Design of Chitosan-Coated, Quercetin-Loaded PLGA Nanoparticles for Enhanced PSMA-Specific Activity on LnCap Prostate Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
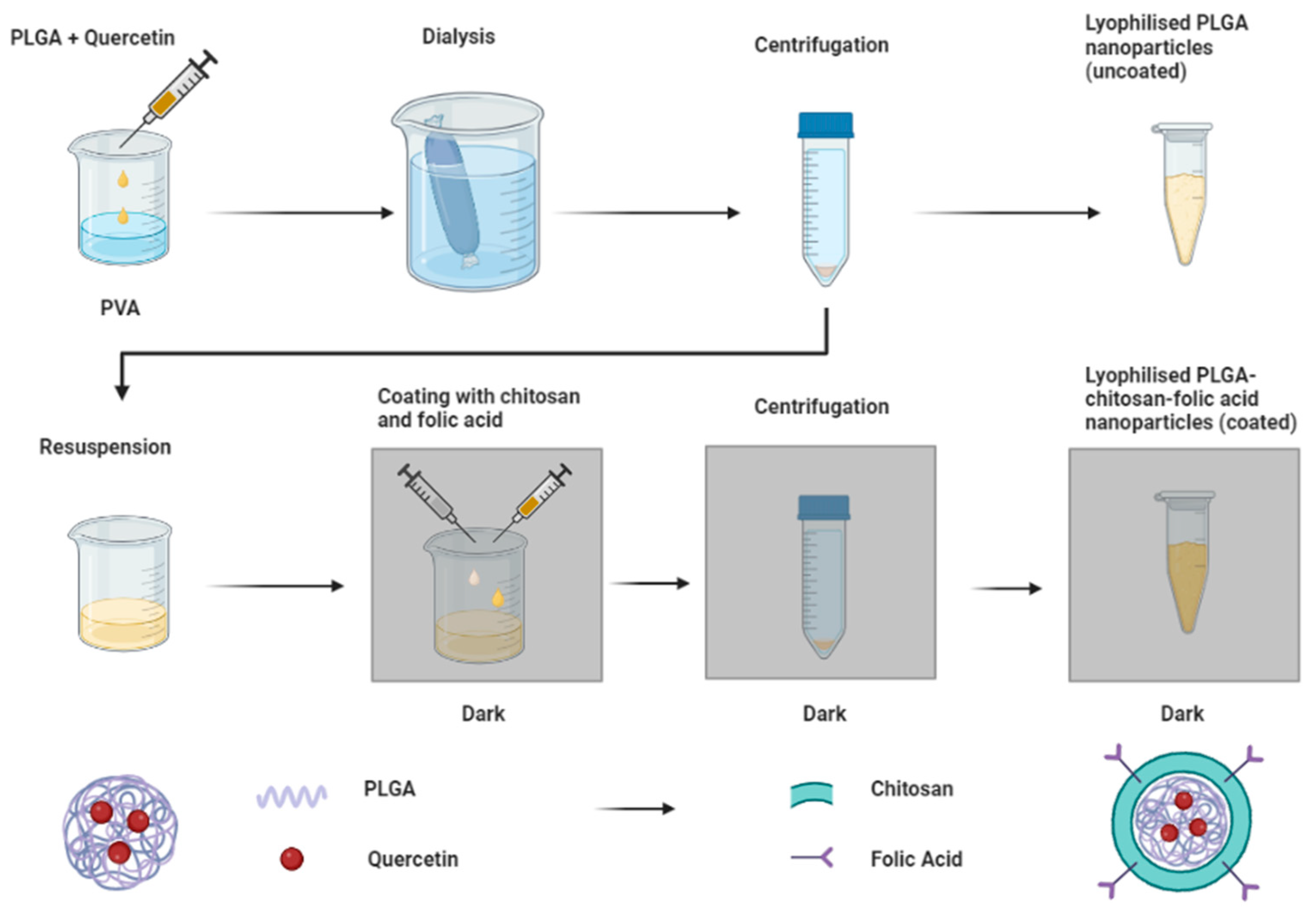
2.2. Preparation of Nanoparticles
Optimization by Design of Experiments
2.3. Particle Size, Polydispersity Index, and Zeta Potential Analysis Using Dynamic Light Scattering
2.4. Determination of Folic Acid Content by Ultra-Violet (UV) Spectrophotometry
2.5. Characterization Using Fourier Transform Infra-Red (FTIR)
2.6. Investigation of Thermal Degradation by Thermogravimetric Analysis (TGA)
2.7. Surface and Crystallinity Experiments Using Powder X-Ray Diffraction (XRD)
2.8. Phase Transition Studies Employing Differential Scanning Calorimetry (DSC)
2.9. Scanning Electron Microscopy (SEM) of Sample Suspensions
2.10. UV Spectrophotometric Analysis of Quercetin Loading in Nanoparticle Systems
2.11. In Vitro Release of Quercetin
2.12. Cell Culture Conditions and Cytotoxicity Studies
2.13. Cellular Uptake of Nanoparticles
2.14. Statistical Analysis
3. Results
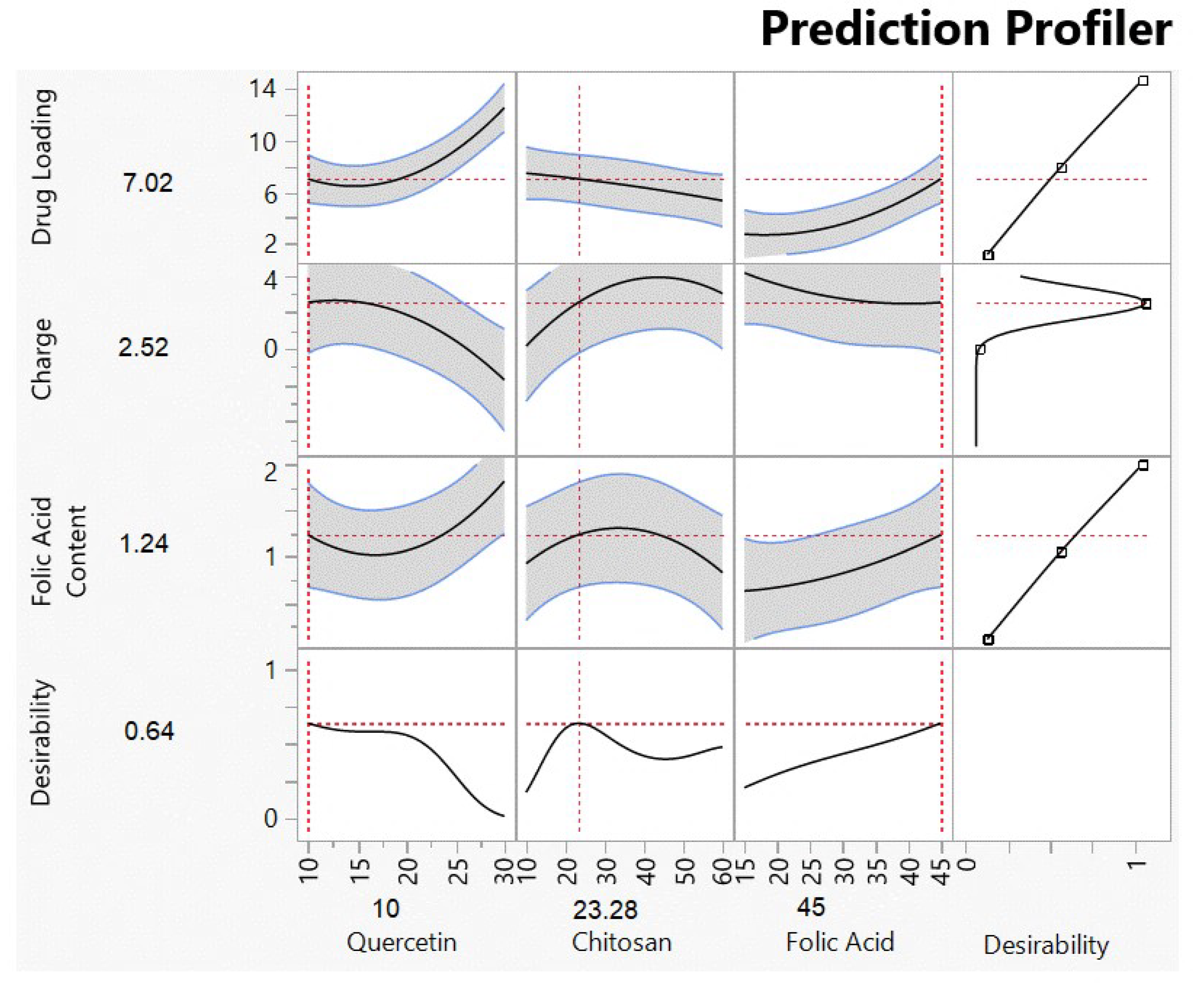
3.1. Optimized Formulation Results Using DOE
3.1.1. Quercetin Loading, Size, and Potential and Folic Acid Conjugation of Formulations
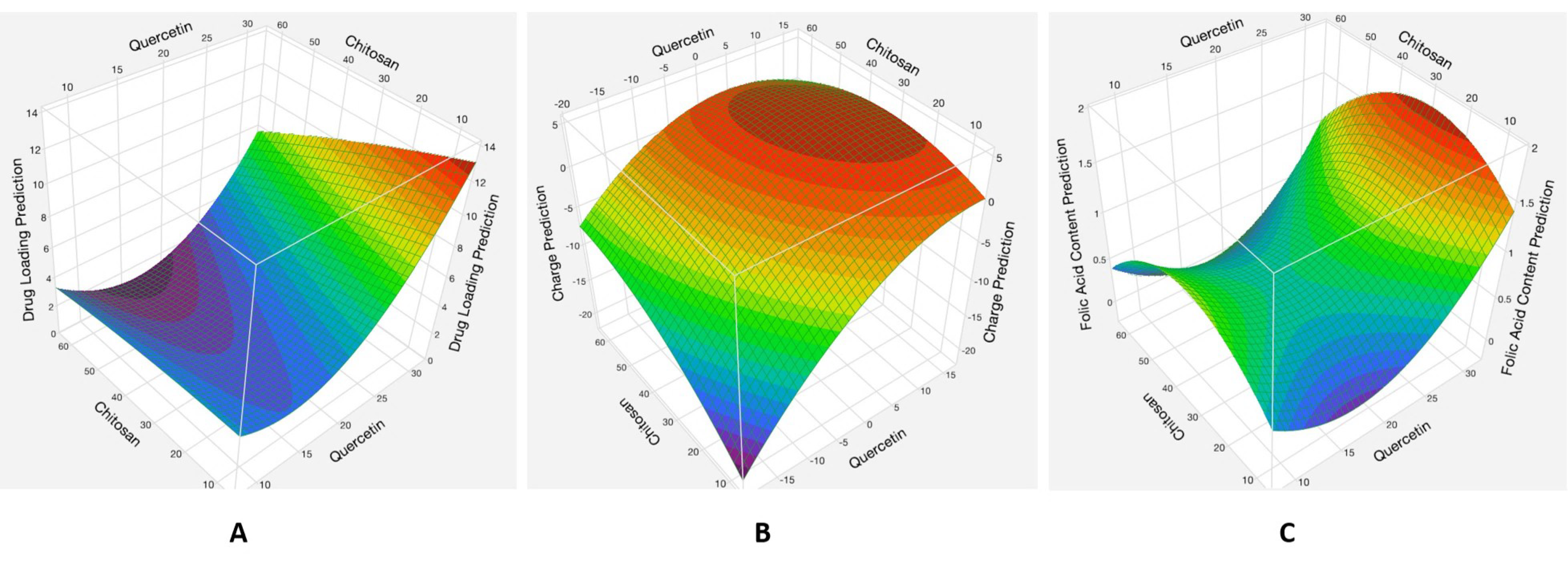
3.1.2. Analysis of Responses
Effects of the Interaction between the Quercetin and Chitosan Factors
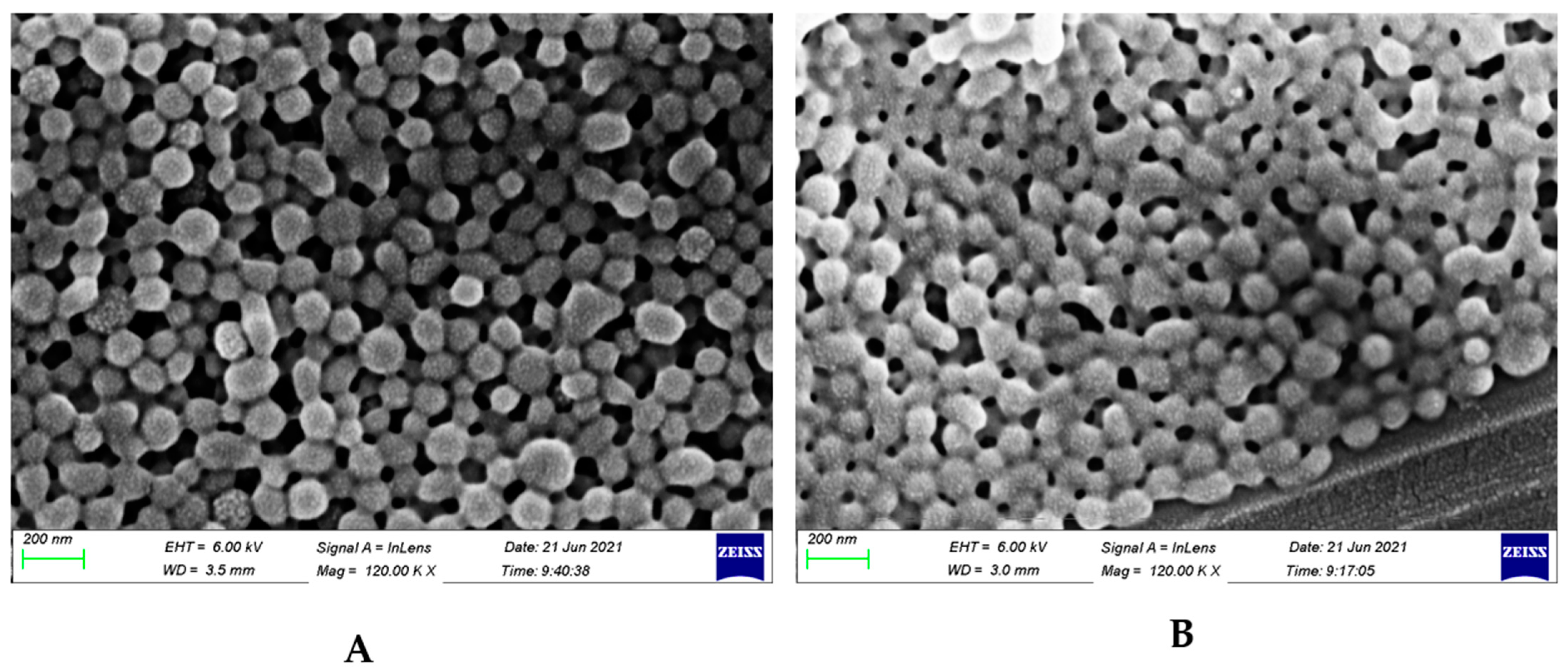
3.2. Size and Potential Data Show Spherical Coated Particles with Positive Surface Charge
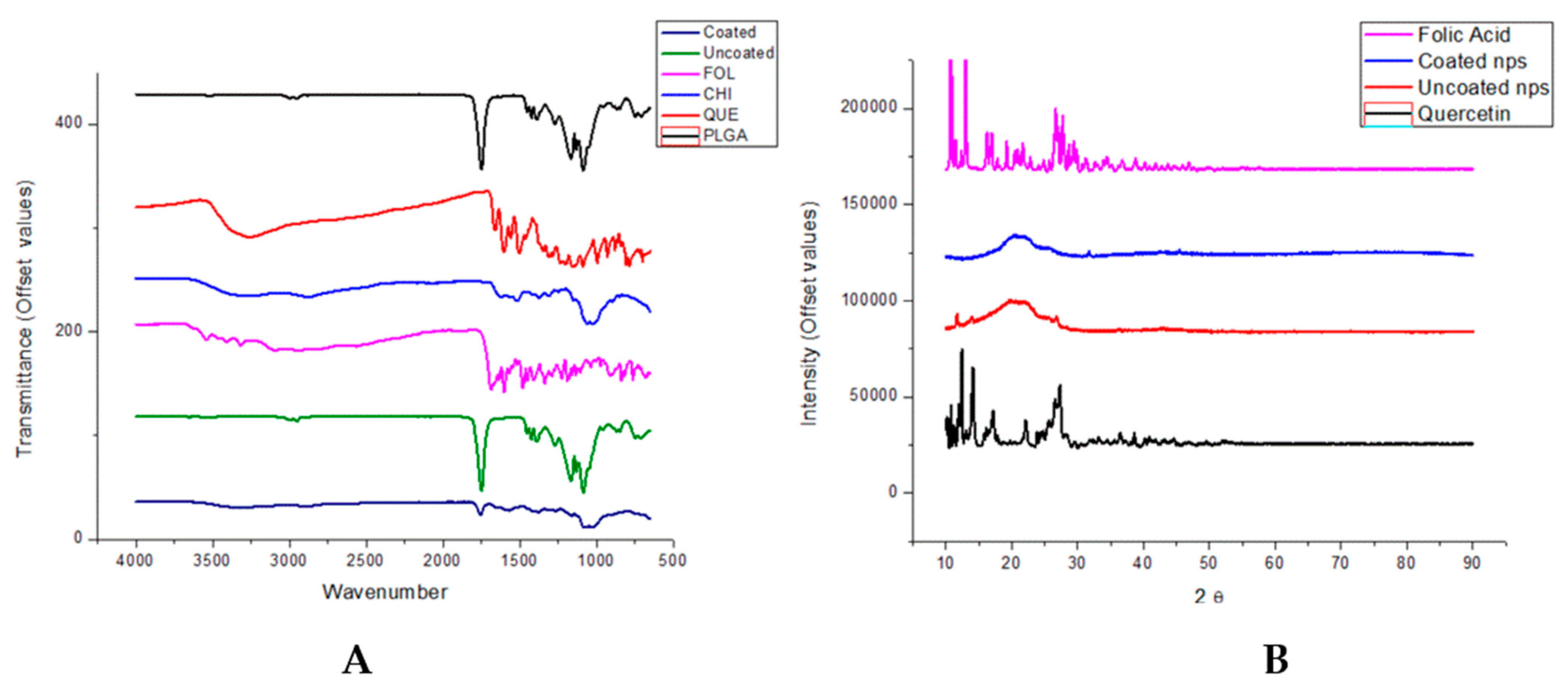
3.3. Molecular Structure by FTIR Shows Adsorption Interactions and XRD Show Amorphous Nanoparticle Structure
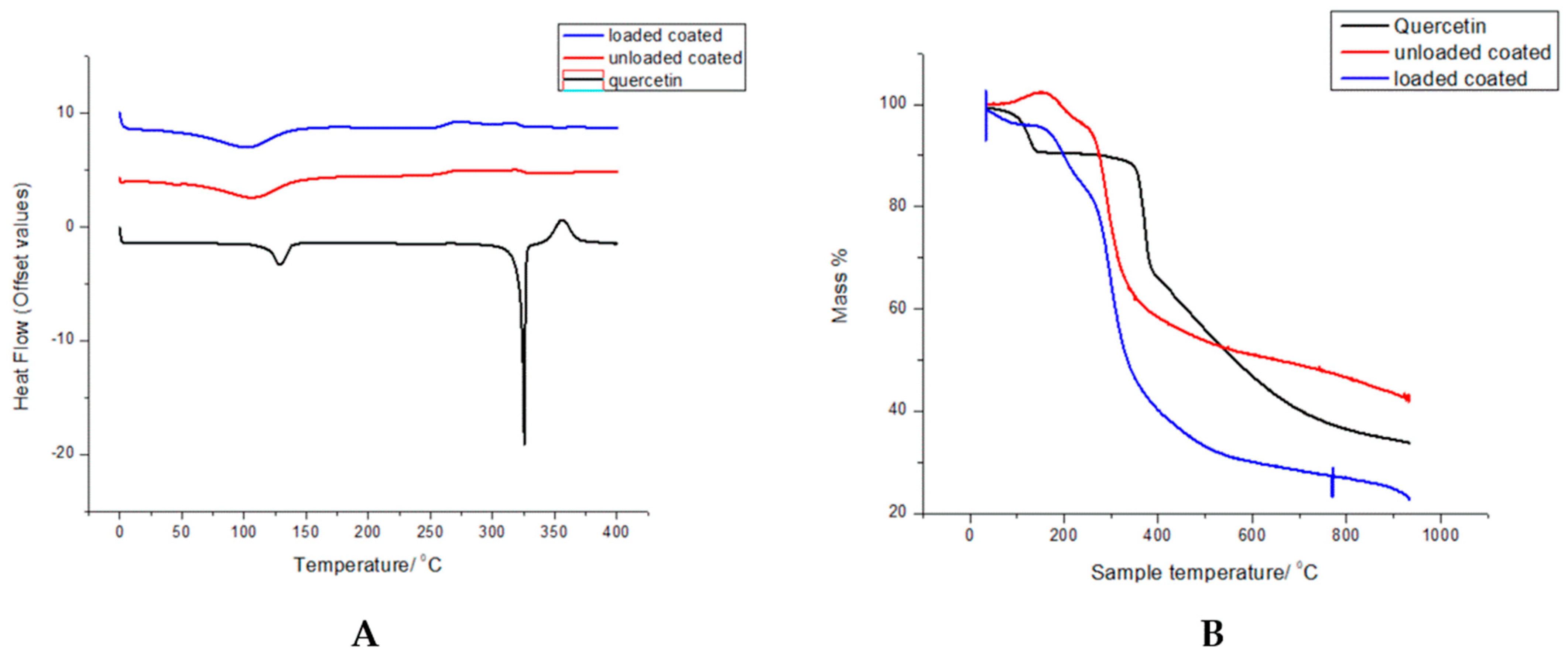
3.4. Thermal Degradation and Phase Transition Show Retention of Polymer Properties
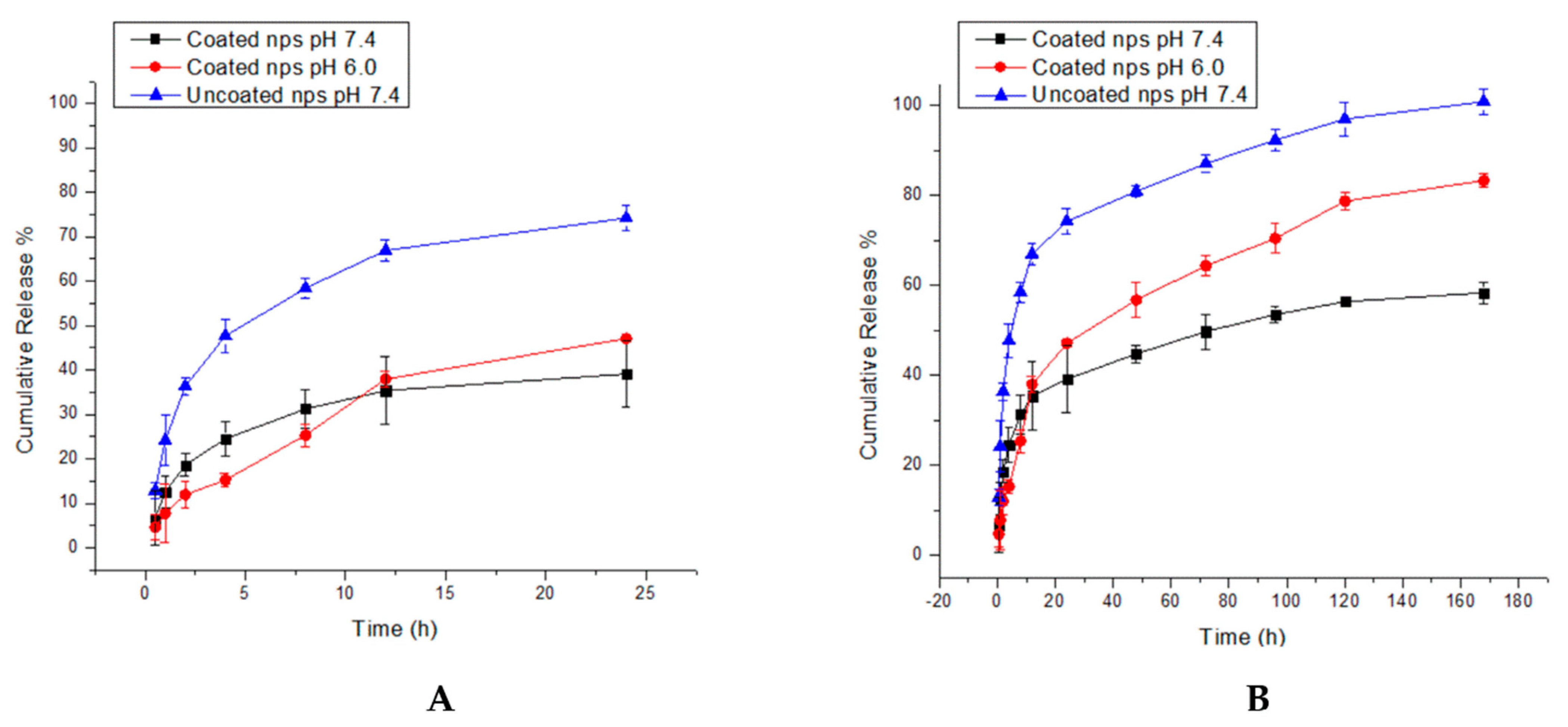
3.5. In Vitro Kinetic Study of Nanoparticle Systems Shows Efficient Quercetin Release
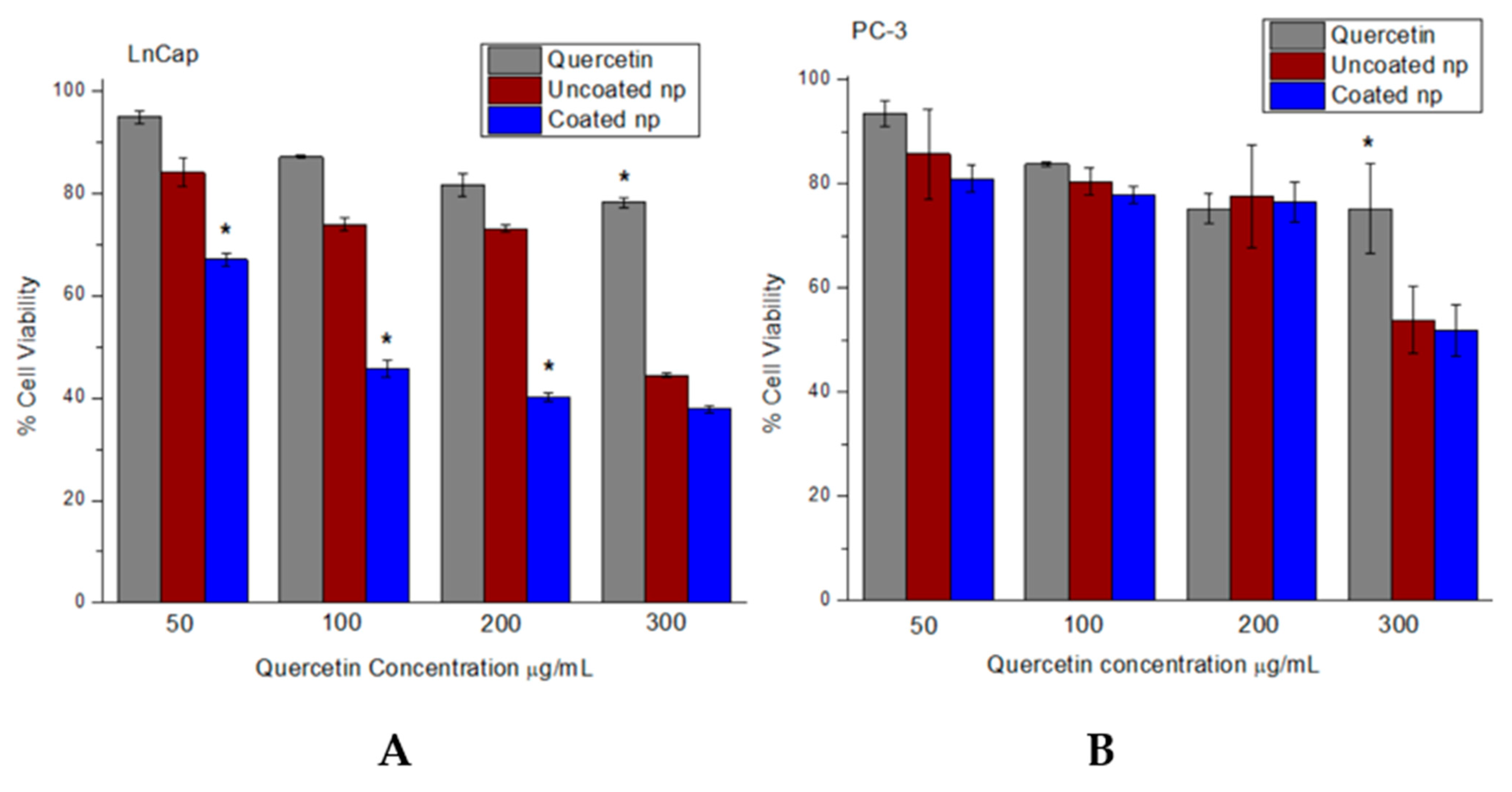
3.6. Increased Cytotoxicity of Quercetin in Nanoparticle System
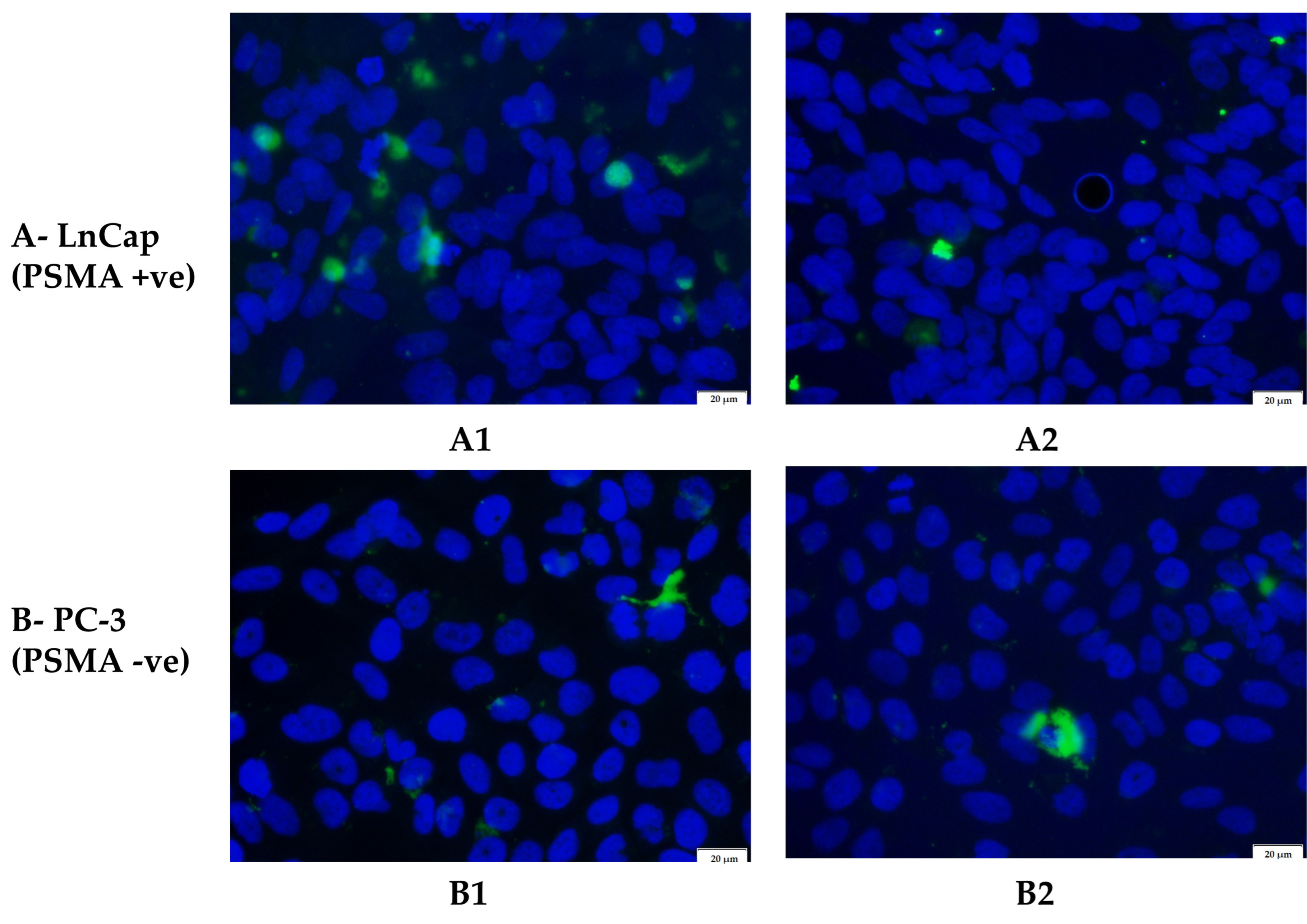
3.7. Greater Cellular Uptake of Targeted Nanoparticles in a PSMA-Positive Cell Line
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Lehto, U.-S.; Tenhola, H.; Taari, K.; Aromaa, A. Patients’ Perceptions of the Negative Effects Following Different Prostate Cancer Treatments and the Impact on Psychological Well-Being: A Nationwide Survey. Br. J. Cancer 2017, 116, 864–873. [Google Scholar] [CrossRef]
- Wolfram, J.; Ferrari, M. Clinical Cancer Nanomedicine. Nano Today 2019, 25, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. Progress in Nanomedicine: Approved and Investigational Nanodrugs. Pharm. Ther. 2017, 42, 742–755. [Google Scholar]
- Han, Y.; Wen, P.; Li, J.; Kataoka, K. Targeted Nanomedicine in Cisplatin-Based Cancer Therapeutics. J. Control. Release 2022, 345, 709–720. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Riccardi, C.; Napolitano, F.; Montesarchio, D.; Sampaolo, S.; Melone, M.A.B. Nanoparticle-Guided Brain Drug Delivery: Expanding the Therapeutic Approach to Neurodegenerative Diseases. Pharmaceutics 2021, 13, 1897. [Google Scholar] [CrossRef]
- Naqvi, S.; Panghal, A.; Flora, S.J.S. Nanotechnology: A Promising Approach for Delivery of Neuroprotective Drugs. Front. Neurosci. 2020, 14, 494. [Google Scholar] [CrossRef]
