The Effects of Antipsychotics in Experimental Models of Krabbe Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Compounds and Antibodies
2.2. Human Astrocytes Cell Culture Studies
2.3. Mouse Organotypic Cerebellar Slice Culture Studies
2.4. Twitcher Model In Vivo Studies
2.5. Statistical Analysis
3. Results
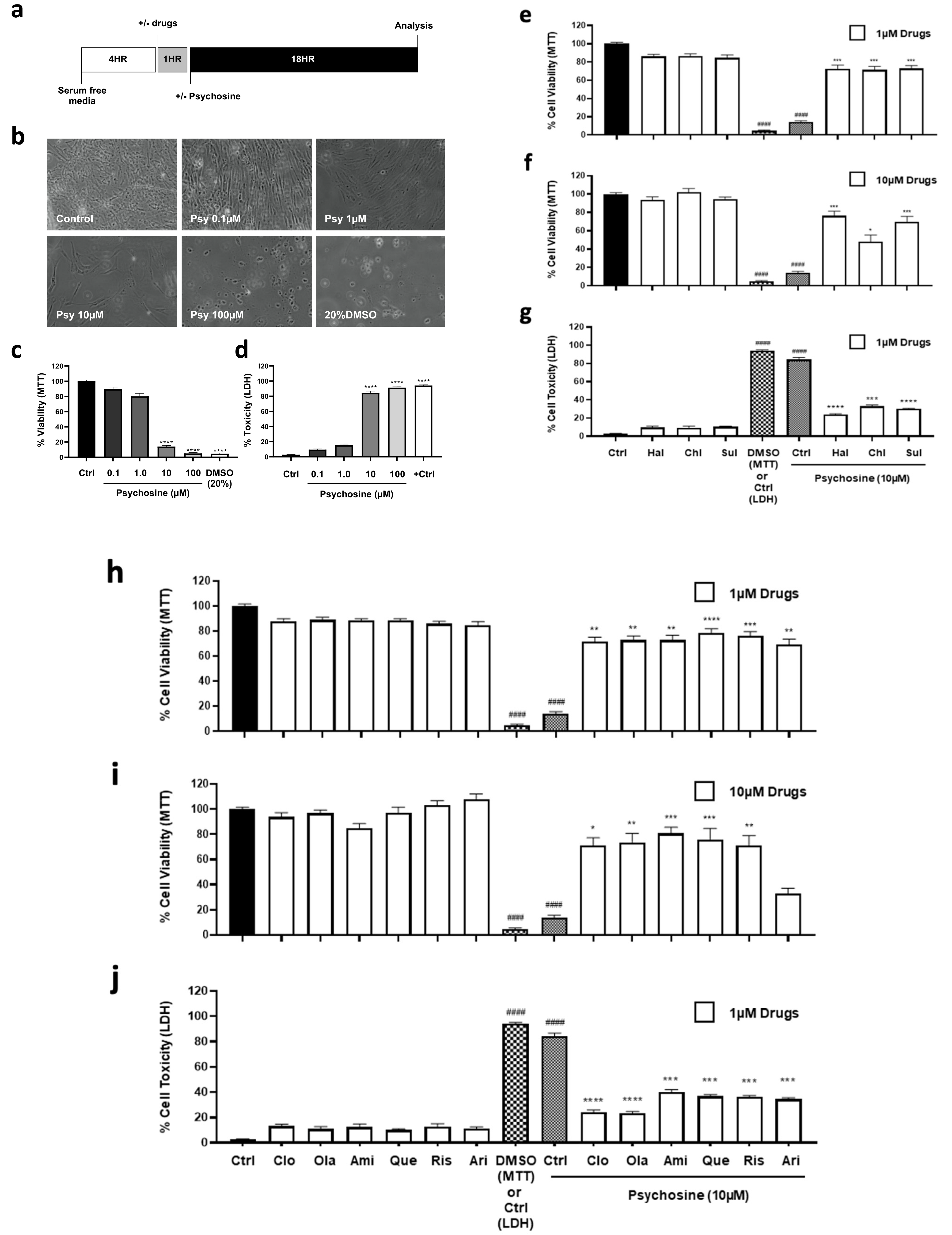
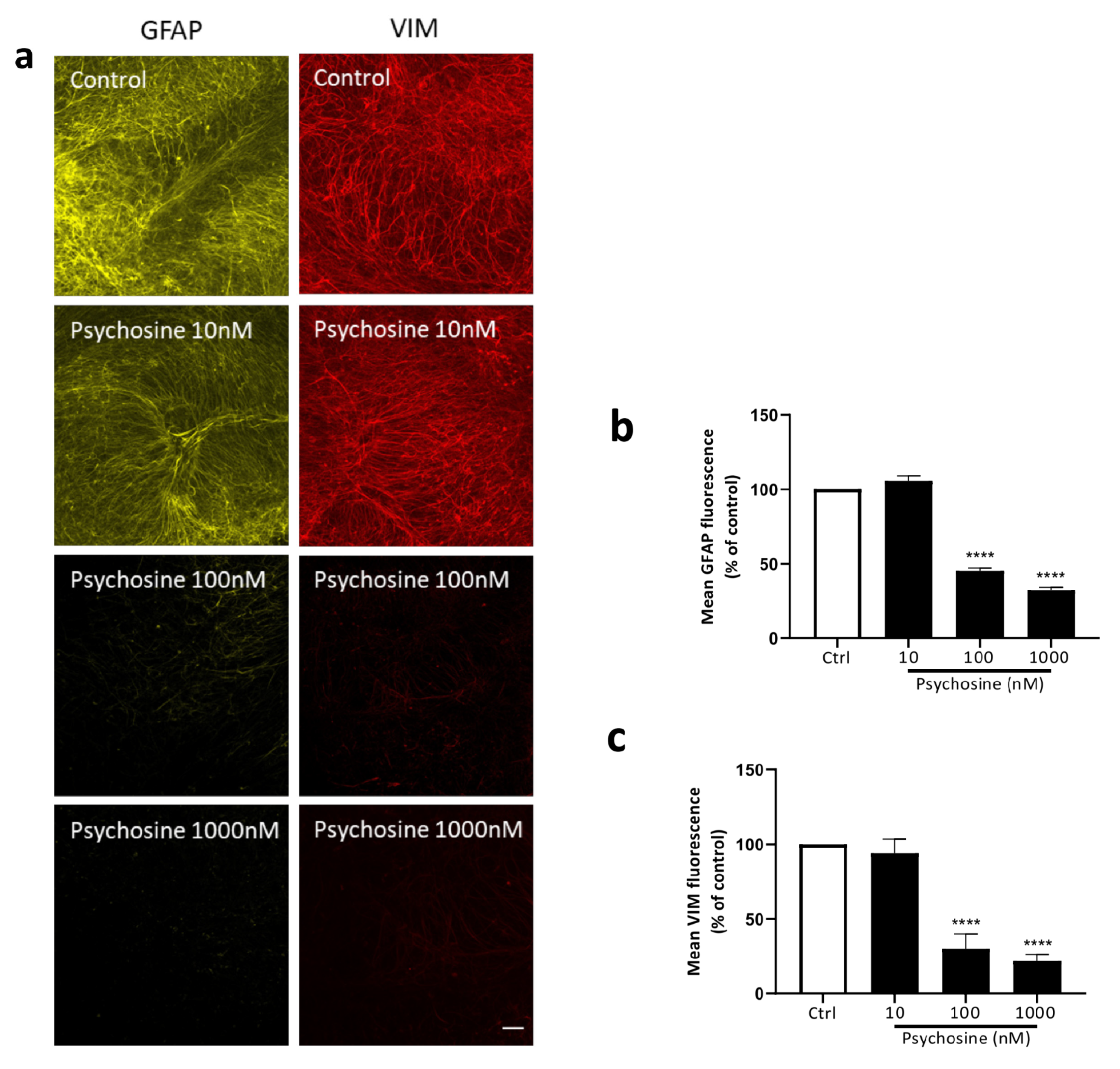
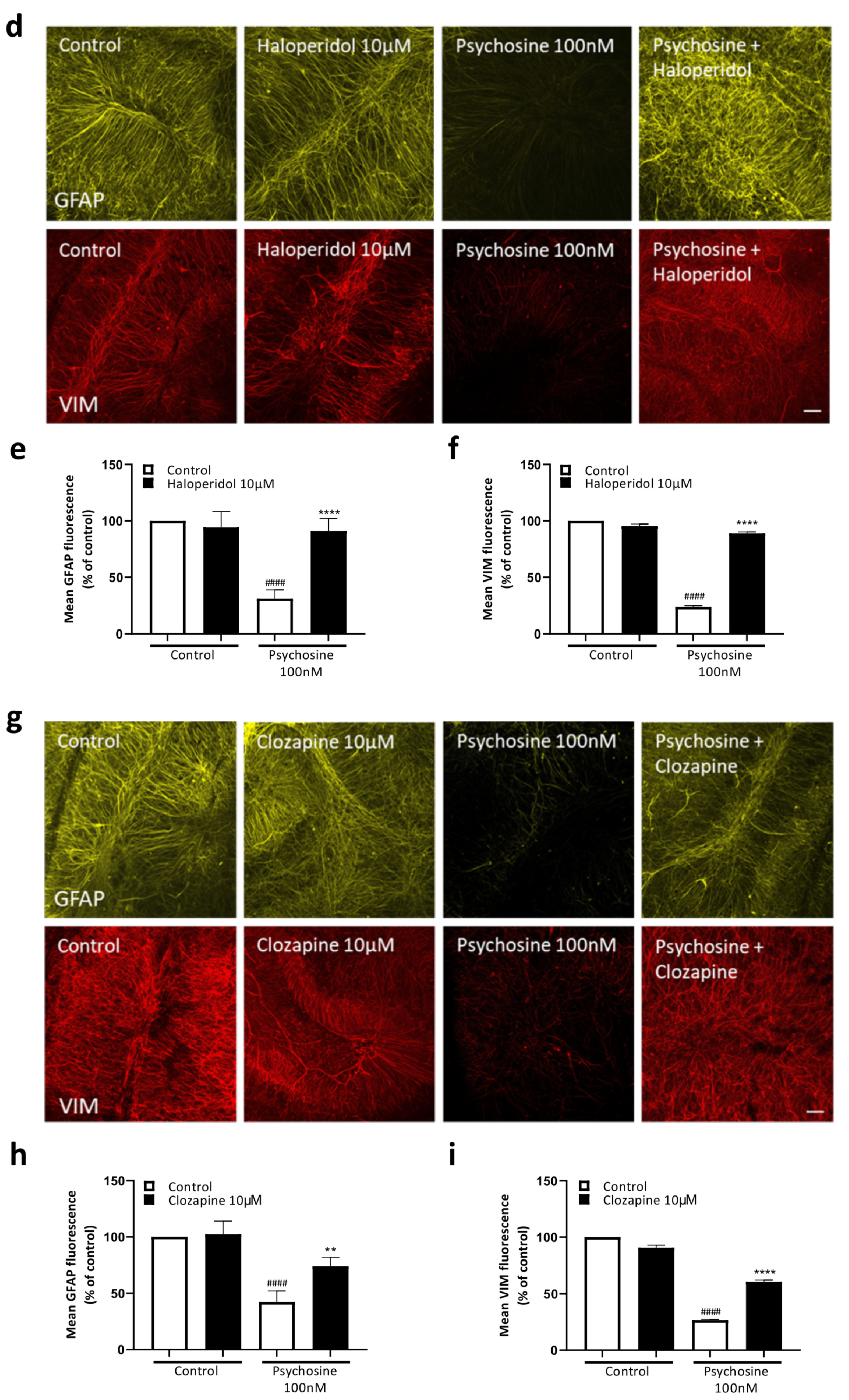
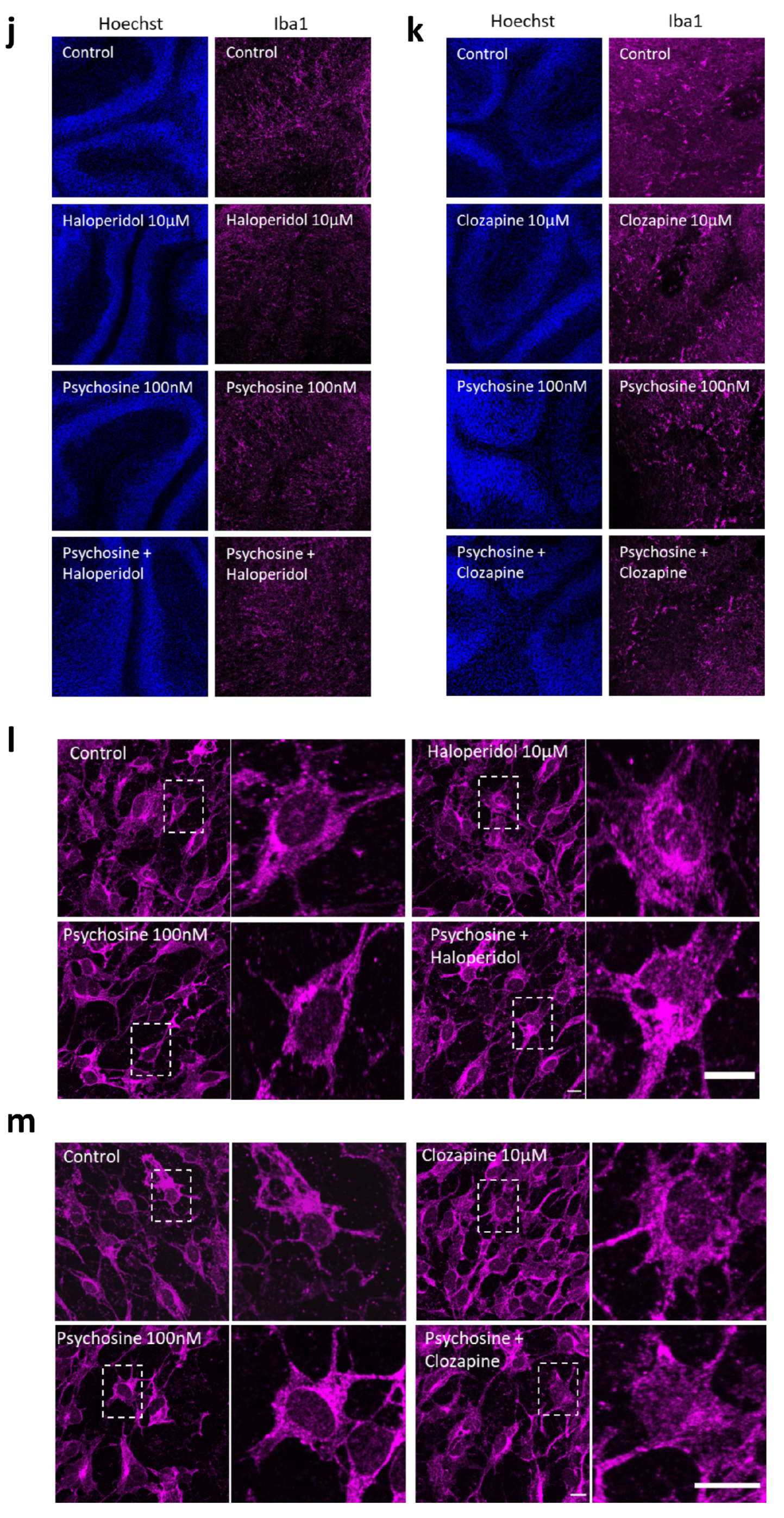
3.1. Antipsychotics Prevent Psychosine-Induced Toxicity in Human Astrocytes
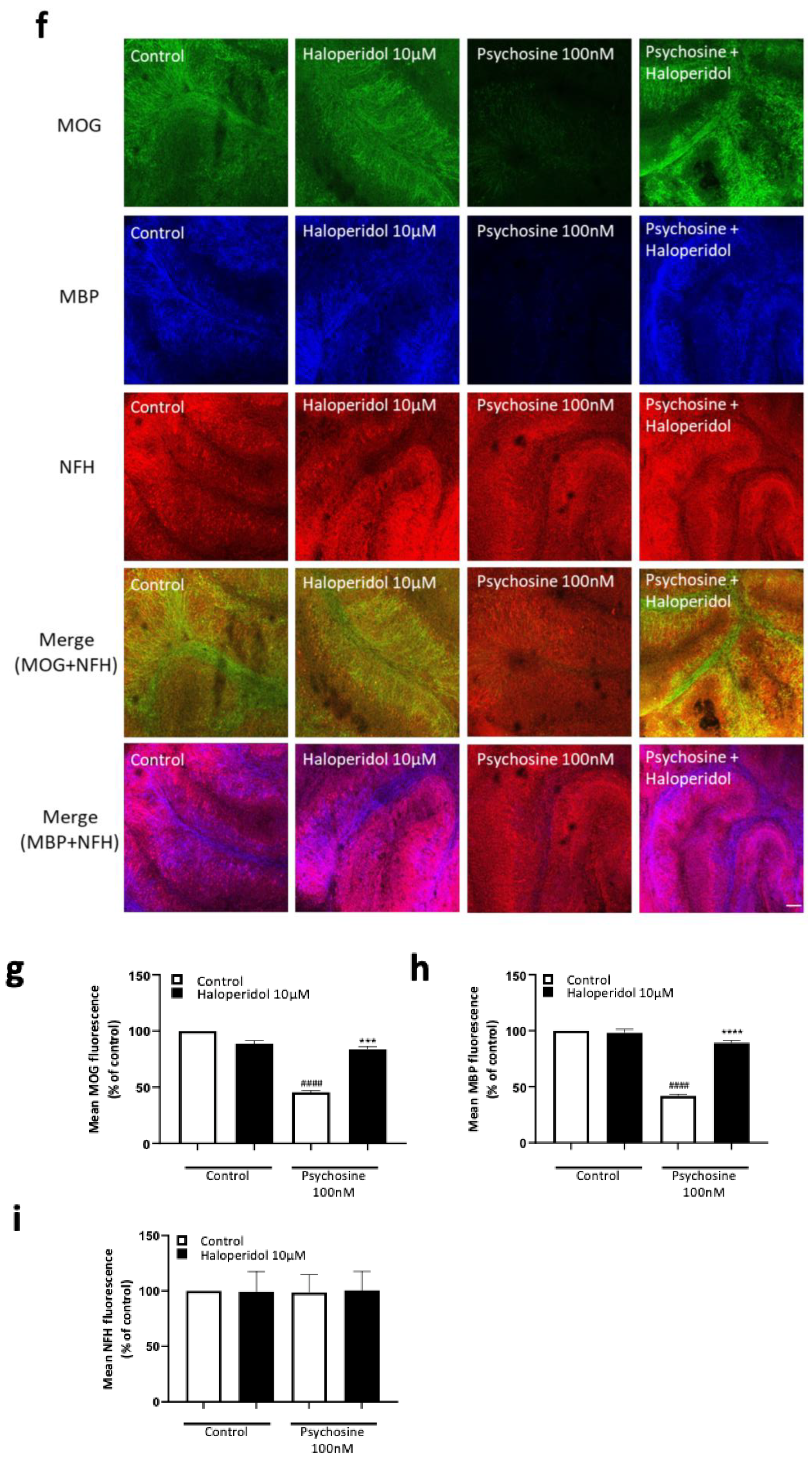
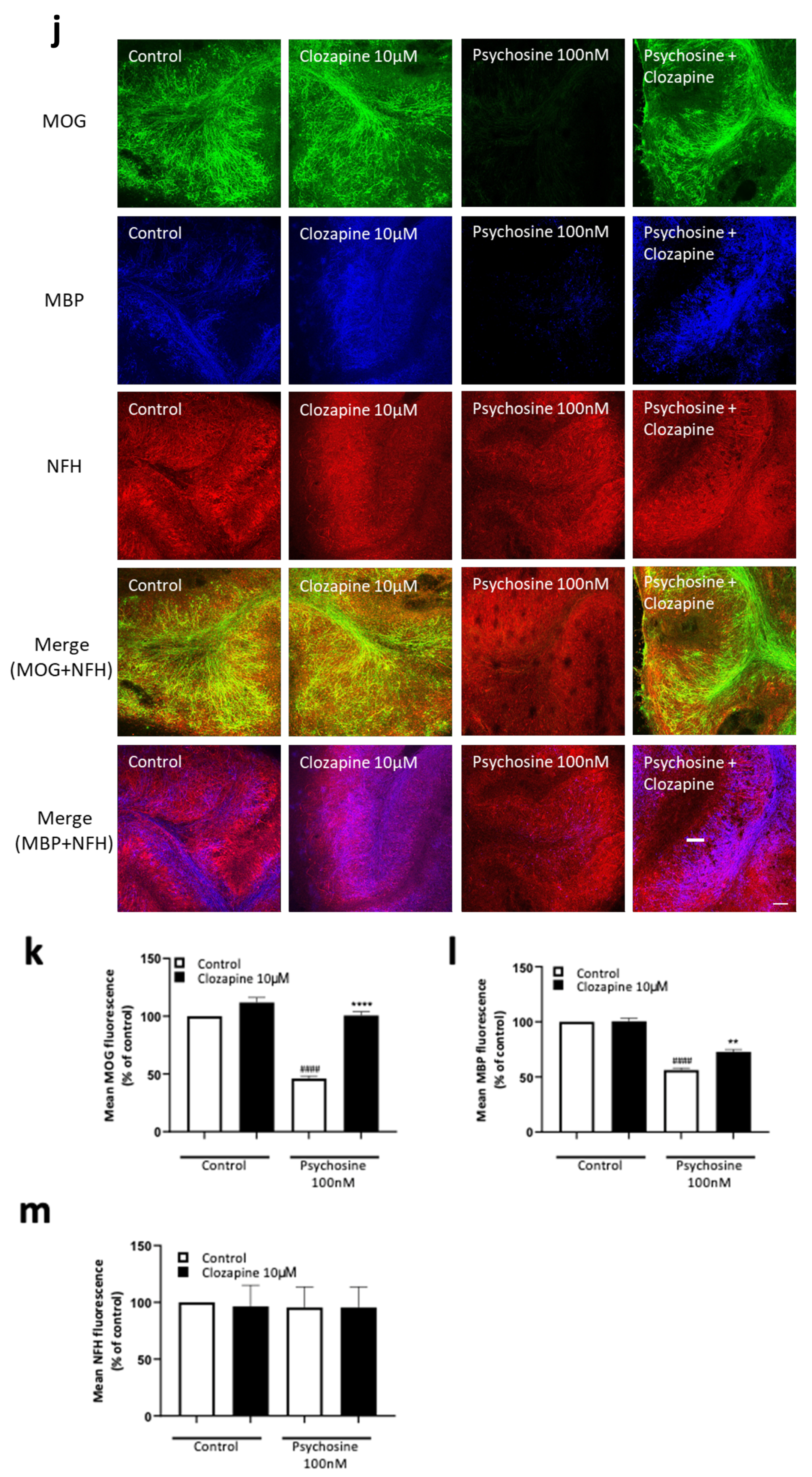
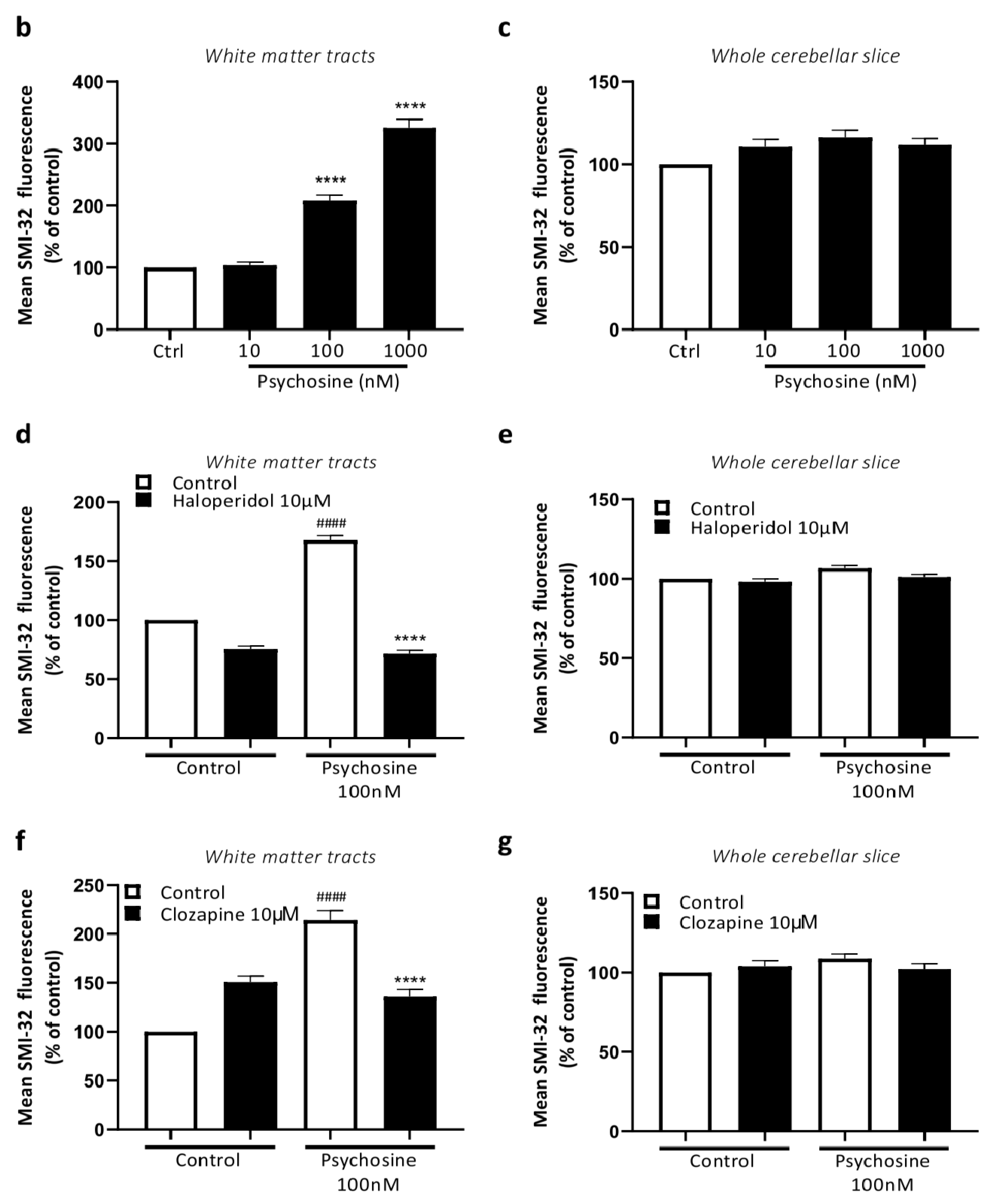
3.2. Haloperidol and Clozapine Attenuate Psychosine Induced Demyelination in Slice Cultures
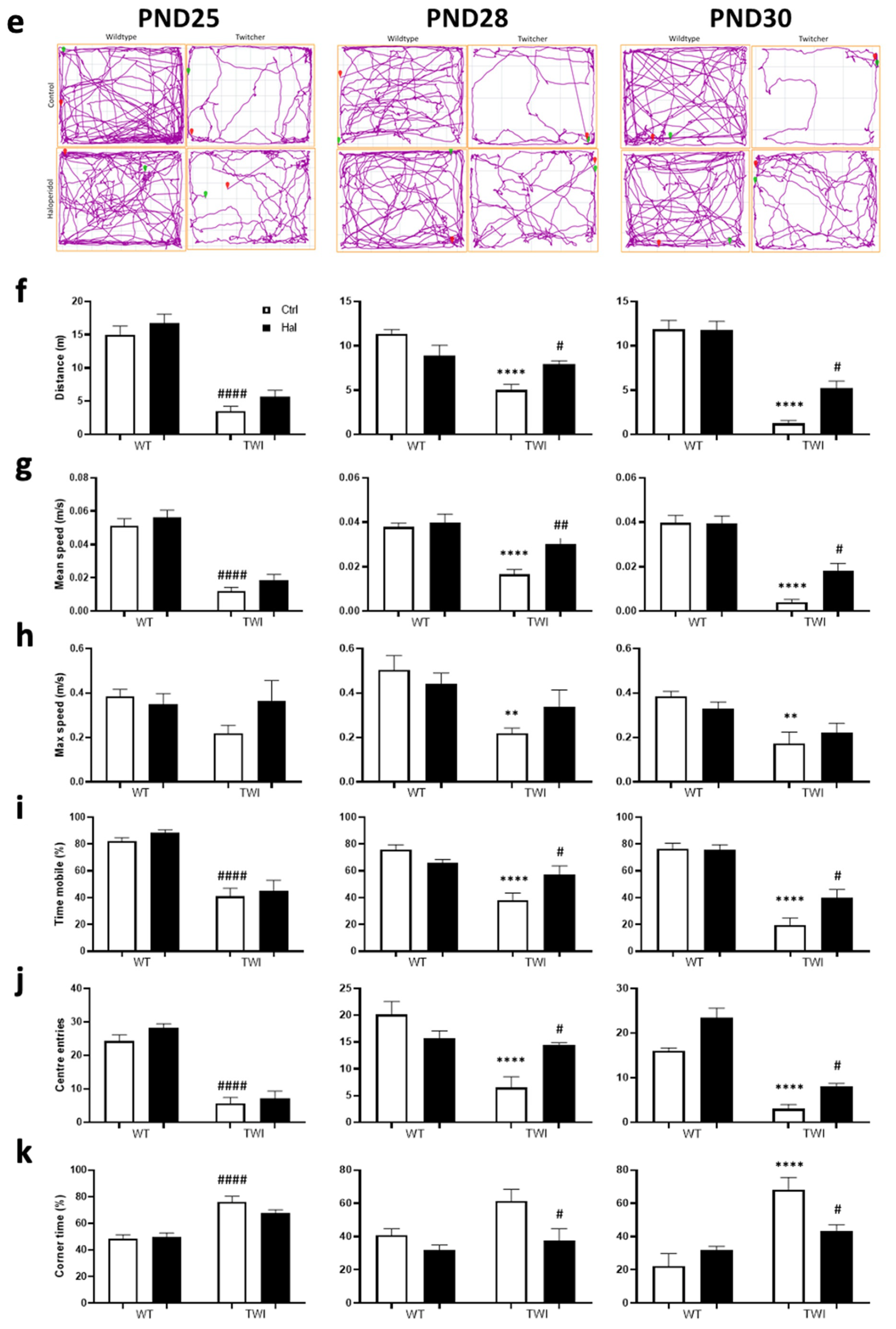
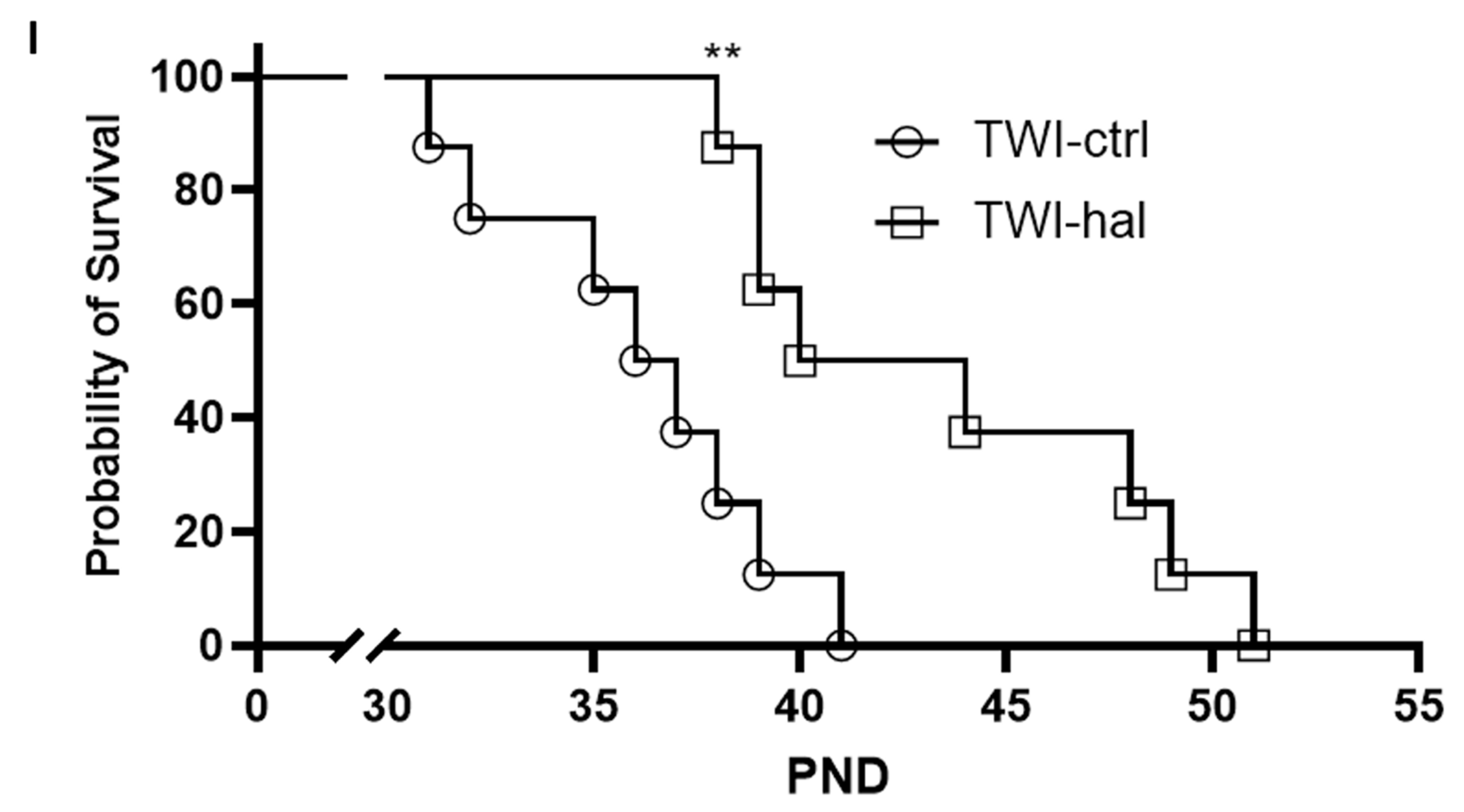
3.3. Haloperidol Improves Survival in Twitcher Mice
4. Discussion
4.1. The Regulation of Glia Cells by Antipsychotics
4.2. Psychosine Toxicity as a Model of Glial Cell Dysfunction
4.3. Limitations and Future Directions
4.4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- O’Connell, K.E.; Thakore, J.; Dev, K.K. Pro-inflammatory cytokine levels are raised in female schizophrenia patients treated with clozapine. Schizophr. Res. 2014, 156, 1–8. [Google Scholar] [CrossRef]
- Schwab, S.G.; Wildenauer, D.B. Genetics of psychiatric disorders in the GWAS era: An update on schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2013, 263, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Karlsgodt, K.H.; Sun, D.; Cannon, T.D. Structural and Functional Brain Abnormalities in Schizophrenia. Curr. Dir. Psychol. Sci. 2010, 19, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, H.G.; Steiner, J.; Guest, P.C.; Dobrowolny, H.; Bogerts, B. Glial cells as key players in schizophrenia pathology: Recent insights and concepts of therapy. Schizophr. Res. 2015, 161, 4–18. [Google Scholar] [CrossRef]
- Chew, L.-J.; Fusar-Poli, P.; Schmitz, T. Oligodendroglial alterations and the role of microglia in white matter injury: Relevance to schizophrenia. Dev. Neurosci. 2013, 35, 102–129. [Google Scholar] [CrossRef]
- Kroken, R.A.; Løberg, E.-M.; Drønen, T.; Grüner, R.; Hugdahl, K.; Kompus, K.; Skrede, S.; Johnsen, E. A critical review of pro-cognitive drug targets in psychosis: Convergence on myelination and inflammation. Front. Psychiatry 2014, 5, 11. [Google Scholar] [CrossRef]
