GLP-1 Increases Circulating Leptin Levels in Truncal Vagotomized Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Vagotomy Procedure
2.2. Confirmation of the Vagotomy Procedure
2.3. Resting Energy Expenditure Assessment and Feeding Studies
2.4. Experimental Endpoint
2.5. Blood Glucose and Hormone Measurements
2.6. Hypothalamic Neuropeptide and UCP-1 Expression
2.7. In Vitro Stimulation of Human VAT Explants with GLP-1
2.8. Data and Statistical Analysis
3. Results
3.1. Confirmation of the Vagotomy Procedure
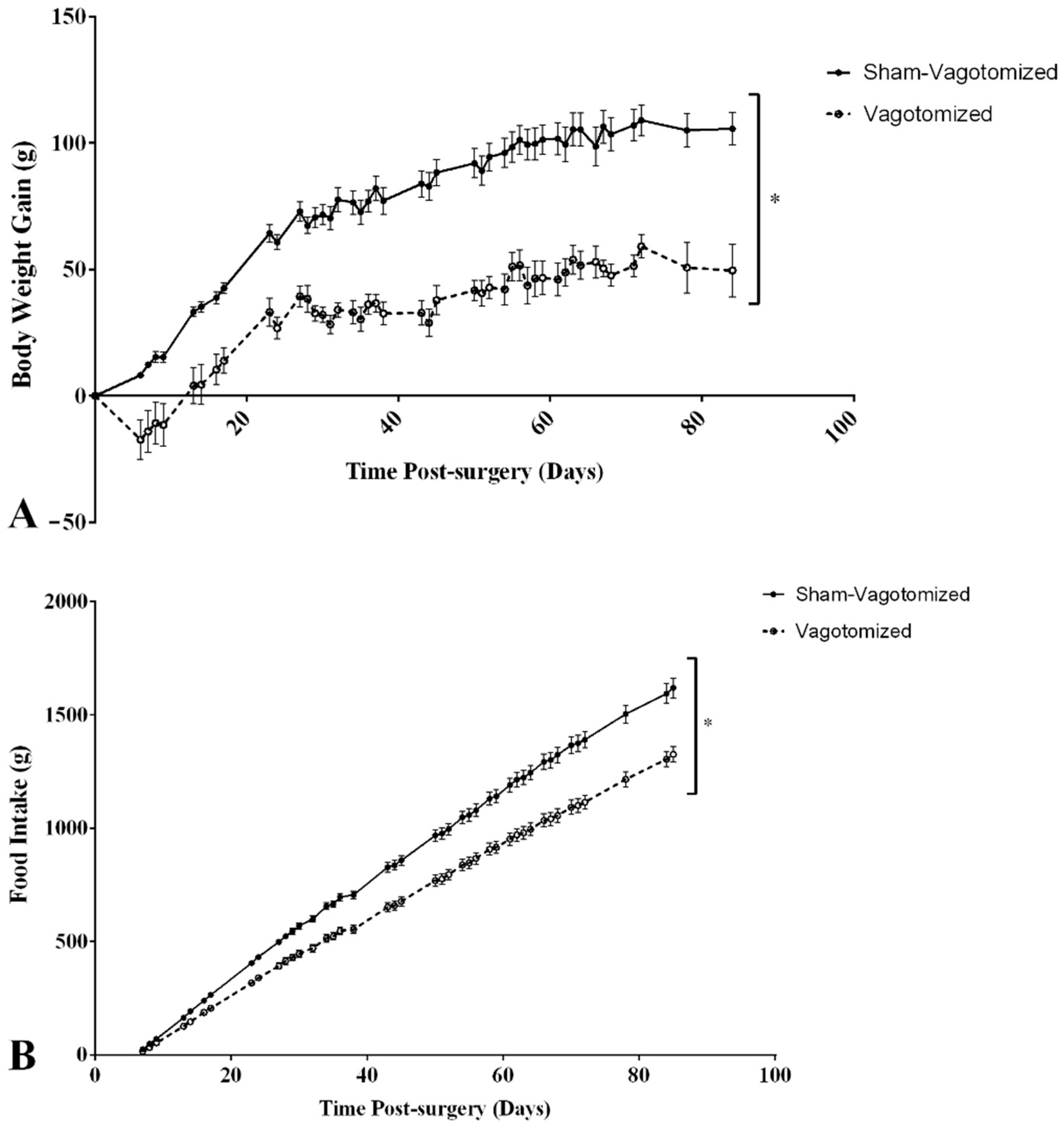
3.2. Body Weight and Food Intake
3.3. Feeding Response to Peripheral Acute GLP-1 Administration
3.4. Adipose Tissue
3.5. Resting Energy Expenditure
3.6. Hypothalamic Gene Expression
3.7. Glucose and Hormone Plasma Levels
3.7.1. Fasting Blood Glucose, Plasma Insulin Levels and HOMA-IR
3.7.2. GI Hormones
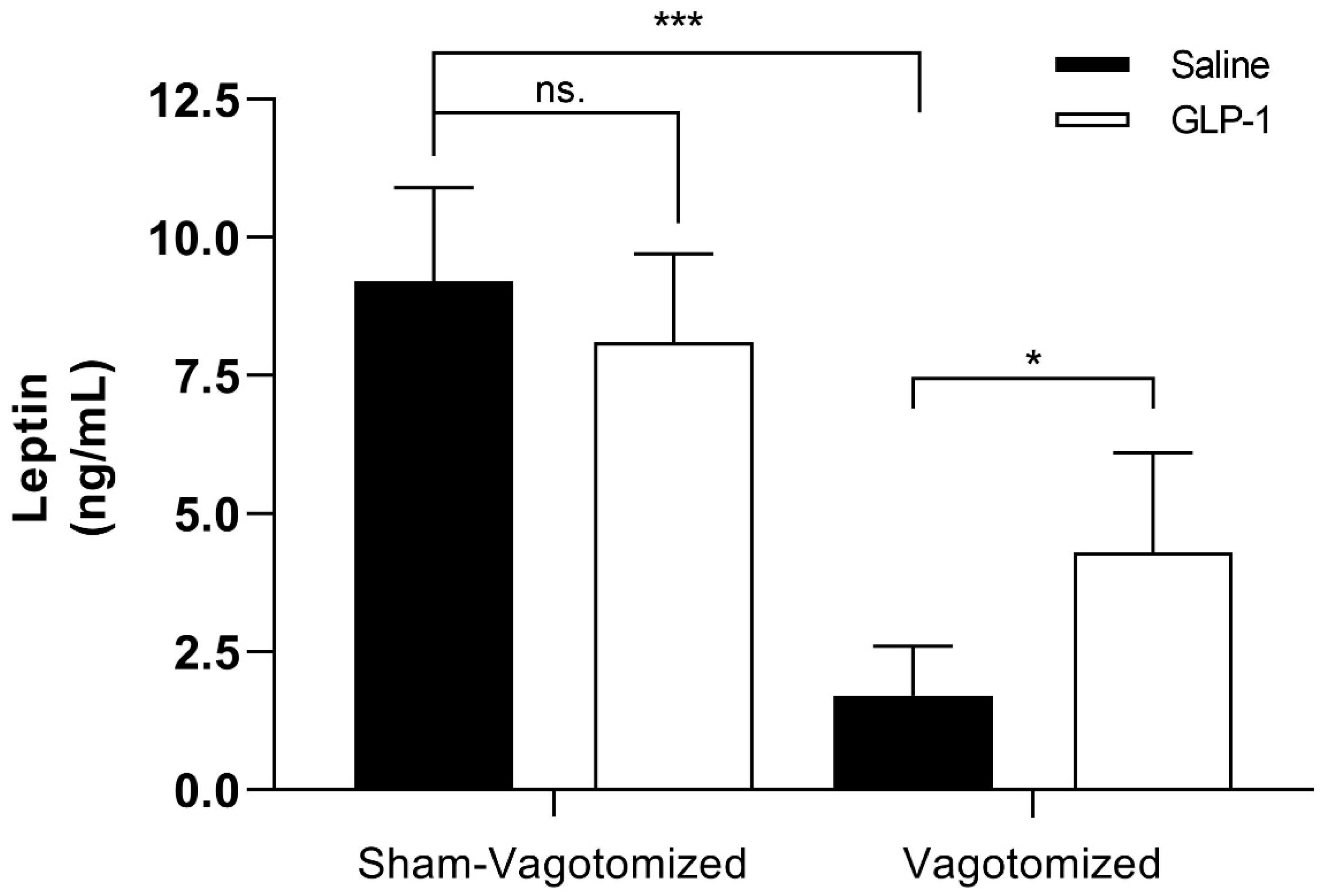
3.7.3. Leptin
3.8. In Vitro Stimulation of Human VAT Explants with GLP-1
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sam, A.H.; Troke, R.C.; Tan, T.M.; Bewick, G.A. The role of the gut/brain axis in modulating food intake. Neuropharmacology 2012, 63, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Abbott, C.R.; Monteiro, M.; Small, C.J.; Sajedi, A.; Smith, K.L.; Parkinson, J.R.; Ghatei, M.A.; Bloom, S.R. The inhibitory effects of peripheral administration of peptide YY(3-36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res. 2005, 1044, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Cork, S.C. The role of the vagus nerve in appetite control: Implications for the pathogenesis of obesity. J. Neuroendocr. 2018, 30, e12643. [Google Scholar] [CrossRef] [PubMed]
- Kodama, Y.; Zhao, C.M.; Kulseng, B.; Chen, D. Eating behavior in rats subjected to vagotomy, sleeve gastrectomy, and duodenal switch. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 2010, 14, 1502–1510. [Google Scholar] [CrossRef] [PubMed]
- Bueter, M.; Lowenstein, C.; Ashrafian, H.; Hillebrand, J.; Bloom, S.R.; Olbers, T.; Lutz, T.; le Roux, C.W. Vagal sparing surgical technique but not stoma size affects body weight loss in rodent model of gastric bypass. Obes. Surg. 2010, 20, 616–622. [Google Scholar] [CrossRef]
- Ruiz-Tovar, J.; Llavero, C. Metabolic Effect of the Hepatic Branch of the Vagal Nerve in One-Anastomosis Gastric Bypass (OAGB). World J. Surg. 2020, 44, 1939–1944. [Google Scholar] [CrossRef]
- Kakei, M.; Yada, T.; Nakagawa, A.; Nakabayashi, H. Glucagon-like peptide-1 evokes action potentials and increases cytosolic Ca2+ in rat nodose ganglion neurons. Auton. Neurosci. Basic Clin. 2002, 102, 39–44. [Google Scholar] [CrossRef]
