Effect of Low Protein Diet Supplemented with Ketoanalogs on Endothelial Function and Protein-Bound Uremic Toxins in Patients with Chronic Kidney Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Subjects
2.2. FMD Measurements
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1151–1210. [Google Scholar] [CrossRef]
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D. Global Prevalence of Chronic Kidney Disease—A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef] [PubMed]
- Hillege, H.L.; Girbes, A.R.; de Kam, P.J.; Boomsma, F.; de Zeeuw, D.; Charlesworth, A.; Hampton, J.R.; van Veldhuisen, D.J. Renal function, neurohormonal activation, and survival in patients with chronic heart failure. Circulation 2000, 102, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Sarnak, M.J.; Levey, A.S.; Schoolwerth, A.C.; Coresh, J.; Culleton, B.; Hamm, L.L.; McCullough, P.A.; Kasiske, B.L.; Kelepouris, E.; Klag, M.J.; et al. Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension 2003, 42, 1050–1065. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, K.; van der Velde, M.; Astor, B.C.; Woodward, M.; Levey, A.S.; de Jong, P.E.; Coresh, J.; Gansevoort, R.T. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 2010, 375, 2073–2081. [Google Scholar] [CrossRef] [PubMed]
- Mafham, M.; Emberson, J.; Landray, M.J.; Wen, C.P.; Baigent, C. Estimated glomerular filtration rate and the risk of major vascular events and all-cause mortality: A meta-analysis. PLoS ONE 2011, 6, e25920. [Google Scholar] [CrossRef] [PubMed]
- Mende, C.W. Chronic Kidney Disease and SGLT2 Inhibitors: A Review of the Evolving Treatment Landscape. Adv. Ther. 2022, 39, 148–164. [Google Scholar] [CrossRef]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef]
- Brenner, B.M.; Meyer, T.W.; Hostetter, T.H. Dietary protein intake and the progressive nature of kidney disease: The role of hemodynamically mediated glomerular injury in the pathogenesis of progressive glomerular sclerosis in aging, renal ablation, and intrinsic renal disease. N. Engl. J. Med. 1982, 307, 652–659. [Google Scholar] [CrossRef]
- Kasiske, B.L.; Lakatua, J.D.; Ma, J.Z.; Louis, T.A. A meta-analysis of the effects of dietary protein restriction on the rate of decline in renal function. Am. J. Kidney Dis. 1998, 31, 954–961. [Google Scholar] [CrossRef]
- Malvy, D.; Maingourd, C.; Pengloan, J.; Bagros, P.; Nivet, H. Effects of severe protein restriction with ketoanalogues in advanced renal failure. J. Am. Coll. Nutr. 1999, 18, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Di Iorio, B.; Di Micco, L.; Torraca, S.; Sirico, M.L.; Russo, L.; Pota, A.; Mirenghi, F.; Russo, D. Acute effects of very-low-protein diet on FGF23 levels: A randomized study. Clin. J. Am. Soc. Nephrol. 2012, 7, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.L.; Fan, P.C.; Lee, C.C.; Kuo, G.; Tu, K.H.; Chen, J.J.; Lee, T.H.; Hsu, H.H.; Tian, Y.C.; Chang, C.H. Advanced Chronic Kidney Disease with Low and Very Low GFR: Can a Low-Protein Diet Supplemented with Ketoanalogues Delay Dialysis? Nutrients 2020, 12, 3358. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.L.; Fan, P.C.; Chen, J.J.; Kuo, G.; Hsiao, C.C.; Chen, C.Y.; Tu, Y.R.; Hsu, H.H.; Chen, Y.C.; Chang, C.H. Ketoanalogues Supplemental Low Protein Diet Safely Decreases Short-Term Risk of Dialysis among CKD Stage 4 Patients. Nutrients 2022, 14, 4020. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, X.; Yang, L.; Li, Z.; Qin, W. Effect of restricted protein diet supplemented with keto analogues in chronic kidney disease: A systematic review and meta-analysis. Int. Urol. Nephrol. 2016, 48, 409–418. [Google Scholar] [CrossRef]