- McCullumsmith, R.E.; Gupta, D.; Beneyto, M.; Kreger, E.; Haroutunian, V.; Davis, K.L.; Meador-Woodruff, J.H. Expression of transcripts for myelination-related genes in the anterior cingulate cortex in schizophrenia. Schizophr. Res. 2007, 90, 15–27. [Google Scholar] [CrossRef]
- Oertel-Knöchel, V.; Lancaster, T.M.; Knöchel, C.; Stäblein, M.; Storchak, H.; Reinke, B.; Jurcoane, A.; Kniep, J.; Prvulovic, D.; Mantripragada, K.; et al. Schizophrenia risk variants modulate white matter volume across the psychosis spectrum: Evidence from two independent cohorts. Neuroimage Clin. 2015, 7, 764–770. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Wang, H.; Xiao, L. Improving myelin/oligodendrocyte-related dysfunction: A new mechanism of antipsychotics in the treatment of schizophrenia? Int. J. Neuropsychopharmacol. 2013, 16, 691–700. [Google Scholar] [CrossRef]
- Ceylan, U.; Haupeltshofer, S.; Kämper, L.; Dann, J.; Ambrosius, B.; Gold, R.; Faissner, S. Clozapine Regulates Microglia and Is Effective in Chronic Experimental Autoimmune Encephalomyelitis. Front. Immunol. 2021, 12, 656941. [Google Scholar] [CrossRef]
- Templeton, N.; Kivell, B.; McCaughey-Chapman, A.; Connor, B.; La Flamme, A.C. Clozapine administration enhanced functional recovery after cuprizone demyelination. PLoS ONE 2019, 14, e0216113. [Google Scholar] [CrossRef]
- Stahl, S.M. Antipsychotics and Mood Stabilizers: Stahl’s Essential Psychopharmacology, 3rd ed.; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Rosin, C.; Colombo, S.; Calver, A.A.; Bates, T.E.; Skaper, S.D. Dopamine D2 and D3 receptor agonists limit oligodendrocyte injury caused by glutamate oxidative stress and oxygen/glucose deprivation. Glia 2005, 52, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Bongarzone, E.R.; Howard, S.G.; Schonmann, V.; Campagnoni, A.T. Identification of the dopamine D3 receptor in oligodendrocyte precursors: Potential role in regulating differentiation and myelin formation. J. Neurosci. Off. J. Soc. Neurosci. 1998, 18, 5344–5353. [Google Scholar] [CrossRef]
- Howard, S.; Landry, C.; Fisher, R.; Bezouglaia, O.; Handley, V.; Campagnoni, A. Postnatal localization and morphogenesis of cells expressing the dopaminergic D2 receptor gene in rat brain: Expression in non-neuronal cells. J. Comp. Neurol. 1998, 391, 87–98. [Google Scholar] [CrossRef]
- Lieberknecht, V.; Junqueira, S.C.; Cunha, M.P.; Barbosa, T.A.; de Souza, L.F.; Coelho, I.S.; Santos, A.R.S.; Rodrigues, A.L.S.; Dafré, A.L.; Dutra, R.C. Pramipexole, a Dopamine D2/D3 Receptor-Preferring Agonist, Prevents Experimental Autoimmune Encephalomyelitis Development in Mice. Mol. Neurobiol. 2016, 54, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Steiner, J.; Sarnyai, Z.; Westphal, S.; Gos, T.; Bernstein, H.-G.; Bogerts, B.; Keilhoff, G. Protective effects of haloperidol and clozapine on energy-deprived OLN-93 oligodendrocytes. Eur. Arch. Psychiatry Clin. Neurosci. 2011, 261, 477–482. [Google Scholar] [CrossRef]
