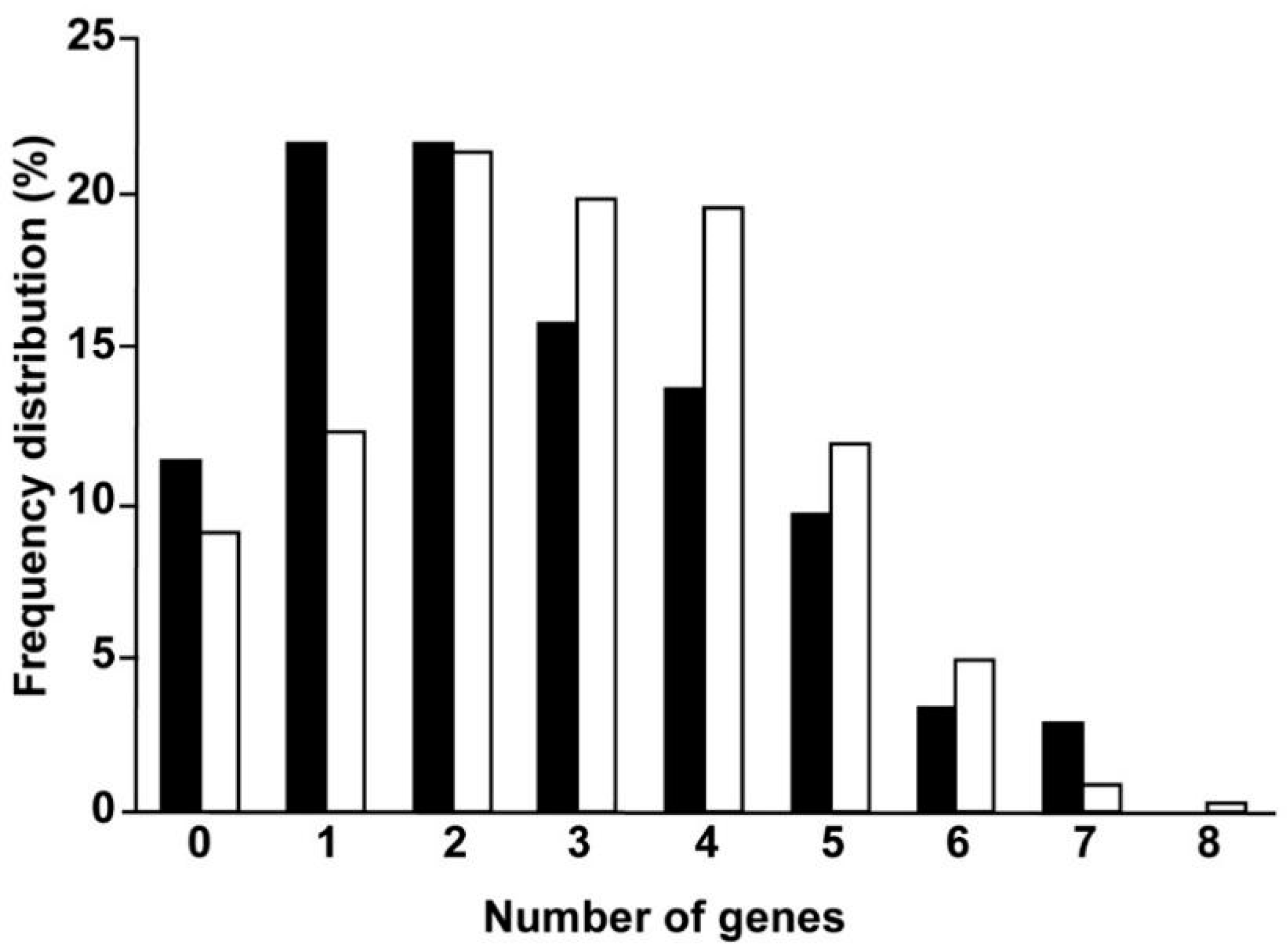


| Type of Tumor | WHO Grade | Main Molecular Alterations | Codified Proteins Affected by Lipid Metabolism or Lipid Composition Regulation | Percentage of Cases |
|---|---|---|---|---|
| GLIOMAS, GLIONEURONAL TUMORS AND NEURONAL TUMORS (excluding adult-type diffuse glioma) | ||||
| Pediatric-type diffuse high-grade glioma | 11.1 | |||
| Diffuse midline glioma H3 K27-altered | 3 | H3 K27, TP53, ACVR1, PDGFRA, EGFR, EZHIP | P53—regulator of lipid metabolism in cancer [11]. Mutations on TP53 provide lipolytic activity to P53 [12]. EGFR is regulated by palmytoilation at Cys1049 and Cys1146 [13]. | |
| Diffuse hemispheric glioma, H3 G34-mutant | 4 | H3 G34, TP53, ATRX | P53—regulator of lipid metabolism in cancer [11]. Mutations on TP53 provide lipolytic activity to P53 [12]. Atrx—transcriptional factor targeting lipid metabolism mediators. | |
| Diffuse High-grade glioma H3-wild-type and IDH-wild-type | 4 | IDH-wildtype, H3-wildtype, PDGFRA, MYCN, EGFR (methylome) | IDH-wild type—IDH1 activity is critical for lipid biosynthesis and its inactivation compromises tumor growth [14] MCYN—Lipid desaturation-associated endoplasmic reticulum stress regulates MYCN gene expression [15]. EGFR is regulated by palmytoilation at Cys1049 and Cys1146 [13]. | |
| Infant-type Hemispheric glioma | 4 | NTRK family, ALK, ROS, MET | NTRK, Alk, Ros and MET are transmembrane proteins. | |
| Pediatric-type diffuse Low-grade gliomas | 25–30 | |||
| Diffuse astrocytoma, MYB or MYBL1 altered | 1–2 | MYB, MYBL1 | ||
| Angiocentric glioma | 1 | MYB, BRAF V600E mut | BRAF V600E mut—induction of lipid droplet accumulation [16] | |
| Polymorphous low-grade neuroepithelial tumor of the young | 1 | BRAF, FGFR family | BRAF—the lipogenic pathway is a key mediator of oncogenic BRAF. Inhibition of oncogenic BRAF caused an increase in the proportion of poly-unsaturated membrane phospholipid species at the expense of saturated and mono-unsaturated phospholipids [17]. FGFR—transmembrane protein | |
| Diffuse low-grade glioma MAPK pathway-altered | 1 | FGFR1, BRAF | BRAF—the lipogenic pathway is a key mediator of oncogenic BRAF. Inhibition of oncogenic BRAF caused an increase in the proportion of poly-unsaturated membrane phospholipid species at the expense of saturated and mono-unsaturated phospholipids [17]. FGFR1—transmembrane protein. The altered lipid structure allows one to factor in the protein–lipid interactions and the biophysical properties of the resulting membranes into the regulation of signal transduction pathways such as the MAPK pathway [18]. | |
| Circumscribed astrocytic gliomas | 17.6 | |||
| Pilocytic astrocytoma | 1 | KIAA1549-BRAF, BRAF, NF1 | BRAF—the lipogenic pathway is a key mediator of oncogenic BRAF. Inhibition of oncogenic BRAF caused an increase in the proportion of poly-unsaturated membrane phospholipid species at the expense of saturated and mono-unsaturated phospholipids [17]. NF1—phospholipid binding protein [19]. | |
