A Pilot Study on Oxidative Stress during the Recovery Phase in Critical COVID-19 Patients in a Rehabilitation Facility: Potential Utility of the PAOT® Technology for Assessing Total Anti-Oxidative Capacity
Abstract
1. Introduction
2. Materials and Methods
- (a)
- Patient population
- (b)
- Assays for measuring OSS biomarkers
- (C)
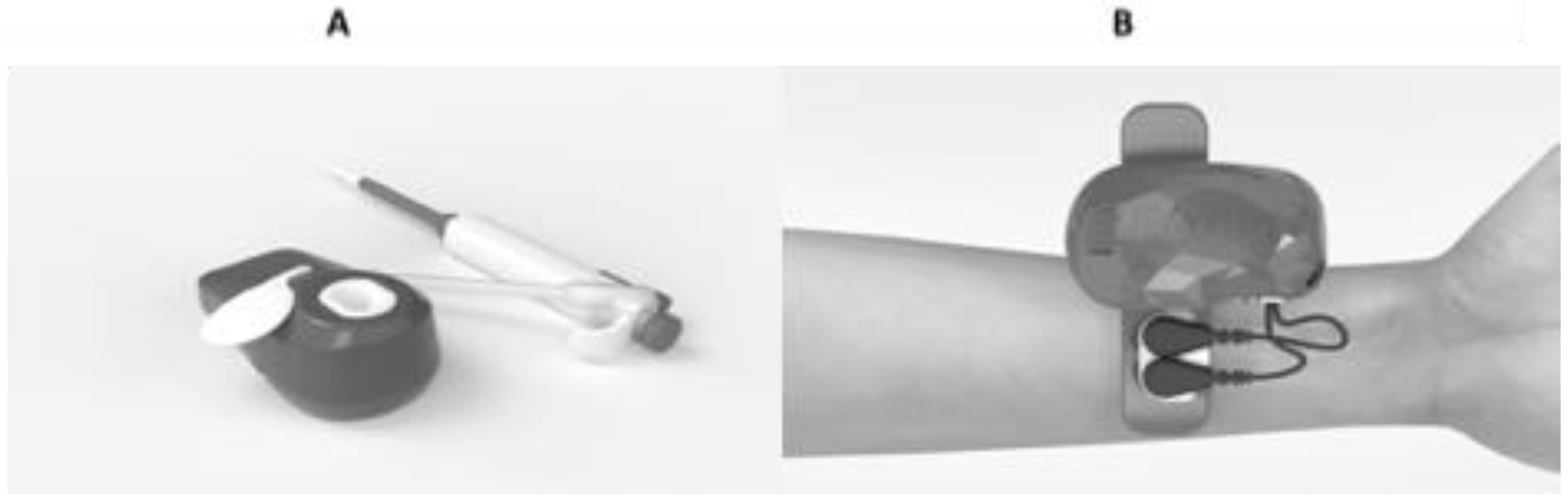
- PAOT®-score determination
Statistical Analysis
3. Results
4. Discussion
- (a)
- Comparison between Plasma OSS Biomarker Levels in Study Population and the Previous Results
- a.1.
- Antioxidants
- a.2.
- Trace Elements
- a.3.
- Oxidative Damage to Lipids
- a.4.
- Inflammation Biomarkers
- (b)
- Correlations between OS Plasma Biomarkers
- (c)
- PAOT®-Scores and Correlations with OS Plasma Biomarkers
5. Limitations of the Study
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sies, H. (Ed.) Oxidative stress: Introductory remarks. In Oxidative Stress; Academic Press: London, UK, 1985; pp. 1–8. [Google Scholar]
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.F.; Harakeh, S.; et al. Oxidative Stress in Human Pathology and Aging: Molecular Mechanisms and Perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef]
- Checa, J.; Aran, J.M. Reactive oxygen species: Drivers of physiological and pathological processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef]
- Jones, D.P. Redefining oxidative stress. Antioxid. Redox Signal. 2006, 8, 1865–1879. [Google Scholar] [CrossRef]
- Nitti, M.; Marengo, B.; Furfaro, A.L.; Pronzato, M.A.; Marinari, U.M.; Domenicotti, C.; Traverso, N. Hormesis and oxidative distress: Pathophysiology of reactive oxygen species and the open question of antioxidant modulation and supplementation. Antioxidants 2022, 11, 1613. [Google Scholar] [CrossRef] [PubMed]
- Steven, S.; Frenis, K.; Oelze, M.; Kalinovic, S.; Kuntic, M.; Jimenez, M.T.; Vujacic-Mirski, K.; Helmstädter, J.; Kröller-Schön, S.; Münzel, T.; et al. Vascular inflammation and oxidative stress: Major triggers for cardiovascular disease. Oxid. Med. Cell. Longev. 2019, 2019, 7092151. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Prather, E.R.; Stetskiv, M.; Garrison, D.E.; Meade, J.R.; Peace, T.I.; Zhou, T. Inflammaging and oxidative stress in human diseases: From molecular mechanisms to novel treatments. Int. J. Mol. Sci. 2019, 20, 4472. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, C.A.; Acosta, O. Inflammatory and oxidative stress in rotavirus infection. World J. Virol. 2016, 5, 38–62. [Google Scholar] [CrossRef]
- Zhang, H.; Penninger, J.M.; Li, Y.; Zhong, N.; Slutsky, A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: Molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020, 46, 586–590. [Google Scholar] [CrossRef]
- Cavezzi, A.; Troiani, E.; Corrao, S. COVID-19: Hemoglobin, iron, and hypoxia beyond inflammation. A narrative review. Clin. Pract. 2020, 10, 1271. [Google Scholar] [CrossRef]
- Cecchini, R.; Cecchini, A.L. SARS-CoV-2 infection pathogenesis is related to oxidative stress as a response to aggression. Med. Hypotheses 2020, 143, 110102. [Google Scholar] [CrossRef]
- Otifi, H.M.; Adiga, B.K. Endothelial dysfunction in COVID-19 infection. Am. J. Med. Sci. 2022, 363, 281–287. [Google Scholar] [CrossRef]
- Fernandes, I.G.; de Brito, C.A.; Silva dos Reis, V.M.; Sato, M.N.; Pereir, N.Z. SARS-CoV-2 and other respiratory viruses: What does oxidative stress have to do with it? Oxid. Med. Cell. Longev. 2020, 2020, 1–13. [Google Scholar] [CrossRef]
- Geng, Y.-J.; Wei, Z.-H.; Qian, H.-Y.; Huay, J.; Lodato, R.; Castriotta, R.J. Pathophysiological characteristics and therapeutic approaches for pulmonary injury and cardiovascular complications of coronavirus disease. Cardiovasc. Pathol. 2020, 47, 107228. [Google Scholar] [CrossRef]