- Burdyga, G.; Varro, A.; Dimaline, R.; Thompson, D.G.; Dockray, G.J. Ghrelin receptors in rat and human nodose ganglia: Putative role in regulating CB-1 and MCH receptor abundance. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G1289–G1297. [Google Scholar] [CrossRef]
- Buyse, M.; Ovesjo, M.L.; Goiot, H.; Guilmeau, S.; Peranzi, G.; Moizo, L.; Walker, F.; Lewin, M.J.; Meister, B.; Bado, A. Expression and regulation of leptin receptor proteins in afferent and efferent neurons of the vagus nerve. Eur. J. Neurosci. 2001, 14, 64–72. [Google Scholar] [CrossRef]
- Andrade, S.; Pinho, F.; Ribeiro, A.M.; Carreira, M.; Casanueva, F.F.; Roy, P.; Monteiro, M.P. Immunization against active ghrelin using virus-like particles for obesity treatment. Curr. Pharm. Des. 2013, 19, 6551–6558. [Google Scholar] [CrossRef]
- Firman, C.; Batterham, R.L. A new era in gut hormone-based pharmacotherapy for people with obesity. Proc. Nutr. Soc. 2022, 81, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Bluher, M.; Aras, M.; Aronne, L.J.; Batterham, R.L.; Giorgino, F.; Ji, L.; Pietilainen, K.H.; Schnell, O.; Tonchevska, E.; Wilding, J.P.H. New insights into the treatment of obesity. Diabetes Obes. Metab. 2023. [Google Scholar] [CrossRef] [PubMed]
- Muller, T.D.; Finan, B.; Bloom, S.R.; D’Alessio, D.; Drucker, D.J.; Flatt, P.R.; Fritsche, A.; Gribble, F.; Grill, H.J.; Habener, J.F.; et al. Glucagon-like peptide 1 (GLP-1). Mol. Metab. 2019, 30, 72–130. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.R.; De Jonghe, B.C.; Kanoski, S.E. Role of the glucagon-like-peptide-1 receptor in the control of energy balance. Physiol. Behav. 2010, 100, 503–510. [Google Scholar] [CrossRef]
- Liu, J.; Li, L.; Deng, K.; Xu, C.; Busse, J.W.; Vandvik, P.O.; Li, S.; Guyatt, G.H.; Sun, X. Incretin based treatments and mortality in patients with type 2 diabetes: Systematic review and meta-analysis. BMJ 2017, 357, j2499. [Google Scholar] [CrossRef]
- Merchenthaler, I.; Lane, M.; Shughrue, P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J. Comp. Neurol. 1999, 403, 261–280. [Google Scholar] [CrossRef]
- Goke, R.; Larsen, P.J.; Mikkelsen, J.D.; Sheikh, S.P. Distribution of GLP-1 binding sites in the rat brain: Evidence that exendin-4 is a ligand of brain GLP-1 binding sites. Eur. J. Neurosci. 1995, 7, 2294–2300. [Google Scholar] [CrossRef]