| High-grade astrocytoma with piloid features | 3–4 | BRAF, NF1, ATRX, CDKN2A/B (methylome) | BRAF—the lipogenic pathway is a key mediator of oncogenic BRAF. Inhibition of oncogenic BRAF caused an increase in the proportion of poly-unsaturated membrane phospholipid species at the expense of saturated and mono-unsaturated phospholipids [17]. NF1—phospholipid binding protein [19]. Atrx—transcriptional factor targeting lipid metabolism mediators. | |
| Pleomorphic xanthoastrocytoma | 2 | BRAF, CDKN2A/B | BRAF—the lipogenic pathway is a key mediator of oncogenic BRAF. Inhibition of oncogenic BRAF caused an increase in the proportion of poly-unsaturated membrane phospholipid species at the expense of saturated and mono-unsaturated phospholipids [17]. | |
| Subependymal giant cell astrocytomas (SEGA) | 1 | TSC1, TSC2 | TSC1—inhibition of lipophagy or its downstream catabolic pathway reverses defective phenotypes caused by Tsc1-null NSCs and reduces tumorigenesis in mouse models [20]. TSC2—TSC2-deficient cells have enhanced choline phospholipid metabolism [21] | |
| Astroblastoma, MN1-altered | 3–4 | MN1 | ||
| Ependymal tumors | 10 | |||
| Subependymoma | 1–2 | |||
| Supratentorial ependymomas ZFTA fusion-positive | 2 | ZFTA, RELA | ||
| Supratentorial ependymomas, YAP1 fusion positive | 2–3 | YAP1, MAML2 | YAP1 positively regulates numerous genes related to cancer stemness and lipid metabolism [22] | |
| Posterior fossa ependymomas, group PFA (EZHIP mutation) | 2–3 | H3 K27me3, EZHIP (methylome) | ||
| Posterior fossa ependymomas, group PFB | 2 | |||
| Spinal ependymomas, MYCN-amplified | 3 | NF2, MYCN | NF2—lipid binding results in the open conformation of neurofibromin 2 [23] MYCN—lipid desaturation-associated endoplasmic reticulum stress regulates MYCN gene expression [15]. | |
| Myxopapillary ependymoma | 2 | |||
| Neuronal and glioneuronal tumors | 4.4 | |||
| Dysembryoplastic neuroepithelial tumors (DNET) | 2 | FGFR1 | FGFR1—transmembrane protein | |
| Gangliogliomas | 1–2 | |||
| Diffuse glioneuronal tumor with oligodendroglioma-like features and nuclear clusters (DGONC) | 2 | Chromosome 14, (methylome) | ||
| Myxoid glioneuronal tumor (MGT) | 2 | PDFGRA | ||
| Multinodular and vacuolating tumor (MVNT) | 1 | MAPK pathway | An altered lipid structure allows one to factor in the protein–lipid interactions and the biophysical properties of the resulting membranes into the regulation of signal transduction pathways such as the MAPK pathway [18] | |
| Rosette-forming glioneuronal tumor | 1 | FGFR1, PIK3CA, NF1 | FGFR1—transmembrane protein. PIK3CA—phospholipid binding protein. NF1—phospholipid binding protein [19]. | |
| Myxoid glioneuronal tumor | 1 | PDFGRA | ||
| Diffuse leptomeningeal glioneuronal tumor | 1–3 | KIAA1549-BRAF fusion, 1p (methylome) | BRAF—the lipogenic pathway is a key mediator of oncogenic BRAF. Inhibition of oncogenic BRAF caused an increase in the proportion of poly-unsaturated membrane phospholipid species at the expense of saturated and mono-unsaturated phospholipids [17]. | |