- Holmes, A.; O’Connor, R.C.; Perry, H.; Tracey, I.; Wessely, S.; Arseneault, L.; Ballard, C.; Christensen, H.; Cohen Silver, R.; Everall, I.; et al. Multidisciplinary research priorities for the COVID-19 pandemic: A call for mental health science. Lancet Psychiatry 2020, 7, 547–560. [Google Scholar] [CrossRef]
- Haute Autorité de la Santé (HAS) Board. Rapid Responses in the Context of COVID-19—Management of COVID+ Patients in Physical Medicine and Rehabilitation (MPR), and on Return Home; Haute Autorité de la Santé (HAS): Saint Denis, France, 2020. [Google Scholar]
- Rousseau, A.-F.; Minguet, P.; Colson, C.; Kellens, I.; Chaabane, S.; Delanaye, P.; Cavalier, E.; Chase, J.G.; Lambermont, B.; Misset, B. Post-intensive care syndrome after a critical COVID-19: Cohort study from a Belgian follow-up clinic. Ann. Intensive Care. 2021, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Joris, M.; Minguet, P.; Colson, C.; Joris, J.; Fadeur, M.; Minguet, G.; Guiot, J.; Misset, B.; Rousseau, A.-F. Cardiopulmonary exercise testing in critically ill coronavirus disease 2019 survivors: Evidence of a sustained exercise intolerance and hypermetabolism. Crit. Care Explor. 2021, 3, e0491. [Google Scholar] [CrossRef] [PubMed]
- Park, S.P.; Kim, S.O.; Lee, Y.C. Impact of oxidative stress on lung diseases. Respirology 2009, 14, 27–38. [Google Scholar] [CrossRef]
- D’Oria, R.; Schipani, R.; Leonardi, A.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. The role of oxidative stress in cardiac disease: From physiological response to injury factor. Oxid. Med. Cell. Longev. 2020, 2020, 5732956. [Google Scholar] [CrossRef] [PubMed]
- Daenen, K.; Andries, A.; Mekahli, D.; Van Schepdael, A.; Jouret, F.; Bammens, B. Oxidative stress in chronic kidney disease. Pediatr. Nephrol. 2019, 34, 975–991. [Google Scholar] [CrossRef] [PubMed]
- Berr, C.; Balansard, B.; Arnaud, J.; Roussel, A.-M.; Alpérovitch, A. Cognitive decline is associated with systemic oxidative stress: The EVA Study. J. Am. Geriatr. Soc. 2015, 48, 1285–1291. [Google Scholar] [CrossRef]
- Pincemail, J.; Cavalier, E.; Charlier, C.; Cheramy-Bien, J.-P.; Brevers, E.; Courtois, A.; Fadeur, M.; Meziane, S.; Le Goff, C.; Misset, B.; et al. Oxidative stress status in COVID-19 patients hospitalized in intensive care unit for severe pneumonia. A pilot study. Antioxidants 2021, 10, 257. [Google Scholar] [CrossRef] [PubMed]
- Holford, P.; Carr, A.C.; Zawari, M.; Vizcaychipi, M.P. Vitamin C intervention for critical COVID-A pragmatic review of the current level of evidence. Life 2021, 11, 1166. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, A.; Heller, R.A.; Sun, Q.; Seelig, J.; Cherkezov, A.; Seibert, L.; Hackler, J.; Seemann, P.; Diegmann, J.; Pilz, M.; et al. Selenium deficiency is associated with mortality risk from COVID-19. Nutrients 2020, 12, 2098. [Google Scholar] [CrossRef] [PubMed]
- Martín-Fernandez, M.; Aller, R.; Heredia-Rodríguez, M.; Gomez-Sanchez, M.; Martínez-Paz, P.; Gonzalo-Benito, H.; Sanchez-de Prada, L.; Gorgojo, O.; Carnicero-Frutos, I.; Tamayo, E.; et al. Lipid peroxidation as a hallmark of severity in COVID-19 patients. Redox Biol. 2021, 48, 102181. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, I.G.; Apetre, C. Analytical methods U-used in D-determining antioxidant activity: A review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Carrion-Garcia, C.J.; Guerra-Hernandez, E.J.; Garcia-Villanova, B.; Serafini, M.; Sanchez, M.-J.; Amiano, P.; Molina-Montes, E. Plasma non-enzymatic antioxidant capacity (NEAC) in relation to dietary NEAC, nutrient antioxidants and inflammation-related biomarkers. Antioxidants 2020, 9, 301. [Google Scholar] [CrossRef]