- Rizvi, S.A.A.; Saleh, A.M. Applications of Nanoparticle Systems in Drug Delivery Technology. Saudi Pharm. J. 2018, 26, 64–70. [Google Scholar] [CrossRef]
- Vinayak, M.; Maurya, A.K. Quercetin Loaded Nanoparticles in Targeting Cancer: Recent Development. Anticancer Agents Med. Chem. 2019, 19, 1560–1576. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Shabestari, F.A.; Vaezi, S.; Abak, A.; Shoorei, H.; Karimi, A.; Taheri, M.; Basiri, A. Emerging Impact of Quercetin in the Treatment of Prostate Cancer. Biomed. Pharmacother. 2021, 138, 111548. [Google Scholar] [CrossRef]
- Sharma, T.; Singh, D.; Mahapatra, A.; Mohapatra, P.; Sahoo, S.; Sahoo, S.K. Advancements in Clinical Translation of Flavonoid Nanoparticles for Cancer Treatment. OpenNano 2022, 8, 100074. [Google Scholar] [CrossRef]
- Saavedra-Leos, M.Z.; Jordan-Alejandre, E.; López-Camarillo, C.; Pozos-Guillen, A.; Leyva-Porras, C.; Silva-Cázares, M.B. Nanomaterial Complexes Enriched with Natural Compounds Used in Cancer Therapies: A Perspective for Clinical Application. Front. Oncol. 2021, 11, 664380. [Google Scholar] [CrossRef] [PubMed]
- Zang, X.; Cheng, M.; Zhang, X.; Chen, X. Quercetin Nanoformulations: A Promising Strategy for Tumor Therapy. Food Funct. 2021, 12, 6664–6681. [Google Scholar] [CrossRef] [PubMed]
- Nan, W.; Ding, L.; Chen, H.; Khan, F.U.; Yu, L.; Sui, X.; Shi, X. Topical Use of Quercetin-Loaded Chitosan Nanoparticles Against Ultraviolet B Radiation. Front. Pharmacol. 2018, 9, 826. [Google Scholar] [CrossRef] [PubMed]
- Tığlı Aydın, R.S.; Pulat, M. 5-Fluorouracil Encapsulated Chitosan Nanoparticles for PH-Stimulated Drug Delivery: Evaluation of Controlled Release Kinetics. J. Nanomater. 2012, 2012, 313961. [Google Scholar] [CrossRef]
- Popat, A.; Liu, J.; Lu, G.Q.M.; Qiao, S.Z. A PH-Responsive Drug Delivery System Based on Chitosan Coated Mesoporous Silica Nanoparticles. J. Mater. Chem. 2012, 22, 11173. [Google Scholar] [CrossRef]
- Shahid, N.; Erum, A.; Zaman, M.; Tulain, U.R.; Shoaib, Q.; Malik, N.S.; Kausar, R.; Rashid, A.; Rehman, U. Synthesis and Evaluation of Chitosan Based Controlled Release Nanoparticles for the Delivery of Ticagrelor. Des. Monomers. Polym. 2022, 25, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Dong, Z.; Tao, D.; Zhang, Y.; Liu, Z. The Acidic Tumor Microenvironment: A Target for Smart Cancer Nano-Theranostics. Natl. Sci. Rev. 2018, 5, 269–286. [Google Scholar] [CrossRef]
- Lin, B.; Chen, H.; Liang, D.; Lin, W.; Qi, X.; Liu, H.; Deng, X. Acidic PH and High-H 2 O 2 Dual Tumor Microenvironment-Responsive Nanocatalytic Graphene Oxide for Cancer Selective Therapy and Recognition. ACS Appl. Mater. Interfaces 2019, 11, 11157–11166. [Google Scholar] [CrossRef]
- Xiong, G.M.; Venkatraman, K.; Venkatraman, S. The Magic Bullet as Cancer Therapeutic—Has Nanotechnology Failed to Find Its Mark? Prog. Biomed. Eng. 2020, 2, 042004. [Google Scholar] [CrossRef]
- Tewabe, A.; Abate, A.; Tamrie, M.; Seyfu, A.; Abdela Siraj, E. Targeted Drug Delivery—From Magic Bullet to Nanomedicine: Principles, Challenges, and Future Perspectives. J. Multidiscip. Healthc. 2021, 14, 1711–1724. [Google Scholar] [CrossRef] [PubMed]
- Donin, N.M.; Reiter, R.E. Why Targeting PSMA Is a Game Changer in the Management of Prostate Cancer. J. Nucl. Med. 2018, 59, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Ma, H.; Shao, M.; Fan, Q.; Lv, H.; Peng, J.; Hao, T.; Li, D.; Zhao, C.; Zong, X. Synthesis of Folate-Chitosan Nanoparticles Loaded with Ligustrazine to Target Folate Receptor Positive Cancer Cells. Mol. Med. Rep. 2017, 16, 1101–1108. [Google Scholar] [CrossRef]
