Fecal and Urinary Adipokines as Disease Biomarkers
Abstract
1. Introduction
2. Adipokines in Urine and Feces
2.1. Adiponectin
2.1.1. Urinary Adiponectin in Diabetic Patients
2.1.2. Urinary Adiponectin in Systemic Lupus Erythematosus (SLE)
2.1.3. Urinary Adiponectin in Patients with Focal Segmental Glomerulosclerosis
2.1.4. Fecal Adiponectin in Colorectal Cancer
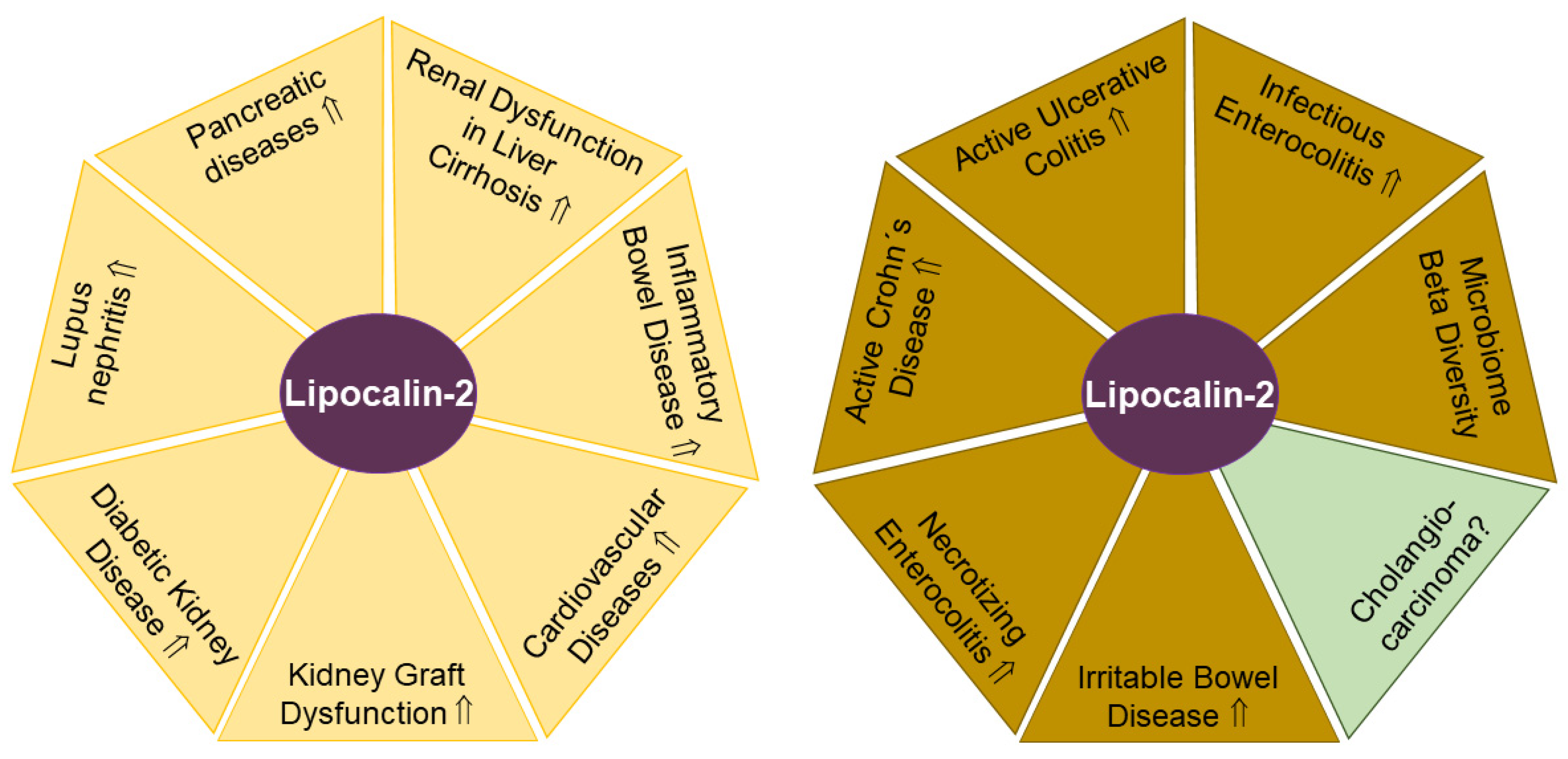
2.2. Lipocalin-2
2.2.1. Urinary Lipocalin-2 in SLE
2.2.2. Urinary Lipocalin-2 in Diabetic Chronic Kidney Disease
2.2.3. Urinary Lipocalin-2 in Patients with Macroproteinuria, Glomerulonephritis, Kidney Transplantation and Sepsis
2.2.4. Urinary Lipocalin-2 in Inflammatory Bowel Disease
2.2.5. Urinary Lipocalin-2 in Pancreatic Diseases
2.2.6. Urinary Lipocalin-2 in Liver Cirrhosis
2.2.7. Fecal Lipocalin-2 in Necrotizing Enterocolitis
2.2.8. Fecal Lipocalin-2 in Inflammatory Bowel Disease
2.2.9. Fecal Lipocalin-2 in Irritable Bowel Disease
2.2.10. Fecal lipocalin-2 in Parkinson’s Disease
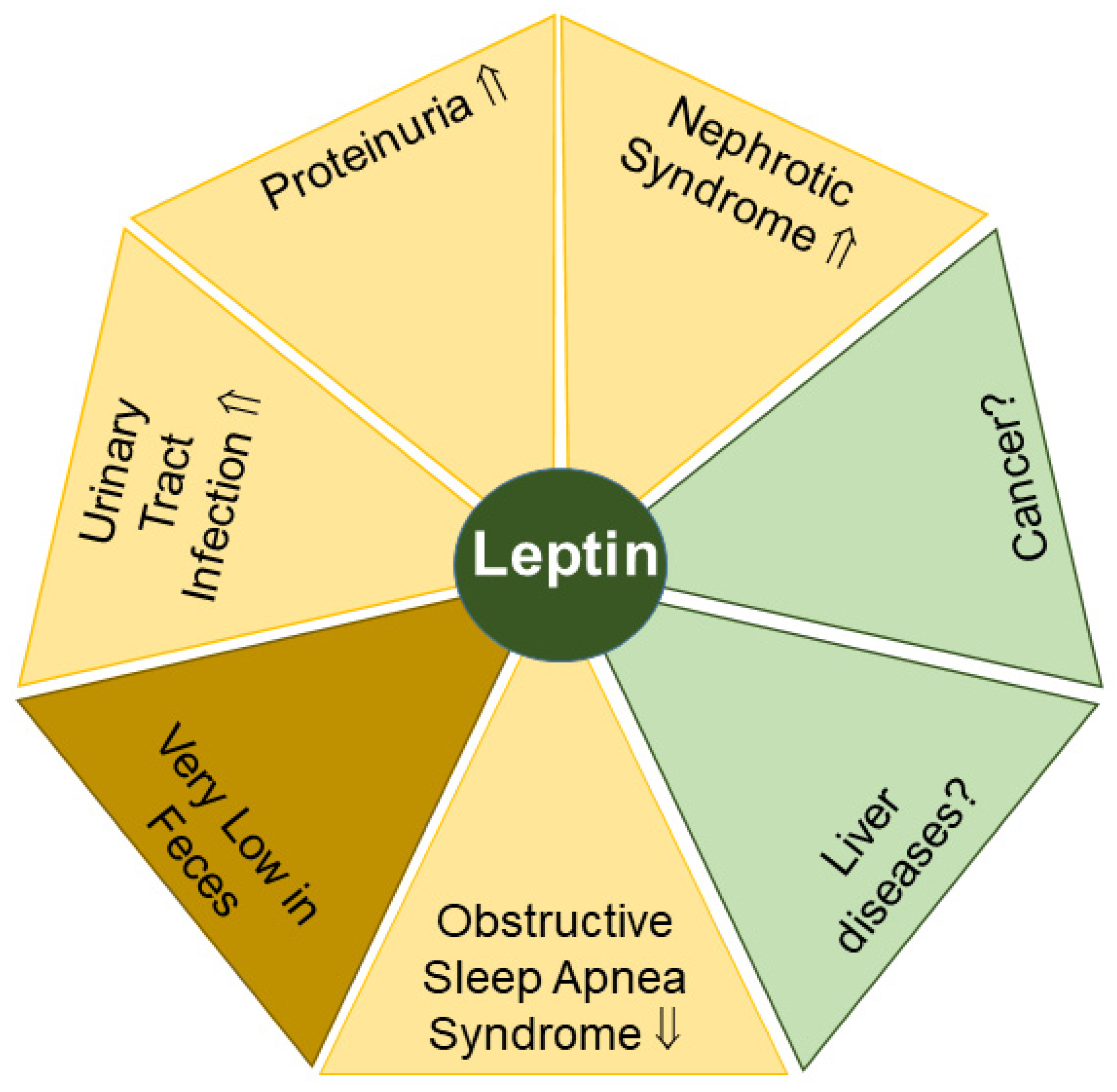
2.3. Leptin
2.3.1. Leptin in Proteinuria and Nephrotic Syndrome
2.3.2. Leptin in Urinary Tract Infection
2.3.3. Leptin in Obstructive Sleep Apnea Syndrome
2.3.4. Leptin in Feces
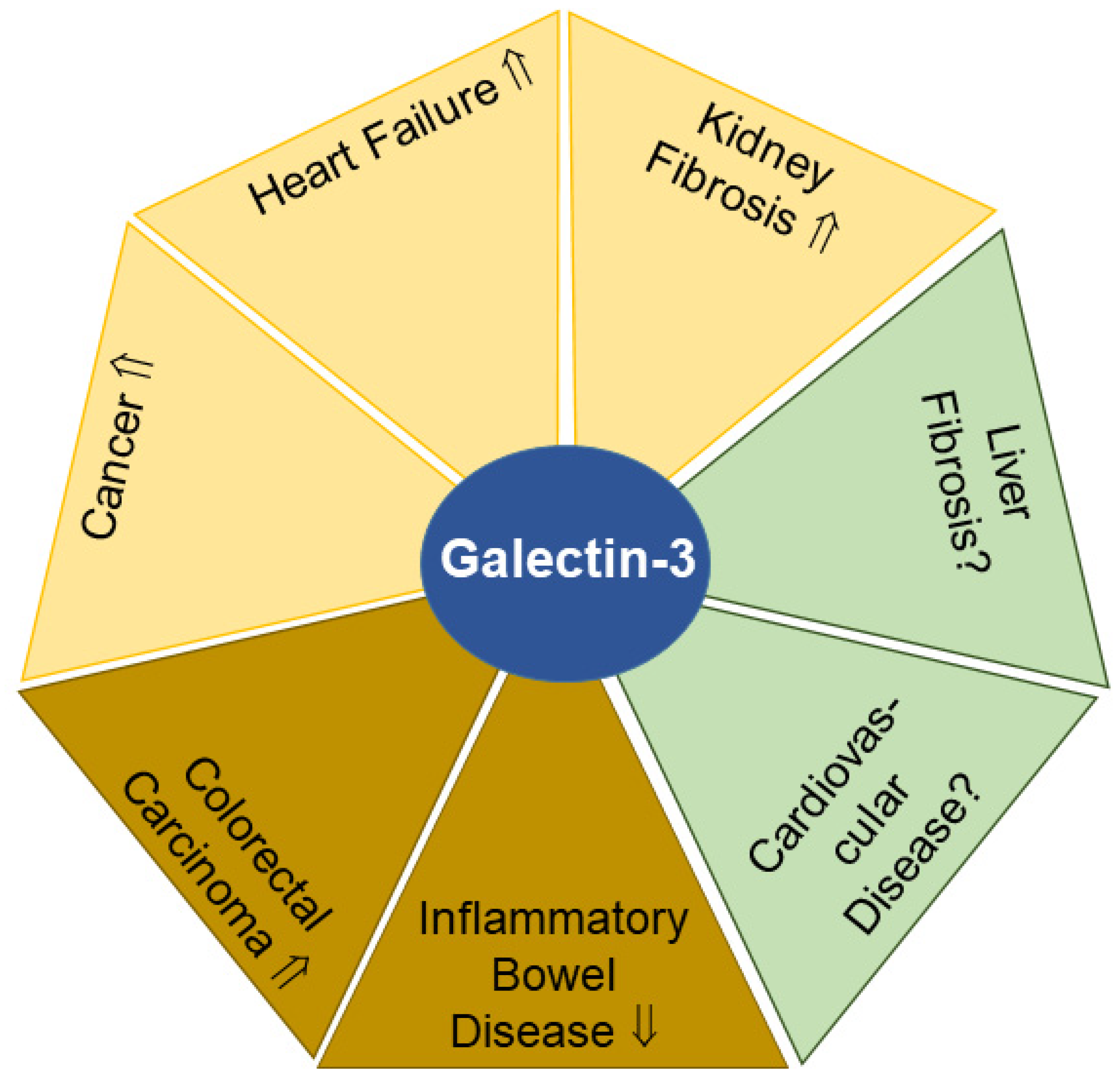
2.4. Galectin-3
2.4.1. Urinary Galectin-3 and Kidney Fibrosis
2.4.2. Urinary Galectin-3 and Heart Failure
2.4.3. Urinary Galectin-3 and Cancer
2.4.4. Fecal Galectin-3 and Cancer
2.4.5. Fecal Galectin-3 and Inflammatory Bowel Disease
2.5. Chemerin
Urinary and Fecal Chemerin in Inflammatory Bowel Disease
2.6. Interleukin-6 (IL-6)
2.6.1. Urinary IL-6 in Rheumatoid Arthritis
2.6.2. Urinary IL-6 and Urinary Tract Infection
2.6.3. Urinary IL-6 in SLE
2.6.4. Urinary IL-6 in IgA Nephropathy
2.6.5. Urinary IL-6 in Diabetic Nephropathy
2.6.6. Urinary IL-6 in Organ Rejection
2.6.7. Fecal IL-6 in Gastroenteritis
2.6.8. Fecal IL-6 in Liver Cirrhosis
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Booth, A.; Magnuson, A.; Fouts, J.; Foster, M. Adipose tissue, obesity and adipokines: Role in cancer promotion. Horm. Mol. Biol. Clin. Investig. 2015, 21, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Buechler, C.; Haberl, E.M.; Rein-Fischboeck, L.; Aslanidis, C. Adipokines in Liver Cirrhosis. Int. J. Mol. Sci. 2017, 18, 1392. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.L.; Yang, Z.; Yang, S.S. Roles of Adipokines in Digestive Diseases: Markers of Inflammation, Metabolic Alteration and Disease Progression. Int. J. Mol. Sci. 2020, 21, 8308. [Google Scholar] [CrossRef]
- Fatima, S.S.; Rehman, R.; Baig, M.; Khan, T.A. New roles of the multidimensional adipokine: Chemerin. Peptides 2014, 62, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Kamada, Y.; Takehara, T.; Hayashi, N. Adipocytokines and liver disease. J. Gastroenterol. 2008, 43, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Schuler-Toprak, S.; Ortmann, O.; Buechler, C.; Treeck, O. The Complex Roles of Adipokines in Polycystic Ovary Syndrome and Endometriosis. Biomedicines 2022, 10, 2503. [Google Scholar] [CrossRef]
- Buechler, C.; Schaffler, A. Does global gene expression analysis in type 2 diabetes provide an opportunity to identify highly promising drug targets? Endocr. Metab. Immune Disord. Drug Targets 2007, 7, 250–258. [Google Scholar] [CrossRef]
- Harb, E.; Kheder, O.; Poopalasingam, G.; Rashid, R.; Srinivasan, A.; Izzi-Engbeaya, C. Brown adipose tissue and regulation of human body weight. Diabetes Metab. Res. Rev. 2023, 39, e3594. [Google Scholar] [CrossRef]
- Beekman, K.M.; Duque, G.; Corsi, A.; Tencerova, M.; Bisschop, P.H.; Paccou, J. Osteoporosis and Bone Marrow Adipose Tissue. Curr. Osteoporos. Rep. 2022, 21, 45–55. [Google Scholar] [CrossRef]
- D’Oria, R.; Genchi, V.A.; Caccioppoli, C.; Calderoni, I.; Marrano, N.; Biondi, G.; Borrelli, A.; Di Gioia, L.; Giorgino, F.; Laviola, L. Impact of Dysfunctional Adipose Tissue Depots on the Cardiovascular System. Int. J. Mol. Sci. 2022, 23, 14296. [Google Scholar] [CrossRef]
- Velingkar, A.; Vuree, S.; Prabhakar, P.K.; Kalshikam, R.R.; Kondeti, S. Fibroblast Growth Factor 21 as a Potential Master Regulator in Metabolic Disorders. Am. J. Physiol. Endocrinol. Metab. 2023; online ahead of print. [Google Scholar] [CrossRef]
- Aparecida Silveira, E.; Vaseghi, G.; de Carvalho Santos, A.S.; Kliemann, N.; Masoudkabir, F.; Noll, M.; Mohammadifard, N.; Sarrafzadegan, N.; de Oliveira, C. Visceral Obesity and Its Shared Role in Cancer and Cardiovascular Disease: A Scoping Review of the Pathophysiology and Pharmacological Treatments. Int. J. Mol. Sci. 2020, 21, 9042. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, M.; Barone, S.; Luo, H.; Zahedi, K. Pathogenesis of Hypertension in Metabolic Syndrome: The Role of Fructose and Salt. Int. J. Mol. Sci. 2023, 24, 4294. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Eagon, J.C.; Trujillo, M.E.; Scherer, P.E.; Klein, S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes 2007, 56, 1010–1013. [Google Scholar] [CrossRef]
- Wiest, R.; Weigert, J.; Wanninger, J.; Neumeier, M.; Bauer, S.; Schmidhofer, S.; Farkas, S.; Scherer, M.N.; Schaffler, A.; Scholmerich, J.; et al. Impaired hepatic removal of interleukin-6 in patients with liver cirrhosis. Cytokine 2011, 53, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Adams-Huet, B.; Devaraj, S.; Siegel, D.; Jialal, I. Increased Adipose Tissue Insulin Resistance in Metabolic Syndrome: Relationship to Circulating Adipokines. Metab. Syndr. Relat. Disord. 2014, 12, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Motawi, T.M.; Mahdy, S.G.; El-Sawalhi, M.M.; Ali, E.N.; El-Telbany, R.F.A. Serum levels of chemerin, apelin, vaspin, and omentin-1 in obese type 2 diabetic Egyptian patients with coronary artery stenosis. Can. J. Physiol. Pharmacol. 2018, 96, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Rabe, K.; Lehrke, M.; Parhofer, K.G.; Broedl, U.C. Adipokines and insulin resistance. Mol. Med. 2008, 14, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Adolph, T.E.; Grander, C.; Grabherr, F.; Tilg, H. Adipokines and Non-Alcoholic Fatty Liver Disease: Multiple Interactions. Int. J. Mol. Sci. 2017, 18, 1649. [Google Scholar] [CrossRef]
- Baltieri, L.; Chaim, E.A.; Chaim, F.D.M.; Utrini, M.P.; Gestic, M.A.; Cazzo, E. Correlation between Nonalcoholic Fatty Liver Disease Features and Levels of Adipokines and Inflammatory Cytokines among Morbidly Obese Individuals. Arq. Gastroenterol. 2018, 55, 247–251. [Google Scholar] [CrossRef]
- Buechler, C.; Wanninger, J.; Neumeier, M. Adiponectin, a key adipokine in obesity related liver diseases. World J. Gastroenterol. 2011, 17, 2801–2811. [Google Scholar] [CrossRef]
- Shi, J.T.; Zhang, Y.; She, Y.; Goyal, H.; Wu, Z.Q.; Xu, H.G. Diagnostic Utility of Non-invasive Tests for Inflammatory Bowel Disease: An Umbrella Review. Front. Med. 2022, 9, 920732. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y. Urine; Promising Biomarker Source for Early Disease Detection; Springer: Singapore, 2019. [Google Scholar] [CrossRef]
- Siddiqui, I.; Majid, H.; Abid, S. Update on clinical and research application of fecal biomarkers for gastrointestinal diseases. World J. Gastrointest. Pharmacol. Ther. 2017, 8, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Ruilope, L.M.; Ortiz, A.; Lucia, A.; Miranda, B.; Alvarez-Llamas, G.; Barderas, M.G.; Volpe, M.; Ruiz-Hurtado, G.; Pitt, B. Prevention of cardiorenal damage: Importance of albuminuria. Eur. Heart J. 2022, 44, 1112–1123. [Google Scholar] [CrossRef] [PubMed]