- De Silva, A.; Salem, V.; Long, C.J.; Makwana, A.; Newbould, R.D.; Rabiner, E.A.; Ghatei, M.A.; Bloom, S.R.; Matthews, P.M.; Beaver, J.D.; et al. The gut hormones PYY 3-36 and GLP-1 7-36 amide reduce food intake and modulate brain activity in appetite centers in humans. Cell Metab. 2011, 14, 700–706. [Google Scholar] [CrossRef]
- Meeran, K.; O’Shea, D.; Edwards, C.M.; Turton, M.D.; Heath, M.M.; Gunn, I.; Abusnana, S.; Rossi, M.; Small, C.J.; Goldstone, A.P.; et al. Repeated intracerebroventricular administration of glucagon-like peptide-1-(7-36) amide or exendin-(9-39) alters body weight in the rat. Endocrinology 1999, 140, 244–250. [Google Scholar] [CrossRef]
- Kanoski, S.E.; Fortin, S.M.; Arnold, M.; Grill, H.J.; Hayes, M.R. Peripheral and central GLP-1 receptor populations mediate the anorectic effects of peripherally administered GLP-1 receptor agonists, liraglutide and exendin-4. Endocrinology 2011, 152, 3103–3112. [Google Scholar] [CrossRef]
- Morais, T.; Patricio, B.; Pereira, S.S.; Andrade, S.; Carreira, M.; Casanueva, F.F.; Monteiro, M.P. GLP-1 induces alpha cell proliferation and overrides leptin suppression induced by negative energy balance in vagotomized rats. J. Cell Biochem. 2019, 120, 14573–14584. [Google Scholar] [CrossRef] [PubMed]
- Abbott, C.R.; Small, C.J.; Sajedi, A.; Smith, K.L.; Parkinson, J.R.; Broadhead, L.L.; Ghatei, M.A.; Bloom, S.R. The importance of acclimatisation and habituation to experimental conditions when investigating the anorectic effects of gastrointestinal hormones in the rat. Int. J. Obes. 2006, 30, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.J.; Powley, T.L. Gastric volume detection after selective vagotomies in rats. Am. J. Physiol. 1998, 274, R1626–R1638. [Google Scholar] [CrossRef] [PubMed]
- Morais, T.; Seabra, A.L.; Patrício, B.G.; Guimarães, M.; Nora, M.; Oliveira, P.F.; Alves, M.G.; Monteiro, M.P. Visceral Adipose Tissue Displays Unique Metabolomic Fingerprints in Obesity, Pre-Diabetes and Type 2 Diabetes. Int. J. Mol. Sci. 2021, 22, 5695. [Google Scholar] [CrossRef] [PubMed]
- Dezfuli, G.; Gillis, R.A.; Tatge, J.E.; Duncan, K.R.; Dretchen, K.L.; Jackson, P.G.; Verbalis, J.G.; Sahibzada, N. Subdiaphragmatic Vagotomy With Pyloroplasty Ameliorates the Obesity Caused by Genetic Deletion of the Melanocortin 4 Receptor in the Mouse. Front. Neurosci. 2018, 12, 104. [Google Scholar] [CrossRef] [PubMed]
- Balbo, S.L.; Ribeiro, R.A.; Mendes, M.C.; Lubaczeuski, C.; Maller, A.C.; Carneiro, E.M.; Bonfleur, M.L. Vagotomy diminishes obesity in cafeteria rats by decreasing cholinergic potentiation of insulin release. J. Physiol. Biochem. 2016, 72, 625–633. [Google Scholar] [CrossRef]
