Osteopontin as a Biomarker in Chronic Kidney Disease
Abstract
1. Introduction
1.1. Molecular Structure and Function
1.2. N-Terminal Osteopontin (ntOPN)
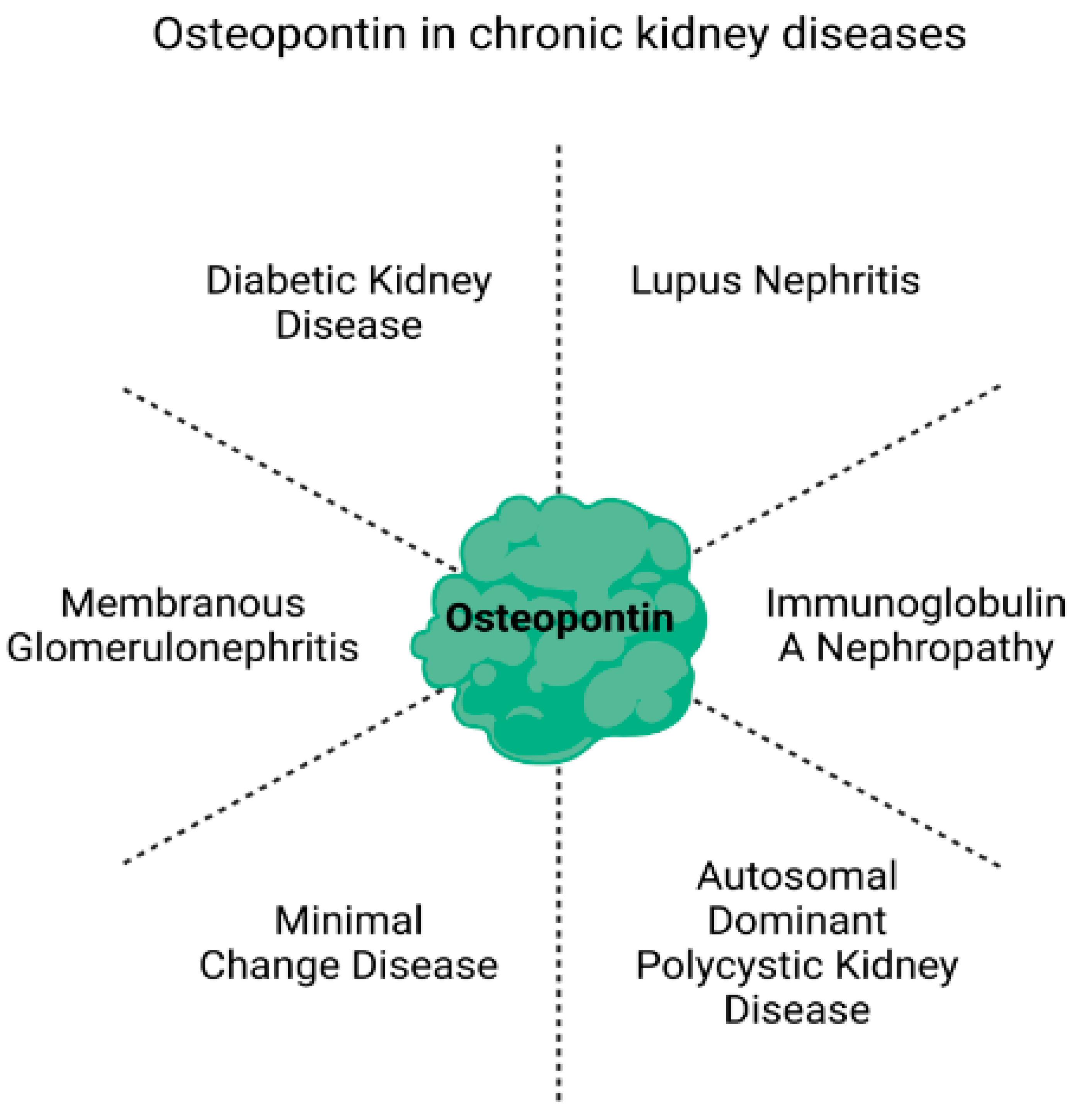
2. Potential of Osteopontin as a Biomarker in Chronic Kidney Disease
2.1. Diabetic Kidney Disease (DKD)
2.2. Lupus Nephritis
2.3. Immunoglobulin A Nephropathy (IgAN)
2.4. Autosomal Dominant Polycystic Kidney Disease (ADPKD)
2.5. Minimal Change Disease (MCD)
2.6. Membranous Glomerulonephritis
2.7. End Stage Renal Disease (ESRD)
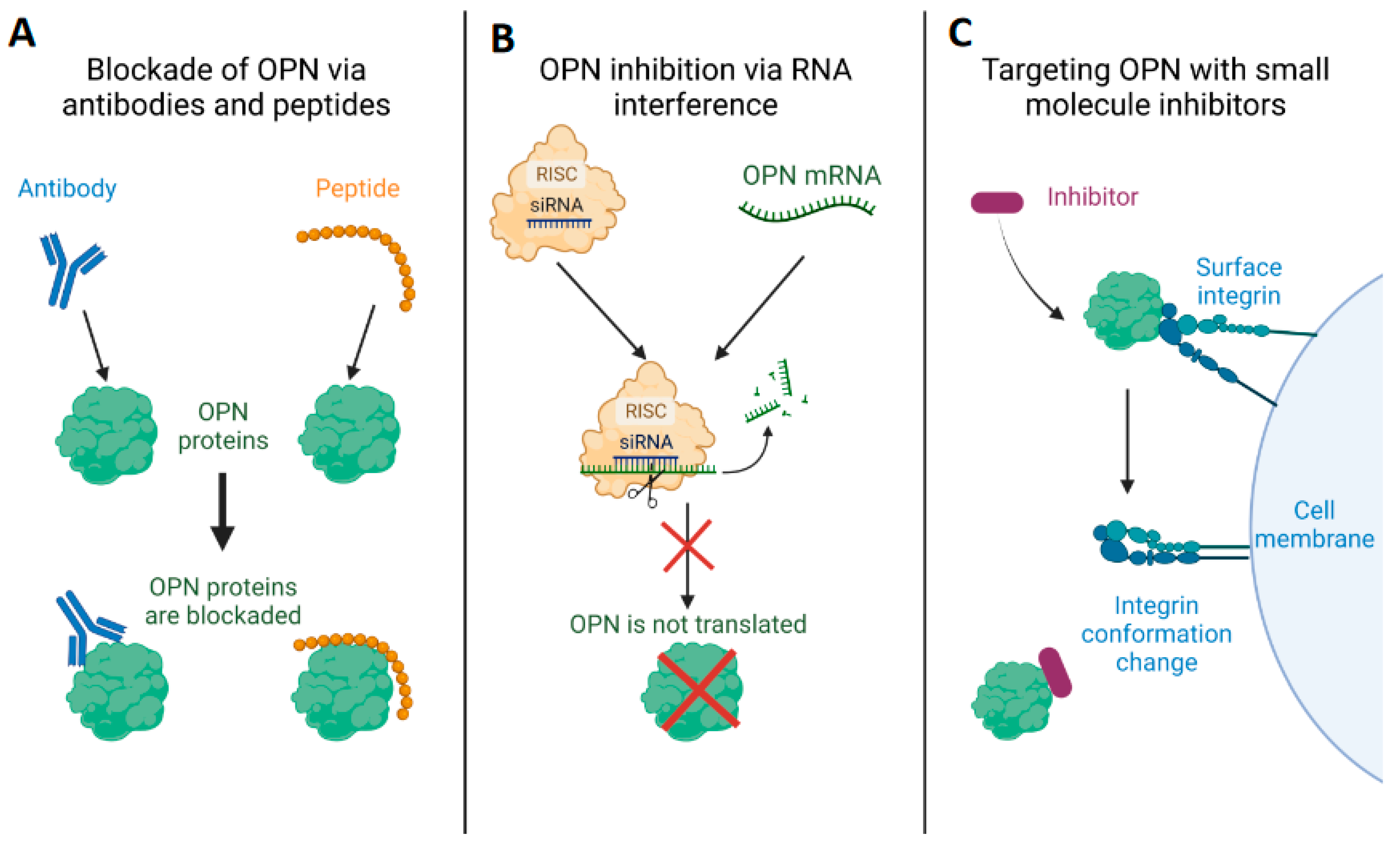
3. Therapeutic Approaches to Modulate OPN Expression and Function
3.1. Blocking of OPN and Its Receptors, Integrins and CD44, by Specific Antibodies/Peptides
3.2. Employment of RNAi against OPN as a Potential Therapeutic Strategy
3.3. Targeting OPN Using Small-Molecule Inhibitors
4. Conclusions
5. Future Direction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Senger, D.R.; Wirth, D.F.; Hynes, R.O. Transformed mammalian cells secrete specific proteins and phosphoproteins. Cell 1979, 16, 885–893. [Google Scholar] [CrossRef]
- Brown, L.F.; Berse, B.; Van de Water, L.; Papadopoulos-Sergiou, A.; Perruzzi, C.A.; Manseau, E.J.; Dvorak, H.F.; Senger, D.R. Expression and distribution of osteopontin in human tissues: Widespread association with luminal epithelial surfaces. Mol. Biol. Cell 1992, 3, 1169–1180. [Google Scholar] [CrossRef]
- Giachelli, C.M.; Steitz, S. Osteopontin: A versatile regulator of inflammation and biomineralization. Matrix Biol. 2000, 19, 615–622. [Google Scholar] [CrossRef]
- Kiss, T.; Jambor, K.; Koroknai, V.; Szasz, I.; Bardos, H.; Mokanszki, A.; Adany, R.; Balazs, M. Silencing Osteopontin Expression Inhibits Proliferation, Invasion and Induce Altered Protein Expression in Melanoma Cells. Pathol. Oncol. Res. 2021, 27, 581395. [Google Scholar] [CrossRef]
- Tan, Y.; Zhao, L.; Yang, Y.G.; Liu, W. The Role of Osteopontin in Tumor Progression Through Tumor-Associated Macrophages. Front. Oncol. 2022, 12, 953283. [Google Scholar] [CrossRef]
- Lamort, A.S.; Giopanou, I.; Psallidas, I.; Stathopoulos, G.T. Osteopontin as a Link between Inflammation and Cancer: The Thorax in the Spotlight. Cells 2019, 8, 815. [Google Scholar] [CrossRef]
- Pagel, C.N.; Wasgewatte Wijesinghe, D.K.; Taghavi Esfandouni, N.; Mackie, E.J. Osteopontin, inflammation and myogenesis: Influencing regeneration, fibrosis and size of skeletal muscle. J. Cell Commun. Signal. 2014, 8, 95–103. [Google Scholar] [CrossRef]
- Syn, W.K.; Choi, S.S.; Liaskou, E.; Karaca, G.F.; Agboola, K.M.; Oo, Y.H.; Mi, Z.; Pereira, T.A.; Zdanowicz, M.; Malladi, P.; et al. Osteopontin is induced by hedgehog pathway activation and promotes fibrosis progression in nonalcoholic steatohepatitis. Hepatology 2011, 53, 106–115. [Google Scholar] [CrossRef]
- Wei, R.; Wong, J.P.C.; Kwok, H.F. Osteopontin–A promising biomarker for cancer therapy. J. Cancer 2017, 8, 2173–2183. [Google Scholar] [CrossRef]
- Lin, E.Y.; Xi, W.; Aggarwal, N.; Shinohara, M.L. Osteopontin (OPN)/SPP1: From its biochemistry to biological functions in the innate immune system and the central nervous system (CNS). Int. Immunol. 2022, 35, 171–180. [Google Scholar] [CrossRef]
- Si, J.; Wang, C.; Zhang, D.; Wang, B.; Zhou, Y. Osteopontin in Bone Metabolism and Bone Diseases. Med. Sci. Monit. 2020, 26, e919159. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, Y.; Scherer, N.; Grundner-Culemann, F.; Lehtimaki, T.; Mishra, B.H.; Raitakari, O.T.; Nauck, M.; Eckardt, K.U.; Sekula, P.; et al. Genetics of osteopontin in patients with chronic kidney disease: The German Chronic Kidney Disease study. PLoS Genet. 2022, 18, e1010139. [Google Scholar] [CrossRef]
- Xie, Y.; Sakatsume, M.; Nishi, S.; Narita, I.; Arakawa, M.; Gejyo, F. Expression, roles, receptors, and regulation of osteopontin in the kidney. Kidney Int. 2001, 60, 1645–1657. [Google Scholar] [CrossRef]
- Alicic, R.Z.; Rooney, M.T.; Tuttle, K.R. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin. J. Am. Soc. Nephrol. 2017, 12, 2032–2045. [Google Scholar] [CrossRef]
- Deng, Y.; Li, N.; Wu, Y.; Wang, M.; Yang, S.; Zheng, Y.; Deng, X.; Xiang, D.; Zhu, Y.; Xu, P.; et al. Global, Regional, and National Burden of Diabetes-Related Chronic Kidney Disease From 1990 to 2019. Front. Endocrinol. 2021, 12, 672350. [Google Scholar] [CrossRef]
- Kamalabadi-Farahani, M.; Atashi, A.; Jabbarpour, Z.; Aghayan, S.S. Expression of osteopontin-5 splice variant in the mouse primary and metastatic breast cancer cells. BMC Res. Notes 2022, 15, 286. [Google Scholar] [CrossRef]
- Kaleta, B. Osteopontin (OPN) Gene Polymorphisms and Autoimmune Diseases. In Genetic Polymorphisms; Parine, N.R., Ed.; IntechOpen Limited: London, UK, 2017. [Google Scholar]
- Gimba, E.R.P.; Brum, M.C.M.; Nestal De Moraes, G. Full-length osteopontin and its splice variants as modulators of chemoresistance and radioresistance (Review). Int. J. Oncol. 2019, 54, 420–430. [Google Scholar] [CrossRef]
- Hattori, T.; Iwasaki-Hozumi, H.; Bai, G.; Chagan-Yasutan, H.; Shete, A.; Telan, E.F.; Takahashi, A.; Ashino, Y.; Matsuba, T. Both Full-Length and Protease-Cleaved Products of Osteopontin Are Elevated in Infectious Diseases. Biomedicines 2021, 9, 1006. [Google Scholar] [CrossRef]
- Lok, Z.S.Y.; Lyle, A.N. Osteopontin in Vascular Disease. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 613–622. [Google Scholar] [CrossRef]