| Gangliocytoma | 1 | |||
| Dysplastic cerebellar gangliocytoma (Lhermitte-Duclos disease) | 1 | PTEN | PTEN—phospholipid binding protein which also interacts with FABP4 [24] | |
| Central neurocytoma | 2 | |||
| Extraventricular neurocytoma | 2 | FGFR (FGFR1-TACC1 fusion), IDH-wild type | FGFR—transmembrane protein | |
| Cerebellar liponeurocytoma | 2 | |||
| CNS EMBRYONAL TUMORS | ||||
| Medulloblastoma | 20.0 | |||
| Medulloblastoma, molecularly defined | 4 | |||
| Medulloblastoma, WNT-activated | 4 | CTNNB1, APC | CTNNB1—ß-catenin strongly promotes ß-oxidation [25] | |
| Medulloblastoma, SHH-activated and TP53-wild-type | 4 | PTCH1, SUFU, SMO, MYCN, GLI2 (methylome) | PTCH1, GLI2—lipid metabolism has a profound influence on both hedgehog signal transduction and the properties of the ligands themselves [26] SMO—Hh signaling transduces to SMO through modulating its cholesterylation [27]. | |
| Medulloblastoma, SHH-activated and TP53-mutant | 4 | TP53, PTCH1, SUFU, SMO, MYCN, GLI2 (methylome) | TP53—mutations on TP53 provide lipolytic activity to P53 [12]. PTCH1, GLI2—Lipid metabolism has a profound influence on both hedgehog signal transduction and the properties of the ligands themselves [26] SMO—Hh signaling transduces to SMO through modulating its cholesterylation [27]. | |
| Medulloblastoma, non-WNT/non-SHH | 3–4 | MYC, MYCN, PRDM6, KDM6A (methylome) | MYC—fatty acids are inhibitors of the DNA binding of c-Myc/Max dimer [28] MCYN—lipid desaturation-associated endoplasmic reticulum stress regulates MYCN gene expression [15,29]. | |
| Medulloblastoma, histologically defined | 3–4 | |||
| Other CNS embryonal tumors | ||||
| Atypical teratoid/rhabdoid tumor (ATRT) | 4 | SMARCB1, SMARCA4 | SMARCB1—also known as SWI/SNF-related matrix-associated protein, related also to SMARCA4—BAF60a and BAF60c, two subunits of the SWI/SNF chromatin-remodeling complexes, are important for maintaining hepatic lipid metabolism. SWI/SNF complex might be targeted to develop drugs aimed at regulation of lipid homeostasis in hepatic steatosis [30]. | |
| Cribriform neuroepithelial tumor (provisional type) | 3–4 | |||
| Embryonal Tumor with Multilayer Rosettes (ETMR) | 4 | C19MC, DICER1 | DICER1—the loss of miRNAs resulting from Dicer1 deficiency greatly contributes to the progression of many diseases, including lipid dysregulation [31]. | |
| Neuroblastoma, FOXR2-activated | 4 | FOXR2 | ||
| CNS tumor with BCOR internal tandem duplication | 4 | BCOR | ||
| Embryonal tumor NEC/NOS | 4 | |||
| TUMORS OF THE SELLAR REGION | ||||
| Craniopharyngioma | 4.0 | |||
| Adamantinomatous craniopharyngioma | 1 | CTNNB1 | CTNNB1—ß-catenin strongly promotes ß-oxidation [25] | |