- Béchet, S.; O’Sullivan, S.A.; Yssel, J.; Fagan, S.G.; Dev, K.K. Fingolimod Rescues Demyelination in a Mouse Model of Krabbe’s Disease. J. Neurosci. Off. J. Soc. Neurosci. 2020, 40, 3104–3118. [Google Scholar] [CrossRef]
- Elain, G.; Jeanneau, K.; Rutkowska, A.; Mir, A.K.; Dev, K.K. The selective anti-IL17A monoclonal antibody secukinumab (AIN457) attenuates IL17A-induced levels of IL6 in human astrocytes. Glia 2014, 62, 725–735. [Google Scholar] [CrossRef]
- Healy, L.M.; Sheridan, G.K.; Pritchard, A.J.; Rutkowska, A.; Mullershausen, F.; Dev, K.K. Pathway specific modulation of S1P1 receptor signalling in rat and human astrocytes. Br. J. Pharmacol. 2013, 169, 1114–1129. [Google Scholar] [CrossRef]
- Misslin, C.; Velasco-Estevez, M.; Albert, M.; O’Sullivan, S.A.; Dev, K.K. Phospholipase A2 is involved in galactosylsphingosine-induced astrocyte toxicity, neuronal damage and demyelination. PLoS ONE 2017, 12, e0187217. [Google Scholar] [CrossRef]
- Mullershausen, F.; Craveiro, L.M.; Shin, Y.; Cortes-Cros, M.; Bassilana, F.; Osinde, M.; Wishart, W.L.; Guerini, D.; Thallmair, M.; Schwab, M.E.; et al. Phosphorylated FTY720 promotes astrocyte migration through sphingosine-1-phosphate receptors. J. Neurochem. 2007, 102, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, C.; Dev, K.K. Galactosylsphingosine (psychosine)-induced demyelination is attenuated by sphingosine 1-phosphate signalling. J. Cell Sci. 2015, 128, 3878–3887. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, C.; Schubart, A.; Mir, A.K.; Dev, K.K. The dual S1PR1/S1PR5 drug BAF312 (Siponimod) attenuates demyelination in organotypic slice cultures. J. Neuroinflammation 2016, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, S.A.; O’Sullivan, C.; Healy, L.M.; Dev, K.K.; Sheridan, G.K. Sphingosine 1-phosphate receptors regulate TLR4-induced CXCL5 release from astrocytes and microglia. J. Neurochem. 2018, 144, 736–747. [Google Scholar] [CrossRef] [PubMed]
- Rutkowska, A.; O’Sullivan, S.A.; Christen, I.; Zhang, J.; Sailer, A.W.; Dev, K.K. The EBI2 signalling pathway plays a role in cellular crosstalk between astrocytes and macrophages. Sci. Rep. 2016, 6, 25520. [Google Scholar] [CrossRef]