- Turpeinen, U.; Hamalainen, E. Determination of cortisol in serum, saliva and urine. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 795–801. [Google Scholar] [CrossRef]
- Florent, C.; L’Hirondel, C.; Desmazures, C.; Aymes, C.; Bernier, J.J. Intestinal clearance of alpha 1-antitrypsin. A sensitive method for the detection of protein-losing enteropathy. Gastroenterology 1981, 81, 777–780. [Google Scholar] [CrossRef]
- Moein, S.; Qujeq, D.; Vaghari Tabari, M.; Kashifard, M.; Hajian-Tilaki, K. Diagnostic accuracy of fecal calprotectin in assessing the severity of inflammatory bowel disease: From laboratory to clinic. Caspian J. Intern. Med. 2017, 8, 178–182. [Google Scholar] [CrossRef]
- van Rheenen, P.F.; Van de Vijver, E.; Fidler, V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: Diagnostic meta-analysis. BMJ 2010, 341, c3369. [Google Scholar] [CrossRef]
- Yui, S.; Nakatani, Y.; Mikami, M. Calprotectin (S100A8/S100A9), an inflammatory protein complex from neutrophils with a broad apoptosis-inducing activity. Biol. Pharm. Bull. 2003, 26, 753–760. [Google Scholar] [CrossRef]
- Sydor, S.; Dandyk, C.; Schwerdt, J.; Manka, P.; Benndorf, D.; Lehmann, T.; Schallert, K.; Wolf, M.; Reichl, U.; Canbay, A.; et al. Discovering Biomarkers for Non-Alcoholic Steatohepatitis Patients with and without Hepatocellular Carcinoma Using Fecal Metaproteomics. Int. J. Mol. Sci. 2022, 23, 8841. [Google Scholar] [CrossRef]
- Drljaca, J.; Milosevic, N.; Milanovic, M.; Abenavoli, L.; Milic, N. When the microbiome helps the brain-current evidence. CNS Neurosci. Ther. 2023; online ahead of print. [Google Scholar] [CrossRef]
- Dranse, H.J.; Rourke, J.L.; Stadnyk, A.W.; Sinal, C.J. Local chemerin levels are positively associated with DSS-induced colitis but constitutive loss of CMKLR1 does not protect against development of colitis. Physiol. Rep. 2015, 3, e12497. [Google Scholar] [CrossRef]
- Dranse, H.J.; Zheng, A.; Comeau, A.M.; Langille MG, I.; Zabel, B.A.; Sinal, C.J. The impact of chemerin or chemokine-like receptor 1 loss on the mouse gut microbiome. PeerJ 2018, 6, e5494. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Fan, C.; Lu, Y.; Fan, X.; Xia, L.; Li, P.; Wang, R.; Tang, T.; Wang, Y.; Qi, K. Alteration of gut microbiota affects expression of adiponectin and resistin through modifying DNA methylation in high-fat diet-induced obese mice. Genes. Nutr. 2020, 15, 12. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wang, H.; Xie, L.; Hu, F. Cross-Talk Between Gut Microbiota and Adipose Tissues in Obesity and Related Metabolic Diseases. Front. Endocrinol. 2022, 13, 908868. [Google Scholar] [CrossRef] [PubMed]
- Gathercole, J.L.; Grosvenor, A.J.; Lee, E.; Thomas, A.; Mitchell, C.J.; Zeng, N.; D’Souza, R.F.; Ramzan, F.; Sharma, P.; Knowles, S.O.; et al. Analysis of Human Faecal Host Proteins: Responsiveness to 10-Week Dietary Intervention Modifying Dietary Protein Intake in Elderly Males. Front. Nutr. 2020, 7, 595905. [Google Scholar] [CrossRef]
- Watanabe, E.; Kawashima, Y.; Suda, W.; Kakihara, T.; Takazawa, S.; Nakajima, D.; Nakamura, R.; Nishi, A.; Suzuki, K.; Ohara, O.; et al. Discovery of Candidate Stool Biomarker Proteins for Biliary Atresia Using Proteome Analysis by Data-Independent Acquisition Mass Spectrometry. Proteomes 2020, 8, 36. [Google Scholar] [CrossRef]
- Debyser, G.; Mesuere, B.; Clement, L.; Van de Weygaert, J.; Van Hecke, P.; Duytschaever, G.; Aerts, M.; Dawyndt, P.; De Boeck, K.; Vandamme, P.; et al. Faecal proteomics: A tool to investigate dysbiosis and inflammation in patients with cystic fibrosis. J. Cyst. Fibros. 2016, 15, 242–250. [Google Scholar] [CrossRef]
- Zhao, M.; Yang, Y.; Guo, Z.; Shao, C.; Sun, H.; Zhang, Y.; Sun, Y.; Liu, Y.; Song, Y.; Zhang, L.; et al. A Comparative Proteomics Analysis of Five Body Fluids: Plasma, Urine, Cerebrospinal Fluid, Amniotic Fluid, and Saliva. Proteom. Clin. Appl. 2018, 12, e1800008. [Google Scholar] [CrossRef]
- Ertl, V.M.; Horing, M.; Schott, H.F.; Blucher, C.; Kjolbaek, L.; Astrup, A.; Burkhardt, R.; Liebisch, G. Quantification of diacylglycerol and triacylglycerol species in human fecal samples by flow injection Fourier transform mass spectrometry. Anal. Bioanal. Chem. 2020, 412, 2315–2326. [Google Scholar] [CrossRef]
- Matysik, S.; Le Roy, C.I.; Liebisch, G.; Claus, S.P. Metabolomics of fecal samples: A practical consideration. Trends Food Sci. Technol. 2016, 57, 244–255. [Google Scholar] [CrossRef]
- Trevethan, R. Sensitivity, Specificity, and Predictive Values: Foundations, Pliabilities, and Pitfalls in Research and Practice. Front. Public. Health 2017, 5, 307. [Google Scholar] [CrossRef]
- Guo, X.; Huang, C.; Xu, J.; Xu, H.; Liu, L.; Zhao, H.; Wang, J.; Huang, W.; Peng, W.; Chen, Y.; et al. Gut Microbiota Is a Potential Biomarker in Inflammatory Bowel Disease. Front. Nutr. 2021, 8, 818902. [Google Scholar] [CrossRef] [PubMed]
- Harpole, M.; Davis, J.; Espina, V. Current state of the art for enhancing urine biomarker discovery. Expert. Rev. Proteom. 2016, 13, 609–626. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tucci, M.; Narain, S.; Barnes, E.V.; Sobel, E.S.; Segal, M.S.; Richards, H.B. Urinary biomarkers in lupus nephritis. Autoimmun. Rev. 2006, 5, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Funcke, J.B.; Scherer, P.E. Beyond adiponectin and leptin: Adipose tissue-derived mediators of inter-organ communication. J. Lipid Res. 2019, 60, 1648–1684. [Google Scholar] [CrossRef]
- Buechler, C.; Feder, S.; Haberl, E.M.; Aslanidis, C. Chemerin Isoforms and Activity in Obesity. Int. J. Mol. Sci. 2019, 20, 3887. [Google Scholar] [CrossRef] [PubMed]
- Kern, L.; Mittenbuhler, M.J.; Vesting, A.J.; Ostermann, A.L.; Wunderlich, C.M.; Wunderlich, F.T. Obesity-Induced TNFalpha and IL-6 Signaling: The Missing Link between Obesity and Inflammation-Driven Liver and Colorectal Cancers. Cancers 2018, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Menini, S.; Iacobini, C.; Blasetti Fantauzzi, C.; Pesce, C.M.; Pugliese, G. Role of Galectin-3 in Obesity and Impaired Glucose Homeostasis. Oxid. Med. Cell. Longev. 2016, 2016, 9618092. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, Y.; Zhang, Y.; Leroith, D.; Bernlohr, D.A.; Chen, X. The role of lipocalin 2 in the regulation of inflammation in adipocytes and macrophages. Mol. Endocrinol. 2008, 22, 1416–1426. [Google Scholar] [CrossRef]
- Wang, Z.V.; Scherer, P.E. Adiponectin, the past two decades. J. Mol. Cell. Biol. 2016, 8, 93–100. [Google Scholar] [CrossRef]
- Arita, Y.; Kihara, S.; Ouchi, N.; Takahashi, M.; Maeda, K.; Miyagawa, J.; Hotta, K.; Shimomura, I.; Nakamura, T.; Miyaoka, K.; et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem. Biophys. Res. Commun. 1999, 257, 79–83. [Google Scholar] [CrossRef]
- Fisher, F.F.; Trujillo, M.E.; Hanif, W.; Barnett, A.H.; McTernan, P.G.; Scherer, P.E.; Kumar, S. Serum high molecular weight complex of adiponectin correlates better with glucose tolerance than total serum adiponectin in Indo-Asian males. Diabetologia 2005, 48, 1084–1087. [Google Scholar] [CrossRef] [PubMed]
- Asano, T.; Watanabe, K.; Kubota, N.; Gunji, T.; Omata, M.; Kadowaki, T.; Ohnishi, S. Adiponectin knockout mice on high fat diet develop fibrosing steatohepatitis. J. Gastroenterol. Hepatol. 2009, 24, 1669–1676. [Google Scholar] [CrossRef] [PubMed]
- Iwabu, M.; Okada-Iwabu, M.; Yamauchi, T.; Kadowaki, T. Adiponectin/adiponectin receptor in disease and aging. NPJ Aging Mech. Dis. 2015, 1, 15013. [Google Scholar] [CrossRef] [PubMed]
- Yano, W.; Kubota, N.; Itoh, S.; Kubota, T.; Awazawa, M.; Moroi, M.; Sugi, K.; Takamoto, I.; Ogata, H.; Tokuyama, K.; et al. Molecular mechanism of moderate insulin resistance in adiponectin-knockout mice. Endocr. J. 2008, 55, 515–522. [Google Scholar] [CrossRef]