| Papillary craniopharyngioma | 1 | BRAF | BRAF—the lipogenic pathway is a key mediator of oncogenic BRAF. Inhibition of oncogenic BRAF caused an increase in the proportion of poly-unsaturated membrane phospholipid species at the expense of saturated and mono-unsaturated phospholipids [17]. | |
| Pituitary endocrine tumors | 3.9 | |||
| Pituitary blastoma | 1–4 | DICER1 | Dicer—Dicer disruption caused a marked decrease in microsomal triglyceride transfer protein, long-chain fatty acyl-CoA ligase 5, fatty acid binding protein, and very-long-chain fatty acyl-CoA dehydrogenase [32]. | |
| MELANOCYTIC TUMORS | ||||
| Meningeal melanocytosis and melanomatosis | 1–3 | 2.5 | ||
| GERM CELL TUMORS | ||||
| 1 | 3.7 | |||
| MENINGIOMAS | ||||
| Meningioma | 1–3 | NF2, AKT1, TRAF7, SMO, PIK3CA; KLF4, SMARCE1, BAP1 in subtypes; H3K27me3; TERT promoter, CDKN2A/B in CNS WHO grade 3 | NF2—lipid binding results in the open conformation of neurofibromin 2 [23]. SMO—Hh signaling transduces to SMO through modulating its cholesterylation [27]. PIK3CA—phospholipid binding protein. KLF4—regulates cholesterol metabolism by endothelial cells [33]. | 2.9 |
| CHOROID PLEXUS TUMORS | ||||
| 2.3 | ||||
| Plexus papilloma | 1 | |||
| Atypical plexus papilloma | 2 | |||
| Plexus carcinoma | 3 | |||
| Plexus papilloma | 1 | |||
| PINEAL TUMORS | ||||
| 3–11 | ||||
| Pineocytoma | 1 | |||
| Pineoblastoma | 4 | |||
| Papillary tumor of pineal region | 2–3 | |||
| OTHER/UNCLASSIFIED TUMORS | ||||
| 4.9 | ||||
| Official Symbol | Enzyme Name |
|---|---|
| ACER1 | ASAH1; Alkaline Ceramidase 1 |
| ACER3 | ASAH3; Alkaline Ceramidase 3 |
| ACSL1 | Acyl-CoA Synthetase Long-Chain Family Member 1 |
| ACSL3 | Acyl-coA synthetase Long Chain Family member 3 |
| ACSL4 | Acyl-coA synthetase Long Chain Family member 4 |
| ACSL5 | Acyl-coA synthetase Long Chain Family member 5 |
| AHR | Aryl Hydrocarbon Receptor |
| ALDH3A2 | Fatty Aldehyde dehydrogenase |
| ASAH2 | Ceramidase, non-lysosomal |
| CD36 | CD36 Molecule |
| CEPT1 | Choline/Ethanolamine Phosphotransferase 1 |
| CERS1 | LASS1, Ceramide Synthase 1 |
| DEGS1 | Delta 4-Desaturase, Sphingolipid 1 |
| FA2H | Fatty Acid 2-hydroxylase |
| FABP5 | Fatty Acid Binding Protein 5 |
| FABP7 | Fatty Acid Binding Protein 7 |
| FADS1 | Fatty Acid Desaturase 1 |
| FADS2 | Fatty Acid Desaturase 2 |
| FASN | Fatty Acid synthase |
| FFAR1 | Free Fatty Acid Receptor 1; GPR40 |
| FFAR2 | Free Fatty Acid Receptor 2; GPR43 |
| FFAR3 | Free Fatty Acid Receptor 3; GPR41 |
| FFAR4 | Free Fatty Acid Receptor 4; GPR120; O3FAR1 |
| GALC | Galactosylceramidase |
| GPR42 | G Protein-Coupled Receptor 42 (Gene/Pseudogene); FFAR1L |
| HACL1 | 2-hydroxypythanoyl-coA-lyase, 2-hydroxyacyl-CoA lyase 1 |
| HSPA5 | BiP, GRP78; Heat Shock Protein Family A (Hsp70) Member 5 |
| LPAR1 | LPA1; Lysophosphatidic Acid Receptor 1 |
| LPAR2 | LPA2; Lysophosphatidic Acid Receptor 2 |
| LPAR3 | LPA3; Lysophosphatidic Acid Receptor 3 |
| LPAR4 | LPA4; Lysophosphatidic Acid Receptor 4 |
| LPAR5 | LPA1; Lysophosphatidic Acid Receptor 5 |