- Chewcharat, A.; Takkavatakarn, K.; Wongrattanagorn, S.; Panrong, K.; Kittiskulnam, P.; Eiam-Ong, S.; Susantitaphong, P. The Effects of Restricted Protein Diet Supplemented With Ketoanalogue on Renal Function, Blood Pressure, Nutritional Status, and Chronic Kidney Disease-Mineral and Bone Disorder in Chronic Kidney Disease Patients: A Systematic Review and Meta-Analysis. J. Ren. Nutr. 2020, 30, 189–199. [Google Scholar] [CrossRef]
- Bellizzi, V.; Garofalo, C.; Ferrara, C.; Calella, P. Ketoanalogue Supplementation in Patients with Non-Dialysis Diabetic Kidney Disease: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 441. [Google Scholar] [CrossRef]
- Vanholder, R.; Baurmeister, U.; Brunet, P.; Cohen, G.; Glorieux, G.; Jankowski, J. A bench to bedside view of uremic toxins. J. Am. Soc. Nephrol. 2008, 19, 863–870. [Google Scholar] [CrossRef]
- Vanholder, R.; Van Laecke, S.; Glorieux, G. What is new in uremic toxicity? Pediatr. Nephrol. 2008, 23, 1211–1221. [Google Scholar] [CrossRef]
- Poesen, R.; Viaene, L.; Verbeke, K.; Claes, K.; Bammens, B.; Sprangers, B.; Naesens, M.; Vanrenterghem, Y.; Kuypers, D.; Evenepoel, P.; et al. Renal clearance and intestinal generation of p-cresyl sulfate and indoxyl sulfate in CKD. Clin. J. Am. Soc. Nephrol. 2013, 8, 1508–1514. [Google Scholar] [CrossRef]
- Lin, C.J.; Chen, H.H.; Pan, C.F.; Chuang, C.K.; Wang, T.J.; Sun, F.J.; Wu, C.J. p-Cresylsulfate and indoxyl sulfate level at different stages of chronic kidney disease. J. Clin. Lab. Anal. 2011, 25, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.J.; Liu, H.L.; Pan, C.F.; Chuang, C.K.; Jayakumar, T.; Wang, T.J.; Chen, H.H.; Wu, C.J. Indoxyl sulfate predicts cardiovascular disease and renal function deterioration in advanced chronic kidney disease. Arch. Med. Res. 2012, 43, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.J.; Pan, C.F.; Chuang, C.K.; Sun, F.J.; Wang, D.J.; Chen, H.H.; Liu, H.L.; Wu, C.J. P-cresyl sulfate is a valuable predictor of clinical outcomes in pre-ESRD patients. Biomed. Res. Int. 2014, 2014, 526932. [Google Scholar] [CrossRef]
- Lin, C.J.; Wu, V.; Wu, P.C.; Wu, C.J. Meta-Analysis of the Associations of p-Cresyl Sulfate (PCS) and Indoxyl Sulfate (IS) with Cardiovascular Events and All-Cause Mortality in Patients with Chronic Renal Failure. PLoS ONE 2015, 10, e0132589. [Google Scholar] [CrossRef]
- Di Iorio, B.R.; Rocchetti, M.T.; De Angelis, M.; Cosola, C.; Marzocco, S.; Di Micco, L.; di Bari, I.; Accetturo, M.; Vacca, M.; Gobbetti, M.; et al. Nutritional Therapy Modulates Intestinal Microbiota and Reduces Serum Levels of Total and Free Indoxyl Sulfate and P-Cresyl Sulfate in Chronic Kidney Disease (Medika Study). J. Clin. Med. 2019, 8, 1424. [Google Scholar] [CrossRef]
- Rocchetti, M.T.; Di Iorio, B.R.; Vacca, M.; Cosola, C.; Marzocco, S.; di Bari, I.; Calabrese, F.M.; Ciarcia, R.; De Angelis, M.; Gesualdo, L. Ketoanalogs’ Effects on Intestinal Microbiota Modulation and Uremic Toxins Serum Levels in Chronic Kidney Disease (Medika2 Study). J. Clin. Med. 2021, 10, 840. [Google Scholar] [CrossRef]
- Lim, Y.J.; Sidor, N.A.; Tonial, N.C.; Che, A.; Urquhart, B.L. Uremic Toxins in the Progression of Chronic Kidney Disease and Cardiovascular Disease: Mechanisms and Therapeutic Targets. Toxins 2021, 13, 142. [Google Scholar] [CrossRef]