- Lee, G.S.; Salazar, H.F.; Joseph, G.; Lok, Z.S.Y.; Caroti, C.M.; Weiss, D.; Taylor, W.R.; Lyle, A.N. Osteopontin isoforms differentially promote arteriogenesis in response to ischemia via macrophage accumulation and survival. Lab. Investig. 2019, 99, 331–345. [Google Scholar] [CrossRef]
- Goparaju, C.M.; Pass, H.I.; Blasberg, J.D.; Hirsch, N.; Donington, J.S. Functional heterogeneity of osteopontin isoforms in non-small cell lung cancer. J. Thorac. Oncol. 2010, 5, 1516–1523. [Google Scholar] [CrossRef] [PubMed]
- Ho, N.T.; Lin, S.W.; Lee, Y.R.; Tzeng, C.R.; Kao, S.H. Osteopontin Splicing Isoforms Contribute to Endometriotic Proliferation, Migration, and Epithelial-Mesenchymal Transition in Endometrial Epithelial Cells. Int. J. Mol. Sci. 2022, 23, 15328. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Hu, M.; Niu, H.; Wang, J.; Si, Y.; Cheng, S.; Ding, W. Osteopontin isoform c promotes the survival of cisplatin-treated NSCLC cells involving NFATc2-mediated suppression on calcium-induced ROS levels. BMC Cancer 2021, 21, 750. [Google Scholar] [CrossRef] [PubMed]
- Tilli, T.M.; Franco, V.F.; Robbs, B.K.; Wanderley, J.L.; da Silva, F.R.; de Mello, K.D.; Viola, J.P.; Weber, G.F.; Gimba, E.R. Osteopontin-c splicing isoform contributes to ovarian cancer progression. Mol. Cancer Res. 2011, 9, 280–293. [Google Scholar] [CrossRef]
- Ashkar, S.; Weber, G.F.; Panoutsakopoulou, V.; Sanchirico, M.E.; Jansson, M.; Zawaideh, S.; Rittling, S.R.; Denhardt, D.T.; Glimcher, M.J.; Cantor, H. Eta-1 (osteopontin): An early component of type-1 (cell-mediated) immunity. Science 2000, 287, 860–864. [Google Scholar] [CrossRef]
- Jayaprakash, N.G.; Surolia, A. Role of glycosylation in nucleating protein folding and stability. Biochem. J. 2017, 474, 2333–2347. [Google Scholar] [CrossRef]
- Oyama, M.; Kariya, Y.; Kariya, Y.; Matsumoto, K.; Kanno, M.; Yamaguchi, Y.; Hashimoto, Y. Biological role of site-specific O-glycosylation in cell adhesion activity and phosphorylation of osteopontin. Biochem. J. 2018, 475, 1583–1595. [Google Scholar] [CrossRef]
- Higashikawa, F.; Eboshida, A.; Yokosaki, Y. Enhanced biological activity of polymeric osteopontin. FEBS Lett. 2007, 581, 2697–2701. [Google Scholar] [CrossRef]
- Nishimichi, N.; Higashikawa, F.; Kinoh, H.H.; Tateishi, Y.; Matsuda, H.; Yokosaki, Y. Polymeric osteopontin employs integrin alpha9beta1 as a receptor and attracts neutrophils by presenting a de novo binding site. J. Biol. Chem. 2009, 284, 14769–14776. [Google Scholar] [CrossRef]
- Wolak, T.; Sion-Vardi, N.; Novack, V.; Greenberg, G.; Szendro, G.; Tarnovscki, T.; Nov, O.; Shelef, I.; Paran, E.; Rudich, A. N-terminal rather than full-length osteopontin or its C-terminal fragment is associated with carotid-plaque inflammation in hypertensive patients. Am. J. Hypertens. 2013, 26, 326–333. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.; Song, W.; Sun, Y.; Jiang, Y. Osteopontin N-Terminal Function in an Abdominal Aortic Aneurysm From Apolipoprotein E-Deficient Mice. Front. Cell Dev. Biol. 2021, 9, 681790. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Chen, J.; Wu, Z.; Huang, H.; Wang, L.; Liang, Y.; Zeng, P.; Yang, J.; Uede, T.; Diao, H. Thrombin cleavage of osteopontin controls activation of hepatic stellate cells and is essential for liver fibrogenesis. J. Cell Physiol. 2019, 234, 8988–8997. [Google Scholar] [CrossRef]
- Mi, Z.; Oliver, T.; Guo, H.; Gao, C.; Kuo, P.C. Thrombin-cleaved COOH(-) terminal osteopontin peptide binds with cyclophilin C to CD147 in murine breast cancer cells. Cancer Res. 2007, 67, 4088–4097. [Google Scholar] [CrossRef]
- Icer, M.A.; Gezmen-Karadag, M. The multiple functions and mechanisms of osteopontin. Clin. Biochem. 2018, 59, 17–24. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, Q.; Alam, A.; Cui, J.; Suen, K.C.; Soo, A.P.; Eguchi, S.; Gu, J.; Ma, D. The role of osteopontin in the progression of solid organ tumour. Cell Death Dis. 2018, 9, 356. [Google Scholar] [CrossRef]
- Senbanjo, L.T.; Chellaiah, M.A. CD44: A Multifunctional Cell Surface Adhesion Receptor Is a Regulator of Progression and Metastasis of Cancer Cells. Front. Cell Dev. Biol. 2017, 5, 18. [Google Scholar] [CrossRef]
- Kamranvar, S.A.; Rani, B.; Johansson, S. Cell Cycle Regulation by Integrin-Mediated Adhesion. Cells 2022, 11, 2521. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; He, X.; Qiu, Z.; Zhang, H.; Xie, R.; Liu, Z.; Gu, Y.; Zhao, N.; Xiang, Q.; Cui, Y. Targeting integrin pathways: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2023, 8, 1. [Google Scholar] [CrossRef]
- Cao, J.; Li, J.; Sun, L.; Qin, T.; Xiao, Y.; Chen, K.; Qian, W.; Duan, W.; Lei, J.; Ma, J.; et al. Hypoxia-driven paracrine osteopontin/integrin alphavbeta3 signaling promotes pancreatic cancer cell epithelial-mesenchymal transition and cancer stem cell-like properties by modulating forkhead box protein M1. Mol. Oncol. 2019, 13, 228–245. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.H.; Quan, Y.J.; Chen, J.H.; Wang, T.F.; Xu, M.; Ye, M.; Yuan, H.; Zhang, C.J.; Liu, X.J.; Min, Z.J. Osteopontin Promotes Cell Migration and Invasion, and Inhibits Apoptosis and Autophagy in Colorectal Cancer by activating the p38 MAPK Signaling Pathway. Cell Physiol. Biochem. 2017, 41, 1851–1864. [Google Scholar] [CrossRef] [PubMed]