- Bartosz, G. Non-enzymatic capacity assays: Limitations of use in medicine. Free Radic. Res. 2010, 44, 711–720. [Google Scholar] [CrossRef]
- Pincemail, J.; Cillard, J.; Nève, J.; Defraigne, J.-O. Mesure de la capacité antioxydante globale du plasma: Une revue critique. Ann. Biol. Clin. 2014, 72, 413–421. [Google Scholar]
- Le Gall, J.R.; Lemeshow, S.; Saulnier, F. A new simplified acute physiology score (SAPS II) based on a European/North American multicenter study. JAMA 1993, 270, 2957–2963. [Google Scholar] [CrossRef]
- Omaye, S.T.; Tumbull, J.D.; Sauerlich, H.E. Selected methods for the determination of ascorbic acid in animal cells, tissues and fluids. Methods Enzym. 1979, 62, 3–11. [Google Scholar]
- Zhao, B.; Tham, S.Y.; Lu, J.; Lai, M.H.; Lee, L.; Moochhala, S.M. Simultaneous determination of vitamins C, E and carotene in human plasma by high-performance liquid chromatography with photodiode-array detection. J. Pharm. Pharm. Sci. 2004, 7, 200–204. [Google Scholar]
- Gey, K.J. Optimum plasma levels of antioxidant micronutrients—Ten years of antioxidant hypothesis on atherosclerosis. New Asp. Nutr. Status 1994, 51, 84–89. [Google Scholar]
- Butterworth, P.H.; Baum, H.; Porter, J.W. A modification of the Ellman procedure for the estimation of protein sulfhydryl groups. Arch. Biochem. Biophys. 1967, 118, 716–723. [Google Scholar] [CrossRef]
- Sturup, S.; Hayes, R.B.; Peters, U. Development and application of a simple routine for the determination of selenium in serum by octopole reaction with ICPMS. Anal. Bioanal. Chem. 2005, 381, 686–694. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institut (Ed.) Defining, Stabling and Verifying Reference Intervals in the Clinical Laboratory; Approved Guideline, 3rd ed.; EP-28-A3c, 28, n°30; Clinical and Laboratory Standards Institut: Wayne, PA, USA, 2010. [Google Scholar]
- Pincemail, J.; Le Goff, C.; Charlier, C.; Gillion, P.; Cheramy-Bien, J.-P.; Van Honacker, E.; Chapelle, J.-P.; Defraigne, J.-O. Evaluation biologique du stress oxydant. Application en routine clinique. Nutr. Et Endocrinol. 2010, Édition Spéciale, 16–31. [Google Scholar]
- Pincemail, J.; Defraigne, J.O.; Cheramy-Bien, J.P.; Dardenne, N.; Donneau, A.F.; Albert, A.; Labropoulos, N.; Sakalihasan, N. On the potential increase of the oxidative stress status in patients with abdominal aortic aneurysm. Redox Rep. 2012, 17, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Turki, A.; Hayot, M.; Carnac, G.; Pillard, F.; Passerieux, E.; Bommart, S.; de Mauverger, E.R.; Hugon, G.; Pincemail, J.; Pietri, S.; et al. Functional muscle impairment in facioscapulohumeral muscular dystrophy is correlated with oxidative stress and mitochondrial dysfunction. Free Radic. Biol. Med. 2012, 53, 1068–1079. [Google Scholar] [CrossRef] [PubMed]
- Pincemail, J.; Kaci, M.-M.; Kevers, C.; Tabart, J.; Elle, R.E.; Meziane, S. PAOT-Liquid® Technology: An easy electrochemical method for evaluating antioxidant capacity of wines. Diseases 2019, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Kaci, M.; Belhaffef, A.; Meziane, S.; Dostert, G.; Menu, P.; Velot, E.; Desobry, S.; Arab-Tehranv, E. Nanoemulsions and topical creams for the safe and effective delivery of lipophilic antioxidant coenzyme Q10. J. Food Process Technol. 2016, 7, 12. [Google Scholar] [CrossRef]
- Pincemail, J.; Kaci, M.-M.; Cheramy-Bien, J.-P.; Defraigne, J.-O. Electrochemical methodology for evaluating skin oxidative stress status (OSS). Diseases 2019, 7, 40. [Google Scholar] [CrossRef]
- Siegel, S. Nonparametric Statistics for the Behavioral Sciences; McGraw-Hill: New York, NY, USA, 1956; pp. 36–42, 250. [Google Scholar]
- Lindstrom, D.P.; Murray, R.P. Schaum’s Easy Outline of Statistics, 2nd ed.; McGraw-Hill Education: New York, NY, USA, 2010. [Google Scholar]