- Bellotti, E.; Cascone, M.G.; Barbani, N.; Rossin, D.; Rastaldo, R.; Giachino, C.; Cristallini, C. Targeting Cancer Cells Overexpressing Folate Receptors with New Terpolymer-Based Nanocapsules: Toward a Novel Targeted DNA Delivery System for Cancer Therapy. Biomedicines 2021, 9, 1275. [Google Scholar] [CrossRef]
- Angelopoulou, A.; Kolokithas-Ntoukas, A.; Fytas, C.; Avgoustakis, K. Folic Acid-Functionalized, Condensed Magnetic Nanoparticles for Targeted Delivery of Doxorubicin to Tumor Cancer Cells Overexpressing the Folate Receptor. ACS Omega 2019, 4, 22214–22227. [Google Scholar] [CrossRef]
- Hattori, Y.; Maitani, Y. Enhanced in Vitro DNA Transfection Efficiency by Novel Folate-Linked Nanoparticles in Human Prostate Cancer and Oral Cancer. J. Control. Release 2004, 97, 173–183. [Google Scholar] [CrossRef]
- Essa, D.; Kondiah, P.P.D.; Choonara, Y.E.; Pillay, V. The Design of Poly(Lactide-Co-Glycolide) Nanocarriers for Medical Applications. Front. Bioeng. Biotechnol. 2020, 8, 48. [Google Scholar] [CrossRef]
- Wilkosz, N.; Łazarski, G.; Kovacik, L.; Gargas, P.; Nowakowska, M.; Jamróz, D.; Kepczynski, M. Molecular Insight into Drug-Loading Capacity of PEG–PLGA Nanoparticles for Itraconazole. J. Phys. Chem. B 2018, 122, 7080–7090. [Google Scholar] [CrossRef]
- Wischke, C.; Schwendeman, S.P. Principles of Encapsulating Hydrophobic Drugs in PLA/PLGA Microparticles. Int. J. Pharm. 2008, 364, 298–327. [Google Scholar] [CrossRef]
- Yadav, N.; Tripathi, A.K.; Parveen, A. PLGA-Quercetin Nano-Formulation Inhibits Cancer Progression via Mitochondrial Dependent Caspase-3,7 and Independent FoxO1 Activation with Concomitant PI3K/AKT Suppression. Pharmaceutics 2022, 14, 1326. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Kaur, C.D.; Saraf, S.; Saraf, S. Formulation, Characterization, and Evaluation of Ligand-Conjugated Biodegradable Quercetin Nanoparticles for Active Targeting. Artif. Cells Nanomed. Biotechnol. 2016, 44, 960–970. [Google Scholar] [CrossRef] [PubMed]
- Baksi, R.; Singh, D.P.; Borse, S.P.; Rana, R.; Sharma, V.; Nivsarkar, M. In Vitro and in Vivo Anticancer Efficacy Potential of Quercetin Loaded Polymeric Nanoparticles. Biomed. Pharmacother. 2018, 106, 1513–1526. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, J.; Watanabe, W. Physical and Chemical Stability of Drug Nanoparticles. Adv. Drug Deliv. Rev. 2011, 63, 456–469. [Google Scholar] [CrossRef]
- Tavares Luiz, M.; Santos Rosa Viegas, J.; Palma Abriata, J.; Viegas, F.; Testa Moura de Carvalho Vicentini, F.; Lopes Badra Bentley, M.V.; Chorilli, M.; Maldonado Marchetti, J.; Tapia-Blácido, D.R. Design of Experiments (DoE) to Develop and to Optimize Nanoparticles as Drug Delivery Systems. Eur. J. Pharm. Biopharm. 2021, 165, 127–148. [Google Scholar] [CrossRef]
- Fasehee, H.; Dinarvand, R.; Ghavamzadeh, A.; Esfandyari-Manesh, M.; Moradian, H.; Faghihi, S.; Ghaffari, S.H. Delivery of Disulfiram into Breast Cancer Cells Using Folate-Receptor-Targeted PLGA-PEG Nanoparticles: In Vitro and in Vivo Investigations. J. Nanobiotechnol. 2016, 14, 32. [Google Scholar] [CrossRef]