- Behera, R.; Kumar, V.; Lohite, K.; Karnik, S.; Kundu, G.C. Activation of JAK2/STAT3 signaling by osteopontin promotes tumor growth in human breast cancer cells. Carcinogenesis 2010, 31, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Wei, Y.Y.; Chen, H.T.; Fong, Y.C.; Hsu, C.J.; Tsai, C.H.; Hsu, H.C.; Liu, S.H.; Tang, C.H. Osteopontin increases migration and MMP-9 up-regulation via alphavbeta3 integrin, FAK, ERK, and NF-kappaB-dependent pathway in human chondrosarcoma cells. J. Cell Physiol. 2009, 221, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Guo, M.; Chen, J.H.; Wang, Z.; Du, X.F.; Liu, P.X.; Li, W.H. Osteopontin knockdown inhibits alphav, beta3 integrin-induced cell migration and invasion and promotes apoptosis of breast cancer cells by inducing autophagy and inactivating the PI3K/Akt/mTOR pathway. Cell Physiol. Biochem. 2014, 33, 991–1002. [Google Scholar] [CrossRef] [PubMed]
- Urtasun, R.; Lopategi, A.; George, J.; Leung, T.M.; Lu, Y.; Wang, X.; Ge, X.; Fiel, M.I.; Nieto, N. Osteopontin, an oxidant stress sensitive cytokine, up-regulates collagen-I via integrin alpha(V)beta(3) engagement and PI3K/pAkt/NFkappaB signaling. Hepatology 2012, 55, 594–608. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Shao, Z.; Sharif, S.; Du, X.Y.; Myles, T.; Merchant, M.; Harsh, G.; Glantz, M.; Recht, L.; Morser, J.; et al. Thrombin-cleaved fragments of osteopontin are overexpressed in malignant glial tumors and provide a molecular niche with survival advantage. J. Biol. Chem. 2013, 288, 3097–3111. [Google Scholar] [CrossRef]
- Grassinger, J.; Haylock, D.N.; Storan, M.J.; Haines, G.O.; Williams, B.; Whitty, G.A.; Vinson, A.R.; Be, C.L.; Li, S.; Sorensen, E.S.; et al. Thrombin-cleaved osteopontin regulates hemopoietic stem and progenitor cell functions through interactions with alpha9beta1 and alpha4beta1 integrins. Blood 2009, 114, 49–59. [Google Scholar] [CrossRef]
- Kale, S.; Raja, R.; Thorat, D.; Soundararajan, G.; Patil, T.V.; Kundu, G.C. Osteopontin signaling upregulates cyclooxygenase-2 expression in tumor-associated macrophages leading to enhanced angiogenesis and melanoma growth via alpha9beta1 integrin. Oncogene 2014, 33, 2295–2306. [Google Scholar] [CrossRef]
- Meng, L.; Liu, X.; Teng, X.; Gu, H.; Yuan, W.; Meng, J.; Li, J.; Zheng, Z.; Wei, Y.; Hu, S. Osteopontin plays important roles in pulmonary arterial hypertension induced by systemic-to-pulmonary shunt. FASEB J. 2019, 33, 7236–7251. [Google Scholar] [CrossRef]
- Jia, D.; Zhu, Q.; Liu, H.; Zuo, C.; He, Y.; Chen, G.; Lu, A. Osteoprotegerin Disruption Attenuates HySu-Induced Pulmonary Hypertension Through Integrin alphavbeta3/FAK/AKT Pathway Suppression. Circ. Cardiovasc. Genet. 2017, 10, e001591. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, Y.; Lei, Z.; Liu, T.; Cai, T.; Wang, A.; Du, W.; Zeng, Y.; Zhu, J.; Liu, Z.; et al. Abnormally activated OPN/integrin alphaVbeta3/FAK signalling is responsible for EGFR-TKI resistance in EGFR mutant non-small-cell lung cancer. J. Hematol. Oncol. 2020, 13, 169. [Google Scholar] [CrossRef]
- Agnihotri, R.; Crawford, H.C.; Haro, H.; Matrisian, L.M.; Havrda, M.C.; Liaw, L. Osteopontin, a novel substrate for matrix metalloproteinase-3 (stromelysin-1) and matrix metalloproteinase-7 (matrilysin). J. Biol. Chem. 2001, 276, 28261–28267. [Google Scholar] [CrossRef] [PubMed]
- Yokosaki, Y.; Tanaka, K.; Higashikawa, F.; Yamashita, K.; Eboshida, A. Distinct structural requirements for binding of the integrins alphavbeta6, alphavbeta3, alphavbeta5, alpha5beta1 and alpha9beta1 to osteopontin. Matrix Biol. 2005, 24, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Boggio, E.; Dianzani, C.; Gigliotti, C.L.; Soluri, M.F.; Clemente, N.; Cappellano, G.; Toth, E.; Raineri, D.; Ferrara, B.; Comi, C.; et al. Thrombin Cleavage of Osteopontin Modulates Its Activities in Human Cells In Vitro and Mouse Experimental Autoimmune Encephalomyelitis In Vivo. J. Immunol. Res. 2016, 2016, 9345495. [Google Scholar] [CrossRef] [PubMed]
- Lopez, B.; Gonzalez, A.; Lindner, D.; Westermann, D.; Ravassa, S.; Beaumont, J.; Gallego, I.; Zudaire, A.; Brugnolaro, C.; Querejeta, R.; et al. Osteopontin-mediated myocardial fibrosis in heart failure: A role for lysyl oxidase? Cardiovasc. Res. 2013, 99, 111–120. [Google Scholar] [CrossRef]
- Uchinaka, A.; Hamada, Y.; Mori, S.; Miyagawa, S.; Saito, A.; Sawa, Y.; Matsuura, N.; Yamamoto, H.; Kawaguchi, N. SVVYGLR motif of the thrombin-cleaved N-terminal osteopontin fragment enhances the synthesis of collagen type III in myocardial fibrosis. Mol. Cell. Biochem. 2015, 408, 191–203. [Google Scholar] [CrossRef]
- Morales-Ibanez, O.; Dominguez, M.; Ki, S.H.; Marcos, M.; Chaves, J.F.; Nguyen-Khac, E.; Houchi, H.; Affo, S.; Sancho-Bru, P.; Altamirano, J.; et al. Human and experimental evidence supporting a role for osteopontin in alcoholic hepatitis. Hepatology 2013, 58, 1742–1756. [Google Scholar] [CrossRef]
- Herum, K.M.; Romaine, A.; Wang, A.; Melleby, A.O.; Strand, M.E.; Pacheco, J.; Braathen, B.; Duner, P.; Tonnessen, T.; Lunde, I.G.; et al. Syndecan-4 Protects the Heart From the Profibrotic Effects of Thrombin-Cleaved Osteopontin. J. Am. Heart Assoc. 2020, 9, e013518. [Google Scholar] [CrossRef]
- Gang, X.; Ueki, K.; Kon, S.; Maeda, M.; Naruse, T.; Nojima, Y. Reduced urinary excretion of intact osteopontin in patients with IgA nephropathy. Am. J. Kidney Dis. 2001, 37, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Kitagori, K.; Yoshifuji, H.; Oku, T.; Sasaki, C.; Miyata, H.; Mori, K.P.; Nakajima, T.; Ohmura, K.; Kawabata, D.; Yukawa, N.; et al. Cleaved Form of Osteopontin in Urine as a Clinical Marker of Lupus Nephritis. PLoS ONE 2016, 11, e0167141. [Google Scholar] [CrossRef]
- Han, X.; Wang, W.; He, J.; Jiang, L.; Li, X. Osteopontin as a biomarker for osteosarcoma therapy and prognosis. Oncol. Lett. 2019, 17, 2592–2598. [Google Scholar] [CrossRef]