- Dou, L.; Bertrand, E.; Cerini, C.; Faure, V.; Sampol, J.; Vanholder, R.; Berland, Y.; Brunet, P. The uremic solutes p-cresol and indoxyl sulfate inhibit endothelial proliferation and wound repair. Kidney Int. 2004, 65, 442–451. [Google Scholar] [CrossRef]
- Deanfield, J.E.; Halcox, J.P.; Rabelink, T.J. Endothelial function and dysfunction: Testing and clinical relevance. Circulation 2007, 115, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Gallo, G.; Volpe, M.; Savoia, C. Endothelial Dysfunction in Hypertension: Current Concepts and Clinical Implications. Front. Med. 2021, 8, 798958. [Google Scholar] [CrossRef]
- Lim, J.; Yu, C.J.; Yu, H.; Ha, S.J. Erythropoietin therapy improves endothelial function in patients with non-dialysis chronic kidney disease and anemia (EARNEST-CKD): A clinical study. Medicine 2021, 100, e27601. [Google Scholar] [CrossRef] [PubMed]
- Duranton, F.; Cohen, G.; De Smet, R.; Rodriguez, M.; Jankowski, J.; Vanholder, R.; Argiles, A. Normal and pathologic concentrations of uremic toxins. J. Am. Soc. Nephrol. 2012, 23, 1258–1270. [Google Scholar] [CrossRef] [PubMed]
- Brevetti, G.; Silvestro, A.; Schiano, V.; Chiariello, M. Endothelial dysfunction and cardiovascular risk prediction in peripheral arterial disease: Additive value of flow-mediated dilation to ankle-brachial pressure index. Circulation 2003, 108, 2093–2098. [Google Scholar] [CrossRef] [PubMed]
- Ras, R.T.; Streppel, M.T.; Draijer, R.; Zock, P.L. Flow-mediated dilation and cardiovascular risk prediction: A systematic review with meta-analysis. Int. J. Cardiol. 2013, 168, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Wischmann, P.; Kuhn, V.; Suvorava, T.; Muessig, J.M.; Fischer, J.W.; Isakson, B.E.; Haberkorn, S.M.; Flögel, U.; Schrader, J.; Jung, C.; et al. Anaemia is associated with severe RBC dysfunction and a reduced circulating NO pool: Vascular and cardiac eNOS are crucial for the adaptation to anaemia. Basic Res. Cardiol. 2020, 115, 43. [Google Scholar] [CrossRef] [PubMed]
- Caruso, C.; Fay, M.E.; Cheng, X.; Liu, A.Y.; Park, S.I.; Sulchek, T.A.; Graham, M.D.; Lam, W.A. Pathologic mechanobiological interactions between red blood cells and endothelial cells directly induce vasculopathy in iron deficiency anemia. iScience 2022, 25, 104606. [Google Scholar] [CrossRef]
- Brownlee, M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef]
- Xu, J.; Zou, M.H. Molecular insights and therapeutic targets for diabetic endothelial dysfunction. Circulation 2009, 120, 1266–1286. [Google Scholar] [CrossRef]
- Garneata, L.; Stancu, A.; Dragomir, D.; Stefan, G.; Mircescu, G. Ketoanalogue-Supplemented Vegetarian Very Low-Protein Diet and CKD Progression. J. Am. Soc. Nephrol. 2016, 27, 2164–2176. [Google Scholar] [CrossRef]
- Yen, C.L.; Tu, K.H.; Lin, M.S.; Chang, S.W.; Fan, P.C.; Hsiao, C.C.; Chen, C.Y.; Hsu, H.H.; Tian, Y.C.; Chang, C.H. Does a Supplemental Low-Protein Diet Decrease Mortality and Adverse Events After Commencing Dialysis? A Nationwide Cohort Study. Nutrients 2018, 10, 1035. [Google Scholar] [CrossRef]