- Nguyen, C.N.; Tran, B.N.; Thi, H.N.; Huu, P.P.; Thi, H.N. Physical Absorption of Folic Acid and Chitosan on Dihydroartemisinin-Loaded Poly-Lactic-Co-Glycolic Acid Nanoparticles via Electrostatic Interaction for Their Enhanced Uptake and Anticancer Effect. J. Nanomater. 2019, 2019, 6808530. [Google Scholar] [CrossRef]
- Musalli, A.H.; Talukdar, P.D.; Roy, P.; Kumar, P.; Wong, T.W. Folate-Induced Nanostructural Changes of Oligochitosan Nanoparticles and Their Fate of Cellular Internalization by Melanoma. Carbohydr. Polym. 2020, 244, 116488. [Google Scholar] [CrossRef]
- Chaudhari, S.P.; Bangar, J.V.; Akuskar, G.K.; Ratnaparkhi, M.P. Development and Validation of UV Spectrophotometric Method for Simultaneous Estimation of Rutin and Quercetin in Niosome Formulation. Pharm. Lett. 2014, 6, 271–276. [Google Scholar]
- Anwer, M.K.; Al-Mansoor, M.A.; Jamil, S.; Al-Shdefat, R.; Ansari, M.N.; Shakeel, F. Development and Evaluation of PLGA Polymer Based Nanoparticles of Quercetin. Int. J. Biol. Macromol. 2016, 92, 213–219. [Google Scholar] [CrossRef]
- Dandamudi, M.; McLoughlin, P.; Behl, G.; Rani, S.; Coffey, L.; Chauhan, A.; Kent, D.; Fitzhenry, L. Chitosan-Coated PLGA Nanoparticles Encapsulating Triamcinolone Acetonide as a Potential Candidate for Sustained Ocular Drug Delivery. Pharmaceutics 2021, 13, 1590. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Lv, X.; Le, Y. Chitosan-Modified PLGA Nanoparticles for Control-Released Drug Delivery. Polymers 2019, 11, 304. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Giottonini, K.Y.; Rodríguez-Córdova, R.J.; Gutiérrez-Valenzuela, C.A.; Peñuñuri-Miranda, O.; Zavala-Rivera, P.; Guerrero-Germán, P.; Lucero-Acuña, A. PLGA Nanoparticle Preparations by Emulsification and Nanoprecipitation Techniques: Effects of Formulation Parameters. RSC Adv. 2020, 10, 4218–4231. [Google Scholar] [CrossRef]
- Fissan, H.; Ristig, S.; Kaminski, H.; Asbach, C.; Epple, M. Comparison of Different Characterization Methods for Nanoparticle Dispersions before and after Aerosolization. Anal. Methods 2014, 6, 7324. [Google Scholar] [CrossRef]
- Al-Nemrawi, N.; Alshraiedeh, N.; Zayed, A.; Altaani, B. Low Molecular Weight Chitosan-Coated PLGA Nanoparticles for Pulmonary Delivery of Tobramycin for Cystic Fibrosis. Pharmaceuticals 2018, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Abd El Hady, W.E.; Mohamed, E.A.; Soliman, O.A.E.-A.; el Sabbagh, H.M. In Vitro–in Vivo Evaluation of Chitosan-PLGA Nanoparticles for Potentiated Gastric Retention and Anti-Ulcer Activity of Diosmin. Int. J. Nanomed. 2019, 14, 7191–7213. [Google Scholar] [CrossRef] [PubMed]
- Al-Nemrawi, N.K.; Altawabeyeh, R.M.; Darweesh, R.S. Preparation and Characterization of Docetaxel-PLGA Nanoparticles Coated with Folic Acid-Chitosan Conjugate for Cancer Treatment. J. Pharm. Sci. 2022, 111, 485–494. [Google Scholar] [CrossRef]
- Li, X.-Y.; Li, Y.-C.; Yu, D.-G.; Liao, Y.-Z.; Wang, X. Fast Disintegrating Quercetin-Loaded Drug Delivery Systems Fabricated Using Coaxial Electrospinning. Int. J. Mol. Sci. 2013, 14, 21647–21659. [Google Scholar] [CrossRef]
- Hassan, M.; Farid, D.; Mahdi, A.; Masood, K.; Hossein, A.; Morteza, K. Methotrexate-Loaded PLGA Nanoparticles: Preparation, Characterization and Their Cytotoxicity Effect on Human Glioblastoma U87MG Cells. Int. J. Med. Nano Res. 2017, 4, 20. [Google Scholar] [CrossRef]