- Nigro, E.; Daniele, A.; Salzillo, A.; Ragone, A.; Naviglio, S.; Sapio, L. AdipoRon and Other Adiponectin Receptor Agonists as Potential Candidates in Cancer Treatments. Int. J. Mol. Sci. 2021, 22, 5569. [Google Scholar] [CrossRef]
- Rovin, B.H.; Song, H.; Hebert, L.A.; Nadasdy, T.; Nadasdy, G.; Birmingham, D.J.; Yung Yu, C.; Nagaraja, H.N. Plasma, urine, and renal expression of adiponectin in human systemic lupus erythematosus. Kidney Int. 2005, 68, 1825–1833. [Google Scholar] [CrossRef]
- Przybycinski, J.; Dziedziejko, V.; Puchalowicz, K.; Domanski, L.; Pawlik, A. Adiponectin in Chronic Kidney Disease. Int. J. Mol. Sci. 2020, 21, 9375. [Google Scholar] [CrossRef]
- Loghman, M.; Haghighi, A.; Broumand, B.; Ataipour, Y.; Tohidi, M.; Marzbani, C.; Fakharran, M. Association between urinary adiponectin level and renal involvement in systemic lupus erythematous. Int. J. Rheum. Dis. 2016, 19, 678–684. [Google Scholar] [CrossRef]
- Yamamoto, M.; Fujimoto, Y.; Hayashi, S.; Hashida, S. A study of high-, middle- and low-molecular weight adiponectin in urine as a surrogate marker for early diabetic nephropathy using ultrasensitive immune complex transfer enzyme immunoassay. Ann. Clin. Biochem. 2018, 55, 525–534. [Google Scholar] [CrossRef]
- Sahakyan, G.; Vejux, A.; Sahakyan, N. The Role of Oxidative Stress-Mediated Inflammation in the Development of T2DM-Induced Diabetic Nephropathy: Possible Preventive Action of Tannins and Other Oligomeric Polyphenols. Molecules 2022, 27, 9035. [Google Scholar] [CrossRef]
- Watanabe, T.; Fujimoto, Y.; Morimoto, A.; Nishiyama, M.; Kawai, A.; Okada, S.; Aiba, M.; Kawano, T.; Kawahigashi, M.; Ishizu, M.; et al. Development of fully automated and ultrasensitive assays for urinary adiponectin and their application as novel biomarkers for diabetic kidney disease. Sci. Rep. 2020, 10, 15869. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.Y.; Hughes, J.T.; Charlesworth, J.A.; Kelly, J.J.; Peake, P.W. Adiponectin is present in the urine in its native conformation, and specifically reduces the secretion of MCP-1 by proximal tubular cells. Nephrology 2008, 13, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Koshimura, J.; Fujita, H.; Narita, T.; Shimotomai, T.; Hosoba, M.; Yoshioka, N.; Kakei, M.; Fujishima, H.; Ito, S. Urinary adiponectin excretion is increased in patients with overt diabetic nephropathy. Biochem. Biophys. Res. Commun. 2004, 316, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Ishizu, M.; Mori, H.; Ohishi, M.; Kuroda, A.; Akehi, Y.; Yoshida, S.; Aihara, K.I.; Aiba, M.; Kawano, T.; Hashida, S.; et al. Urinary adiponectin excretion is an early predictive marker of the decline of the renal function in patients with diabetes mellitus. J. Diabetes Complicat. 2021, 35, 107848. [Google Scholar] [CrossRef]
- Kopf, S.; Oikonomou, D.; von Eynatten, M.; Kieser, M.; Zdunek, D.; Hess, G.; Morcos, M.; Forsblom, C.; Bierhaus, A.; Groop, P.H.; et al. Urinary excretion of high molecular weight adiponectin is an independent predictor of decline of renal function in type 2 diabetes. Acta Diabetol. 2014, 51, 479–489. [Google Scholar] [CrossRef]
- Nishida, M.; Funahashi, T.; Shimomura, I. Pathophysiological significance of adiponectin. Med. Mol. Morphol. 2007, 40, 55–67. [Google Scholar] [CrossRef]
- Frystyk, J.; Tarnow, L.; Hansen, T.K.; Parving, H.H.; Flyvbjerg, A. Increased serum adiponectin levels in type 1 diabetic patients with microvascular complications. Diabetologia 2005, 48, 1911–1918. [Google Scholar] [CrossRef]
- von Eynatten, M.; Liu, D.; Hock, C.; Oikonomou, D.; Baumann, M.; Allolio, B.; Korosoglou, G.; Morcos, M.; Campean, V.; Amann, K.; et al. Urinary adiponectin excretion: A novel marker for vascular damage in type 2 diabetes. Diabetes 2009, 58, 2093–2099. [Google Scholar] [CrossRef]
- Vichinsartvichai, P.; Teeramara, R.; Jirasawas, T.; Sakoonwatanyoo, P. Comparison of urinary adiponectin in the presence of metabolic syndrome in peri- and postmenopausal women. BMC Womens Health 2022, 22, 70. [Google Scholar] [CrossRef]
- Whittall-Garcia, L.; Goliad, K.; Kim, M.; Bonilla, D.; Gladman, D.; Urowitz, M.; Fortin, P.R.; Atenafu, E.G.; Touma, Z.; Wither, J. Identification and Validation of a Urinary Biomarker Panel to Accurately Diagnose and Predict Response to Therapy in Lupus Nephritis. Front. Immunol. 2022, 13, 889931. [Google Scholar] [CrossRef]
- Ramirez, G.A.; Efthymiou, M.; Isenberg, D.A.; Cohen, H. Under crossfire: Thromboembolic risk in systemic lupus erythematosus. Rheumatology 2019, 58, 940–952. [Google Scholar] [CrossRef]
- de Cos, M.; Meliambro, K.; Campbell, K.N. Novel Treatment Paradigms: Focal Segmental Glomerulosclerosis. Kidney Int. Rep. 2023, 8, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Sethna, C.B.; Boone, V.; Kwok, J.; Jun, D.; Trachtman, H. Adiponectin in children and young adults with focal segmental glomerulosclerosis. Pediatr. Nephrol. 2015, 30, 1977–1985. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Fu, X.; Li, J.; Liu, L.; Alkohlani, A.; Tan, S.C.; Low, T.Y.; Hou, Y. Association of circulating leptin and adiponectin levels with colorectal cancer risk: A systematic review and meta-analysis of case-control studies. Cancer Epidemiol. 2021, 73, 101958. [Google Scholar] [CrossRef]
- Yoon, Y.S.; Kwon, A.R.; Lee, Y.K.; Oh, S.W. Circulating adipokines and risk of obesity related cancers: A systematic review and meta-analysis. Obes. Res. Clin. Pract. 2019, 13, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.S.; Liu, X.; Nagel, J.M.; Chamberland, J.P.; Diakopoulos, K.N.; Brinkoetter, M.T.; Hatziapostolou, M.; Wu, Y.; Robson, S.C.; Iliopoulos, D.; et al. Salutary effects of adiponectin on colon cancer: In vivo and in vitro studies in mice. Gut 2013, 62, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Chumanevich, A.; Fletcher, E.; Larsen, B.; Lattwein, K.; Kaur, K.; Fayad, R. Adiponectin deficiency: Role in chronic inflammation induced colon cancer. Biochim. Biophys. Acta 2012, 1822, 527–536. [Google Scholar] [CrossRef] [PubMed]
- An, W.; Bai, Y.; Deng, S.X.; Gao, J.; Ben, Q.W.; Cai, Q.C.; Zhang, H.G.; Li, Z.S. Adiponectin levels in patients with colorectal cancer and adenoma: A meta-analysis. Eur. J. Cancer Prev. 2012, 21, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Feder, S.; Kandulski, A.; Schacherer, D.; Weiss, T.S.; Buechler, C. Serum Adiponectin Levels Do Not Distinguish Primary from Metastatic Liver Tumors. Anticancer. Res. 2020, 40, 143–151. [Google Scholar] [CrossRef]
- Kaser, S.; Moschen, A.; Cayon, A.; Kaser, A.; Crespo, J.; Pons-Romero, F.; Ebenbichler, C.F.; Patsch, J.R.; Tilg, H. Adiponectin and its receptors in non-alcoholic steatohepatitis. Gut 2005, 54, 117–121. [Google Scholar] [CrossRef]
- Rasouli, N.; Kern, P.A. Adipocytokines and the metabolic complications of obesity. J. Clin. Endocrinol. Metab. 2008, 93, S64–S73. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Vanarsa, K.; Zhang, T.; Soomro, S.; Cicalese, P.A.; Duran, V.; Dasari, S.; Lee, K.H.; Pedroza, C.; Kisiel, J.B.; et al. Comprehensive aptamer-based screen of 1317 proteins uncovers improved stool protein markers of colorectal cancer. J. Gastroenterol. 2021, 56, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Czauderna, C.; Luley, K.; von Bubnoff, N.; Marquardt, J.U. Tailored Systemic Therapy for Colorectal Cancer Liver Metastases. Int. J. Mol. Sci. 2021, 22, 11780. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, H.; Bauer, C.; Hu, Y.; Lewis, J.R.; Xu, A.; Levinger, I.; Wang, Y. Lipocalin-2 Variants and Their Relationship With Cardio-Renal Risk Factors. Front. Endocrinol. 2021, 12, 781763. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Rubin, J.; Han, W.; Venge, P.; Xu, S. The origin of multiple molecular forms in urine of HNL/NGAL. Clin. J. Am. Soc. Nephrol. 2010, 5, 2229–2235. [Google Scholar] [CrossRef]