- Mukaka, M.M. A guide to appropriate use of correlation coefficient in medical research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar]
- Karkhanei, E.; Ghane, E.T.; Mehri, F. Evaluation of oxidative stress level: Total antioxidant capacity, total oxidant status and glutathione activity in patients with COVID-19. New Microbes New Infect. 2021, 42, 100897. [Google Scholar] [CrossRef]
- Muhammad, Y.; Kani, Y.A.; Iliya, S.; Muhammad, J.B.; Binji, A.; El-Fulaty Ahmad, A.; Kabir, M.B.; Umar Bindawa, K.; Ahmed, A.U. Deficiency of antioxidants and increased oxidative stress in COVID-19 patients: A cross-sectional comparative study in Jigawa, Northwestern Nigeria. SAGE Open Med. 2021, 9, 2050312121991246. [Google Scholar] [CrossRef] [PubMed]
- Bartolini, D.; Stabile, A.M.; Bastianelli, S.; Giustarini, D.; Pierucci, S.; Busti, C.; Vacca, C.; Gidari, A.; Francisci, D.; Castronari, R.; et al. SARS-2-CoV2 infection impairs the metabolism and redox function of cellular glutathione. Redox Biol. 2021, 45, 102041. [Google Scholar] [CrossRef]
- Cekerevac, I.; Turnic, T.N.; Draginic, N.; Andjic, M.; Zivkovic, V.; Simovic, S.; Susa, R.; Novkovic, L.; Mijailovic, Z.; Andjelkovic, M.; et al. Predicting severity and intrahospital mortality in COVID-19; the place and role of oxidative stress. Oxidative Med. Cell. Longev. 2021, 2021, 6615787. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.; Khurasany, K.; Nguyen, T.; Kim, J.; Guilford, F.; Mehta, R.; Gray, D.; Saviola, B.; Venketaraman, V. Glutathione and infection. Biochem. Biophys. Acta 2013, 1830, 3329–3349. [Google Scholar] [CrossRef]
- Wayner, D.D.M.; Burton, G.W.; Ingold, K.U.; Barclay, L.R.C.; Locke, S.J. The relative contributions of vitamin E, urate, ascorbate and proteins to the total peroxyl radical-trapping antioxidant capacity of human blood plasma. BBA 1987, 924, 408–419. [Google Scholar]
- Vlasova, I.I.; Sokolov, A.V.; Kostevich, V.A.; Mikhalchik, E.V.; Vasilyev, V.B. Myeloperoxidase-induced oxidation of albumin and ceruloplasmin: Role of Tyrosines. Biochemistry 2019, 84, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.D.; Cooney, R.V. The potential physiological role of γ-tocopherol in human health: A qualitative review. Nutr. Cancer 2020, 72, 808–825. [Google Scholar] [CrossRef]
- Lindblad, M.; Tveden-Nyborg, P.; Lykkesfeldt, J. Regulation of vitamin C homeostasis during deficiency. Nutrients 2013, 5, 2860–2879. [Google Scholar] [CrossRef]
- McKay, G.J.; Lyner, N.; Linden, G.; Kee, F.; Moitry, M.; Biasch, K.; Amouyel, P.; Dallongeville, J.; Bongard, V.; Ferrières, J.; et al. Association of low plasma antioxidant levels with all-cause mortality and coronary events in healthy middle-aged men from France and Northern Ireland in the PRIME study. Eur. J. Nutr. 2021, 60, 2631–2641. [Google Scholar] [CrossRef] [PubMed]
- Duthie, S.J.; Duthie, G.G.; Russell, W.R.; Kyle, J.A.M.; Macdiarmid, J.I.; Rungapamestry, V.; Stephen, S.; Megias-Baeza, C.; Kaniewska, J.J.; Shaw, L.; et al. Effect of increasing fruit and vegetable intake by dietary intervention on nutritional biomarkers and attitudes to dietary change: A randomised trial. Eur. J. Nutr. 2018, 57, 1855–1872. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.; Eck, P.; Vitamin, C. Working on the x-axis1,2. Am. J. Clin. Nutr. 2009, 90, 1121–1123. [Google Scholar] [CrossRef]
- Saldeen, K.; Saldeen, T. Importance of tocopherols beyond α-tocopherol: Evidence from animal and human studies. Nutr. Res. 2005, 25, 877–889. [Google Scholar] [CrossRef]