- Moradi, H.; Sica, D.A.; Kalantar-Zadeh, K. Cardiovascular burden associated with uremic toxins in patients with chronic kidney disease. Am. J. Nephrol. 2013, 38, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, A.; Takeda, M.; Tojo, A.; Sekine, T.; Cha, S.H.; Khamdang, S.; Takayama, F.; Aoyama, I.; Nakamura, S.; Endou, H.; et al. Role of organic anion transporters in the tubular transport of indoxyl sulfate and the induction of its nephrotoxicity. J. Am. Soc. Nephrol. 2002, 13, 1711–1720. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Watanabe, H.; Noguchi, T.; Kotani, S.; Nakajima, M.; Kadowaki, D.; Otagiri, M.; Maruyama, T. Organic anion transporters play an important role in the uptake of p-cresyl sulfate, a uremic toxin, in the kidney. Nephrol. Dial. Transplant. 2011, 26, 2498–2502. [Google Scholar] [CrossRef] [PubMed]
- Harlacher, E.; Wollenhaupt, J.; Baaten, C.; Noels, H. Impact of Uremic Toxins on Endothelial Dysfunction in Chronic Kidney Disease: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 531. [Google Scholar] [CrossRef] [PubMed]
- Fliser, D.; Wiecek, A.; Suleymanlar, G.; Ortiz, A.; Massy, Z.; Lindholm, B.; Martinez-Castelao, A.; Agarwal, R.; Jager, K.J.; Dekker, F.W.; et al. The dysfunctional endothelium in CKD and in cardiovascular disease: Mapping the origin(s) of cardiovascular problems in CKD and of kidney disease in cardiovascular conditions for a research agenda. Kidney Int. Suppl. 2011, 1, 6–9. [Google Scholar] [CrossRef]
- Kang, D.H.; Kanellis, J.; Hugo, C.; Truong, L.; Anderson, S.; Kerjaschki, D.; Schreiner, G.F.; Johnson, R.J. Role of the microvascular endothelium in progressive renal disease. J. Am. Soc. Nephrol. 2002, 13, 806–816. [Google Scholar] [CrossRef]
- Maciel, R.A.P.; Cunha, R.S.; Busato, V.; Franco, C.R.C.; Gregório, P.C.; Dolenga, C.J.R.; Nakao, L.S.; Massy, Z.A.; Boullier, A.; Pecoits-Filho, R.; et al. Uremia Impacts VE-Cadherin and ZO-1 Expression in Human Endothelial Cell-to-Cell Junctions. Toxins 2018, 10, 404. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. Endothelial Barrier and Its Abnormalities in Cardiovascular Disease. Front. Physiol. 2015, 6, 365. [Google Scholar] [CrossRef]
- Padberg, J.S.; Wiesinger, A.; di Marco, G.S.; Reuter, S.; Grabner, A.; Kentrup, D.; Lukasz, A.; Oberleithner, H.; Pavenstädt, H.; Brand, M.; et al. Damage of the endothelial glycocalyx in chronic kidney disease. Atherosclerosis 2014, 234, 335–343. [Google Scholar] [CrossRef]
- Ito, S.; Osaka, M.; Higuchi, Y.; Nishijima, F.; Ishii, H.; Yoshida, M. Indoxyl sulfate induces leukocyte-endothelial interactions through up-regulation of E-selectin. J. Biol. Chem. 2010, 285, 38869–38875. [Google Scholar] [CrossRef]
- Jing, Y.J.; Ni, J.W.; Ding, F.H.; Fang, Y.H.; Wang, X.Q.; Wang, H.B.; Chen, X.N.; Chen, N.; Zhan, W.W.; Lu, L.; et al. p-Cresyl sulfate is associated with carotid arteriosclerosis in hemodialysis patients and promotes atherogenesis in apoE-/- mice. Kidney Int. 2016, 89, 439–449. [Google Scholar] [CrossRef]
- Faure, V.; Dou, L.; Sabatier, F.; Cerini, C.; Sampol, J.; Berland, Y.; Brunet, P.; Dignat-George, F. Elevation of circulating endothelial microparticles in patients with chronic renal failure. J. Thromb. Haemost. 2006, 4, 566–573. [Google Scholar] [CrossRef]
- Buendía, P.; Montes de Oca, A.; Madueño, J.A.; Merino, A.; Martín-Malo, A.; Aljama, P.; Ramírez, R.; Rodríguez, M.; Carracedo, J. Endothelial microparticles mediate inflammation-induced vascular calcification. FASEB J. 2015, 29, 173–181. [Google Scholar] [CrossRef]
- Seliger, S.L.; Salimi, S.; Pierre, V.; Giffuni, J.; Katzel, L.; Parsa, A. Microvascular endothelial dysfunction is associated with albuminuria and CKD in older adults. BMC Nephrol. 2016, 17, 82. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Ren, L.; Wei, Q.; Shao, H.; Chen, L.; Liu, N. Advanced glycation end-products decreases expression of endothelial nitric oxide synthase through oxidative stress in human coronary artery endothelial cells. Cardiovasc. Diabetol. 2017, 16, 52. [Google Scholar] [CrossRef] [PubMed]