- De la Chesnaye, E.; Manuel-Apolinar, L.; Oviedo-de Anda, N.; Revilla-Monsalve, M.C.; Islas-Andrade, S. Gender differences in lipocalin 2 plasmatic levels are correlated with age and the triglyceride/high-density lipoprotein ratio in healthy individuals. Gac. Med. Mex. 2016, 152, 612–617. [Google Scholar]
- Helanova, K.; Spinar, J.; Parenica, J. Diagnostic and prognostic utility of neutrophil gelatinase-associated lipocalin (NGAL) in patients with cardiovascular diseases—Review. Kidney Blood Press. Res. 2014, 39, 623–629. [Google Scholar] [CrossRef]
- Asimakopoulou, A.; Borkham-Kamphorst, E.; Henning, M.; Yagmur, E.; Gassler, N.; Liedtke, C.; Berger, T.; Mak, T.W.; Weiskirchen, R. Lipocalin-2 (LCN2) regulates PLIN5 expression and intracellular lipid droplet formation in the liver. Biochim. Biophys. Acta 2014, 1842, 1513–1524. [Google Scholar] [CrossRef]
- Meyers, K.; Lopez, M.; Ho, J.; Wills, S.; Rayalam, S.; Taval, S. Lipocalin-2 deficiency may predispose to the progression of spontaneous age-related adiposity in mice. Sci. Rep. 2020, 10, 14589. [Google Scholar] [CrossRef]
- Mosialou, I.; Shikhel, S.; Luo, N.; Petropoulou, P.I.; Panitsas, K.; Bisikirska, B.; Rothman, N.J.; Tenta, R.; Cariou, B.; Wargny, M.; et al. Lipocalin-2 counteracts metabolic dysregulation in obesity and diabetes. J. Exp. Med. 2020, 217, e20191261. [Google Scholar] [CrossRef]
- Srinivasan, G.; Aitken, J.D.; Zhang, B.; Carvalho, F.A.; Chassaing, B.; Shashidharamurthy, R.; Borregaard, N.; Jones, D.P.; Gewirtz, A.T.; Vijay-Kumar, M. Lipocalin 2 deficiency dysregulates iron homeostasis and exacerbates endotoxin-induced sepsis. J. Immunol. 2012, 189, 1911–1919. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.M.; Kim, T.N.; Yoo, H.J.; Lee, K.W.; Cho, G.J.; Hwang, T.G.; Baik, S.H.; Choi, D.S.; Kim, S.M. Effect of exercise training on A-FABP, lipocalin-2 and RBP4 levels in obese women. Clin. Endocrinol. 2009, 70, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Malyszko, J.; Bachorzewska-Gajewska, H.; Malyszko, J.S.; Pawlak, K.; Dobrzycki, S. Serum neutrophil gelatinase-associated lipocalin as a marker of renal function in hypertensive and normotensive patients with coronary artery disease. Nephrology 2008, 13, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Stejskal, D.; Karpisek, M.; Humenanska, V.; Hanulova, Z.; Stejskal, P.; Kusnierova, P.; Petzel, M. Lipocalin-2: Development, analytical characterization, and clinical testing of a new ELISA. Horm. Metab. Res. 2008, 40, 381–385. [Google Scholar] [CrossRef]
- Malyszko, J.; Tesar, V.; Macdougall, I.C. Neutrophil gelatinase-associated lipocalin and hepcidin: What do they have in common and is there a potential interaction? Kidney Blood Press. Res. 2010, 33, 157–165. [Google Scholar] [CrossRef]
- Meier, E.M.; Pohl, R.; Rein-Fischboeck, L.; Schacherer, D.; Eisinger, K.; Wiest, R.; Krautbauer, S.; Buechler, C. Circulating lipocalin 2 is neither related to liver steatosis in patients with non-alcoholic fatty liver disease nor to residual liver function in cirrhosis. Cytokine 2016, 85, 45–50. [Google Scholar] [CrossRef]
- De la Chesnaye, E.; Manuel-Apolinar, L.; Zarate, A.; Damasio, L.; Espino, N.; Revilla-Monsalve, M.C.; Islas-Andrade, S. Lipocalin-2 plasmatic levels are reduced in patients with long-term type 2 diabetes mellitus. Int. J. Clin. Exp. Med. 2015, 8, 2853–2859. [Google Scholar]
- Crescenzi, E.; Leonardi, A.; Pacifico, F. NGAL as a Potential Target in Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 12333. [Google Scholar] [CrossRef]
- Chiang, K.C.; Yeh, T.S.; Wu, R.C.; Pang, J.S.; Cheng, C.T.; Wang, S.Y.; Juang, H.H.; Yeh, C.N. Lipocalin 2 (LCN2) is a promising target for cholangiocarcinoma treatment and bile LCN2 level is a potential cholangiocarcinoma diagnostic marker. Sci. Rep. 2016, 6, 36138. [Google Scholar] [CrossRef]
- Czauderna, C.; Kirstein, M.M.; Tews, H.C.; Vogel, A.; Marquardt, J.U. Molecular Subtypes and Precision Oncology in Intrahepatic Cholangiocarcinoma. J. Clin. Med. 2021, 10, 2803. [Google Scholar] [CrossRef]
- Marakala, V. Neutrophil gelatinase-associated lipocalin (NGAL) in kidney injury—A systematic review. Clin. Chim. Acta 2022, 536, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Haase-Fielitz, A.; Haase, M.; Bellomo, R. Instability of urinary NGAL during long-term storage. Am. J. Kidney Dis. 2009, 53, 564–565. [Google Scholar] [CrossRef] [PubMed]
- Qing, X.; Zavadil, J.; Crosby, M.B.; Hogarth, M.P.; Hahn, B.H.; Mohan, C.; Gilkeson, G.S.; Bottinger, E.P.; Putterman, C. Nephritogenic anti-DNA antibodies regulate gene expression in MRL/lpr mouse glomerular mesangial cells. Arthritis Rheum. 2006, 54, 2198–2210. [Google Scholar] [CrossRef] [PubMed]
- Pitashny, M.; Schwartz, N.; Qing, X.; Hojaili, B.; Aranow, C.; Mackay, M.; Putterman, C. Urinary lipocalin-2 is associated with renal disease activity in human lupus nephritis. Arthritis Rheum. 2007, 56, 1894–1903. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, B.; Cao, J.; Feng, S.; Liu, B. Elevated Urinary Neutrophil Gelatinase-Associated Lipocalin Is a Biomarker for Lupus Nephritis: A Systematic Review and Meta-Analysis. Biomed. Res. Int. 2020, 2020, 2768326. [Google Scholar] [CrossRef]
- Brunner, H.I.; Mueller, M.; Rutherford, C.; Passo, M.H.; Witte, D.; Grom, A.; Mishra, J.; Devarajan, P. Urinary neutrophil gelatinase-associated lipocalin as a biomarker of nephritis in childhood-onset systemic lupus erythematosus. Arthritis Rheum. 2006, 54, 2577–2584. [Google Scholar] [CrossRef]
- Woo, K.S.; Choi, J.L.; Kim, B.R.; Kim, J.E.; An, W.S.; Han, J.Y. Urinary neutrophil gelatinase-associated lipocalin levels in comparison with glomerular filtration rate for evaluation of renal function in patients with diabetic chronic kidney disease. Diabetes Metab. J. 2012, 36, 307–313. [Google Scholar] [CrossRef]
- Greco, M.; Chiefari, E.; Mirabelli, M.; Salatino, A.; Tocci, V.; Cianfrone, P.; Foti, D.P.; Brunetti, A. Plasma or Urine Neutrophil Gelatinase-Associated Lipocalin (NGAL): Which Is Better at Detecting Chronic Kidney Damage in Type 2 Diabetes? Endocrines 2022, 3, 175–186. [Google Scholar] [CrossRef]
- Zylka, A.; Gala-Bladzinska, A.; Dumnicka, P.; Ceranowicz, P.; Kuzniewski, M.; Gil, K.; Olszanecki, R.; Kusnierz-Cabala, B. Is Urinary NGAL Determination Useful for Monitoring Kidney Function and Assessment of Cardiovascular Disease? A 12-Month Observation of Patients with Type 2 Diabetes. Dis. Markers 2016, 2016, 8489543. [Google Scholar] [CrossRef]
- Bolignano, D.; Coppolino, G.; Campo, S.; Aloisi, C.; Nicocia, G.; Frisina, N.; Buemi, M. Urinary neutrophil gelatinase-associated lipocalin (NGAL) is associated with severity of renal disease in proteinuric patients. Nephrol. Dial. Transplant. 2008, 23, 414–416. [Google Scholar] [CrossRef]
- Coppolino, G.; Comi, N.; Bolignano, D.; Patella, G.; Comi, A.; Provenzano, M.; Rivoli, L.; Andreucci, M.; Fuiano, G. Urinary Neutrophil Gelatinase-Associated Lipocalin (NGAL) Predicts Renal Function Decline in Patients with Glomerular Diseases. Front. Cell. Dev. Biol. 2020, 8, 336. [Google Scholar] [CrossRef] [PubMed]
- Floege, J.; Amann, K. Primary glomerulonephritides. Lancet 2016, 387, 2036–2048. [Google Scholar] [CrossRef]