- Moorman, H.R.; Poschel, D.; Klement, J.D.; Lu, C.; Redd, P.S.; Liu, K. Osteopontin: A Key Regulator of Tumor Progression and Immunomodulation. Cancers 2020, 12, 3379. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Hou, J.; Wang, L.; Fu, H.; Zhang, Y.; Song, Y.; Wang, X. Regulatory roles of osteopontin in human lung cancer cell epithelial-to-mesenchymal transitions and responses. Clin. Transl. Med. 2021, 11, e486. [Google Scholar] [CrossRef] [PubMed]
- Kaleta, B. The role of osteopontin in kidney diseases. Inflamm. Res. 2019, 68, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, S.B.; Liu, J.; Kim, J.; Ren, Y.; Collins, A.R.; Nguyen, L.; Hsueh, W.A. Critical role for osteopontin in diabetic nephropathy. Kidney Int. 2010, 77, 588–600. [Google Scholar] [CrossRef]
- Moszczuk, B.; Krata, N.; Rudnicki, W.; Foroncewicz, B.; Cysewski, D.; Paczek, L.; Kaleta, B.; Mucha, K. Osteopontin-A Potential Biomarker for IgA Nephropathy: Machine Learning Application. Biomedicines 2022, 10, 734. [Google Scholar] [CrossRef]
- Wirestam, L.; Enocsson, H.; Skogh, T.; Padyukov, L.; Jonsen, A.; Urowitz, M.B.; Gladman, D.D.; Romero-Diaz, J.; Bae, S.C.; Fortin, P.R.; et al. Osteopontin and Disease Activity in Patients with Recent-onset Systemic Lupus Erythematosus: Results from the SLICC Inception Cohort. J. Rheumatol. 2019, 46, 492–500. [Google Scholar] [CrossRef]
- Steinbrenner, I.; Sekula, P.; Kotsis, F.; von Cube, M.; Cheng, Y.; Nadal, J.; Schmid, M.; Schneider, M.P.; Krane, V.; Nauck, M.; et al. Association of osteopontin with kidney function and kidney failure in chronic kidney disease patients: The GCKD study. Nephrol. Dial. Transplant 2022, gfac173. [Google Scholar] [CrossRef]
- Sinha, S.K.; Sun, L.; Didero, M.; Martins, D.; Norris, K.C.; Lee, J.E.; Meng, Y.X.; Sung, J.H.; Sayre, M.; Carpio, M.B.; et al. Vitamin D3 Repletion Improves Vascular Function, as Measured by Cardiorenal Biomarkers in a High-Risk African American Cohort. Nutrients 2022, 14, 3331. [Google Scholar] [CrossRef]
- Trostel, J.; Truong, L.D.; Roncal-Jimenez, C.; Miyazaki, M.; Miyazaki-Anzai, S.; Kuwabara, M.; McMahan, R.; Andres-Hernando, A.; Sato, Y.; Jensen, T.; et al. Different effects of global osteopontin and macrophage osteopontin in glomerular injury. Am. J. Physiol. Renal Physiol. 2018, 315, F759–F768. [Google Scholar] [CrossRef]
- Gordin, D.; Forsblom, C.; Panduru, N.M.; Thomas, M.C.; Bjerre, M.; Soro-Paavonen, A.; Tolonen, N.; Sandholm, N.; Flyvbjerg, A.; Harjutsalo, V.; et al. Osteopontin is a strong predictor of incipient diabetic nephropathy, cardiovascular disease, and all-cause mortality in patients with type 1 diabetes. Diabetes Care 2014, 37, 2593–2600. [Google Scholar] [CrossRef]
- Kelly, D.J.; Wilkinson-Berka, J.L.; Ricardo, S.D.; Cox, A.J.; Gilbert, R.E. Progression of tubulointerstitial injury by osteopontin-induced macrophage recruitment in advanced diabetic nephropathy of transgenic (mRen-2)27 rats. Nephrol. Dial Transplant. 2002, 17, 985–991. [Google Scholar] [CrossRef]
- Susztak, K.; Bottinger, E.; Novetsky, A.; Liang, D.; Zhu, Y.; Ciccone, E.; Wu, D.; Dunn, S.; McCue, P.; Sharma, K. Molecular profiling of diabetic mouse kidney reveals novel genes linked to glomerular disease. Diabetes 2004, 53, 784–794. [Google Scholar] [CrossRef]
- Kelly, D.J.; Chanty, A.; Gow, R.M.; Zhang, Y.; Gilbert, R.E. Protein kinase Cbeta inhibition attenuates osteopontin expression, macrophage recruitment, and tubulointerstitial injury in advanced experimental diabetic nephropathy. J. Am. Soc. Nephrol. 2005, 16, 1654–1660. [Google Scholar] [CrossRef]
- Li, C.; Yang, C.W.; Park, C.W.; Ahn, H.J.; Kim, W.Y.; Yoon, K.H.; Suh, S.H.; Lim, S.W.; Cha, J.H.; Kim, Y.S.; et al. Long-term treatment with ramipril attenuates renal osteopontin expression in diabetic rats. Kidney Int. 2003, 63, 454–463. [Google Scholar] [CrossRef]
- Wong, C.K.; Lit, L.C.; Tam, L.S.; Li, E.K.; Lam, C.W. Elevation of plasma osteopontin concentration is correlated with disease activity in patients with systemic lupus erythematosus. Rheumatology 2005, 44, 602–606. [Google Scholar] [CrossRef]
- Ma, R.; Jiang, W.; Li, Z.; Sun, Y.; Wei, Z. Intrarenal macrophage infiltration induced by T cells is associated with podocyte injury in lupus nephritis patients. Lupus 2016, 25, 1577–1586. [Google Scholar] [CrossRef]
- Sano, N.; Kitazawa, K.; Sugisaki, T. Localization and roles of CD44, hyaluronic acid and osteopontin in IgA nephropathy. Nephron 2001, 89, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Wasilewska, A.; Taranta-Janusz, K.; Kuroczycka-Saniutycz, E.; Zoch-Zwierz, W. Urinary OPN excretion in children with glomerular proteinuria. Adv. Med. Sci. 2011, 56, 193–199. [Google Scholar] [CrossRef]
- Kim, H.; Sung, J.; Bae, J.Y.; Lee, P.; Oh, Y.K.; Kim, H. Identification of osteopontin as a urinary biomarker for autosomal dominant polycystic kidney disease progression. Kidney Res. Clin. Pract. 2022, 41, 730–740. [Google Scholar] [CrossRef] [PubMed]
- Mezzano, S.A.; Barria, M.; Droguett, M.A.; Burgos, M.E.; Ardiles, L.G.; Flores, C.; Egido, J. Tubular NF-kappaB and AP-1 activation in human proteinuric renal disease. Kidney Int. 2001, 60, 1366–1377. [Google Scholar] [CrossRef] [PubMed]