- Jiang, Q. Natural forms of vitamin E: Metabolism, antioxidant and anti-inflammatory activities and the role in disease prevention 543 and therapy. Free Radic. Biol. Med. 2014, 72, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Deth, R.; Clarke, A.; Ni, J.; Trivedi, M. Clinical evaluation of glutathione concentrations after consumption of milk containing different subtypes of β-casein: Results from a randomized, cross-over clinical trial. Nutr. J. 2016, 15, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Puto, C.A. N-Acetylcysteine to combat COVID-19: An evidence review. Ther. Clin. Risk Manag. 2020, 16, 1047–1055. [Google Scholar] [CrossRef]
- Huang, Z.; Rose, A.H.; Hoffmann, P.R. The role of selenium in inflammation and immunity: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2012, 16, 705–743. [Google Scholar] [CrossRef]
- Bomer, N.; Beverborg, N.G.; Hoes, M.F.; Streng, K.W.; Vermeer, M.; Dokter, M.M.; IJmker, J.; Anker, S.D.; Cleland, J.G.F.; Hillege, H.L.; et al. Selenium and outcome in heart failure. Eur. J. Heart Fail. 2022, 22, 1415–1423. [Google Scholar] [CrossRef]
- Steinbrenner, H.; Duntas, L.H.; Rayman, M.P. The role of selenium in type-2 diabetes mellitus and its metabolic comorbidities. Redox Biol. 2022, 50, 102236. [Google Scholar] [CrossRef]
- Gaetke, L.M.; Chow, C.K. Copper toxicity, oxidative stress and antioxidant nutrients. Toxicology 2003, 189, 147–163. [Google Scholar] [CrossRef]
- Jarosz, M.; Olbert, M.; Wyszogrodzka, G.; Młyniec, K.; Librowski, T. Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-κB signaling. Inflammopharmacology 2017, 25, 11–24. [Google Scholar] [CrossRef]
- Mezzetti, A.; Pierdomenico, S.D.; Costantini, F.; Romano, F.; De Cesare, D.; Cuccurullo, F.; Imbastaro, T.; Riario-Sforza, G.; Di Giacomo, F.; Zuliani, G.; et al. Copper/zinc ratio and systemic oxidant load: Effect of aging and aging-related degenerative diseases. Free Radic. Biol. Med. 1998, 25, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.-H.; Chen, P.-C.; Yeh, M.-S.; der-Yun, H.; Wang, C.-L. Cu/Zn Ratios are associated with nutritional status, oxidative stress, inflammation and Immune abnormalities in patients on peritoneal dialysis. Clin. Biochem. 2011, 44, 275–280. [Google Scholar] [CrossRef]
- Ozturk, P.; Kurutos, E.B.; Ataseven, A. Copper/zinc and copper/selenium ratios, and oxidative stress as biochemical markers in recurrent aphthous stomatitis. J. Trace Elem. Med. Biol. 2013, 27, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.H.; Wang, C.L. Effects of zinc supplementation on plasma Copper/Zinc ratios, oxidative stress, and immunological status in hemodialysis patients. Int. J. Med. Sci. 2013, 10, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.D. Protein oxidation and peroxidation. Biochem. J. 2016, 473, 805–825. [Google Scholar] [CrossRef]
- Moore, K.; Roberts, J. Measurement of lipid peroxidation. Free Radic. Res. 1998, 28, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Singh, V.; Maniar, K.; Chawdhary, S.; Bhattacharyya, R.; Banerjee, D. Plasma hydrogen peroxide: A myth or reality? Int. J. Clin. Biochem. 2019, 34, 118–119. [Google Scholar] [CrossRef]
- Ganini, D.; Canistro, D.; Jang, J.; Sadler, K.; Mason, R.P.; Kadiiska, M.B. Ceruloplasmin (ferroxidase) oxidized hydroxylamine probes: Deceptive implications for free radical detection. Free Radic. Biol. Med. 2012, 53, 1514–1521. [Google Scholar] [CrossRef]
- Savic-Radojevic, A.; Pljesa-Ercegovac, M.; Matic, M.; Simic, D.; Radovanovic, S.; Simic, T. Chapter Four—Novel Biomarkers of Heart Failure. Adv. Clin. Chem. 2017, 79, 93–152. [Google Scholar]
- Ehrenwald, E.; Chislom, G.M.; Fox, P.L. Intact ceruloplasmin oxidatively modifies low density lipoprotein. J. Clin. Investig. 1994, 93, 1493–1501. [Google Scholar] [CrossRef] [PubMed]
- Ozgünes, H.; Gürer, H.; Tuner, S. Correlation between plasma malondialdehyde and ceruloplasmin activity values in rheumatoid arthritis. Clin. Biochem. 1995, 28, 193–194. [Google Scholar] [CrossRef]