- Kielar, M.; Dumnicka, P.; Gala-Bladzinska, A.; Bedkowska-Prokop, A.; Ignacak, E.; Maziarz, B.; Ceranowicz, P.; Kusnierz-Cabala, B. Urinary NGAL Measured after the First Year Post Kidney Transplantation Predicts Changes in Glomerular Filtration over One-Year Follow-Up. J. Clin. Med. 2020, 10, 43. [Google Scholar] [CrossRef]
- Reese, P.P.; Doshi, M.D.; Hall, I.E.; Besharatian, B.; Bromberg, J.S.; Thiessen-Philbrook, H.; Jia, Y.; Kamoun, M.; Mansour, S.G.; Akalin, E.; et al. Deceased-Donor Acute Kidney Injury and Acute Rejection in Kidney Transplant Recipients: A Multicenter Cohort. Am. J. Kidney Dis. 2023, 81, 222–231.e1. [Google Scholar] [CrossRef] [PubMed]
- Gomes, B.C.; Silva Junior, J.M.; Tuon, F.F. Evaluation of Urinary NGAL as a Diagnostic Tool for Acute Kidney Injury in Critically Ill Patients with Infection: An Original Study. Can. J. Kidney Health Dis. 2020, 7, 2054358120934215. [Google Scholar] [CrossRef] [PubMed]
- Martensson, J.; Bellomo, R. The rise and fall of NGAL in acute kidney injury. Blood Purif. 2014, 37, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pollok, R.; Goldsmith, D. Renal and Urological Disorders Associated with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2022; online ahead of print. [Google Scholar] [CrossRef]
- Mahmud, N.; Stinson, J.; O’Connell, M.A.; Mantle, T.J.; Keeling, P.W.; Feely, J.; Weir, D.G.; Kelleher, D. Microalbuminuria in inflammatory bowel disease. Gut 1994, 35, 1599–1604. [Google Scholar] [CrossRef]
- Bolignano, D.; Della Torre, A.; Lacquaniti, A.; Costantino, G.; Fries, W.; Buemi, M. Neutrophil gelatinase-associated lipocalin levels in patients with crohn disease undergoing treatment with infliximab. J. Investig. Med. 2010, 58, 569–571. [Google Scholar] [CrossRef]
- Scholmerich, J. Inflammatory bowel disease: Pandora’s box, present and future. Ann. N. Y. Acad. Sci. 2006, 1072, 365–378. [Google Scholar] [CrossRef]
- Oikonomou, K.A.; Kapsoritakis, A.N.; Theodoridou, C.; Karangelis, D.; Germenis, A.; Stefanidis, I.; Potamianos, S.P. Neutrophil gelatinase-associated lipocalin (NGAL) in inflammatory bowel disease: Association with pathophysiology of inflammation, established markers, and disease activity. J. Gastroenterol. 2012, 47, 519–530. [Google Scholar] [CrossRef]
- Gumpper, K.; Dangel, A.W.; Pita-Grisanti, V.; Krishna, S.G.; Lara, L.F.; Mace, T.; Papachristou, G.I.; Conwell, D.L.; Hart, P.A.; Cruz-Monserrate, Z. Lipocalin-2 expression and function in pancreatic diseases. Pancreatology 2020, 20, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Hogendorf, P.; Durczynski, A.; Skulimowski, A.; Kumor, A.; Poznanska, G.; Strzelczyk, J. Neutrophil Gelatinase-Associated Lipocalin (NGAL) concentration in urine is superior to CA19-9 and Ca 125 in differentiation of pancreatic mass: Preliminary report. Cancer Biomark. 2016, 16, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, M.; Rydzewska-Rosolowska, A.; Rydzewski, A.; Rydzewska, G. Urinary neutrophil gelatinase-associated lipocalin as an early predictor of disease severity and mortality in acute pancreatitis. Pancreas 2015, 44, 448–452. [Google Scholar] [CrossRef]
- Sporek, M.; Dumnicka, P.; Gala-Bladzinska, A.; Ceranowicz, P.; Warzecha, Z.; Dembinski, A.; Stepien, E.; Walocha, J.; Drozdz, R.; Kuzniewski, M.; et al. Angiopoietin-2 Is an Early Indicator of Acute Pancreatic-Renal Syndrome in Patients with Acute Pancreatitis. Mediat. Inflamm. 2016, 2016, 5780903. [Google Scholar] [CrossRef] [PubMed]
- Bucsics, T.; Krones, E. Renal dysfunction in cirrhosis: Acute kidney injury and the hepatorenal syndrome. Gastroenterol. Rep. 2017, 5, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Allegretti, A.S.; Parada, X.V.; Endres, P.; Zhao, S.; Krinsky, S.; St Hillien, S.A.; Kalim, S.; Nigwekar, S.U.; Flood, J.G.; Nixon, A.; et al. Urinary NGAL as a Diagnostic and Prognostic Marker for Acute Kidney Injury in Cirrhosis: A Prospective Study. Clin. Transl. Gastroenterol. 2021, 12, e00359. [Google Scholar] [CrossRef]
- Campos-Martinez, A.M.; Exposito-Herrera, J.; Gonzalez-Bolivar, M.; Fernandez-Marin, E.; Uberos, J. Evaluation of Risk and Preventive Factors for Necrotizing Enterocolitis in Premature Newborns. A Systematic Review of the Literature. Front. Pediatr. 2022, 10, 874976. [Google Scholar] [CrossRef]
- Chassaing, B.; Srinivasan, G.; Delgado, M.A.; Young, A.N.; Gewirtz, A.T.; Vijay-Kumar, M. Fecal lipocalin 2, a sensitive and broadly dynamic non-invasive biomarker for intestinal inflammation. PLoS ONE 2012, 7, e44328. [Google Scholar] [CrossRef]
- Thibault, M.P.; Tremblay, E.; Horth, C.; Fournier-Morin, A.; Grynspan, D.; Babakissa, C.; Levy, E.; Ferretti, E.; Bertelle, V.; Beaulieu, J.F. Lipocalin-2 and calprotectin as stool biomarkers for predicting necrotizing enterocolitis in premature neonates. Pediatr. Res. 2022, 91, 129–136. [Google Scholar] [CrossRef]
- Mao, R.; Xiao, Y.L.; Gao, X.; Chen, B.L.; He, Y.; Yang, L.; Hu, P.J.; Chen, M.H. Fecal calprotectin in predicting relapse of inflammatory bowel diseases: A meta-analysis of prospective studies. Inflamm. Bowel Dis. 2012, 18, 1894–1899. [Google Scholar] [CrossRef]
- Thorsvik, S.; Damas, J.K.; Granlund, A.V.; Flo, T.H.; Bergh, K.; Ostvik, A.E.; Sandvik, A.K. Fecal neutrophil gelatinase-associated lipocalin as a biomarker for inflammatory bowel disease. J. Gastroenterol. Hepatol. 2017, 32, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, O.H.; Gionchetti, P.; Ainsworth, M.; Vainer, B.; Campieri, M.; Borregaard, N.; Kjeldsen, L. Rectal dialysate and fecal concentrations of neutrophil gelatinase-associated lipocalin, interleukin-8, and tumor necrosis factor-alpha in ulcerative colitis. Am. J. Gastroenterol. 1999, 94, 2923–2928. [Google Scholar] [CrossRef] [PubMed]
- Buisson, A.; Vazeille, E.; Minet-Quinard, R.; Goutte, M.; Bouvier, D.; Goutorbe, F.; Pereira, B.; Barnich, N.; Bommelaer, G. Fecal Matrix Metalloprotease-9 and Lipocalin-2 as Biomarkers in Detecting Endoscopic Activity in Patients With Inflammatory Bowel Diseases. J. Clin. Gastroenterol. 2018, 52, e53–e62. [Google Scholar] [CrossRef] [PubMed]
- Magro, F.; Lopes, S.; Coelho, R.; Cotter, J.; de Castro, F.D.; de Sousa, H.T.; Salgado, M.; Andrade, P.; Vieira, A.I.; Figueiredo, P.; et al. Accuracy of Faecal Calprotectin and Neutrophil Gelatinase B-associated Lipocalin in Evaluating Subclinical Inflammation in UlceRaTIVE Colitis-the ACERTIVE study. J. Crohn’s Colitis 2017, 11, 435–444. [Google Scholar] [CrossRef]
- Abdulganieva, D.I.; Mukhametova, D.D.; Valeeva, I.K.; Blatt, N.; Zinkevich, O.D.; Safina, N.A.; Odintsova, A.K.; Korovina, M.O. Faecal Neutrophil Gelatinase-associated Lipocalin as a Non-invasive Biomarker in Inflammatory Bowel Disease. BioNanoScience 2022, 12, 1381–1387. [Google Scholar] [CrossRef]
- Park, J.M.; Choi, M.G.; Kim, Y.S.; Choi, C.H.; Choi, S.C.; Hong, S.J.; Jeong, J.J.; Lee, D.H.; Lee, J.S.; Lee, K.J.; et al. Quality of life of patients with irritable bowel syndrome in Korea. Qual. Life Res. 2009, 18, 435–446. [Google Scholar] [CrossRef]
- El-Haggar, S.M.; Hegazy, S.K.; SM, A.-E.; Bahaa, M.M. Open-label pilot study of ethosuximide as adjunctive therapy for relieving abdominal pain related to Irritable Bowel Syndrome. J. Clin. Pharm. Ther. 2022, 47, 306–312. [Google Scholar] [CrossRef]
- Aho, V.T.E.; Houser, M.C.; Pereira PA, B.; Chang, J.; Rudi, K.; Paulin, L.; Hertzberg, V.; Auvinen, P.; Tansey, M.G.; Scheperjans, F. Relationships of gut microbiota, short-chain fatty acids, inflammation, and the gut barrier in Parkinson’s disease. Mol. Neurodegener. 2021, 16, 6. [Google Scholar] [CrossRef]