| LPAR6 | LPA1; Lysophosphatidic Acid Receptor 6 |
| NR1H3 | LXRA; Liver X Nuclear Receptor Alpha Variant 1 |
| NSMAF | N-Smase; Neutral Sphingomyelinase Activation Associated Factor |
| PEMT | Phosphatidylethanolamine N-Methyltransferase |
| PHYH | Phytanoyl-CoA 2-hydroxylase |
| PPARa | Peroxisome Proliferator Activated Receptor Alpha |
| PPARb | Peroxisome Proliferator Activated Receptor Beta |
| PPARd | Peroxisome Proliferator Activated Receptor Delta |
| PPARg | Peroxisome Proliferator Activated Receptor Gamma |
| S1P1 | Sphingosine-1-Phosphate Receptor 1 |
| S1P2 | Sphingosine-1-Phosphate Receptor 2 |
| S1P3 | Sphingosine-1-Phosphate Receptor 3 |
| S1P4 | Sphingosine-1-Phosphate Receptor 4 |
| S1P5 | Sphingosine-1-Phosphate Receptor 5 |
| SAMD8 | SMSr; CEP Synthase; Sterile Alpha Motif Domain Containing 8 |
| SCD | Stearoyl CoA desaturase |
| SMS1 | Sphingomyelin synthase 1 |
| SMS2 | Sphingomyelin synthase 2 |
| SGPL1 | Sphingosine-1-Phosphate Lyase 1 |
| SMPD1 | Acid Sphingomyelinase |
| SMPD2 | Neutral sphingomyelinase 1 |
| SMPD3 | Neutral sphingomyelinase 2 |
| SMPD4 | Neutral sphingomyelinase 3 |
| SMPDL3A | Acid Sphingomyelinase-Like Phosphodiesterase 3a |
| SMPDL3B | Acid Sphingomyelinase-Like Phosphodiesterase 3b+C1:C63 |
| SPHK1 | Sphingosine kinase 1 |
| SPHK2 | Sphingosine kinase 2 |
| SPTLC3 | Serine palmitoyl Transferase, long chain subunit 3 |
| TLR2 | Toll-Like Receptor 2 |
| UGCG | UDP-Glucose Cer Glucosyltransferase (GluCer synthase) |
| Official Symbol | Transcription Factor Name | Target Lipid Metabolism Mediator |
|---|---|---|
| AHR | Aryl Hydrocarbon Receptor | To be determined [94] |
| AP-1 | Activator protein 1 | ASAH2 [95] SPHK1 [96] |
| AP-2 | Transcription Factor AP-2 Alpha | ASAH2 [95] |
| Atrx | Alpha Thalassemia/Mental Retardation Syndrome X-Linked | Several complexes along the chromosome maintain different states of chromatin [97] |
| Atf-4 | Activating Transcription Factor 4 | SPHK2 [98,99] |
| BCL11B | B-Cell Lymphoma/Leukaemia 11B | SMPD2 [100] |
| CREB | CAMP Responsive Element Binding Protein 1 | SPHK2 [98] |
| E2F | E2F Transcription Factor 1 | SPHK1 [101] |
| Fos | FBJ Murine Osteosarcoma Viral Oncogene Homolog | SMPD3 [102,103,104,105] |
| GATA | GATA Transcription Factor | ASAH2 [95] |
| Hey1 | Hes Related Family BHLH Transcription Factor With YRPW Motif 1 | ACVR1 [106] |
| HIF1α | Hypoxia-inducible factor 1-alpha | PDGFRA [107] |
| HIF2α | Hypoxia-inducible factor 2-alpha | SPHK1 [108] |
| IRF1 | Interferon-regulatory factor-1 | ASCL4 [109,110] |
| LMO2 | LIM domain only 2 rhombotin-like 1 | SPHK1 [111] |
| NF-Y | Nuclear factor Y | ASAH2 [95] FASN [112,113] |
| Oct-1 | POU Class 2 Homeobox 1 | ASAH2 [95] |
| SP1 | Specificity Protein 1 | ASAH2 [95] UGCG [95,114] |
| ZBTB7A/LRF | Zinc Finger And BTB Domain Containing 7A/Lymphoma Related Factor | ACVR1 [106] |
| Drug Type | Example Agents | Target | Disease | Pediatric Clinical Trial |
|---|---|---|---|---|