- Jardim, K.V.; Siqueira, J.L.N.; Báo, S.N.; Parize, A.L. In Vitro Cytotoxic and Antioxidant Evaluation of Quercetin Loaded in Ionic Cross-Linked Chitosan Nanoparticles. J. Drug Deliv. Sci. Technol. 2022, 74, 103561. [Google Scholar] [CrossRef]
- Hu, F.; Liu, W.; Yan, L.; Kong, F.; Wei, K. Optimization and Characterization of Poly(Lactic-Co-Glycolic Acid) Nanoparticles Loaded with Astaxanthin and Evaluation of Anti-Photodamage Effect in Vitro. R. Soc. Open Sci. 2019, 6, 191184. [Google Scholar] [CrossRef] [PubMed]
- Gathirwa, J.W.; Omwoyo, W.; Ogutu, B.; Oloo, F.; Swai, H.; Kalombo, L.; Melariri, P.; Maroa, G. Preparation, Characterization, and Optimization of Primaquine-Loaded Solid Lipid Nanoparticles. Int. J. Nanomed. 2014, 9, 3865–3874. [Google Scholar] [CrossRef] [PubMed]
- de Lima, I.A.; Khalil, N.M.; Tominaga, T.T.; Lechanteur, A.; Sarmento, B.; Mainardes, R.M. Mucoadhesive Chitosan-Coated PLGA Nanoparticles for Oral Delivery of Ferulic Acid. Artif Cells Nanomed. Biotechnol. 2018, 46, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Chronopoulou, L.; Massimi, M.; Giardi, M.F.; Cametti, C.; Devirgiliis, L.C.; Dentini, M.; Palocci, C. Chitosan-Coated PLGA Nanoparticles: A Sustained Drug Release Strategy for Cell Cultures. Colloids. Surf. B Biointerfaces 2013, 103, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Homayouni Tabrizi, M. Fabrication of Folic Acid-Conjugated Chitosan-Coated PLGA Nanoparticles for Targeted Delivery of Peganum Harmala Smoke Extract to Breast Cancer Cells. Nanotechnology 2022, 33, 495101. [Google Scholar] [CrossRef] [PubMed]
- Flores, O.; Santra, S.; Kaittanis, C.; Bassiouni, R.; Khaled, A.S.; Khaled, A.R.; Grimm, J.; Perez, J.M. PSMA-Targeted Theranostic Nanocarrier for Prostate Cancer. Theranostics 2017, 7, 2477–2494. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response Surface Methodology (RSM) as a Tool for Optimization in Analytical Chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef]
- Lee, R. Statistical Design of Experiments for Screening and Optimization. Chem. Ing. Tech. 2019, 91, 191–200. [Google Scholar] [CrossRef]
- Pandey, P.; Chellappan, D.K.; Tambuwala, M.M.; Bakshi, H.A.; Dua, K.; Dureja, H. Central Composite Designed Formulation, Characterization and in Vitro Cytotoxic Effect of Erlotinib Loaded Chitosan Nanoparticulate System. Int. J. Biol. Macromol. 2019, 141, 596–610. [Google Scholar] [CrossRef]
- Dhas, N.L.; Ige, P.P.; Kudarha, R.R. Design, Optimization and in-Vitro Study of Folic Acid Conjugated-Chitosan Functionalized PLGA Nanoparticle for Delivery of Bicalutamide in Prostate Cancer. Powder Technol. 2015, 283, 234–245. [Google Scholar] [CrossRef]
- Duranoğlu, D.; Uzunoglu, D.; Mansuroglu, B.; Arasoglu, T.; Derman, S. Synthesis of Hesperetin-Loaded PLGA Nanoparticles by Two Different Experimental Design Methods and Biological Evaluation of Optimized Nanoparticles. Nanotechnology 2018, 29, 395603. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Lin, J.; Hong, J.; Han, D.; Zhang, A.D.; Lan, R.; Fu, L.; Wu, Z.; Lin, J.; Zhang, W.; et al. Potential Toxicity of Quercetin: The Repression of Mitochondrial Copy Number via Decreased POLG Expression and Excessive TFAM Expression in Irradiated Murine Bone Marrow. Toxicol. Rep. 2014, 1, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Wu, Y.; Liu, Y.; Wu, D. High Drug-Loading Nanomedicines: Progress, Current Status, and Prospects. Int. J. Nanomed. 2017, 12, 4085–4109. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Liu, Y.; Jin, S.; Hui, Y.; Wang, X.; Xu, L.; Chen, D.; Weitz, D.; Zhao, C. Phase Separation-induced Nanoprecipitation for Making Polymer Nanoparticles with High Drug Loading. Aggregate 2023, e314. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, G.; Jin, S.; Xu, L.; Zhao, C. Development of High-Drug-Loading Nanoparticles. Chempluschem 2020, 85, 2143–2157. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E. The Role of Surface Charge in Cellular Uptake and Cytotoxicity of Medical Nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef] [PubMed]
- Foroozandeh, P.; Aziz, A.A. Insight into Cellular Uptake and Intracellular Trafficking of Nanoparticles. Nanoscale Res. Lett. 2018, 13, 339. [Google Scholar] [CrossRef]
- Sabourian, P.; Yazdani, G.; Ashraf, S.S.; Frounchi, M.; Mashayekhan, S.; Kiani, S.; Kakkar, A. Effect of Physico-Chemical Properties of Nanoparticles on Their Intracellular Uptake. Int. J. Mol. Sci. 2020, 21, 8019. [Google Scholar] [CrossRef]
- Duskey, J.T.; Rice, K.G. Nanoparticle Ligand Presentation for Targeting Solid Tumors. AAPS PharmSciTech 2014, 15, 1345–1354. [Google Scholar] [CrossRef]
- Yoo, J.; Park, C.; Yi, G.; Lee, D.; Koo, H. Active Targeting Strategies Using Biological Ligands for Nanoparticle Drug Delivery Systems. Cancers 2019, 11, 640. [Google Scholar] [CrossRef]
- Mesquita, B.S.; Fens, M.H.A.M.; di Maggio, A.; Bosman, E.D.C.; Hennink, W.E.; Heger, M.; Oliveira, S. The Impact of Nanobody Density on the Targeting Efficiency of PEGylated Liposomes. Int. J. Mol. Sci. 2022, 23, 14974. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.S.; Schorzman, A.N.; Finniss, M.C.; Bowerman, C.J.; Peng, L.; Luft, J.C.; Madden, A.J.; Wang, A.Z.; Zamboni, W.C.; DeSimone, J.M. Nanoparticle Drug Loading as a Design Parameter to Improve Docetaxel Pharmacokinetics and Efficacy. Biomaterials 2013, 34, 8424–8429. [Google Scholar] [CrossRef] [PubMed]
- Lestari, W.A.; Wahyuningsih, S.; Gomez-Ruiz, S.; Wibowo, F.R. Drug Loading Ability and Release Study of Various Size Small Mesoporous Silica Nanoparticle as Drug Carrier. J. Phys. Conf. Ser. 2022, 2190, 012032. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.; Gao, Y.; Liu, S.; Li, K.; Wang, S.; Gao, L.; Shi, M.; Liu, Z.; Han, Z.; et al. Effect of a Drug Delivery System Made of Quercetin Formulated into PEGylation Liposomes on Cervical Carcinoma In Vitro and In Vivo. J. Nanomater. 2021, 2021, 9389934. [Google Scholar] [CrossRef]
- Davarnejad, R.; Layeghy, K.; Soleymani, M.; Ayazi, A. Encapsulation of Quercetin in a Mixed Nanomicellar System to Enhance Its Cytotoxicity against Breast Cancer Cells. Chem. Eng. Technol. 2022, 45, 1100–1105. [Google Scholar] [CrossRef]
- Xiao, K.; Li, Y.; Luo, J.; Lee, J.S.; Xiao, W.; Gonik, A.M.; Agarwal, R.G.; Lam, K.S. The Effect of Surface Charge on in Vivo Biodistribution of PEG-Oligocholic Acid Based Micellar Nanoparticles. Biomaterials 2011, 32, 3435–3446. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.S.; Radharani, N.N.V.; Gorain, M.; Bulbule, A.; Shetti, D.; Roy, G.; Baby, T.; Kundu, G.C. RGD Functionalized Chitosan Nanoparticle Mediated Targeted Delivery of Raloxifene Selectively Suppresses Angiogenesis and Tumor Growth in Breast Cancer. Nanoscale 2020, 12, 10664–10684. [Google Scholar] [CrossRef] [PubMed]