- Bargut, T.C.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. Brown adipose tissue: Updates in cellular and molecular biology. Tissue Cell 2016, 48, 452–460. [Google Scholar] [CrossRef]
- Vijgen, G.H.; Bouvy, N.D.; Leenen, L.; Rijkers, K.; Cornips, E.; Majoie, M.; Brans, B.; van Marken Lichtenbelt, W.D. Vagus nerve stimulation increases energy expenditure: Relation to brown adipose tissue activity. PLoS ONE 2013, 8, e77221. [Google Scholar] [CrossRef]
- Li, H.; Zhang, J.B.; Xu, C.; Tang, Q.Q.; Shen, W.X.; Zhou, J.Z.; Chen, J.D.; Wang, Y.P. Effects and mechanisms of auricular vagus nerve stimulation on high-fat-diet--induced obese rats. Nutrition 2015, 31, 1416–1422. [Google Scholar] [CrossRef]
- Madden, C.J.; Santos da Conceicao, E.P.; Morrison, S.F. Vagal afferent activation decreases brown adipose tissue (BAT) sympathetic nerve activity and BAT thermogenesis. Temperature 2017, 4, 89–96. [Google Scholar] [CrossRef]
- Szekely, M. The vagus nerve in thermoregulation and energy metabolism. Auton. Neurosci. Basic Clin. 2000, 85, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Balbo, S.L.; Grassiolli, S.; Ribeiro, R.A.; Bonfleur, M.L.; Gravena, C.; Brito Mdo, N.; Andreazzi, A.E.; Mathias, P.C.; Torrezan, R. Fat storage is partially dependent on vagal activity and insulin secretion of hypothalamic obese rat. Endocrine 2007, 31, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Minokoshi, Y.; Kim, Y.B.; Peroni, O.D.; Fryer, L.G.; Muller, C.; Carling, D.; Kahn, B.B. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature 2002, 415, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Morton, G.J.; Gelling, R.W.; Niswender, K.D.; Morrison, C.D.; Rhodes, C.J.; Schwartz, M.W. Leptin regulates insulin sensitivity via phosphatidylinositol-3-OH kinase signaling in mediobasal hypothalamic neurons. Cell Metab. 2005, 2, 411–420. [Google Scholar] [CrossRef]
- McMinn, J.E.; Baskin, D.G.; Schwartz, M.W. Neuroendocrine mechanisms regulating food intake and body weight. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2000, 1, 37–46. [Google Scholar] [CrossRef]
- Cummings, D.E.; Weigle, D.S.; Frayo, R.S.; Breen, P.A.; Ma, M.K.; Dellinger, E.P.; Purnell, J.Q. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N. Engl. J. Med. 2002, 346, 1623–1630. [Google Scholar] [CrossRef] [PubMed]
- Plamboeck, A.; Veedfald, S.; Deacon, C.F.; Hartmann, B.; Wettergren, A.; Svendsen, L.B.; Meisner, S.; Hovendal, C.; Vilsboll, T.; Knop, F.K.; et al. The effect of exogenous GLP-1 on food intake is lost in male truncally vagotomized subjects with pyloroplasty. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G1117–G1127. [Google Scholar] [CrossRef]