- Eidson, L.N.; Kannarkat, G.T.; Barnum, C.J.; Chang, J.; Chung, J.; Caspell-Garcia, C.; Taylor, P.; Mollenhauer, B.; Schlossmacher, M.G.; Ereshefsky, L.; et al. Candidate inflammatory biomarkers display unique relationships with alpha-synuclein and correlate with measures of disease severity in subjects with Parkinson’s disease. J. Neuroinflamm. 2017, 14, 164. [Google Scholar] [CrossRef]
- Acharya, C.; Bajaj, J.S. Altered Microbiome in Patients with Cirrhosis and Complications. Clin. Gastroenterol. Hepatol. 2019, 17, 307–321. [Google Scholar] [CrossRef]
- Niederreiter, L.; Adolph, T.E.; Tilg, H. Food, microbiome and colorectal cancer. Dig. Liver Dis. 2018, 50, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Lustig, R.H.; Fennoy, I. The History of Obesity Research. Horm. Res. Paediatr. 2022, 95, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J. The long road to leptin. J. Clin. Invest. 2016, 126, 4727–4734. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Leclercq, I.; Brymora, J.M.; Xu, N.; Ramezani-Moghadam, M.; London, R.M.; Brigstock, D.; George, J. Kupffer cells mediate leptin-induced liver fibrosis. Gastroenterology 2009, 137, 713–723.e1. [Google Scholar] [CrossRef]
- Wauman, J.; Zabeau, L.; Tavernier, J. The Leptin Receptor Complex: Heavier Than Expected? Front. Endocrinol. 2017, 8, 30. [Google Scholar] [CrossRef]
- Jimenez-Cortegana, C.; Lopez-Saavedra, A.; Sanchez-Jimenez, F.; Perez-Perez, A.; Castineiras, J.; Virizuela-Echaburu, J.A.; de la Cruz-Merino, L.; Sanchez-Margalet, V. Leptin, Both Bad and Good Actor in Cancer. Biomolecules 2021, 11, 913. [Google Scholar] [CrossRef]
- Cheng, J.; Luo, Y.; Li, Y.; Zhang, F.; Zhang, X.; Zhou, X.; Ji, L. Sex- and body mass index-specific reference intervals for serum leptin: A population based study in China. Nutr. Metab. 2022, 19, 54. [Google Scholar] [CrossRef]
- Meyer, C.; Robson, D.; Rackovsky, N.; Nadkarni, V.; Gerich, J. Role of the kidney in human leptin metabolism. Am. J. Physiol. 1997, 273, E903–E907. [Google Scholar] [CrossRef]
- Chorostowska-Wynimko, J.; Plywaczewski, R.; Jonczak, L.; Bednarek, M.; Stepniewska, A.; Borowska, A.; Gorecka, D. Leptin measurement in urine is a reliable method of monitoring its secretion in patients with obstructive sleep apnea syndrome. J. Physiol. Pharmacol. 2007, 58 (Suppl. S5), 105–115. [Google Scholar]
- Maqsood, A.R.; Trueman, J.A.; Whatmore, A.J.; Westwood, M.; Price, D.A.; Hall, C.M.; Clayton, P.E. The relationship between nocturnal urinary leptin and gonadotrophins as children progress towards puberty. Horm. Res. 2007, 68, 225–230. [Google Scholar] [CrossRef]
- Zaman, N.; Hall, C.M.; Gill, M.S.; Jones, J.; Tillmann, V.; Westwood, M.; Whatmore, A.J.; Clayton, P.E. Leptin measurement in urine in children and its relationship to other growth peptides in serum and urine. Clin. Endocrinol. 2003, 58, 78–85. [Google Scholar] [CrossRef]
- Sharifian, M.; Shohadaee, S.; Esfandiar, N.; Mohkam, M.; Dalirani, R.; Akhavan Sepahi, M. Serum and Urine Leptin Concentrations in Children Before and After Treatment of Urinary Tract Infection. Iran. J. Kidney Dis. 2015, 9, 374–378. [Google Scholar] [PubMed]
- Schroth, M.; Groschl, M.; Dorr, H.G.; Blum, W.F.; Rascher, W.; Dotsch, J. Renal loss of leptin in patients with nephrotic syndrome. Eur. J. Endocrinol. 2001, 145, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Wasilewska, A.; Tomaszewska, B.; Zoch-Zwierz, W.; Biernacka, A.; Klewinowska, K.; Koput, A. Serum and urine leptin concentration in children with nephrotic syndrome. Pediatr. Nephrol. 2005, 20, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Buyan, N.; Ozkaya, O.; Bideci, A.; Soylemezoglu, O.; Cinaz, P.; Gonen, S.; Kalman, S.; Bakkaloglu, S.; Hasanoglu, E. Leptin, soluble leptin receptor, and transforming growth factor-beta1 levels in minimal change nephrotic syndrome. Pediatr. Nephrol. 2003, 18, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, J. The Association Between Serum/Plasma Leptin Levels and Obstructive Sleep Apnea Syndrome: A Meta-Analysis and Meta-Regression. Front. Endocrinol. 2021, 12, 696418. [Google Scholar] [CrossRef]
- Van Harmelen, V.; Reynisdottir, S.; Eriksson, P.; Thorne, A.; Hoffstedt, J.; Lonnqvist, F.; Arner, P. Leptin secretion from subcutaneous and visceral adipose tissue in women. Diabetes 1998, 47, 913–917. [Google Scholar] [CrossRef]
- Brignardello, J.; Morales, P.; Diaz, E.; Romero, J.; Brunser, O.; Gotteland, M. Pilot study: Alterations of intestinal microbiota in obese humans are not associated with colonic inflammation or disturbances of barrier function. Aliment. Pharmacol. Ther. 2010, 32, 1307–1314. [Google Scholar] [CrossRef]
- Dong, R.; Zhang, M.; Hu, Q.; Zheng, S.; Soh, A.; Zheng, Y.; Yuan, H. Galectin-3 as a novel biomarker for disease diagnosis and a target for therapy (Review). Int. J. Mol. Med. 2018, 41, 599–614. [Google Scholar] [CrossRef]
- Krautbauer, S.; Eisinger, K.; Hader, Y.; Buechler, C. Free fatty acids and IL-6 induce adipocyte galectin-3 which is increased in white and brown adipose tissues of obese mice. Cytokine 2014, 69, 263–271. [Google Scholar] [CrossRef]
- Weigert, J.; Neumeier, M.; Wanninger, J.; Bauer, S.; Farkas, S.; Scherer, M.N.; Schnitzbauer, A.; Schaffler, A.; Aslanidis, C.; Scholmerich, J.; et al. Serum galectin-3 is elevated in obesity and negatively correlates with glycosylated hemoglobin in type 2 diabetes. J. Clin. Endocrinol. Metab. 2010, 95, 1404–1411. [Google Scholar] [CrossRef] [PubMed]
- Hsu, D.K.; Dowling, C.A.; Jeng, K.C.; Chen, J.T.; Yang, R.Y.; Liu, F.T. Galectin-3 expression is induced in cirrhotic liver and hepatocellular carcinoma. Int. J. Cancer 1999, 81, 519–526. [Google Scholar] [CrossRef]
- Wanninger, J.; Weigert, J.; Wiest, R.; Bauer, S.; Karrasch, T.; Farkas, S.; Scherer, M.N.; Walter, R.; Weiss, T.S.; Hellerbrand, C.; et al. Systemic and hepatic vein galectin-3 are increased in patients with alcoholic liver cirrhosis and negatively correlate with liver function. Cytokine 2011, 55, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Henderson, N.C.; Mackinnon, A.C.; Farnworth, S.L.; Poirier, F.; Russo, F.P.; Iredale, J.P.; Haslett, C.; Simpson, K.J.; Sethi, T. Galectin-3 regulates myofibroblast activation and hepatic fibrosis. Proc. Natl. Acad. Sci. USA 2006, 103, 5060–5065. [Google Scholar] [CrossRef]
- Gudowska, M.; Gruszewska, E.; Cylwik, B.; Panasiuk, A.; Rogalska, M.; Flisiak, R.; Szmitkowski, M.; Chrostek, L. Galectin-3 Concentration in Liver Diseases. Ann. Clin. Lab. Sci. 2015, 45, 669–673. [Google Scholar]
- Li, Y.; Li, T.; Zhou, Z.; Xiao, Y. Emerging roles of Galectin-3 in diabetes and diabetes complications: A snapshot. Rev. Endocr. Metab. Disord. 2022, 23, 569–577. [Google Scholar] [CrossRef]
- Boutin, L.; Depret, F.; Gayat, E.; Legrand, M.; Chadjichristos, C.E. Galectin-3 in Kidney Diseases: From an Old Protein to a New Therapeutic Target. Int. J. Mol. Sci. 2022, 23, 3124. [Google Scholar] [CrossRef]
- Jeftic, I.; Jovicic, N.; Pantic, J.; Arsenijevic, N.; Lukic, M.L.; Pejnovic, N. Galectin-3 Ablation Enhances Liver Steatosis, but Attenuates Inflammation and IL-33-Dependent Fibrosis in Obesogenic Mouse Model of Nonalcoholic Steatohepatitis. Mol. Med. 2015, 21, 453–465. [Google Scholar] [CrossRef]
- An, Y.; Xu, S.; Liu, Y.; Xu, X.; Philips, C.A.; Chen, J.; Mendez-Sanchez, N.; Guo, X.; Qi, X. Role of Galectins in the Liver Diseases: A Systematic Review and Meta-Analysis. Front. Med. 2021, 8, 744518. [Google Scholar] [CrossRef]
- Ou, S.M.; Tsai, M.T.; Chen, H.Y.; Li, F.A.; Lee, K.H.; Tseng, W.C.; Chang, F.P.; Lin, Y.P.; Yang, R.B.; Tarng, D.C. Urinary Galectin-3 as a Novel Biomarker for the Prediction of Renal Fibrosis and Kidney Disease Progression. Biomedicines 2022, 10, 585. [Google Scholar] [CrossRef]