- Mezzano, S.A.; Droguett, M.A.; Burgos, M.E.; Ardiles, L.G.; Aros, C.A.; Caorsi, I.; Egido, J. Overexpression of chemokines, fibrogenic cytokines, and myofibroblasts in human membranous nephropathy. Kidney Int. 2000, 57, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Desai, R.; Singh, S.; Syed, M.H.; Dave, H.; Hasnain, M.; Zahid, D.; Haider, M.; Jilani, S.M.A.; Mirza, M.A.; Kiran, N.; et al. Temporal Trends in the Prevalence of Diabetes Decompensation (Diabetic Ketoacidosis and Hyperosmolar Hyperglycemic State) Among Adult Patients Hospitalized with Diabetes Mellitus: A Nationwide Analysis Stratified by Age, Gender, and Race. Cureus 2019, 11, e4353. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Liu, S.; Bastacky, S.I.; Wang, X.; Tian, X.J.; Zhou, D. Diabetic kidney diseases revisited: A new perspective for a new era. Mol. Metab. 2019, 30, 250–263. [Google Scholar] [CrossRef]
- Gonzalez-Perez, A.; Saez, M.; Vizcaya, D.; Lind, M.; Garcia Rodriguez, L. Incidence and risk factors for mortality and end-stage renal disease in people with type 2 diabetes and diabetic kidney disease: A population-based cohort study in the UK. BMJ Open Diabetes Res. Care 2021, 9, e002146. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Kim, N.H. Renoprotective Mechanism of Sodium-Glucose Cotransporter 2 Inhibitors: Focusing on Renal Hemodynamics. Diabetes Metab. J. 2022, 46, 543–551. [Google Scholar] [CrossRef]
- Sawaf, H.; Thomas, G.; Taliercio, J.J.; Nakhoul, G.; Vachharajani, T.J.; Mehdi, A. Therapeutic Advances in Diabetic Nephropathy. J. Clin. Med. 2022, 11, 378. [Google Scholar] [CrossRef]
- Chen, Y.; Lee, K.; Ni, Z.; He, J.C. Diabetic Kidney Disease: Challenges, Advances, and Opportunities. Kidney Dis. 2020, 6, 215–225. [Google Scholar] [CrossRef]
- Tuttle, K.R.; Agarwal, R.; Alpers, C.E.; Bakris, G.L.; Brosius, F.C.; Kolkhof, P.; Uribarri, J. Molecular mechanisms and therapeutic targets for diabetic kidney disease. Kidney Int. 2022, 102, 248–260. [Google Scholar] [CrossRef]
- Alicic, R.; Nicholas, S.B. Diabetic Kidney Disease Back in Focus: Management Field Guide for Health Care Professionals in the 21st Century. Mayo Clin. Proc. 2022, 97, 1904–1919. [Google Scholar] [CrossRef] [PubMed]
- Oellgaard, J.; Gaede, P.; Rossing, P.; Persson, F.; Parving, H.H.; Pedersen, O. Intensified multifactorial intervention in type 2 diabetics with microalbuminuria leads to long-term renal benefits. Kidney Int. 2017, 91, 982–988. [Google Scholar] [CrossRef]
- Colhoun, H.M.; Marcovecchio, M.L. Biomarkers of diabetic kidney disease. Diabetologia 2018, 61, 996–1011. [Google Scholar] [CrossRef]
- Klessens, C.Q.; Woutman, T.D.; Veraar, K.A.; Zandbergen, M.; Valk, E.J.; Rotmans, J.I.; Wolterbeek, R.; Bruijn, J.A.; Bajema, I.M. An autopsy study suggests that diabetic nephropathy is underdiagnosed. Kidney Int. 2016, 90, 149–156. [Google Scholar] [CrossRef]
- Gluck, C.; Ko, Y.A.; Susztak, K. Precision Medicine Approaches to Diabetic Kidney Disease: Tissue as an Issue. Curr. Diab. Rep. 2017, 17, 30. [Google Scholar] [CrossRef]
- Zou, L.X.; Sun, L.; Nicholas, S.B.; Lu, Y.; Sinha, K.S.; Hua, R. Comparison of bias and accuracy using cystatin C and creatinine in CKD-EPI equations for GFR estimation. Eur. J. Intern. Med. 2020, 80, 29–34. [Google Scholar] [CrossRef]
- Ide, H.; Iwase, M.; Fujii, H.; Ohkuma, T.; Kaizu, S.; Jodai, T.; Kikuchi, Y.; Idewaki, Y.; Sumi, A.; Nakamura, U.; et al. Comparison of cystatin C- and creatinine-based estimated glomerular filtration rates for predicting all-cause mortality in Japanese patients with type 2 diabetes: The Fukuoka Diabetes Registry. Clin. Exp. Nephrol. 2017, 21, 383–390. [Google Scholar] [CrossRef]
- Burns, K.D.; Lytvyn, Y.; Mahmud, F.H.; Daneman, D.; Deda, L.; Dunger, D.B.; Deanfield, J.; Dalton, R.N.; Elia, Y.; Har, R.; et al. The relationship between urinary renin-angiotensin system markers, renal function, and blood pressure in adolescents with type 1 diabetes. Am. J. Physiol. Renal Physiol. 2017, 312, F335–F342. [Google Scholar] [CrossRef] [PubMed]
- Villela-Torres, M.L.; Higareda-Mendoza, A.E.; Gomez-Garcia, A.; Alvarez-Paredes, A.R.; Garcia-Lopez, E.; Stenvikel, P.; Gu, H.F.; Rashid-Qureshi, A.; Lindholm, B.; Alvarez-Aguilar, C. Copeptin Plasma Levels are Associated with Decline of Renal Function in Patients with Type 2 Diabetes Mellitus. Arch. Med. Res. 2018, 49, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Dieter, B.P.; McPherson, S.M.; Afkarian, M.; de Boer, I.H.; Mehrotra, R.; Short, R.; Barbosa-Leiker, C.; Alicic, R.Z.; Meek, R.L.; Tuttle, K.R. Serum amyloid a and risk of death and end-stage renal disease in diabetic kidney disease. J. Diabetes Complicat. 2016, 30, 1467–1472. [Google Scholar] [CrossRef] [PubMed]
- Murakoshi, M.; Gohda, T.; Suzuki, Y. Circulating Tumor Necrosis Factor Receptors: A Potential Biomarker for the Progression of Diabetic Kidney Disease. Int. J. Mol. Sci. 2020, 21, 1957. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Sano, M.; Lu, L.; Wang, W.; Zhang, Q.; Zhang, R.; Wang, L.; Chen, Q.; Fukuda, K.; Shen, W. Plasma concentrations of osteopontin, but not thrombin-cleaved osteopontin, are associated with the presence and severity of nephropathy and coronary artery disease in patients with type 2 diabetes mellitus. Cardiovasc. Diabetol. 2010, 9, 70. [Google Scholar] [CrossRef] [PubMed]