- Rutkowska, A.; Preuss, I.; Gessier, F.; Sailer, A.W.; Dev, K.K. EBI2 regulates intracellular signaling and migration in human astrocyte. Glia 2014, 63, 341–351. [Google Scholar] [CrossRef]
- Velasco-Estevez, M.; Gadalla, K.K.; Liñan-Barba, N.; Cobb, S.; Dev, K.K.; Sheridan, G.K. Inhibition of Piezo1 attenuates demyelination in the central nervous system. Glia 2020, 68, 356–375. [Google Scholar] [CrossRef] [PubMed]
- Clementino, A.; Velasco-Estevez, M.; Buttini, F.; Sonvico, F.; Dev, K.K. Hybrid nanoparticles as a novel tool for regulating psychosine-induced neuroinflammation and demyelination in vitro and ex vivo. Neurotherapeutics 2021, 18, 2608–2622. [Google Scholar] [CrossRef]
- O’Sullivan, S.; Dev, K.K. Sphingosine-1-phosphate receptor therapies: Advances in clinical trials for CNS-related diseases. Neuropharmacology 2017, 113, 597–607. [Google Scholar] [CrossRef]
- O’Sullivan, S.A.; Dev, K.K. The chemokine fractalkine (CX3CL1) attenuates H2O2-induced demyelination in cerebellar slices. J. Neuroinflammation 2017, 14, 159. [Google Scholar] [CrossRef]
- Rutkowska, A.; Shimshek, D.R.; Sailer, A.W.; Dev, K.K. EBI2 regulates pro-inflammatory signalling and cytokine release in astrocytes. Neuropharmacology 2018, 133, 121–128. [Google Scholar] [CrossRef]
- Dietz, A.G.; Goldman, S.A.; Nedergaard, M. Glial cells in schizophrenia: A unified hypothesis. Lancet Psychiatry 2019, 7, 272–281. [Google Scholar] [CrossRef]
- Akkouh, I.A.; Hribkova, H.; Grabiec, M.; Budinska, E.; Szabo, A.; Kasparek, T.; Andreassen, O.A.; Sun, Y.M.; Djurovic, S. Derivation and Molecular Characterization of a Morphological Subpopulation of Human iPSC Astrocytes Reveal a Potential Role in Schizophrenia and Clozapine Response. Schizophr. Bull. 2022, 48, 190–198. [Google Scholar] [CrossRef]
- Fukuyama, K.; Okada, M. Effects of Atypical Antipsychotics, Clozapine, Quetiapine and Brexpiprazole on Astroglial Transmission Associated with Connexin43. Int. J. Mol. Sci. 2021, 22, 5623. [Google Scholar] [CrossRef]
- Okazaki, S.; Boku, S.; Otsuka, I.; Horai, T.; Kimura, A.; Shimmyo, N.; Yamaki, N.; Hishimoto, A. Clozapine increases macrophage migration inhibitory factor (MIF) expression via increasing histone acetylation of MIF promoter in astrocytes. J. Psychiatr. Res. 2021, 135, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Fukuyama, K.; Okubo, R.; Murata, M.; Shiroyama, T.; Okada, M. Activation of Astroglial Connexin is Involved in Concentration-Dependent Double-Edged Sword Clinical Action of Clozapine. Cells 2020, 9, 414. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Sakurai, T. Roles of glial cells in schizophrenia: Possible targets for therapeutic approaches. Neurobiol. Dis. 2013, 53, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Tanahashi, S.; Yamamura, S.; Nakagawa, M.; Motomura, E.; Okada, M. Clozapine, but not haloperidol, enhances glial D-serine and L-glutamate release in rat frontal cortex and primary cultured astrocytes. Br. J. Pharmacol. 2012, 165, 1543–1555. [Google Scholar] [CrossRef]