- Meijers, W.C.; Schroten, N.F.; Ruifrok, W.P.; Assa, S.; Dokter, M.M.; Damman, K.; Gansevoort, R.T.; Van Gilst, W.H.; Sillje, H.H.; De Boer, R.A. Urinary and plasma galectin-3 in heart failure—Insights in renal handling. Eur. Heart J. 2013, 34, 782. [Google Scholar] [CrossRef]
- Ahmad, T.; Rao, V.; Chunara, Z.; Mahoney, D.; Jackson, K.; Hodson, D.; Tarleton, C.; Thomas, D.; Chen, M.; Jacoby, D.; et al. Urine Galectin-3 Levels Identify High Risk Renal Dysfunction in Patients with Heart Failure. J. Card. Fail. 2017, 23, S32. [Google Scholar] [CrossRef]
- Balasubramanian, K.; Vasudevamurthy, R.; Venkateshaiah, S.U.; Thomas, A.; Vishweshwara, A.; Dharmesh, S.M. Galectin-3 in urine of cancer patients: Stage and tissue specificity. J. Cancer Res. Clin. Oncol. 2009, 135, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, M.; Gajovic, N.; Zdravkovic, N.; Jovanovic, M.; Jurisevic, M.; Vojvodic, D.; Maric, V.; Arsenijevic, A.; Jovanovic, I. Fecal Galectin-3: A New Promising Biomarker for Severity and Progression of Colorectal Carcinoma. Mediat. Inflamm. 2018, 2018, 8031328. [Google Scholar] [CrossRef] [PubMed]
- Volarevic, V.; Zdravkovic, N.; Harrell, C.R.; Arsenijevic, N.; Fellabaum, C.; Djonov, V.; Lukic, M.L.; Simovic Markovic, B. Galectin-3 Regulates Indoleamine-2,3-dioxygenase-Dependent Cross-Talk between Colon-Infiltrating Dendritic Cells and T Regulatory Cells and May Represent a Valuable Biomarker for Monitoring the Progression of Ulcerative Colitis. Cells 2019, 8, 709. [Google Scholar] [CrossRef] [PubMed]
- Lippert, E.; Stieber-Gunckel, M.; Dunger, N.; Falk, W.; Obermeier, F.; Kunst, C. Galectin-3 Modulates Experimental Colitis. Digestion 2015, 92, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, M.; Simovic Markovic, B.; Gajovic, N.; Jurisevic, M.; Djukic, A.; Jovanovic, I.; Arsenijevic, N.; Lukic, A.; Zdravkovic, N. Metabolic syndrome attenuates ulcerative colitis: Correlation with interleukin-10 and galectin-3 expression. World J. Gastroenterol. 2019, 25, 6465–6482. [Google Scholar] [CrossRef] [PubMed]
- Akamata, K.; Asano, Y.; Taniguchi, T.; Yamashita, T.; Saigusa, R.; Nakamura, K.; Noda, S.; Aozasa, N.; Toyama, T.; Takahashi, T.; et al. Increased expression of chemerin in endothelial cells due to Fli1 deficiency may contribute to the development of digital ulcers in systemic sclerosis. Rheumatology 2015, 54, 1308–1316. [Google Scholar] [CrossRef]
- Chua, S.K.; Shyu, K.G.; Lin, Y.F.; Lo, H.M.; Wang, B.W.; Chang, H.; Lien, L.M. Tumor Necrosis Factor-Alpha and the ERK Pathway Drive Chemerin Expression in Response to Hypoxia in Cultured Human Coronary Artery Endothelial Cells. PLoS ONE 2016, 11, e0165613. [Google Scholar] [CrossRef]
- Weigert, J.; Neumeier, M.; Wanninger, J.; Filarsky, M.; Bauer, S.; Wiest, R.; Farkas, S.; Scherer, M.N.; Schaffler, A.; Aslanidis, C.; et al. Systemic chemerin is related to inflammation rather than obesity in type 2 diabetes. Clin. Endocrinol. 2010, 72, 342–348. [Google Scholar] [CrossRef]
- Ferland, D.J.; Watts, S.W. Chemerin: A comprehensive review elucidating the need for cardiovascular research. Pharmacol. Res. 2015, 99, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Rourke, J.L.; Dranse, H.J.; Sinal, C.J. Towards an integrative approach to understanding the role of chemerin in human health and disease. Obes. Rev. 2013, 14, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Bondue, B.; Wittamer, V.; Parmentier, M. Chemerin and its receptors in leukocyte trafficking, inflammation and metabolism. Cytokine Growth Factor. Rev. 2011, 22, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Mariani, F.; Roncucci, L. Chemerin/chemR23 axis in inflammation onset and resolution. Inflamm. Res. 2015, 64, 85–95. [Google Scholar] [CrossRef]
- Goralski, K.B.; Jackson, A.E.; McKeown, B.T.; Sinal, C.J. More Than an Adipokine: The Complex Roles of Chemerin Signaling in Cancer. Int. J. Mol. Sci. 2019, 20, 4778. [Google Scholar] [CrossRef]
- Treeck, O.; Buechler, C.; Ortmann, O. Chemerin and Cancer. Int. J. Mol. Sci. 2019, 20, 3750. [Google Scholar] [CrossRef]
- Jacenik, D.; Fichna, J. Chemerin in immune response and gastrointestinal pathophysiology. Clin. Chim. Acta 2020, 504, 146–153. [Google Scholar] [CrossRef]
- Horn, P.; von Loeffelholz, C.; Forkert, F.; Stengel, S.; Reuken, P.; Aschenbach, R.; Stallmach, A.; Bruns, T. Low circulating chemerin levels correlate with hepatic dysfunction and increased mortality in decompensated liver cirrhosis. Sci. Rep. 2018, 8, 9242. [Google Scholar] [CrossRef]
- Peschel, G.; Grimm, J.; Gulow, K.; Muller, M.; Buechler, C.; Weigand, K. Chemerin Is a Valuable Biomarker in Patients with HCV Infection and Correlates with Liver Injury. Diagnostics 2020, 10, 974. [Google Scholar] [CrossRef]
- Bonomini, M.; Pandolfi, A. Chemerin in renal dysfunction and cardiovascular disease. Vascul Pharmacol. 2016, 77, 28–34. [Google Scholar] [CrossRef]
- Abais-Battad, J.M.; Lund, H.; Fehrenbach, D.J.; Dasinger, J.H.; Alsheikh, A.J.; Mattson, D.L. Parental Dietary Protein Source and the Role of CMKLR1 in Determining the Severity of Dahl Salt-Sensitive Hypertension. Hypertension 2019, 73, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Gunawan, S.; Elger, T.; Loibl, J.; Fererberger, T.; Sommersberger, S.; Kandulski, A.; Muller, M.; Tews, H.C.; Buechler, C. Urinary chemerin as a potential biomarker for inflammatory bowel disease. Front. Med. 2022, 9, 1058108. [Google Scholar] [CrossRef]
- Mocker, A.; Hilgers, K.F.; Cordasic, N.; Wachtveitl, R.; Menendez-Castro, C.; Woelfle, J.; Hartner, A.; Fahlbusch, F.B. Renal Chemerin Expression is Induced in Models of Hypertensive Nephropathy and Glomerulonephritis and Correlates with Markers of Inflammation and Fibrosis. Int. J. Mol. Sci. 2019, 20, 6240. [Google Scholar] [CrossRef]
- Levey, A.S.; Perrone, R.D.; Madias, N.E. Serum Creatinine and Renal-Function. Annu. Rev. Med. 1988, 39, 465–490. [Google Scholar] [CrossRef]
- Wang, T.; He, C.; Yu, X. Pro-Inflammatory Cytokines: New Potential Therapeutic Targets for Obesity-Related Bone Disorders. Curr. Drug. Targets 2017, 18, 1664–1675. [Google Scholar] [CrossRef] [PubMed]
- Nilsonne, G.; Lekander, M.; Akerstedt, T.; Axelsson, J.; Ingre, M. Diurnal Variation of Circulating Interleukin-6 in Humans: A Meta-Analysis. PLoS ONE 2016, 11, e0165799. [Google Scholar] [CrossRef] [PubMed]
- Sothern, R.B.; Roitman-Johnson, B.; Kanabrocki, E.L.; Yager, J.G.; Fuerstenberg, R.K.; Weatherbee, J.A.; Young, M.R.; Nemchausky, B.M.; Scheving, L.E. Circadian characteristics of interleukin-6 in blood and urine of clinically healthy men. In Vivo 1995, 9, 331–339. [Google Scholar]
- Yao, X.; Huang, J.; Zhong, H.; Shen, N.; Faggioni, R.; Fung, M.; Yao, Y. Targeting interleukin-6 in inflammatory autoimmune diseases and cancers. Pharmacol. Ther. 2014, 141, 125–139. [Google Scholar] [CrossRef]
- Kumari, N.; Dwarakanath, B.S.; Das, A.; Bhatt, A.N. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumour Biol. 2016, 37, 11553–11572. [Google Scholar] [CrossRef]
- Lowin, T.; Straub, R.H. Synovial fibroblasts integrate inflammatory and neuroendocrine stimuli to drive rheumatoid arthritis. Expert. Rev. Clin. Immunol. 2015, 11, 1069–1071. [Google Scholar] [CrossRef]
- Herrmann, M.; Scholmerich, J.; Straub, R.H. Stress and rheumatic diseases. Rheum. Dis. Clin. North. Am. 2000, 26, 737–763. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Yoo, S.A.; Kim, G.R.; Cho, C.S.; Kim, W.U. Urinary interleukin-6 as a predictor of radiographic progression in rheumatoid arthritis: A 3-year evaluation. Sci. Rep. 2016, 6, 35242. [Google Scholar] [CrossRef] [PubMed]