- Gianazza, E.; Brioschi, M.; Fernandez, A.F.; Casalnuovo, F.; Altomare, A.; Aldini, G.; Banfi, C. Lipid Peroxidation in Atherosclerotic Cardiovascular Diseases. Antioxid. Redox Signal. 2021, 34, 49–98. [Google Scholar] [CrossRef] [PubMed]
- MillerIII, E.R.; Appel, L.J.; Risby, T.H. Effect of dietary patterns on measures of lipid peroxidation. Results from a randomized Clinical trial. Circulation 1998, 98, 2390–2395. [Google Scholar] [CrossRef]
- Virella, G.; Carter, R.E.; Saad, A.; Crosswell, E.G.; Game, A.; DCCT/EDIC Study Group; Lopes-Virella, M.F. Distribution of IgM and IgG antibodies to oxidized LDL in immune complexes isolated from patients with type 1 diabetes and its relationship with nephropathy. Distribution of IgM and IgG antibodies to oxidized LDL in immune complexes isolated from patients with type 1 diabetes and its relationship with nephropathy. Clin. Immunol. 2008, 127, 394–400. [Google Scholar] [PubMed]
- Tsimikas, S.; Brilakis, E.S.; Lennon, R.J.; Miller, E.R.; Witztum, J.L.; McConnell, J.P.; Kornman, K.S.; Berger, P.B. Relationship of IgG and IgM autoantibodies to oxidized low density lipoprotein with coronary artery disease and cardiovascular events. J. Lipid Res. 2007, 48, 425–433. [Google Scholar] [CrossRef]
- Xie, Y.; Xu, E.; Bowe, B.; Al-Aly, Z. Long-term cardiovascular outcomes of COVID-19. Nat. Med. 2022, 28, 583–590. [Google Scholar] [CrossRef]
- Davies, M.J.; Hawkins, C. The role of myeloperoxidase in biomolecule modification, chronic inflammation, and disease. Antioxid. Redox Signal. 2020, 32, 957–981. [Google Scholar] [CrossRef]
- Pincemail, J.; Vanbelle, S.; Gaspard, U.; Collette, G.; Haleng, J.; Cheramy-Bien, J.P.; Charlier, C.; Chapelle, J.P.; Giet, D.; Albert, A.; et al. Effect of different contraceptive methods on the oxidative stress status in women aged 40–48 years from the ELAN study in the province of Liège, Belgium. Hum. Reprod. 2007, 22, 2335–2343. [Google Scholar] [CrossRef]
- Prasad, P.; Tyagi, A.T.; Aggarwal, B.B. Detection of inflammatory biomarkers in saliva and urine: Potential in diagnosis, prevention, and treatment for chronic diseases. Exp. Biol. Med. 2016, 241, 783–799. [Google Scholar] [CrossRef]
- II’yasova, D.; Scarbrough, P.; Spasojevic, I. Urinary biomarkers of oxidative stress. Clin. Chim. Acta 2012, 413, 1446–1453. [Google Scholar] [CrossRef] [PubMed]
- Peluso, I.; Raguzzini, A. Salivary and urinary total antioxidant capacity as biomarkers of oxidative stress in humans. Pathol. Res. Int. 2016, 2016, 5480267. [Google Scholar] [CrossRef] [PubMed]
- Maciejczyk, M.; Szulimowska, J.; Taranta-Janusz, K.; Werbel, K.; Wasileewska, A.; Zalewska, A. Salivary FRAP as a marker of chronic kidney disease progression in children. Antioxidants 2019, 8, 409. [Google Scholar] [CrossRef] [PubMed]
- Macieczyk, M.; Zalewska, A.; Ladny, J.R. Salivary antioxidant barrier, redox status, and oxidative damage to proteins and lipids in healthy children, adults, and the elderly. Oxid. Med. Cell. Longev. 2019, 2019, 1–12. [Google Scholar] [CrossRef]
- Evan, L.W.; Omaye, S.T. Use of saliva biomarkers to monitor efficacy of vitamin C in exercise-induced oxidative stress. Antioxidants 2017, 6, 5. [Google Scholar] [CrossRef]
- da Silva Marques, D.; da Mata, M.; Silveira, J.; Marques, J.; Amaral, J.; Guilherme, N. Hydrogen peroxide release kinetics into saliva from different whitening products: A double-blind, randomized clinical trial. Clin. Oral. Investig. 2011, 16, 155–163. [Google Scholar] [CrossRef]