- Corretti, M.C.; Anderson, T.J.; Benjamin, E.J.; Celermajer, D.; Charbonneau, F.; Creager, M.A.; Deanfield, J.; Drexler, H.; Gerhard-Herman, M.; Herrington, D.; et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: A report of the International Brachial Artery Reactivity Task Force. J. Am. Coll. Cardiol. 2002, 39, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Niwa, T. Uremic toxicity of indoxyl sulfate. Nagoya J. Med. Sci. 2010, 72, 1–11. [Google Scholar]
- Neirynck, N.; Vanholder, R.; Schepers, E.; Eloot, S.; Pletinck, A.; Glorieux, G. An update on uremic toxins. Int. Urol. Nephrol. 2013, 45, 139–150. [Google Scholar] [CrossRef]
- Wu, I.W.; Hsu, K.H.; Lee, C.C.; Sun, C.Y.; Hsu, H.J.; Tsai, C.J.; Tzen, C.Y.; Wang, Y.C.; Lin, C.Y.; Wu, M.S. p-Cresyl sulphate and indoxyl sulphate predict progression of chronic kidney disease. Nephrol. Dial. Transplant. 2011, 26, 938–947. [Google Scholar] [CrossRef]
- Sun, C.Y.; Chang, S.C.; Wu, M.S. Uremic toxins induce kidney fibrosis by activating intrarenal renin-angiotensin-aldosterone system associated epithelial-to-mesenchymal transition. PLoS ONE 2012, 7, e34026. [Google Scholar] [CrossRef]
- Bolati, D.; Shimizu, H.; Yisireyili, M.; Nishijima, F.; Niwa, T. Indoxyl sulfate, a uremic toxin, downregulates renal expression of Nrf2 through activation of NF-κB. BMC Nephrol. 2013, 14, 56. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Miyamoto, Y.; Enoki, Y.; Ishima, Y.; Kadowaki, D.; Kotani, S.; Nakajima, M.; Tanaka, M.; Matsushita, K.; Mori, Y.; et al. p-Cresyl sulfate, a uremic toxin, causes vascular endothelial and smooth muscle cell damages by inducing oxidative stress. Pharmacol. Res. Perspect. 2015, 3, e00092. [Google Scholar] [CrossRef] [PubMed]
- Mircescu, G.; Gârneaţă, L.; Stancu, S.H.; Căpuşă, C. Effects of a supplemented hypoproteic diet in chronic kidney disease. J. Ren. Nutr. 2007, 17, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Di Iorio, B.R.; Di Micco, L.; Marzocco, S.; De Simone, E.; De Blasio, A.; Sirico, M.L.; Nardone, L. Very Low-Protein Diet (VLPD) Reduces Metabolic Acidosis in Subjects with Chronic Kidney Disease: The “Nutritional Light Signal” of the Renal Acid Load. Nutrients 2017, 9, 69. [Google Scholar] [CrossRef]
- Milovanova, L.; Fomin, V.; Moiseev, S.; Taranova, M.; Milovanov, Y.; Lysenko Kozlovskaya, L.; Kozlov, V.; Kozevnikova, E.; Milovanova, S.; Lebedeva, M.; et al. Effect of essential amino acid кetoanalogues and protein restriction diet on morphogenetic proteins (FGF-23 and Кlotho) in 3b-4 stages chronic кidney disease patients: A randomized pilot study. Clin. Exp. Nephrol. 2018, 22, 1351–1359. [Google Scholar] [CrossRef]
- Rigalleau, V.; Blanchetier, V.; Combe, C.; Guillot, C.; Deleris, G.; Aubertin, J.; Aparicio, M.; Gin, H. A low-protein diet improves insulin sensitivity of endogenous glucose production in predialytic uremic patients. Am. J. Clin. Nutr. 1997, 65, 1512–1516. [Google Scholar] [CrossRef]
- Teplan, V.; Schück, O.; Racek, J.; Mareckova, O.; Stollova, M.; Hanzal, V.; Malý, J. Reduction of plasma asymmetric dimethylarginine in obese patients with chronic kidney disease after three years of a low-protein diet supplemented with keto-amino acids: A randomized controlled trial. Wien. Klin. Wochenschr. 2008, 120, 478–485. [Google Scholar] [CrossRef]