- Sakulkhu, U.; Mahmoudi, M.; Maurizi, L.; Coullerez, G.; Hofmann-Amtenbrink, M.; Vries, M.; Motazacker, M.; Rezaee, F.; Hofmann, H. Significance of Surface Charge and Shell Material of Superparamagnetic Iron Oxide Nanoparticle (SPION) Based Core/Shell Nanoparticles on the Composition of the Protein Corona. Biomater. Sci. 2015, 3, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Bozkir, A.; Saka, O.M. Chitosan Nanoparticles for Plasmid DNA Delivery: Effect of Chitosan Molecular Structure on Formulation and Release Characteristics. Drug Deliv. 2004, 11, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, B.; Leonick, N.; Gade, V.; Frey, D.M.; Zhang, F.; Linhardt, R.J.; Gross, R.A. Ionically Complexed Nanoparticles for Heparin Oral Delivery. Precis. Nanomed. 2022, 5, 918–929. [Google Scholar] [CrossRef]
- Wang, F.; Li, J.; Tang, X.; Huang, K.; Chen, L. Polyelectrolyte Three Layer Nanoparticles of Chitosan/Dextran Sulfate/Chitosan for Dual Drug Delivery. Colloids. Surf. B Biointerfaces 2020, 190, 110925. [Google Scholar] [CrossRef] [PubMed]
- Escareño, N.; Hassan, N.; Kogan, M.J.; Juárez, J.; Topete, A.; Daneri-Navarro, A. Microfluidics-Assisted Conjugation of Chitosan-Coated Polymeric Nanoparticles with Antibodies: Significance in Drug Release, Uptake, and Cytotoxicity in Breast Cancer Cells. J. Colloid. Interface Sci. 2021, 591, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Yao, V.; Berkman, C.E.; Choi, J.K.; O’Keefe, D.S.; Bacich, D.J. Expression of Prostate-Specific Membrane Antigen (PSMA), Increases Cell Folate Uptake and Proliferation and Suggests a Novel Role for PSMA in the Uptake of the Non-Polyglutamated Folate, Folic Acid. Prostate 2010, 70, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Jivrajani, M.; Nivsarkar, M. Ligand-Targeted Bacterial Minicells: Futuristic Nano-Sized Drug Delivery System for the Efficient and Cost Effective Delivery of ShRNA to Cancer Cells. Nanomedicine 2016, 12, 2485–2498. [Google Scholar] [CrossRef] [PubMed]
| Formulation | Parameters | Q/mg | Chi/mg | Fol/mg |
|---|---|---|---|---|
| P1 | (1, 0, 0) | 30 | 35 | 30 |
| P2 | (−1, −1, −1) | 10 | 10 | 15 |
| P3 | (0, 0, −1) | 20 | 35 | 10 |
| P4 | (1, 1, 1) | 30 | 60 | 45 |
| P5 | (0, 0, 0) | 20 | 35 | 30 |
| P6 | (0, 1, 0) | 20 | 60 | 30 |
| P7 | (−1, 1, −1) | 10 | 60 | 15 |
| P8 | (1, 1, −1) | 30 | 60 | 15 |
| P9 | (1, −1, −1) | 30 | 10 | 15 |
| P10 | (1, −1, 1) | 30 | 10 | 45 |
| P11 | (0, −1, 0) | 20 | 10 | 30 |
| P12 | (0, 0, 0) | 20 | 35 | 30 |
| P13 | (−1, −1, 1) | 10 | 10 | 45 |
| P14 | (0, 0, 1) | 20 | 35 | 45 |
| P15 | (−1, 1, 1) | 10 | 60 | 45 |
| P16 | (−1, 0, 0) | 10 | 35 | 30 |
| Formulation | Parameters | QL/% | Ζ Potential | FA/% |
|---|---|---|---|---|
| P1 | (1, 0, 0) | 9.04 ± 2.15 | −1.60 ± 1.45 | 1.90 ± 0.15 |
| P2 | (−1, −1, −1) | 3.01 ± 1.50 | +2.33 ± 0.87 | 0.37 ± 0.15 |
| P3 | (0, 0, −1) | 3.90 ± 1.05 | +2.40 ± 0.08 | 0.77 ± 0.26 |
| P4 | (1, 1, 1) | 8.27 ± 1.30 | −5.03 ± 0.67 | 1.20 ± 0.25 |
| P5 | (0, 0, 0) | 2.78 ± 1.71 | +3.79 ± 1.21 | 0.44 ± 0.06 |
| P6 | (0, 1, 0) | 1.83 ± 1.30 | −2.17 ± 0.63 | 0.21 ± 0.17 |
| P7 | (−1, 1, −1) | 1.71 ± 2.86 | +3.83 ± 1.34 | 0.18 ± 0.15 |
| P8 | (1, 1, −1) | 6.72 ± 1.30 | −4.93 ± 0.17 | 0.66 ± 0.46 |
| P9 | (1, −1, −1) | 8.94 ± 1.30 | +1.07 ± 0.35 | 0.91 ± 0.10 |
| P10 | (1, −1, 1) | 14.2 ± 2.39 | −3.37 ± 0.31 | 1.48 ± 0,59 |
| P11 | (0, −1, 0) | 5.18 ± 0.80 | +1.58 ± 1.56 | 0.61 ± 0.21 |