- Benson, M.; Jodal, U.; Andreasson, A.; Karlsson, A.; Rydberg, J.; Svanborg, C. Interleukin 6 response to urinary tract infection in childhood. Pediatr. Infect. Dis. J. 1994, 13, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.; Ahmadzadeh, H.; Toloui, A.; Ahmadzadeh, K.; Madani Neishaboori, A.; Rafiei Alavi, S.N.; Gubari, M.I.M.; Jones, M.E.; Ataei, F.; Yousefifard, M.; et al. The value of interleukin levels in the diagnosis of febrile urinary tract infections in children and adolescents; a systematic review and meta-analysis. J. Pediatr. Urol. 2022, 18, 211–223. [Google Scholar] [CrossRef]
- Otto, G.; Braconier, J.; Andreasson, A.; Svanborg, C. Interleukin-6 and disease severity in patients with bacteremic and nonbacteremic febrile urinary tract infection. J. Infect. Dis. 1999, 179, 172–179. [Google Scholar] [CrossRef]
- Shores, D.R.; Everett, A.D. Children as Biomarker Orphans: Progress in the Field of Pediatric Biomarkers. J. Pediatr. 2018, 193, 14–20.e31. [Google Scholar] [CrossRef]
- Peterson, E.; Robertson, A.D.; Emlen, W. Serum and urinary interleukin-6 in systemic lupus erythematosus. Lupus 1996, 5, 571–575. [Google Scholar] [CrossRef]
- Dima, A.; Jurcut, C.; Balanescu, P.; Balanescu, E.; Badea, C.; Caraiola, S.; Miler, I.; Ramba, D.; Ionescu, R.; Baicus, C.; et al. Clinical significance of serum and urinary interleukin-6 in systemic lupus erythematosus patients. Egypt. Rheumatol. 2017, 39, 1–6. [Google Scholar] [CrossRef]
- El-Shereef, R.R.; Lotfi, A.; Abdel-Naeam, E.A.; Tawfik, H. Serum and Urinary Interleukin-6 in Assessment of Renal Activity in Egyptian Patients with Systemic Lupus Erythematosus. Clin. Med. Insights Arthritis Musculoskelet Disord. 2016, 9, 29–36. [Google Scholar] [CrossRef]
- Horii, Y.; Iwano, M.; Hirata, E.; Shiiki, M.; Fujii, Y.; Dohi, K.; Ishikawa, H. Role of interleukin-6 in the progression of mesangial proliferative glomerulonephritis. Kidney Int. Suppl. 1993, 39, S71–S75. [Google Scholar]
- Kalliakmani, P.; Nakopoulou, L.; Tsakas, S.; Gerolymos, M.; Papasotiriou, M.; Goumenos, D.S. Urinary interleukin-6 (IL-6) and transforming growth factor (TGF-beta) levels in corticosteroidtreated patients with IgA nephropathy. Clin. Nephrol. 2011, 76, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Cove-Smith, A.; Hendry, B.M. The regulation of mesangial cell proliferation. Nephron Exp. Nephrol. 2008, 108, e74–e79. [Google Scholar] [CrossRef] [PubMed]
- Hirata, E.; Iwano, M.; Hirayama, T.; Horii, Y.; Kitamura, Y.; Kishimoto, T.; Hanatani, M.; Dohi, K. Rapid measurement of urinary IL-6 by ELISA: Urinary IL-6 as a marker of mesangial proliferation. Jpn. J. Nephrol. 1994, 36, 33–37. [Google Scholar]
- Dohi, K.; Iwano, M.; Muraguchi, A.; Horii, Y.; Hirayama, T.; Ogawa, S.; Shiiki, H.; Hirano, T.; Kishimoto, T.; Ishikawa, H. The prognostic significance of urinary interleukin 6 in IgA nephropathy. Clin. Nephrol. 1991, 35, 1–5. [Google Scholar] [PubMed]
- Maniwa, K.; Ogushi, F.; Haku, T.; Sone, S.; Ohmoto, Y. Sarcoidosis associated with acute renal failure and increased levels of interleukin-6 in urine. Intern. Med. 1998, 37, 757–761. [Google Scholar] [CrossRef]
- Shikano, M.; Sobajima, H.; Yoshikawa, H.; Toba, T.; Kushimoto, H.; Katsumata, H.; Tomita, M.; Kawashima, S. Usefulness of a highly sensitive urinary and serum IL-6 assay in patients with diabetic nephropathy. Nephron 2000, 85, 81–85. [Google Scholar] [CrossRef]
- Zubair, H.; Azim, S.; Maluf, D.G.; Mas, V.R.; Martins, P.N. Contribution of Proteomics in Transplantation: Identification of Injury and Rejection Markers. Transplantation, 2023; e004542, online ahead of print. [Google Scholar] [CrossRef]
- Casiraghi, F.; Ruggenenti, P.; Noris, M.; Locatelli, G.; Perico, N.; Perna, A.; Remuzzi, G. Sequential monitoring of urine-soluble interleukin 2 receptor and interleukin 6 predicts acute rejection of human renal allografts before clinical or laboratory signs of renal dysfunction. Transplantation 1997, 63, 1508–1514. [Google Scholar] [CrossRef]
- Chen, S.M.; Lin, C.P.; Tsai, J.D.; Chao, Y.H.; Sheu, J.N. The significance of serum and fecal levels of interleukin-6 and interleukin-8 in hospitalized children with acute rotavirus and norovirus gastroenteritis. Pediatr. Neonatol. 2014, 55, 120–126. [Google Scholar] [CrossRef]
- Zaki, M.E.S.; Alsayed, M.A.L.; Shrief, R. Study of the diagnostic value of interleukin-6 and interleukin-8 in children with acute gastroenteritis. Germs 2020, 10, 27–33. [Google Scholar] [CrossRef]
- Riva, A.; Gray, E.H.; Azarian, S.; Zamalloa, A.; McPhail, M.J.W.; Vincent, R.P.; Williams, R.; Chokshi, S.; Patel, V.C.; Edwards, L.A. Faecal cytokine profiling as a marker of intestinal inflammation in acutely decompensated cirrhosis. JHEP Rep. 2020, 2, 100151. [Google Scholar] [CrossRef]
- Bozaoglu, K.; Bolton, K.; McMillan, J.; Zimmet, P.; Jowett, J.; Collier, G.; Walder, K.; Segal, D. Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology 2007, 148, 4687–4694. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Li, C.; Shen, L.; Wang, S.; Li, X.; Fu, C.; Li, T.; Liu, B.; Gu, Y.; Wang, W.; et al. The Intestinal Effect of Atorvastatin: Akkermansia muciniphila and Barrier Function. Front. Microbiol. 2021, 12, 797062. [Google Scholar] [CrossRef] [PubMed]


| Disease | Urinary/Fecal Biomarkers |
|---|---|
| Cancer | Urinary Galectin-3 ⇑ |
| Cardiovascular Disease | Urinary Adiponectin ⇑ Urinary Lipocalin-2 ⇑ |
| Colorectal Carcinoma | Fecal Adiponectin ⇑ Fecal Galectin-3 ⇑ |
| Decompensated Liver Cirrhosis | Fecal IL-6 ⇑ |
| Diabetic Kidney Disease | Urinary Adiponectin ⇑ Urinary IL-6 ⇑ Urinary Lipocalin-2 ⇑ |
| Focal Segmental Glomerulosclerosis | Urinary Adiponectin ⇑ |
| Gastroenteritis | Fecal IL-6 ⇑ |
| Heart Failure | Urinary Galectin-3 ⇑ |
| IgA Nephropathy | Urinary IL-6 ⇑ |
| Infectious Enterocolitis | Fecal Lipocalin-2 ⇑ |
| Inflammatory Bowel Disease | Urinary Chemerin ⇑ Fecal Galectin-3 ⇓ Fecal Lipocalin-2 ⇑ Urinary Lipocalin-2 ⇑ |
| Irritable Bowel Disease | Fecal Lipocalin-2 ⇑ |
| Kidney Fibrosis | Urinary Galectin-3 ⇑ |
| Kidney Graft Dysfunction | Urinary IL-6 ⇑ Urinary Lipocalin-2 ⇑ |
| Liver Cirrhosis-Related Kidney Dysfunction | Urinary Lipocalin-2 ⇑ |
| Lupus Nephritis | Urinary Adiponectin ⇑ Urinary IL-6 ⇑ Urinary Lipocalin-2 ⇑ |
| Necrotizing Enterocolitis | Fecal Lipocalin-2 ⇑ |
| Nephrotic Syndrome | Urinary Leptin ⇑ |
| Obstructive Sleep Apnea Syndrome | Urinary Leptin ⇓ |
| Pancreatic Diseases | Urinary Lipocalin-2 ⇑ |
| Proteinuria | Urinary Leptin ⇑ |
| Rheumatoid Arthritis | Urinary IL-6 ⇑ |
| Urinary Tract Infection | Urinary IL-6 ⇑ Urinary Leptin ⇑ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tews, H.C.; Elger, T.; Grewal, T.; Weidlich, S.; Vitali, F.; Buechler, C. Fecal and Urinary Adipokines as Disease Biomarkers. Biomedicines 2023, 11, 1186. https://doi.org/10.3390/biomedicines11041186
Tews HC, Elger T, Grewal T, Weidlich S, Vitali F, Buechler C. Fecal and Urinary Adipokines as Disease Biomarkers. Biomedicines. 2023; 11(4):1186. https://doi.org/10.3390/biomedicines11041186
Chicago/Turabian StyleTews, Hauke C., Tanja Elger, Thomas Grewal, Simon Weidlich, Francesco Vitali, and Christa Buechler. 2023. "Fecal and Urinary Adipokines as Disease Biomarkers" Biomedicines 11, no. 4: 1186. https://doi.org/10.3390/biomedicines11041186
APA StyleTews, H. C., Elger, T., Grewal, T., Weidlich, S., Vitali, F., & Buechler, C. (2023). Fecal and Urinary Adipokines as Disease Biomarkers. Biomedicines, 11(4), 1186. https://doi.org/10.3390/biomedicines11041186