- Lorenzen, J.; Shah, R.; Biser, A.; Staicu, S.A.; Niranjan, T.; Garcia, A.M.; Gruenwald, A.; Thomas, D.B.; Shatat, I.F.; Supe, K.; et al. The role of osteopontin in the development of albuminuria. J. Am. Soc. Nephrol. 2008, 19, 884–890. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Igarashi, M.; Hirata, A.; Tsuchiya, H.; Sugiyama, K.; Morita, Y.; Jimbu, Y.; Ohnuma, H.; Daimon, M.; Tominaga, M.; et al. Progression of diabetic nephropathy enhances the plasma osteopontin level in type 2 diabetic patients. Endocr. J. 2004, 51, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Chagan-Yasutan, H.; Hanan, F.; Niki, T.; Bai, G.; Ashino, Y.; Egawa, S.; Telan, E.F.O.; Hattori, T. Plasma Osteopontin Levels is Associated with Biochemical Markers of Kidney Injury in Patients with Leptospirosis. Diagnostics 2020, 10, 439. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.A.; Holder, D.J.; Ennulat, D.; Gautier, J.C.; Sauer, J.M.; Yang, Y.; McDuffie, E.; Sonee, M.; Gu, Y.Z.; Troth, S.P.; et al. Rat Urinary Osteopontin and Neutrophil Gelatinase-Associated Lipocalin Improve Certainty of Detecting Drug-Induced Kidney Injury. Toxicol. Sci. 2016, 151, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Giles, B.M.; Boackle, S.A. Linking complement and anti-dsDNA antibodies in the pathogenesis of systemic lupus erythematosus. Immunol. Res. 2013, 55, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Marks, S.D.; Tullus, K. Autoantibodies in systemic lupus erythematosus. Pediatr. Nephrol. 2012, 27, 1855–1868. [Google Scholar] [CrossRef] [PubMed]
- Ikeuchi, H.; Hiromura, K.; Kayakabe, K.; Tshilela, K.A.; Uchiyama, K.; Hamatani, H.; Sakairi, T.; Kaneko, Y.; Maeshima, A.; Nojima, Y. Renal outcomes in mixed proliferative and membranous lupus nephritis (Class III/IV + V): A long-term observational study. Mod. Rheumatol. 2016, 26, 908–913. [Google Scholar] [CrossRef] [PubMed]
- Kaleta, B. Role of osteopontin in systemic lupus erythematosus. Arch. Immunol. Ther. Exp. 2014, 62, 475–482. [Google Scholar] [CrossRef]
- Salimi, S.; Noora, M.; Nabizadeh, S.; Rezaei, M.; Shahraki, H.; Milad, M.K.; Naghavi, A.; Farajian-Mashhadi, F.; Zakeri, Z.; Sandoughi, M. Association of the osteopontin rs1126616 polymorphism and a higher serum osteopontin level with lupus nephritis. Biomed. Rep. 2016, 4, 355–360. [Google Scholar] [CrossRef]
- Metwally, R.M.; Hasan, A.S. Association of Osteopontin gene single nucleotide polymorphism with lupus nephritis. Int. J. Rheum. Dis. 2022, 25, 571–575. [Google Scholar] [CrossRef]
- Xu, A.P.; Liang, Y.Y.; Lu, J.; Li, J.G.; Wang, Z. Association of osteopontin gene polymorphism with lupus nephritis in Chinese Han population. Nan Fang Yi Ke Da Xue Xue Bao 2007, 27, 1348–1351. [Google Scholar] [PubMed]
- Rajasekaran, A.; Julian, B.A.; Rizk, D.V. IgA Nephropathy: An Interesting Autoimmune Kidney Disease. Am. J. Med. Sci. 2021, 361, 176–194. [Google Scholar] [CrossRef]
- Kaimori, J.Y.; Takenaka, M.; Nagasawa, Y.; Nakajima, H.; Izumi, M.; Akagi, Y.; Imai, E.; Hori, M. Quantitative analyses of osteopontin mRNA expression in human proximal tubules isolated from renal biopsy tissue sections of minimal change nephrotic syndrome and IgA glomerulonephropathy patients. Am. J. Kidney Dis. 2002, 39, 948–957. [Google Scholar] [CrossRef]
- Wenderfer, S.E.; Gaut, J.P. Glomerular Diseases in Children. Adv. Chronic. Kidney Dis. 2017, 24, 364–371. [Google Scholar] [CrossRef]
- Bertelli, R.; Bonanni, A.; Caridi, G.; Canepa, A.; Ghiggeri, G.M. Molecular and Cellular Mechanisms for Proteinuria in Minimal Change Disease. Front. Med. 2018, 5, 170. [Google Scholar] [CrossRef]
- Hoxha, E.; von Haxthausen, F.; Wiech, T.; Stahl, R.A.K. Membranous nephropathy-one morphologic pattern with different diseases. Pflugers Arch. 2017, 469, 989–996. [Google Scholar] [CrossRef]
- Cattran, D.C.; Brenchley, P.E. Membranous nephropathy: Integrating basic science into improved clinical management. Kidney Int. 2017, 91, 566–574. [Google Scholar] [CrossRef]
- Glassock, R.J. Human idiopathic membranous nephropathy--a mystery solved? N. Engl. J. Med. 2009, 361, 81–83. [Google Scholar] [CrossRef] [PubMed]
- Couser, W.G. Basic and translational concepts of immune-mediated glomerular diseases. J. Am. Soc. Nephrol. 2012, 23, 381–399. [Google Scholar] [CrossRef]
- Chang, K.H.; Wang, C.H.; Hsu, B.G.; Tsai, J.P. Serum Osteopontin Level Is Positively Associated with Aortic Stiffness in Patients with Peritoneal Dialysis. Life 2022, 12, 397. [Google Scholar] [CrossRef] [PubMed]
- Contenti, J.; Durand, M.; Vido, S.; Declemy, S.; Raffort, J.; Carboni, J.; Bonnet, S.; Koelsch, C.; Hassen-Khodja, R.; Gual, P.; et al. Plasmatic osteopontin and vascular access dysfunction in hemodialysis patients: A cross-sectional, case-control study (The OSMOSIS Study). J. Nephrol. 2022, 35, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Batko, K.; Krzanowski, M.; Gajda, M.; Dumnicka, P.; Fedak, D.; Woziwodzka, K.; Sulowicz, W.; Kuzniewski, M.; Litwin, J.A.; Krzanowska, K. Endothelial injury is closely related to osteopontin and TNF receptor-mediated inflammation in end-stage renal disease. Cytokine 2019, 121, 154729. [Google Scholar] [CrossRef] [PubMed]
- Druck, A.; Patel, D.; Bansal, V.; Hoppensteadt, D.; Fareed, J. Osteopontin Levels in Patients With Chronic Kidney Disease Stage 5 on Hemodialysis Directly Correlate With Intact Parathyroid Hormone and Alkaline Phosphatase. Clin. Appl. Thromb. Hemost. 2019, 25, 1076029619896621. [Google Scholar] [CrossRef] [PubMed]
- Joseph, S.B.; Castrillo, A.; Laffitte, B.A.; Mangelsdorf, D.J.; Tontonoz, P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat. Med. 2003, 9, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, D.; Stone, J.F.; Takata, Y.; Blaschke, F.; Chu, V.H.; Towler, D.A.; Law, R.E.; Hsueh, W.A.; Bruemmer, D. Liver × receptor agonists inhibit cytokine-induced osteopontin expression in macrophages through interference with activator protein-1 signaling pathways. Circ. Res. 2005, 96, e59–e67. [Google Scholar] [CrossRef]