- Lafaurie, G.L.; Zaror, C.; Díaz-Báez, D.; Castillo, D.M.; De Ávila, J.; Trujillo, T.G.; Calderón-Mendoza, J. Evaluation of substantivity of hypochlorous acid as an antiplaque agent: A randomized controlled trial. Int. J. Dent. Hyg. 2018, 16, 527–534. [Google Scholar] [CrossRef]
- Polizzi, A.; Torrisi, S.; Santonocito, S.; Di Stefano, M.; Indelicato, F.; Lo Giudice, A. Influence of myeloperoxidase levels on periodontal disease: An applied clinical study. App. Sci. 2020, 10, 1037. [Google Scholar] [CrossRef]
- Trouba, K.J.; Hamadeh, H.K.; Amin, R.P.; Germolec, D.R. Oxidative stress and its role in skin disease. Redox Signal. 2002, 4, 665–673. [Google Scholar] [CrossRef]
- Poljšak, B.; Dahmane, R.; Godic, A. Intrinsic skin aging: The role of oxidative stress. Acta Derm. Alp. Pannonica Adriat. 2012, 21, 33–36. [Google Scholar]
- Poljšak, B.; Dahmane, R. Free radicals and extrinsic skin aging. Dermatol. Res. Pract. 2012, 2012, 135206. [Google Scholar] [CrossRef] [PubMed]
- Brainina, K.Z.; Gerasimova, E.L.; Varzakova, D.P.; Kazakov, Y.E.; Galperin, L.G. Noninvasive method of determining skin antioxidant/oxidant activity: Clinical and cosmetics applications. Anal. Bioanal. Electrochem. 2013, 5, 528–542. [Google Scholar]
- Sinclair, A.J.; Taylor, P.B.; Lunec, J.; Girling, A.J.; Barnett, A.H. Low plasma ascorbate levels in patients with type 2 diabetes mellitus consuming adequate dietary vitamin C. Diabet. Med. 1994, 11, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Ran, L.; Zhao, W.; Tan, X.; Wang, H.; Mizuno, K.; Takagi, K.; Zhao, Y.; Bu, H. Association between serum vitamin C and the blood pressure: A systematic review and meta-analysis of observational studies. Cardiovasc. Ther. 2020, 2020, 4940673. [Google Scholar] [CrossRef]
| Variable | Median (Range) |
|---|---|
| Age (y) | 64 (47–76) |
| Male gender | 9 (75) |
| Weight (kg) | 94 (50–118) |
| Height (cm) | 177 (152–196) |
| BMI (kg/m2) | 29.8 (21.9–33.9) |
| Active smoking | 0 (0) |
| Active alcoholism | 0 (0) |
| Pre-existing medical conditions | |
| -Type 2 diabetes | 5 (42) |
| -Arterial hypertension | 9 (75) |
| -Hypothyroidism | 4 (33) |
| SAPS II | 33 (20–82) |
| ICU LOS (d) | 42 (15–57) |
| Hospital LOS (d) | 53 (41–138) |
| Mechanical ventilation duration (d) | 27 (8–50) |
| CVVH, n (%) | 2 (17) |
| Complete regression * | 5(41.6) |
| Partial regression * | 7 (58.3) |
| Presence of fibrosis * | 3 (25) |
| COVID-Patients in ICU (N = 9) | COVID-19 Patients in Rehabilitation Facility (N = 12) | ||||
|---|---|---|---|---|---|
| Parameter | Median (Range) | p Value * | Median (Range) | p Value | Reference Interval (N=897) [38,39,40] |
| Antioxidants | |||||
| Vitamin C (µg/mL) | 3.91 (3.06–6.14) | 0.004 | 5.43 (2.51–10.90) | 0.006 | 6.0–18 |
| Vitamin E as α-tocopherol (µg/mL) | 17.90 (13.3–21.1) | 1.0 | 17.3 (11.1–26.6) | 1.0 | 8.6–19.2 |
| γ-tocopherol (µg/mL) | 0.84 (0.57–1.28) | 0.040 | 0.95 (0.39–2.60) | 0.006 | 0.39–2.42 |
| β-carotene (µg/mL) | 0.14 (0.06–0.68) | 0.004 | 0.22 (0.09–0.0.40) | 0.006 | 0.06–0.68 |
| Thiol proteins (PSH) (µM) | 250 (204–258) | 0.004 | 242 (197–357) | <0.0005 | 314–516 |
| Total glutathione (tGSH) (µM) | 629 (508–697) | 0.040 | 614 (450–721) | <0.0005 | 717–1110 |
| Oxidized glutathione (GSSG) (µM) | <0.96 | 1.0 | <0.96 | 1.0 | 0.96–10 |
| PAOT®-Skin Score | 67.7 (11.5–115) | 1.0 | 7.86–62.9 | ||
| PAOT®-Plasma Score | 10.52 (6.63–10.77) | 0.04 | 29.4 (12.2–79.2) | 1.0 | 1.42–36.78 |
| PAOT®-Saliva Score | 7.03 (0.88–22.1) | 1.0 | 1.52–14.14 | ||
| PAOT®-Urinary Score | 11.8 (8.6–26.4) | <0.0005 | 43–105 | ||
| Glutathione peroxidase (GPx) (UI/g Hb) | 69.55 (61.9–78.27) | 0.004 | 78 (51–103) | <0.0005 | 20–56 |
| Albumin (g/L) | 28 (27.5–33.0) | 0.04 | 38 (32–44) | 1.0 | 32–46 |
| Trace elements | |||||
| Copper (Cu) (mg/mL) | 1.16 (0.66–1.47) | 1.0 | 1.10 (0.76–1.56) | 0.15 | 0.70–1.1 |