- Vallejo-Illarramendi, A.; Torres-Ramos, M.; Melone, M.; Conti, F.; Matute, C. Clozapine reduces GLT-1 expression and glutamate uptake in astrocyte cultures. Glia 2005, 50, 276–279. [Google Scholar] [CrossRef]
- Dorph-Petersen, K.-A.; Pierri, J.N.; Perel, J.M.; Sun, Z.; Sampson, A.R.; Lewis, D.A. The influence of chronic exposure to antipsychotic medications on brain size before and after tissue fixation: A comparison of haloperidol and olanzapine in macaque monkeys. Neuropsychopharmacol. 2005, 30, 1649–1661. [Google Scholar] [CrossRef]
- Bian, Q.; Kato, T.; Monji, A.; Hashioka, S.; Mizoguchi, Y.; Horikawa, H.; Kanba, S. The effect of atypical antipsychotics, perospirone, ziprasidone and quetiapine on microglial activation induced by interferon-γ. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2008, 32, 42–48. [Google Scholar] [CrossRef]
- Hou, Y.; Wu, C.F.; Yang, J.Y.; He, X.; Bi, X.L.; Yu, L.; Guo, T. Effects of clozapine, olanzapine and haloperidol on nitric oxide production by lipopolysaccharide-activated N9 cells. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2006, 30, 1523–1528. [Google Scholar] [CrossRef]
- Mizoguchi, Y.; Kato, T.A.; Horikawa, H.; Monji, A. Microglial intracellular Ca2+ signaling as a target of antipsychotic actions for the treatment of schizophrenia. Front. Cell. Neurosci. 2014, 8, 370. [Google Scholar] [CrossRef] [PubMed]
- Cotel, M.-C.; Lenartowicz, E.M.; Natesan, S.; Modo, M.M.; Cooper, J.D.; Williams, S.C.; Kapur, S.; Vernon, A.C. Microglial activation in the rat brain following chronic antipsychotic treatment at clinically relevant doses. Eur. Neuropsychopharmacol. 2015, 25, 2098–2107. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, B.M.; do Carmo, M.R.; Freire, R.S.; Rocha, N.F.; Borella, V.C.; de Menezes, A.T.; Monte, A.S.; Gomes, P.X.; de Sousa, F.C.; Vale, M.L.; et al. Evidences for a progressive microglial activation and increase in iNOS expression in rats submitted to a neurodevelopmental model of schizophrenia: Reversal by clozapine. Schizophr. Res. 2013, 151, 12–19. [Google Scholar] [CrossRef]
- Domercq, M.; Matute, C. Neuroprotection by tetracyclines. Trends Pharmacol. Sci. 2004, 25, 609–612. [Google Scholar] [CrossRef]