| Immunomodulators | APX005M | CD40 agonist | GBM, A, CNST, E, DIPG, MB | NCT03389802 |
| Pomalidomide | TNFa | CNSTS | NCT03257631 | |
| Indoximod | IDO, mTOR | E, MB, GBM, DIPG | NCT05106296 NCT04049669 | |
| NKTR-214 | CD122 IL2 pathway agonist | E, HGG, MB, PBTs | NCT04730349 | |
| Antibodies | Magrolimab | CD47 | PBTs | NCT05169944 |
| Avelumab | PD-L1 | CNSTs | NCT05081180 | |
| Nivolumab | PD-1 receptor | CNSTs | NCT03838042 NCT04500548 | |
| Ipilimumab | CTLA-4 | CNSTs | NCT04500548 | |
| Bevacizumab | VEGF-A | PBTs | NCT02698254 | |
| CAR T Cells and other cellular immunotherapies | HER2-specific CAR T cell locoregional Immunotherapy | HER2 | G, E, MB, GCT, ATRT, PB | NCT03500991 |
| EGFR806-specific CAR T cell locoregional Immunotherapy | EGFR | G, E, MB, GCT, ATRT, PNET, CPC, PB | NCT03638167 | |
| B7-H3-specific CAR T Cell locoregional Immunotherapy | B7H3 | DIPG, DMG, E, MB, GCT, ATRT, CPC, PB, G | NCT04185038 | |
| GD2-CART01 (iC9-GD2-CAR T-cells) | Disialoganglioside GD2 | MB, PBTs | NCT05298995 | |
| IL13Ralpha2-specific hinge-optimized 41BB-co-stimulatory CAR truncated CD19 | IL13Ralpha2 | PBTs | NCT04510051 | |
| Haploidentical transplant and donor NK cell infusion | CNSTs | NCT02100891 | ||
| Bone marrow-derived allogenic mesenchymal stem cells infected with an oncolytic adenovirus, ICOVIR-5 | pRB pathway | DIPG, MB | NCT04758533 | |
| Vaccines | PEP-CMV | CMV antigen | HGG, DIPG, MB | NCT03299309 NCT05096481 |
| Personalized neoantigen DNA vaccine | DMG, DIPG | NCT03988283 | ||
| rHSC-DIPGVax (neo-antigen heat schock protein vaccine) | DMG, DIPG | NCT04943848 | ||
| Dendritic cell vaccination: WT1 mRNA-loaded autologous monocyte-derived DCs | HGG DIPG | NCT04911621 | ||
| Immunomodulatory DC vaccine | DIPG, GBM | NCT03914768 | ||
| SurVaxM | Survivin | MB, GBM, AA, A, NOS, AO, AE, E, DIPG | NCT04978727 | |
| K27M peptide | DIPG, DMG | NCT02960230 | ||
| Viral Therapy | HSV G207 oncolytic herpes simplex virus-1 (HSV) | CNSTs | NCT03911388 NCT02457845 | |
| Wild-type reovirus (reolysin) | HGGs | NCT02444546 | ||
| Polio/rhinovirus recombinant (PVSRIPO) | CD155 nectin-like molecule-5 | CNSTs | NCT03043391 | |
| DNX-2401 oncolytic adenovirus | Integrins | BSG, DIPG | NCT03178032 | |
| Conventional chemotherapeutics | Mebendazole: | Tubulin | MB, A, GB, AA, Brain Stem Neoplasms, O, AO, G | NCT02644291 |
| PTC596 | Tubulin | DIPG, HGG | NCT03605550 | |
| Antimetabolites | Pemetrexed | Folate analog | MB | NCT01878617 |
| Hydroxyurea | RRM2 | G, GBM | NCT03463733 | |
| New chemotherapeutics | Marizomib | Proteasome | DIPG, BSG, PBTs | NCT04341311 |
| ALRN-6924 | MDM2/MDMX | PBTs | NCT03654716 | |
| Curaxin CBL0137 | FACT | DMG, DIPG, CNSTs | NCT04870944 | |
| Kinase Inhibitors | CX-4945 silmitasertib | CK2 | MB | NCT03904862 |
| Prexasertib | Chk1 | MB | NCT04023669 | |
| 9-ING-41 | GSK 3β | PBTs | NCT04239092 | |
| Trametinib | MEK1, MEK2 | PBTs | NCT03434262 | |