| Zinc (Zn) (mg/mL) | 0.84 (0.81–1.01) | 1.0 | 0.95 (0.56–1.18) | 1.0 | 0.70–1.20 |
| Selenium (Se) (µg/mL) | 74 (59–103) | 1.0 | 76.3 (52.1–102) | 0.39 | 73–110 |
| Biomarkers of oxidation | |||||
| Total hydroperoxides (tROOH) (µM) | 674 (181–1415) | 0.50 | 1061 (162–1905) | 0.006 | 0–432 |
| Oxidized LDL (ox-LDL) (ng/mL) | 50 (36–70) | 1.0 | 67 (31–114) | 0.39 | 28–70 |
| Antibodies against oxidized LDL (Ig G Ab-ox-LDL) (IU/L) | 306 (64–1200) | 1.0 | 870 (70–8190) | 0.39 | 200–600 |
| Inflammatory biomarkers | |||||
| Copper/zinc ratio (Cu/Zn) | 1.55 (0.79–1.69) | 1.0 | 1.24 (0.74–1.68) | 0.038 | 1–1.17 |
| Myeloperoxidase (MPO) (ng/mL) | 88 (60–191) | 0.04 | 72 (40–124) | 0.038 | 27–72 |
| C-reactive protein (CRP) (mg/L) | 32.8 (9.6–59.8) | 0.04 | 10.2 (1.2–45.4) | 0.038 | 0–5 |
| White blood cells 103/mm3 | 8.42 (7.07–13.03) | 0.04 | 6.9 (3.0–9.0) | 1.0 | 4.6–10.1 |
| Parameter | Parameter | Spearman Correlation | Determination Coefficient | p-Value |
|---|---|---|---|---|
| Cu | Cu/Zn | 0.63 | 0.39 | 0.028 |
| Cu | γ-tocopherol | −0.66 | 0.43 | 0.020 |
| Cu | tROOH | 0.95 | 0.90 | 0.001 |
| Cu/Zn | tROOH | 0.66 | 0.43 | 0.020 |
| γ-tocopherol | ROOH | −0.61 | 0.37 | 0.034 |
| ox-LDL | vitamin C | −0.72 | 0.51 | 0.017 |
| PAOT®-Plasma Score | selenium | 0.68 | 0.46 | 0.015 |
| PAOT®-Urinary Score | Cu | −0.72 | 0.51 | 0.008 |
| PAOT®-Urinary Score | ROOH | −0.69 | 0.47 | 0.013 |
| PAOT®-Saliva Score | ROOH | −0.78 | 0.60 | 0.005 |
| PAOT®-Saliva Score | Cu | −0.81 | 0.65 | 0.003 |
| PAOT®-Saliva Score | Cu/Zn | −0.59 | 0.34 | 0.055 |
| PAOT®-Saliva Score | γ-tocopherol | 0.59 | 0.34 | 0.055 |
| PAOT®-Skin Score | ROOH | −0.77 | 0.59 | 0.005 |
| PAOT®-Skin Score | Cu | −0.76 | 0.57 | 0.007 |
| PAOT®-Skin Score | vitamin C | 0.62 | 0.38 | 0.043 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pincemail, J.; Rousseau, A.-F.; Kaux, J.-F.; Cheramy-Bien, J.-P.; Bruyère, C.; Prick, J.; Stern, D.; Kaci, M.-M.; Maertens De Noordhout, B.; Albert, A.; et al. A Pilot Study on Oxidative Stress during the Recovery Phase in Critical COVID-19 Patients in a Rehabilitation Facility: Potential Utility of the PAOT® Technology for Assessing Total Anti-Oxidative Capacity. Biomedicines 2023, 11, 1308. https://doi.org/10.3390/biomedicines11051308
Pincemail J, Rousseau A-F, Kaux J-F, Cheramy-Bien J-P, Bruyère C, Prick J, Stern D, Kaci M-M, Maertens De Noordhout B, Albert A, et al. A Pilot Study on Oxidative Stress during the Recovery Phase in Critical COVID-19 Patients in a Rehabilitation Facility: Potential Utility of the PAOT® Technology for Assessing Total Anti-Oxidative Capacity. Biomedicines. 2023; 11(5):1308. https://doi.org/10.3390/biomedicines11051308
Chicago/Turabian StylePincemail, Joël, Anne-Françoise Rousseau, Jean-François Kaux, Jean-Paul Cheramy-Bien, Christine Bruyère, Jeanine Prick, David Stern, Mouna-Messaouda Kaci, Benoît Maertens De Noordhout, Adelin Albert, and et al. 2023. "A Pilot Study on Oxidative Stress during the Recovery Phase in Critical COVID-19 Patients in a Rehabilitation Facility: Potential Utility of the PAOT® Technology for Assessing Total Anti-Oxidative Capacity" Biomedicines 11, no. 5: 1308. https://doi.org/10.3390/biomedicines11051308
APA StylePincemail, J., Rousseau, A.-F., Kaux, J.-F., Cheramy-Bien, J.-P., Bruyère, C., Prick, J., Stern, D., Kaci, M.-M., Maertens De Noordhout, B., Albert, A., Eubelen, C., Goff, C. L., Misset, B., Cavalier, E., Charlier, C., & Meziane, S. (2023). A Pilot Study on Oxidative Stress during the Recovery Phase in Critical COVID-19 Patients in a Rehabilitation Facility: Potential Utility of the PAOT® Technology for Assessing Total Anti-Oxidative Capacity. Biomedicines, 11(5), 1308. https://doi.org/10.3390/biomedicines11051308