- Dohi, Y.; Thiel, M.A.; Bühler, F.R.; Lüscher, T.F. Activation of endothelial L-arginine pathway in resistance arteries. Effect of age and hypertension. Hypertension 1990, 16, 170–179. [Google Scholar] [CrossRef]
- Hermann, M.; Flammer, A.; Lüscher, T.F. Nitric oxide in hypertension. J. Clin. Hypertens. 2006, 8, 17–29. [Google Scholar] [CrossRef]
- Cai, H.; Harrison, D.G. Endothelial dysfunction in cardiovascular diseases: The role of oxidant stress. Circ. Res. 2000, 87, 840–844. [Google Scholar] [CrossRef]
- Dijkhorst-Oei, L.T.; Stroes, E.S.; Koomans, H.A.; Rabelink, T.J. Acute simultaneous stimulation of nitric oxide and oxygen radicals by angiotensin II in humans in vivo. J. Cardiovasc. Pharmacol. 1999, 33, 420–424. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Kurz, S.; Münzel, T.; Tarpey, M.; Freeman, B.A.; Griendling, K.K.; Harrison, D.G. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J. Clin. Investig. 1996, 97, 1916–1923. [Google Scholar] [CrossRef]
- Hegde, L.G.; Srivastava, P.; Kumari, R.; Dikshit, M. Alterations in the vasoreactivity of hypertensive rat aortic rings: Role of nitric oxide and superoxide radicals. Clin. Exp. Hypertens. 1998, 20, 885–901. [Google Scholar] [CrossRef] [PubMed]
- Virdis, A.; Neves, M.F.; Amiri, F.; Viel, E.; Touyz, R.M.; Schiffrin, E.L. Spironolactone improves angiotensin-induced vascular changes and oxidative stress. Hypertension 2002, 40, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Sakima, A.; Arima, H.; Matayoshi, T.; Ishida, A.; Ohya, Y. Effect of Mineralocorticoid Receptor Blockade on Arterial Stiffness and Endothelial Function: A Meta-Analysis of Randomized Trials. Hypertension 2021, 77, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Lacy, P.S.; Thom, S.M.; Cruickshank, K.; Stanton, A.; Collier, D.; Hughes, A.D.; Thurston, H.; O’Rourke, M. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: Principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation 2006, 113, 1213–1225. [Google Scholar] [CrossRef]
- Guo, W.; Diao, Z.; Liu, W. Asymmetric dimethylarginine downregulates sarco/endoplasmic reticulum calcium-ATPase 3 and induces endoplasmic reticulum stress in human umbilical vein endothelial cells. Mol. Med. Rep. 2017, 16, 7541–7547. [Google Scholar] [CrossRef]
- Stühlinger, M.C.; Oka, R.K.; Graf, E.E.; Schmölzer, I.; Upson, B.M.; Kapoor, O.; Szuba, A.; Malinow, M.R.; Wascher, T.C.; Pachinger, O.; et al. Endothelial dysfunction induced by hyperhomocyst(e)inemia: Role of asymmetric dimethylarginine. Circulation 2003, 108, 933–938. [Google Scholar] [CrossRef]
- Garibotto, G.; Sofia, A.; Parodi, E.L.; Ansaldo, F.; Bonanni, A.; Picciotto, D.; Signori, A.; Vettore, M.; Tessari, P.; Verzola, D. Effects of Low-Protein, and Supplemented Very Low-Protein Diets, on Muscle Protein Turnover in Patients With CKD. Kidney Int. Rep. 2018, 3, 701–710. [Google Scholar] [CrossRef]
- DeFronzo, R.A. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: The missing links. The Claude Bernard Lecture 2009. Diabetologia 2010, 53, 1270–1287. [Google Scholar] [CrossRef]
- Kim, J.A.; Montagnani, M.; Koh, K.K.; Quon, M.J. Reciprocal relationships between insulin resistance and endothelial dysfunction: Molecular and pathophysiological mechanisms. Circulation 2006, 113, 1888–1904. [Google Scholar] [CrossRef] [PubMed]