- Tachibana, H.; Ogawa, D.; Matsushita, Y.; Bruemmer, D.; Wada, J.; Teshigawara, S.; Eguchi, J.; Sato-Horiguchi, C.; Uchida, H.A.; Shikata, K.; et al. Activation of liver X receptor inhibits osteopontin and ameliorates diabetic nephropathy. J. Am. Soc. Nephrol. 2012, 23, 1835–1846. [Google Scholar] [CrossRef]
- Diao, H.; Iwabuchi, K.; Li, L.; Onoe, K.; Van Kaer, L.; Kon, S.; Saito, Y.; Morimoto, J.; Denhardt, D.T.; Rittling, S.; et al. Osteopontin regulates development and function of invariant natural killer T cells. Proc. Natl. Acad. Sci. USA 2008, 105, 15884–15889. [Google Scholar] [CrossRef]
- Tsuji, H.; Shimizu, N.; Nozawa, M.; Umekawa, T.; Yoshimura, K.; De Velasco, M.A.; Uemura, H.; Khan, S.R. Osteopontin knockdown in the kidneys of hyperoxaluric rats leads to reduction in renal calcium oxalate crystal deposition. Urolithiasis 2014, 42, 195–202. [Google Scholar] [CrossRef]
- Tang, M.; Guo, C.; Sun, M.; Zhou, H.; Peng, X.; Dai, J.; Ding, Q.; Wang, Y.; Yang, C. Effective delivery of osteopontin small interference RNA using exosomes suppresses liver fibrosis via TGF-beta1 signaling. Front. Pharmacol. 2022, 13, 882243. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.T.; Guo, C.; Ding, X.; Fan, W.J.; Zhang, F.H.; Xu, W.L.; Ma, Y.C. Role of osteopontin in the regulation of human bladder cancer proliferation and migration in T24 cells. Mol. Med. Rep. 2015, 11, 3701–3707. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, Y.; Shen, Y.; Xu, Y.; Li, X. Osteopontin silencing by small interfering RNA induces apoptosis and suppresses invasion in human renal carcinoma Caki-1 cells. Med. Oncol. 2010, 27, 1179–1184. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Park, C.W.; Choi, Y.; Lin, J.; Seo, D.H.; Kim, H.S.; Lee, S.Y.; Kang, I.C. A novel small-molecule PPI inhibitor targeting integrin alphavbeta3-osteopontin interface blocks bone resorption in vitro and prevents bone loss in mice. Biomaterials 2016, 98, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Desai, B.; Rogers, M.J.; Chellaiah, M.A. Mechanisms of osteopontin and CD44 as metastatic principles in prostate cancer cells. Mol. Cancer 2007, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Zagani, R.; Hamzaoui, N.; Cacheux, W.; de Reynies, A.; Terris, B.; Chaussade, S.; Romagnolo, B.; Perret, C.; Lamarque, D. Cyclooxygenase-2 inhibitors down-regulate osteopontin and Nr4A2-new therapeutic targets for colorectal cancers. Gastroenterology 2009, 137, 1358–1366.e3. [Google Scholar] [CrossRef]
- Kumar, S.; Patil, H.S.; Sharma, P.; Kumar, D.; Dasari, S.; Puranik, V.G.; Thulasiram, H.V.; Kundu, G.C. Andrographolide inhibits osteopontin expression and breast tumor growth through down regulation of PI3 kinase/Akt signaling pathway. Curr. Mol. Med. 2012, 12, 952–966. [Google Scholar] [CrossRef]
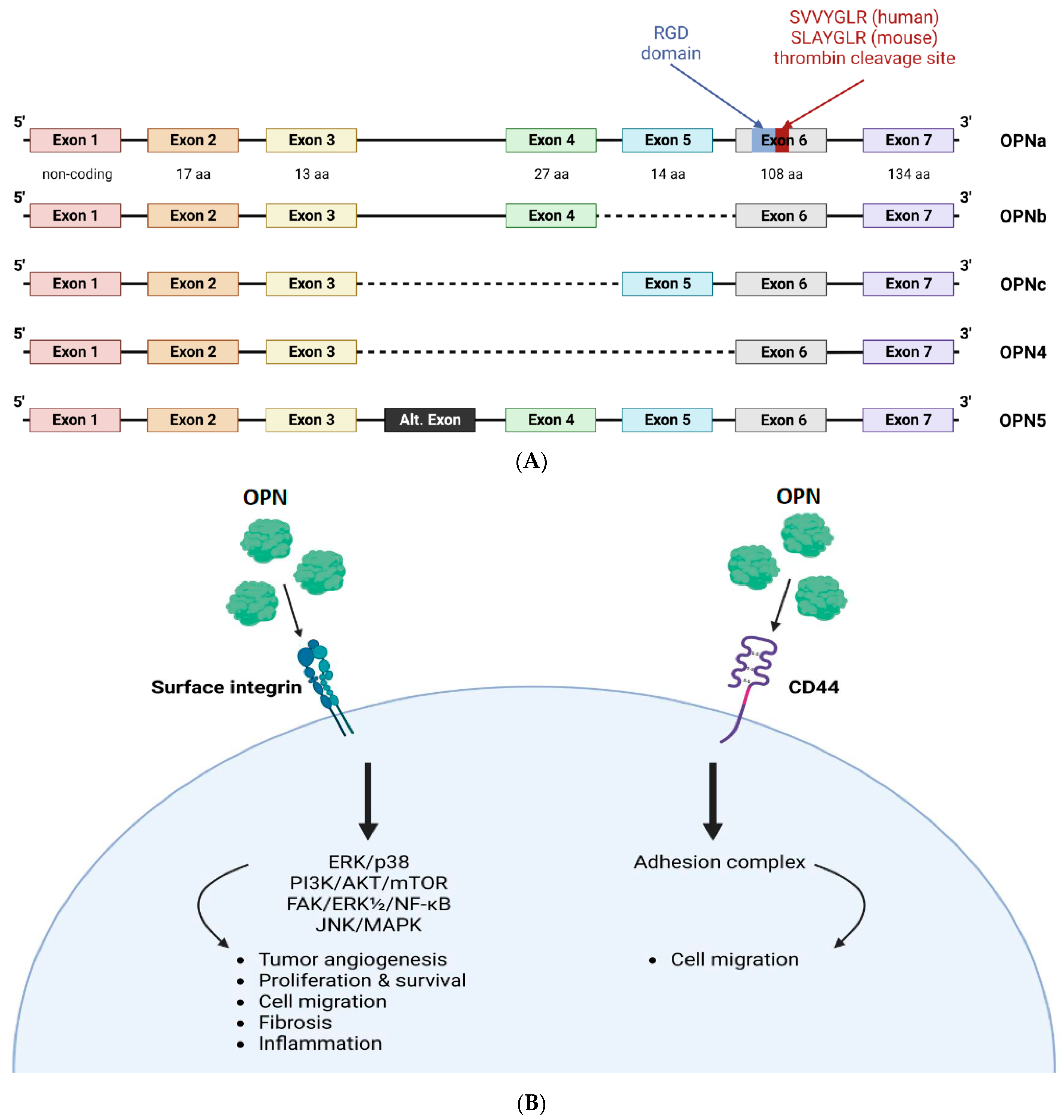
| Modifications of OPN | Functional Characteristics | References | |
|---|---|---|---|
| Post-transcription modifications | OPNa | 1. In a murine model of post-ischemic neovascularization, OPNa significantly boosted macrophage migration | [21] |
| 2. In non-small cell lung cancer (NSCLC), OPNa promoted cell migration, proliferation, colony formation, and invasion. | [22] | ||
| 3. OPNa was shown to significantly increase functional collateral vessel formation in vivo | [21] | ||
| OPNb | 1. In NSCLC, OPNb had a less significant effect as a modulator of proliferation, colony formation, and invasion. | [22] | |
| 2. The overexpression of OPNb spliced variants in endometriotic cells activated the PI3K and NF-κB pathways. This led to endothelial mesenchymal transformation, cell migration, proliferation, morphological changes, and actin remodeling. | [23] | ||
| OPNc | 1. OPNc was shown to exhibit a more significant enhancement of macrophage migration compared to OPNa in post-ischemic neovascularization. | [21] | |
| 2. The activation of cellular calcium signals and subsequent nuclear translocation of nuclear factor of activated T-cells, cytoplasmic 2, induced by secretory OPNc, increased the survival of NSCLC cells treated with cisplatin | [24] | ||
| 3. OPNc spliced isoform has been shown to contribute to ovarian cancer progression | [25] | ||
| 4. In NSCLC, OPNc reduced cell proliferation, colony formation, and invasion. | [22] | ||
| 5. Elevated expression of OPNc activated PI3K and NF-κB pathways in endometriotic cells resulting in various cellular changes | [23] | ||
| 6. OPNc has been shown to increase functional collateral vessel formation in vivo | [21] | ||
| Post-translational modifications | Phosphorylation | Phosphorylation of OPN is a prerequisite for inducing IL-12 expression in macrophages, and its dephosphorylation nullifies this effect. | [26] |
| Glycosylation | The folding structure, proteolytic cleavage, and functional characteristics of OPN are influenced by the presence of glycosylation. Removing several O-glycosylation sites from OPN affects cell adhesion activity and phosphorylation status. | [27,28] | |
| Transglutamination | Transglutaminase 2, a calcium-dependent enzyme, can utilize OPN as a substrate and catalyze the cross-linking of glutamine and lysine residues. This process can enable polymeric OPN to bind to the α9β1 receptor without relying on the SVVYGLR sequence. | [29,30] | |
| Proteolytic cleavage | ntOPN | 1. ntOPN has been shown to be associated with greater degrees of inflammation in carotid plaques in patients with hypertension | [31] |