- Jeppesen, R.; Christensen, R.H.; Pedersen, E.M.; Nordentoft, M.; Hjorthøj, C.; Köhler-Forsberg, O.; Benros, M.E. Efficacy and safety of anti-inflammatory agents in treatment of psychotic disorders–A comprehensive systematic review and meta-analysis. Brain Behav. Immun. 2020, 90, 364–380. [Google Scholar] [CrossRef] [PubMed]
- Davenport, A.; Williamson, P.; Taylor, R. Pathophysiology of Krabbe disease. Orbit Univ. Syd. Undergrad. Res. J. 2011, 2–10. [Google Scholar]
- Suzuki, K. Twenty five years of the “psychosine hypothesis”: A personal perspective of its history and present status. Neurochem. Res. 1998, 23, 251–259. [Google Scholar] [CrossRef]
- Giri, S.; Khan, M.; Nath, N.; Singh, I.; Singh, A.K. The role of AMPK in psychosine mediated effects on oligodendrocytes and astrocytes: Implication for Krabbe disease. J. Neurochem. 2008, 105, 1820–1833. [Google Scholar] [CrossRef]
- Giri, S.; Khan, M.; Rattan, R.; Singh, I.; Singh, A.K. Krabbe disease: Psychosine-mediated activation of phospholipase A2 in oligodendrocyte cell death. J. Lipid Res. 2006, 47, 1478–1492. [Google Scholar] [CrossRef] [PubMed]
- Haq, E.; Giri, S.; Singh, I.; Singh, A.K. Molecular mechanism of psychosine-induced cell death in human oligodendrocyte cell line. J. Neurochem. 2003, 86, 1428–1440. [Google Scholar] [CrossRef]
- Tohyama, J.; Matsuda, J.; Suzuki, K. Psychosine Is as Potent an Inducer of Cell Death as C6-Ceramide in Cultured Fibroblasts and in MOCH-1 Cells. Neurochem. Res. 2001, 26, 667–671. [Google Scholar] [CrossRef]
- Zaka, M.; Wenger, D.A. Psychosine-induced apoptosis in a mouse oligodendrocyte progenitor cell line is mediated by caspase activation. Neurosci. Lett. 2004, 358, 205–209. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Bell, R.M. Lysosphingolipids Inhibit Protein Kinase C: Implications for the Sphingolipidoses. Science 1987, 235, 670–674. [Google Scholar] [CrossRef] [PubMed]
- White, A.B.; Galbiati, F.; Givogri, M.I.; Lopez Rosas, A.; Qiu, X.; van Breemen, R.; Bongarzone, E.R. Persistence of psychosine in brain lipid rafts is a limiting factor in the therapeutic recovery of a mouse model for Krabbe disease. J. Neurosci. Res. 2011, 89, 352–364. [Google Scholar] [CrossRef]
- Yamada, H.; Martin, P.; Suzuki, K. Impairment of protein kinase C activity in twitcher Schwann cells in vitro. Brain Res. 1996, 718, 138–144. [Google Scholar] [CrossRef]
- Cantuti-Castelvetri, L.; Zhu, H.; Givogri, M.I.; Chidavaenzi, R.L.; Lopez-Rosas, A.; Bongarzone, E.R. Psychosine induces the dephosphorylation of neurofilaments by deregulation of PP1 and PP2A phosphatases. Neurobiol. Dis. 2012, 46, 325–335. [Google Scholar] [CrossRef]