- Vogel, R.A.; Corretti, M.C.; Plotnick, G.D. Effect of a single high-fat meal on endothelial function in healthy subjects. Am. J. Cardiol. 1997, 79, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Feron, O.; Dessy, C.; Moniotte, S.; Desager, J.P.; Balligand, J.L. Hypercholesterolemia decreases nitric oxide production by promoting the interaction of caveolin and endothelial nitric oxide synthase. J. Clin. Investig. 1999, 103, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Koh, K.K.; Quon, M.J.; Han, S.H.; Lee, Y.; Ahn, J.Y.; Kim, S.J.; Koh, Y.; Shin, E.K. Simvastatin improves flow-mediated dilation but reduces adiponectin levels and insulin sensitivity in hypercholesterolemic patients. Diabetes Care 2008, 31, 776–782. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Study Group, Mean ± SD (n = 11) | Control Group, Mean ± SD (n = 11) | p |
|---|---|---|---|
| gender (male) | 5 (45.5%) | 8 (72.7%) | 0.387 |
| age (yrs) | 50.36 ± 8.30 | 57.82 ± 7.28 | 0.037 |
| FMD (%) | 4.16 ± 0.79 | 3.87 ± 0.40 | 0.291 |
| TPCS (mg/L) | 12.84 ± 14.54 | 7.65 ± 4.63 | 0.281 |
| FPCS (mg/L) | 0.42 ± 0.78 | 0.09 ± 0.10 | 0.191 |
| TIS (mg/L) | 9.51 ± 9.82 | 4.53 ± 2.21 | 0.129 |
| FIS (mg/L) | 0.23 ± 0.31 | 0.10 ± 0.07 | 0.175 |
| SBP (mmHg) | 127.91 ± 8.26 | 143.64 ± 4.48 | p < 0.001 |
| DBP (mmHg) | 73.82 ± 5.95 | 86.55 ± 4.52 | p < 0.001 |
| eGFR (mL/min) | 22.83 ± 8.73 | 20.97 ± 3.71 | 0.524 |
| HB (g/dL) | 13.14 ± 3.45 | 10.62 ± 1.37 | 0.046 |
| Hct (%) | 35.55 ± 7.26 | 31.49 ± 3.46 | 0.132 |
| BUN (mg/dL) | 34.50 ± 8.89 | 43.40 ± 12.38 | 0.081 |
| Creatinine (mg/dL) | 2.84 ± 1.02 | 3.04 ± 0.42 | 0.554 |
| Na (mEq/L) | 141.38 ± 1.41 | 139.09 ± 2.21 | 0.020 |
| K (mEq/L) | 4.40 ± 0.69 | 4.72 ± 0.36 | 0.261 |
| Ca (mg/dL) | 9.27 ± 0.44 | 9.01 ± 0.48 | 0.227 |
| P (mg/dL) | 4.27 ± 0.49 | 3.79 ± 0.61 | 0.064 |
| HCO3 (mEq/L) | 21.96 ± 2.06 | 21.73 ± 2.05 | 0.790 |
| Albumin (g/dL) | 4.37 ± 0.48 | 4.02 ± 0.24 | 0.044 |
| Cholesterol (mg/dL) | 162.20 ± 22.44 | 177.50 ± 54.37 | 0.562 |
| Triglyceride (mg/dL) | 242.60 ± 70.40 | 178.64 ± 63.48 | 0.092 |
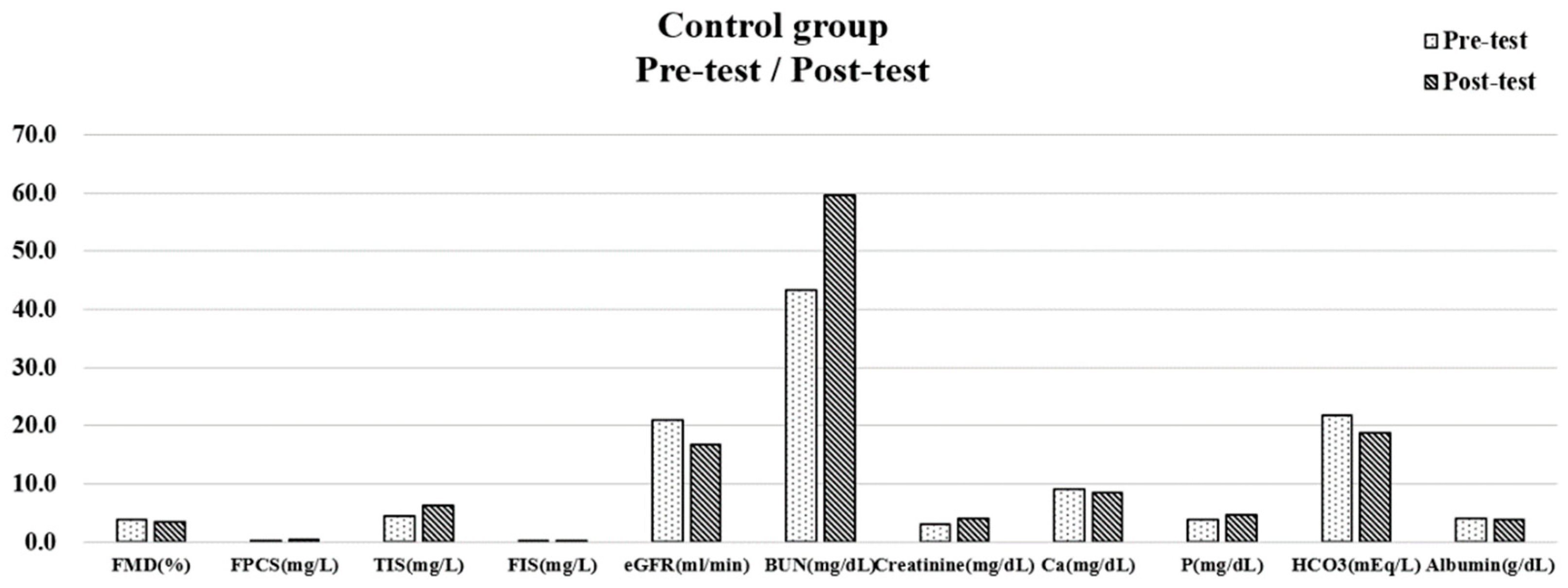
| Study Group | Control Group | Post-Pre Test | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameters | Pre-Test, Mean ± SD | Post-Test, Mean ± SD | p | Pre-Test, Mean ± SD | Post-Test, Mean ± SD | p | Study Group, Mean ± SD | Control Group, Mean ± SD | p |
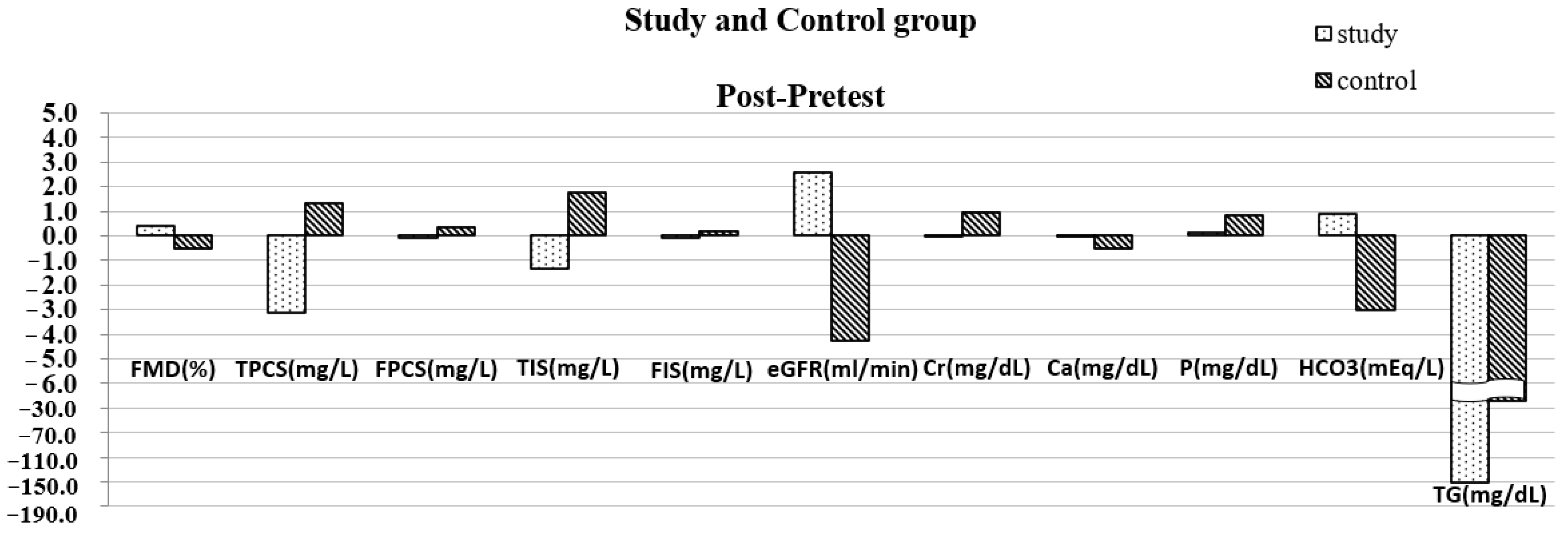
| FMD (%) | 4.16 ± 0.79 | 4.54 ± 0.64 | 0.045 | 3.87 ± 0.40 | 3.38 ± 0.43 | 0.001 | 0.38 ± 0.56 | −0.50 ± 0.38 | p < 0.001 |
| TPCS (mg/L) | 12.84 ± 14.54 | 9.73 ± 11.03 | 0.020 | 7.65 ± 4.63 | 8.95 ± 4.96 | 0.057 | −3.11 ± 3.73 | 1.31 ± 2.01 | 0.002 |
| FPCS (mg/L) | 0.42 ± 0.78 | 0.33 ± 0.58 | 0.191 | 0.09 ± 0.10 | 0.45 ± 0.34 | 0.006 | −0.09 ± 0.21 | 0.36 ± 0.35 | 0.002 |
| TIS (mg/L) | 9.51 ± 9.82 | 8.15 ± 9.69 | 0.027 | 4.53 ± 2.21 | 6.32 ± 1.91 | 0.003 | −1.36 ± 1.74 | 1.79 ± 1.50 | p < 0.001 |
| FIS (mg/L) | 0.23 ± 0.31 | 0.16 ± 0.22 | 0.104 | 0.10 ± 0.07 | 0.29 ± 0.13 | 0.001 | −0.07 ± 0.13 | 0.20 ± 0.14 | p < 0.001 |
| SBP (mmHg) | 127.91 ± 8.26 | 128.00 ± 11.66 | 0.968 | 143.64 ± 4.48 | 143.82 ± 7.35 | 0.921 | 0.09 ± 7.41 | 0.18 ± 5.93 | 0.975 |
| DBP (mmHg) | 73.82 ± 5.95 | 76.36 ± 5.78 | 0.295 | 86.55 ± 4.52 | 89.00 ± 3.07 | 0.121 | 2.55 ± 7.65 | 2.45 ± 4.80 | 0.974 |
| eGFR (mL/min) | 22.83 ± 8.73 | 25.42 ± 11.28 | 0.037 | 20.97 ± 3.71 | 16.69 ± 3.96 | 0.001 | 2.59 ± 3.57 | −4.28 ± 3.03 | p < 0.001 |
| HB (g/dL) | 13.14 ± 3.45 | 12.52 ± 1.93 | 0.385 | 10.62 ± 1.37 | 9.83 ± 1.38 | 0.056 | −1.24 ± 4.06 | −0.67 ± 0.96 | 0.669 |