- Krieger, J.P.; Arnold, M.; Pettersen, K.G.; Lossel, P.; Langhans, W.; Lee, S.J. Knockdown of GLP-1 Receptors in Vagal Afferents Affects Normal Food Intake and Glycemia. Diabetes 2016, 65, 34–43. [Google Scholar] [CrossRef]
- Secher, A.; Jelsing, J.; Baquero, A.F.; Hecksher-Sorensen, J.; Cowley, M.A.; Dalboge, L.S.; Hansen, G.; Grove, K.L.; Pyke, C.; Raun, K.; et al. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J. Clin. Investig. 2014, 124, 4473–4488. [Google Scholar] [CrossRef]
- Imeryuz, N.; Yegen, B.C.; Bozkurt, A.; Coskun, T.; Villanueva-Penacarrillo, M.L.; Ulusoy, N.B. Glucagon-like peptide-1 inhibits gastric emptying via vagal afferent-mediated central mechanisms. Am. J. Physiol. 1997, 273, G920–G927. [Google Scholar]
- Wettergren, A.; Wojdemann, M.; Holst, J.J. Glucagon-like peptide-1 inhibits gastropancreatic function by inhibiting central parasympathetic outflow. Am. J. Physiol. 1998, 275, G984–G992. [Google Scholar] [CrossRef]
- Farr, O.M.; Tsoukas, M.A.; Triantafyllou, G.; Dincer, F.; Filippaios, A.; Ko, B.J.; Mantzoros, C.S. Short-term administration of the GLP-1 analog liraglutide decreases circulating leptin and increases GIP levels and these changes are associated with alterations in CNS responses to food cues: A randomized, placebo-controlled, crossover study. Metabolism 2016, 65, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Pastel, E.; McCulloch, L.J.; Ward, R.; Joshi, S.; Gooding, K.M.; Shore, A.C.; Kos, K. GLP-1 analogue-induced weight loss does not improve obesity-induced AT dysfunction. Clin. Sci. 2017, 131, 343–353. [Google Scholar] [CrossRef]
- Rondanelli, M.; Perna, S.; Astrone, P.; Grugnetti, A.; Solerte, S.B.; Guido, D. Twenty-four-week effects of liraglutide on body composition, adherence to appetite, and lipid profile in overweight and obese patients with type 2 diabetes mellitus. Patient Prefer. Adher. 2016, 10, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Iepsen, E.W.; Lundgren, J.; Dirksen, C.; Jensen, J.E.; Pedersen, O.; Hansen, T.; Madsbad, S.; Holst, J.J.; Torekov, S.S. Treatment with a GLP-1 receptor agonist diminishes the decrease in free plasma leptin during maintenance of weight loss. Int. J. Obes. 2015, 39, 834–841. [Google Scholar] [CrossRef]
- Naslund, E.; Bogefors, J.; Skogar, S.; Gryback, P.; Jacobsson, H.; Holst, J.J.; Hellstrom, P.M. GLP-1 slows solid gastric emptying and inhibits insulin, glucagon, and PYY release in humans. Am. J. Physiol. 1999, 277, R910–R916. [Google Scholar] [CrossRef]