- Castelvetri, L.C.; Givogri, M.I.; Zhu, H.; Smith, B.; Lopez-Rosas, A.; Qiu, X.; van Breemen, R.; Bongarzone, E.R. Axonopathy is a compounding factor in the pathogenesis of Krabbe disease. Acta Neuropathol. 2011, 122, 35–48. [Google Scholar] [CrossRef]
- Claycomb, K.I.; Johnson, K.M.; Winokur, P.N.; Sacino, A.V.; Crocker, S.J. Astrocyte regulation of CNS inflammation and remyelination. Brain Sci. 2013, 3, 1109–1127. [Google Scholar] [CrossRef]
- Kondo, Y.; Wenger, D.A.; Gallo, V.; Duncan, I.D. Galactocerebrosidase-deficient oligodendrocytes maintain stable central myelin by exogenous replacement of the missing enzyme in mice. Proc. Natl. Acad. Sci. USA 2005, 102, 18670–18675. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, M.; Turnbull, T.; Sebastian, S.; Kempson, I. The MTT Assay: Utility, Limitations, Pitfalls, and Interpretation in Bulk and Single-Cell Analysis. Int. J. Mol. Sci. 2021, 22, 12827. [Google Scholar] [CrossRef] [PubMed]





| WTCtrl | WTHal | TWICtrl | TWIHal | WTCtr vs. WTHal | WTCtr vs. TWICtrl | WTCtr vs. TWIHal | WTHal vs. TWICtrl | WTHal vs. TWIHal | TWICtrl vs. TWIHal | |
|---|---|---|---|---|---|---|---|---|---|---|
| Metric | Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | ||||||
| No. of animals | 8 | 8 | 8 | 8 | ||||||
| Behavioural metrics for each treatment group for PND 25 | ||||||||||
| Distance (m) | 15.00 ± 1.3 | 16.81 ± 1.29 | 3.54 ± 0.70 | 5.67 ± 0.97 | ns | **** p < 0.0001 | **** p < 0.0001 | **** p < 0.0001 | **** p < 0.0001 | ns |
| Mean speed (cm/s) | 5.11 ± 0.43 | 5.61 ± 0.49 | 1.19 ± 0.24 | 1.89 ± 0.32 | ns | **** p < 0.0001 | **** p < 0.0001 | **** p < 0.0001 | **** p < 0.0001 | ns |
| Max speed (cm/s) | 38.53 ± 3.06 | 35.04 ± 4.67 | 21.88 ± 3.53 | 36.65 ± 8.90 | ns | ns | ns | ns | ns | ns |
| Time mobile (%) | 82.15 ± 2.67 | 88.33 ± 2.35 | 40.95 ± 6.07 | 45.08 ± 7.87 | ns | **** p < 0.0001 | **** p = 0.0002 | **** p < 0.0001 | **** p < 0.0001 | ns |
| Centre entries | 24.25 ± 1.88 | 28.25 ± 1.19 | 5.63 ± 1.82 | 7.25 ± 2.05 | ns | **** p < 0.0001 | **** p < 0.0001 | **** p < 0.0001 | **** p < 0.0001 | ns |
| Corner time (%) | 48.48 ± 2.86 | 49.95 ± 2.83 | 76.03 ± 4.28 | 67.60 ± 2.57 | ns | **** p < 0.0001 | *** p = 0.0013 | **** p < 0.0001 | * p = 0.003 | ns |
| Behavioural metrics for each treatment group for PND 28 | ||||||||||
| Distance (m) | 11.31 ± 0.53 | 8.92 ± 1.14 | 5.01 ± 0.65 | 7.93 ± 0.38 | ns | **** p < 0.0001 | * p = 0.0146 | ** p = 0.004 | ns | * p = 0.0412 |