| Hct (%) | 35.55 ± 7.26 | 36.31 ± 6.95 | 0.708 | 31.49 ± 3.46 | 29.17 ± 3.58 | 0.060 | 0.37 ± 3.03 | −1.82 ± 2.67 | 0.104 |
| BUN (mg/dL) | 34.50 ± 8.89 | 35.18 ± 16.50 | 0.477 | 43.40 ± 12.38 | 59.60 ± 12.75 | 0.013 | 2.90 ± 12.36 | 13.78 ± 13.04 | 0.079 |
| Creatinine (mg/dL) | 2.84 ± 1.02 | 2.80 ± 1.42 | 0.818 | 3.04 ± 0.42 | 4.01 ± 1.11 | 0.013 | −0.04 ± 0.51 | 0.94 ± 0.96 | 0.008 |
| Na (mEq/L) | 141.38 ± 1.41 | 140.70 ± 1.16 | 0.222 | 139.09 ± 2.21 | 137.20 ± 3.39 | 0.252 | −0.75 ± 1.58 | −1.90 ± 4.91 | 0.500 |
| K (mEq/L) | 4.40 ± 0.69 | 4.67 ± 0.64 | 0.197 | 4.72 ± 0.36 | 4.98 ± 0.61 | 0.234 | 0.15 ± 0.30 | 0.26 ± 0.64 | 0.640 |
| Ca (mg/dL) | 9.27 ± 0.44 | 9.25 ± 0.43 | 0.909 | 9.01 ± 0.48 | 8.48 ± 0.38 | 0.001 | −0.01 ± 0.27 | −0.50 ± 0.30 | 0.002 |
| P (mg/dL) | 4.27 ± 0.49 | 4.33 ± 0.77 | 0.609 | 3.79 ± 0.61 | 4.73 ± 0.54 | 0.002 | 0.13 ± 0.78 | 0.84 ± 0.63 | 0.037 |
| HCO3 (mEq/L) | 21.96 ± 2.06 | 22.85 ± 1.59 | 0.003 | 21.73 ± 2.05 | 18.73 ± 3.04 | p < 0.001 | 0.88 ± 0.75 | −3.00 ± 1.61 | p < 0.001 |
| Albumin (g/dL) | 4.37 ± 0.48 | 4.42 ± 0.19 | 0.669 | 4.02 ± 0.24 | 3.79 ± 0.27 | 0.002 | 0.06 ± 0.43 | −0.23 ± 0.18 | 0.056 |
| Choleterol (mg/dL) | 162.20 ± 22.44 | 213.67 ± 58.36 | 0.856 | 177.50 ± 54.37 | 199.60 ± 33.85 | 0.280 | 1.50 ± 9.19 | 18.78 ± 48.61 | 0.642 |
| Trglyceide (mg/dL) | 242.60 ± 70.40 | 98.83 ± 37.99 | 0.071 | 178.64 ± 63.48 | 151.09 ± 34.99 | 0.260 | −188.00 ± 29.70 | −27.55 ± 76.51 | 0.016 |
| Parameters | Adjusted For Baseline and Age | Adjusted for Baseline, Age, SBP, Na, and Albumin | Adjusted for Baseline, Age, SBP, DBP, Na, and Albumin | ||||||
|---|---|---|---|---|---|---|---|---|---|
| B | 95% C.I. | p | B | 95% C.I. | p | B | 95% C.I. | p | |
| FMD (%) | 1.00 | 0.62~1.38 | <0.001 | 0.96 | 0.50~1.41 | <0.001 | 0.96 | 0.50~1.41 | <0.001 |
| TPCS (mg/L) | −3.25 | −4.91~−1.59 | <0.001 | −3.01 | −4.79~−1.22 | <0.001 | |||
| FPCS (mg/L) | −0.36 | −0.60~−0.13 | <0.001 | −0.37 | −0.65~−0.09 | 0.012 | −0.37 | −0.65~−0.09 | 0.012 |
| TIS (mg/L) | −2.92 | −4.47~−1.37 | <0.001 | −3.23 | −4.61~−1.84 | <0.001 | −3.23 | −4.61~−1.84 | <0.001 |
| FIS (mg/L) | −0.22 | −0.33~−0.11 | <0.001 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, G.; Shih, H.-M.; Pan, C.-F.; Wu, C.-J.; Lin, C.-J. Effect of Low Protein Diet Supplemented with Ketoanalogs on Endothelial Function and Protein-Bound Uremic Toxins in Patients with Chronic Kidney Disease. Biomedicines 2023, 11, 1312. https://doi.org/10.3390/biomedicines11051312
Chang G, Shih H-M, Pan C-F, Wu C-J, Lin C-J. Effect of Low Protein Diet Supplemented with Ketoanalogs on Endothelial Function and Protein-Bound Uremic Toxins in Patients with Chronic Kidney Disease. Biomedicines. 2023; 11(5):1312. https://doi.org/10.3390/biomedicines11051312
Chicago/Turabian StyleChang, George, Hong-Mou Shih, Chi-Feng Pan, Chih-Jen Wu, and Cheng-Jui Lin. 2023. "Effect of Low Protein Diet Supplemented with Ketoanalogs on Endothelial Function and Protein-Bound Uremic Toxins in Patients with Chronic Kidney Disease" Biomedicines 11, no. 5: 1312. https://doi.org/10.3390/biomedicines11051312
APA StyleChang, G., Shih, H.-M., Pan, C.-F., Wu, C.-J., & Lin, C.-J. (2023). Effect of Low Protein Diet Supplemented with Ketoanalogs on Endothelial Function and Protein-Bound Uremic Toxins in Patients with Chronic Kidney Disease. Biomedicines, 11(5), 1312. https://doi.org/10.3390/biomedicines11051312