- Seo, S.; Ju, S.; Chung, H.; Lee, D.; Park, S. Acute effects of glucagon-like peptide-1 on hypothalamic neuropeptide and AMP activated kinase expression in fasted rats. Endocr. J. 2008, 55, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.P.; Ronveaux, C.C.; de Lartigue, G.; Geary, N.; Asarian, L.; Raybould, H.E. Deletion of leptin receptors in vagal afferent neurons disrupts estrogen signaling, body weight, food intake and hormonal controls of feeding in female mice. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E568–E577. [Google Scholar] [CrossRef]
- Grabauskas, G.; Wu, X.; Zhou, S.; Li, J.; Gao, J.; Owyang, C. High-fat diet-induced vagal afferent dysfunction via upregulation of 2-pore domain potassium TRESK channel. JCI Insight 2019, 4, e130402. [Google Scholar] [CrossRef]
- Zhao, S.; Kanoski, S.E.; Yan, J.; Grill, H.J.; Hayes, M.R. Hindbrain leptin and glucagon-like-peptide-1 receptor signaling interact to suppress food intake in an additive manner. Int. J. Obes. 2012, 36, 1522–1528. [Google Scholar] [CrossRef]
- Williams, D.L.; Baskin, D.G.; Schwartz, M.W. Leptin regulation of the anorexic response to glucagon-like peptide-1 receptor stimulation. Diabetes 2006, 55, 3387–3393. [Google Scholar] [CrossRef] [PubMed]
- Charpentier, J.; Waget, A.; Klopp, P.; Magnan, C.; Cruciani-Guglielmacci, C.; Lee, S.J.; Burcelin, R.; Grasset, E. Lixisenatide requires a functional gut-vagus nerve-brain axis to trigger insulin secretion in controls and type 2 diabetic mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G671–G684. [Google Scholar] [CrossRef]
- Vendrell, J.; El Bekay, R.; Peral, B.; Garcia-Fuentes, E.; Megia, A.; Macias-Gonzalez, M.; Fernandez Real, J.; Jimenez-Gomez, Y.; Escote, X.; Pachon, G.; et al. Study of the potential association of adipose tissue GLP-1 receptor with obesity and insulin resistance. Endocrinology 2011, 152, 4072–4079. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, H.; Ma, X.; Zhang, Y.; Lu, S.; Wang, Y.; Zong, C.; Qin, D.; Wang, Y.; Yingfeng Yang, Y.; et al. GLP-1/GLP-1R Signaling in Regulation of Adipocyte Differentiation and Lipogenesis. Cell Physiol. Biochem. 2017, 42, 1165–1176. [Google Scholar] [CrossRef]
- Xu, F.; Cao, H.; Chen, Z.; Gu, H.; Guo, W.; Lin, B.; Weng, J. Short-term GLP-1 receptor agonist exenatide ameliorates intramyocellular lipid deposition without weight loss in ob/ob mice. Int. J. Obes. 2020, 44, 937–947. [Google Scholar] [CrossRef]