| 2. ntOPN promotes abdominal aortic aneurysm by increasing the expression of pyroptosis-related inflammatory factors through the NF-κB pathway, inflammation, and extracellular matrix degradation. | [32] | ||
| 3. ntOPN controls activation of hepatic stellate cells and is essential for liver fibrogenesis. | [33] | ||
| ctOPN | 1. Studies have revealed that modifying the ctOPN can regulate its interaction with the widely expressed αVβ3-integrin. | [25] | |
| 2. In vitro studies have shown that thrombin cleaved ctOPN can affect the migration and invasion of breast cancer cells. | [34] | ||
| 3. The ctOPN is reported to be involved in macrophage chemotaxis | [35] |
| Forms of Chronic Kidney Disease | Main Findings | References |
|---|---|---|
| Diabetic kidney disease (DKD) | 1. High expression of OPN was reported in the tubular epithelium of the renal cortex and in glomeruli in rat and mouse models of diabetic nephropathy. | [72,73] |
| 2. OPN deletion prevented disease progression while OPN expression increased glomerular damage, possibly through the production of transforming growth factor-β, indicating that OPN might be a therapeutic target. | [65] | |
| 3. OPN overexpression and macrophage recruitment were associated with considerable macrophage buildup in the renal interstitium in diabetic nephropathy, which may also be contributing to the tubulo-interstitial damage. | [72,74] | |
| 4. Long-acting angiotensin-converting enzyme inhibitor, perindopril, significantly decreased the accumulation of macrophages and the expression of OPN, induced by diabetes, in the renal interstitium of diabetic rats. | [72] | |
| 5. Blockade of the renin-angiotensin system by ramipril may confer renoprotection by decreasing OPN expression in non-insulin-dependent diabetic nephropathy. | [75] | |
| Lupus Nephritis (LN) | 1. A significant difference in the plasma concentration of OPN was seen in patients with systemic lupus erythematosus (SLE) and kidney impairment compared to healthy controls. The differences correlated with the levels of IL-18 and the SLE Disease Activity Index. | [76] |
| 2. Full-length OPN and ntOPN concentrations have been reported to be considerably greater in patients with LN. However, urine ntOPN was related to renal inflammation and thought to be a reliable prognostic indicator for LN. | [60,77] | |
| 3. It was reported that intrarenal macrophage infiltration and higher OPN expression were positively associated in patients with LN. | [77] | |
| Immunoglobulin A Nephropathy (IgAN) | 1. Studies suggest that OPN is involved in the development of IgAN | [59] |
| 2. OPN had an accuracy of 87% in distinguishing IgAN from other glomerulopathies, and thus appeared to be a valuable biomarker. | [66] | |
| 3. In this pathology both, OPN and CD44 receptor are highly expressed in cells in areas of tubulointerstitial injury. | [78] | |
| 4. Another study conducted in children with IgAN showed that the high urinary level of OPN was associated with high OPN-to-creatinine ratio. | [79] | |
| 5. Studies have shown that during the development of IgAN, increased OPN mRNA correlated with macrophage infiltration. | [79] | |
| Autosomal dominant polycystic kidney disease (ADPKD) | 1. OPN has been identified as a urinary biomarker for autosomal dominant polycystic kidney disease progression. | [80] |
| 2. The urinary OPN excretion levels were reported to be lower in rapid progressors than in slow progressors, suggesting that it may be a useful urinary biomarker for predicting ADPKD progression. | [80] | |
| Minimal change disease (MCD) | 1. A study in children with MCD revealed higher urinary OPN-to-creatinine ratio compared to the control group. | [79] |
| 2. A positive correlation of OPN mRNA expression in proximal tubules, and urinary OPN were observed in patients with MCD. | [59] | |
| 3. Patients with MCD have higher urinary ntOPN, which was also associated with higher levels of albuminuria. | [59] | |
| Membranous glomerulonephritis (MGN) | 1. Recent studies demonstrated higher expression of OPN in the proximal tubules in patients with progressive and nonprogressive MGN. | [81,82] |
| 2. In this kidney disease, a strong correlation between the mRNA and OPN has been demonstrated. In a murine model, high expression of OPN in the kidney was associated with increased infiltration of macrophages and other immune cells, like CD4+ and CD8+ T lymphocytes. | [82] | |
| 3. Overexpression of OPN in the kidney was also correlated with activation of NF-ĸB, increasing the expression of proinflammatory cytokines, which can contribute to glomerular damage. | [82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sinha, S.K.; Mellody, M.; Carpio, M.B.; Damoiseaux, R.; Nicholas, S.B. Osteopontin as a Biomarker in Chronic Kidney Disease. Biomedicines 2023, 11, 1356. https://doi.org/10.3390/biomedicines11051356
Sinha SK, Mellody M, Carpio MB, Damoiseaux R, Nicholas SB. Osteopontin as a Biomarker in Chronic Kidney Disease. Biomedicines. 2023; 11(5):1356. https://doi.org/10.3390/biomedicines11051356
Chicago/Turabian StyleSinha, Satyesh K., Michael Mellody, Maria Beatriz Carpio, Robert Damoiseaux, and Susanne B. Nicholas. 2023. "Osteopontin as a Biomarker in Chronic Kidney Disease" Biomedicines 11, no. 5: 1356. https://doi.org/10.3390/biomedicines11051356
APA StyleSinha, S. K., Mellody, M., Carpio, M. B., Damoiseaux, R., & Nicholas, S. B. (2023). Osteopontin as a Biomarker in Chronic Kidney Disease. Biomedicines, 11(5), 1356. https://doi.org/10.3390/biomedicines11051356