| P12 | (0, 0, 0) | 2.45 ± 1.67 | +3.15 ± 0,47 | 0.54 ± 0.17 |
| P13 | (−1, −1, 1) | 7.01 ± 1.05 | −1.20 ± 0.61 | 0.95 ± 0.25 |
| P14 | (0, 0, 1) | 6.12 ± 1.30 | +2.39 ± 0.85 | 1.13 ± 0.21 |
| P15 | (−1, 1, 1) | 6.05 ± 0.80 | +3.28 ± 0.36 | 0.95 ± 0.15 |
| P16 | (−1, 0, 0) | 3.42 ± 1.30 | +2.26 ± 0.29 | 0.70 ± 0.25 |
| Response | Predicted | Actual | Bias |
|---|---|---|---|
| Quercetin Loading | 7.02 | 7.11 ± 1.60 | +0.0126 |
| Surface Charge | +2.52 | +1.84 ± 0.40 | −0.370 |
| Folic Acid Content | 1.24 | 1.61 ± 0.35 | +0.236 |
| Formulation | Size/nm | PDI | ζ Potential/mV |
|---|---|---|---|
| Uncoated nps | 159.8 ± 2.0 | 0.068 ± 0.01 | −21.0 ± 1.6 |
| Coated nps | 206.2 ± 1.7 | 0.069 ± 0.002 | +1.84 ± 0.4 |
| Cell Line | Treatment | % Fluorescence |
|---|---|---|
| LnCap | Coated nps | 2.79 ± 0.50 |
| PC-3 | Uncoated nps | 1.86 ± 0.22 |
| Coated nps | 1.72 ± 0.37 | |
| Uncoated nps | 1.99 ± 0.39 |
| System | ζ Potential/mV | Drug Release | Biological Activity |
|---|---|---|---|
| Silica nanoparticles | Varied | 7% after 72 h | Not specified |
| Pegylated quercetin liposomes | −13.1 | 85% after 96 h | Increased cytotoxicity in cervical cancer cells and greater reduction in tumor size in a mouse model compared to free quercetin |
| Nanomicelles | Not specified | 83.6% after 120 h | Increased cytotoxicity on breast cancer cells compared to free quercetin |
| Chitosan-quercetin nps | +22.53 | 76% after 12 h | Increased cytotoxicity on lung and breast cancer cells and greater reduction in tumor volume in a mouse model compared to free quercetin |
| SPIONS | Varied | Not applicable | Increased protein binding of positively charged particles |
| Chitosan nps | +9 | 100% after 24 h | Not specified |
| Folate-linked nps | +19 | 70% after 4 h | PSMA binding on LnCap cells |
| Folic acid-conjugated nps | Not specified | 90% after 24 h | Increased cytotoxicity of therapeutic peptide and greater cellular uptake in LnCap cells |
| Folic acid minicells | Not specified | Not applicable | Increased cellular uptake in LnCap cells |
| PLGA-quercetin chitosan folic acid nps | +1.84 | 78% after 168 h | Increased cytotoxicity of quercetin and greater cellular uptake in LnCap cells |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Essa, D.; Kondiah, P.P.D.; Kumar, P.; Choonara, Y.E. Design of Chitosan-Coated, Quercetin-Loaded PLGA Nanoparticles for Enhanced PSMA-Specific Activity on LnCap Prostate Cancer Cells. Biomedicines 2023, 11, 1201. https://doi.org/10.3390/biomedicines11041201
Essa D, Kondiah PPD, Kumar P, Choonara YE. Design of Chitosan-Coated, Quercetin-Loaded PLGA Nanoparticles for Enhanced PSMA-Specific Activity on LnCap Prostate Cancer Cells. Biomedicines. 2023; 11(4):1201. https://doi.org/10.3390/biomedicines11041201
Chicago/Turabian StyleEssa, Divesha, Pierre P. D. Kondiah, Pradeep Kumar, and Yahya E. Choonara. 2023. "Design of Chitosan-Coated, Quercetin-Loaded PLGA Nanoparticles for Enhanced PSMA-Specific Activity on LnCap Prostate Cancer Cells" Biomedicines 11, no. 4: 1201. https://doi.org/10.3390/biomedicines11041201
APA StyleEssa, D., Kondiah, P. P. D., Kumar, P., & Choonara, Y. E. (2023). Design of Chitosan-Coated, Quercetin-Loaded PLGA Nanoparticles for Enhanced PSMA-Specific Activity on LnCap Prostate Cancer Cells. Biomedicines, 11(4), 1201. https://doi.org/10.3390/biomedicines11041201