- Cammisotto, P.G.; Gelinas, Y.; Deshaies, Y.; Bukowiecki, L.J. Regulation of leptin secretion from white adipocytes by free fatty acids. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E521–E526. [Google Scholar] [CrossRef] [PubMed]
- Kolaczynski, J.W.; Nyce, M.R.; Considine, R.V.; Boden, G.; Nolan, J.J.; Henry, R.; Mudaliar, S.R.; Olefsky, J.; Caro, J.F. Acute and chronic effect of insulin on leptin production in humans: Studies in vivo and in vitro. Diabetes 1996, 45, 699–701. [Google Scholar] [CrossRef] [PubMed]


| Sham-Vagotomized | Vagotomized | |||
|---|---|---|---|---|
| Saline (n = 6) | GLP-1 (n = 5) | Saline (n = 6) | GLP-1 (n = 5) | |
| Glucose (mg/dL) | 175.3 ± 78.2 | 126.3 ± 49.1 | 91.6 ± 23.3 * | 122.8 ± 46.1 |
| Insulin (ng/mL) | 1.5 ± 0.3 | 1.4 ± 1.2 | 0.5 ± 0.1 ** | 1.4 ± 0.8 |
| HOMA-IR | 16.4 ± 7.7 | 10.7 ± 8.4 | 2.8 ± 1.2 * | 11.0 ± 6.5 |
| PYY (ng/mL) | 0.3 ± 0.2 | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 |
| OXM (ng/mL) | 2.1 ± 1.6 | 2.2 ± 1.0 | 2.2 ± 1.7 | 2.0 ± 1.2 |
| GLP-1 Active (pmol/L) | 3.8 ± 0.4 | 62.1 ± 0.9 *** | 3.8 ± 0.1 | 39.7 ± 0.1 ††† |
| GLP-1 Total (pmol/L) | 24.1 ± 11.1 | 434.9 ± 172.7 ** | 21.8 ± 7.1 | 459.2 ± 352.6 † |
| Active/Total GLP-1 | 0.18 ± 0.03 | 0.17 ± 0.04 | 0.16 ± 0.03 | 0.11 ± 0.03 |
| Ghrelin (ng/mL) | 1.0 ± 0.3 | 0.8 ± 0.3 | 2.1 ± 0.7 ** | 1.4 ± 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morais, T.; Pereira, S.S.; Andrade, S.; Neves, D.; Guimarães, M.; Nora, M.; Carreira, M.C.; Casanueva, F.F.; Monteiro, M.P. GLP-1 Increases Circulating Leptin Levels in Truncal Vagotomized Rats. Biomedicines 2023, 11, 1322. https://doi.org/10.3390/biomedicines11051322
Morais T, Pereira SS, Andrade S, Neves D, Guimarães M, Nora M, Carreira MC, Casanueva FF, Monteiro MP. GLP-1 Increases Circulating Leptin Levels in Truncal Vagotomized Rats. Biomedicines. 2023; 11(5):1322. https://doi.org/10.3390/biomedicines11051322
Chicago/Turabian StyleMorais, Tiago, Sofia S. Pereira, Sara Andrade, Diogo Neves, Marta Guimarães, Mário Nora, Marcos C. Carreira, Felipe F. Casanueva, and Mariana P. Monteiro. 2023. "GLP-1 Increases Circulating Leptin Levels in Truncal Vagotomized Rats" Biomedicines 11, no. 5: 1322. https://doi.org/10.3390/biomedicines11051322
APA StyleMorais, T., Pereira, S. S., Andrade, S., Neves, D., Guimarães, M., Nora, M., Carreira, M. C., Casanueva, F. F., & Monteiro, M. P. (2023). GLP-1 Increases Circulating Leptin Levels in Truncal Vagotomized Rats. Biomedicines, 11(5), 1322. https://doi.org/10.3390/biomedicines11051322