| Ibrutinib | Bruton’s tyrosine Kinase | E, MB, GBM | NCT05106296 | |
| Lenvatinib | VEGFR1, 2 and 3, FGFR1, 2, 3 and 4, PDGFR alpha, c-Kit, RET proto-oncogene | CNSTs | NCT05081180 NCT03245151 | |
| Alectinib | ALK | CNSTs | NCT04774718 | |
| Larotrectinib | Tropomyosin receptor kinases | CNSTs | NCT03213704 NCT03834961 NCT03155620 | |
| Repotrectinib (TPX-0005) | ALK, ROS | CNSTs | NCT04094610 | |
| Downstream signaling pathway inhibitors | Vemurafenib | B-Raf. BRAFV600 | G | NCT01748149 NCT03220035 |
| Entrectinib | TRKA, TRKB, TRKC, ROS1, ALK | CNSTs | NCT02650401 | |
| ONC206 | Stress response, DRD2/ClpP | DMG, CNSTs | NCT04732065 | |
| Everolimus immunosupr | mTor, FKBP-12 | HGG, PNET | NCT03245151 | |
| Sirolimus immunosupr | mTor, FKBP-12 | CNSTs | NCT02574728 | |
| GDC-0084 | PI3K/mTor | CNSTs | NCT03696355 | |
| WP1066 | JAK/STAT3 | PBTs | NCT04334863 | |
| Indoximod | IDO, mTOR | E, MB, GBM | NCT05106296 | |
| MEK162 | Ras/Raf/MEK | LGG | NCT02285439 | |
| Trametinib | MEK1/2 | PBTs | NCT04485559 NCT03363217 NCT05180825 NCT02684058 NCT04201457 | |
| Developmental pathway inhibitors | Vismodegib | SMO | MB | NCT01878617 |
| Cell Death Pathway inducers | ONC201 | TRAIL, ISR | DIPG, DMG, HGG | NCT05009992 NCT05580562 |
| Angiogenesis inhibitor | Recombinant human endostatin (rh-ES) | Ras, Raf, VEGF, VEGFR2 | LGG | NCT04659421 |
| Epigenetic therapy | BMS-986158 and BMS-986378 | Bromodomain (BRD) and extra-terminal domain (BET) | PBTs | NCT03936465 |
| RRx-001 | DNMT and global methylation | PBTs | NCT04525014 | |
| Panobinostat | HDAC | DIPG, BSG, PBTs | NCT02717455 NCT04341311 | |
| MRT/ATRT | NCT04897880 | |||
| Entinostat | Class I and IV HDAC | CNSTs | NCT03838042 | |
| Tazemetostat | EZH2 | CNSTs | NCT03213665 | |
| Vorinostat | HDAC | BSG, A, CAA, CSCN | NCT01236560 | |
| BMS-986158 | Bromodomain and extra-terminal (BET) proteins | PBTs | NCT03936465 | |
| Melitherapy | 2-hydroxyoleic acid | Plasma membrane composition | PBTs | NCT04299191 |
| BXQ-350 | Plasma membrane sphingolipid modulation | DIPG, DMG PBTs | NCT04771897 NCT04404569 | |
| Radiolabeled drugs | Radiolabeled phospholipid drug conjugate: CLR 131 radioiodinated phospholipid ethers (PLEs) | Lipid rafts of cancer cell membranes | PBTs, | NCT03478462 |
| Peptide receptor radionuclide: lutathera (177Lu-DOTATATE) | Somatostatin receptors | CNSTs | NCT05278208 | |
| Radiolabelled monoclonal antibody: iodine I 131 MOAB 8H9 | 4Ig-B7-H3 | CNSTs | NCT00089245 |
| Category | Drug Agent | Family | Target | Affected Pathways | Disease | Model | Reference |
|---|---|---|---|---|---|---|---|
| Pediatric | Cordycepin | Nucleoside derivative | miR-33 | Lipid metabolism | MB | Orthotropic xenograft | [172] |
| GSK126+ Atorvastatin | Small molecule inhibitors | EZH2 | Cholesterol synthesis | DIPG | Murine orthotopic model | [173] | |
| ABC294640 | Small molecule inhibitor | SphK2 | Sphingolipid metabolism | DIPG | SF8628 and SF7761 Soft agar | [174] | |