| Mean speed (cm/s) | 3.78 ± 0.18 | 3.99 ± 0.38 | 1.66 ± 0.22 | 3.01 ± 0.30 | ns | **** p < 0.0001 | ns | **** p < 0.0001 | ns | *** p = 0.0099 |
| Max speed (cm/s) | 50.41 ± 6.43 | 44.26 ± 4.74 | 21.86 ± 2.38 | 33.86 ± 7.51 | ns | ** p = 0.0063 | ns | * p = 0.0407 | ns | ns |
| Time mobile (%) | 75.68 ± 3.88 | 65.89 ± 2.53 | 38.16 ± 5.27 | 57.14 ± 6.45 | ns | **** p < 0.0001 | p = 0.0477 | ** p = 0.0017 | ns | * p = 0.0412 |
| Centre entries | 20.13 ± 2.43 | 15.63 ± 1.46 | 6.50 ± 2.02 | 14.38 ± 0.46 | ns | **** p < 0.0001 | ns | ** p = 0.0052 | ns | * p = 0.0181 |
| Corner time (%) | 40.81 ± 3.99 | 32.06 ± 2.93 | 61.56 ± 6.87 | 37.53 ± 7.24 | ns | ns | ns | ** p = 0.0043 | ns | * p = 0.0241 |
| Behavioural metrics for each treatment group for PND 30 | ||||||||||
| Distance (m) | 11.87 ± 1.00 | 11.77 ± 0.10 | 1.26 ± 0.32 | 5.22 ± 0.80 | ns | **** p < 0.0001 | **** p < 0.0001 | **** p < 0.0001 | **** p < 0.0001 | * p = 0.0107 |
| Mean speed (cm/s) | 3.98 ± 0.33 | 3.95 ± 0.33 | 0.41 ± 0.11 | 1.81 ± 0.33 | ns | **** p < 0.0001 | **** p < 0.0001 | **** p < 0.0001 | *** p = 0.0001 | * p = 0.0114 |
| Max speed (cm/s) | 38.45 ± 2.32 | 33.09 ± 2.83 | 17.18 ± 5.25 | 22.28 ± 4.09 | ns | ** p = 0.0025 | * p = 0.0264 | * p = 0.0296 | ns | ns |
| Time mobile (%) | 76.66 ± 3.98 | 75.72 ± 3.81 | 19.76 ± 5.11 | 39.77 ± 6.25 | ns | **** p < 0.0001 | **** p < 0.0001 | **** p < 0.0001 | **** p < 0.0001 | * p = 0.0346 |
| Centre entries | 16.00 ± 0.66 | 23.50 ± 2.10 | 3.00 ± 1.02 | 8.00 ± 0.73 | ns | **** p < 0.0001 | *** p = 0.0007 | **** p < 0.0001 | **** p < 0.0001 | * p = 0.0439 |
| Corner time (%) | 22.24 ± 7.52 | 32.22 ± 1.92 | 68.09 ± 7.49 | 43.58 ± 3.56 | ns | **** p < 0.0001 | ns | *** p = 0.0007 | ns | * p = 0.0241 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, K.; Dev, K.K. The Effects of Antipsychotics in Experimental Models of Krabbe Disease. Biomedicines 2023, 11, 1313. https://doi.org/10.3390/biomedicines11051313
Sharma K, Dev KK. The Effects of Antipsychotics in Experimental Models of Krabbe Disease. Biomedicines. 2023; 11(5):1313. https://doi.org/10.3390/biomedicines11051313
Chicago/Turabian StyleSharma, Kapil, and Kumlesh K. Dev. 2023. "The Effects of Antipsychotics in Experimental Models of Krabbe Disease" Biomedicines 11, no. 5: 1313. https://doi.org/10.3390/biomedicines11051313
APA StyleSharma, K., & Dev, K. K. (2023). The Effects of Antipsychotics in Experimental Models of Krabbe Disease. Biomedicines, 11(5), 1313. https://doi.org/10.3390/biomedicines11051313