| Carbenoxolone + palbociclib | Small molecule inhibitors | HSD11β2- CDK4/6 | Oxysterol biosynthesis | MB | Transgenic | [175] | |
| GW9662 | Small molecule agonist | BLBP | Fatty acid uptake | E | 3D spheroid | [176] | |
| ω3-LCPUFA | Fatty acids | CRYAB | Protein folding | MB | Xenograft | [177] | |
| Erucylphosphocholine | Ether lipid | Membrane | Apoptosis | MB | D283 Med | [178] | |
| General | Fluoxetine | Small molecule inhibitor | SMDP-1 | Sphingolipid metabolism | GBM | Orthotropic xenograft | [179] |
| Triacsin C + Etoximir | Small molecule inhibitors | ACSL1- ACSL3-CPT1 | Lipid biosynthesis and fatty acid oxidation | Mesenchymal GBM | Xenograft | [180] | |
| LAU-0901 + Avastin + Elovanoids | Small molecule + synthetic lipids | PAFR | Tumor cell proliferation | GBM | Orthotropic xenograft | [181] | |
| Arachidonyl trifluoromethyl ketone | Small molecule inhibitor | PTRF(cavin-1) | Phospholipid metabolism | GBM | Intracranial Patient-Derived Xenograft Model | [182] | |
| CAY10566 | Small molecule inhibitor | SCD1 | Lipogenesis | GBM | Xenograft | [183] | |
| YTX-7739 | Small molecule inhibitor | SCD | Lipogenesis | GBM | Orthotropic xenograft | [184] | |
| Etomoxir | Small molecule inhibitor | CPT1 | Fatty acid oxidation | GBM | Syngeneic | [185] | |
| Azathioprine | Purine analogue | EGFR-AKT | Lipid metabolism | GBM | Orthotropic xenograft | [186] | |
| Ophiobolin A | Terpenoid antagonist | PE | Membrane Destabilization | GBM | orthotopic U251-LUC xenograft | [187] | |
| LXR-623 | Small molecule agonist | LXR | Cholesterol metabolism | GBM | Orthotropic xenograft | [188] | |
| GM3 | Ganglioside | VEGF | Tumor angiogenesis. | A | CT-2A Matrigel | [189] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-García, P.; Malet-Engra, G.; Torres, M.; Hanson, D.; Rosselló, C.A.; Román, R.; Lladó, V.; Escribá, P.V. Evolving Diagnostic and Treatment Strategies for Pediatric CNS Tumors: The Impact of Lipid Metabolism. Biomedicines 2023, 11, 1365. https://doi.org/10.3390/biomedicines11051365
Fernández-García P, Malet-Engra G, Torres M, Hanson D, Rosselló CA, Román R, Lladó V, Escribá PV. Evolving Diagnostic and Treatment Strategies for Pediatric CNS Tumors: The Impact of Lipid Metabolism. Biomedicines. 2023; 11(5):1365. https://doi.org/10.3390/biomedicines11051365
Chicago/Turabian StyleFernández-García, Paula, Gema Malet-Engra, Manuel Torres, Derek Hanson, Catalina A. Rosselló, Ramón Román, Victoria Lladó, and Pablo V. Escribá. 2023. "Evolving Diagnostic and Treatment Strategies for Pediatric CNS Tumors: The Impact of Lipid Metabolism" Biomedicines 11, no. 5: 1365. https://doi.org/10.3390/biomedicines11051365
APA StyleFernández-García, P., Malet-Engra, G., Torres, M., Hanson, D., Rosselló, C. A., Román, R., Lladó, V., & Escribá, P. V. (2023). Evolving Diagnostic and Treatment Strategies for Pediatric CNS Tumors: The Impact of Lipid Metabolism. Biomedicines, 11(5), 1365. https://doi.org/10.3390/biomedicines11051365








